Abstract
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in recent years not only caused a global pandemic but resulted in enormous social, economic, and health burdens worldwide. Despite considerable efforts to combat coronavirus disease 2019 (COVID-19), various SARS-CoV-2 variants have emerged, and their underlying mechanisms of pathogenicity remain largely unknown. Furthermore, effective therapeutic drugs are still under development. Thus, an ideal animal model is crucial for studying the pathogenesis of COVID-19 and for the preclinical evaluation of vaccines and antivirals against SARS-CoV-2 and variant infections. Currently, several animal models, including mice, hamsters, ferrets, and non-human primates (NHPs), have been established to study COVID-19. Among them, ferrets are naturally susceptible to SARS-CoV-2 infection and are considered suitable for COVID-19 study. Here, we summarize recent developments and application of SARS-CoV-2 ferret models in studies on pathogenesis, therapeutic agents, and vaccines, and provide a perspective on the role of these models in preventing COVID-19 spread.
Keywords: SARS-CoV-2, COVID-19, Animal models, Ferret, Angiotensin-converting enzyme 2 (ACE2) receptor
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), emerged in Wuhan, Hubei Province, China, in December 2019, and spread globally thereafter (Bedford et al., 2020; Zhu et al., 2020). Today, COVID-19 remains a serious threat to human health, with over 600 million confirmed cases and 6.4 million deaths across nearly 200 countries as of 1 September 2022 (https://covid19.who.int). SARS-CoV-2 infection is characterized by respiratory disease ranging in severity from mild upper respiratory tract illness to acute respiratory distress syndrome (ARDS) and severe interstitial pneumonia, leading to potential multiple organ failure and death in severely ill patients (Wang et al., 2020a). In addition to respiratory symptoms, SARS-CoV-2 can also cause myocarditis, thromboembolism, liver dysfunction, sepsis, and anosmia (Wiersinga et al., 2020). Given the large number of mutations, multiple SARS-CoV-2 variants, including variants of interest (VOIs) and concern (VOC), have challenged global efforts to control COVID-19 due to their potential effects on viral transmissibility, epidemiology, virulence, and pathogenicity, as well as the reduced effectiveness of COVID-19 vaccines (immune escape) (Beavis et al., 2022; Sun et al., 2022a). Thus, understanding the pathogenicity of SARS-CoV-2 is necessary to develop novel antiviral drugs and therapeutic strategies against COVID-19.
Establishing an ideal animal model for COVID-19 is of great significance for clarifying the pathogenic mechanisms of disease and testing the efficacy of vaccines and antiviral agents. To date, mice, hamsters, ferrets, minks, tree shrews, and several non-human primates (NHPs) have been used to study SARS-CoV-2 infection (Ma et al., 2022; Xu et al., 2020). Among them, ferrets are naturally susceptible to SARS-CoV-2 and have been widely used in respiratory virus research (Kim et al., 2020; Wong et al., 2019). Thus, in this review, we discuss ferret models of COVID-19 and their ability to mimic natural COVID-19 infection and pathogenesis, thereby providing relevant information for further preclinical research and testing of COVID-19 vaccines and antiviral agents.
SARS-CoV-2 AND HOST CELL RECEPTORS
SARS-CoV-2 is an enveloped and pleomorphic virus belonging to the β-coronavirus genus, with round or oval viral particles about 60–140 nm in diameter (Zhu et al., 2020). The virus contains a single positive-sense RNA genome, which shares 79% and 50% sequence homology with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), respectively (Zhou et al., 2020). The spike (S) protein is a vital protein located on the surface of the virus envelope and can be cleaved into S1 and S2 subunits by host cell transmembrane serine protease 2 (TMPRSS2) (Baughn et al., 2020). Subunit S1 is involved in host receptor binding, while subunit S2 participates in membrane fusion to promote viral entry into host cells by endocytosis. After entry, virus RNA is subsequently released into the cytoplasm to begin its life cycle and produce more virus particles (Li, 2016; Walls et al., 2020).
Similar to SARS-CoV, SARS-CoV-2 enters host cells primarily by targeting the human angiotensin I converting enzyme 2 (ACE2) receptor. The affinity of the S protein to ACE2 is 10–20 times stronger than that of SARS-CoV, which may be responsible for its high infectivity (Wrapp et al., 2020). Single-cell RNA sequencing has shown that ACE2 is mainly expressed in type Ⅱ alveolar epithelial cells (AT2) (Zhao et al., 2020). About 83% of cells expressing ACE2 in lung tissue are AT2 cells, suggesting that SARS-CoV-2 mainly infects the lower respiratory tract. However, in addition to respiratory epithelial cells, ACE2 is also highly expressed in cardiomyocytes, renal proximal convoluted tubule epithelial cells, bladder epithelial cells, and esophageal, ileal, and Leydig cells, making these tissues and organs permissible to SARS-COV-2 infection (Hamming et al., 2004; He et al., 2020; Liu et al., 2020).
SARS-CoV-2 ANIMAL MODELS
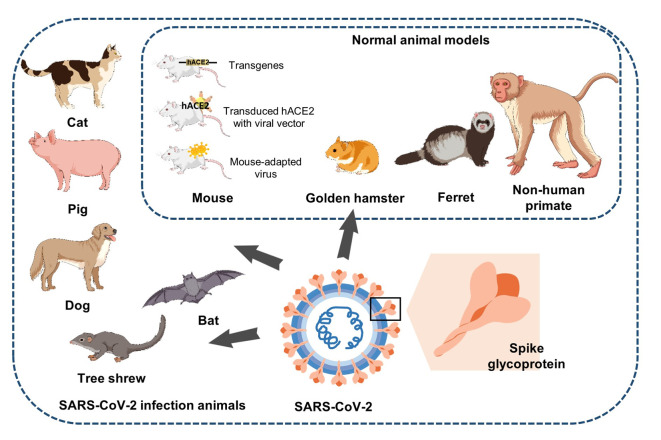
Animal models play a central role in COVID-19 research. SARS-CoV-2 can recognize ACE2 from transgenic mice, hamsters, rats, pigs, ferrets, and cats, suggesting their potential as animal models of SARS-CoV-2 infection. NHP models can accurately simulate the pathogenesis of human COVID-19 and are ideal models for studying SARS-CoV-2. Various species and transgenic animal models have been developed and used to facilitate the testing of vaccines and therapeutics for the prevention and treatment of COVID-19 (Lin et al., 2022) (Figure 1; Table 1).
Figure 1.
Animal models of SARS-CoV-2
Mice, golden hamsters, ferrets, and NHPs are often used to study pathogenesis, transmission, and countermeasure evaluation. Other animals, such as cats, pigs, dogs, and bats, can also be infected with SARS-CoV-2.
Table 1. Comparison of commonly used animal models of SARS-CoV-2.
| Animal model | Pathology | Application | Advantages | Disadvantages |
| Transgenic mouse (Bao et al., 2020a; Bao et al., 2020b; Jiang et al., 2020; Sun et al., 2020b) | Moderate interstitial pneumonia; infiltration of inflammatory cells; weight loss; immune factors can be detected | Drug screening and vaccine evaluation. | Expressing hACE2; susceptible to SARS-CoV-2. | High cost; long breeding time; development of few cases with severe symptoms or death. |
| Non-transgenic mouse (Israelow et al., 2020; Rathnasinghe et al., 2020; Sun et al., 2020a) | Inflammatory response develops in lungs; weight loss; immune factors can be detected. | Drug screening and vaccine evaluation | Simple to construct; easy to repeat; suitable for widespread popularization | High cost; few subjects develop severe symptoms or death |
| Hamster (Chan et al., 2020; Imai et al., 2020; Sia et al., 2020) | Weight loss; severe lung injury, including severe, bilateral, peripheral, multi-lobular ground glass opacity and lung consolidation. | Study of infection and transmission routes. | Low cost; susceptible to SARS-CoV-2; symptoms and manifestations are very similar to that in human infection | Few subjects develop severe symptoms or death |
| Ferret (Kim et al., 2020; Pulit-Penaloza et al., 2022; Ryan et al., 2021) | Acute bronchiolitis in lungs; obvious virus replication; rise in temperature. | Drug screening, vaccine evaluation, and study of immune responses | Anatomical structure of upper and lower respiratory tracts of ferrets is very similar to that of humans; susceptible to SARS-CoV-2 | Showing mild clinical symptoms; virus titer in lungs is relatively low |
| NHPs (Rockx et al., 2020; Shan et al., 2020; Song et al., 2020; Wang et al., 2020b; Yu et al., 2020) | Typical interstitial pneumonia; diffuse alveolar damage; immune factors can be detected | Drug screening and vaccine evaluation. | Very close genetic relationship with humans, with similar anatomy, physiology, and pathology | High cost; difficult operation; small number of subjects. |
FERRET MODEL OF SARS-CoV-2
The domestic ferret (Mustela putorius furo) is a popular animal model for evaluating viral pathogenesis and transmission as well as the efficacy of antiviral agents (Schiffman et al., 2022). Given their similar anatomical structure and physiology to the human respiratory system and their ability to cough and sneeze, ferrets are considered a suitable model for studying respiratory virus transmission and pathogenesis (Sun et al., 2010). Ferrets are highly susceptible to the influenza virus, and exhibit many of the clinical symptoms observed in humans following infection. As such, they are an excellent animal model for evaluating the pathogenesis and transmission of influenza viruses as well as testing countermeasure efficacy (Belser et al., 2020; Si et al., 2022). Ferrets are also valuable models for other respiratory viruses, including human respiratory syncytial virus (Prince and Porter, 1976; Stittelaar et al., 2016) and SARS-CoV (Martina et al., 2003; Van Den Brand et al., 2008). They have also been used to characterize filovirus infections, showing high susceptibility to lethal diseases caused by Ebola, Sudan, Bundibugyo, and Reston viruses and recapitulating many aspects of human filovirus disease, including systemic viral replication, coagulation abnormalities, and dysregulated immune response (Schiffman et al., 2022). In addition, their relatively small size and availability make ferrets an ideal choice for countermeasure evaluation and pathogenesis modeling of certain viruses.
Ferrets have also been shown to support SARS-CoV-2 infection and have been used extensively in COVID-19 research, including transmission, pathogenesis, and treatment.
Ferrets and transmission of SARS-CoV-2
Ferrets provide an ideal model to study SARS-CoV-2 transmission. Richard et al. (2020) provided the first empirical evidence of SARS-CoV-2 transmission via direct contact and respiratory droplets/aerosols among exposed ferrets, as evidenced by prolonged viral shedding and the occurrence of infectious virus in secondary recipient animals. This discovery aligns with the outcomes of other research, such as Kim et al. (2020) and Kutter et al. (2021), who documented airborne transmission of SARS-CoV-2 between ferrets over more than 1 m. However, Sawatzki et al. (2021) reported that ferrets may have a host barrier that limits natural infection and transmission after finding no evidence of infection in 29 ferrets constantly exposed to one confirmed and one suspected case of symptomatic COVID-19. Furthermore, based on genetic sequences of viruses and host, Lehtinen et al. (2022) suggested that ferrets may possess genetic factors that confer resistance to natural SARS-CoV-2 infection. Patel et al. (2021) demonstrated that ferrets are semi-permissive to SARS-CoV-2 USA-WA1/2020 isolate infection, showing efficient transmission via direct contact but poor transmission by respiratory droplets. Zhou et al. (2021) found that D614G substitution in the S protein markedly increases replication and transmissibility in hamster and ferret models of SARS-CoV-2. In addition, Ulrich et al. (2022) reported that the SARS-CoV-2 Alpha variant exhibits a clear replication advantage over wt-S614G and is easily transmitted among ferrets. Peacock et al. (2021) found that, unlike wild-type virus, SARS-CoV-2 lacking the S1/S2 furin cleavage site sheds at lower titers from infected ferrets and is not transmitted to co-housed sentinel animals. Based on the ferret model, they also revealed that the furin cleavage site is an important determinant of SARS-CoV-2 transmission.
Several inhibitors also appear to prevent SARS-CoV-2 transmission in ferret models (Cox et al., 2021b). For example, twice daily treatment of infected ferrets with MK-4482/EIDD-2801, an orally efficacious ribonucleoside analog inhibitor of influenza virus, significantly reduces SARS-CoV-2 load in the upper respiratory tract and suppresses transmission to untreated contact animals (Cox et al., 2021b; Toots et al., 2019). Oral remdesivir parent prodrug GS-441524 shows efficacious effects against SARS-CoV-2 in ferrets by blocking viral replication and preventing transmission to untreated contact animals (Cox et al., 2021a). Daily intranasal administration of lipopeptide fusion inhibitors, which block membrane fusion between the virus and host cell, appears to prevent direct contact transmission of SARS-CoV-2 during 24 h co-housing with infected animals under stringent conditions that result in the infection of 100% of untreated animals (De Vries et al., 2021). Thus, ferrets may serve as sensitive animal models to study the transmission dynamics and molecular basis of SARS-CoV-2 transmissibility, as well as interventions aimed at preventing viral transmission.
SARS-CoV-2 pathogenesis in ferrets
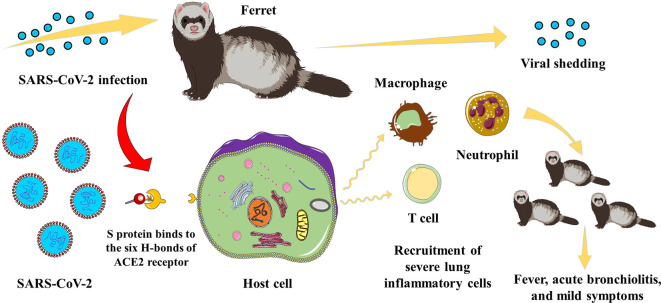
Ferrets are also good animal models for exploring the pathogenesis of COVID-19 (Figure 2). A recent study proposed a novel bioinformatics framework to systematically trace animals susceptible to SARS-CoV-2 and predict the binding affinities between mutated/un-mutated ACE2 receptors of susceptible animals. The researchers discovered that the ACE2 gene in ferrets is phylogenetically identical to that in humans, and their ACE2 protein forms six hydrogen bonds with the SARS-CoV-2 S protein, creating a strong binding force (Sun et al., 2022b) (Figure 2). Shi et al. (2020) found that SARS-CoV-2 can efficiently infect the upper respiratory tract of ferrets for up to 8 days without causing severe disease or death, with all ferrets developing SARS-CoV-2-specific antibodies. The features of SARS-CoV-2 replication and pathogenesis in ferrets makes them an ideal candidate animal model for evaluating the efficacy of antiviral drugs or vaccines against COVID-19 (Shi et al., 2020) (Figure 2). Similarly, Kim et al. (2020) found that ferrets are susceptible to SARS-CoV-2 infection and exhibit elevated body temperatures and acute bronchiolitis (Figure 2), with viral shedding in nasal, saliva, urine, and fecal samples up to 8 days post-infection (dpi). Furthermore, ferrets can effectively transmit the virus to naïve ferrets by direct or indirect contact, as evidenced by the detection of SARS-CoV-2 in all naïve direct contact ferrets 2 days after exposure and in several naïve indirect contact ferrets, suggesting that the virus can be transmitted via the air (Kim et al., 2020). Analysis of age-related disease severity, as observed in COVID-19 patients, also indicates that all ferrets can be infected by SARS-CoV-2 regardless of age, but aged ferrets (≥3 years old) exhibit higher viral loads, longer nasal virus shedding, and more severe lung inflammatory cell infiltration (Figure 2) and clinical symptoms compared to juveniles (≤6 months) and young adults (1–2 years) (Richard et al., 2020). Therefore, age is a critical factor in COVID-19 severity. Although no neurological symptoms or fatalities have been observed in SARS-CoV-2-infected ferrets, various clinical signs and modes of transmission found in COVID-19 patients are also observed in ferrets, indicating that ferrets represent an ideal animal model that should facilitate SARS-CoV-2 research.
Figure 2.
Schematic of pathogenesis in SARS-CoV-2-infected ferret
After SARS-CoV-2 infection in the ferret, the S protein binds to the six H-bonds of the host cell ACE2 receptor. Infected cells are triggered to recruit severe lung inflammatory cells, causing fever, acute bronchiolitis, and other mild symptoms. Aged infected ferrets also show longer nasal viral shedding.
Many studies have also suggested that ferrets are suitable animal models for asymptomatic or mild SARS-CoV-2 infection (Au et al., 2022; Everett et al., 2021; Van De Ven et al., 2021). Notably, intranasal SARS-CoV-2 challenge in ferrets does not manifest in overt clinical symptoms but does generate productive infection within the nasal turbinate mucosae, as evidenced by the detection of viral RNA in nasal wash and throat swab samples (Everett et al., 2021). Antibody responses to the S protein and nucleoprotein can be observed from 21 dpi, despite low virus neutralizing activity. Although low levels of viral RNA can also be detected in the fur of some ferrets, infectious virus cannot be re-isolated, highlighting the potential importance of indirect means of transmission, as observed in clinical settings (Everett et al., 2021). Ferrets can also serve as valuable animal models for studying asymptomatic SARS-CoV-2 infection. Au et al. (2022) conducted a natural history/time course study of SARS-CoV-2 infection in ferrets to characterize and assess their suitability as an animal model. In brief, 10 ferrets of each sex were challenged intranasally with 4.64×104 TCID50 of SARS-CoV-2 isolate Australia/VIC01/2020 and monitored for clinical signs of disease and viral shedding, with tissues collected post-mortem for histopathological and virological assessment at set intervals. SARS-CoV-2 was found to replicate in the upper respiratory tract with consistent viral shedding in nasal wash samples and oral swab samples until 9 dpi. Infectious SARS-CoV-2 was recovered from nasal wash, oral swab, nasal turbinate, pharynx, and olfactory bulb samples within 3–7 dpi. However, only viral RNA was detected in samples collected from the trachea, lung, and parts of the gastrointestinal tract. Viral antigen was observed exclusively in nasal epithelium and associated sloughed cells and in draining lymph nodes upon immunohistochemical staining (Au et al., 2022). Van De Ven et al. (2021) also revealed that intranasally infected ferrets exhibit asymptomatic COVID-19 and possibly aspects of long-COVID. Therefore, ferrets can be experimentally infected with SARS-CoV-2 to model human asymptomatic infection. All infected ferrets develop antibodies against SARS-CoV-2 (Shi et al., 2020) and re-challenge of recovered ferrets shows reduced upper respiratory tract viral shedding and transmission as well as lung pathology (Kim et al., 2021c). Transcriptome analysis of aged ferret lungs revealed strong enrichment in gene sets related to type I interferon, activated T cells, and M1 macrophage responses (Figure 2), suggesting that the severity of COVID-19 in aged population is linked with severe inflammatory response (Kim et al., 2022b). Francis et al. (2021) showed that older males may play a role in viral transmission due to decreased antiviral responses. Martins et al. (2022) also demonstrated that age can affect susceptibility of ferrets to SARS-CoV-2 and confirmed that aged ferrets are more likely to get infected when exposed to lower infectious doses than young ferrets, with enhanced viral replication in the upper respiratory tract as well as viral shedding. These findings suggest that ferrets can be used as a model for studying age-related SARS-CoV-2 infection dynamics and viral replication. Ferret models can also be used to evaluate metabolic profiling of minimally invasive biological samples collected from SARS-CoV-2-infected ferrets during viral shedding and post-shedding periods. Based on multivariate analysis, Beale et al. (2021) identified 29 significant metabolites and three lipids subjected to pathway enrichment and impact analysis. The presence of viral shedding coincided with the challenge dose administered and significant changes in the citric acid cycle, purine metabolism, and pentose phosphate pathways. An elevated immune response in the host was also observed between the two isolates studied. These results support other metabolomic-based findings in clinical observational studies and indicate the utility of metabolomics in ferrets for further COVID-19 research (Beale et al., 2021). Co-infection of ferrets with H1N1 and SARS-CoV-2 extends the clinical duration of COVID-19 and enhances pulmonary damage, but does not reduce viral shedding in throat swabs or viral loads in lungs (Bao et al., 2021). A dose titration study of SARS-CoV-2 in ferrets showed that high (5×106 plaque-forming units (pfu)) and medium dose (5×104 pfu) challenge can induce upper respiratory tract RNA shedding associated with lower respiratory tract viral RNA and lung pathology, while re-challenge of recovered ferrets shows reduced upper respiratory tract viral shedding and lung pathology (Ryan et al., 2021). Therefore, the use of such models may aid our understanding of SARS-CoV-2 pathogenesis and naturally acquired immunity.
Ferrets and countermeasure evaluation
The ferret immune system shares many similarities to that of humans. As such, ferrets have been widely used to test vaccines, therapeutics, and antivirals against SARS-CoV-2 (Table 2) (Pandey et al., 2021). A recently developed receptor-binding domain (RBD) protein-based vaccine candidate against SARS-CoV-2 using self-assembling bullfrog ferritin nanoparticles as an antigen delivery system was shown to induce potent neutralizing antibodies against SARS-CoV-2 in ferrets and provide efficient protection from virus challenge (Kim et al., 2021b). Additionally, single vaccination with Ad5-nCoV was found to protect ferrets from wild-type SARS-CoV-2 infection in the upper respiratory tract (Wu et al., 2020). Vaccination with ChAdOx1 nCoV-19, a replication-deficient simian adenoviral vector expressing codon-optimized full-length SARS-CoV-2 S protein, was also found to reduce both viral shedding and lung pathology in ferrets, with a second vaccination increasing antibody titers (Lambe et al., 2021; Marsh et al., 2021). Ferret models have also been used to evaluate the efficacy of protein-based subunit vaccines. Toll-like receptor (TLR)1/2 and TLR3 agonists (L-pampo) can be potent adjuvants for SARS-CoV-2 subunit vaccines in ferrets; notably, SARS-CoV-2 antigens with L-pampo can elicit a neutralizing antibody response and antigen-specific cellular immune response against SARS-CoV-2, resulting in substantially decreased viral load in ferret nasal washes (Jeong et al., 2021; Kim et al., 2021a). Recombinant SARS-CoV-2 S protein formulated with an Advax-SM adjuvant also protects ferrets against COVID-19 infection (Li et al., 2021). COVID-eVax, a DNA plasmid encoding a secreted monomeric form of the SARS-CoV-2 S protein RBD, also confers significant protection to ferrets upon SARS-CoV-2 challenge and can induce a potent T-cell immune response and relatively low neutralizing antibody titers (Compagnone et al., 2022).
Table 2. Vaccine and antiviral drug candidates evaluated using ferret models for SARS-CoV-2 prevention and treatment.
| Category | Name | Features | Effect evaluation | Reference |
| Vaccines | Self-assembling RBD-nanoparticles | Protein-based vaccine | Antibodies | Kim et al., 2021b |
| Ad5-nCoV | Adenoviral-vectored vaccine | Antibodies; T-cell responses | Wu et al., 2020 | |
| ChAdOx1 nCoV-19 | Adenoviral-vectored vaccine | Antibodies; T-cell responses | Lambe et al., 2021;Marsh et al., 2021 | |
| SARS-CoV-2 antigens with L-pampo | Protein-based vaccine | Antibodies; T-cell responses | Jeong et al., 2021 | |
| Covax-19TM | Protein-based vaccine | Antibodies | Li et al., 2021 | |
| COVID-eVax | DNA vaccine | Antibodies; T-cell responses | Conforti et al., 2022;Compagnone et al., 2022 | |
| Antiviral drugs | Lopinavir/Ritonavir | Anti-HIV drugs, protease inhibitor | Clinical symptom (CS) values | Park et al., 2020 |
| Hydroxychloroquine sulfate | Autophagy inhibitor | Clinical symptom (CS) values | Park et al., 2020 | |
| Emtricitabine/Tenofovir | Nucleotide analog that inhibits RNA-dependent RNA polymerase activity | Clinical symptom (CS) values; virus titers | Park et al., 2020 | |
| Azathioprine | Immunosuppressive drug | Park et al., 2020 | ||
| Human placenta hydrolysate (hPH) (Laennec®) | Virus titers | Kim et al., 2021a | ||
| Selinexor | XPO1 inhibitor | Viral load; inflammatory cytokine | Kashyap et al., 2021 | |
| GS-621763 | Oral prodrug of remdesivir parent nucleoside | Viral burden | Cox et al., 2021a | |
| EIDD-2749 | Ribonucleoside analog | Viral burden; Viral titers | Sourimant et al., 2022 | |
| CT-P59 | Monoclonal antibody | Viral load | Ryu et al., 2021 | |
| Molnupiravir | Orally bioavailable prodrug of ribonucleoside analog EIDD-1931 | Viral shedding | Lieber et al., 2022 | |
| OL-1 and OL-2 | Probiotic consortia | Viral titers | Lehtinen et al., 2022 |
The antiviral effects of certain FDA-approved drugs against SARS-CoV-2, including lopinavir-ritonavir, hydroxychloroquine sulfate, emtricitabine-tenofovir, and azathioprine, have also been assessed in ferrets (Park et al., 2020). Of note, all tested antiviral drugs have been shown to marginally reduce the overall clinical score in infected ferrets but have no significant effects on in vivo virus titers. The antiviral effects of human placenta hydrolysate (hPH) against SARS-CoV-2 have also been evaluated in ferrets, resulting in minimal body weight loss, attenuated viral replication in nasal washes, turbinates, and lungs, and markedly up-regulated gene expression of type I (IFN-α and IFN-β) and II (IFN-γ) interferons (IFNs) in SARS-CoV-2-infected ferrets (Kim et al., 2021a). Selinexor, an FDA-approved XPO1 inhibitor, was shown to reduce viral load in the lungs and protect against tissue damage in the nasal turbinates and lungs of ferrets (Kashyap et al., 2021). Interestingly, SARS-CoV-2 challenge in ferrets has also been used to assess the effects of probiotics against the virus. In ferrets challenged with SARS-CoV-2, the probiotic consortia OL-1 and OL-2 were found to significantly reduce viral load, modulate immune response, and regulate viral receptor expression compared to placebo (Lehtinen et al., 2022), indicating that the potential benefits of probiotics against SARS-CoV-2 infection should be further explored in human clinical trials.
Ferrets and SARS-CoV-2 variants
Many SARS-CoV-2 variants, including VOIs and VOCs, have evolved since the initial outbreak of COVID-19, posing an increased risk to global public health (https://www.who.int/activities/tracking-SARS-CoV-2-variants). VOIs and VOCs can affect viral traits, including transmissibility, clinical manifestations, and immune escape, with various VOCs also associated with an increase in global epidemiology and disease severity and a decrease in efficacy of current vaccines and therapeutics. Therefore, it is important to understand the transmission and pathogenesis of these SARS-CoV-2 variants using various animal models, including ferrets.
Using a ferret-based model, Kim et al. (2022a) demonstrated that natural temperature differences between the upper (33 °C) and lower (37 °C) respiratory tract can have profound effects on SARS-CoV-2 replication and transmission. They found that SARS-CoV-2 variants containing the P323L or P323L/G671S mutation in NSP12 RNA-dependent RNA polymerase (RdRp) exhibited enhanced RdRp enzymatic activity at 33 °C compared to 37 °C and high transmissibility in ferrets. They further suggested that the evolutionarily forced NSP12 P323L and P323L/G671S mutations of recent SARS-CoV-2 VOC strains may be associated with increased RdRp complex stability and enzymatic activity, thus promoting the high transmissibility (Kim et al., 2022a). Ryan et al. (2021) found that all ferrets (6/6) challenged intranasally with high (5×106 pfu) and medium (5×104 pfu) doses of the Victoria/1/202026 SARS-CoV-2 variant exhibited viral RNA shedding in the upper respiratory tract, whereas only one of the six ferrets showed similar signs after low dose (5×102 pfu) challenge. Mild multifocal bronchopneumonia in approximately 5%–15% of the lung was also observed on day 3 in the high- and medium-dosed groups. Pulit-Penaloza et al. (2022) performed a comparative analysis of four SARS-CoV-2 strains, including an early pandemic isolate from the United States (WA1) and representatives of the Alpha, Beta, and Delta lineages. Their results showed that the Beta virus failed to replicate in the ferrets, whereas the WA1, Alpha, and Delta viruses replicated efficiently in the upper respiratory tract, causing mild disease with no overt histopathological changes. They also revealed that the WA1 and Delta viruses transmitted in a direct contact setting, whereas the Delta virus was also capable of limited airborne transmission in ferrets.
Ferret models have also been used to test anti-SARS-CoV-2 VOC efficacy of certain drugs. Cox et al. (2021a) assessed the anti-SARS-CoV-2 VOC efficacy of GS-621763, an oral prodrug of the remdesivir parent nucleoside GS-441524, and found that VOC γ did not invade the ferret host more aggressively than WA1/2020 and oral GS-621763 was highly efficacious at reducing viral burden and tissue titers to undetectable levels and at lowering viral RNA copies in nasal lavages and turbinates. The oral antiviral 4’-fluorouridine (EIDD-274) also shows efficacy against SARS-CoV-2 VOCs in ferrets and CT-P59 monoclonal antibodies can reduce viral load of the South African (SA) variant B.1.351 in vivo (Ryu et al., 2021; Sourimant et al., 2022). Recently, Lieber et al. (2022) showed that molnupiravir can consistently reduce upper respiratory VOC shedding and prevent viral transmission in ferrets, whereas Omicron-infected dwarf hamsters show significant individual variation in response to treatment, suggesting that approved antivirals should be continuously re-assessed in vivo as new VOCs emerge. Furthermore, differences in animal models need to be considered in the development of antivirals and vaccines and different models should be applied to ensure full evaluation.
CONCLUSIONS
The ferret model provides a suitable platform to facilitate the development of SARS-CoV-2 therapeutics and vaccines, and a useful animal model for studying the transmission of SARS-CoV-2. However, although such models can successfully simulate human infection and transmission of SARS-CoV-2, SARS-CoV-2 infected ferrets only show mild clinical symptoms without weight loss or mortality and with relatively low viral titers in the lungs, which may limit their application in the study of severe and critical COVID-19 patients. Consequently, ferrets may be more suitable for studying the pathogenesis of mild SARS-CoV-2 infections. Moreover, as variants continue to evolve worldwide, studies on the transmission, pathogenesis, and countermeasures against emerging SARS-CoV-2 variants using ferret models are warranted.
Funding Statement
This work was supported by the S&T Program of Hebei (20277705D and 20372601D), Natural Science Foundation of Hebei Province, China (H2020206352), Science and Technology Project of Hebei Education Department (QN2018150), Hebei Medical Science Research Project (20220973), and Chinese Medicine Research Program of Hebei Province (2021119)
References
- Au GG, Marsh GA, Mcauley AJ, et al Characterisation and natural progression of SARS-CoV-2 infection in ferrets. Scientific Reports. 2022;12(1):5680. doi: 10.1038/s41598-022-08431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao LL, Deng W, Huang BY, et al The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020a;583(7818):830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- Bao LL, Deng W, Qi FF, et al Sequential infection with H1N1 and SARS-CoV-2 aggravated COVID-19 pathogenesis in a mammalian model, and co-vaccination as an effective method of prevention of COVID-19 and influenza. Signal Transduction and Targeted Therapy. 2021;6(1):200. doi: 10.1038/s41392-021-00618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao LL, Gao H, Deng W, et al Transmission of severe acute respiratory syndrome coronavirus 2 via close contact and respiratory droplets among human angiotensin-converting enzyme 2 mice. The Journal of Infectious Diseases. 2020b;222(4):551–555. doi: 10.1093/infdis/jiaa281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn LB, Sharma N, Elhaik E, et al Targeting TMPRSS2 in SARS-CoV-2 infection. Mayo Clinic Proceedings. 2020;95(9):1989–1999. doi: 10.1016/j.mayocp.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale DJ, Shah R, Karpe AV, et al Metabolic profiling from an asymptomatic ferret model of SARS-CoV-2 infection. Metabolites. 2021;11(5):327. doi: 10.3390/metabo11050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis AC, Li Z, Briggs K, et al. 2022. Efficacy of parainfluenza virus 5 (PIV5)-vectored intranasal COVID-19 vaccine as a single dose vaccine and as a booster against SARS-CoV-2 variants. bioRxiv,doi: 10.1101/2022.06.07.495215.
- Bedford J, Enria D, Giesecke J, et al COVID-19: towards controlling of a pandemic. The Lancet. 2020;395(10229):1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Pulit-Penaloza JA, Maines TR Ferreting out influenza virus pathogenicity and transmissibility: past and future risk assessments in the ferret model. Cold Spring Harbor Perspectives in Medicine. 2020;10(7):a038323. doi: 10.1101/cshperspect.a038323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JFW, Zhang AJ, Yuan SF, et al Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a golden syrian hamster model: implications for disease pathogenesis and transmissibility. Clinical Infectious Diseases. 2020;71(9):2428–2446. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone M, Pinto E, Salvatori E, et al DNA-vaccine-induced immune response correlates with lower viral SARS-CoV-2 titers in a ferret model. Vaccines. 2022;10(8):1178. doi: 10.3390/vaccines10081178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti A, Marra E, Palombo F, et al COVID-eVax, an electroporated DNA vaccine candidate encoding the SARS-CoV-2 RBD, elicits protective responses in animal models. Molecular Therapy. 2022;30(1):311–326. doi: 10.1016/j.ymthe.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RM, Wolf JD, Lieber CM, et al Oral prodrug of remdesivir parent GS-441524 is efficacious against SARS-CoV-2 in ferrets. Nature Communications. 2021a;12(1):6415. doi: 10.1038/s41467-021-26760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RM, Wolf JD, Plemper RK Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nature Microbiology. 2021b;6(1):11–18. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries RD, Schmitz KS, Bovier FT, et al Intranasal fusion inhibitory lipopeptide prevents direct-contact SARS-CoV-2 transmission in ferrets. Science. 2021;371(6536):1379–1382. doi: 10.1126/science.abf4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett HE, Lean FZX, Byrne AMP, et al Intranasal infection of ferrets with SARS-CoV-2 as a model for asymptomatic human infection. Viruses. 2021;13(1):113. doi: 10.3390/v13010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis ME, Richardson B, Goncin U, et al Sex and age bias viral burden and interferon responses during SARS-CoV-2 infection in ferrets. Scientific Reports. 2021;11(1):14536. doi: 10.1038/s41598-021-93855-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis MLC, et al Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He QY, Mok TN, Yun L, et al Single-cell RNA sequencing analysis of human kidney reveals the presence of ACE2 receptor: A potential pathway of COVID-19 infection. Molecular Genetics & Genomic Medicine. 2020;8(10):e1442. doi: 10.1002/mgg3.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, Iwatsuki-Horimoto K, Hatta M, et al Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(28):16587–16595. doi: 10.1073/pnas.2009799117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelow B, Song E, Mao TY, et al Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. The Journal of Experimental Medicine. 2020;217(12):e20201241. doi: 10.1084/jem.20201241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SK, Heo YK, Jeong JH, et al COVID-19 subunit vaccine with a combination of TLR1/2 and TLR3 agonists induces robust and protective immunity. Vaccines. 2021;9(9):957. doi: 10.3390/vaccines9090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang RD, Liu MQ, Chen Y, et al Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell. 2020;182(1):50–58.e8. doi: 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap T, Murray J, Walker CJ, et al. 2021. Selinexor, a novel selective inhibitor of nuclear export, reduces SARS-CoV-2 infection and protects the respiratory system in vivo. Antiviral Research, 192: 105115.
- Kim EH, Kim YI, Jang SG, et al Antiviral effects of human placenta hydrolysate (Laennec®) against SARS-CoV-2 in vitro and in the ferret model. Journal of Microbiology. 2021a;59(11):1056–1062. doi: 10.1007/s12275-021-1367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Kim EH, Casel MAB, et al. 2022a. SARS-CoV-2 variants show temperature-dependent enhanced polymerase activity in the upper respiratory tract and high transmissibility. bioRxiv,doi: 10.1101/2022.09.27.509689.
- Kim YI, Kim D, Yu KM, et al Development of spike receptor-binding domain nanoparticles as a vaccine candidate against SARS-CoV-2 infection in ferrets. mBio. 2021b;12(2):e00230–21. doi: 10.1128/mBio.00230-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Kim SG, Kim SM, et al Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host & Microbe. 2020;27(5):704–709.e2. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Kim SM, Park SJ, et al Critical role of neutralizing antibody for SARS-CoV-2 reinfection and transmission. Emerging Microbes & Infections. 2021c;10(1):152–160. doi: 10.1080/22221751.2021.1872352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Yu KM, Koh JY, et al Age-dependent pathogenic characteristics of SARS-CoV-2 infection in ferrets. Nature Communications. 2022b;13(1):21. doi: 10.1038/s41467-021-27717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter JS, De Meulder D, Bestebroer TM, et al SARS-CoV and SARS-CoV-2 are transmitted through the air between ferrets over more than one meter distance. Nature Communications. 2021;12(1):1653. doi: 10.1038/s41467-021-21918-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe T, Spencer AJ, Thomas KM, et al ChAdOx1 nCoV-19 protection against SARS-CoV-2 in rhesus macaque and ferret challenge models. Communications Biology. 2021;4(1):915. doi: 10.1038/s42003-021-02443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen MJ, Kumar R, Zabel B, et al. 2022. The effect of the probiotic consortia on SARS-CoV-2 infection in ferrets and on human immune cell response in vitro. iScience, 25(6): 104445.
- Li F Structure, function, and evolution of coronavirus spike proteins. Annual Review of Virology. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Honda-Okubo Y, Huang Y, et al Immunisation of ferrets and mice with recombinant SARS-CoV-2 spike protein formulated with Advax-SM adjuvant protects against COVID-19 infection. Vaccine. 2021;39(40):5940–5953. doi: 10.1016/j.vaccine.2021.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CM, Cox RM, Sourimant JD, et al SARS-CoV-2 VOC type and biological sex affect molnupiravir efficacy in severe COVID-19 dwarf hamster model. Nature Communications. 2022;13(1):4416. doi: 10.1038/s41467-022-32045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin QS, Lu CN, Hong YQ, et al Animal models for studying coronavirus infections and developing antiviral agents and vaccines. Antiviral Research. 2022;203:105345. doi: 10.1016/j.antiviral.2022.105345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HN, Gai SJ, Wang XY, et al Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart. Cardiovascular Research. 2020;116(10):1733–1741. doi: 10.1093/cvr/cvaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Ma WJ, Song TZ, et al Single-nucleus transcriptomic profiling of multiple organs in a rhesus macaque model of SARS-CoV-2 infection. Zoological Research. 2022;43(6):1041–1062. doi: 10.24272/j.issn.2095-8137.2022.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh GA, Mcauley AJ, Au GG, et al ChAdOx1 nCoV-19 (AZD1222) vaccine candidate significantly reduces SARS-CoV-2 shedding in ferrets. npj Vaccines. 2021;6(1):67. doi: 10.1038/s41541-021-00315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina BEE, Haagmans BL, Kuiken T, et al Virology: SARS virus infection of cats and ferrets. Nature. 2003;425(6961):915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M, Fernandes MHV, Joshi LR, et al Age-related susceptibility of ferrets to SARS-CoV-2 infection. Journal of Virology. 2022;96(3):e0145521. doi: 10.1128/jvi.01455-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey K, Acharya A, Mohan M, et al Animal models for SARS-CoV-2 research: a comprehensive literature review. Transboundary and Emerging Diseases. 2021;68(4):1868–1885. doi: 10.1111/tbed.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Yu KM, Kim YI, et al Antiviral efficacies of FDA-approved drugs against SARS-CoV-2 infection in ferrets. mBio. 2020;11(3):e01114–20. doi: 10.1128/mBio.01114-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DP, Field CJ, Septer KM, et al Transmission and protection against reinfection in the ferret model with the SARS-CoV-2 USA-WA1/2020 reference isolate. Journal of Virology. 2021;95(13):e0223220. doi: 10.1128/JVI.02232-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock TP, Goldhill DH, Zhou J, et al The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nature Microbiology. 2021;6(7):899–909. doi: 10.1038/s41564-021-00908-w. [DOI] [PubMed] [Google Scholar]
- Prince GA, Porter DD The pathogenesis of respiratory syncytial virus infection in infant ferrets. The American Journal of Pathology. 1976;82(2):339–352. [PMC free article] [PubMed] [Google Scholar]
- Pulit-Penaloza JA, Belser JA, Sun XJ, et al Comparative assessment of severe acute respiratory syndrome coronavirus 2 variants in the ferret model. mBio. 2022;13(5):e0242122. doi: 10.1128/mbio.02421-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnasinghe R, Strohmeier S, Amanat F, et al Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerging Microbes & Infections. 2020;9(1):2433–2445. doi: 10.1080/22221751.2020.1838955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M, Kok A, De Meulder D, et al SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nature Communications. 2020;11(1):3496. doi: 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B, Kuiken T, Herfst S, et al Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368(6494):1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KA, Bewley KR, Fotheringham SA, et al Dose-dependent response to infection with SARS-CoV-2 in the ferret model and evidence of protective immunity. Nature Communications. 2021;12(1):81. doi: 10.1038/s41467-020-20439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu DK, Song RN, Kim M, et al Therapeutic effect of CT-P59 against SARS-CoV-2 South African variant. Biochemical and Biophysical Research Communications. 2021;566:135–140. doi: 10.1016/j.bbrc.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatzki K, Hill NJ, Puryear WB, et al Host barriers to SARS-CoV-2 demonstrated by ferrets in a high-exposure domestic setting. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(18):e2025601118. doi: 10.1073/pnas.2025601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman Z, Liu GD, Cao WG, et al The ferret as a model for filovirus pathogenesis and countermeasure evaluation. ILAR Journal. 2022;61(1):62–71. doi: 10.1093/ilar/ilab011. [DOI] [PubMed] [Google Scholar]
- Shan C, Yao YF, Yang XL, et al Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques. Cell Research. 2020;30(8):670–677. doi: 10.1038/s41422-020-0364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi JZ, Wen ZY, Zhong GX, et al Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si LL, Shen Q, Li J, et al Generation of a live attenuated influenza A vaccine by proteolysis targeting. Nature Biotechnology. 2022;40(9):1370–1377. doi: 10.1038/s41587-022-01381-4. [DOI] [PubMed] [Google Scholar]
- Sia SF, Yan LM, Chin AWH, et al Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583(7818):834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song TZ, Zheng HY, Han JB, et al Delayed severe cytokine storm and immune cell infiltration in SARS-CoV-2-infected aged Chinese rhesus macaques. Zoological Research. 2020;41(5):503–516. doi: 10.24272/j.issn.2095-8137.2020.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourimant J, Lieber CM, Aggarwal M, et al 4'-Fluorouridine is an oral antiviral that blocks respiratory syncytial virus and SARS-CoV-2 replication. Science. 2022;375(6577):161–167. doi: 10.1126/science.abj5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stittelaar KJ, De Waal L, Van Amerongen G, et al Ferrets as a novel animal model for studying human respiratory syncytial virus infections in immunocompetent and immunocompromised hosts. Viruses. 2016;8(6):168. doi: 10.3390/v8060168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Xie C, Bu GL, et al Molecular characteristics, immune evasion, and impact of SARS-CoV-2 variants. Signal Transduction and Targeted Therapy. 2022a;7(1):202. doi: 10.1038/s41392-022-01039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HL, Wang AL, Wang LX, et al Systematic tracing of susceptible animals to SARS-CoV-2 by a bioinformatics framework. Frontiers in Microbiology. 2022b;13:781770. doi: 10.3389/fmicb.2022.781770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhuang Z, Zheng J, et al Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell. 2020a;182(3):734–743.e5. doi: 10.1016/j.cell.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SH, Chen Q, Gu HJ, et al A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host & Microbe. 2020b;28(1):124–133.e4. doi: 10.1016/j.chom.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XS, Sui H, Fisher JT, et al Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. The Journal of Clinical Investigation. 2010;120(9):3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toots M, Yoon JJ, Cox RM, et al Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Science Translational Medicine. 2019;11(515):eaax5866. doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich L, Halwe NJ, Taddeo A, et al Enhanced fitness of SARS-CoV-2 variant of concern Alpha but not Beta. Nature. 2022;602(7896):307–313. doi: 10.1038/s41586-021-04342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Ven K, Van Dijken H, Wijsman L, et al Pathology and immunity after SARS-CoV-2 infection in male ferrets is affected by age and inoculation route. Frontiers in Immunology. 2021;12:750229. doi: 10.3389/fimmu.2021.750229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Brand JMA, Haagmans BL, Leijten L, et al Pathology of experimental SARS coronavirus infection in cats and ferrets. Veterinary Pathology. 2008;45(4):551–562. doi: 10.1354/vp.45-4-551. [DOI] [PubMed] [Google Scholar]
- Walls AC, Park YJ, Tortorici MA, et al Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DW, Hu B, Hu C, et al Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020a;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang YT, Huang BY, et al Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020b;182(3):713–721.e9. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga WJ, Rhodes A, Cheng AC, et al Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Wong J, Layton D, Wheatley AK, et al Improving immunological insights into the ferret model of human viral infectious disease. Influenza and Other Respiratory Viruses. 2019;13(6):535–546. doi: 10.1111/irv.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D, Wang NS, Corbett KS, et al Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SP, Zhong GX, Zhang J, et al A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nature Communications. 2020;11(1):4081. doi: 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yu DD, Ma YH, et al COVID-19-like symptoms observed in Chinese tree shrews infected with SARS-CoV-2. Zoological Research. 2020;41(5):517–526. doi: 10.24272/j.issn.2095-8137.2020.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Qi FF, Xu YF, et al Age-related rhesus macaque models of COVID-19. Animal Models and Experimental Medicine. 2020;3(1):93–97. doi: 10.1002/ame2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhao ZX, Wang YJ, et al Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. American Journal of Respiratory and Critical Care Medicine. 2020;202(5):756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Thao TTN, Hoffmann D, et al SARS-CoV-2 spike D614G change enhances replication and transmission. Nature. 2021;592(7852):122–127. doi: 10.1038/s41586-021-03361-1. [DOI] [PubMed] [Google Scholar]
- Zhou P, Yang XL, Wang XG, et al A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang DY, Wang WL, et al A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]