Abstract
Adult hippocampal neurogenesis (AHN) is crucial for learning, memory, and emotion. Deficits of AHN may lead to reduced cognitive abilities and neurodegenerative disorders, such as Alzheimer's disease. Extensive studies on rodent AHN have clarified the developmental and maturation processes of adult neural stem/progenitor cells. However, to what extent these findings apply to primates remains controversial. Recent advances in next-generation sequencing technologies have enabled in-depth investigation of the transcriptome of AHN-related populations at single-cell resolution. Here, we summarize studies of AHN in primates. Results suggest that neurogenesis is largely shared across species, but substantial differences also exist. Marker gene expression patterns in primates differ from those of rodents. Compared with rodents, the primate hippocampus has a higher proportion of immature dentate granule cells and a longer maturation period of newly generated granule cells. Future research on species divergence may deepen our understanding of the mechanisms underlying adult neurogenesis in primates.
Keywords: Neural precursor cells, Immature granule cells, Sc-RNA-seq
INTRODUCTION
Adult hippocampal neurogenesis (AHN) plays a crucial role in spatial learning, memory formation, and mood regulation (Deng et al., 2010; Kim et al., 2022; Sahay et al., 2011). However, whether AHN persists in primates remains controversy. Histological studies have suggested that a sharp decline in neurogenesis occurs during the early postnatal period, with undetectable neurogenesis in the adult human hippocampus (Sorrells et al., 2018). However, other studies have reported that AHN is persistent in humans (Moreno-Jiménez et al., 2019). To date, immunobiology-based studies have largely relied on rodent-derived immature neuronal markers such as doublecortin (DCX) (Boldrini et al., 2018; Cipriani et al., 2018; Dennis et al., 2016; Flor-García et al., 2020; Moreno-Jiménez et al., 2019; Sorrells et al., 2018; Tobin et al., 2019), and the existence, abundance, and molecular properties of human AHN are often inferred from rodents. At present, research with human samples still lacks concrete biomarkers to measure AHN. Thus, detailed profiling of adult neurogenesis in primates is necessary to bridge the gaps in adult neurogenesis between rodents and humans.
New technologies, such as single-cell RNA sequencing (RNA-seq), have provided new insight into the expression profile, cellular diversity, and heterogeneity of tissues at the single-cell level (Berg et al., 2021; Cadwell et al., 2016; Hochgerner et al., 2018; Hodge et al., 2019; Huang et al., 2022; Lake et al., 2018; Pollen et al., 2015; Schmitz et al., 2022; Wei et al., 2022; Zhong et al., 2018, 2020; Zhu et al., 2018; Zywitza et al., 2018). Furthermore, large-scale transcriptomic datasets have shed light on proliferating neural progenitors, newly formed neurons, and rare cell populations, while adult neurogenesis research has provided insight into cellular heterogeneity and lineages (Artegiani et al., 2017; Bakken et al., 2021a, 2021b; Harris et al., 2021; Hochgerner et al., 2018), as well as quiescent neural stem cell (NSC) maintenance and activation in the hippocampus of mice (Habib et al., 2016; Hodge et al., 2020; Kozareva et al., 2021; Welch et al., 2019; Yao et al., 2021; Ziffra et al., 2021). To date, however, little is known about the dynamic regulation of postnatal hippocampal development in primates.
In this review, we summarize research on AHN in primates in recent decades. We also discuss the similarities and differences between adult rodents and primates to elucidate the distinct physiological roles and self-repair mechanisms of the primate brain.
HISTORY AND CONTROVERSY OF AHN IN PRIMATES
In the 1980s, 3H-thymidine labeling studies reported a lack of adult neurogenesis in non-human primate (NHP) brains (Eckenhoff & Rakic, 1988; Rakic, 1985). In the 1990s, however, BrdU labeling combined with immunohistochemistry revealed persistent neurogenesis in the dentate gyrus (DG) of adult primates (Aizawa et al., 2011; Boldrini et al., 2018; Dennis et al., 2016; Knoth et al., 2010; Ngwenya et al., 2015; Spalding et al., 2013; Yuan et al., 2014). Adult neurogenesis in NHPs is considered less robust after the juvenile period compared to that in rodents and other mammals, with longer cycles of turnover and maturation (Amrein et al., 2004, 2011; Gould et al., 1999; Knoth et al., 2010; Perera et al., 2007; Spalding et al., 2013). Given that maturation of new neurons in NHPs can last up to six months, newly formed cells may not have yet differentiated at the point of detection. Compared with adult neurogenesis in rodents, the age-dependent decline in neuronal production in NHPs appears to be regulated by absolute age, not by relative age (Aizawa et al., 2009, 2011; Amrein et al., 2011; Leuner et al., 2007; Ngwenya et al., 2006, 2008). Adult neurogenesis in humans was first characterized in the 1990s (Eriksson et al., 1998; Gould et al., 1999, 2001). Since then, neuronal formation in the human adult DG of the hippocampus and subventricular zone (SVZ) has been widely reported, with growing interest in alternative approaches for estimating neurogenesis. For example, measuring nuclear bomb test-derived 14C concentrations in genomic DNA has shown that humans and mice have similar levels of AHN (Spalding et al., 2005, 2008). Furthermore, compared with immunobiological methods, the 14C birth dating approach has reported the existence of neurogenesis in the adult striatum and much higher levels of neurogenesis in the human hippocampus (Ernst et al., 2014; Kempermann, 2014).
Immunostaining studies have provided evidence both for the existence of adult-born granule cells in humans (Ammothumkandy et al., 2022; Boldrini et al., 2018; Moreno-Jiménez et al., 2019, 2021; Terreros-Roncal et al., 2021; Tobin et al., 2019) as well as for against (Cipriani et al., 2018; Sorrells et al., 2018, 2021). The conclusions drawn from these studies are predicated on morphological characteristics and the detection of specific markers such as DCX and polysialylated neuronal cell adhesion molecule (PSA-NCAM). As a microtubule-associated protein involved in the extension of neuronal processes, DCX is the most widely utilized proxy marker for AHN. Nestin+/Sox2+/Ki67+ neural progenitors, DCX+/PCNA+ neuroblasts, and DCX+ immature neurons have been detected in the adult human DG, providing further evidence of persistent neurogenesis. However, the expression of DCX is not exclusive to neuroblasts in the DG but has also been observed in mature neurons and glial cells, indicating immaturity or plasticity among both newly generated neurons and non-newly generated neurons. Consequently, the mere presence of DCX expression is insufficient to confirm adult neurogenesis. In addition to DCX, several rodent-derived markers have been used to detect AHN in primates. Radial glia-like cells (RGLs), neural stem cells and neural progenitor cells can be labeled using markers such as phosphine (PH3), glial fibrillary acidic protein (GFAP), nestin, vimentin, brain lipid binding protein (BLBP), and sex determining region Y-box 2 (SOX2) (Cipriani et al., 2018; Moreno-Jiménez et al., 2019; Tobin et al., 2019). Proliferating cells can be labeled by nestin, KI67, SRY-box transcription factor 1 (SOX1), SOX2, and minichromosome maintenance complex component 2 (MCM2) (Cipriani et al., 2018; Moreno-Jiménez et al., 2019; Sorrells et al., 2021; Tobin et al., 2019). DCX, PSA-NCAM, calretinin (CR), β-III-tubulin, and prospero homeobox protein 1 (PROX1) support the existence of early differentiated and immature granule cells (Boldrini et al., 2018; Cipriani et al., 2018; Kempermann et al., 2018; Moreno-Jiménez et al., 2021; Sorrells et al., 2018, 2021; Terreros-Roncal et al., 2021; Tobin et al., 2019), while NeuN, tau, calbindin (CB), microtubule-associated protein 2 (MAP2), and PROX1 label late differentiated and mature neurons (Moreno-Jiménez et al., 2021; Tobin et al., 2019).
Whether more robust and reliable markers exist in primates remains controversial. Immunostaining based on pre-selected markers derived from rodents is insufficient to determine the extent to which adult neurogenesis occurs in primates. These controversies highlight the major gaps in our knowledge regarding AHN in primates based on limited markers and suggest the need for new approaches to identify AHN.
EVIDENCE OF AHN IN PRIMATES BASED ON NEXT-GENERATION SEQUENCING TECHNOLOGIES
Single-cell/single-nucleus RNA sequencing (sc/sn-RNA-seq) is a powerful technique for evaluation of the expression profile, cellular diversity, and heterogeneity of tissues at single-cell resolution (Franjic et al., 2022; Han et al., 2022; Hao et al., 2022; Shin et al., 2015; van Galen et al., 2019; Wang et al., 2022; Zeisel et al., 2015; Zhang et al., 2021; Zhong et al., 2018, 2020; Zhou et al., 2022). A systematic investigation of postnatal hippocampal development in primates should provide more definitive information on the dynamic regulation of postnatal hippocampal development and adult neurogenesis.
Sc/Sn-RNA-seq has been extensively used in rodent AHN studies (Artegiani et al., 2017; Bakken et al., 2021a, 2021b; Habib et al., 2016; Hochgerner et al., 2018; Hodge et al., 2019, 2020; Kozareva et al., 2021; Welch et al., 2019; Yao et al., 2021), while limited research has been conducted in primates. Despite evidence suggesting the persistence of AHN in the macaque hippocampus, the extent of adult neurogenesis in the human brain remains controversy. Zhang et al. (2021) provided the first sn-RNA-seq analysis of frozen post-mortem hippocampus samples from cynomolgus macaques, which revealed aging-specific differences and impaired neuronal regeneration. Franjic et al. (2022) performed sn-RNA-seq analysis of the hippocampal-entorhinal system in adult humans, macaques, and pigs and reported a lack of neurogenic cell populations in the adult human DG. Wang et al. (2022) conducted a comprehensive evaluation of the molecular and cellular dynamics of the hippocampus in macaques across their lifespan and in aged humans using high-throughput sn-RNA-seq and demonstrated adult neurogenesis in humans. Hao et al. (2022) established a large-scale dataset of adult macaque hippocampal cells and demonstrated robust adult neurogenesis in macaques. Thus, these studies provide support for the existence of key neurogenic precursor cell populations in the adult macaque hippocampus, but the extent of adult neurogenesis in the human brain remains contentious.
Variability in analysis methodology of AHN in primates
Sc/Sn-RNA-seq can provide new insights into the study of AHN in primates. Sc-RNA-seq captures cytosolic and nuclear RNA, resulting in higher unique transcripts per cell. Enrichment of unique RNA enables higher resolution during cell-type identification and subsequent bioinformatics analysis. However, considering that some cell types are more vulnerable to tissue dissociation, sc-RNA-seq analysis may be biased towards certain cell types that are resilient to treatment. In contrast, nuclei are more resistant to mechanical assault and can be isolated from frozen tissue. As such, sn-RNA-seq can provide a more robust and convenient tool for studying AHN in humans, as fresh human brain samples are difficult to obtain. The isolation of high-fidelity single cells and nuclei and the generation of single-cell suspensions are crucial. Modified SPLiT-seq has been applied to isolate nuclei from snap-frozen hippocampal tissue (Qian et al., 2020; Rosenberg et al., 2018), while droplet-based and plate-based methods have also been used in the field of adult neurogenesis (Habib et al., 2016, 2017). For droplet-based methodologies, the 10x Genomics platform offers high-throughput capability and improved capture of rare populations. Various analysis pipelines have been developed to determine the biological significance of sc-RNA-seq data. After clustering and annotation, each cell type can be characterized based on differential gene expression, pathway dynamics, pseudo temporal resolution, and RNA velocity (La Manno et al., 2018; Trapnell et al., 2014). Multiple platforms for cell clustering and differential gene expression have been developed, including R-based (e.g., Seurat from the Satija lab and Monocle from the Trapnel lab), Python-based (SCANPY) (Wolf et al., 2018), and Matlab-based analyses. Zhou et al. (2022) employed a validated machine learning-based approach to identify and quantify immature dentate granule cells in the human hippocampus at different stages across lifespan. Through the utilization of high-throughput datasets, bioinformatics has the potential to facilitate a more precise identification of cell populations and key regulatory pathways during neurogenesis.
Identification of cell types associated with AHN in primates
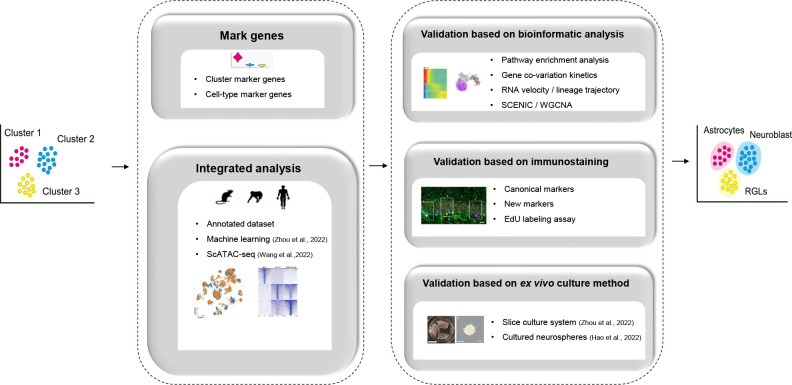
Identification of critical neurogenic populations using sc/sn-RNA-seq analyses can help disentangle high-dimensional information during AHN (Figure 1). Franjic et al. (2022) performed an integrated analysis across the mouse, pig, macaque, and human, and identified five clusters including astrocytes, quiescent stem cells (qNSCs), activated stem cells (aNSCs), neural intermediate progenitor cells, neuroblasts and granule cells. RNA velocity further identified progenitor and neuroblast trajectories for adult neurogenesis in the mice, pigs, and macaques, while these trajectories were absent in humans. Wang et al. (2022) observed a cohort of cells expressing immature neuronal markers in human tissue samples and identified 12 clusters using unsupervised clustering, detecting immature neurons expressing neuroblast markers not detected in Franjic et al. (2022). All other major cell types, except for the immature neurons, were identified in the two studies, indicating that newborn neurons exist in human samples.
Figure 1.
Identifying cell types from sc/sn-RNA-seq data in primates
Cell-type annotation from sc/sn-RNA-seq data in primates. WGCNA, weighted gene co-expression network analysis; SCENIC, single-cell regulatory network inference and clustering.
Hao et al. (2022) performed an integrated analysis of large-scale sc-RNA-seq datasets of adult macaque hippocampal cells and identified all key neurogenic precursor cell populations, including RGLs, intermediate progenitor cells, neuroblasts, as well as abundant immature granule cells. Transcriptomic analysis has also revealed substantial disparities in neurogenic processes between rodents and NHPs, as evidenced by the different marker gene expression patterns and substantially higher proportion of immature granule cells in the adult macaque hippocampus than in the adult mouse hippocampus, consistent with Wang et al. (2022). Using sn-RNA-seq, Zhou et al. (2022) also demonstrated the existence of neurogenesis in the adult human hippocampus based on the presence of rare dentate granule cell fate-specific proliferating neural progenitors. They speculated that the continuous generation of new neurons at low frequencies is accompanied by a prolonged period of neuronal maturation, resulting in the accumulation of a substantial number of neurons with immature neuronal characteristics in the adult human hippocampus at any given time. Thus, the function of adult neurogenesis arises primarily from the unique properties of immature neurons, rather than proliferating neural progenitors. Future larger-scale datasets analysis may capture these rare proliferating neural progenitors for molecular analyses.
Additional bioinformatics approaches to identify key neurogenic populations
Using expression patterns of rodent-derived marker genes as a starting point for identifying primate neurogenic populations, sc/sn-RNA-seq can provide additional information to define cell types in silico based on the whole transcriptome rather than relying on a few pre-selected markers. Taking advantage of the rich resources accumulated for rodents, primate studies have confirmed cell identification based on shared gene expression patterns at the transcriptome level. After removal of batch effects, Korsunsky et al. (2019) confirmed that datasets from two species can be integrated and clustered based on their transcriptomic similarities. Hao et al. (2022) showed that, consistent with their identification, macaque IPCs, neuroblasts, and granule cells cluster with their counterparts in rodents and developing humans. Similarly, Zhang et al. (2021) captured a broad spectrum of cell types in the human hippocampus, including neurogenic lineage cells, oligodendrocyte lineage cells, microglia, and other niche cells. Franjic et al. (2022) only detected one cell with the neuroblast transcriptomic profile among 32 067 granule cells (0.003%) in humans, with much higher proportions of neuroblasts in mice (6.6%), pigs (55.6%), macaques (2.0%), suggesting a lack of neurogenic cell populations in the adult human DG (Table 1).
Table 1. Summary of adult primate hippocampal neurogenesis studies using sc-RNA-seq or sn-RNA-seq.
| Method | Species | Tissue | Number of cells | Cell population | Platform | Neurogenesis | Notes | Studies |
| Sn-RNA-seq | Cynomolgus monkey | Eight young and aged CA1, CA3, and DG | 8 000 nuclei | 12 clusters | 10x Genomics | Yes | Neural transiently amplifying progenitor cell and microglia were most affected by aging | Zhang et al., 2021 |
| Sn-RNA-seq | Human | 38 human post-mortem hippocampi | 32 103 nuclei | 14 clusters | SPLiT-Seq | Yes | Novel candidate gene STMN1 in human immature neurons | Zhou et al., 2022 |
| Sn-RNA-seq Sc-ATAC-seq | Human,macaque | 13 macaque and four human donor hippocampi | 132 524 nuclei | 13 clusters | 10x Genomics | Yes for both | ETNPPL as a primate-specific NSC marker. STMN1 and STMN2 as immature neuronal markers in primates | Wang et al., 2022 |
| Sc-RNA-seq | Macaque | Eight adult macaque hippocampi | 207 785 cells | 34 clusters | 10x Genomics | Yes | HMGB2 as a novel intermediate progenitor cell marker | Hao et al., 2022 |
| Sn-RNA-seq | Human, macaque, pig | Human post-mortem DG, CA2–CA4, CA1, Sub, and EC | 219 058 nuclei | 69 Clusters | 10x Genomics | Yes for macaques,No for humans | METTL7B-defined subregion-specific excitatory neurons and astrocytes in primates | Franjic et al., 2022 |
Advances in data analysis approaches for high-throughput studies have provided details on previously obscured physiological properties. For example, network enrichment analysis combined with RNA velocity recapitulated the transition from quiescent RGL to activated RGLs, suggesting several shifted metabolic processes and providing a list of candidate transcriptomic factors crucial for that shift (Hao et al., 2022). Furthermore, pseudotime plot and subclustering analyses indicated that astrocytes and NSCs exhibit strong interactions and distinct astrocyte subtypes exert distinct functions by providing distinct signals to adult NSCs (Wang et al., 2022).
Identification of novel marker genes
The validation of neurogenic cell populations in primate species through immunostaining using rodent-derived marker genes presents a significant challenge given the substantial differences between species. The expression of classical neurogenesis marker genes, such as NES or DCX, at the transcriptome level is low in adult primates compared to NPCs in the developing hippocampus (Kempermann et al., 2018). However, SLC1A3 (also known as GLAST) has demonstrated greater efficacy than NES or other canonical developmental RGL markers (Harris et al., 2021). The primate-specific NSC marker ETNPPL has been documented in humans and macaques (Wang et al., 2022). STMN1 and STMN2 have been identified as novel markers of immature granule cells (Zhou et al., 2022). Furthermore, HMGB2 has been identified as a novel intermediate progenitor cell marker in the macaque hippocampus (Hao et al., 2022). The identification of these novel markers should facilitate our understanding of adult hippocampal neurogenesis in primates (Figure 2).
Figure 2.
Schematic representation of AHN in adult primate hippocampus
Schematic representation of AHN in adult primate hippocampus in subgranular zone. Left-most is radial glia-like cells, followed by intermediate progenitor cells and finally immature and mature neurons. Important biomarkers of each cell type are labeled. RGL, radial glia-like cell; IPCs, intermediate progenitor cells; NB, neuroblasts; GC, granule cells.
EFFECTS OF AGING AND DISEASE ON AHN IN PRIMATES
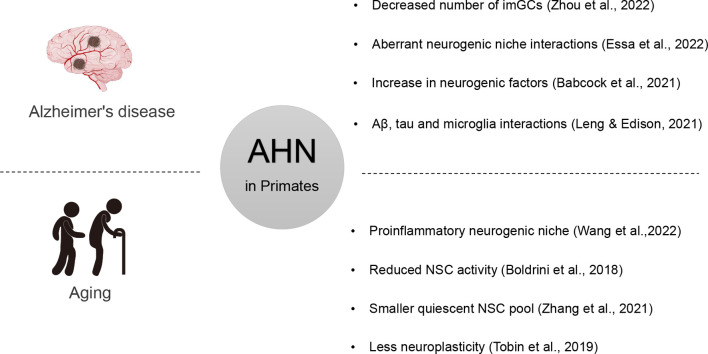
As previously reported, neurogenesis is profoundly influenced by the neurogenic niche, which is comprised of microglia, oligodendrocytes, neurovascular elements, and a complex network of cytokines and chemokines (Artegiani et al., 2017; Nicola et al., 2015; Sultan et al., 2015). Zhang et al. (2021) recently highlighted a range of new aging-associated phenotypic changes in the primate hippocampus. Wang et al. (2022) conducted a comprehensive study of the macaque hippocampus throughout postnatal developmental stages and identified a cluster of active astrocytes and microglia exhibiting inflammatory signatures, with active astrocytes present in aged samples and active microglia found in both young and aged samples. NSCs first differentiate into neural transiently amplifying progenitor cells (TAPCs), which give rise to neuroblasts that differentiate into immature and then mature granule neurons. Several studies have indicated that aging can have a profound impact on both TAPCs and microglia. An in-depth analysis of gene expression dynamics revealed that aging impairs TAPC division and compromises neuronal function along the neurogenesis trajectory (Zhang et al.,2021). Furthermore, aged microglia and oligodendrocytes exhibit elevated pro-inflammatory responses and aged endothelial cells exhibit dysregulated coagulation pathways, which may contribute to a hostile microenvironment for neurogenesis (Wang et al., 2022). The impact of extrinsic factors such as environmental enrichment, exercise, stress, and diet on adult hippocampal neurogenesis is also well documented (Zhang et al., 2021). At aged stages, both microglia and astrocytes contribute to the neuroinflammatory response, which has been linked to detrimental effects on brain function. Impairment of AHN may contribute to memory and cognitive dificits and neurodegenerative disorders, such as Alzheimer's disease (Babcock et al., 2021; Essa et al., 2022; Leng & Edison, 2021) (Figure 3) Impairment of AHN may contribute to memory and cognitive dificits and neurodegenerative disorders, such as Alzheimer's disease (Babcock et al., 2021; Essa et al., 2022; Leng & Edison, 2021) (Figure 3)
Figure 3.
Effects of aging and Alzheimer’s disease on AHN in primates
Summary of potential effects of aging and Alzheimer’s disease on AHN in primates. ImGCs, immature dentate granule cells; NSCs, neural stem cells.
How active astrocytes and microglia affect other cell types is an interesting question. Analysis of the interaction dynamics among microglia, astrocytes, and NSCs has demonstrated that astrocytes and NSCs have a more pronounced interaction, suggesting that active astrocytes exert direct effects on NSCs. Transcriptome dynamics of astrocytes and analysis of the interaction between astrocytes and NSCs have provided evidence that different astrocyte subtypes play distinct roles by mediating differential signals to adult NSCs (Cassé et al., 2018; Luo et al., 2015; Masuda et al., 2020). Previous studies in mice have also indicated that active astrocytes are capable of inducing the death of neurons and oligodendrocytes (Artegiani et al., 2017).
CONCLUSIONS AND PROSPECTS FOR FUTURE WORK
At present, the validity of AHN in adult primates is primarily based on immunohistochemical analysis of traditional markers. These staining results are susceptible to various experimental factors, such as fixatives, post-mortem intervals, variability in antibody batches, and antigen retrieval methods (Dennis et al., 2016; Flor-García et al., 2020; Moreno-Jiménez et al., 2021). Sc/Sn-RNA-seq provides a robust and sensitive tool that circumvents the limitations of traditional techniques, such as RNA in situ hybridization and immunohistochemistry, to reveal rare cell types. Indeed, the identification of key neurogenic precursor cell populations in sc/sn-RNA-seq datasets provides strong support for the robustness of neurogenesis in the adult macaque hippocampus. However, the extent of neurogenesis in the adult human hippocampus remains controversial. Further investigation of the nature of these key populations should deepen our understanding of adult neurogenesis.
The existence of immature neurons has been a matter of contention for the past several decades. The utilization of sn-RNA-seq should provide further molecular details regarding the expression profiles of various cell types, which may lead to the identification of additional primate-specific markers for immature granule cells. Further comparative studies between primates and rodents may also help clarify the mechanisms underlying adult neurogenesis in primates. Although research focusing on the primate hippocampus has provided evidence for the broad existence of AHH in primates, controversy still exists. Furthermore, accumulating evidence on the differences between rodents and primates highlights the need for further comparative studies. One potential direction is to develop better and more reliable endogenous markers for the characterization of neural precursors and neurogenesis in post-mortem human tissues. Newly developed spatial transcriptomic analysis (Maynard et al., 2021) may provide a potential resolution for measuring transcriptional profiles of previously undetectable cells. The development of new imaging methods for high-resolution longitudinal analysis of neurogenesis in humans should also be explored. One study using magnetic resonance imaging identified neural precursors in the primate hippocampus through complex signal processing (Sierra et al., 2011), although further validation is required large-scale sn-RNA-seq analysis may also enable the capture of rare proliferating neural progenitors for molecular analyses. Furthermore, comprehensive analyses of postnatal neurons, both in infants and adults, should enable a deeper understanding of the state and function of human AHN and plasticity.
AHN is not a cell-replacement mechanism. In rodents, it helps maintain plasticity in hippocampal neuronal circuits via the continuous addition of immature neurons for circuit integration (Harris et al., 2018; Hochgerner et al., 2018; Zeisel et al., 2018). Once successfully integrated, adult newborn granule cells exhibit unique features, suggesting that these newborn cells may play a role in hippocampal function, including learning and memory as well as anxiety and stress regulation (Abrous & Wojtowicz, 2015; Deng et al., 2010; Hernández-Rabaza et al., 2009; Kent et al., 2016; Lemaire et al., 2000). However, the contribution of newly formed neurons to network function in primates is unknown. Despite the difficulties, validation of the functional integration of newborn cells is a crucial step for understanding the functional consequences of adult neurogenesis in primates. Cutting-edge in vivo lineage tracing may be a good approach to further clarify the controversy regarding AHN in primates. Genetically modified NHPs with specific markers could be created to meet this requirement and provide resources for future research.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
S.L. conceived the review. S.L., Y.L., N.N.X., and Z.Z.H. prepared the manuscript and tables. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the Natural Science Foundation of China (81961128021, 81870682), National Key R&D Program of China (2022YEF0203200), Guangdong Provincial Key R&D Programs (2018B030335001), and Science and Technology Program of Guangzhou (202007030011, 202007030010,202007030001)
References
- Abrous DN, Wojtowicz JM Interaction between neurogenesis and hippocampal memory system: new vistas. Cold Spring Harbor Perspectives in Biology. 2015;7(6):a018952. doi: 10.1101/cshperspect.a018952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa K, Ageyama N, Terao K, et al Primate-specific alterations in neural stem/progenitor cells in the aged hippocampus. Neurobiology of Aging. 2011;32(1):140–150. doi: 10.1016/j.neurobiolaging.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Aizawa K, Ageyama N, Yokoyama C, et al Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Experimental Animals. 2009;58(4):403–407. doi: 10.1538/expanim.58.403. [DOI] [PubMed] [Google Scholar]
- Ammothumkandy A, Ravina K, Wolseley V, et al Altered adult neurogenesis and gliogenesis in patients with mesial temporal lobe epilepsy. Nature Neuroscience. 2022;25(4):493–503. doi: 10.1038/s41593-022-01044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein I, Isler K, Lipp HP Comparing adult hippocampal neurogenesis in mammalian species and orders: influence of chronological age and life history stage. European Journal of Neuroscience. 2011;34(6):978–987. doi: 10.1111/j.1460-9568.2011.07804.x. [DOI] [PubMed] [Google Scholar]
- Amrein I, Slomianka L, Poletaeva II, et al Marked species and age-dependent differences in cell proliferation and neurogenesis in the hippocampus of wild-living rodents. Hippocampus. 2004;14(8):1000–1010. doi: 10.1002/hipo.20018. [DOI] [PubMed] [Google Scholar]
- Artegiani B, Lyubimova A, Muraro M, et al A single-cell RNA sequencing study reveals cellular and molecular dynamics of the hippocampal neurogenic niche. Cell Reports. 2017;21(11):3271–3284. doi: 10.1016/j.celrep.2017.11.050. [DOI] [PubMed] [Google Scholar]
- Babcock KR, Page JS, Fallon JR, et al Adult Hippocampal Neurogenesis in Aging and Alzheimer's Disease. Stem Cell Reports. 2021;16(4):681–693. doi: 10.1016/j.stemcr.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken TE, Jorstad NL, Hu QW, et al Comparative cellular analysis of motor cortex in human, marmoset and mouse. Nature. 2021a;598(7879):111–119. doi: 10.1038/s41586-021-03465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken TE, van Velthoven CTJ, Menon V, et al Single-cell and single-nucleus RNA-seq uncovers shared and distinct axes of variation in dorsal LGN neurons in mice, non-human primates, and humans. eLife. 2021b;10:e64875. doi: 10.7554/eLife.64875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J, Sorensen SA, Ting JT, et al Human neocortical expansion involves glutamatergic neuron diversification. Nature. 2021;598(7879):151–158. doi: 10.1038/s41586-021-03813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, et al Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22(4):589–599.e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell CR, Palasantza A, Jiang XL, et al Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nature Biotechnology. 2016;34(2):199–203. doi: 10.1038/nbt.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassé F, Richetin K, Toni N Astrocytes' contribution to adult neurogenesis in physiology and Alzheimer's disease. Frontiers in Cellular Neuroscience. 2018;12:432. doi: 10.3389/fncel.2018.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S, Ferrer I, Aronica E, et al Hippocampal radial glial subtypes and their neurogenic potential in human fetuses and healthy and Alzheimer's disease adults. Cerebral Cortex. 2018;28(7):2458–2478. doi: 10.1093/cercor/bhy096. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature Reviews Neuroscience. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CV, Suh LS, Rodriguez ML, et al Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathology and Applied Neurobiology. 2016;42(7):621–638. doi: 10.1111/nan.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenhoff MF, Rakic P Nature and fate of proliferative cells in the hippocampal dentate gyrus during the life span of the rhesus monkey. The Journal of Neuroscience. 1988;8(8):2729–2747. doi: 10.1523/JNEUROSCI.08-08-02729.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, et al Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, et al Neurogenesis in the striatum of the adult human brain. Cell. 2014;156(5):1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Essa H, Peyton L, Hasan W, et al. 2022. Implication of Adult Hippocampal Neurogenesis in Alzheimer's Disease and Potential Therapeutic Approaches. Cells, 11(2).
- Flor-García M, Terreros-Roncal J, Moreno-Jiménez EP, et al Unraveling human adult hippocampal neurogenesis. Nature Protocols. 2020;15(2):668–693. doi: 10.1038/s41596-019-0267-y. [DOI] [PubMed] [Google Scholar]
- Franjic D, Skarica M, Ma S, et al Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron. 2022;110(3):452–469.e14. doi: 10.1016/j.neuron.2021.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, et al Hippocampal neurogenesis in adult Old World primates. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(9):5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Vail N, Wagers M, et al Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(19):10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib N, Avraham-Davidi I, Basu A, et al Massively parallel single-nucleus RNA-seq with DroNc-seq. Nature Methods. 2017;14(10):955–958. doi: 10.1038/nmeth.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib N, Li YQ, Heidenreich M, et al Div-Seq: single-nucleus RNA-Seq reveals dynamics of rare adult newborn neurons. Science. 2016;353(6302):925–928. doi: 10.1126/science.aad7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Wei XY, Liu CY, et al. 2022. Cell transcriptomic atlas of the non-human primate Macaca fascicularis. Nature, 604(7907): 723–731.
- Hao ZZ, Wei JR, Xiao DC, et al Single-cell transcriptomics of adult macaque hippocampus reveals neural precursor cell populations. Nature Neuroscience. 2022;25(6):805–817. doi: 10.1038/s41593-022-01073-x. [DOI] [PubMed] [Google Scholar]
- Harris KD, Hochgerner H, Skene NG, et al Classes and continua of hippocampal CA1 inhibitory neurons revealed by single-cell transcriptomics. PLoS Biology. 2018;16(6):e2006387. doi: 10.1371/journal.pbio.2006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L, Rigo P, Stiehl T, et al Coordinated changes in cellular behavior ensure the lifelong maintenance of the hippocampal stem cell population. Cell Stem Cell. 2021;28(5):863–876.e6. doi: 10.1016/j.stem.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Rabaza V, Llorens-Martín M, Velázquez-Sánchez C, et al Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159(1):59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Hochgerner H, Zeisel A, Lönnerberg P, et al Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nature Neuroscience. 2018;21(2):290–299. doi: 10.1038/s41593-017-0056-2. [DOI] [PubMed] [Google Scholar]
- Hodge RD, Bakken TE, Miller JA, et al Conserved cell types with divergent features in human versus mouse cortex. Nature. 2019;573(7772):61–68. doi: 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, Miller JA, Novotny M, et al Transcriptomic evidence that von Economo neurons are regionally specialized extratelencephalic-projecting excitatory neurons. Nature Communications. 2020;11(1):1172. doi: 10.1038/s41467-020-14952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WJ, Xu Q, Su J, et al Linking transcriptomes with morphological and functional phenotypes in mammalian retinal ganglion cells. Cell Reports. 2022;40(11):111322. doi: 10.1016/j.celrep.2022.111322. [DOI] [PubMed] [Google Scholar]
- Kempermann G Off the beaten track: new neurons in the adult human striatum. Cell. 2014;156(5):870–871. doi: 10.1016/j.cell.2014.02.027. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH, Aigner L, et al Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23(1):25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BA, Hvoslef-Eide M, Saksida LM, et al The representational-hierarchical view of pattern separation: not just hippocampus, not just space, not just memory? Neurobiology of Learning and Memory. 2016;129:99–106. doi: 10.1016/j.nlm.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Kim TA, Syty MD, Wu K, et al Adult hippocampal neurogenesis and its impairment in Alzheimer's disease. Zoological Research. 2022;43(3):481–496. doi: 10.24272/j.issn.2095-8137.2021.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, et al Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5(1):e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsunsky I, Millard N, Fan J, et al Fast, sensitive and accurate integration of single-cell data with Harmony. Nature Methods. 2019;16(12):1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozareva V, Martin C, Osorno T, et al A transcriptomic atlas of mouse cerebellar cortex comprehensively defines cell types. Nature. 2021;598(7879):214–219. doi: 10.1038/s41586-021-03220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Manno G, Soldatov R, Zeisel A, et al RNA velocity of single cells. Nature. 2018;560(7719):494–498. doi: 10.1038/s41586-018-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake BB, Chen S, Sos BC, et al Integrative single-cell analysis of transcriptional and epigenetic states in the Human Adult Brain. Nature Biotechnology. 2018;36(1):70–80. doi: 10.1038/nbt.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, et al Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng F, Edison P Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here. Nature Reviews Neurology. 2021;17(3):157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, et al Diminished adult neurogenesis in the marmoset brain precedes old age. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YP, Coskun V, Liang AB, et al Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell. 2015;161(5):1175–1186. doi: 10.1016/j.cell.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Sankowski R, Staszewski O, et al Microglia heterogeneity in the single-cell era. Cell Reports. 2020;30(5):1271–1281. doi: 10.1016/j.celrep.2020.01.010. [DOI] [PubMed] [Google Scholar]
- Maynard KR, Collado-Torres L, Weber LM, et al Transcriptome-scale spatial gene expression in the human dorsolateral prefrontal cortex. Nature Neuroscience. 2021;24(3):425–436. doi: 10.1038/s41593-020-00787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, et al Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nature Medicine. 2019;25(4):554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- Moreno-Jiménez EP, Terreros-Roncal J, Flor-García M, et al Evidences for adult hippocampal neurogenesis in humans. Journal of Neuroscience. 2021;41(12):2541–2553. doi: 10.1523/JNEUROSCI.0675-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwenya LB, Peters A, Rosene DL Maturational sequence of newly generated neurons in the dentate gyrus of the young adult rhesus monkey. Journal of Comparative Neurology. 2006;498(2):204–216. doi: 10.1002/cne.21045. [DOI] [PubMed] [Google Scholar]
- Ngwenya LB, Rosene DL, Peters A An ultrastructural characterization of the newly generated cells in the adult monkey dentate gyrus. Hippocampus. 2008;18(2):210–220. doi: 10.1002/hipo.20384. [DOI] [PubMed] [Google Scholar]
- Ngwenya LB, Heyworth NC, Shwe Y, et al Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Frontiers in systems neuroscience. 2015;9:102–102. doi: 10.3389/fnsys.2015.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola Z, Fabel K, Kempermann G Development of the adult neurogenic niche in the hippocampus of mice. Frontiers in Neuroanatomy. 2015;9:53. doi: 10.3389/fnana.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, et al Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. Journal of Neuroscience. 2007;27(18):4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen AA, Nowakowski TJ, Chen JD, et al Molecular identity of human outer radial glia during cortical development. Cell. 2015;163(1):55–67. doi: 10.1016/j.cell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian XY, Su YJ, Adam CD, et al Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell. 2020;26(5):766–781.e9. doi: 10.1016/j.stem.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P Limits of neurogenesis in primates. Science. 1985;227(4690):1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- Rosenberg AB, Roco CM, Muscat RA, et al Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science. 2018;360(6385):176–182. doi: 10.1126/science.aam8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, et al Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz MT, Sandoval K, Chen CP, et al The development and evolution of inhibitory neurons in primate cerebrum. Nature. 2022;603(7903):871–877. doi: 10.1038/s41586-022-04510-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Berg DA, Zhu YH, et al Single-cell RNA-Seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell. 2015;17(3):360–372. doi: 10.1016/j.stem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Maletic-Savatic M Adult human neurogenesis: from microscopy to magnetic resonance imaging. Frontiers in Neuroscience. 2011;5:47. doi: 10.3389/fnins.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Cebrian-Silla A, et al Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555(7696):377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Zhang ZZ, et al Positive controls in adults and children support that very few, if any, new neurons are born in the adult human hippocampus. Journal of Neuroscience. 2021;41(12):2554–2565. doi: 10.1523/JNEUROSCI.0676-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, et al Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, et al Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bhardwaj RD, Buchholz BA, et al Retrospective birth dating of cells in humans. Cell. 2005;122(1):133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Sultan S, Li LY, Moss J, et al Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron. 2015;88(5):957–972. doi: 10.1016/j.neuron.2015.10.037. [DOI] [PubMed] [Google Scholar]
- Terreros-Roncal J, Moreno-Jiménez EP, Flor-García M, et al Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science. 2021;374(6571):1106–1113. doi: 10.1126/science.abl5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MK, Musaraca K, Disouky A, et al Human hippocampal neurogenesis persists in aged adults and Alzheimer's disease patients. Cell Stem Cell. 2019;24(6):974–982.e3. doi: 10.1016/j.stem.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Cacchiarelli D, Grimsby J, et al The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nature Biotechnology. 2014;32(4):381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Galen P, Hovestadt V, Wadsworth II MH, et al Single-cell RNA-Seq reveals AML hierarchies relevant to disease progression and immunity. Cell. 2019;176(6):1265–1281.e24. doi: 10.1016/j.cell.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang MD, Yang M, et al Transcriptome dynamics of hippocampal neurogenesis in macaques across the lifespan and aged humans. Cell Research. 2022;32(8):729–743. doi: 10.1038/s41422-022-00678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei JR, Hao ZZ, Xu C, et al Identification of visual cortex cell types and species differences using single-cell RNA sequencing. Nature Communications. 2022;13(1):6902–6902. doi: 10.1038/s41467-022-34590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JD, Kozareva V, Ferreira A, et al Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell. 2019;177(7):1873–1887.e17. doi: 10.1016/j.cell.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf FA, Angerer P, Theis FJ SCANPY: large-scale single-cell gene expression data analysis. Genome Biology. 2018;19(1):15. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ZZ, van Velthoven CTJ, Nguyen TN, et al A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell. 2021;184(12):3222–3241.e26. doi: 10.1016/j.cell.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Hochgerner H, Lönnerberg P, et al Molecular architecture of the mouse nervous system. Cell. 2018;174(4):999–1014.e22. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Muñoz-Manchado AB, Codeluppi S, et al Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347(6226):1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li JM, Ren J, et al Single-nucleus transcriptomic landscape of primate hippocampal aging. Protein & Cell. 2021;12(9):695–716. doi: 10.1007/s13238-021-00852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong SJ, Ding WY, Sun L, et al Decoding the development of the human hippocampus. Nature. 2020;577(7791):531–536. doi: 10.1038/s41586-019-1917-5. [DOI] [PubMed] [Google Scholar]
- Zhong SJ, Zhang S, Fan XY, et al A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature. 2018;555(7697):524–528. doi: 10.1038/nature25980. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Su YJ, Li SY, et al Molecular landscapes of human hippocampal immature neurons across lifespan. Nature. 2022;607(7919):527–533. doi: 10.1038/s41586-022-04912-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Sousa AMM, Gao TLY, et al Spatiotemporal transcriptomic divergence across human and macaque brain development. Science. 2018;362(6420):eaat8077. doi: 10.1126/science.aat8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziffra RS, Kim CN, Ross JM, et al Single-cell epigenomics reveals mechanisms of human cortical development. Nature. 2021;598(7879):205–213. doi: 10.1038/s41586-021-03209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zywitza V, Misios A, Bunatyan L, et al Single-cell transcriptomics characterizes cell types in the subventricular zone and uncovers molecular defects impairing adult neurogenesis. Cell Reports. 2018;25(9):2457–2469.e8. doi: 10.1016/j.celrep.2018.11.003. [DOI] [PubMed] [Google Scholar]