Abstract
Chronic skin wound healing, especially in diabetes mellitus, is still unsolved. Although many efforts have been made to treat diabetic skin wounds, current strategies have achieved limited effectiveness. Nowadays, a great number of studies have shown that exosomes might be a promising approach for treating diabetic wounds. Many studies and reviews have focused on investigating and discussing the effectiveness and mechanism of exosomes. However, maximizing its value in treating skin wounds in diabetes mellitus requires further consideration. In this review, we reviewed and discussed the aspects that could be further improved in this process, including finding a better source of exosomes, engineering exosomes, adjusting dosage and frequency, and combining more efficient delivery methods. This review provided an overview and idea of what we can do to improve the therapeutic effect of exosomes on skin wounds in diabetes mellitus. Only by combining all the factors that affect the effectiveness of exosomes in diabetic wound healing can we further promote their clinical usefulness.
Keywords: diabetes, skin wounds, exosomes, maximize, therapeutic effect
1. Introduction
The healing of chronic skin wounds, especially diabetic skin wounds, is one of the most intractable problems for clinicians and a heavy burden for patients, both physically and financially (1, 2). To date, there are numerous strategies and methods to treat diabetic wounds, and however, these are not exempt from limitations (3, 4). Hence, there is a crucial and urgent need for effective and safe methods to promote diabetic wound healing. Exosomes are one type of extracellular vesicles (EVs) secreted by various cells and show a double-layer membrane structure and a particle size ranging from 30 to 200 nm. They are involved in cell-cell communication and intracellular signaling. Exosomes show a lot of advantages, such as being stable, easily stored, and not rejected by the immune system, offering a homing effect, and the dosage can be easily controlled (5). Recent research results indicated that exosomes participated in the development and outcome of diabetes and its related complications (6). For the difficult-to-heal skin wounds caused by diabetes, exogenous exosome therapy could promote the functional recovery of multiple essential cells. They effectively promoted angiogenesis, collagen synthesis, and modulating inflammation (7), so exosome therapy might become very important in wound healing strategies in order to enhance antimicrobial stewardship (8).
Although exosome therapies hold great potential for facilitating diabetic skin wound healing and regeneration, for any drug, its clinical efficacy will also depend on many other factors, like dosage, application frequency, and delivery methods (9, 10). In this review, according to published studies on the application of exosomes in diabetic wound healing, we first summarized the characterization of skin wounds in diabetes mellitus and the role of exosomes in promoting this type of wound healing. More importantly, we reviewed and discussed the aspects that could be further improved to maximize the value of exosomes in detail.
2. Characterization of skin wounds in diabetes mellitus
The healing of skin wounds follows four steps, hemostasis, inflammation, proliferation, and remodeling. However, in diabetes mellitus, several factors impair these processes, making healing longer and more difficult. For example, the high glucose in diabetic wounds can lead to the gathering of bacteria and the weakening of leukocyte phagocytosis, ultimately leading to serious local infection and inflammation. The neurons are the most sensitive and initially affected cells in diabetes mellitus, and diabetic neuropathy is one of the major causes of diabetic ulcers (11). Moreover, the blood vessels of diabetic wounds are damaged, and their angiogenic capacity is weak, leading to insufficient nutrition supply and low oxygen concentration in diabetic wounds (12). In addition to an inadequate oxygen supply, high oxygen consumption by wound cells during inflammation also induces hypoxia. Likewise, hypoxia further amplifies the inflammatory response, thereby prolonging injury by increasing the levels of oxygen radicals (13). Therefore, improving these factors is the key to treating skin wounds in diabetes mellitus.
3. Effect of exosomes on promoting skin wound healing in diabetes mellitus
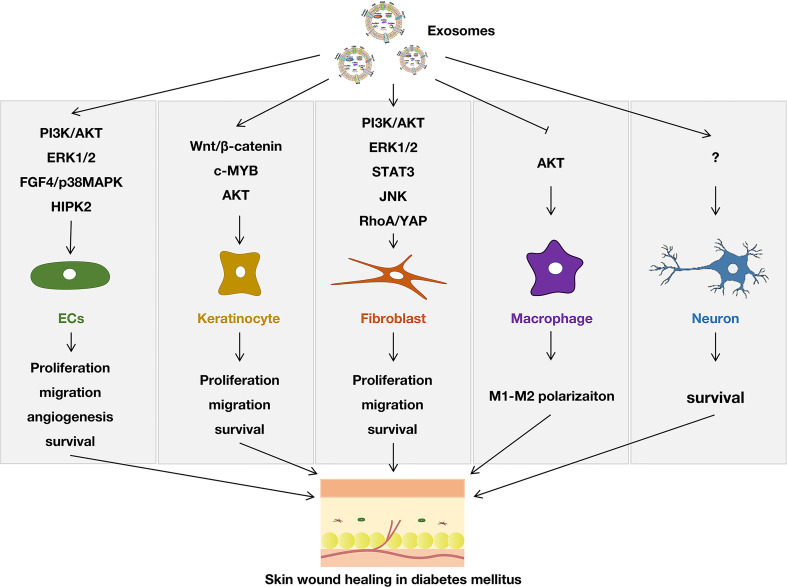
It has been reported that endothelial cells (ECs), fibroblasts, macrophages, and keratinocytes participate in angiogenesis, collagen synthesis, and anti-inflammatory processes, which are significant in diabetic wound healing. However, in the diabetic environment, the number and function of these cells are restricted to varying degrees, and the wound-healing process is delayed or interrupted. Studies found that exosomes could greatly promote survival and inhibit the apoptosis of ECs (14–17), fibroblasts (11), keratocytes (11), and neurons (11). Mostly, exosomes were reported to promote the proliferation, migration, and angiogenesis of endothelial cells, and a variety of pathways were involved, like PI3K/AKT pathway, ERK1/2 pathway, FGF4/p38MAPK pathway and HIPK2 pathway (15, 17–29). This greatly enhances the ability of local vascular regeneration in diabetic wound healing. Exosomes could also promote the proliferation and migration of keratinocytes (28, 30, 31) and fibroblasts (20, 25, 28, 32–35), and the associated pathway could be seen in Figure 1 . It was reported that exosomes played a role in polarizing pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages (36–38), and the inhabitation of the phosphorylation of AKT might contribute to this progress (37). Especially, the increase of nerve fiber density and the functional recovery of neurons induced by exosomes played an important role in diabetic skin wound healing (39). All the functions of exosomes confirmed the potential application value of exosomes for wound healing in diabetes mellitus. However, how to maximize or further improve the therapeutic effect of exosomes is still a problem that needs a further breakthrough.
Figure 1.
Effect of exosomes on modulating different cells in diabetic skin wound healing.
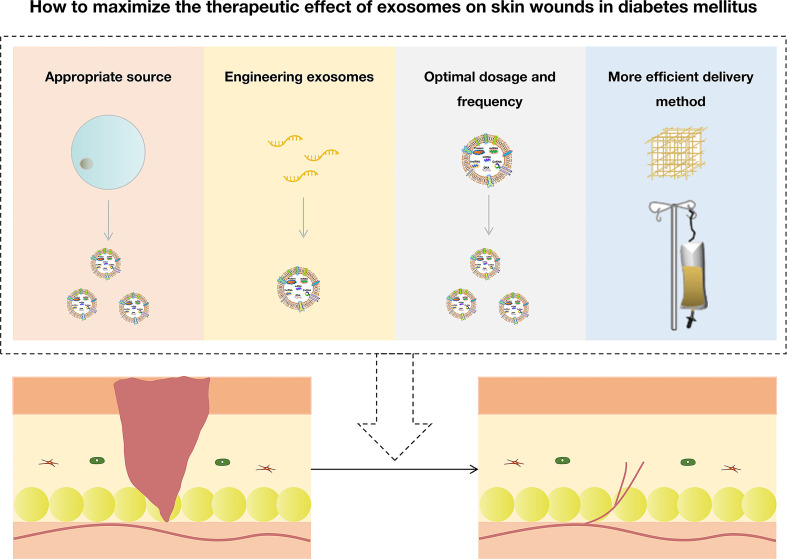
4. How to maximize the therapeutic effect of exosomes
4.1. Which is the better source of exosomes
Up to now, exosomes from various cell sources have been used to promote wound healing in diabetes. Stem cells are the most studied, including adipose stem cells (ADSCs) (11, 17, 28, 31, 35, 38, 40–47), bone marrow mesenchymal stem cells (BMSCs) (15, 16, 23, 26, 32, 33, 37, 44, 48–51), human umbilical cord-derived mesenchymal stem cells (hUCMSCs) (27, 52, 53), synovium mesenchymal stem cells (SMSCs) (18, 21), gingival mesenchymal stem cells (GMSCs) (39), human urine-derived stem cells (USCs) (22), menstrual blood-derived mesenchymal stem cells (MenSCs) (36), placental mesenchymal stem cells (PMSCs) (54), human endometrial stem cells (hEnSCs) (34), hair follicle-derived mesenchymal stromal cells (55), epidermal stem cells (ESCs) (56, 57). Other cells, like fibrocytes (58), human umbilical cord blood endothelial progenitor cells (19, 59), human umbilical cord blood mononuclear cells (hUCBMNCs) (30), macrophages (24), human amniotic epithelial cells (25), dermal fibroblasts (DFs) (14), M2 macrophages (60), human umbilical vein endothelial cells (HUVECs) (61), also were used for exosome isolation. Among these sources, ADSCs and BMSCs were chosen by most researchers. Considering the abundant sources and guaranteed effectiveness, these two kinds of stem cells might be the most reliable source of exosomes. It was reported that there were differences in the efficacy of the two types of stem cell-derived exosomes. For example, M. Pomatto et al. found that BMSCs-derived exosomes were shown to mainly promote cell proliferation, whereas ADSCs-derived exosomes demonstrated a major effect on angiogenesis (44). Therefore, the better source of exosomes still needs more comprehensive assessments.
Of course, the source of exosomes might not be limited to cells. For example, Chen et al. found that serum exosomes could accelerate diabetic wound healing by promoting angiogenesis and extracellular matrix formation (62). Guo et al. and Xu et al. isolated exosomes from platelet-rich plasma (PRP) and found this type of exosome could effectively induce the proliferation and migration of endothelial cells and fibroblasts to improve angiogenesis and re-epithelialization in diabetic skin wounds (20, 63). Besides, milk was also reported to be a source of exosome isolation (64). In our view, serum, PRP, and milk were abundant sources of exosomes. However, due to insufficient studies, its effectiveness and stability need to be further confirmed. Especially, a recent study reported that plant-derived exosomes were of therapeutic value (65); although it was not applied to skin wounds, it provided an excellent idea for exosome isolation. Perhaps it is a new direction for us to find exosomes from the proper kind of plants because many drugs contain plant extracts.
4.2. Engineering exosomes
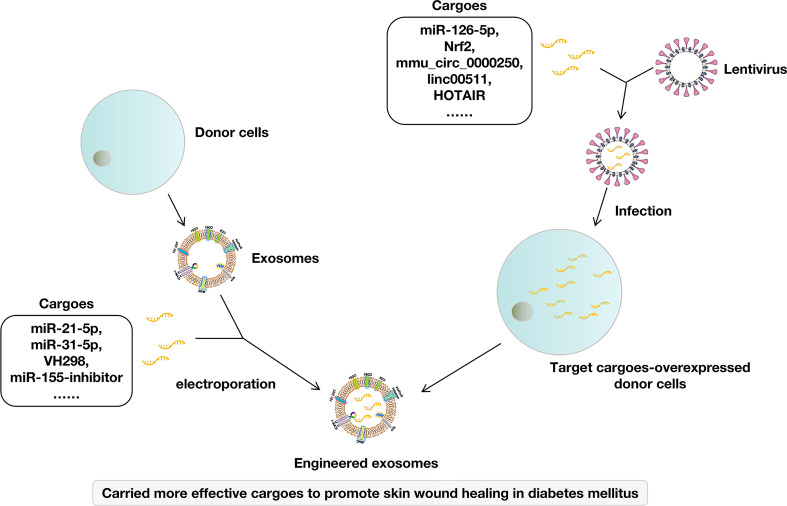
In addition to finding more effective natural exosomes, due to their structural characteristics, exosomes are also highly engineerable. Engineering of exosomal surface confers cell and tissue specificity. Besides, exosomes are considered delivery vehicles of diverse biological molecules, including the delivery of nucleic acid, proteins, and lipids. Studies showed that some molecules in the exosomes were particularly beneficial to wound healing, so increasing these components in exosomes through various engineering technologies could enhance the function of exosomes. Engineering strategy could be divided into direct engineering of exosomes (chemical modification and physical modification) and indirect engineering of exosomes (genetic modification of exosome-donor cells) (66). To enhance the therapeutic effect of exosomes in diabetic wound healing, some researchers have tried to use an engineering strategy ( Figure 2 ).
Figure 2.
Engineering exosomes for skin wound healing in diabetes mellitus.
Directly loading cargoes to exosomes was adopted by many studies. For example, direct loading miR-21-5p to ADSCs-derived exosomes by electroporation exhibited excellent effects on promoting the proliferation and migration of keratinocytes and accelerating diabetic wound healing by increasing re-epithelialization, collagen remodeling, angiogenesis, and vessel maturation (31). Loading miR-155 inhibitor to BMSCs-derived exosomes showed synergistic effects in keratinocyte migration and anti-inflammatory action, leading to accelerated wound healing by negative regulation of miR-155 (50). ESCs-derived exosomes loaded with VH298 were also found to have a better therapeutic effect on wound healing and angiogenesis in diabetes mellitus (56). Yan et al. used milk-derived exosomes as a novel system for miR-31-5p delivery and successfully encapsulated miR-31-5p mimics into milk exosomes through electroporation. Then, they proved that the miR-31-5p loaded in exosomes achieved higher cell uptake and improved endothelial cell functions in vitro, promoting angiogenesis and enhanced skin wound healing in vivo (64).
Genetic modification of donor cells was also adopted because it was a convenient and stable method. Briefly, donor cells were infected by lentivirus carrying target cargoes and stably expressed these cargoes. Then, target cargoes-carried exosomes were isolated from these donor cells. For example, SMSCs were infected by lentivirus carrying miR-126-5p. Then, miR-126-3p overexpressed exosomes (SMSCs-126-Exos) were isolated. SMSCs-126-Exos showed more effectiveness in promoting the proliferation of endothelial cells and fibroblasts and more effective in promoting angiogenesis in diabetic wound healing (18, 21). Similarly, exosomes isolated from Nrf2-overexpressed ADSCs could increase the granulation tissue formation and the levels of growth factor expression and reduce the levels of inflammation and oxidative stress-related proteins (40). Exosomes derived from mmu_circ_0000250-overexpressed ADSCs enhanced the therapeutic effect of exosomes to promote wound healing in diabetes by absorption of miR-128-3p and upregulation of sirtuin (SIRT)1 (43). Exosomes from linc00511-overexpressed ADSCs accelerated angiogenesis in diabetic foot ulcer healing by suppressing PAQR3-induced Twist1 degradation (45). Long non-coding RNA HOX transcript antisense RNA (HOTAIR)-overexpressed BMSCs produce exosomes with increased HOTAIR content that promote angiogenesis and wound healing in diabetes (48). Exosomes from mmu_circ_0001052-overexpressed ADSCs promote angiogenesis of DFU via miR-106a-5p and FGF4/p38MAPK pathway (17).
Other studies have enhanced the role of exosomes by changing the cultural environment of donor cells. Although it was not targeted to modify certain cargoes, it did change the cargoes in exosomes, thereby enhancing the role of exosomes in promoting diabetic wound healing. For example, Melatonin-pretreated MSCs-derived exosomes increased the ratio of M2 polarization to M1 polarization by upregulating the expression of PTEN and inhibiting the phosphorylation of AKT in diabetic wound healing (37). Exosomes derived from atorvastatin-pretreated MSCs accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway (26). Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing by enhancing angiogenesis (15). Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wounds involving activation of PI3K/Akt pathways (35) and to improve wound healing in diabetic mice via delivery of circ-Snhg11 and induction of M2-like macrophage polarization (38).
Although exosome engineering used by different studies showed benefits to the therapeutic effect of exosomes, the contents of exosomes were diverse and complex. Therefore, avoiding ineffective and even harmful ingredients being transferred to the wound is difficult. In our view, the ultimate goal of exosome engineering might be to maximize the valuable components and minimize the useless components rather than focus on a single component. In addition, exosomes mainly play a role in regulating the function of cells and show no direct antibacterial effect. Therefore, it may be an effective strategy to increase its antibacterial ability to use engineering technology to wrap antibacterial drugs in exosomes.
4.3. Adjust the dosage and frequency
The dosage and frequency are unavoidable issues for any drug use. Some studies show that a better therapeutic effect can be achieved simply by increasing the dosage of exosomes, especially in some in vitro studies. For example, the proliferation and migration of fibroblasts induced by exosomes could be increased by increasing the dose of exosomes (21, 24, 32, 46). The uptake of exosomes by endothelial cells also resulted in dose-dependent increases in tube formation and angiogenesis (19, 32). For the frequency, Helena et al. reported that multiple carefully timed applications of exosomes had superior regeneration than a single dose of the same total concentration of exosomes (30). Although there are few exploratory experiments and discussions on dosage and frequency up to now, they are critical factors in the process of exosome application. They should be discussed together with the content of active cargoes in the exosomes. Therefore, it is necessary to test the dose and frequency in the application of exosomes in the same way as conventional drugs are tested (minimum effective dosage, therapeutic dosage, maximum dosage, lethal dosage, etc.).
4.4. Improve the delivery methods
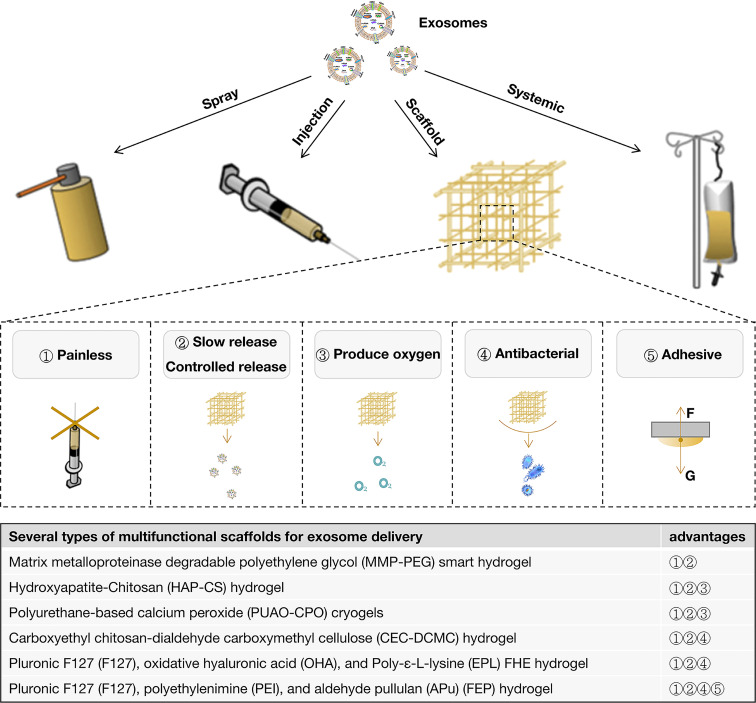
Through the above aspects, exosomes could solve some problems of diabetic wounds that are difficult to heal, such as difficulties in angiogenesis, nerve damage, and some inflammation problems. However, the hypoxia, bacteria, and high glucose levels have not been resolved. This requires better delivery methods to assist the therapeutic effect of exosomes. Drug delivery methods for treating skin wounds can be divided into four types: spraying, local injection, application combined with scaffold materials, and systemic application ( Figure 3 ).
Figure 3.
Exosome delivery methods for diabetic wounds and the advantages of scaffolds.
4.4.1. Local application
Most studies delivered exosomes by subcutaneous injection around the wounds at 2 points (62), at 4 points (14, 17, 19, 22, 23, 35, 38, 43, 51, 56, 59), at multiple points (15, 26, 37) or points unknown (24, 47, 48, 50). Others combined subcutaneous injection around the wounds and injection onto the wound bed to deliver exosomes for diabetic wound healing (58). Moreover, some studies indicated that exosomes were delivered by injection onto the wound bed only (31, 57). Some studies applied exosomes by intradermal injection around the wounds (33, 36), which was regarded as a drug delivery method that could directly stimulate the active cells in the dermis. No matter which injection method was used, it can only maximize the function of exosomes themselves.
To assist the therapeutic effect of exosomes, diverse scaffolds were used to deliver exosomes. Different scaffolds played different regulatory and auxiliary roles in the function of exosomes. In general, the use of all scaffolds reduces the iatrogenic trauma and pain caused by the local injection. It has the effect of slowly releasing exosomes to varying degrees, including some simple (20, 21, 44), thermosensitive (52), photosensitive (56, 61), pH-responsive (41), and biomimetic (67) scaffolds. To control the release of exosomes, Jiang et al. fabricated a matrix metalloproteinase degradable polyethylene glycol (MMP-PEG) smart hydrogel, which could release exosomes by reacting to MMP stimulating (28). Both slow and controlled releases are designed to prolong the exosome’s action time and maintain the wounds’ local drug concentration.
Many studies improved the performance of the scaffolds, including increasing the release of oxygen (11, 18, 60) and improving the antibacterial (41, 49) and adhesive properties (42), which were important in diabetic wound healing ( Figure 3 ). Hydroxyapatite (HAP) was reported to release oxygen (68, 69). Therefore, Li et al. combined HAP and Chitosan (HAP-CS) to form a hydrogel loaded with exosomes to enhance bioactivities, support angiogenesis and promote diabetic wound healing (18). Parvaiz et al. fabricated polyurethane-based oxygen-releasing antioxidant scaffolds (PUAO-CPO) to load exosomes by incorporating calcium peroxide (CPO) in polyurethane (PUAO) cryogels, which showed the sustained release of oxygen and exosomes for more than 10 days. This exosome-loaded scaffold could increase cell survival under hypoxic conditions (11). It was also reported that manganese dioxide (MnO2) could induce the decomposition of endogenous ROS (H2O2) into oxygen and effectively ameliorate oxidative stress and a hypoxic environment. Thus, integrating MnO2 into antibacterial injectable hydrogels fulfill multiple requirements, such as ROS depletion, oxygen production, and antibacterial property. Therefore, loading exosomes to this scaffold is helpful for the repair of diabetic skin wounds (60). Geng et al. indicated that carboxyethyl chitosan-dialdehyde carboxymethyl cellulose (CEC-DCMC) hydrogel showed excellent antibacterial properties and provided a physical and chemical barrier for further infection of diabetes wounds, which played an auxiliary role in the function of exosomes (49). Wang et al. also developed an injectable self-healing polypeptide-based hydrogel that exhibited inherent antibacterial activity (41). In addition to the above characteristics, some studies included the viscosity of scaffold materials to achieve good adhesion to wounds (42).
To maximize the therapeutic effect of exosomes, Wang Min et al. fabricated a thermosensitive, injectable, self-healing, and adhesive polysaccharide-based multifunctional hydrogel scaffold that exhibited efficient antibacterial activity, fast hemostatic ability, good UV-shielding performance, and pH-responsive exosome release for promoting diabetic wounds. These biomedical functions for exosomes-loaded FEP dressing probably enhance their high capability in angiogenesis and wound healing (42). Although this kind of scaffold material with extremely rich functions shows various excellent properties, it is difficult to avoid adding more complex non-medical components, which will delay its clinical transformation. Therefore, how to achieve the balance between effectiveness and safety might need to be comprehensively evaluated.
4.4.2. Systemic application
For diabetic wound healing, we only found one study that delivered exosomes by systemic application via tail vein injection (16). For non-diabetic wound healing, one study has compared the effect of exosomes on wounds by topical injection and intravenous injection and interestingly found that intravenous injection of exosomes could enhance the healing of skin wounds compared to local injection (70). In another study, Zhou et al. systematically compared the effect of different exosome delivery methods for non-diabetic wound healing, and the results showed that the combined application of local smearing and intravenous administration offered the optimal impact on promoting wound healing, accelerating re-epithelialization, reducing scar widths, and enhancing angiogenesis and collagen synthesis (71).
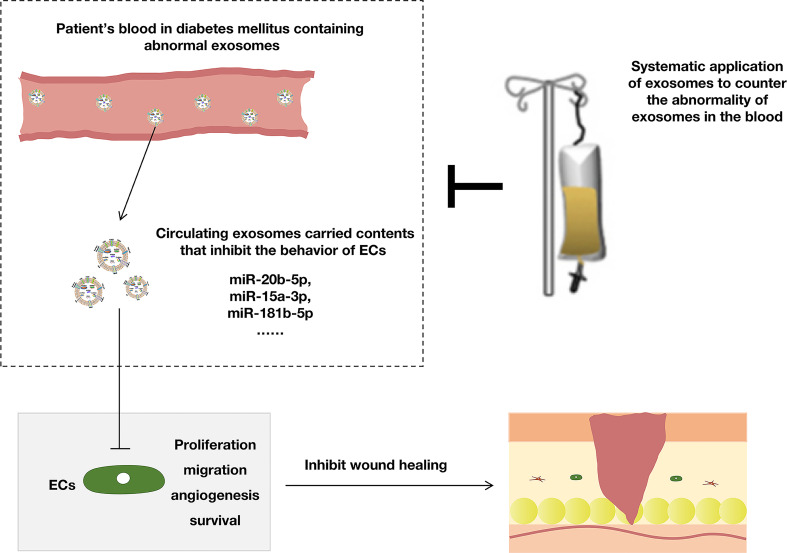
Although the local application can play a good role in treating diabetes wounds, diabetes, as a metabolic-disorder disease, not only causes skin wounds to be difficult to heal but also faces some other physical problems, such as kidney disease, retinopathy, and neuropathy. Moreover, some studies reported that the significant upregulation of miRNAs (miR-20b-5p (72, 73), miR-15a-3p (74), miR-181b-5p (75) were observed in exosomes isolated from patients with diabetes mellitus), and these miRNAs could suppress the angiogenesis of ECs via different signaling pathways. Inhibition of circulating exosomal miRNAs accelerates diabetic wound repair ( Figure 4 ). Therefore, we speculated that the combined local and systemic application of exosomes might benefit diabetic wound healing, and this requires further research.
Figure 4.
The systemic application of exosomes was beneficial to diabetic wounds.
5. Conclusions
Exosomes are a promising therapy for wounds in diabetes, and various ways to maximize their value are discussed in this paper. In this article, we reviewed and discussed the aspects that could be improved, including choosing appropriate donor cells, engineering exosomes, mediating dosage and frequency, and combining more efficient delivery methods ( Figure 5 ). This review might provide an overview and idea for better-using exosomes to treat skin wounds in diabetes mellitus. Further reviews will be necessary to stay up to date with this rapidly evolving area of research.
Figure 5.
How to maximize the therapeutic effect of exosomes on skin wounds in diabetes mellitus.
Author contributions
JD, BW, and WT jointly conceived and discussed the manuscript. JD wrote the original manuscript. WT revised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We gratefully acknowledge the members of the Tian Lab for their helpful discussions and assistance.
Funding Statement
This project was funded by Shenzhen Science and Technology Program (JCYJ20220530165014032), China Postdoctoral Science Foundation (2022M722257), and Construction Funds of Key Medical Disciplines in Longhua District, Shenzhen (MKD202007090212).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis (†). Ann Med (2017) 49(2):106–16. doi: 10.1080/07853890.2016.1231932 [DOI] [PubMed] [Google Scholar]
- 3. Okur ME, Karantas ID, Şenyiğit Z, Üstündağ Okur N, Siafaka PI. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J Pharm Sci (2020) 15(6):661–84. doi: 10.1016/j.ajps.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han G, Ceilley R. Chronic wound healing: A review of current management and treatments. Adv Ther (2017) 34(3):599–610. doi: 10.1007/s12325-017-0478-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Théry C, Witwer K, Aikawa E, Alcaraz M, Anderson J, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles (2018) 7(1):1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castaño C, Novials A, Párrizas M. Exosomes and diabetes. Diabetes Metab Res Rev (2019) 35(3):e3107. doi: 10.1002/dmrr.3107 [DOI] [PubMed] [Google Scholar]
- 7. Li D, Wu N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res Clin Pract (2022) 187:109882. doi: 10.1016/j.diabres.2022.109882 [DOI] [PubMed] [Google Scholar]
- 8. Dehghanbanadaki H, Aazami H, Razi F, Nasli-Esfahani E, Norouzi P, Hashemi E. The global trend of exosome in diabetes research: A bibliometric approach. Diabetes Metab Syndr (2022) 16(4):102450. doi: 10.1016/j.dsx.2022.102450 [DOI] [PubMed] [Google Scholar]
- 9. Johnson NR, Wang Y. Drug delivery systems for wound healing. Curr Pharm Biotechnol (2015) 16(7):621–9. doi: 10.2174/1389201016666150206113720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCrudden MT, McAlister E, Courtenay AJ, González-Vázquez P, Singh TR, Donnelly RF. Microneedle applications in improving skin appearance. Exp Dermatol (2015) 24(8):561–6. doi: 10.1111/exd.12723 [DOI] [PubMed] [Google Scholar]
- 11. Shiekh P, Singh A, Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials (2020) 249:120020. doi: 10.1016/j.biomaterials.2020.120020 [DOI] [PubMed] [Google Scholar]
- 12. Fu W, Liang D, Wu X, Chen H, Hong X, Wang J, et al. Long noncoding RNA LINC01435 impedes diabetic wound healing by facilitating YY1-mediated HDAC8 expression. iScience (2022) 25(4):104006. doi: 10.1016/j.isci.2022.104006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desmet CM, Préat V, Gallez B. Nanomedicines and gene therapy for the delivery of growth factors to improve perfusion and oxygenation in wound healing. Adv Drug Deliv Rev (2018) 129:262–84. doi: 10.1016/j.addr.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 14. Han X, Wu P, Li L, Sahal H, Ji C, Zhang J, et al. Exosomes derived from autologous dermal fibroblasts promote diabetic cutaneous wound healing through the akt/β-catenin pathway. Cell Cycle (Georgetown Tex) (2021) 20:616–29. doi: 10.1080/15384101.2021.1894813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu Y, Tao R, Chen L, Xiong Y, Xue H, Hu L, et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology (2021) 19(1):150. doi: 10.1186/s12951-021-00894-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han Z, Cao J, Liu Z, Yang Z, Qi R, Xu H. Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res Clin Practice (2022) 183:109126. doi: 10.1016/j.diabres.2021.109126 [DOI] [PubMed] [Google Scholar]
- 17. Liang Z, Pan N, Lin S, Qiu Z, Liang P, Wang J, et al. Exosomes from mmu_circ_0001052-modified adipose-derived stem cells promote angiogenesis of DFU via miR-106a-5p and FGF4/p38MAPK pathway. Stem Cell Res Ther (2022) 13(1):336. doi: 10.1186/s13287-022-03015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li M, Ke QF, Tao SC, Guo SC, Rui BY, Guo YP. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J Mater Chem B (2016) 4(42):6830–41. doi: 10.1039/c6tb01560c [DOI] [PubMed] [Google Scholar]
- 19. Zhang J, Chen C, Hu B, Niu X, Liu X, Zhang G, et al. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci (2016) 12(12):1472–87. doi: 10.7150/ijbs.15514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics (2017) 7(1):81–96. doi: 10.7150/thno.16803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tao S, Guo S, Li M, Ke Q, Guo Y, Zhang C. Chitosan wound dressings incorporating exosomes derived from MicroRNA-126-Overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Trans Med (2017) 6(3):736–47. doi: 10.5966/sctm.2016-0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen C, Rao S, Ren L, Hu X, Tan Y, Hu Y, et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics (2018) 8(6):1607–23. doi: 10.7150/thno.22958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding J, Wang X, Chen B, Zhang J, Xu J. Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. BioMed Res Int (2019) 2019:9742765. doi: 10.1155/2019/9742765 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Li M, Wang T, Tian H, Wei G, Zhao L, Shi Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cells Nanomedicine Biotechnol (2019) 47(1):3793–803. doi: 10.1080/21691401.2019.1669617 [DOI] [PubMed] [Google Scholar]
- 25. Wei P, Zhong C, Yang X, Shu F, Xiao S, Gong T, et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burns Trauma (2020) 8:tkaa020. doi: 10.1093/burnst/tkaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu M, Liu W, Li J, Lu J, Lu H, Jia W, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther (2020) 11(1):350. doi: 10.1186/s13287-020-01824-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Zhang P, Gao X, Chang L, Chen Z, Mei X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C Mater Biol Appl (2021) 120:111671. doi: 10.1016/j.msec.2020.111671 [DOI] [PubMed] [Google Scholar]
- 28. Jiang T, Liu S, Wu Z, Li Q, Ren S, Chen J, et al. ADSC-exo@MMP-PEG smart hydrogel promotes diabetic wound healing by optimizing cellular functions and relieving oxidative stress. Materials Today Bio (2022) 16:100365. doi: 10.1016/j.mtbio.2022.100365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu Q, Liu L, Zhang X, Chang H, Ma S, Xie Z, et al. MiR-221-3p targets HIPK2 to promote diabetic wound healing. Microvasc Res (2022) 140:104306. doi: 10.1016/j.mvr.2021.104306 [DOI] [PubMed] [Google Scholar]
- 30. Henriques-Antunes H, Cardoso R, Zonari A, Correia J, Leal E, Jiménez-Balsa A, et al. The kinetics of small extracellular vesicle delivery impacts skin tissue regeneration. ACS Nano (2019) 13(8):8694–707. doi: 10.1021/acsnano.9b00376 [DOI] [PubMed] [Google Scholar]
- 31. Lv Q, Deng J, Chen Y, Wang Y, Liu B, Liu J. Engineered human adipose stem-Cell-Derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol Pharm (2020) 17(5):1723–33. doi: 10.1021/acs.molpharmaceut.0c00177 [DOI] [PubMed] [Google Scholar]
- 32. Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev (2015) 24(14):1635–47. doi: 10.1089/scd.2014.0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li B, Luan S, Chen J, Zhou Y, Wang T, Li Z, et al. The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids (2020) 19:814–26. doi: 10.1016/j.omtn.2019.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nooshabadi VT, Khanmohamadi M, Valipour E, Mahdipour S, Salati A, Malekshahi ZV, et al. Impact of exosome-loaded chitosan hydrogel in wound repair and layered dermal reconstitution in mice animal model. J BioMed Mater Res A (2020) 108(11):2138–49. doi: 10.1002/jbm.a.36959 [DOI] [PubMed] [Google Scholar]
- 35. Wang J, Wu H, Peng Y, Zhao Y, Qin Y, Zhang Y, et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnol (2021) 19(1):202. doi: 10.1186/s12951-021-00942-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regener Med (2019) 13(4):555–68. doi: 10.1002/term.2799 [DOI] [PubMed] [Google Scholar]
- 37. Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther (2020) 11(1):259. doi: 10.1186/s13287-020-01756-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi R, Jin Y, Zhao S, Yuan H, Shi J, Zhao H. Hypoxic ADSC-derived exosomes enhance wound healing in diabetic mice via delivery of circ-Snhg11 and induction of M2-like macrophage polarization. Biomedicine Pharmacother = Biomedecine Pharmacotherapie (2022) 153:113463. doi: 10.1016/j.biopha.2022.113463 [DOI] [PubMed] [Google Scholar]
- 39. Shi Q, Qian Z, Liu D, Sun J, Wang X, Liu H, et al. GMSC-derived exosomes combined with a Chitosan/Silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol (2017) 8:904. doi: 10.3389/fphys.2017.00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li X, Xie X, Lian W, Shi R, Han S, Zhang H, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med (2018) 50(4):1–14. doi: 10.1038/s12276-018-0058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W, et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics (2019) 9(1):65–76. doi: 10.7150/thno.29766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang M, Wang C, Chen M, Xi Y, Cheng W, Mao C, et al. Efficient angiogenesis-based diabetic wound Healing/Skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano (2019) 13(9):10279–93. doi: 10.1021/acsnano.9b03656 [DOI] [PubMed] [Google Scholar]
- 43. Shi R, Jin Y, Hu W, Lian W, Cao C, Han S, et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol (2020) 318(5):C848–56. doi: 10.1152/ajpcell.00041.2020 [DOI] [PubMed] [Google Scholar]
- 44. Pomatto M, Gai C, Negro F, Cedrino M, Grange C, Ceccotti E, et al. Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. Int J Mol Sci (2021) 22(8). doi: 10.3390/ijms22083851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qiu J, Shu C, Li X, Ye C, Zhang WC. Exosomes from linc00511-overexpressing ADSCs accelerates angiogenesis in diabetic foot ulcers healing by suppressing PAQR3-induced Twist1 degradation. Diabetes Res Clin Pract (2021) 180:109032. doi: 10.1016/j.diabres.2021.109032 [DOI] [PubMed] [Google Scholar]
- 46. Xiao S, Xiao C, Miao Y, Wang J, Chen R, Fan Z, et al. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Ther (2021) 12(1):255. doi: 10.1186/s13287-021-02333-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y, Bai X, Shen K, Luo L, Zhao M, Xu C, et al. Exosomes derived from adipose mesenchymal stem cells promote diabetic chronic wound healing through SIRT3/SOD2. Cells (2022) 11(16). doi: 10.3390/cells11162568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Born LJ, Chang KH, Shoureshi P, Lay F, Bengali S, Hsu ATW, et al. HOTAIR-loaded mesenchymal Stem/Stromal cell extracellular vesicles enhance angiogenesis and wound healing. Adv Healthc Mater (2022) 11(5):e2002070. doi: 10.1002/adhm.202002070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Geng X, Qi Y, Liu X, Shi Y, Li H, Zhao L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomaterials Advances (2022) 133:112613. doi: 10.1016/j.msec.2021.112613 [DOI] [PubMed] [Google Scholar]
- 50. Gondaliya P, Sayyed A, Bhat P, Mali M, Arya N, Khairnar A, et al. Mesenchymal stem cell-derived exosomes loaded with miR-155 inhibitor ameliorate diabetic wound healing. Mol Pharm (2022) 19(5):1294–308. doi: 10.1021/acs.molpharmaceut.1c00669 [DOI] [PubMed] [Google Scholar]
- 51. Wang L, Cai Y, Zhang Q, Zhang Y. Pharmaceutical activation of Nrf2 accelerates diabetic wound healing by exosomes from bone marrow mesenchymal stem cells. Int J Stem Cells (2022) 15(2):164–72. doi: 10.15283/ijsc21067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomedicine (2020) 15:5911–26. doi: 10.2147/ijn.S249129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yan C, Xv Y, Lin Z, Endo Y, Xue H, Hu Y, et al. viaHuman umbilical cord mesenchymal stem cell-derived exosomes accelerate diabetic wound healing ameliorating oxidative stress and promoting angiogenesis. Front Bioeng Biotechnol (2022) 10:829868. doi: 10.3389/fbioe.2022.829868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang C, Liang C, Wang R, Yao X, Guo P, Yuan W, et al. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomaterials Sci (2019) 8(1):313–24. doi: 10.1039/c9bm01207a [DOI] [PubMed] [Google Scholar]
- 55. Las Heras K, Royo F, Garcia-Vallicrosa C, Igartua M, Santos-Vizcaino E, Falcon-Perez J, et al. Extracellular vesicles from hair follicle-derived mesenchymal stromal cells: isolation, characterization and therapeutic potential for chronic wound healing. Stem Cell Res Ther (2022) 13(1):147. doi: 10.1186/s13287-022-02824-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Y, Cao Z, Wei Q, Ma K, Hu W, Huang Q, et al. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta Biomater (2022) 147:342–55. doi: 10.1016/j.actbio.2022.05.018 [DOI] [PubMed] [Google Scholar]
- 57. Xu J, Bai S, Cao Y, Liu L, Fang Y, Du J, et al. miRNA-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diabetes Metab Syndr Obes Targets Ther (2020) 13:1259–70. doi: 10.2147/dmso.S243549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Geiger A, Walker A, Nissen E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem Biophys Res Commun (2015) 467(2):303–9. doi: 10.1016/j.bbrc.2015.09.166 [DOI] [PubMed] [Google Scholar]
- 59. Li X, Jiang C, Zhao J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J Diabetes Complications (2016) 30(6):986–92. doi: 10.1016/j.jdiacomp.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 60. Xiong Y, Chen L, Liu P, Yu T, Lin C, Yan C, et al. All-in-One: Multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small (Weinheim an der Bergstrasse Germany) (2022) 18(1):e2104229. doi: 10.1002/smll.202104229 [DOI] [PubMed] [Google Scholar]
- 61. Yuan M, Liu K, Jiang T, Li S, Chen J, Wu Z, et al. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and tazarotene promote diabetic wound healing. J Nanobiotechnol (2022) 20(1):147. doi: 10.1186/s12951-022-01354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen L, Qin L, Chen C, Hu Q, Wang J, Shen J. Serum exosomes accelerate diabetic wound healing by promoting angiogenesis and ECM formation. Cell Biol Int (2021) 45(9):1976–85. doi: 10.1002/cbin.11627 [DOI] [PubMed] [Google Scholar]
- 63. Xu N, Wang L, Guan J, Tang C, He N, Zhang W, et al. Wound healing effects of a curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol (2018) 117:102–7. doi: 10.1016/j.ijbiomac.2018.05.066 [DOI] [PubMed] [Google Scholar]
- 64. Yan C, Chen J, Wang C, Yuan M, Kang Y, Wu Z, et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv (2022) 29(1):214–28. doi: 10.1080/10717544.2021.2023699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Del Pozo-Acebo L, López de Las Hazas MC, Tomé-Carneiro J, Del Saz-Lara A, Gil-Zamorano J, Balaguer L, et al. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol Res (2022), 185:106472. doi: 10.1016/j.phrs.2022.106472 [DOI] [PubMed] [Google Scholar]
- 66. Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo JK, Choi C. Biodistribution of exosomes and engineering strategies for targeted delivery of therapeutic exosomes. Tissue Eng Regener Med (2021) 18(4):499–511. doi: 10.1007/s13770-021-00361-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dong J, Yu M, Zhang Y, Yin Y, Tian W. Recent developments and clinical potential on decellularized adipose tissue. J BioMed Mater Res A (2018) 106(9):2563–74. doi: 10.1002/jbm.a.36435 [DOI] [PubMed] [Google Scholar]
- 68. Meagher MJ, Weiss-Bilka HE, Best ME, Boerckel JD, Wagner DR, Roeder RK. Acellular hydroxyapatite-collagen scaffolds support angiogenesis and osteogenic gene expression in an ectopic murine model: Effects of hydroxyapatite volume fraction. J BioMed Mater Res A (2016) 104(9):2178–88. doi: 10.1002/jbm.a.35760 [DOI] [PubMed] [Google Scholar]
- 69. Lee SH, Elias PM, Proksch E, Menon GK, Mao-Quiang M, Feingold KR. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest (1992) 89(2):530–8. doi: 10.1172/jci115617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep (2016), 6:32993. doi: 10.1038/srep32993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou Y, Zhao B, Zhang X, Lu Y, Lu S, Cheng J, et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther (2021) 12(1):257. doi: 10.1186/s13287-021-02287-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xiong Y, Chen L, Yan C, Zhou W, Endo Y, Liu J, et al. Circulating exosomal miR-20b-5p inhibition restores Wnt9b signaling and reverses diabetes-associated impaired wound healing. Small (2020) 16(3):e1904044. doi: 10.1002/smll.201904044 [DOI] [PubMed] [Google Scholar]
- 73. Chen K, Yu T, Wang X. Inhibition of circulating exosomal miRNA-20b-5p accelerates diabetic wound repair. Int J Nanomedicine (2021) 16:371–81. doi: 10.2147/ijn.S287875 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74. Xiong Y, Chen L, Yu T, Yan C, Zhou W, Cao F, et al. Inhibition of circulating exosomal microRNA-15a-3p accelerates diabetic wound repair. Aging (Albany NY) (2020) 12(10):8968–86. doi: 10.18632/aging.103143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang S, Shi M, Zhou J, Wang W, Zhang Y, Li Y. Circulating exosomal miR-181b-5p promoted cell senescence and inhibited angiogenesis to impair diabetic foot ulcer via the nuclear factor erythroid 2-related factor 2/Heme oxygenase-1 pathway. Front Cardiovasc Med (2022) 9:844047. doi: 10.3389/fcvm.2022.844047 [DOI] [PMC free article] [PubMed] [Google Scholar]