Abstract
Background
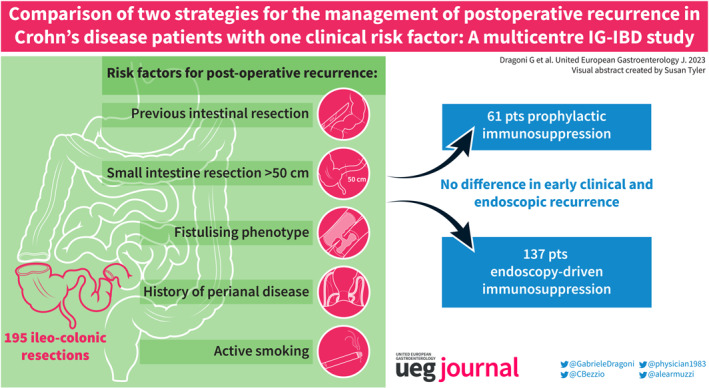
The management of postoperative recurrence (POR) in Crohn's disease (CD) after ileo‐colonic resection is a highly debated topic. Prophylactic immunosuppression after surgery is currently recommended in the presence of at least one clinical risk factor.
Objective
Our aim was to determine whether early immunosuppression can be avoided and guided by endoscopy in CD patients with only one risk factor.
Methods
CD patients with only one risk factor for POR, including previous intestinal resection, extensive small intestine resection (>50 cm), fistulising phenotype, history of perianal disease, and active smoking, were retrospectively included. Two groups were formed based on whether immunosuppression was started immediately after surgery (“prophylaxis group”) or guided by endoscopy (“endoscopy‐driven group”). Primary endpoints were rates of any endoscopic recurrence (Rutgeerts ≥ i2a) and severe endoscopic recurrence (i4) within 12 months after surgery. Secondary outcomes were clinical recurrence rates at 6, 12 and 24 months after surgery.
Results
A total of 195 patients were enroled, of whom 61 (31.3%) received immunoprophylaxis. No differences between immunoprophylaxis and the endoscopy‐driven approach were found regarding any endoscopic recurrence (36.1% vs. 45.5%, respectively, p = 0.10) and severe endoscopic recurrence (9.8% vs. 15.7%, respectively, p = 0.15) at the first endoscopic evaluation. Clinical recurrence rates were also not statistically different (p = 0.43, p = 0.09, and p = 0.63 at 6, 12, and 24 months, respectively).
Conclusions
In operated CD patients with only one risk factor for POR, immediate immunoprophylaxis does not decrease the rate of early clinical and endoscopic recurrence. Prospective studies are needed to confirm our results.
Keywords: Crohn's disease, endoscopic recurrence, ileo‐colonic resection, immunoprophylaxis, inflammatory bowel disease, postoperative Crohn's disease, postoperative recurrence, Rutgeerts Score
Key Summary.
Management of Crohn's disease (CD) postoperative recurrence (POR) after ileo‐colonic resection is a highly debated topic. Although postoperative prophylactic administration of either thiopurines or biologics seems appropriate in patients at high risk of POR, data are missing about the proper approach of patients at intermediate risk.
There is no clear evidence supporting the administration of early prophylactic therapy over the endoscopy‐guided approach in patients with only one risk factor for POR.
In this study, we report that systematic immunoprophylaxis does not seem superior to endoscopy‐driven approach to prevent POR in CD patients with only one risk factor.
Our findings are irrespective of previous exposure to biological agents and the type of risk factor, among which active smoking, previous resections, extensive resection, perianal disease and penetrating disease were considered.
INTRODUCTION
Crohn's disease (CD) is a chronic inflammatory condition of the bowel with a complex pathogenesis in which environmental triggers and gut microbiota shape the immune response towards the intestinal tissue in individuals with genetic predisposition. 1 All gastrointestinal (GI) tracts can be involved in a discontinuous manner with the terminal ileum and right colon being the most frequently involved. 2 Although most CD patients present with an inflammatory phenotype at diagnosis, approximately half of them will need at least one intestinal resection during their lifetime due to treatment failure or development of stricturing or penetrating complications. 3 After resective surgery, new lesions in the neo‐terminal ileum have been observed in up to 73% of patients at 1 year, with many of them requiring medical treatment or even re‐resection. 4 The postoperative recurrence (POR) of CD is more likely to occur in the presence of any of the known clinical risk factors, such as smoking, prior intestinal surgery, penetrating disease at index surgery, perianal location, and extensive small bowel resection (>50 cm). 5 , 6 , 7 , 8 , 9 On the other hand, it has been shown that early treatment with thiopurines or anti‐TNF 10 as well as the use of Kono‐S anastomosis 11 play some protective role.
To date, the prevention of POR after ileo‐colonic (IC) resection is a highly debated topic in CD management. Prophylactic immunosuppression with either thiopurines or anti‐TNFs is recommended by current European Crohn's and Colitis Organization (ECCO) guidelines to prevent POR in the presence of at least one of the abovementioned clinical risk factors. 12 Nevertheless, there is still no clear evidence showing that the administration of an early prophylactic therapy would be superior to starting a treatment on an endoscopy‐guided approach. The POCER trial suggested that a treat‐to‐target strategy (with ileocolonoscopy at 6 months and treatment step‐up if recurrence was detected) was superior to a symptoms‐driven approach in patients at high risk of recurrence, irrespective of the initiation of the prescribed immunoprophylaxis immediately after surgery. 13 Moreover, the PREVENT trial showed the superiority of infliximab over placebo administered within 45 days after surgery, for the prevention of endoscopic POR at 1 year, although no clear advantage being shown in the primary endpoint of clinical recurrence. 14 In this context, the 2019 Y‐ECCO/ClinCom Survey showed that the clinical management of POR prevention is highly heterogeneous among European gastroenterologists. 15
Although prophylactic immunosuppression after surgery in CD patients with many POR risk factors seems appropriate, data are missing about the proper approach of patients with only one risk factor for POR. As immunosuppressive medications are not free of side effects and some of them still present a non‐negligible cost for the healthcare systems, 16 , 17 we aimed to determine whether the prevention of POR with immunomodulating agents is superior to the endoscopy‐driven management of CD recurrence in patients with only one risk factor.
MATERIALS AND METHODS
Study design
A multicentre, retrospective, observational study was conducted in 13 centres across Italy under the endorsement of the Italian Group for the study of Inflammatory Bowel Disease (IG‐IBD).
The study was approved by the Ethical Committee of the Coordinating Centre (Careggi University Hospital, Florence, Italy) on 24 June 2021 (Ref CEAVC‐19591). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Because of its retrospective nature, informed consent of patients was not mandatory at the time of data collection. Every Centre independently uploaded patients' information in specific electronic case report forms (e‐CRF) of the IG‐IBD registry platform from November 2021 to June 2022.
Patient characteristics
All CD patients who underwent curative IC resection and who had only one risk factor for POR among previous intestinal resection, extensive small intestine resection (>50 cm), fistulising phenotype (presence of abscess, fistulae, or bowel perforation), history of perianal disease, or active smoking were included in the study. At least one colonoscopy between 6 and 12 months (±2 months) after surgery was deemed mandatory to be eligible for the study. Exclusion criteria were as follows: age <18 years at the time of surgery, none or more than one POR risk factor, persisting macroscopic disease activity after surgical resection (e.g., colonic, upper GI, perianal disease), surgical anastomosis different from IC anastomosis, presence of an end stoma (ileostomy or colostomy), endoscopically inaccessible anastomosis by standard colonoscopy, and pregnancy.
Patients were divided into two groups depending on whether they started immunoprophylaxis within 6 weeks of surgery (“prophylaxis group”) or treatment was guided by subsequent ileocolonoscopy (“endoscopy‐driven group”). The decision of starting immunoprophylaxis or waiting for the results of postoperative endoscopy was taken locally by the treating physicians of each Centre and based on the individual assessment of each patient. Therapies considered in the “prophylaxis group” were infliximab (5 mg/kg intravenous [i.v.] at weeks 0, 2, 6 followed by 5 mg/kg i.v. every 8 weeks); adalimumab (160 mg subcutaneous [s.c.] at week 0, 80 mg s.c. at week 2 followed by 40 mg s.c. every other week); ustekinumab (6 mg/kg i.v. at week 0 followed by 90 mg s.c. at week 8 and then every 12 weeks); vedolizumab 300 mg i.v. at week 0, 2, 6 followed by 300 mg i.v. every 8 weeks; azathioprine 2–2.5 mg/kg/day orally. In the “endoscopy‐driven group”, one of the same treatments could have been administered only in case of endoscopic recurrence. No treatment intensification was allowed in the “prophylaxis group” before the first endoscopic evaluation.
Demographic and clinical variables considered were age, gender, age at diagnosis, age at surgery, disease duration at surgery, the disease phenotype based on the Montreal classification, 18 smoking status, previous thiopurine or biological drug administration, previous intestinal surgery, specific risk factor for POR, and information at index surgery (i.e., type of anastomosis and urgent vs. elective surgery).
Clinical activity was scored with Harvey‐Bradshaw Index (HBI), 19 with clinical recurrence defined as HBI ≥ 5. Ileocolonoscopy between 6 and 12 months after surgery was assessed with Rutgeerts Score 4 ; endoscopic recurrence was defined as Rutgeerts score ≥ i2a 20 with severe recurrence identified by Rutgeerts score of i4.
Outcomes
The primary endpoint was to compare the prevalence of any endoscopic recurrence and severe endoscopic recurrence at the time of first postoperative colonoscopy between the two groups. Secondary endpoints were as follows: short‐term and long‐term clinical recurrence rates (12 and 24 months after surgery, respectively), long‐term endoscopic recurrence rates in case patients had performed a second colonoscopy at least 18 months after surgery, CD‐related re‐surgery rates, and long‐term CD‐related rates of intestinal complications (development of stenosis, fistulae, and abscesses).
Statistical analysis
Continuous variables were expressed as medians and interquartile ranges (IQR), while categorical variables were presented as percentages. Homogeneity between the two study groups and clinical recurrence rates at fixed timepoints were tested with Pearson's Chi‐square test and Fisher's exact test for small samples for categorical variables, while Kruskal–Wallis Test and Mann–Whitney U Test were used for continuous variables. Kaplan‐Meier curves with Log rank test were performed to compare endoscopic recurrence and treatment persistence in the two groups. Binary logistic regression analysis and Cox proportional‐hazard regression model were used for multivariate analysis of potential confounding factors (i.e., the five clinical risk factor for POR, previous experience with biologics before surgery, the use of Kono‐S anastomosis, and the need for urgent surgery). A two‐sided p value < 0.05 was considered significant. All statistical analyses were performed using SPSS Statistics version 25.0 (IBM, Armonk, NY, USA).
RESULTS
One hundred and ninety‐five patients were enroled, 61 (31.3%) of whom were administered immunoprophylactic therapy. Risk factors for POR were homogeneously distributed between the two groups. Baseline patient characteristics are detailed in Table 1. Patients (n = 61) in the “prophylaxis group” started their therapy after a median time of 32 (IQR 26–55) days after surgery, with 21 cases that had to postpone the first administration beyond 6 weeks due to personal reasons. Among them, 14 patients were on infliximab, 40 on adalimumab (of which 3 patients were in combination‐therapy with azathioprine), 4 on azathioprine monotherapy, and 3 on ustekinumab.
TABLE 1.
Baseline characteristics of the population.
| Total | Prophylaxis group | Endoscopy‐driven group | p values | |
|---|---|---|---|---|
| (n = 195) | (n = 61) | |||
| (n = 134) | ||||
| Females, n (%) | 89 (45.6) | 27 (44.3) | 62 (46.3) | 0.92 |
| Median age at surgery, years (IQR) | 35 (25–47) | 31 (23.5–41) | 28.5 (26.5–50) | 0.004 |
| Median disease duration at surgery, years (IQR) | 5 (1–12) | 6 (2–13.5) | 5 (1–5) | 0.31 |
| Montreal A n (%) | ||||
| A1 | 27 (13.8) | 12 (19.7) | 15 (11.2) | 0.17 |
| A2 | 113 (57.9) | 42 (68.8) | 71 (53.0) | 0.05 |
| A3 | 55 (28.2) | 7 (11.5) | 48 (35.8) | 0.001 |
| Montreal B n (%) | ||||
| B1 | 13 (6.7) | 2 (3.3) | 11 (8.2) | 0.33 |
| B2 | 94 (48.2) | 35 (57.4) | 59 (49.3) | 0.11 |
| B3 | 88 (45.1) | 24 (39.3) | 64 (42.5) | 0.35 |
| Montreal L n (%) | ||||
| L1 | 94 (48.2) | 25 (41.0) | 69 (51.5) | 0.23 |
| L2 | 6 (3.1) | 3 (4.9) | 3 (2.2) | 0.58 |
| L3 | 95 (48.7) | 33 (54.1) | 62 (46.3) | 0.39 |
| Experienced to biological agents, n (%) | 70 (35.9) | 26 (42.6) | 44 (32.8) | 0.25 |
| Active smokers, n (%) | 67 (34.4) | 18 (29.5) | 49 (36.6) | 0.42 |
| Quit smoking at surgery, n (%) | 6 (3.1) | 0 (0.0) | 6 (4.5) | 0.42 |
| Risk factors for POR n (%) | ||||
| Active smoker after surgery | 61 (31.3) | 18 (29.5) | 43 (32.1) | 0.85 |
| Penetrating disease | 88 (45.1) | 24 (39.3) | 64 (47.8) | 0.35 |
| Previous resection | 25 (12.8) | 10 (16.4) | 15 (11.2) | 0.44 |
| History of perianal disease | 14 (7.2) | 7 (11.5) | 7 (5.2) | 0.20 |
| Large resection (>50 cm) | 7 (3.6) | 2 (3.3) | 5 (3.7) | 0.80 |
Abbreviations: IQR, interquartile range; POR, postoperative recurrence.
The first colonoscopy was performed after a median time of 8 months (IQR 6–11) in the “endoscopy‐driven group” and after a median time of 10 months (IQR 7–11) in the “prophylaxis group”. No differences between primary prophylaxis and endoscopy‐driven approach were found regarding any endoscopic recurrence (22/61 vs. 61/134, 36.1% vs. 45.5%, respectively, p = 0.10, Figure 1a) and severe endoscopic recurrence (6/61 vs. 21/134, 9.8% vs. 15.7%, respectively, p = 0.15, Figure 1b). In the “prophylaxis group”, there were no differences in recurrence rates between patients treated with anti‐TNF agents and other treatments (20/54 vs. 2/7, 58.8% vs. 28.6%, p = 0.98). Thirty‐two (16.4%) patients had a second colonoscopy 30.5 (IQR 22–43.75) months after surgery, 5 in the “prophylaxis group” and 27 in the “endoscopy‐driven group”. In these patients, any endoscopic recurrence (1.6% vs. 11.9%, p = 0.55, Figure 2a) and severe endoscopic recurrence (0.0% vs. 5.2%, p = 0.43, Figure 2b) were also not significantly different.
FIGURE 1.
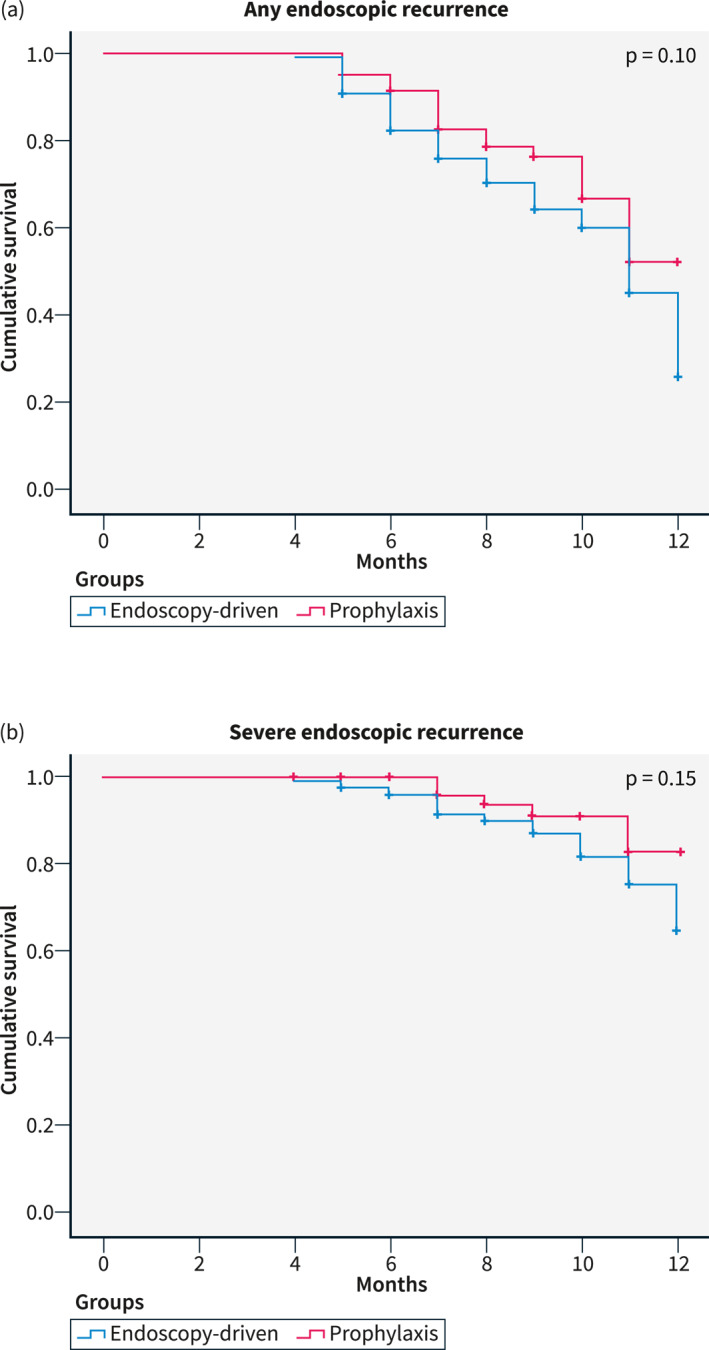
Kaplan‐Meier curves for early endoscopic recurrence.
FIGURE 2.
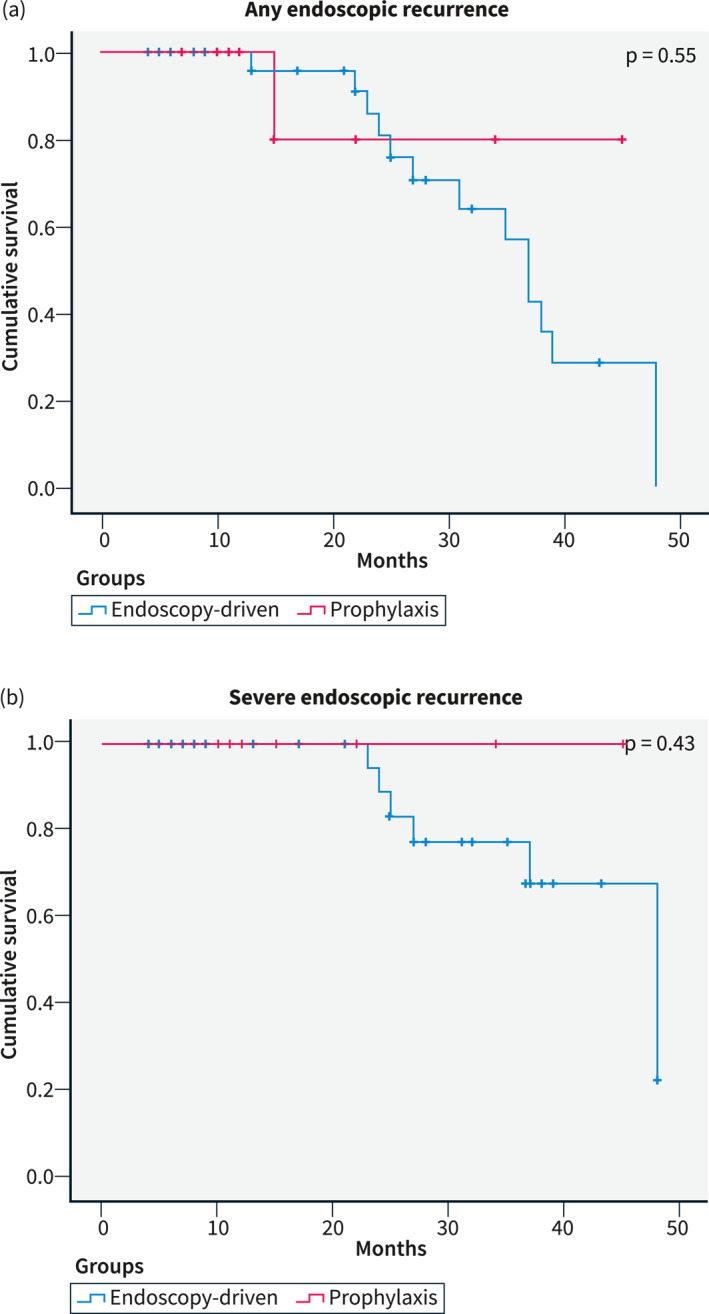
Kaplan‐Meier curves for late endoscopic recurrence.
Clinical data were available for 136 (69.7%) patients at 6 months, for 128 (65.6%) patients at 12 months, and for 97 (49.7%) patients at 24 months after surgery. Early clinical recurrence (i.e., 6 months) was reported in 11/47 (23.4%) patients on immunoprophylaxis versus 28/89 (31.5%) in patients who were not (p = 0.43). Clinical recurrence rates between the two groups were also not statistically different at 12 months (7/39 “prophylaxis group” vs. 31/89 “endoscopy‐driven group”, 17.9% vs. 34.8%, p = 0.09) and at 24 months (7/39 “prophylaxis group” vs. 14/58 “endoscopy‐driven group”, 17.9% vs. 24.1%, p = 0.63).
As reported in Table 2, no differences regarding early endoscopic recurrence rates between the two groups were found after stratification based on the type of risk factor for POR. Of the whole population, 35.9% of patients experienced biological therapy before surgery, equally distributed between “prophylaxis” and “endoscopy‐driven” groups (42.6% vs. 32.8%, respectively, p = 0.25, Table 1). Similar endoscopic recurrence rates at the first timepoint were reported in patients experienced or naïve to biologics before surgery (34/70 vs. 49/125, respectively, 48.6% vs. 39.2%, p = 0.26).
TABLE 2.
Recurrence rates divided per risk factor.
| Prophylaxis group | Endoscopy‐driven group | p values | |
|---|---|---|---|
| (n = 22, 36.1% a ) | (n = 61, 45.5% a ) | ||
| Active smoker after surgery | 7 (11.5% b ) | 17 (12.7%) | 0.81 |
| Penetrating disease | 10 (16.4%) | 30 (22.4%) | 0.84 |
| Previous resection | 2 (3.3%) | 7 (5.2%) | 0.23 |
| History of perianal disease | 1 (1.6%) | 3 (2.2%) | 0.56 |
| Extensive small intestine resection (>50 cm) | 2 (3.3%) | 4 (3.0%) | 1 |
Recurrence rates.
Percentage of total recurrences.
In multivariate analysis, extensive small intestine resection was the only risk factor significantly associated with any and with severe endoscopic recurrence at the first timepoint (p = 0.03 and p = 0.04, respectively, see Supplementary Table S1). On the other hand, the use of previous biologics was the only factor influencing the occurrence of any (but not severe) endoscopic recurrence at the second timepoint (p = 0.02, Supplementary Table S2). In all analyses, the use of early immunoprophylaxis was not associated with a clear benefit on endoscopic outcomes. No factor was independently associated with clinical recurrence by logistic regression analysis (Supplementary Table S3).
Among the 61 patients in the “prophylaxis group”, the median time of treatment at the time of analysis was 32 (IQR 22–55) months. Twenty‐seven patients of this group (44.3%) interrupted the treatment during the observational period for the following reasons: 6 (9.8%) CD recurrence after dose escalation, 18 (29.5%) adverse events, 2 (3.3%) sustained remission and 1 (1.6%) for patient decision. Forty‐nine (80.3%) out of the 61 patients who had an endoscopic recurrence in the “endoscopy‐driven group” after the first colonoscopy started a therapy (24 adalimumab, 8 ustekinumab, 7 azathioprine, 4 infliximab, 4 vedolizumab, 2 combinations of anti‐TNF plus azathioprine), and the median treatment survival was 22.5 (IQR 14–48.5) months. In this group, 69.4% of the patients who started a therapy were still receiving the same medication at the time of data collection. Two patients stopped the treatment because of primary nonresponse, 5 because of secondary loss of response, 5 due to adverse events and 3 for unspecified reasons. The probability of treatment survival was numerically higher in the “prophylaxis group”, but without reaching statistical significance (p = 0.06), as shown in Figure 3.
FIGURE 3.
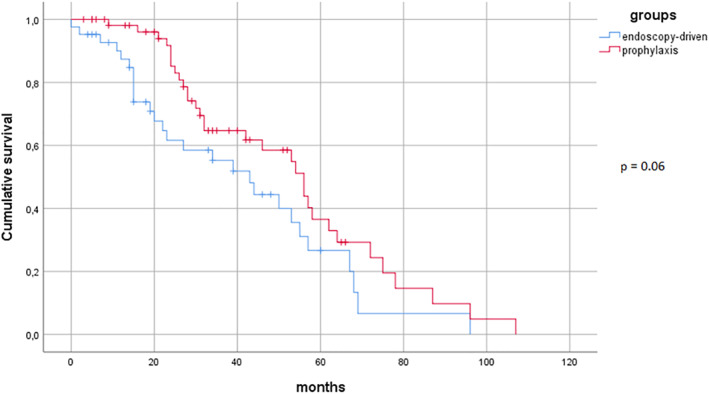
Kaplan‐Meier curves for treatment persistence.
The median overall follow‐up period was 25 (IQR 18–48) months in the “prophylaxis group” and 18 (IQR 11–24) months in the “endoscopy‐driven group”. Data on re‐surgeries and long‐term CD complications (fistulas, abscesses, strictures) were not available for most of the patients and were excluded from the analysis.
DISCUSSION
Despite the increasing number of available medications for the management of CD, surgery remains an essential therapeutic option for the treatment of its stricturing and penetrating complications. Around one out of two CD patients will require a surgical resection during their lifetime, 3 and repeated surgery with additional resection is nowadays reported as high as 8.8% within 10 years from the index surgery. 21 Although current guidelines suggest administering a prophylactic therapy with thiopurines or anti‐TNFs in patients with at least one clinical risk factor for recurrence, 12 it is still unclear whether this would be the best fitting approach to prevent POR in all patient subgroups. Clinical risk factors are often considered together and there is little evidence regarding the weighed impact of each risk factor. A recent review suggests an approach with a short course of metronidazole or treatment with thiopurines or anti‐TNF in patients with one risk factor, whereas anti‐TNF agents (or newer biologics in case of anti‐drug antibodies) should be considered in case that two or more risk factors are present. 22 However, studies providing data for patients with a single risk factor for POR are currently lacking, and guidelines do not suggest different treatment strategies based on the number of risk factors. 12
A recent survey among European gastroenterologists has shown that the management of recently operated CD patients is still heterogeneous across Centres; particularly, the approach may differ based on the number of risk factors for POR that the patient presents after surgery. 15 For this reason, we aimed to perform a retrospective, multicentre, observational study to compare the two main approaches in this scenario, that is, the systematic immunoprophylactic therapy with thiopurines or biological agents immediately after surgery, and the endoscopy‐driven approach based on the first colonoscopy performed between 6 and 12 months after surgery.
In our cohort, the two strategies resulted comparable with regard to endoscopic recurrence at first ileocolonoscopy and clinical recurrence at all timepoints (6, 12 and 24 months after surgery). The occurrence of POR was independent of all baseline characteristics considered, including previous exposure to biologics. Most importantly, the type of risk factor does not significantly impact the comparison of recurrence rates in the two populations.
A few years ago, the POCER trial showed that a colonoscopy‐driven approach 6 months after surgery and treatment step‐up in case of Rutgeerts score ≥ i2 reduces the rate of endoscopic recurrence at 18 months compared to clinical follow‐up alone (adjusted odds ratio [OR] 0.45, 95% confidence interval [CI] 0.22–0.93, p = 0.03). 13 In this cohort, smoking was an independent risk factor for POR (OR 2.4, 95% CI 1.2–4.8; p = 0.02), whereas previous resections and penetrating disease were not; most interestingly, patients with two or more risk factors had a 2.8 times higher risk of endoscopic recurrence compared with patients with no or one risk factor. Thus, in this large and controlled cohort study, having only one risk factor was identified as a “grey zone” of possible low‐risk patients.
In accordance with the evidence of the POCER trial, ECCO guidelines suggest colonoscopy 6–12 months after surgery as the best approach to guide clinical management of CD after intestinal resection. 12 Further results have confirmed that the most informative evaluation is the one performed within one year and that late endoscopy (>36 months) has no prognostic value. 23
Both biologics and thiopurines may be used for POR prevention. The PREVENT trial did not show significant benefit of infliximab over placebo with regard to the primary outcome of clinical recurrence before or at week 76 (p = 0.097), but infliximab was found superior in reducing the risk of endoscopic recurrence defined as Rutgeerts score ≥ i2a, before or at week 76 (22.4% vs. 51.3%, p < 0.001). 14 Thiopurines are an alternative to anti‐TNFs, with azathioprine that showed similar efficacy to adalimumab in preventing endoscopic recurrence at 1 year in a multicentre randomised trial (APPRECIA study). 24 Despite this evidence, subsequent meta‐analyses reported that anti‐TNFs are the best medication to prevent endoscopic POR when compared to azathioprine, antibiotics and mesalamine. 25 , 26 Recent data have also shown that both vedolizumab and ustekinumab may be used in this setting with results comparable to anti‐TNFs. 10 , 27 In our “prophylaxis group”, no difference was found in endoscopic recurrence rates between patients administered with anti‐TNF agents and those with other immunomodulators, although it is difficult to draw conclusions in the absence of adequate power for this analysis.
To our knowledge, this is the first study that retrospectively compares the two main postoperative strategies in CD patients with only one risk factor for POR. A similar study comparing systematic immunoprophylaxis versus endoscopy‐driven approach has already been attempted with azathioprine in patients with one or more risk factors for POR. Although the trial was prematurely interrupted due to slow recruitment, early postoperative azathioprine was not superior to no treatment regarding endoscopic recurrence 6 months after surgery. 28 The study was not powered for subgroup analysis based on the number of risk factors that were present, but the conclusions are comparable to our work.
In support of our data, a multicentre retrospective study observed the long‐term outcomes of a cohort of 86 CD patients who underwent IC resection and did not have signs of recurrence at the first‐evaluation colonoscopy performed between 6 and 12 months after surgery. 29 Approximately half of the patients received the immunoprophylactic therapy before the first endoscopic evaluation. The authors analysed the occurrence of a composite outcome including clinical recurrence, hospitalisation for CD and other recurrence surrogates. In patients who experienced recurrence after a median time of 14.2 months (IQR 6.3–26.1), this event was independent of initial medical prophylaxis (p = 0.90). However, in this study, the number of risk factors was not considered in the analysis. 29
Recently, Joustra et al. analysed retrospective data from a large cohort of 376 CD patients undergoing ileocecal resection and with at least 3 years of follow‐up. 30 Although endoscopic POR at 1 year was more frequent in high‐risk patients (defined as ≥1 risk factor) that were not on immunoprophylactic treatment, a larger albeit not significant difference in 3‐year clinical POR was mainly observed in patients with three or more risk factors for POR (62.5% vs. 28.6% of the remainder, p = 0.11). 30 Considering this evidence, the authors concluded that immunoprophylaxis may present clinical benefit in patients with ≥3 risk factor for POR, whereas an endoscopy‐driven approach can be the preferred strategy in the remainder to avoid unnecessary exposure to treatment side effects.
Our study has some important limitations. First, its retrospective design is inevitably associated with missing data, including some clinical timepoints, biomarkers of disease activity (i.e., faecal calprotectin), and information about serum drug levels and anti‐drug antibodies. Particularly, the small amount of available information on hospitalisation and additional surgeries reduces the strength of our findings regarding which strategy may be preferable for the long‐term POR prevention and control. In addition, a small group of patients in the “prophylaxis group” postponed the beginning of immunosuppression beyond 6 weeks for personal reasons, representing another potential limitation. Moreover, despite a large multicentre cohort, some of the comparisons may not be adequately powered. Finally, selection bias may have influenced the result and prospective trials would be needed to confirm our data.
In conclusion, this study shows that systematic immunoprophylaxis does not seem superior to the endoscopy‐driven approach to prevent POR in CD patients with only one risk factor for POR. Moreover, previous exposure to biologicals and the type of risk factor did not influence the outcome. Based on our results, it appears probable that the setting of high POR risk that deserves immunosuppressive prophylaxis is not when only one risk factor is present but when more than one concur in the same patient. This may avoid unnecessary exposure to immunosuppressants, that is also more difficult to accept for patients who have no active disease at the time the treatment is suggested.
AUTHOR CONTRIBUTIONS
Gabriele Dragoni and Gionata Fiorino designed the study. Gabriele Dragoni, Gionata Fiorino and Tommaso Innocenti performed statistical analysis. Gabriele Dragoni and Tommaso Innocenti wrote the manuscript. All authors collected patients' data, interpreted the results, critically revised the article, and approved the final version to be submitted. GF is the guarantor of the article.
CONFLICT OF INTEREST STATEMENT
Gabriele Dragoni reports speaker fees from Janssen and Novartis. Fabiana Castiglione reports advisory boards for AbbVie, Fresenius, Janssen, Pfizer and Takeda. Cristina Bezzio received lecture fees and served as a consultant for Ferring, Galapagos, Janssen, MSD and Takeda. Daniela Pugliese received speaker fees and/or advisory board fees from AbbVie, Janssen, MSD, Pfizer and Takeda. Annalisa Aratari received consultancy fees from Galapagos. Edoardo Vincenzo Savarino has served as a speaker for AbbVie, AGPharma, Alfasigma, EG Stada Group, Fresenius Kabi, Grifols, Janssen, Innovamedica, Malesci, Pfizer, Reckitt Benckiser, Sandoz, SILA, Sofar, Takeda, Unifarco; has served as consultant for Alfasigma, Amgen, Biogen, Bristol‐Myers Squibb, Celltrion, Diadema Farmaceutici, Falk, Fresenius Kabi, Janssen, Merck & Co, Reckitt Benckiser, Regeneron, Sanofi, Shire, SILA, Sofar, Synformulas GmbH, Takeda, Unifarco; he received research support from Reckitt Benckiser, SILA, Sofar, Unifarco. Paola Balestrieri has participated to advisory boards for Janssen and Takeda. Sara Onali received speaker fees from AbbVie, Galapagos, Janssen, Norgine, and Takeda. DGR reports consultancies with AbbVie, Galapagos, Janssen and Takeda. Anna Testa reports advisory boards for Galapagos, Janssen, Pfizer, Takeda. Simone Saibeni received lecture fees from Janssen and Takeda and served as a consultant and advisory board member for AbbVie and Janssen. Giuseppe Privitera reports consultancy fees from Alphasigma and Janssen. Annalisa Aratari has received consulting/advisory board fees from AbbVie, Allergan, Amgen, Arena, Biogen, Bristol‐Myers Squibb, Celgene, Celltrion, Eli‐Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz, Takeda; speaker's fees from AbbVie, Amgen, Arena, Biogen, Bristol‐Myers Squibb, Eli‐Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda, Tigenix; research support from Biogen, MSD, Pfizer and Takeda. MCF received consultancy fees from AbbVie, Biogen, Bristol‐Myers Squibb, Galapagos, Janssen‐Cilag, Pfizer, Sandoz, Takeda, and research grants from Janssen‐Cilag and Pfizer. Gionata Fiorino served as a Consultant for AbbVie, Amgen, Celltrion, Ferring, Galapagos, Janssen, Pfizer, Sandoz and Takeda. The other authors have no conflicts of interest to declare.
ETHICAL APPROVAL
The study was approved by the Ethical Committee of the Coordinating Centre (Careggi University Hospital, Florence, Italy) on 24 June 2021 (Ref CEAVC‐19591).
Supporting information
Supporting Information S1
ACKNOWLEDGEMENTS
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
STROBE Statement—checklist of items that should be included in reports of observational studies
| Item No | Recommendation | Page No | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study's design with a commonly used term in the title or the abstract | 1 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 4–5 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 6–7 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 7 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 8 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow‐up, and data collection | 8–9 |
| Participants | 6 | (a) Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow‐up | 8–9 |
| Case‐control study—Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls | |||
| Cross‐sectional study—Give the eligibility criteria, and the sources and methods of selection of participants | |||
| (b) Cohort study—For matched studies, give matching criteria and number of exposed and unexposed | 8–9 | ||
| Case‐control study—For matched studies, give matching criteria and the number of controls per case | |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 8–9 |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe the comparability of assessment methods if there is more than one group | 8–9 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 8–10 |
| Study size | 10 | Explain how the study size was arrived at | 8–10 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 10 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 10 |
| (b) Describe any methods used to examine subgroups and interactions | 10 | ||
| (c) Explain how missing data were addressed | 10 | ||
| (d) Cohort study—If applicable, explain how loss to follow‐up was addressed | NA | ||
| Case‐control study—If applicable, explains how matching of cases and controls was addressed | |||
| Cross‐sectional study—If applicable, describes analytical methods taking into account the sampling strategy | |||
| (e) Describe any sensitivity analyses | 10 | ||
| Results | |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study, e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow‐up, and analysed | 11 |
| (b) Give reasons for non‐participation at each stage | 11 | ||
| (c) Consider the use of a flow diagram | NA | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 11 |
| (b) Indicate the number of participants with missing data for each variable of interest | 11 | ||
| (c) Cohort study—Summarise follow‐up time (e.g. average and total amount) | 11 | ||
| Outcome data | 15* | Cohort study—Report numbers of outcome events or summary measures over time | NA |
| Case‐control study—Report numbers in each exposure category or summary measures of exposure | 11–13 | ||
| Cross‐sectional study—Report numbers of outcome events or summary measures | NA | ||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder‐adjusted estimates and their precision (e.g. 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 11–13 |
| (b) Report category boundaries when continuous variables were categorized | 11–13 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk of a meaningful time period | NA | ||
| Other analyses | 17 | Report other analyses conducted, analyses of subgroups and interactions, and sensitivity analyses | 11–13 |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 14–15 |
| Limitations | 19 | Discuss the limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 17 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 14–17 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 16–17 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | NA |
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at www.strobe-statement.org.
*Give information separately for cases and controls in case‐control studies and, if applicable, for exposed and unexposed groups in cohort and cross‐sectional studies.
Dragoni G, Castiglione F, Bezzio C, Pugliese D, Spagnuolo R, Viola A, et al. Comparison of two strategies for the management of postoperative recurrence in Crohn’s disease patients with one clinical risk factor: a multicentre IG‐IBD study. United European Gastroenterol J. 2023;11(3):271–81. 10.1002/ueg2.12367
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. De Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13(1):13–27. Review. 10.1038/nrgastro.2015.186 [DOI] [PubMed] [Google Scholar]
- 2. Torres J, Mehandru S, Colombel J.‐F, Peyrin‐Biroulet L. Crohn's disease. Lancet. 2017;389(10080):1741–55. 10.1016/S0140-6736(16)31711-1 [DOI] [PubMed] [Google Scholar]
- 3. Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, et al. ECCO guidelines on therapeutics in Crohn's disease: surgical treatment. J Crohns Colitis. 2020;14(2):155–68. 10.1093/ecco-jcc/jjz187 [DOI] [PubMed] [Google Scholar]
- 4. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99(4):956–63. 10.1016/0016-5085(90)90613-6 [DOI] [PubMed] [Google Scholar]
- 5. To N, Gracie DJ, Ford AC. Systematic review with meta‐analysis: the adverse effects of tobacco smoking on the natural history of Crohn's disease. Aliment Pharmacol Ther. 2016;43(5):549–61. 20160107. 10.1111/apt.13511 [DOI] [PubMed] [Google Scholar]
- 6. Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn's disease. Br J Surg. 2000;87(12):1697–701. 10.1046/j.1365-2168.2000.01589.x [DOI] [PubMed] [Google Scholar]
- 7. Simillis C, Yamamoto T, Reese GE, Umegae S, Matsumoto K, Darzi AW, et al. A meta‐analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn's disease. Am J Gastroenterol. 2008;103(1):196–205. 20070925. 10.1111/j.1572-0241.2007.01548.x [DOI] [PubMed] [Google Scholar]
- 8. Riss S, Schuster I, Papay P, Mittlbock M, Stift A. Repeat intestinal resections increase the risk of recurrence of Crohn's disease. Dis Colon Rectum. 2013;56(7):881–7. 10.1097/DCR.0b013e31828cb80c [DOI] [PubMed] [Google Scholar]
- 9. McLeod RS, Wolff BG, Ross S, Parkes R, McKenzie M. Recurrence of Crohn's disease after ileocolic resection is not affected by anastomotic type: results of a multicenter, randomized, controlled trial. Dis Colon Rectum. 2009;52(5):919–27. 10.1007/DCR.0b013e3181a4fa58 [DOI] [PubMed] [Google Scholar]
- 10. Yanai H, Kagramanova A, Knyazev O, Sabino J, Haenen S, Mantzaris GJ, et al. Endoscopic postoperative recurrence in Crohn's disease after curative ileocecal resection with early prophylaxis by anti‐Tnf, vedolizumab or ustekinumab: a real‐world multicenter European study. J Crohns Colitis. 2022;16(12):1882–92. 20220727. 10.1093/ecco-jcc/jjac100 [DOI] [PubMed] [Google Scholar]
- 11. Luglio G, Rispo A, Imperatore N, Giglio MC, Amendola A, Tropeano FP, et al. Surgical prevention of anastomotic recurrence by excluding mesentery in Crohn's disease: the SuPREMe‐CD study – a randomized clinical trial. Ann Surg. 2020;272(2):210–17. 10.1097/SLA.0000000000003821 [DOI] [PubMed] [Google Scholar]
- 12. Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, Lakatos PL, et al. European evidence‐based consensus on the diagnosis and management of Crohn's disease 2016: Part 2: surgical management and special situations. J Crohns Colitis. 2017;11(2):135–49. 20160922. 10.1093/ecco-jcc/jjw169 [DOI] [PubMed] [Google Scholar]
- 13. De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. Crohn's disease management after intestinal resection: a randomised trial. Lancet. 2015;385(9976):1406–17. 20141224. 10.1016/S0140-6736(14)61908-5 [DOI] [PubMed] [Google Scholar]
- 14. Regueiro M, Feagan BG, Zou B, Johanns J, Blank MA, Chevrier M, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn's disease after ileocolonic resection. Gastroenterology. 2016;150(7):1568–78. 20160303. 10.1053/j.gastro.2016.02.072 [DOI] [PubMed] [Google Scholar]
- 15. Dragoni G, Ding N, Gecse KB, Mansfield JC, Kopylov U, Beaugerie L, et al. The prevention and management of Crohn's disease postoperative recurrence: results from the Y‐ECCO/ClinCom 2019 Survey. Eur J Gastroenterol Hepatol. 2020;32(8):1062–6. 10.1097/MEG.0000000000001729 [DOI] [PubMed] [Google Scholar]
- 16. Zhao M, Gönczi L, Lakatos PL, Burisch J. The burden of inflammatory bowel disease in Europe in 2020. J Crohns Colitis. 2021;15(9):1573–87. 10.1093/ecco-jcc/jjab029 [DOI] [PubMed] [Google Scholar]
- 17. Quezada SM, McLean LP, Cross RK. Adverse events in IBD therapy: the 2018 update. Expert Rev Gastroenterol Hepatol. 2018;12:1183–91. 10.1080/17474124.2018.1545574 [DOI] [PubMed] [Google Scholar]
- 18. Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl a):5A–36A. 10.1155/2005/269076 [DOI] [PubMed] [Google Scholar]
- 19. Harvey RF, Bradshaw JM. A simple index of Crohn's‐disease activity. Lancet. 1980;1(8167):514. 10.1016/s0140-6736(80)92767-1 [DOI] [PubMed] [Google Scholar]
- 20. Domènech E, Mañosa M, Bernal I, Garcia‐Planella E, Cabre E, Pinol M, et al. Impact of azathioprine on the prevention of postoperative Crohn's disease recurrence: results of a prospective, observational, long‐term follow‐up study. Inflamm Bowel Dis. 2008;14(4):508–13. 10.1002/ibd.20359 [DOI] [PubMed] [Google Scholar]
- 21. Kalman TD, Everhov AH, Nordenvall C, Sachs MC, Halfvarson J, Ekbom A, et al. Decrease in primary but not in secondary abdominal surgery for Crohn's disease: nationwide cohort study, 1990‐2014. Br J Surg. 2020;107(11):1529–38. 2020/05/27. 10.1002/bjs.11659 [DOI] [PubMed] [Google Scholar]
- 22. Valibouze C, Desreumaux P, Zerbib P. Post‐surgical recurrence of Crohn's disease: situational analysis and future prospects. J Visc Surg. 2021;158(5):401–10. 20210412. 10.1016/j.jviscsurg.2021.03.012 [DOI] [PubMed] [Google Scholar]
- 23. Dal Piaz G, Mendolaro M, Mineccia M, Randazzo C, Massucco P, Cosimato M, et al. Predictivity of early and late assessment for post‐surgical recurrence of Crohn's disease: data from a single‐center retrospective series. Dig Liver Dis. 2021;53(8):987–95. 20201022. 10.1016/j.dld.2020.09.018 [DOI] [PubMed] [Google Scholar]
- 24. López‐Sanromán A, Vera‐Mendoza I, Domènech E, Taxonera C, Vega Ruiz V, Marin‐Jimenez I, et al. Adalimumab vs azathioprine in the prevention of postoperative Crohn's disease recurrence. A GETECCU randomised trial. J Crohns Colitis. 2017;11:1293–301. 10.1093/ecco-jcc/jjx051 [DOI] [PubMed] [Google Scholar]
- 25. Burr NE, Hall B, Hamlin PJ, Selinger CP, Ford AC, O’Connor A. Systematic review and network meta‐analysis of medical therapies to prevent recurrence of post‐operative Crohn's disease. J Crohns Colitis. 2019;13(6):693–701. 10.1093/ecco-jcc/jjy216 [DOI] [PubMed] [Google Scholar]
- 26. Beelen EMJ, Nieboer D, Arkenbosch JHC, Regueiro MD, Satsangi J, Ardizzone S, et al. Risk prediction and comparative efficacy of anti‐TNF vs thiopurines, for preventing postoperative recurrence in Crohn's disease: a pooled analysis of 6 trials. Clin Gastroenterol Hepatol. 2021;20(12):2741–52.e6. 20211020. 10.1016/j.cgh.2021.10.021 [DOI] [PubMed] [Google Scholar]
- 27. Yamada A, Komaki Y, Patel N, Komaki F, Pekow J, Dalal S, et al. The use of vedolizumab in preventing postoperative recurrence of Crohn's disease. Inflamm Bowel Dis. 2018;24(3):502–9. 10.1093/ibd/izx054 [DOI] [PubMed] [Google Scholar]
- 28. Ferrante M, Papamichael K, Duricova D, D’Haens G, Vermeire S, Archavlis E, et al. Systematic versus endoscopy‐driven treatment with azathioprine to prevent postoperative ileal Crohn's disease recurrence. J Crohns Colitis. 2015;9(8):617–24. 20150429. 10.1093/ecco-jcc/jjv076 [DOI] [PubMed] [Google Scholar]
- 29. Pouillon L, Remen T, Amicone C, Louis E, Maes S, Reenaers C, et al. Risk of late postoperative recurrence of Crohn's disease in patients in endoscopic remission after ileocecal resection, over 10 Years at multiple Centers. Clin Gastroenterol Hepatol. 2021;19(6):1218–25.e1214. 20200520. 10.1016/j.cgh.2020.05.027 [DOI] [PubMed] [Google Scholar]
- 30. Joustra V, van Sabben J, van der Does de Willebois E, Duijvestein M, de Boer N, Jansen J, et al. Benefit of risk‐stratified prophylactic treatment on clinical outcome in post‐operative Crohn's disease. J Crohns Colitis. 2022. Sep 17:jjac139. 10.1093/ecco-jcc/jjac139 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


