Abstract
Previous studies revealed a latitudinal gradient of multiple sclerosis (MS) prevalence, increasing by moving from the equator to the poles. The duration and quality of an individual’s exposure to sunlight vary with latitude. Skin exposure to sunlight activates vitamin D synthesis, while light absence, as perceived by the eyes, activates melatonin synthesis in the pineal gland. Vitamin D or melatonin deficiency/insufficiency or overdose can occur at any latitude due to specific lifestyles and diets. Moving away from the equator, especially beyond 37°, decreases vitamin D while raising melatonin. Furthermore, melatonin synthesis increases in cold habitats like northern countries. Since melatonin's beneficial role was shown in MS, it is expected that northern countries whose individuals have higher endogenous melatonin should show a lower MS prevalence; however, these are ranked with the highest scores. In addition, countries like the United States and Canada have uncontrolled over-the-counter usage. In high latitudes, vitamin D deficiency and a higher MS prevalence persist even though vitamin D is typically compensated for by supplementation and not sunlight. Recently, we found that prolonged darkness increased MS melatonin levels, mimicking the long-term increase in northern countries. This caused a reduction in cortisol and increased infiltration, inflammation, and demyelination, which were all rescued by constant light therapy. In this review, we explain melatonin and vitamin D's possible roles in MS prevalence. The possible causes in northern countries are then discussed. Finally, we suggest strategies to treat MS by manipulating vitamin D and melatonin, preferably with sunlight or darkness, not supplements.
Keywords: multiple sclerosis, melatonin, latitude, vitamin D, MS
Background
Multiple sclerosis (MS), an inflammatory-mediated demyelinating disease, is on the rise globally, and there is currently no treatment. While the etiology of MS is still unknown, combinations of risk factors have been identified. Since the environment is considered as one of the most vital risk factors for MS, latitude and the geographical area in which people live are strongly associated with MS prevalence. Vitamin D and melatonin hormones can be affected considerably by latitude and the environment. Some adequately documented studies showed an increase in MS susceptibility by moving from the equator to the poles (1, 2). Here, we aimed to report the current knowledge regarding the roles of latitude and seasons in MS susceptibility and then to discuss how they affect vitamin D and melatonin levels. We will then summarize the studies that reported immunoenhancing and detrimental roles of melatonin in MS. In fact, the beneficial roles of melatonin have been previously reported by several review papers, while negative studies were disregarded. We will support the idea that melatonin is not a universal remedy and that taking it as a supplement does not necessarily improve our health, especially in the absence of studies on the long-term effects of melatonin. Then, we explain why this universal remedy failed to decline the high MS prevalence in northern countries, where both endogenous melatonin and the use of melatonin supplement are high. Finally, we propose a therapeutic strategy based on our recently published research on melatonin and MS and the current knowledge about vitamin D, melatonin, and latitude.
MS Prevalence Increase by Living at Higher Latitude
Several studies have shown that MS prevalence is related to latitude, with people living closer to the equator being at a lower risk, while those living at higher latitudes being at a higher risk. For instance, while only about 15% of the world population lives at latitudes beyond 40th parallels north and south, including Europe, Canada, the north of the United States, and Russia in the northern hemisphere versus New Zealand, Tasmania, and Patagonia in the southern hemisphere, these countries show almost twofold higher MS prevalence than 85% of the remaining populations living between 40th parallels north and south (3). An updated meta-analysis found that latitude is significantly related to the MS prevalence and that moving to higher latitudes increases the MS susceptibility, possibly due to lower sun exposure (4). Another systematic review, covering ten studies, found a clear trend in MS prevalence when moving away from the equator, from Panama (8.5°N) to Argentina (38°S) (5). Another study in Newfoundland, Canada (53°N), showed that while ultraviolet (UV) exposure flows south to north gradient, more at lower latitudes, MS incidence follows north to south gradient, more at higher latitudes (6). In parallel, a study in North American regions and the continental United States showed that the latitudinal prevalence gradient of MS could be due to UV radiation (7). This was further confirmed when a latitude gradient of MS was found by comparing the MS prevalence between the north and south of France (46°N) (8) and New Zealand (40°S) (9).
These studies became even more interesting since a study examined the latitudinal gradient prevalence of MS by taking patient migration history into account. Indeed, they found that 1,587 of 2,127 MS patients in New Zealand were born in New Zealand and that the prevalence latitude gradient was stronger at birth when the birthplace of the patients was considered. This slope of the gradient remained consistent until the age of 12 y, and, it started decreasing afterward. This study helped clarify the hypothesis that early life environmental exposures is the main risk factor for MS and that this exposure can be at birth or even in utero (1). In Denmark, a northern country with a high MS prevalence, Munk Nielsen and colleagues also revealed the same idea (2).
Melatonin Increases at Higher Latitudes by Cold Habitats and Low Exposure to Sunlight
Melatonin levels were found to be higher and to last for longer periods in winter in locations with seasonal variations and significant changes in photoperiod length (10–13). It is widely accepted that bright light exposure is significantly higher in the summer than in the winter, a difference that is more pronounced at higher latitudes (11, 14). Annual variations in melatonin is reported in northern latitude countries including Sweden (60°N), Estonia (59°N) (11), and Finland (67°N) (15) among others. Investigating the melatonin profile in Estonia, a North European country, and Italy (41°N), a South European country, revealed significantly higher melatonin in Estonia (16), confirming the trend of melatonin increase from the equator to poles.
Temperature change is another factor that varies when moving from the equator to the north. Since an increase in pineal gland size is associated with a rise in melatonin synthesis, it has been shown that moving away from the equator causes an increase in pineal gland size (17). In accordance, physicians recommend keeping cold the bedroom at night to boost melatonin synthesis and thus have a better sleep (18). In addition, the cold temperature was revealed to induce the expression of AANAT, a key enzyme in melatonin synthesis (19).
Pathological Roles of Melatonin in Neuroinflammation and Autoimmune Diseases
Pineal melatonin secretion has a seasonal rhythm, increasing in the winter when the nights are longer and decreasing in the summer when the nights are shorter (20). Several investigations on nocturnal and diurnal plasma levels of cytokines clearly showed that most proinflammatory cytokines involved in autoimmune diseases, including IFN-γ, TNF, interleukin1 (IL-1), and IL-12, reach the peak of their secretion at night, between midnight and 3 to 4 AM, when melatonin is at its highest level and cortisol is at the lowest value. Indeed, the administration of cortisone acetate keeps morning cortisol levels at the physiological range and causes a decline in these cytokines (21–24).
Peripheral blood mononuclear cells (PBMCs) showed that melatonin stimulates the release of IL-6, a potent inducer of the acute phase response in MS. This IL-6 was found to be monocyte dependent since CD14+ cell–depleted PBMC monocytes failed to affect its release. Interestingly, this stimulatory effect of melatonin on IL-6 was observed only in inactivated or slightly mitogen phytohemagglutinin (PHA)-activated PBMC, not in high PHA–activated cells (25). Furthermore, it has been proposed that monocyte chemoattractant protein-1 [MCP-1, also known as chemokine ligand 2 (CCL2)], plays a key role in MS pathogenesis, most likely by recruiting peripheral monocytes via the blood–brain barrier (BBB) and parenchyma (26). This idea was examined in our recent study, in which we discovered that keeping mice in constant darkness results in an increased melatonin production by the pineal gland which causes a dramatic rise in the number of infiltrating monocytes into the brain of a MS model (27). Furthermore, investigating the correlation between daytime saliva melatonin and inflammatory cytokines uncovered a positive correlation between increased melatonin levels and MCP-1/CCL2, a finding that highlighted the bidirectional interaction between melatonin and the immune system (28).
On the other hand, Hansson et al. study on collagen-induced arthritis, as an autoimmune model that mimics rheumatoid arthritis, showed that keeping this mouse in constant darkness caused an exacerbation in disease severity associated with an increase in anti–type II collagen antibodies and enlarged spleen. Hansson et al. also proposed that darkness melatonin affects the disease by its impact on neurohumoral compound via gonadal-independent mechanisms that can enhance T cell priming (29, 30). Another relevant autoimmune disease is colitis in which it was shown that acute melatonin therapy reduced its severity; however, long-term melatonin increased its severity in rats (31). In addition, they performed a pinealectomy to stop melatonin secretion. They discovered that maintaining pinealectomized mice in complete darkness did not raise the levels of melatonin; however, it delayed the development and symptoms of induced arthritis. They concluded that increased physiological melatonin levels brought on by darkness stimulate the immune system, aggravating autoimmune diseases (32).
In accordance, Cutolo and colleagues reported a correlation for melatonin with another autoimmune disease, rheumatoid arthritis, by considering the latitude. They found that patients from Estonia, northern Europe, have higher levels of night melatonin and an earlier peak than patients from Italy, southern Europe. This was positively correlated with higher levels of the potent proinflammatory cytokine TNF-α in Estonian patients (16).
Melatonin’s Adverse Effects in MS and Its Models.
A pioneering study by Reuven Sandyk in 1992 investigated the role of melatonin in a patient with chronic progressive MS (33). Since the artificial magnetic field was reported to inhibit the secretion of melatonin by the pineal gland (34), he used it for the treatment of a 50-y-old MS patient and observed a remarkable and sustained improvement in disease severity. He then treated the patient with 3 mg melatonin after applying the magnetic field and observed, surprisingly, an extreme increase in disease severity within 1 h of melatonin administration (33).
Janković and colleagues showed, for the first time, that pinealectomy in neonatal rats caused an extreme development in experimental autoimmune encephalomyelitis (EAE), while pinealectomized adult rats of 6 wk of age prevented EAE development (35, 36). They suggested an involvement of the pineal gland in the function of BBB and that there is an age-dependent function of melatonin. We tested this hypothesis and found that EAE development is exacerbated following melatonin therapy in younger rats (37). This negative effect of melatonin in EAE was also reported earlier by Constantinescu and colleagues who showed that functional inhibition of melatonin receptors by luzindole, an antagonist, prevented EAE development in mice; hence, they concluded that melatonin is an immunoenhancing agent for autoimmune demyelination (38). Later, we found that melatonin improved the severity of EAE, associated with a side effect which is to suppress the function of pyruvate dehydrogenase complex (PDC) by pyruvate dehydrogenase kinase (PDK), critical for fatty acid synthesis during the remyelination process. However, we found that cells use an alternative source for fatty acid synthesis in myelination (39). We then eliminated this side effect by an inhibitor of PDK (40). On the other hand, a study on the cuprizone model of MS showed that melatonin caused a significant increase in nuclear factor kappa B (NFκB) directly correlated with oligodendrocyte death and failed to improve remyelination (41).
Role of Pineal Melatonin in the Function of the Immune System
Accumulating evidence indicating the direct and indirect involvement of melatonin in the function of immune system has been summarized in SI Appendix.
Simulating Higher Endogenous Melatonin in Murine Models of MS Exacerbated the Disease
Most recently, our team conducted an experimental study on the cuprizone model of MS. Data showed that this model has the potential to mimic the higher levels of melatonin seen in the habitants of higher latitudes. While the EAE model of MS showed a reduction in melatonin levels, the cuprizone model of demyelination showed a tendency to increase them. Furthermore, the cuprizone model mimics several characteristics of progressive MS, for which there is no FDA-approved medication. In addition, the current treatment for RRMS does not show efficient outcomes on these patients. Moreover, we observed that keeping mice in constant darkness, to boost endogenous melatonin, or treating with exogenous melatonin caused amplification of all disease pathological outcomes including higher monocyte infiltration into the brain, suppression of neural stem cell proliferation and their differentiation to oligodendrocyte precursor cells, and inhibition of maturation and recruitment of oligodendrocytes to the sites of demyelination. Surprisingly, keeping mice in constant light, to suppress the pineal melatonin synthesis, and treating with an antagonist of melatonin receptors, to suppress the function of melatonin, caused a rescue of all the abovementioned events and improved the severity of the disease. Besides, this was associated with suppression of nighttime melatonin, near physiological concentration, and boosting cortisol levels (42). Therefore, we suggest the suppression of melatonin for improvement of MS; however, we claim that keeping melatonin at physiological concentrations is vital for improving the disease.
Early Life Exposure to Low or High Melatonin in MS Susceptibility.
To date, it is shown that melatonin synthesis increases by moving to higher latitude and by high-melatonin diet. Besides, melatonin synthesis and rhythm are markedly affected by melatonin disturbance risk factors such as light at night, LED screens, night shifts, and so forth (43). Pineal melatonin synthesis begins at 4 to 6 mo of age and is produced in a light-dependent circadian rhythm. It reaches its peak during early childhood, between the ages of 5 to 10 y, and then gradually declines with age (43). A sharp decline in melatonin level has been reported during the transition from childhood to puberty (44). In addition to the endogenous melatonin synthesis, food can greatly increase melatonin, which is abundant in animal foods, such as eggs and fish, and in plant foods, such as nuts (45).
In the absence of research on how melatonin supplements can affect the development of fetal immune and nervous systems, it is best to avoid taking melatonin during pregnancy and gestation unless advised to by a physician. Although melatonin receptors are expressed in the embryo and fetus, the fetus and newborn depend on the mother’s melatonin via the placenta and milk, respectively. Any change in mother’s melatonin level, either during pregnancy or gestation, quickly affects the development of the fetus or growth of neonates. In fact, photoperiodic information perceived by the mother is transferred to the fetus (46). Melatonin levels naturally increase throughout gestation and decline right after delivery. For instance, while normal nonpregnant women have a melatonin level of 41.7 pmol/L, it increases by twofold to reach 76.6 pmol/L at the third semester (47).
While some reports showed beneficial roles of melatonin in fetus development, we focus in this review on the negative studies. A group studied the effect of low to high doses of melatonin in fetus development. Indeed, they administered melatonin daily during the whole gestation period and found that melatonin caused lower maternal weight gain during pregnancy, smaller pups’ size, and lower birth weight. Furthermore, the pups’ mortalities increased in high melatonin–treated rats (48). Furthermore, using H9c2 embryonic rat cardiac cells, Zhao et al showed that only higher doses of melatonin suppressed the cell growth and induced cell cycle arrest and apoptosis which was associated with a reduction in gene expression related to heart development (49).
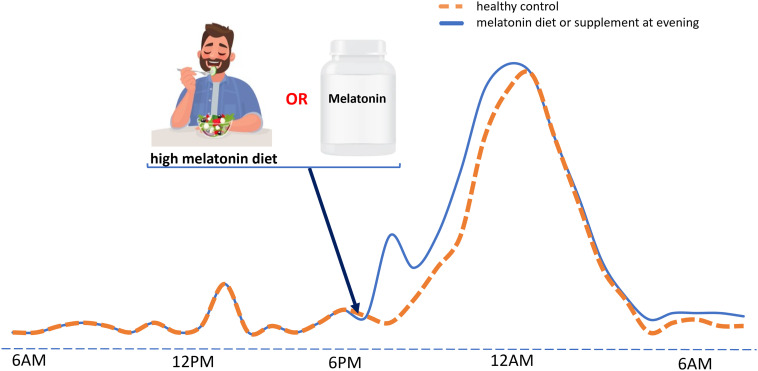
It has been reported that taking evening melatonin increases melatonin secretion and its synthesis duration (50). This was also studied for foods containing high levels of melatonin (51, 52). Considering that melatonin is not a synthetic drug that naturally cannot be found in nature or diet, it is important to know the patient’s lifestyle and diet before prescribing melatonin or melatonin supplements. For instance, a person who consumes melatonin-rich foods in the evening will have an increase in circulating melatonin within 3 h; therefore, adding a melatonin supplement may result in an unexpected melatonin peak, usually 2 h after use (51). In the absence of relevant studies, it is strongly advised that physicians consider this possibility (Fig. 1).
Fig. 1.
Schematic representation of the effect of melatonin supplements or high-melatonin food intake on the levels of melatonin in the circulation. Orange line: Melatonin production from the pineal gland begins with the start of the dark phase, here 7 PM, and reaches its peak at midnight or about 1 to 2 AM. Melatonin secretion then starts to drop and reaches its minimum level at sunrise, here 6 AM. On the other hand, melatonin has a transient peak in the afternoon, about 1 PM, and continues at a low level for the entire day. Blue line: Eating a high-melatonin food diet in the evening not only induces a fast rise in melatonin levels, that persist for 2 to 3 h, but also appears to increase the amplitude and duration of the midnight peak of melatonin. In addition, melatonin supplements can cause a similar impact as diets. In the absence of studies on the effects of simultaneous consumption of melatonin supplements along with a melatonin-rich meal, it is believed that this can synergistically lead to a significantly higher amplitude and duration of the melatonin midnight peak that may disrupt brain hemostasis.
How does maternal and fetal melatonin increase by latitude, lifestyle, and diet can affect the development of immune and nervous systems? This still remains to be answered. However, based on the current knowledge that melatonin doses usually affect the expression of its receptor by the fetus, we expect that long-term increase or decrease in melatonin levels can significantly alter the normal development, which may have an impact on the onset of disease, especially autoimmunity and multiple sclerosis, in the upcoming years of life.
MS Seasonality Might Be Caused by Variability in Vitamin D Synthesis by Sunlight Exposure
Although vitamin D deficiency can result from low consumption of food sources of vitamin D or low skin exposure to sunlight at any latitude, it is well documented that living at higher latitudes, above 37°N and below −37°S, is a risk factor for vitamin D deficiency. Indeed, countries located at high latitudes have very cold autumn, spring, and winter that do not allow people to expose their skin to sunlight (53). Furthermore, a cross-sectional survey of 2,301 participants with MS showed a direct correlation between moving away from the equator and a reduction in sun exposure and vitamin D synthesis, proposing supplementary vitamin D for improving MS (54). Despite the Farez study in Argentina (38°S) which did not show any significant correlation between UV exposure, vitamin D, and MS severity (55), studies in Sweden (60°N) (56, 57), Germany (51°N) (58), and Australia (27° to 43°S) (59) showed that low exposure to sunlight and vitamin D insufficiency considerably increase the risk of MS. A study in Denmark (56°N) also showed that lower sunlight exposure in adolescence is associated with younger age of MS onset (60). The importance of sunlight exposure and latitude in MS was also revealed by reviewing the relevant literature and comparing the map of UV index in the United States (i.e., the amount of sunlight received by different regions) in addition to the prevalence of vitamin D deficiency and MS in the north and south of the country (61).
Furthermore, a study in the United States investigated the role of residential UV exposure from sunlight in MS patients and reported that a reduction in average lifetime UV exposure in winter is associated with increased MS risk (62). Recently, in their study on the pediatric onset of MS in the United States, Sebastian and colleagues showed that spending half an hour outside in summer clearly reduced MS development compared to the most recent summer as control (63).
Early Life Exposure to Insufficient Vitamin D Is Linked to MS Susceptibility
Although diet and lifestyle should not be ignored, sunlight exposure is the main variable for several months after birth. Oral vitamin D and to a greater extent sunlight exposure are the two main sources of vitamin D. A systematic review on the relationship between the season of birth and MS revealed that MS prevalence is higher in the spring and lower in the autumn. The authors proposed that the month of birth could be another risk factor for MS and that sunlight exposure may be the most effective agent (64). In accordance, a low level of vitamin D during pregnancy is reported to be involved in seasonality of month of birth of MS cases (65). This idea is supported by a study on dried blood spot samples of 521 patients with MS, which showed that MS patients had significantly lower levels of vitamin D. Briefly, using data from the nationwide Danish Multiple Sclerosis Registry, they identified MS patients born after 1918 and whose disease onset was before 2012. Then, they measured vitamin D levels in the neonatal dried blood spot samples of MS patients that were stored at birth in the Danish Newborn Screening Biobank. Results showed that individuals whose vitamin D levels were below 20.7 nmol/L had a high risk of MS development, whereas those with above 48.9 nmol/L had the lowest risk. Indeed, an increase of 25 nmol/L in neonatal vitamin D caused a 30% reduction in MS risk (66). This study confirmed that early life environmental exposure, here sunlight, is involved in MS. Another meta-analysis systematic review found a reduction in MS (11/15 studies) in children born in late autumn in the northern hemisphere. Interestingly, people who migrated from high to low MS risk factor environments experienced a significant decrease in MS incidence, only if the migration occurred before the age of 15 y (67). In parallel, a study in Newfoundland and Labrador, Canada (53°N), investigated vitamin D in pregnant women, newborns (umbilical cord blood), and children. Vitamin D insufficiency was found to be very high since more than 90% of children and 80% of maternal and cord blood showed vitamin D insufficiency at winter; however, this slightly decreased at summer (68).
Role of Vitamin D in the Function of the Immune System
Although the mechanistic pathway by which vitamin D affects the function of the immune system is not well studied, previous reports showed that vitamin D plays key roles in both adaptive and innate immune systems via nuclear vitamin D receptors (VDRs) expressed on B and T lymphocytes, monocytes, dendritic cells, and neutrophils. In general, vitamin D is recognized as a modulator of the immune system playing beneficial roles in fighting against autoimmune disease initiation and progression. However, vitamin D binds to VDRs, and the formed complex interacts with vitamin D responsive elements (VDREs), a DNA regulatory element, allowing to recruit other coregulatory elements and DNA-binding proteins that would ultimately change the expression of a wide range of genes involved in cell proliferation, differentiation, and apoptosis and hence control the cell function by calcium and phosphate metabolism. In addition, vitamin D can bind to membrane VDRs to affect intracellular signaling molecules including PKC, PI3K, and MAP kinases (69).
Regarding the adaptive immune system, it can switch between Th1 cells, which mediate a cellular immune response and the release of proinflammatory cytokines, and Th2 cells, which potentiate a humoral response and the release of anti-inflammatory cytokines (69). It is found that vitamin D regulates the release of cytokines via upregulation of IκBα, an NF-κB inhibitory protein, leading to blockage of NF-κB (70). On the other hand, while vitamin D increases differentiation of monocytes to macrophages (71), it also affects macrophage polarization by shifting it to an anti-inflammatory M2 phenotype (72). Differentiation of monocytes to macrophages is accompanied with a reduction in VDR expression. Vitamin D reduces proinflammatory cytokines IL-12 and TNF-α and the expression of class II MHC on antigen-presenting dendritic cells, inhibiting their differentiation. While autoimmune diseases are mediated by the synthesis of antibodies directed to self-antigens, vitamin D can inhibit the cell proliferation and differentiation in immunoglobulin-producing B cells (73). Furthermore, it was found that vitamin D reduces the stimulation of T cells by decreasing CD86 expression of B cells (74).
Vitamin D and Calcium Insufficiency as Risk Factors for MS Could Be Mediated by Gastric Stasis
There are some evidences that vitamin D deficiency leads to gastric stasis due to a reduction in intestinal calcium absorption. This allows more LPS translocation into the blood stream and causes disruption in the integrity of BBB following several signaling cascades. We have provided a thorough explanation of this in SI Appendix.
Sunlight or Vitamin D Supplement May Affect MS via Melatonin-Dependent Pathway
The synthesis pattern of vitamin D and melatonin is opposite to each other’s. The skin starts to produce vitamin D during daylight and upon exposure to sunshine, whereas the pineal gland starts melatonin synthesis in the absence of any light, at night. This means that light (or darkness) will only boost one of these two agents while suppressing the other one. An elegant study by Irving and colleagues in 2019, using vitamin D receptor knockout mice that were also unable to produce vitamin D, showed that UV light improves EAE severity independent of vitamin D and its receptor. This study clearly demonstrated that sunshine might improve MS via a vitamin D–independent mechanism (75). One of the possible mechanisms by which sunshine or vitamin D affect MS is reported by Golan and colleagues when they treated MS patients with vitamin D. Results showed that after a year of daily treatment of MS patients, high doses of vitamin D significantly suppressed the nighttime melatonin and that there is a negative correlation between vitamin D and melatonin. They suggested that vitamin D mediates its effect via melatonin in MS (76).
Sunlight May Not Only Compensate for Vitamin D Insufficiency But Also Boost Cortisol Immunosuppressant
Although the dietary sources of vitamin D are very few, it is estimated that 90% of vitamin D requirement is efficiently produced by the body after exposure to sunshine. Compared to 100 gram of oily fish salmon as high diet source of vitamin D, which produce 360 IU of vitamin D, only 10 to 15 min of sun exposure in adult during the spring or summer, with 22% uncovered skin, can make 1,000 IU (77). Taking vitamin D supplement is only designed to boost the blood vitamin D level but boosting it with sunlight would be a multipurpose strategy. For instance, it is reported that boosting vitamin D levels by supplements cause a significant reduction in cortisol levels (78). This finding was further confirmed by Abu-Samak and colleagues who reported that people with vitamin D deficiency have higher levels of cortisol (79). In contrast, boosting by light is well reported to increase the cortisol level in human skin ex vivo (80). Leproult and colleagues demonstrated that the morning transition from dim to bright light leads to an immediate rise in cortisol levels while suppressing melatonin (81). However, another study reported that acute exposure to light plays an inhibitory role for cortisol release (82). Interestingly, another study indicated that summer exposure to natural sunlight not only shortened the nighttime melatonin but also advanced the morning cortisol rise (13). Investigating the correlation between sunlight exposure and cortisol levels in different seasons in Poland (52°N) indicated significantly increased levels in summer than in winter, with daily sunlight duration of 7.8 h compared to 2.9 h, respectively, which was another reason for the increase in cortisol by sunlight (83). Accumulating evidence demonstrates the beneficial role of cortisol, the main endogenous glucocorticoid hormone, in MS. Corticosteroids, which are widely used MS medications, are synthetic versions of cortisol (84).
On the other hand, it was reported that adrenalectomy or metyrapone therapy, an inhibitor of steroid synthesis, decreased night melatonin in chronic inflammatory conditions (85). Besides, acute stress may cause corticosteroids to suppress melatonin synthesis (86). In addition, our previous study on EAE rats showed that treating these mice with methylprednisolone, a systemic synthetic corticosteroid and MS medication, caused a significant increase in melatonin levels (87). Another study showed that administration of prednisone, another synthetic corticosteroid and MS medication, to zebrafish, whose pineal gland is considered to function as the master clock, was associated with sleep disorders and reduction in melatonin secretion (88). Does the reduction of melatonin by MS medications, especially corticosteroids, play a beneficial role in MS improvement is a question that still remains to be answered?
Melatonin Global Usage in Developed Countries
A recently published study by Bliddal and colleagues analyzed the melatonin use from 2012 to 2019 in Denmark. They reported a sharp increase in the consummation rate from 2.4 to 3.9 in every 1,000 people. Among those, 31% of long-term consumers are 5 to 13 y old. Indeed, 75% of melatonin consumers had registered complaints about psychopathological problems within 2 y of their first melatonin prescription. Anxiety disorder was reported as the most common psychiatric diagnosis, especially among 14 to 17 y old (89). This high use among children was also reported in Stockholm, Sweden (90). Besides, after receiving 90 cases of adverse effects following the intake of these supplements, the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) filed an internal request to conduct an expert appraisal on the risks associated with the use of melatonin-enriched foods. ANSES recommended to limit the use of melatonin supplements to occasional use due to the lack of studies on the long-term effects of melatonin supplements on human health. In addition, they prohibited the prescription of melatonin supplements to pregnant and breastfeeding women. Furthermore, an interview survey in the United States showed that melatonin consumption among children aged 4 to 17 y has increased from 0.1% in 2007 to 0.7% in 2012. Melatonin is reported as the second most used natural product among American children, after fish oil, and fourth among adults (91). Recently, the trend in consumption of melatonin supplements among 55,021 people in the United States showed a roughly fivefold increase from 0.4% in 1999 to 2.1% in 2018. This rise in the use of melatonin in the absence of enough evidence of its safety is highly worrying. Consistent with the expert’s recommendations in taking doses below 5 mg/d as a safe dose, the use of melatonin above this dose was not reported until 2006 (0.08%) in the United States, while higher doses increased by threefold (0.28%) in 2018 (92). In Canada, Erland and Saxena analyzed the melatonin content in 31 supplements. Surprisingly, they found that the actual melatonin in each melatonin supplement showed a huge variability compared to the labeled doses. Indeed, some doses were 83% below or 478% above the labeled dosage. For instance, among the different types of supplements, chewable tablets labeled as 1.5 mg, mostly used by children, showed an actual content of 9 mg (93).
It is worth noting that some food products are enriched with melatonin in European countries. These countries limited melatonin supplements to low doses of usually 1 mg, while doses of 5 mg are made available only by prescription. Although some countries, such as Denmark and the Czech Republic, even prohibited melatonin as food supplements, there is roughly a twofold increase in the number of users of prescribed melatonin in Denmark from 2012 to 2017, according to Statista, a well-known German company specialized in market and consumer data. However, the United States and Canada are selling melatonin in different forms over the counter at doses even as high as 10 mg. Based on Statista, the total global melatonin supplements’ market size has increased from 851 million USD in 2016 to 1.5 billion USD in 2021 worldwide.
Melatonin and Vitamin D, Two Sides of the Same Coin
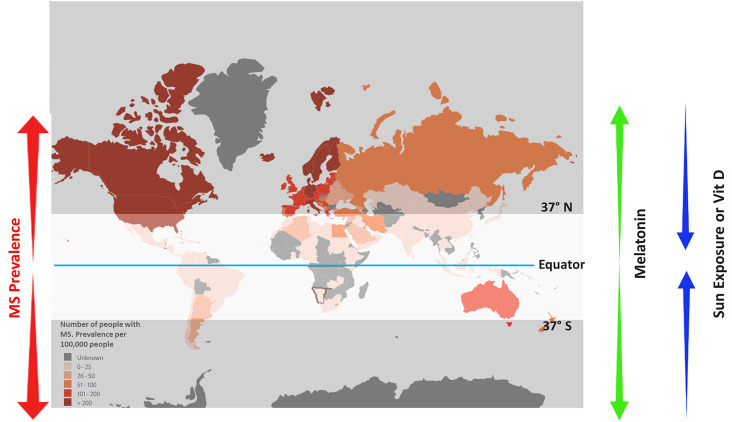
We consider melatonin and vitamin D as two sides of the same coin that affect life through diet, seasons, and day–night cycles. During the summer, vitamin D is produced in higher amounts due to more exposure to sunlight, while melatonin is further suppressed either by longer day length or boosted vitamin D, which seems to apply inhibitory effects on melatonin release. This trend is reversed in nonsummer seasons, especially during winter when the night is longer, and skin exposure to sunlight is extremely reduced. This reduction in sun exposure is due to cold temperature, which necessitates body coverage, as well as to light angle, which does not come straight, resulting in boosting melatonin and reducing vitamin D synthesis. Indeed, evidence shows that a cold climate causes an increase in melatonin synthesis enzyme (Fig. 2).
Fig. 2.
Schematic diagram demonstrating the epidemiological prevalence of MS. MS prevalence is increased by moving from the equator to poles, which is associated with a decline in sunlight exposure and vitamin D levels with an opposite rise in melatonin levels.
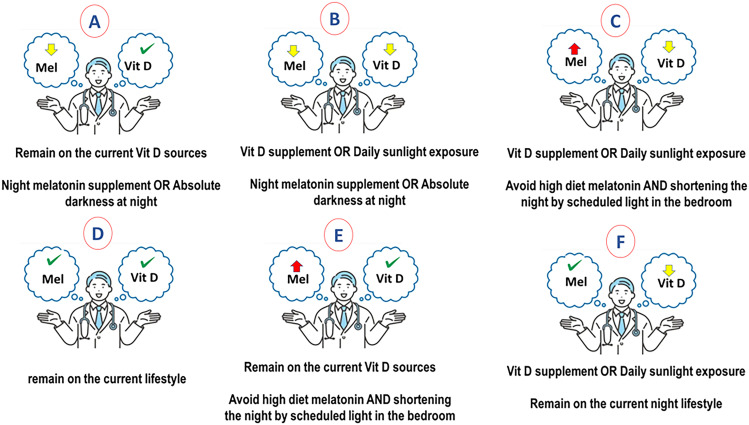
Therefore, when the coin lands on melatonin’s side during summer, it is more likely that vitamin D be normal and melatonin drops. This mimics the situation in countries located near the equator where longer, warmer days, as well as high and easy exposure to sunlight, are beneficial in boosting vitamin D and increasing the natural immune suppression of cortisol in patients. A low-melatonin diet and shorter nights may decrease melatonin levels. It may be suggested to patients to change their night diet and lifestyle to boost melatonin (Fig. 3A); for instance, by sleeping 7 to 8 h in absolute darkness (SI Appendix, Fig. S1, Upper). However, if patients are both below the normal range of melatonin and vitamin D, they will need a daytime vitamin D booster by taking a vitamin D supplement or preferably exposure to sunlight and nighttime melatonin booster by having melatonin-enriched diet, melatonin supplement, or having absolute darkness at night when sleep (Fig. 3B and SI Appendix, Fig. S1, Lower).
Fig. 3.
Proposed strategy for adjusting vitamin D and melatonin levels to reach a balance for the proper function of immune system in MS. (A) Individuals with low melatonin but normal vitamin D levels may only take night melatonin supplements or high melatonin meals. (B) Individuals with low melatonin and vitamin D should take night melatonin supplements or high-melatonin meals and increase their sun exposure or take vitamin D supplements to normalize their vitamin D level. (C) Individuals with high melatonin but low vitamin D levels, as expected in northern countries, should control their melatonin by replacing high-melatonin meals with low-melatonin ones and by increasing their exposure to sunlight or by taking vitamin D supplements to normalize their vitamin D levels. (D) Individuals with normal melatonin and vitamin D levels should remain in their current lifestyles. (E) Individuals with high melatonin and normal vitamin D may only control their melatonin level by changing their diet or the length of darkness phase. (F) Individuals with normal melatonin, but vitamin D insufficiency, should remain in their current lifestyle but increase their sun exposure or take vitamin D supplements to normalize their vitamin D level.
When the coin lands on the vitamin D side during winter, it is more likely that vitamin D declines and melatonin rise (Fig. 3C). This mimics the situation in northern countries where the night is longer, and skin exposure to sunlight is extremely reduced due to cold temperature, causing a surge in melatonin levels. A high-melatonin diet can synergistically boost melatonin levels. This uncontrolled increase in melatonin may be associated with an immunoenhancing role in MS and an increase in inflammatory cytokines at night by the pineal gland. It is more likely that recovering the declined vitamin D for these patients improves the disease for a short term; however, it would be more efficient to induce a balance in vitamin D and melatonin levels, as seen in healthy cases. Therefore, boosting vitamin D levels, preferably by exposure to sunlight or vitamin D supplements, and avoiding the melatonin-enhancing lifestyle and diet may work for balancing these two when the coin lands on its edge (Fig. 3D). Based on our most recent experimental study, keeping the murine model of MS at light during night inhibited the surge of night melatonin and caused a remarkable improvement in disease severity. To translate this study to the clinical level, we propose that habitants in higher latitude, whose melatonin levels are high for a long term, to sleep in a lighted room without sleep disturbance, which is important for the proper function of the brain (SI Appendix, Fig. S1, Upper). It is already revealed that our pineal gland can perceive light even with closed eyelids when we are at either REM or no-REM phase, which accounts for roughly 50% in nighttime melatonin (94). On the other hand, vitamin D provided by lifestyle and diet do not need adjustment if it is already at the normal range (Fig. 3E). However, patients should remain in their lifestyle in case they have normal melatonin levels; instead, they may only increase vitamin D levels (Fig. 3F).
Since vitamin D toxicity is rare to happen especially in MS patients, we did not include in this review the cases whose vitamin D level is higher than the normal. Nevertheless, if the cases with higher vitamin D are reported, vitamin D level needs to be adjusted using the aforementioned approaches in addition to melatonin control. Furthermore, instead of prescribing vitamin D or melatonin supplements, we highly suggest asking the patient to adjust vitamin D and melatonin levels through sunlight or night, which seems to be more efficient and with less side effects. It should not be forgotten that sunlight may recover the dropped cortisol level in MS patients, which is expected to significantly suppress disease progression.
Conclusion
The current knowledge regarding the role of vitamin D and, in particular, melatonin in autoimmune diseases such as MS is scant and insufficient. This is due to the lack of long-term studies on both humans and animal models, especially during early life. Indeed, the fetus and neonates are highly susceptible to environmental factors and to very small changes in hormones and vitamins. Growing evidence suggests that an early event in life increases the probability of MS incidence years later. Both melatonin and vitamin D are sold over the counter, and their use has increased dramatically over the last few years. It is worrying that vitamin D and melatonin are not controlled in mothers during gestation and that monitoring their balance is ignored. Similarly, it is essential to balance vitamin D and melatonin in MS patients as, together, they play crucial roles in regulating the function of the immune system.
It is generally accepted that vitamin D is helpful based on previous studies on animal models and patients. Currently, vitamin D is prescribed to MS patients to relieve the symptoms and to slow down the progression of the disease. On the other hand, while more than 50% of MS patients experience sleep disorders, these patients may receive prescribed or over-the-counter melatonin for sleep improvements. More recently, it was suggested that melatonin therapy may be beneficial for MS amelioration. Here, we propose that taking vitamin D or melatonin may play beneficial roles in MS; however, this can disrupt their balance at the long term, which is crucial for the fine-tuning function of immune cells. On the other hand, an increase or a decrease in the average levels of vitamin D and melatonin in a specific population, and at a particular time, does not mean that all individuals have the same trend. Instead, every person has his own diet and lifestyle, which also vary over time. Therefore, vitamin D or melatonin may improve MS symptoms, but the effects are variable from patient to patient. Hence, instead of using vitamin D and melatonin supplements, it should be better to check their oscillation in patients and to consider the most efficient strategy for adjusting their levels. Indeed, balancing these factors is expected to show their optimal efficiency at the long term in MS, a long-standing disease.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (no. 143279, Foundation Scheme Program) and Les Fonds de recherche du Québec - Santé via the research center funding grant. S.R. was supported by a Canada Research Chair in Neuroimmunology.
Author contributions
M.G., K.Z., and S.R. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
There are no data underlying this work.
Supporting Information
References
- 1.Sabel C. E., et al. , The latitude gradient for multiple sclerosis prevalence is established in the early life course. Brain 144, 2038–2046 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Munk Nielsen N., et al. , Multiple sclerosis among first- and second-generation immigrants in Denmark: A population-based cohort study. Brain 142, 1587–1597 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Pierrot-Deseilligny C., Souberbielle J.-C., Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis? Brain 133, 1869–1888 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Simpson S. Jr., et al. , Latitude continues to be significantly associated with the prevalence of multiple sclerosis: an updated meta-analysis. J. Neurol. Neurosurg. Psychiatry. 90, 1193–1200 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Risco J., et al. , Latitudinal prevalence gradient of multiple sclerosis in Latin America. Mult. Scler. 17, 1055–1059 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Sloka J. S., Pryse-Phillips W. E., Stefanelli M., The relation of ultraviolet radiation and multiple sclerosis in Newfoundland. Can. J. Neurol. Sci. 35, 69–74 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Beretich B. D., Beretich T. M., Explaining multiple sclerosis prevalence by ultraviolet exposure: A geospatial analysis. Mult. Scler. 15, 891–898 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Dalla Costa G., et al. , Digital epidemiology confirms a latitude gradient of MS in France. Mult. Scler. Relat. Disord. 20, 129–131 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Taylor B. V., et al. , MS prevalence in New Zealand, an ethnically and latitudinally diverse country. Mult. Scler. 16, 1422–1431 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Kauppila A., Kivelä A., Pakarinen A., Vakkuri O., Inverse seasonal relationship between melatonin and ovarian activity in humans in a region with a strong seasonal contrast in luminosity. J. Clin. Endocrinol. Metab. 65, 823–828 (1987). [DOI] [PubMed] [Google Scholar]
- 11.Adamsson M., Laike T., Morita T., Annual variation in daily light exposure and circadian change of melatonin and cortisol concentrations at a northern latitude with large seasonal differences in photoperiod length. J. Physiol. Anthropol. 36, 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stokkan K. A., Reiter R. J., Melatonin rhythms in Arctic urban residents. J. Pineal. Res. 16, 33–36 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Vondrasová D., Hájek I., Illnerová H., Exposure to long summer days affects the human melatonin and cortisol rhythms. Brain. Res. 759, 166–170 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Cole R. J., et al. , Seasonal variation in human illumination exposure at two different latitudes. J. Biol. Rhythms. 10, 324–334 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Leppäluoto J., Sikkilä K., Meyer-Rochow V. B., Hassi J., Low melatonin secretion associates with albedo in circumpolar environments. J. Pineal. Res. 35, 158–162 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Cutolo M., et al. , Circadian melatonin and cortisol levels in rheumatoid arthritis patients in winter time: a north and south Europe comparison. Ann. Rheum. Dis. 64, 212–216 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ralph C. L., The pineal gland and geographical distribution of animals. Int. J. Biometeorol. 19, 289–303 (1975). [DOI] [PubMed] [Google Scholar]
- 18.Fu J., et al. , Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Sci. Rep. 7, 39865 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X., et al. , Association of melatonin production with seasonal changes, low temperature, and immuno-responses in hamsters. Molecules 23, 703 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arendt J., Role of the pineal gland and melatonin in seasonal reproductive function in mammals. Oxf Rev. Reprod. Biol. 8, 266–320 (1986). [PubMed] [Google Scholar]
- 21.Petrovsky N., Harrison L. C., The chronobiology of human cytokine production. Int. Rev. Immunol. 16, 635–649 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Gudewill S., et al. , Nocturnal plasma levels of cytokines in healthy men. Eur. Arch. Psychiatry. Clin. Neurosci. 242, 53–56 (1992). [DOI] [PubMed] [Google Scholar]
- 23.Petrovsky N., McNair P., Harrison L. C., Diurnal rhythms of pro-inflammatory cytokines: Regulation by plasma cortisol and therapeutic implications. Cytokine 10, 307–312 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Petrovsky N., et al. , Macrophage migration inhibitory factor exhibits a pronounced circadian rhythm relevant to its role as a glucocorticoid counter-regulator. Immunol. Cell Biol. 81, 137–143 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Mauriño S., et al. , Melatonin enhances IL-2, IL-6, and IFN-gamma production by human circulating CD4+ cells: a possible nuclear receptor-mediated mechanism involving T helper type 1 lymphocytes and monocytes. J. Immunol. 159, 574–581 (1997). [PubMed] [Google Scholar]
- 26.Mahad D. J., Ransohoff R. M., The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Semin. Immunol. 15, 23–32 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Ghareghani M., Pons V., Laflamme N., Zibara K., Rivest S., Inhibiting nighttime melatonin and boosting cortisol increase patrolling monocytes, phagocytosis, and myelination in a murine model of multiple sclerosis. Exp. Mol. Med. 55, 215–227 (2023), 10.1038/s12276-023-00925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundberg I., et al. , Daytime melatonin levels in saliva are associated with inflammatory markers and anxiety disorders. Psychoneuroendocrinology 112, 104514 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Hansson I., Holmdahl R., Mattsson R., Constant darkness enhances autoimmunity to type II collagen and exaggerates development of collagen-induced arthritis in DBA/1 mice. J. Neuroimmunol. 27, 79–84 (1990). [DOI] [PubMed] [Google Scholar]
- 30.Hansson I., Holmdahl R., Mattsson R., The pineal hormone melatonin exaggerates development of collagen-induced arthritis in mice. J. Neuroimmunol. 39, 23–30 (1992). [DOI] [PubMed] [Google Scholar]
- 31.Marquez E., Sánchez-Fidalgo S., Calvo J. R., la de Lastra C. A., Motilva V., Acutely administered melatonin is beneficial while chronic melatonin treatment aggravates the evolution of TNBS-induced colitis. J. Pineal. Res. 40, 48–55 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Hansson I., Holmdahl R., Mattsson R., Pinealectomy ameliorates collagen II-induced arthritis in mice. Clin. Exp. Immunol. 92, 432–436 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandyk R., Clinical case report: Successful treatment of multiple sclerosis with magnetic fields. Int. J. Neurosci. 66, 237–250 (1992). [PubMed] [Google Scholar]
- 34.Welker H. A., et al. , Effects of an artificial magnetic field on serotonin N-acetyltransferase activity and melatonin content of the rat pineal gland. Exp. Brain. Res. 50, 426–432 (1983). [DOI] [PubMed] [Google Scholar]
- 35.Janković B. D., Isaković K., Petrović S., Effect of pinealectomy on immune reactions in the rat. Immunology 18, 1–6 (1970). [PMC free article] [PubMed] [Google Scholar]
- 36.Sandyk R., Influence of the pineal gland on the expression of experimental allergic encephalomyelitis: possible relationship to the aquisition of multiple sclerosis. Int. J. Neurosci. 90, 129–133 (1997). [DOI] [PubMed] [Google Scholar]
- 37.Ghareghani M., et al. , Melatonin exacerbates acute experimental autoimmune encephalomyelitis by enhancing the serum levels of lactate: A potential biomarker of multiple sclerosis progression. Clin. Exp. Pharmacol. Physiol. 44, 52–61 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Constantinescu C. S., Hilliard B., Ventura E., Rostami A., Luzindole, a melatonin receptor antagonist, suppresses experimental autoimmune encephalomyelitis. Pathobiology 65, 190–194 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Ghareghani M., et al. , Melatonin therapy modulates cerebral metabolism and enhances remyelination by increasing PDK4 in a mouse model of multiple sclerosis. Front. Pharmacol. 10, 147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghareghani M., Farhadi Z., Rivest S., Zibara K., PDK4 inhibition ameliorates melatonin therapy by modulating cerebral metabolism and remyelination in an EAE demyelinating mouse model of multiple sclerosis. Front. Immunol. 13, 862316 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vakilzadeh G., et al. , The effect of melatonin on behavioral, molecular, and histopathological changes in cuprizone model of demyelination. Mol. Neurobiol. 53, 4675–4684 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Ghareghani M., Pons V., Laflamme N., Zibara K., Rivest S., "Inhibiting nighttime melatonin and boosting cortisol increase patrolling monocytes" in Phagocytosis, and Myelination in a Murine Model of Multiple Sclerosis (Exp Mol Med, 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghareghani M., Zibara K., Reiter R. J., Rivest S., Reduced melatonin levels may facilitate glioblastoma initiation in the subventricular zone. Expert. Rev. Mol. Med. 24, e24. (2022). [DOI] [PubMed] [Google Scholar]
- 44.Sandyk R., Multiple sclerosis: The role of puberty and the pineal gland in its pathogenesis. Int. J. Neurosci. 68, 209–225 (1993). [DOI] [PubMed] [Google Scholar]
- 45.Meng X., et al. , Dietary sources and bioactivities of melatonin. Nutrients 9, 367 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu C. N., Tain Y. L., Light and circadian signaling pathway in pregnancy: Programming of adult health and disease. Int. J. Mol. Sci. 21, 2232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kivelä A., Serum melatonin during human pregnancy. Acta Endocrinol. 124, 233–237 (1991). [PubMed] [Google Scholar]
- 48.Singh H. J., Saleh H. I., Gupalo S., Omar E., Effect of melatonin supplementation on pregnancy outcome in Wistar-Kyoto and Sprague-Dawley rats. Sheng Li Xue Bao 65, 149–157 (2013). [PubMed] [Google Scholar]
- 49.Zhao A., et al. , Melatonin inhibits embryonic rat H9c2 cells growth through induction of apoptosis and cell cycle arrest via PI3K-AKT signaling pathway. Birth Defects Res. 113, 1171–1181 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Grivas T. B., Savvidou O. D., Melatonin the "light of night" in human biology and adolescent idiopathic scoliosis. Scoliosis 2, 6 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sae-Teaw M., Johns J., Johns N. P., Subongkot S., Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal. Res. 55, 58–64 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Howatson G., et al. , Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 51, 909–916 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Wacker M., Holick M. F., Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol 5, 51–108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jelinek G. A., et al. , Latitude, sun exposure and vitamin D supplementation: Associations with quality of life and disease outcomes in a large international cohort of people with multiple sclerosis. BMC Neurol. 15, 132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farez M. F., et al. , Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162, 1338–1352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bäärnhielm M., et al. , Sunlight is associated with decreased multiple sclerosis risk: no interaction with human leukocyte antigen-DRB1*15. Eur. J. Neurol. 19, 955–962 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Hedström A. K., Olsson T., Kockum I., Hillert J., Alfredsson L., Low sun exposure increases multiple sclerosis risk both directly and indirectly. J. Neurol. 267, 1045–1052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ostkamp P., et al. , Sunlight exposure exerts immunomodulatory effects to reduce multiple sclerosis severity. Proc. Natl. Acad. Sci. U.S.A. 118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tremlett H., Zhu F., Ascherio A., Munger K. L., Sun exposure over the life course and associations with multiple sclerosis. Neurology 90, e1191–e1199 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laursen J. H., Søndergaard H. B., Sørensen P. S., Sellebjerg F., Oturai A. B., Association between age at onset of multiple sclerosis and vitamin D level-related factors. Neurology 86, 88–93 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Maeser D., Maeser N., Sunlight exposure, vitamin D synthesis, and multiple sclerosis in the northern and southern regions of the United States. Minnesota Undergraduate Res. Acad. J. 1 (2018). [Google Scholar]
- 62.Gallagher L. G., et al. , Lifetime exposure to ultraviolet radiation and the risk of multiple sclerosis in the US radiologic technologists cohort study. Mult. Scler. 25, 1162–1169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sebastian P., et al. , Association between time spent outdoors and risk of multiple sclerosis. Neurology 98, e267–e278 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torkildsen O., Grytten N., Aarseth J., Myhr K. M., Kampman M. T., Month of birth as a risk factor for multiple sclerosis: An update. Acta Neurol. Scand. Suppl. 58–62, 10.1111/ane.12040 (2012). [DOI] [PubMed]
- 65.Sidhom Y., et al. , Season of birth and multiple sclerosis in Tunisia. Mult. Scler. Relat. Disord. 4, 491–494 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Nielsen N. M., et al. , Neonatal vitamin D status and risk of multiple sclerosis: A population-based case-control study. Neurology 88, 44–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ismailova K., Poudel P., Parlesak A., Frederiksen P., Heitmann B. L., Vitamin D in early life and later risk of multiple sclerosis-A systematic review, meta-analysis. PLoS One 14, e0221645 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Newhook L. A., et al. , Vitamin D insufficiency common in newborns, children and pregnant women living in Newfoundland and Labrador, Canada. Matern. Child Nutr. 5, 186–191 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colotta F., Jansson B., Bonelli F., Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 85, 78–97 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Chen Y., et al. , Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J. Biol. Chem. 288, 19450–19458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu H., Soruri A., Gieseler R. K., Peters J. H., 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand. J. Immunol. 38, 535–540 (1993). [DOI] [PubMed] [Google Scholar]
- 72.Zhang X., Zhou M., Guo Y., Song Z., Liu B., 1,25-dihydroxyvitamin D3 promotes high glucose-induced M1 macrophage switching to M2 via the VDR-PPARγ signaling pathway. Biomed. Res. Int. 2015, 157834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knippenberg S., et al. , Effect of vitamin D(3) supplementation on peripheral B cell differentiation and isotype switching in patients with multiple sclerosis. Mult. Scler. 17, 1418–1423 (2011). [DOI] [PubMed] [Google Scholar]
- 74.Drozdenko G., Scheel T., Heine G., Baumgrass R., Worm M., Impaired T cell activation and cytokine production by calcitriol-primed human B cells. Clin. Exp. Immunol. 178, 364–372 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Irving A. A., Marling S. J., Seeman J., Plum L. A., DeLuca H. F., UV light suppression of EAE (a mouse model of multiple sclerosis) is independent of vitamin D and its receptor. Proc. Natl. Acad. Sci. U.S.A. 116, 22552–22555 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Golan D., et al. , The influence of vitamin D supplementation on melatonin status in patients with multiple sclerosis. Brain Behav. Immun. 32, 180–185 (2013). [DOI] [PubMed] [Google Scholar]
- 77.Religi A., et al. , Estimation of exposure durations for vitamin D production and sunburn risk in Switzerland. J. Expo. Sci. Environ. Epidemiol. 29, 742–752 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Al-Dujaili E. A., Munir N., Iniesta R. R., Effect of vitamin D supplementation on cardiovascular disease risk factors and exercise performance in healthy participants: a randomized placebo-controlled preliminary study. Ther. Adv. Endocrinol. Metab. 7, 153–165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abu-Samak M. S., AbuRuz M. E., Masa’Deh R., Khuzai R., Jarrah S., Correlation of selected stress associated factors with vitamin D deficiency in Jordanian men and women. Int. J. Gen. Med. 12, 225–233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skobowiat C., Sayre R. M., Dowdy J. C., Slominski A. T., Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br. J. Dermatol. 168, 595–601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leproult R., Colecchia E. F., L’Hermite-Balériaux M., Van Cauter E., Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J. Clin. Endocrinol. Metab. 86, 151–157 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Jung C. M., et al. , Acute effects of bright light exposure on cortisol levels. J. Biol. Rhythms. 25, 208–216 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kanikowska D., et al. , Seasonal differences in rhythmicity of salivary cortisol in healthy adults. J. Appl. Physiol. 126, 764–770 (2019). [DOI] [PubMed] [Google Scholar]
- 84.Melief J., et al. , Glucocorticoid receptor haplotypes conferring increased sensitivity (BclI and N363S) are associated with faster progression of multiple sclerosis. J. Neuroimmunol. 299, 84–89 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Lopes C., Mariano M., Markus R. P., Interaction between the adrenal and the pineal gland in chronic experimental inflammation induced by BCG in mice. Inflamm. Res. 50, 6–11 (2001). [DOI] [PubMed] [Google Scholar]
- 86.Yuwiler A., Effects of steroids on serotonin-N-acetyltransferase activity of pineals in organ culture. J. Neurochem. 52, 46–53 (1989). [DOI] [PubMed] [Google Scholar]
- 87.Dokoohaki S., et al. , Corticosteroid therapy exacerbates the reduction of melatonin in multiple sclerosis. Steroids 128, 32–36 (2017). [DOI] [PubMed] [Google Scholar]
- 88.Jiang Y., Gen N., Wang P., Feng N., Lu X., Prednisolone induces sleep disorders via inhibition of melatonin secretion by the circadian rhythm in zebrafish. Biomed. Pharmacother. 147, 112590 (2022). [DOI] [PubMed] [Google Scholar]
- 89.Bliddal M., et al. , Melatonin use among children, adolescents, and young adults: A Danish nationwide drug utilization study. Eur. Child Adolesc. Psychiatry. (2022), 10.1007/s00787-022-02035-1. [DOI] [PubMed]
- 90.Tedroff K., von Euler M., Dahlén E., Melatonin usage in children and young adults, a registry-based cohort study. Eur. J. Paediatr. Neurol. 39, 30–34 (2022). [DOI] [PubMed] [Google Scholar]
- 91.Black L. I., Clarke T. C., Barnes P. M., Stussman B. J., Nahin R. L., Use of complementary health approaches among children aged 4–17 years in the United States: National health interview survey, 2007–2012. Natl. Health Stat. Rep. 1–19 (2015). [PMC free article] [PubMed] [Google Scholar]
- 92.Li J., Somers V. K., Xu H., Lopez-Jimenez F., Covassin N., Trends in use of melatonin supplements among US adults, 1999–2018. JAMA 327, 483–485 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Erland L. A., Saxena P. K., Melatonin natural health products and supplements: Presence of serotonin and significant variability of melatonin content. J. Clin. Sleep. Med. 13, 275–281 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Figueiro M. G., Rea M. S., Preliminary evidence that light through the eyelids can suppress melatonin and phase shift dim light melatonin onset. BMC Res. Notes 5, 221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
There are no data underlying this work.