Significance
The potent vasopressor angiotensin II is released acutely during blood pressure control, and chronically during heart failure as a physiological strategy to increase cardiac output. We propose a mechanism, whereby angiotensin II signaling in ventricular myocytes stimulates hydrolysis of a membrane phospholipid called PIP2, triggering CaV1.2 channel internalization, and providing a means to acutely tune cellular excitability and modulate cardiac excitation-contraction coupling. Accordingly, this study presents data that supports a novel mechanistic role of PIP2 as a stabilizer of CaV1.2 channel expression on the sarcolemma of ventricular myocytes. Moreover, we provide the evidence that physiological signaling pathways alter cardiac PIP2 levels, and thus this work has implications for the many cardiac ion channels and exchangers regulated by PIP2.
Keywords: L-type calcium channels, PIP2, EC-coupling, ion channel trafficking, angiotensin II
Abstract
CaV1.2 channels are critical players in cardiac excitation–contraction coupling, yet we do not understand how they are affected by an important therapeutic target of heart failure drugs and regulator of blood pressure, angiotensin II. Signaling through Gq-coupled AT1 receptors, angiotensin II triggers a decrease in PIP2, a phosphoinositide component of the plasma membrane (PM) and known regulator of many ion channels. PIP2 depletion suppresses CaV1.2 currents in heterologous expression systems but the mechanism of this regulation and whether a similar phenomenon occurs in cardiomyocytes is unknown. Previous studies have shown that CaV1.2 currents are also suppressed by angiotensin II. We hypothesized that these two observations are linked and that PIP2 stabilizes CaV1.2 expression at the PM and angiotensin II depresses cardiac excitability by stimulating PIP2 depletion and destabilization of CaV1.2 expression. We tested this hypothesis and report that CaV1.2 channels in tsA201 cells are destabilized after AT1 receptor-triggered PIP2 depletion, leading to their dynamin-dependent endocytosis. Likewise, in cardiomyocytes, angiotensin II decreased t-tubular CaV1.2 expression and cluster size by inducing their dynamic removal from the sarcolemma. These effects were abrogated by PIP2 supplementation. Functional data revealed acute angiotensin II reduced CaV1.2 currents and Ca2+ transient amplitudes thus diminishing excitation–contraction coupling. Finally, mass spectrometry results indicated whole-heart levels of PIP2 are decreased by acute angiotensin II treatment. Based on these observations, we propose a model wherein PIP2 stabilizes CaV1.2 membrane lifetimes, and angiotensin II-induced PIP2 depletion destabilizes sarcolemmal CaV1.2, triggering their removal, and the acute reduction of CaV1.2 currents and contractility.
Voltage-gated L-type CaV1.2 Ca2+ channels are depolarization-triggered conduits of Ca2+ entry into excitable cells. In the heart, they are critical players in excitation-contraction (EC) coupling, where they provide the initial, predominantly t-tubule localized entry pathway for Ca2+ (1). During the peak and plateau phases of the ventricular action potential, CaV1.2-conducted Ca2+ influx activates a larger release of sarcoplasmic reticulum (SR) Ca2+ from clusters of type 2 ryanodine receptors (RyR2) on the juxtaposed junctional SR via Ca2+-induced Ca2+-release (CICR). The magnitude of local CICR and its summation across thousands of sites within each cardiomyocyte dictates the amplitude of the global Ca2+ transient and the strength of the subsequent myocardial contraction. As release of SR Ca2+ is graded by the amplitude of Ca2+ entry through CaV1.2 channels, alterations in the number and/or activity of CaV1.2 channels on the t-tubule sarcolemma tunes EC-coupling (2).
Physiological signaling cascades (3–6), post-translational modifications (7–11), and Ca2+ itself (12–16) are well-studied regulators of both CaV1.2 channel activity and sarcolemmal expression in various tissues, including the heart. However, even though CaV1.2 channels are embedded in a lipid environment, and despite the fact that many other ion channels and exchangers are known to be regulated by the membrane phospholipid phosphatidylinositol 4,5 bisphosphate (PI(4,5)P2, henceforth abbreviated as PIP2), its potential effects on CaV1.2 distribution, activity, and expression in cardiomyocytes have been overlooked. There is accumulating evidence that several subtypes of voltage-gated Ca2+ channels are regulated by PIP2; however, the experiments underlying these findings have largely been carried out in heterologous expression systems (17–20), neurons (21), or pancreatic β-cells (22) and were not done in cardiomyocytes. These previous reports indicate that CaV1.2 currents are suppressed upon depletion of PIP2 downstream of Gq-coupled activation of M1-muscarinic receptors (M1R) by oxotremorine-m (Oxo-m) or through activation of a voltage-sensitive 5-phosphatase DR-VSP that removes the 5′ phosphate from PI(4,5)P2 and converts it to PI(4)P (17, 20). However, how PIP2 regulates CaV1.2 remains unknown.
During Gq-coupled physiological signaling cascades, PIP2 levels in the plasma membrane (PM) fall due to its phospholipase C (PLC)-mediated hydrolysis, generating inositol trisphosphate (IP3) and diacylglycerol (DAG). The M1 receptors targeted in the aforementioned Hille group studies are not expressed in ventricular myocytes but several other Gq-coupled receptors are, including angiotensin type 1 receptors (AT1R). Activated by the peptide hormone and vasoconstrictor agonist angiotensin II (AngII) (23), the AT1R/Gq/PLC signaling cascade is triggered during acute blood pressure control and chronically during hypertension and heart failure (HF) where it is implicated in cardiac hypertrophy and fibrosis (24–26). While AngII and its derivative Ang(1-7) can act on other receptors in the heart including angiotensin type 2 receptors, and Ang(1-7)/Mas receptors (27), we focus here on the effects on AT1R since this is the only pathway of the three that is Gq-coupled and thus is the most likely to directly modulate PIP2 levels.
AngII has both indirect and direct effects on the heart. Indirectly, the effects of AngII on the vasculature change hemodynamic loading conditions. Directly, AngII has been demonstrated to affect cardiomyocyte L-type Ca2+ channel currents (ICa) in a species-dependent manner. Accordingly, the regulation of cardiac CaV1.2 by acute AngII treatment has been varyingly reported to cause an increase (in rabbit, cat, and cultured neonatal rat) (28–30), or a decrease in (human, chick, and cultured neonatal rat) (6, 31, 32) cardiomyocyte ICa. However, the underlying mechanism is unclear. Some have implicated channel phosphorylation by protein kinase C (PKC) as the regulatory driver (32, 33), while others have suggested a role for arachidonic acid and a CaVβ isoform-dependent effect (31). Thus, a unifying theory that explains the ICa-depleting effects of AngII on cardiomyocytes remains elusive and is the focus of this study.
We hypothesize that PIP2 depletion is the driver of the regulatory effects of AngII on cardiac ICa. Furthermore, we propose that PIP2 stabilizes CaV1.2 channel expression in the t-tubule sarcolemma and that AngII can acutely depress cardiac excitability by stimulating PIP2 depletion which destabilizes PM CaV1.2 and ultimately leads to their removal from the PM. Using electrophysiology, total internal reflection fluorescence (TIRF) microscopy and single-molecule localization microscopy, biochemistry, and lipid mass spectrometry approaches, our investigation uncovered a novel consequence of AngII signaling, that acute physiological signaling through AngII and the induction of PIP2 depletion results in destabilization of PM CaV1.2 channels, triggering their removal and the acute reduction of ICa and contractility in the heart.
Results
Receptor-Stimulated PIP2 Depletion Destabilizes PM Expression of CaV1.2 Channels.
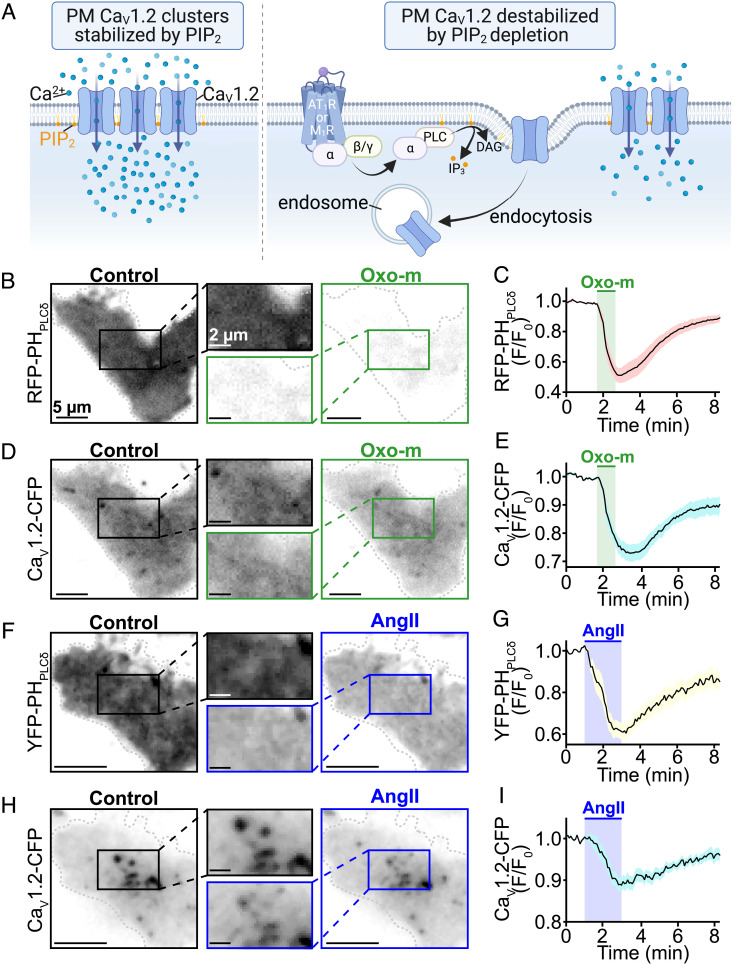
We began our study by testing the hypothesis that PIP2 stabilizes CaV1.2 channel expression in the PM (as illustrated in Fig. 1A) by examining the effects of Gq-coupled receptor-driven PIP2 depletion on PM CaV1.2 channel expression in a reductionist system lacking the complexities of a cardiomyocyte. Accordingly, fluorescence imaging experiments were performed on tsA201 cells transfected with M1R or AT1R. Cells were additionally transfected with a red fluorescent protein tagged PIP2 probe based on the pleckstrin homology domain of PLC [PH-PLC∂1-RFP (34)], and a cyan fluorescent protein (CFP) tagged CaV1.2 (CaV1.2-CFP) to monitor PM PIP2 and channel expression, respectively. Adapting the approach of Suh et al. (17), M1R were activated with a saturating concentration of the agonist Oxo-m (10 µM), triggering the Gq/PLC signaling pathway and resulting in PIP2 hydrolysis. In TIRF time series experiments application of Oxo-m triggered a robust 48.1 ± 4.7% depletion of PHPLC∂1-RFP from the PM, which upon washout was recovered, demonstrating PIP2 hydrolysis and resynthesis (Fig. 1 B and C). A 22.4 ± 3.1% decline in PM CaV1.2-CFP commenced within ~10 s of PIP2 depletion followed by a partial recovery upon washout (Fig. 1 D and E). This delay potentially reflects the time required for channel endocytosis and recycling to begin after PIP2 depletion. Once initiated, the channel removal and recovery phases followed a similar kinetic profile to that of the PH-fluoroprobe (channel: τdepletion = 49.24 ± 4.54 s, τrecovery = 118.92 ± 6.16 s; PIP2: τhydrolysis = 21.67 ± 1.85 s, τrecovery = 139.46 ± 6.83 s). These PIP2 kinetics measurements agree with prior published values following M1R activation in tsA201 cells (35, 36). In AT1R expressing tsA201 cells, the application of AngII (100 nM) resulted in a 39.6 ± 3.6% depletion of PHPLC∂1-YFP signals (PIP2 τhydrolysis = 40.15 ± 17.20 s). This was accompanied by an 11.1 ± 1.6% decline in PM CaV1.2-CFP that began ~30 s after the onset of PIP2 depletion (Fig. 1 F–I). Taken together, these experiments indicate that CaV1.2 channel destabilization and removal from the PM occurs after GqPCR/PLC-mediated PIP2 hydrolysis.
Fig. 1.
Receptor-stimulated PIP2 depletion destabilizes plasma membrane CaV1.2 expression. (A) Illustration of our hypotheses where PIP2 stabilizes membrane expression of CaV1.2 (Left) and Gq-coupled receptor-stimulated PIP2 hydrolysis destabilizes CaV1.2 expression triggering their endocytosis (Right). (B) Representative TIRF images showing localization of the PIP2 biosensor RFP-PH-PLC∂1 before and after 10 µM Oxo-m treatment in M1R and CaV1.2-expressing tsA201 cells (n = 12). (C) Time-course (mean ± SEM) of the Oxo-m stimulated changes in normalized RFP-PH-PLC∂1 fluorescence emission (F/F0) in the TIRF footprint. (D) TIRF images of CaV1.2-CFP in the same cell before and after Oxo-m, and (E) the average time-course of changes in CaV1.2-CFP fluorescence emission (F/F0) over the course of the experiments. (F) Representative TIRF images and (G) average time-course of intensity changes of YFP-PH-PLC∂1 before and after 100 nM AngII treatment in AT1R and CaV1.2-expressing tsA201 cells (n = 13). (H) TIRF images of CaV1.2-CFP in the same cell and, (I) average time-course of the experiments.
Recruitment of a Lipid Phosphatase to the PM Leads to CaV1.2 Endocytosis.
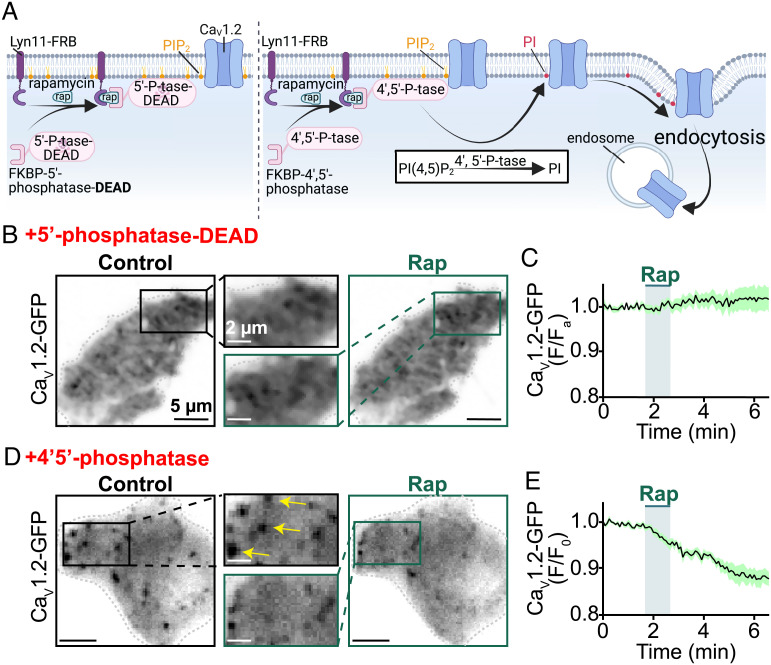
Gq-coupled receptor signaling cascades generate/activate several second messengers and downstream effectors, e.g., arachidonic acid and protein kinase C (PKC), that could themselves affect CaV1.2 channel expression. To isolate the specific effects of PIP2 depletion on PM CaV1.2 expression levels, we utilized a chemical dimerization strategy to deplete PIP2 in a targeted manner that would be independent of receptor signaling. In this previously described system, addition of rapamycin is used to dimerize an FKBP-rapamycin binding (FRB) domain-linked membrane anchor (Lyn11-FRB), to an FK506 binding protein (FKBP) that is coupled to a 4′, 5′ lipid phosphatase enzyme (FKBP-4′ 5′ phosphatase) (37). This irreversible dimerization recruits cytosolic FKBP-4′, 5′ phosphatase to the PM anchor where it removes the 4′ and 5′ phosphate groups from PIP2 to produce PI (Fig. 2A). To control for any rapamycin-specific effects, we initially recruited an enzymatically dead lipid phosphatase to the PM of transfected tsA201 cells and observed no reduction in green fluorescent protein (GFP) tagged CaV1.2 (CaV1.2-GFP) (Fig. 2 B and C). In contrast, PM recruitment of a catalytically active phosphatase produced a 12.4 ± 1.7% reduction in PM CaV1.2-GFP in response to rapamycin-induced PIP2 depletion (Fig. 2 D and E). Together, these experiments show that PIP2 depletion destabilizes PM expression of CaV1.2-GFP.
Fig. 2.
Receptor-independent PIP2 depletion via chemical translocation of a lipid phosphatase destabilizes plasma membrane CaV1.2 expression. (A) Illustration of the FKBP–FRB rapamycin-dependent dimerization system used to irreversibly recruit an enzymatically dead (control; Left) or an active (Right) lipid phosphatase to the PM. The active enzyme depletes PIP2 by metabolizing it into PI. The cartoon graphically illustrates the testable prediction that this would destabilize PM expression of CaV1.2 and trigger their endocytosis. (B) TIRF images and (C) average CaV1.2-GFP (F/F0) time-course showing PM CaV1.2-GFP expression in transfected tsA201 cells before and after rapamycin (1 µM) induced recruitment of enzymatically dead 5′-lipid-phosphatase (n = 7). (D) TIRF images and (E) average CaV1.2-GFP (F/F0) time-course showing modulation of PM CaV1.2-GFP expression in transfected tsA201 cells before and after rapamycin-induced recruitment of active 4′,5′-phosphatase (pseudojanin-FKBP; n = 6).
PIP2 Depletion Triggered CaV1.2 Endocytosis Is Dynamin-Dependent.
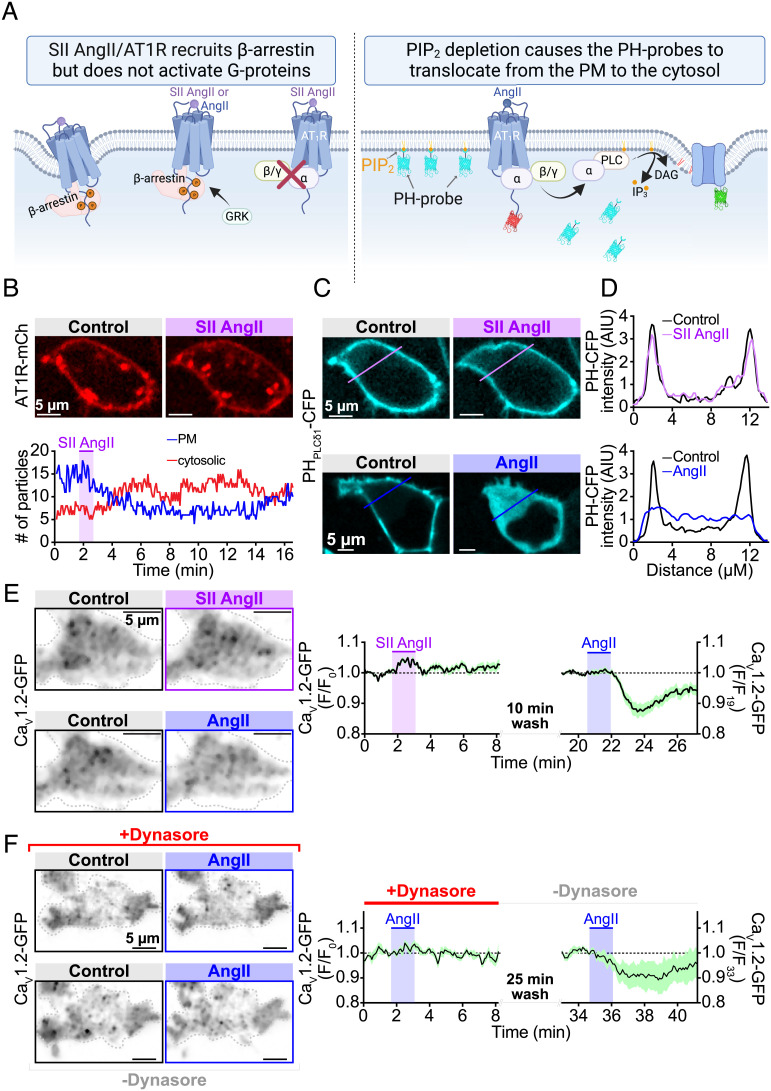
AT1R are known to undergo agonist-promoted internalization in a process that involves β-arrestin and dynamin-dependent endocytosis of the receptors (38). Thus to determine whether AngII-stimulated internalization of CaV1.2-GFP occurred simply due to receptor-adjacent channels being caught up in AT1R sequestration, we stimulated the receptors with [Sar1,Ile4,Ile8]-AngII (SII-AngII), a β-arrestin-biased agonist of AT1R (39). SII-AngII binding to AT1R induces β-arrestin recruitment but fails to engage G-proteins thus allowing discrimination between an internalization that requires β-arrestin and one that is stimulated by Gq signaling (Fig. 3A). In proof-of-reagent experiments, confocal imaging performed on AT1R-mCherry and PHPLC∂1-CFP expressing tsA201 cells revealed AT1R-mCherry internalization in response to SII-AngII (Fig. 3B), but PIP2 depletion only occurred during AngII application (Fig. 3 C and D). As PIP2 hydrolysis occurred, PHPLC∂1-CFP lost its binding partner in the PM, was released from membrane sites and in turn accumulated in the cytosol. These results illustrate the β-arrestin bias of SII-AngII and its failure to engage G-proteins. Importantly, TIRF experiments on cells expressing CaV1.2-GFP revealed no change in TIRF fluorescence of the channel with the SII-AngII treatment. Moreover, after a washout period, these same cells responded to AngII, leading to a 12.7 ± 2.3% reduction in channel fluorescence (Fig. 3E). Collectively, these results indicate that AngII-stimulated CaV1.2 channel internalization does not occur due to β-arrestin-dependent sequestration of neighboring AT1R.
Fig. 3.
AngII/AT1R stimulated CaV1.2 endocytosis is dependent on dynamin, not β-arrestin. (A) Illustration of our experimental design where SII AngII was used to stimulate β-arrestin recruitment but failed to activate G-proteins (Left). (Right) CFP-tagged PH-probes were used to monitor PM PIP2 levels, while CaV1.2-GFP and AT1R-mCherry were used to track the localization of the channels and receptors before and after application of agonists. (B, Top) Representative confocal images showing AT1R-mcherry localization in tsA201 cells, and (Bottom) accompanying time-course showing AT1R-mCherry “particle” tracking in the PM (blue line) and cytosolic (red line) compartments of this cell before and after application of the β-arrestin-biased AT1R agonist, SII AngII (10 µM; n = 8). (C) Confocal images showing localization of the PIP2 biosensor PH-PLC∂1-CFP before and during application of SII AngII (Top), or AngII (Bottom). (D) Plot profiles of normalized PH-PLC∂1-CFP fluorescence intensity across the cell in each condition at the line-regions of interest (ROIs) indicated in panel C. (E) TIRF images showing CaV1.2-GFP localization in a representative tsA cell treated first with SII AngII, and subsequently with 100 nM AngII. (Right) Corresponding mean ± SEM time courses of the normalized CaV1.2-GFP intensity (n = 14). (F) Representative TIRF images and corresponding mean ± SEM time-courses showing CaV1.2-GFP intensity in tsA201 cell TIRF footprints during application of AngII in the presence (Top), and absence (Bottom) of the dynamin-inhibitor dynasore (10 µM; n = 5).
Next, to test the hypothesis that AngII-stimulated CaV1.2 channel endocytosis was dynamin-dependent (SI Appendix, Fig. S1), we pre-treated CaV1.2-GFP and AT1R expressing tsA201 cells with the dynamin inhibitor dynasore (80 µM). In TIRF imaging experiments, this treatment prevented AngII-stimulated CaV1.2 removal from the PM (Fig. 3F). The washout of dynasore and subsequent reapplication of AngII to the same cells revealed the recovery of the CaV1.2 channel removal response (Fig. 3F). Channel endocytosis was determined to be Ca2+ independent, as it persisted when Ba2+ was substituted for Ca2+ (SI Appendix, Fig. S2). These results confirm that AT1R-stimulated PIP2 depletion destabilizes CaV1.2 anchoring in the PM and triggers dynamin-dependent endocytosis of the channel in tsA201 cells.
AngII Reduces CaV1.2 Cluster Size and Sarcolemma Expression in Ventricular Myocytes.
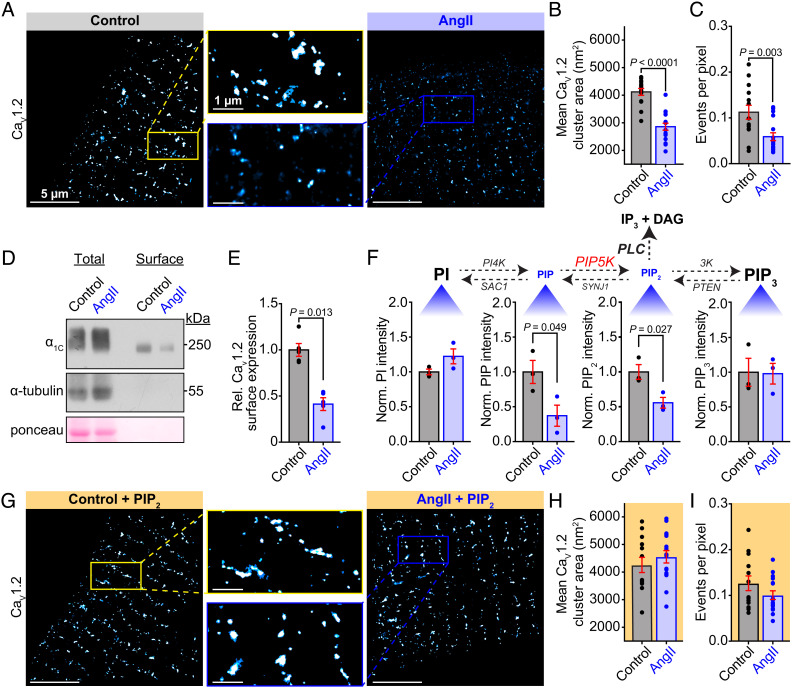
Turning our attention to primary cells from native tissues, we set out to determine whether a similar AngII-triggered CaV1.2 endocytosis exists in freshly isolated ventricular myocytes. If a similar phenomenon exists in these cells, then a testable prediction is that acute treatment with AngII should reduce CaV1.2 channel expression at the sarcolemma. That hypothesis was rigorously tested using a two-pronged approach. First, we examined the nanoscale distribution of immunostained CaV1.2 channels using super-resolution single molecule localization microscopy (SMLM). CaV1.2 channel cluster areas in myocytes acutely treated with AngII were 30.9 ± 4.4% smaller on average compared to controls (Fig. 4 A and B). The number of events per pixel within the cell-occupied area of the localization map provides an indication of the density of labeling and the number of channels. This measure was also reduced by 47.9 ± 14.8% in the AngII-treated cells versus controls (Fig. 4C) suggesting that acute AngII treatment reduced the number of CaV1.2 channels in the t-tubule sarcolemma. Similar results were obtained from female ventricular myocytes suggesting this response is conserved across both sexes (SI Appendix, Fig. S3). It is also noteworthy that this stimulated decrease in CaV1.2 channel clustering and expression appears confined to the t-tubules as crest-localized CaV1.2 clusters were not significantly altered by acute AngII (SI Appendix, Fig. S4).
Fig. 4.
Acute AngII application reduces t-tubular CaV1.2 cluster area and PM expression in a PIP2-depletion–dependent manner. (A) Representative SMLM localization maps showing CaV1.2 channel distribution in the t-tubules of mouse ventricular myocytes with (Right) or without (Left) AngII-stimulation. Boxes indicate the location of the enlarged regions. (B) Aligned dot plots showing mean CaV1.2 channel cluster areas, and (C) events per pixel as an indicator of channel expression density (control: N = 4, n = 14; AngII: N = 3, n = 17). (D) Representative western blot image of the total and biotinylated cell surface fraction of CaV1.2, internal control alpha-tubulin, and total protein ponceau stain, in untreated control, and AngII-treated ventricular myocyte lysates. (E) Aligned dot plots showing relative CaV1.2 surface expression (N = 13, n = 5). (F, Top) The homeostatic phospholipid metabolism pathway with species scaled to illustrate the observed effects of AngII. (Bottom) Histograms summarizing UPLC-MS/MS measurements of PI, PIP, PIP2, and PIP3 in samples of untreated control or AngII-stimulated whole heart lysates, (N = 3 for each). (G) SMLM localization maps showing CaV1.2 channel distribution in control and AngII-treated myocytes supplemented with PIP2 (control + PIP2N = 3, n = 13; AngII + PIP2N = 3, n = 16). (H) Aligned dot plots showing mean CaV1.2 channel cluster area and (I) the events per pixel in each condition. Error bars indicate SEM. Statistical analyses on data summarized in B, C, F, H, and I were performed using unpaired, two-tailed Student’s t-tests. Data in E were compared using a paired, two-tailed Student’s t-test.
Next, in the second prong of our approach, we biotinylated membrane proteins from isolated myocytes and quantified the biotinylated (plasma membrane-localized) fraction of the a11.2 CaV1.2 channel subunit that bound to NeutrAvidin beads in pull-down assays via immunoblotting. Here we determined that surface a11.2 in the AngII-treated myocytes was reduced by 58.8 ± 13.8% in comparison to controls (Fig. 4 D and E). Altogether, these complementary results using two separate techniques reveal that acute treatment of mouse ventricular myocytes with AngII leads to reduced expression of CaV1.2 channels at the PM.
Whole-Heart Phospholipid Species’ Levels Are Altered by Acute AngII Treatment.
To establish that AT1R/Gq/PLC-driven PIP2 depletion drives these changes in CaV1.2 channel distribution, it was necessary to quantify phosphoinositide species in hearts with and without acute AngII-treatment. A simplified view of phosphoinositide metabolism is shown in Fig. 4 F, Top. We measured each of these species using phospholipid mass spectrometry and found that Langendorff perfusion of hearts with 100 nM AngII for just 5 min generated significant alterations in the levels of PIP2 and its precursor PIP (Fig. 4 F, Bottom). Specifically, we observed a 62.8 ± 22.4% decrease in PIP and a 44.2 ± 13.0% decrease in PIP2 (Fig. 4 F, Bottom) after AngII. These results suggest that acute, physiological elevations in AngII in the heart result in PIP and PIP2 depletion.
AngII-Stimulated CaV1.2 Endocytosis in Cardiomyocytes Requires PIP2 Depletion.
To address the question of whether the reductions in t-tubule sarcolemma-localized CaV1.2 were driven by PIP2 depletion-triggered endocytosis, SMLM was performed on cardiomyocytes supplemented with PIP2. We reasoned that if PIP2 stabilizes CaV1.2 channel expression then boosting PIP2 concentration in the membrane might reduce or eliminate the AngII-stimulated endocytosis. In line with that prediction, PIP2 supplementation prevented AngII-mediated decreases in cluster area and expression (Fig. 4 G–I). Furthermore, myocyte treatment with the β-arrestin-biased AT1R agonist SII-AngII failed to trigger the endocytosis response (SI Appendix, Fig. S5). As discussed above, SII-AngII bound AT1R cannot engage G-proteins to activate PLC-mediated hydrolysis of PIP2, thus these results reinforce the idea that AngII stimulation of AT1R triggers CaV1.2 channel endocytosis in a process that requires PIP2 depletion.
CaV1.2 Channels Endocytosed Downstream of AT1R Activation Are Stored in Endosomes.
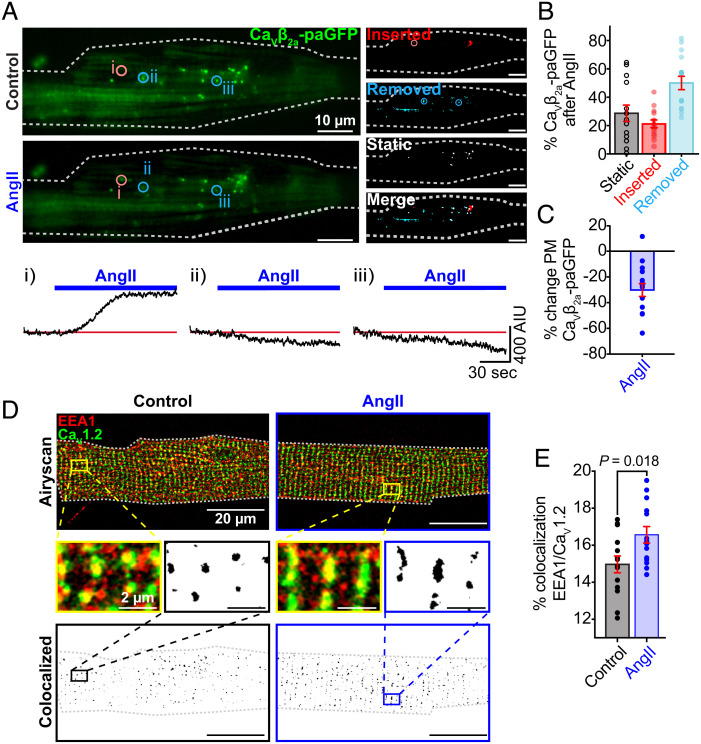
We next sought to visualize the dynamics of AngII-stimulated CaV1.2 endocytosis in live cells. To accomplish this, we utilized transduced myocytes isolated from mice 2 wk after their inoculation with a retro-orbital injection of the AAV9-CaVβ2a-paGFP. The transduced expression construct encodes an auxiliary subunit that finds and binds endogenous pore-forming α1C-subunits on a 1:1 basis and thus acts as a fluorescent biosensor of CaV1.2 channels as has been extensively verified in other studies (4, 5). After photoactivation with 405 nm light, clusters of GFP-tagged channels were visualized and a time series of experiments was performed to track biosensor-tagged channels before and during application of AngII via 150 nm penetration depth TIRF microscopy, an imaging technique that allows detection of the surface and initial portion of the t-tubule sarcolemma. Image analysis was used to identify and quantify subpopulations of channels that were inserted, removed, or static during AngII treatment relative to the control period (Fig. 5 A and B). Our results indicated a strong bias toward channel removal during AngII treatment (Fig. 5B). Since the population of channels at the membrane at any given time is dictated by the balance between their insertion and removal, enhanced removal relative to insertion will lead to reduced expression of channels at the membrane. Accordingly, we found a 28.8 ± 5.2% reduction in biosensor-tagged channels in the TIRF footprints of myocytes after AngII (Fig. 5C). This evident dynamic shift toward channel internalization invites the question, where are these channels going?
Fig. 5.
AngII promotes CaV1.2 channel removal from cardiomyocyte sarcolemmas and sequestration in early endosomes. (A) Representative TIRF images of GFP fluorescence emission from CaVβ2a-paGFP transduced cardiomyocytes before (Top) and after 100 nM AngII (Bottom) (N = 5, n = 14). Images illustrating stable, inserted, and endocytosed channel populations are shown to the Right. Time courses of the changes in CaVβ2a-paGFP intensity in individual ROIs (indicated by circles on TIRF images) are represented below. (B and C) Histograms summarizing the percentage of static, inserted, and removed channel populations after AngII (B) and the percentage change of TIRF-footprint CaVβ2a-paGFP fluorescence after AngII (C). (D) Two-color Airyscan super-resolution images showing distributions of CaV1.2 and EEA1+ early endosomes in representative control (Left) and AngII-stimulated (Right) myocytes. Binary colocalization maps (Bottom) display pixels in which CaV1.2 and endosomal expression fully overlap. (E) Histogram summarizing the percentage colocalization between EEA1 and CaV1.2 in control (N = 3, n = 14) and AngII-stimulated (N = 3, n = 13) conditions. Statistical analyses on data summarized E was performed using unpaired, two-tailed Student’s t-tests. Error bars indicate SEM.
Based on our prior work (4), we hypothesized that internalized channels may populate an endosomal reservoir. To determine the acute fate of these channels in response to AngII, we examined the distribution of CaV1.2 on EEA1-positive early endosomes, Rab7-positive-late endosomes, and Rab11-positive recycling endosomes. Two-color Airyscan super-resolution images were taken on control or AngII-treated AMVMs immunostained for CaV1.2 and these endosomal markers. Colocalization analysis revealed a 10.7 ± 4.2% increase in CaV1.2 localized on early endosomes treated with AngII (Fig. 5 D and E). However, there was no significant difference in the percent of CaV1.2 on recycling endosomes (SI Appendix, Fig. S6 A and B) and late endosomes (SI Appendix, Fig. S6 C and D) between the two groups. This suggests that after only 5 min of AngII signaling, internalized channels are gathered on early endosomes but have not yet been shuttled toward recycling or degradation pathways.
AngII Attenuates ICa, PIP2, and Ca2+ Transient Amplitudes in Cardiomyocytes.
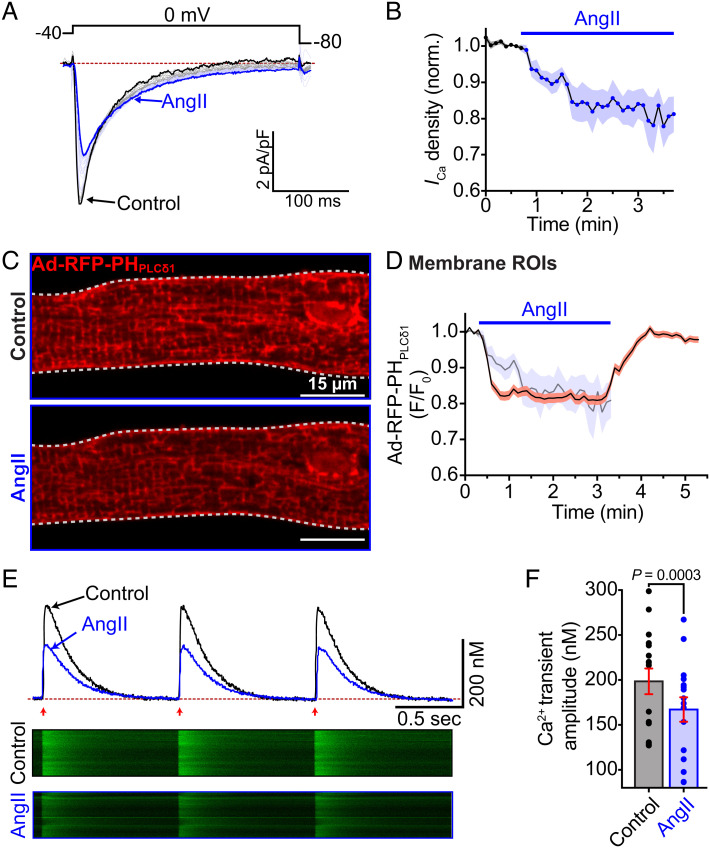
We hypothesized that the apparent deficit in PM CaV1.2 channel expression and clustering would ultimately decrease functional output. To test that, we performed perforated patch clamp experiments and recorded ICa from freshly isolated myocytes, before and during acute AngII-treatment. Accordingly, application of AngII caused an ~20% decrease in ICa density that occurred with a τ of 0.69 min (Fig. 6 A and B). If this AngII-induced current reduction is triggered by PIP2 depletion and destabilization of CaV1.2, then a testable prediction is that PIP2 hydrolysis should occur on a similar timescale. To test this, we transduced ventricular myocytes with adenovirus-packaged RFP-PHPLCδ1. After 48 h in culture, the transduced PH-probe biosensor was predominantly localized to the sarcolemma of the cardiomyocytes (Fig. 6C). Time series confocal experiments revealed an AngII-induced reduction in membrane localization of RFP-PHPLCδ1 due to PIP2 hydrolysis and a subsequent recovery upon washout as PIP2 was resynthesized (Fig. 6D). The τ of PIP2 hydrolysis was, at 0.42 min, slightly faster than that of the AngII-triggered current reduction. Superimposition of the current decay onto the PH-probe measurements revealed the initiation of PIP2 hydrolysis slightly precedes the onset of ICa decay.
Fig. 6.
AngII depression of ventricular myocyte CaV1.2 currents and Ca2+ transient amplitude occurs coincidently with PIP2 depletion. (A) Representative L-type ICa elicited every 6 s by a 300 ms depolarization step from −40 mV to 0 mV. Black traces show the current before, and blue traces the current after AngII. (B) Diary plot of normalized Ca2+ current density summarizing the results from N = 4, n = 6 cells. (C) Confocal images of a cardiomyocyte transduced with Ad-RFP-PHPLCδ1 before and during AngII. (D) Time course of the changes in membrane RFP-PHPLCδ1 intensity (F/F0) in the same cell before, and during AngII and its recovery on washout. Representative of N = 3, n = 4 cells. Time course of the changes in ICa from panel B are superimposed for ease of comparison. (E) Representative EFS-evoked Ca2+ transients recorded under control (black traces) and AngII-stimulated conditions (blue traces). Arrows indicate EFS pulse application at 1 Hz. Corresponding line scans from the fluo4-AM-loaded cells appear below. (F) Histogram summarizing Ca2+ transient amplitude (N = 3, n = 16). Data were analyzed using paired Student’s t-tests. Error bars indicate SEM.
Finally, to investigate the effects of AngII on EC-coupling, we recorded Ca2+ transients from freshly isolated myocytes, before and during acute AngII-treatment. Accordingly, AngII elicited a a 15.8 ± 3.3% reduction in EFS-stimulated Ca2+ transient amplitude (Fig. 6 E and F). In line with our hypothesis, these data collectively show that acute AngII-stimulation tunes and diminishes EC-coupling and does so on a timescale that closely aligns with that of PIP2 hydrolysis.
Discussion
Our data show that PIP2 stabilizes cardiac CaV1.2 channel PM lifetime and that AngII-induced PIP2 depletion destabilizes PM CaV1.2 clusters, triggers their dynamin-dependent endocytosis, results in the acute reduction of whole-cell ICa, and decreases Ca2+ transient amplitudes, which diminishes myocardial excitability and contractility. We provide evidence for this phenomenon using both a heterologous expression system and primary ventricular myocytes. In transfected tsA201 cells, both GqPCR-mediated and receptor-independent PIP2 depletion triggered CaV1.2 removal from the PM. In myocytes, AngII/AT1R signaling triggered sarcolemmal PIP2 hydrolysis, reduced CaV1.2 expression and cluster area, and resulted in the stimulated removal of CaV1.2 from the PM to early endosomes. PIP2 reinforcement stabilized CaV1.2 channel clusters and their sarcolemmal expression. β-arrestin biased AT1R agonism also eliminated this response, specifically implicating Gq activation. Moreover, dynamin inhibition abrogated the internalization response, suggesting internalization proceeded via dynamin-dependent endocytosis.
The finding that CaV1.2 endocytosis is dynamin-dependent agrees with published work from several other groups although none were testing the triggering of this event by AngII-mediated PIP2 depletion (12, 40, 41). In our hands, endocytosed CaV1.2 were sequestered into early endosomes but not recycling or late endosomes. Previous work from our group identified an endosomal reservoir of CaV1.2 channels on early and recycling endosomes that can be mobilized to the t-tubule sarcolemma of cardiomyocytes during periods of acute stress via Rab4-dependent fast and Rab11-dependent slow recycling pathways (4), enhancing CaV1.2 cluster areas and channel expression in t-tubule sarcolemma (5), and facilitating a positive inotropic response to tune cardiac performance to meet physiological demands. Results from the current study reveal activation of AT1R has essentially the opposite effect, invoking an image of a rheostatic system whereby cardiac EC-coupling can be tuned up or down by manipulating sarcolemmal expression of CaV1.2 channels via selective activation of different receptor populations. While our work rules out the possibility that the acute CaV1.2 internalization response we report occurs due to their entrapment in β-arrestin-dependent sequestration of nearby AT1R, others have shown that longer, 1 h applications of AngII produce CaV1.2 internalization that does involve β-arrestin (6). We found that AngII-stimulated CaV1.2 internalization occurred predominantly in t-tubules and not in the sarcolemma crest. It is possible that AT1R are more closely associated with t-tubule CaV1.2 channels than with crest populations, creating signaling microdomains. However, to our knowledge, there are no specific antibodies against AT1R and thus this idea remains speculative.
Prior studies have indicated that neuronal CaV1.2 channel endocytosis occurs upon Ca2+ influx through the channels as the incoming Ca2+ binds to calmodulin (CaM) forming Ca2+. CaM that competes with α-actinin for a common binding site on the α1C C-terminal (12). In that model, binding of α-actinin to an α1C surface-targeting motif promotes CaV1.2 channel expression on the PM. Ca2+.CaM outcompetes α-actinin, displacing it from the channel and favoring endocytosis. Our model describes what appears to be a separate process since it occurs independently of Ca2+ influx, as shown by its persistence with Ba2+ substitution. We find that PIP2 exerts a stabilizing influence on CaV1.2 surface expression and that its depletion by either Gq-receptor stimulation or PM recruitment of a lipid phosphatase favors channel endocytosis. This implies that PIP2 must bind to the channel complex. Rosetta structural modeling has predicted the presence of at least one putative PIP2 binding site formed by four arginines within the I-II loop α-helix region of CaV1.2 (42). Interestingly, neutralization of these residues removed PM binding of the linker and decreased on-gating current (Qon), revealing that there were fewer functional channels at the PM. Thus, there appears to be a role for PIP2 in supporting calcium channel activity and expression at the cardiac sarcolemma. Intriguingly, the putative site is found on the I-II loop that also contains the gain-of-function Timothy syndrome CaV1.2 mutation site at G406R (G436R in mice). Replacing a neutral glycine with a positively charged arginine might further enhance binding to negatively charged phospholipids at this interface. In our model, enhanced PIP2 binding would favor stabilization of CaV1.2 channels at the PM and supporting that idea, we have previously reported that G436R mutant gain-of-function CaV1.2 channels form extremely large, pronounced clusters in ventricular myocytes (43). In CaV2.2 channels, high-resolution cryo-EM structures in the presence of a pore-blocking pain-killer called Ziconotide revealed a PIP2 binding site that stabilizes the down conformation of voltage sensitive domain II (44). This would favor the closed state of the channel and limit Ca2+ influx. However, over 50% of the residues in that site are CaV2-specific, so it remains to be determined whether a homologous site exists in CaV1.2.
Our study provides a quantitative analysis of phosphoinositide species in the heart and reveals that PIP2 levels measurably change during physiological Gq-protein coupled receptor signaling in cardiomyocytes. Specifically, we show that levels of PIP2 and its precursor PIP are both significantly reduced by acute application of AngII. Further experiments on cultured adult mouse ventricular myocytes transduced with an adenovirus-packaged fluorescent PIP2 biosensor (PH-RFP) revealed the kinetics of AngII-stimulated sarcolemmal PIP2 depletion. The hydrolysis of PIP2 was concordant with the observed reduction in ICa supporting the idea that PIP2 depletion destabilizes CaV1.2 channels. The effects of Gq/PLC-dependent pathway activation were previously studied in guinea pig ventricular myocytes but in contrast to our results using different experimental approaches, the tested agonists (endothelin, phenylephrine, and adenosine) elicited no detectable change in total cardiac PIP2 (45). Given that PIP2 is a modulator of many cardiac ion channels and transporters including CaV1.2 (17), KCNQ1/E1 (46), KATP and Na+-Ca2+ exchanger [NCX (47)], our findings have a broad impact. It remains to be determined whether PIP2 exerts a similar stabilizing effect on CaV1.2 in other excitable tissues, including smooth muscle, neurons, and pancreatic islets. Specifically in the heart, elevated production of AngII is associated with cardiac hypertrophy, remodeling, and HF (48–51). Future studies should examine the effects of chronic AngII on phosphoinositide populations in the heart and the ramifications for cardiac ion channel function.
Our finding that PIP2 depletion destabilizes CaV1.2 expression potentially explains prior results indicating voltage-gated Ca2+ channel current depletion by activation of M1Rs or voltage-sensitive phosphatase (DR-VSP) (17, 20). In favor of this stabilization hypothesis, PIP2 supplementation is known to reduce P/Q-type CaV2.1 channel rundown in Xenopus oocytes, while sequestering PIP2 with antibodies increased rundown (19). Rundown describes the poorly understood process of ion channel current decline observed in whole-cell and excised-patch clamp recordings over time. The endocytosis of CaV1.2 channels from the membrane has also been proposed as a factor underlying rundown (12). The supplementation of patch bathing or cell dialyzing solutions with MgATP, CaM, or crude cytoplasmic extracts from cardiac cells (52) have all been reported to reduce ICa rundown (53, 54). MgATP supports PIP2 resynthesis by activating lipid kinases (19, 46, 55), while Mg2+ can guard against PIP2 hydrolysis by a charge shielding effect (56). CaM has also been reported to promote PIP2 generation in cardiac cell membranes although the underlying mechanism underlying this effect is not understood (57). Accordingly, each of these additives support PIP2 synthesis or maintenance and appear to stabilize CaV1.2 channel currents, supporting our hypothesis.
Prior studies have shown PIP2 suppression of CaV currents dependent on the associated CaVβ-subunit isoform with the palmitoylated CaVβ2a conferring the least sensitivity to PIP2 depletion (58). Our model implies the palmitoylation may provide an additional membrane anchor that stabilizes CaV1.2 and reduces its sensitivity to PIP2 depletion. To that end, AngII inhibition of CaV1.2 currents has been shown to be less robust when co-expressed with CaVβ2a versus other CaVβ subunits (31). In the heart, the most abundant isoform is CaVβ2b, a splice variant that lacks the consensus site for palmitoylation (59, 60) and thus cardiac CaV1.2 channels may be vulnerable to PIP2 depletion. Dynamic imaging of CaVβ2a-paGFP transduced myocytes herein revealed a bias toward channel endocytosis with acute AngII-treatment but if palmitoylation of CaVβ2a does in fact confer additional stability that reduces the modulatory effects of PIP2 depletion on PM CaV1.2 expression levels, it is possible that these results represent an underestimation of the true effects in vivo.
In summary, our results show that PIP2 stabilizes CaV1.2 channel expression at the PM and that acute AngII can depress cardiac EC-coupling by stimulating PIP2 depletion and destabilization of PM CaV1.2, leading to their endocytosis from the PM. Given that PIP2 is present in every PM of every cell in the body, our findings have broad ramifications not only for cardiac physiology but for the myriad excitable cells that express CaV1.2.
Materials and Methods
All animal handling and procedures adhered to the NIH Guide for the Care and Use of Laboratory Animals (UC Davis) and were approved by the local Institutional Animal Care and Use Committee. Ventricular myocytes were isolated from 3 to 6-mo-old C57BL/6J mouse hearts via retrograde Langendorff perfusion as previously described (4, 5) and used in microscopy, Ca2+ transient, and/or patch-clamp electrophysiology experiments. In surface biotinylation experiments, isolated myocytes from untreated and AngII-treated hearts were biotinylated, lysed, and probed for protein content using western blot as previously described (61, 62). Phosphoinositide species were quantified using ultra-high-pressure liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) as previously described (63, 64). N and n represent the number of animals and number of cells, respectively. Data are reported as mean ± SEM. Datasets were compared using paired or unpaired Student’s t tests, one-way ANOVAs, or two-way ANOVAs with Tukey’s multiple comparisons post-hoc tests as stated in the figure legends. P < 0.05 was considered statistically significant. Detailed methods can be found in the SI Appendix.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Acknowledgments
Illustrations were generated using Biorender.com. This work was supported by NIH grants R01HL159304 and R01AG063796 to R.E.D. and R01GM127513 to E.J.D.; by T32 GM099608 and later American Heart Association Predoctoral Fellowship 827909 to T.L.V., by R25 GM056765 (Initiative for Maximizing Student Development) and T32 GM113770 to A.M.C. and by R01 NS123050, RF1 AG055357 and a WM Keck foundation award (to J.W.H.).
Author contributions
J.W.H., M.C.H., E.J.D., and R.E.D. designed research; T.L.V., S.G.d.V., M.W., A.D.C., A.M.C., and E.J.D. performed research; T.L.V., S.G.d.V., M.W., A.D.C., A.M.C., and E.J.D. analyzed data; and T.L.V. and R.E.D. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. S.E. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Bers D. M., Cardiac excitation-contraction coupling. Nature 415, 198–205 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Cannell M. B., Berlin J. R., Lederer W. J., Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science 238, 1419–1423 (1987). [DOI] [PubMed] [Google Scholar]
- 3.Liu G., et al. , Mechanism of adrenergic CaV1.2 stimulation revealed by proximity proteomics. Nature 577, 695–700 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Villar S. G., et al. , Adrenergic control of sarcolemmal CaV1.2 abundance by small GTPase Rab proteins. Proc. Natl. Acad. Sci. U.S.A. 118, e2017937118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito D. W., et al. , beta-Adrenergic-mediated dynamic augmentation of sarcolemmal CaV1.2 clustering and co-operativity in ventricular myocytes. J. Physiol. 597, 2139–2162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermosilla T., et al. , Prolonged AT1R activation induces CaV1.2 channel internalization in rat cardiomyocytes. Sci. Rep. 7, 10131 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nystoriak M. A., et al. , Ser 1928 phosphorylation by PKA stimulates the L-type Ca2+ channel CaV1.2 and vasoconstriction during acute hyperglycemia and diabetes. Sci. Signal. 10, eaaf9647 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patriarchi T., et al. , Phosphorylation of CaV1.2 on S1928 uncouples the L-type Ca2+ channel from the beta2 adrenergic receptor. EMBO J. 35, 1330–1345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang H., Viola H. M., Filipovska A., Hool L. C., CaV1.2 calcium channel is glutathionylated during oxidative stress in guinea pig and ischemic human heart. Free Radic. Biol. Med. 51, 1501–1511 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Tetreault M. P., et al. , Identification of glycosylation sites essential for surface expression of the CaVα2δ1 subunit and modulation of the cardiac CaV1.2 channel activity. J. Biol. Chem. 291, 4826–4843 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan W., Bers D. M., Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am. J. Physiol. 267, H982–993 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Hall D. D., et al. , Competition between alpha-actinin and Ca(2) (+)-calmodulin controls surface retention of the L-type Ca2+ channel CaV1.2. Neuron 78, 483–497 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon R. E., et al. , Graded Ca2+/calmodulin-dependent coupling of voltage-gated CaV1.2 channels. Elife 4, e05608 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno C. M., et al. , Ca2+ entry into neurons is facilitated by cooperative gating of clustered CaV1.3 channels. Elife 5, e15744 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imredy J. P., Yue D. T., Mechanism of Ca2+-sensitive inactivation of L-type Ca2+ channels. Neuron 12, 1301–1318 (1994). [DOI] [PubMed] [Google Scholar]
- 16.Peterson B. Z., DeMaria C. D., Adelman J. P., Yue D. T., Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron 22, 549–558 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Suh B. C., Leal K., Hille B., Modulation of high-voltage activated Ca2+ channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron 67, 224–238 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamper N., Reznikov V., Yamada Y., Yang J., Shapiro M. S., Phosphatidylinositol 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J. Neurosci. 24, 10980–10992 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L., Bauer C. S., Zhen X. G., Xie C., Yang J., Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature 419, 947–952 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Suh B. C., Kim D. I., Falkenburger B. H., Hille B., Membrane-localized beta-subunits alter the PIP2 regulation of high-voltage activated Ca2+ channels. Proc. Natl. Acad. Sci. U.S.A. 109, 3161–3166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vivas O., Castro H., Arenas I., Elias-Vinas D., Garcia D. E., PIP2 hydrolysis is responsible for voltage independent inhibition of CaV2.2 channels in sympathetic neurons. Biochem. Biophys. Res. Commun. 432, 275–280 (2013). [DOI] [PubMed] [Google Scholar]
- 22.de la Cruz L., et al. , PIP2 in pancreatic β-cells regulates voltage-gated calcium channels by a voltage-independent pathway. Am. J. Physiol. Cell Physiol. 311, C630–C640 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Kaschina E., Unger T., Angiotensin AT1/AT2 receptors: Regulation, signalling and function. Blood Press. 12, 70–88 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Dorn G. W. II, Force T., Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Invest. 115, 527–537 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burniston J. G., Saini A., Tan L. B., Goldspink D. F., Angiotensin II induces apoptosis in vivo in skeletal, as well as cardiac, muscle of the rat. Exp. Physiol. 90, 755–761 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Schluter K. D., Wenzel S., Angiotensin II: A hormone involved in and contributing to pro-hypertrophic cardiac networks and target of anti-hypertrophic cross-talks. Pharmacol. Ther. 119, 311–325 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Forrester S. J., et al. , Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 98, 1627–1738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichiyanagi O., Ishii K., Endoh M., Angiotensin II increases L-type Ca2+ current in gramicidin D-perforated adult rabbit ventricular myocytes: Comparison with conventional patch-clamp method. Pflugers Arch. 444, 107–116 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Aiello E. A., Cingolani H. E., Angiotensin II stimulates cardiac L-type Ca2+ current by a Ca2+- and protein kinase C-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 280, H1528–H1536 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Allen I. S., et al. , Angiotensin II increases spontaneous contractile frequency and stimulates calcium current in cultured neonatal rat heart myocytes: Insights into the underlying biochemical mechanisms. Circ. Res. 62, 524–534 (1988). [DOI] [PubMed] [Google Scholar]
- 31.Hermosilla T., et al. , L-type calcium channel beta subunit modulates angiotensin II responses in cardiomyocytes. Channels (Austin) 5, 280–286 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Bkaily G., et al. , Angiotensin II-induced increase of T-type Ca2+ current and decrease of L-type Ca2+ current in heart cells. Peptides 26, 1410–1417 (2005). [DOI] [PubMed] [Google Scholar]
- 33.De Mello W. C., Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension 32, 976–982 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Varnai P., Balla T., Visualization of phosphoinositides that bind pleckstrin homology domains: Calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143, 501–510 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falkenburger B. H., Jensen J. B., Hille B., Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J. Gen. Physiol. 135, 99–114 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen J. B., Lyssand J. S., Hague C., Hille B., Fluorescence changes reveal kinetic steps of muscarinic receptor-mediated modulation of phosphoinositides and Kv7.2/7.3 K+ channels. J. Gen. Physiol. 133, 347–359 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickson E. J., Jensen J. B., Hille B., Golgi and plasma membrane pools of PI(4)P contribute to plasma membrane PI(4,5)P2 and maintenance of KCNQ2/3 ion channel current. Proc. Natl. Acad. Sci. U.S.A. 111, E2281–E2290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaborik Z., et al. , Beta-arrestin- and dynamin-dependent endocytosis of the AT1 angiotensin receptor. Mol. Pharmacol. 59, 239–247 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Holloway A. C., et al. , Side-chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol. Pharmacol. 61, 768–777 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Ferron L., Guderyan S. D., Smith E. J., Zamponi G. W., CaVβ-subunit dependence of forward and reverse trafficking of CaV1.2 calcium channels. Mol. Brain 15, 43 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conrad R., et al. , Rapid turnover of the cardiac L-type CaV1.2 channel by endocytic recycling regulates its cell surface availability. iScience 7, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaur G., et al. , A polybasic plasma membrane binding motif in the I-II linker stabilizes voltage-gated CaV1.2 calcium channel function. J. Biol. Chem. 290, 21086–21100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng E. P., et al. , Restoration of normal L-type Ca2+ channel function during Timothy syndrome by ablation of an anchoring protein. Circ. Res. 109, 255–261 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao S., Yao X., Yan N., Structure of human CaV2.2 channel blocked by the painkiller ziconotide. Nature 596, 143–147 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasuhoglu C., et al. , Modulation of cardiac PIP2 by cardioactive hormones and other physiologically relevant interventions. Am. J. Physiol. Cell Physiol. 283, C223–C234 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Suh B. C., Hille B., Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron 35, 507–520 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Hilgemann D. W., Ball R., Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science 273, 956–959 (1996). [DOI] [PubMed] [Google Scholar]
- 48.Swaminathan P. D., Purohit A., Hund T. J., Anderson M. E., Calmodulin-dependent protein kinase II: Linking heart failure and arrhythmias. Circ. Res. 110, 1661–1677 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S., et al. , Angiotensin II induces cardiac phenotypic modulation and remodeling in vivo in rats. Hypertension 25, 1252–1259 (1995). [DOI] [PubMed] [Google Scholar]
- 50.Crowley S. D., et al. , Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci. U.S.A. 103, 17985–17990 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang M., et al. , Contractile function during angiotensin-II activation: Increased Nox2 activity modulates cardiac calcium handling via phospholamban phosphorylation. J. Am. Coll. Cardiol. 66, 261–272 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kameyama A., Yazawa K., Kaibara M., Ozono K., Kameyama M., Run-down of the cardiac Ca2+ channel: Characterization and restoration of channel activity by cytoplasmic factors. Pflugers Arch. 433, 547–556 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Weiss S., Oz S., Benmocha A., Dascal N., Regulation of cardiac L-type Ca2+ channel CaV1.2 via the β-adrenergic-cAMP-protein kinase A pathway: Old dogmas, advances, and new uncertainties. Circ. Res. 113, 617–631 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Yamaoka K., Kameyama M., Regulation of L-type Ca2+ channels in the heart: Overview of recent advances. Mol. Cell. Biochem. 253, 3–13 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Hilgemann D. W., Cytoplasmic ATP-dependent regulation of ion transporters and channels: Mechanisms and messengers. Annu. Rev. Physiol. 59, 193–220 (1997). [DOI] [PubMed] [Google Scholar]
- 56.Seo J. B., Jung S. R., Huang W., Zhang Q., Koh D. S., Charge shielding of PIP2 by cations regulates enzyme activity of phospholipase C. PLoS One 10, e0144432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hilgemann D. W., On the physiological roles of PIP2 at cardiac Na+ Ca2+ exchangers and KATP channels: A long journey from membrane biophysics into cell biology. J. Physiol. 582, 903–909 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suh B. C., Kim D. I., Falkenburger B. H., Hille B., Membrane-localized β-subunits alter the PIP2 regulation of high-voltage activated Ca2+ channels. Proc. Natl. Acad. Sci. U.S.A. 109, 3161–3166 (2012), 10.1073/pnas.1121434109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colecraft H. M., et al. , Novel functional properties of Ca(2+) channel β subunits revealed by their expression in adult rat heart cells. J. Physiol. 541, 435–452 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi S. X., Mittman S., Colecraft H. M., Distinctive modulatory effects of five human auxiliary β2 subunit splice variants on L-type calcium channel gating. Biophys. J. 84, 3007–3021 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartels P., et al. , Half-calcified calmodulin promotes basal activity and inactivation of the L-type calcium channel CaV1.2. J. Biol. Chem. 298, 102701 (2022), 10.1016/j.jbc.2022.102701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turner M., et al. , alpha-Actinin-1 promotes activity of the L-type Ca2+ channel CaV1.2. EMBO J. 39, e106171 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vivas O., Tiscione S. A., Dixon R. E., Ory D. S., Dickson E. J., Niemann-pick type C disease reveals a link between lysosomal cholesterol and PtdIns(4,5)P2 that regulates neuronal excitability. Cell Rep. 27, 2636–2648.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Traynor-Kaplan A., et al. , Fatty-acyl chain profiles of cellular phosphoinositides. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 513–522 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Data Availability Statement
All study data are included in the article and/or SI Appendix.