Abstract
Plant genetic resources (PGR), including collections held in national and international gene banks, provide access to a wide array of genetic diversity and are critical to genomics research, conservation efforts, and applied breeding. Yet, there is a general lack of awareness in the research community about the rules and treaties that govern the use of PGR, about access and benefit sharing obligations contained in international treaties and/or national laws, and about how best to comply with potentially applicable requirements. This article provides a brief history and overview of three key international agreements, namely the Convention on Biological Diversity, the Nagoya Protocol, and the International Treaty on Plant Genetic Resources for Food and Agriculture, which collectively address responsibilities and obligations related to the use of much of the world’s PGR. By highlighting the coverage and key considerations of each agreement, the article provides a guide for those who use PGR in plant genetics research to better understand when and how international agreements apply, and—where the rules are unclear—to suggest best practices for compliance with existing agreements.
Keywords: plant genetic resources (PGR), Plant Treaty, standard material transfer agreement (SMTA), Nagoya Protocol, Convention on Biological Diversity
Plant genetic resources (PGR), andsequence data and information derived from PGR, play a central role in genomics research, conservation efforts, and applied breeding. PGR serve as raw material for exploring biological and evolutionary questions, as a source of variation for traits of interest, for establishing provenance and ancestry, and for addressing unknown future needs (1).
Collections of PGR held in national and international gene banks have contributed to making these diverse resources widely available, but there is a general lack of awareness in the research community about the rules that govern the use of PGR, whether it is in situ or in a gene bank or in a privately held collection and, in particular, about access and benefit-sharing (ABS) obligations contained in international treaties and/or national laws. Further, even when researchers are aware of international agreements and laws, there is often a lack of understanding about how best to comply with potentially applicable requirements.
This article provides an overview of three key international agreements that govern access and use of PGR and related benefit sharing (Table 1) and may serve as a guide for those engaged in plant genetics research to help navigate the agreements and, where the rules are unclear, to suggest best practices for complying with the intent of these agreements.
Table 1.
Key international agreements
| Agreement | Year | Description |
| Convention on Biological Diversity (Convention) | 1993 | An international agreement ratified* by 196 parties including every country except the Holy See and the United States, as of July 2022. It came into force in 1993 and is discussed further in The Convention on Biological Diversity. |
| International Treaty on Plant Genetic Resources for Food and Agriculture (Plant Treaty) | 2004 | An international agreement with 149 Contracting Parties, as of July 2022, that came into force in 2004 and is discussed further in International Treaty on Plant Genetic Resources for Food and Agriculture (Plant Treaty). |
| Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization to the Convention on Biological Diversity (Nagoya Protocol) | 2014 | An agreement that builds on and supports the implementation of the Convention. It has been ratified by 138 parties, as of July 2022. It is discussed in more detail in Nagoya Protocol. |
*We use the term “ratified” generically to include all four modalities (ratification, acceptance, approval, and accession) by which a country can become a Contracting Party to an international agreement. “Signed” has a different purpose: For a limited duration after the text of an international agreement is adopted, the agreement remains open for signature by countries to indicate their intention to ratify, and the agreement comes into force after enough countries have signed. A country that has signed can become a Contracting Party by whichever process (ratification, acceptance, or approval) is appropriate under its constitution. A country that has not signed can become a Contracting Party by accession. Contracting Parties have identical rights and obligations under the agreements regardless of which modality they used.
These international agreements came into being as part of an effort to address long-standing concerns about the privatization of biological resources and the impact of intellectual property rights. Until the mid-20th century PGR were generally treated as global public goods that could be freely collected and exchanged. However, with the development and widespread use of controlled hybridization techniques and the growth of a more robust seed industry, ownership and proprietary protections (i.e., intellectual property rights, or IPR) became available in industrialized countries for developers of agricultural crop varieties (2). Such ownership came about through plant variety protection, and to some extent, patents. These proprietary rights allowed seed companies to flourish but also raised concerns about what this shift would mean for long-standing practices regarding seed sharing in agriculture. Initiatives to protect IPR on crop varieties, first in the United States and the European Union and later in developing countries as promoted through international trade agreements, raised concerns about how to balance the interests of commercial plant breeders with those of society at large.
The largest of these international agreements on IPR of crop varieties is the International Convention for the Protection of New Varieties of Plants (UPOV Convention), first adopted in 1961 (and updated in 1978 and 1991), which sought to provide a harmonized intellectual property framework for plant variety protection at the international level, while aiming to encourage the development of new varieties of plants for the benefit of society. With the advent of molecular biology, biotechnological innovations in plant variety development came to be recognized as inventions worthy of protection with utility patents in certain jurisdictions. This shifted the global landscape for valorization of PGR, expanding opportunities for the development of proprietary products in the life sciences, including biotech crop varieties, but failed to acknowledge the inherent value of the “raw material” (e.g., traditional crop varieties) or to provide any form of compensation to the developers of that material.
The series of international agreements discussed in this article represent an attempt to address this inequity. They underscore that genetic resources—including PGR—are subject to national sovereign control. They establish norms and mechanisms by which PGR users must obtain permission to access and use those resources and share the benefits arising from their use either directly with providers in countries of origin or through multilateral mechanisms. It is noteworthy that these agreements do not seek to vest IPR in the communities of farmer-innovators that developed early crop varieties.
The complexity lies in the fact that not all countries have ratified all three of these international agreements, not all PGR are subject to the agreements, and different rules apply to different users under different circumstances. International agreements are between sovereign nations; the effect of these agreements on individuals and organizations depends primarily on how countries where they operate implement those agreements. As discussed below, a determination of whether and how any of the agreements apply depends on 1) the source of the plant genetic resource (including, for example, in what country the PGR was collected and whether it comes from a national gene bank or other national collection, an international gene bank such as a CGIAR center, a private collection, public lands, etc.), 2) the plant species in question, 3) the user’s purpose for accessing the PGR, and of course 4) the content of the national measures that countries have developed to implement these international agreements (noting that countries often put measures in place that exceed the scope of the international agreements concerned). Specific additional issues may arise and have to be negotiated related to the use of PGR in research and any follow-up applications in breeding (3).
An important issue relates to whether the international agreements also govern ABS of genetic sequence data and information derived from (or arising out of use of) the PGR, often referred to collectively as “digital sequence information” or DSI. As addressed below, the agreements are generally understood to regulate access to physical genetic resources and not data. Under national measures implementing the Convention and Nagoya Protocol, the providers of physical genetic resources can insist upon conditions concerning the use of data derived from the genetic material they provide. Questions about access and use of DSI are a major focus of international debate.
These international agreements and laws are complex and may seem remote to individual researchers. It is nonetheless important to understand the origins and intent of the agreements. Noncompliance can lead to controversy and even litigation over research, research results, and ultimately any commercial output (4). Understanding laws and the intent of international treaties can help researchers frame expectations and outputs that facilitate better and more extensive transnational collaborative efforts that lead to wider, broader, and more extensive social impacts. Key terms used in these international agreements are summarized in SI Appendix, Table S1.
International agreements: Convention on Biological Diversity, International Treaty on Plant Genetic Resources for Food and Agriculture, and Nagoya Protocol
The Convention was the first international legally binding agreement recognizing the sovereignty of countries over their genetic resources and governing access to and use of those genetic resources. It set the stage for contracting parties to put legal measures in place to regulate and create ABS agreements between providers and recipients of genetic resources, subject to the approval of a competent national authority in the providing country. The Convention is very broad and its ABS provisions apply to all genetic resources occurring and originating in the 196 member countries (or acquired by those countries in accordance with the Convention) and to any potential use of those genetic resources, with a few exceptions. Specifically, the Convention does not apply to human genetic diversity, nor does it apply to genetic resources beyond countries’ jurisdictions such as those in the extraterritorial waters and the Antarctic, nor to genetic resources removed from the country of origin before the Convention came into force.
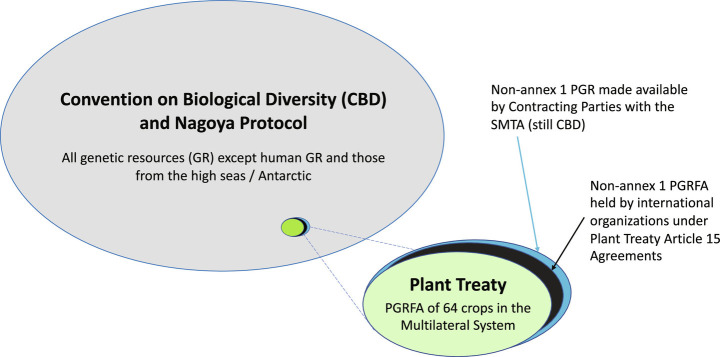
The Plant Treaty was negotiated to update and replace an earlier voluntary agreement (the International Undertaking on Plant Genetic Resources) that had been intended to promote international collection, storage, and use of PGR, because that earlier agreement was incompatible with and rendered outdated by the Convention. The Plant Treaty was designed to be legally binding and in harmony with the Convention. In addition, it was intended to address the fact that, as highlighted in the text of the resolution adopting the Convention, there was “a need to seek solutions to outstanding matters” concerning PGR for food and agriculture (PGRFA, defined in the Plant Treaty as “genetic material of plant origin of actual or potential value for food and agriculture”) and “in particular, access to ex-situ collections not acquired in accordance with this Convention” (5). The Plant Treaty is focused on PGRFA, a subset of both genetic resources and potential purposes for use, enabling it to be tailored specifically to meet the needs of the PGRFA community to undertake the large numbers of material transfers required. As a result, the Plant Treaty is the most important international ABS agreement for plant genetics researchers working in areas of relevance to food and agriculture. Having been designed to be in harmony with the Convention, implementation of the Plant Treaty requires no further legislation or action under the Convention, other than making sure that national legislation implementing the Convention should leave legal space for the implementation of the Plant Treaty. Hence, from a user’s perspective, where ABS applies to PGRFA that is subject to the Plant Treaty, the user does not need to consider legislation implementing the Convention (Fig. 1).
Fig. 1.
Scope of the Convention, Nagoya Protocol, and Plant Treaty.
For transfers of genetic resources that are not covered by the Plant Treaty (because either the genetic resources or the purposes are out of scope), the Nagoya Protocol sets out further details of what countries need to do in order to implement the Convention effectively.
It is worth emphasizing that not all countries have ratified all of these agreements. Further, in the case of the Convention and Nagoya Protocol, implementation requires Contracting Parties to establish requisite measures to implement the agreements. This means that the agreements do not apply in the same way in each country, or when accessing PGR from different countries. This reality makes compliance more confusing—and potentially complex—than it otherwise might be.
Transfer of genetic resources is subject to laws of the provider’s country relating to provision of the material and to laws of the user’s country relating to the use of the material. Thus, for example, the United States has not ratified the Convention or the Nagoya Protocol. However, the European Union has ratified both. When a person in the United States accesses PGR from the European Union that is subject to the Convention and the Nagoya Protocol, the European Union’s implementation of those agreements as providers will apply, as will any additional national measures implemented in the specific European Union country concerned.
Up-to-date lists of the countries that have signed or ratified each of the agreements are available at the following websites: Convention: https://www.cbd.int/information/parties.shtml; Plant Treaty: https://www.fao.org/plant-treaty/countries/membership/en/; Nagoya Protocol: https://www.cbd.int/abs/nagoya-protocol/signatories/.
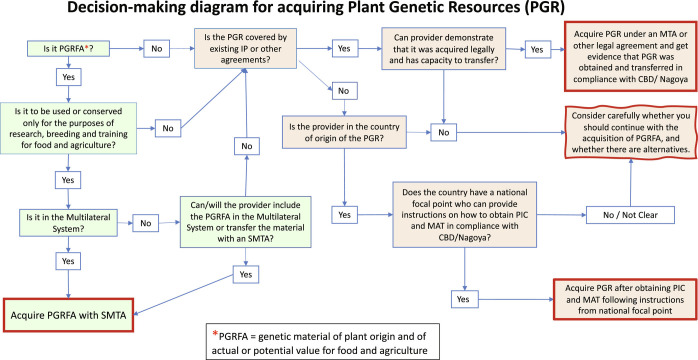
The Plant Treaty, Convention, and Nagoya Protocol are addressed in sequence below. The key decision-making steps needed to guide the acquisition of PGR in compliance with all three of these international agreements are outlined in Fig. 2.
Fig. 2.
Key decision-making steps for acquiring PGR in compliance with international agreements. Green boxes indicate decisions governed by the Plant Treaty; orange boxes indicate decisions governed by the Convention and/or the Nagoya Protocol; boxes outlined in red indicate user outcomes. Figure is modeled on a decision-making diagram found in CGIAR’s 2018 Guidelines on the Nagoya Protocol for CGIAR Research Centers.
International Treaty on Plant Genetic Resources for Food and Agriculture (Plant Treaty).
The Plant Treaty has three objectives, which are a subset of those of the Convention:
-
•
Conservation of plant genetic resources for food and agriculture;
-
•
Sustainable use of plant genetic resources for food and agriculture; and
-
•
The fair and equitable sharing of benefits arising from the use of plant genetic resources for food and agriculture.
To achieve these objectives, the Plant Treaty created a Multilateral System of access and benefit-sharing (MLS). Within the MLS, Contracting Parties to the Plant Treaty agree to virtually pool samples of PGRFA of the 64 crops and forages listed in Annex 1 of the Plant Treaty (see Annex 1 Crops and Forages) and to facilitate access to them for the purposes of research, breeding, and training for food and agriculture (see Research, Breeding, and Training for Food and Agriculture). The MLS includes only certain defined samples of the crops and forages listed in Annex 1 (see Coverage), with significant ambiguity relating to the inclusion of in situ PGRFA (see In Situ PGRFA). Access must be facilitated pursuant to the Standard Material Transfer Agreement (SMTA; see The SMTA). Through mandating use of the SMTA for all transfers, the Plant Treaty eliminates the need, indeed even the possibility, of negotiating new terms and conditions for each transaction, although it provides for some flexibility for access to “PGRFA under Development” as defined in PGRFA under Development.
The Plant Treaty is exceptional among international agreements in that it establishes detailed rules and contract templates (i.e., the SMTA) that can be used by individuals and organizations without implementing legislation. This does not prevent countries from implementing related legislation, for example to regulate inclusion of PGRFA in the MLS. Moreover the Plant Treaty requires countries to take appropriate measures to encourage holders of PGRFA under their jurisdiction to include their materials through the MLS, which may require legislation or at least a public policy instrument confirming their right to do so. Nevertheless, countries cannot alter the ABS conditions or contract templates or require negotiation of terms with users (6).
Annex 1 Crops and Forages.
The list of Annex 1 crops and forages defines the limited set of PGRFA that may be subject to the Plant Treaty’s MLS (SI Appendix, Table S2). These 64 crops and forages were negotiated for inclusion in the Plant Treaty because of their recognized importance to food security and countries’ interdependence. Each crop or forage may span more than one species or in some cases more than one genus and often includes wild relatives. For example, the crop “cassava” includes only the single species Manihot esculenta, whereas the crop “rice” includes all species of the genus Oryza including wild as well as cultivated species, while “brassica complex” encompasses most species of 13 different genera. Annex 1 is considered an integral part of the Treaty; as such, it has not changed since 2002. During negotiations to improve the functioning of the MLS from 2013 to 2019, contracting parties considered broadening the list to include all crops and forages, along with other measures to increase monetary benefit sharing. However, those negotiations were suspended in 2019 without any agreement being reached.
Research, Breeding, and Training for Food and Agriculture.
The applicability of the Plant Treaty’s MLS is limited to PGRFA used for the specific purposes of “research, breeding and training for food and agriculture.” Uses of PGRFA in the MLS for other purposes (including, for example, pharmaceutical development, biofuels, or other industrial ends) are not within the scope of the Plant Treaty’s MLS. Access to and use of Annex 1 PGRFA for such other purposes may therefore be subject to national measures implementing the Convention and Nagoya Protocol (Fig. 2).
Coverage.
PGRFA that are Annex 1 crops and forages under the management and control of the Contracting Parties and in the public domain are automatically included within the MLS. They are generally considered to be those held in national gene banks, national public research organizations, and national public universities. Most Contracting Parties will make clear that a certain resource, such as the Plant Gene Resources of Canada, is part of the MLS and that the SMTA applies to transfers of PGRFA from that collection.*
PGRFA held by individuals, including researchers’ own collections, farmers, and private companies, are not automatically included in the Plant Treaty’s MLS. However, the Plant Treaty does allow (and indeed its Contracting Parties invite) all holders of Annex 1 PGRFA to include those PGRFA in the MLS. Moreover, Contracting Parties must take appropriate measures to encourage “natural and legal persons” (i.e., individuals, companies, and other organizations) within their jurisdiction to include their Annex 1 PGRFA in the MLS. To do so, a person or company can make their plant genetic resources available under the nonnegotiable SMTA. The natural or legal person can also officially communicate, to the appropriate person in their country and also to the Plant Treaty, their decision to include plant genetic resources in the MLS. Finally, a natural or legal person can offer to put their plant genetic resources in an ex situ collection (or to be listed by an ex situ collection) managed by a national public entity so that it comes “under the management and control” of the national government and “in the public domain” and thereby is automatically included in the MLS. (It should be noted that such encouragement to include PGRFA voluntarily comes balanced with the threat that the Governing Body may choose to stop facilitating access to natural and legal persons who do not voluntarily include their PGRFA—other than PGRFA under Development—in the MLS.)
Third, under Article 15 of the Plant Treaty, the MLS also includes Annex 1 PGRFA held in the collections of CGIAR Centers and other international institutions that have signed agreements with the Governing Body to this effect. All 11 CGIAR Centers hosting international collections have signed such agreements.† These agreements stipulate that the Centers will make non-Annex 1 materials in their collections held under their 1994 “in trust” agreements with the FAO available under terms to be approved by the Governing Body. In 2009, the Governing Body agreed that Centers should make such non-Annex 1 materials available under the same SMTA used for transfers for Annex 1 materials in the MLS.
PGRFA in collections under the MLS may have been legally acquired from countries that have not ratified the Plant Treaty. The samples held in such collections will be available under the MLS even though the country of origin is not obliged to facilitate access to samples of the same PGRFA occurring within its boundaries.
In Situ PGRFA.
In situ PGRFA are in a more complex position. Most expert commentators agree that coverage of the Plant Treaty’s MLS (Article 11) makes no distinction between in situ and ex situ materials, and as such includes in situ materials in the same way as ex situ PGRFA. Namely, they are automatically included where they are under the management and control of a Contracting Party and in the public domain (for example in a national park system) or voluntarily at the discretion of the holder for other PGRFA. However, the Plant Treaty’s provisions governing facilitated access to such materials (Article 12) state that “access … will be provided according to national legislation or, in the absence of such legislation, in accordance with such standards as may be set by the Governing Body.” That is, even when in situ PGRFA are included in the MLS, access may require additional authorization, for example rules governing management of protected areas. However, once those additional procedures have been complied with, the in situ materials in the MLS should be made available under the SMTA. Despite this, some contracting parties have taken the position that in situ Annex 1 PGRFA are not included within the scope of the MLS. The net result is that, if the aim is to access in situ material, it may be best to contact a national focal point in the country where the PGRFA are located to inquire about national legislation on collecting PGRFA. Relevant legislation may have been established under the Convention and the Nagoya Protocol.
The SMTA.
The SMTA was adopted by the Governing Body of the Plant Treaty in 2006. It is the standard agreement used for transfer of PGRFA in the MLS, a contract under international law between the provider and recipient of PGRFA, legally binding regardless of the countries of the provider and recipient, and provides rules for access and use of such materials, benefit sharing by the recipient, dispute resolution, and related issues. The SMTA is nonnegotiable and thus eliminates the need to negotiate new terms each time a recipient wants to access and/or use PGRFA. Thus, recipients must agree to and abide by the terms of the SMTA to access and use PGRFA in the MLS. In relation to this, it is important to determine who legally takes on these rights and obligations. Is it the individual researcher or is it the legal entity (laboratory, organization, company, university, etc.) for whom the researcher works? If the latter, does the researcher have the right to bind the researcher’s employer to the SMTA? If not, are there procedures in place to ensure that the legal entity accepts the terms of the SMTA as its corporate responsibilities? Are procedures in place to ensure that those rights and obligations continue to be met in the future, for example even when staff change or retire?
The SMTA requires the recipient to use the SMTA for onward transfers of the material to third parties. Hence the SMTA ultimately binds all users, not only the first recipient.
The Plant Treaty places the burden on the party accessing the PGRFA to ensure that the purpose is use or conservation for research, breeding, and training for food and agriculture. When recipients sign an SMTA to access PGRFA under the MLS, they agree to use the PGRFA for research, breeding, and training for food and agriculture. The research can have an ultimate commercial purpose, as long as that purpose is related to food and agriculture. Accessing and using PGRFA for a different, e.g. pharmaceutical or industrial, purpose under the SMTA is not permitted and signing the SMTA for such a purpose would be considered fraud. Note that breeding for nutritional content of crops intended to be consumed as food (not for medicinal use) falls squarely within the domain of the SMTA. Accessing PGRFA (other than samples of PGRFA already subject to the SMTA) for other purposes is certainly possible, but instead of the Plant Treaty and SMTA such access may be subject to national measures implementing the Convention or Nagoya Protocol or possibly intellectual property agreements. These other uses are not permitted if the PGRFA is being provided by someone who had previously acquired that material with an SMTA.
The Plant Treaty’s SMTA would not normally be used for the transfer of PGRFA that are
-
•
not included in the multilateral system (exceptions: non-Annex 1 PGRFA from the in trust collections of the CGIAR gene banks and from countries that have chosen the SMTA as their preferred instrument under the Nagoya Protocol);
-
•
to be used for industrial, pharmaceutical, or other nonfood or nonagricultural purposes (hence outside the scope of the MLS; there are no exceptions for this case);
-
•
PGRFA Products (as defined in the SMTA) being transferred for the purpose of commercialization by the recipient on behalf of the developer (there are no exceptions in this case);
-
•
under the management and control of national governments that are not Contracting Parties to the Plant Treaty (hence not automatically included in the MLS. Exceptions: subject to relevant legislation and/or authorizations, such entities sometimes do voluntarily choose to provide PGRFA with SMTA);
-
•
proprietary material in private collections (exceptions: as discussed above, material that the owners have voluntarily included in the MLS).
PGRFA from the MLS are available for free or at “minimal cost.” When individuals or institutions sign the SMTA they agree to a list of key terms (included in Section 6 of the SMTA). The key terms are identified and explained further in SI Appendix, Table S4. Note that SI Appendix, Table S4 does not include all the terms in the SMTA but rather those that may be of most interest to researchers.
Under the SMTA (Article 6.9) recipients are required to share nonconfidential research information through the Plant Treaty’s Global Information System (GLIS), which under the Plant Treaty Article 13.2(a) is recognized as a form of benefit sharing. The only other form of benefit sharing that is mandatory for recipients (under certain conditions) is monetary benefit sharing (SMTA Articles 6.7 and 6.11). Under the Plant Treaty and the SMTA nonmonetary benefit sharing, in the form of sharing PGRFA, sharing information, access to and transfer of technology, and capacity-building [Articles 13.1 and 13.2(a)-(c)], is mandatory at the level of countries but is only encouraged at the level of recipients. This stands in contrast to the Nagoya Protocol, which, as discussed in more detail below, makes clear that both monetary and nonmonetary benefits (summarized in SI Appendix, Table S3) can satisfy the benefit-sharing obligation of users.
PGRFA under Development.
In the SMTA, PGRFA under Development refers to material derived from PGRFA accessed from the MLS that is not yet ready for commercialization and which the developer intends to further develop or to transfer to another person or entity for further development—for example, a new crop variety being developed using PGRFA accessed from the MLS as a parent. “Under development” therefore generally refers to breeding material derived from material in the MLS that is in the process of being improved and that has not yet been released as a variety.
This category is important because providers and recipients of PGRFA in the MLS make PGRFA under Development available, if they choose to do so, under the SMTA with modifications that acknowledge the developer’s intellectual and financial investment in developing the material. Access to PGRFA under Development is at the discretion of its developer during the period of its development. One of the clauses of the SMTA does not apply (the clause by which, for all other types of PGRFA, the provider undertakes that “access shall be accorded expeditiously, without the need to track individual accessions and free of charge, or, when a fee is charged, it shall not exceed the minimal cost involved”). Thus, the developer can deny access if they are asked while it is still under development, and the provider can demand feedback on what the recipient does with the material and can charge fees for access. In addition, the provider and recipient have the right to attach to the SMTA additional conditions relating to further product development, as long as these do not conflict with other provisions of the SMTA.
The Convention on Biological Diversity.
The Convention has three main objectives, which are equivalent to those of the Plant Treaty but cover a much broader range of biological and genetic resources and of purposes:
-
•
Conservation of biological diversity;
-
•
Sustainable use of components of biological diversity; and
-
•
The fair and equitable sharing of the benefits arising out of the use of genetic resources.
Unlike the Plant Treaty, the Convention does not define specific rights and obligations of providers and recipients in their use of genetic resources. It defines general principles that should be followed to establish those rights—these must be implemented by member countries through establishing authorities, procedures, criteria, and legislation.
Under the Convention, anyone wishing to obtain genetic resources from their country of origin must obtain prior informed consent (PIC) from the relevant authorities (unless the country has determined otherwise) and establish mutually agreed terms (MAT) governing use of those genetic resources (Fig. 2). The Convention anticipates that contracting parties will develop national laws, as they see fit, to set the terms for access to and use of genetic materials. In certain cases, national laws have been passed that apply ABS conditions to genetic sequence information as well as to physical genetic resources.
According to the Convention, the country of origin for wild genetic resources is the country where the genetic resource exists within ecosystems and natural habitats. For domesticated or cultivated genetic resources, the country of origin is the one where the genetic resource developed its distinctive properties. Although clear for endemic species, in some cases the country of origin may be difficult and expensive to determine; indeed, it is impossible to determine a single country of origin for many crop varieties, which typically have multiple distinctive properties originating in different countries. Note that this definition of “country of origin” differs from the scientific definition of the “center of origin of a species” because the entities are different. The Convention focuses on the country of origin of specific variants of a species, and given the continuous nature of evolution and the huge biogeographical diversity within a species, the Convention’s definition makes the practical assumption that the origin of individual variants is wherever they occur naturally. As explained in the legal interpretation of the Convention, “many species exist in ecosystems as apparently natural, self-maintaining populations outside their original ranges (that is, ranges prior to the recent era of human translocation), and the country where these species are now living in in situ conditions would be considered as the country of origin” (7).
PIC requires 1) consent of the national authorities in the country of the genetic resource provider (an affirmative act), 2) based on information provided by the potential genetic resource user, 3) with information being provided by the potential user prior to consent for access being granted.
MAT are generally understood to imply a negotiation between the country granting access to genetic resources and the party desiring access to and use of the genetic resources. The MAT should include provisions for “sharing in a fair and equitable way the results of research and development and the benefits arising from the commercial and other utilization of genetic resources with the Contracting Party providing such resources.” Theoretically, MAT could be negotiated every time a party wants to access genetic resources. In some countries legislation includes a requirement for benefit sharing based on access to and/or use of digital sequence information.
Those seeking genetic materials from a country that is a party to the Convention would first identify the potential provider of genetic resources to ascertain whether they are willing to provide the materials and on what terms. The provider should then contact their country’s competent national authority, as identified at https://s3.amazonaws.com/absch.documents.abs/contacts.pdf/absch-all-authority-en.pdf, to initiate the national process of seeking PIC and establishing MAT. The competent national authority should be able to inform access seekers and providers what specific national ABS laws apply.
In cases where PGR are being accessed from a country that has ratified the Convention but does not have a national focal point that can provide instructions on how to obtain PIC and MAT, or from a provider who cannot, even with expert help and guidance, demonstrate that the PGR was acquired legally and with rights to transfer it to a third party, a user should consider carefully whether to continue with acquisition of the PGR. Continuing with the acquisition risks the possibility of unwittingly acquiring PGR illegally, or at least of being unable to demonstrate that it was acquired legally, which could jeopardize years of research and development. It may be possible to locate alternative sources of the material that could be acquired in compliance with international agreements and national legislation. If none of this is possible, the safest approach would simply be not to acquire the material.
If the PGR are proprietary materials held in a private collection, the situation is more complex because of the applicability of relevant legislation on intellectual and other property rights as well as the Convention. How countries handle the resulting potential conflict varies between countries. Often national ABS laws are silent on whether or not they extend to privately owned materials. Some countries bring both aspects under one umbrella. For countries that have declared that they do not need PIC there is of course no conflict, but legislation on proprietary materials would still apply: In this case the relevant contact would be a person with authority to determine under what kind(s) of IPR or other agreement(s) the PGR are shared (Fig. 2), which could be within the organization of the provider. Admittedly, this creates a very complex set of steps when accessing genetic resources.
Nagoya Protocol.
The Nagoya Protocol has only one objective: the fair and equitable sharing of the benefits arising from the utilization of genetic resources, thereby advancing the Convention’s third objective. It does so in part by providing greater legal certainty and transparency for both providers and users of genetic resources. It sets out the systems that countries must implement for both sides of a material transfer: not only procedures for providing countries to grant PIC and establish MAT but also procedures for users’ countries to monitor use and compliance with the MAT. The aim is that through the adoption of effective access and monitoring and enforcement measures, provider Parties can capture benefits that result from utilization of genetic resources over which they have sovereign rights.
Parties to the Nagoya Protocol have or are in the process of developing laws that interpret and implement its provisions. The European Union and some of the mega-diverse countries from Asia, Africa, and Latin America have implemented such laws. Canada is not yet a Party to the Nagoya Protocol, and the United States is not eligible to become a Party as it is not a contracting party to the Convention. The status of individual countries and national laws of Parties can be found at https://absch.cbd.int/en/countries/status/party.
The Nagoya Protocol goes beyond the Convention in several other ways. Under the Convention, access must be subject to PIC granted by the national authorities of the country (unless otherwise determined by the country). Under the Nagoya Protocol, countries must also take measures (if appropriate and subject to domestic law) to ensure the PIC or approval and involvement of Indigenous and local communities where they have the established the right to grant access to such resources.
Under the Convention, the MAT must include provisions for benefit sharing with the country providing the genetic resources. Under the Nagoya Protocol countries must also take measures ensure that, where appropriate, benefits are shared with Indigenous and local communities that hold the genetic resources or traditional knowledge provided.
The Nagoya Protocol also requires countries to take measures, where appropriate, to ensure PIC and MAT for access to traditional knowledge associated with genetic resources that is held by indigenous and local communities.
In addition, the Nagoya Protocol provides a definition for “utilization” (a definition omitted from the Convention), which is important, since utilization is the trigger for benefit sharing under both the Convention and the Protocol. The Nagoya Protocol states that utilization of genetic resources means “research and development on the genetic and/or biochemical composition of genetic resources, including through the application of biotechnology.” Biotechnology, in turn, is defined as “any technological application that uses biological systems, living organisms, or derivatives thereof, to make or modify products or processes for specific use.” The Nagoya Protocol defines “derivative,” in this context, as “a naturally occurring biochemical compound resulting from the genetic expression or metabolism of biological or genetic resources, even if it does not contain functional units of heredity.”
The plain language of this definition of “utilization” does not appear to include generation and further use of digital sequence information. However, the Nagoya Protocol does not expressly preclude doing so and, importantly, the parties to the Nagoya Protocol may develop their own interpretation of this term in national law. It is therefore very important to read and understand any agreement negotiated with the provider of access to genetic resources.
The Nagoya Protocol provides an Annex with a nonexhaustive list of possible monetary and nonmonetary benefits that can satisfy the Convention’s requirement for benefit sharing. These are included in SI Appendix, Table S3 and at https://www.cbd.int/abs/text/articles/?sec=abs-37. Note that while all of the items included on the Annex may be considered benefits, it is up to the country negotiating MAT to determine what it considers to be acceptable benefits in any given case. Notably, this list of nonmonetary benefits is very broad and encompasses many activities regularly undertaken by those in the plant genomics and breeding communities.
While most of the current discussions of benefit sharing have focused on monetary benefits, there is an immediate need for further exploration and documentation of nonmonetary benefits, including social, economic, and ecological impacts, that accrue to countries providing PGRFA. Indeed, the monetary value of sharing improved crops and associated technologies, information, and enhanced human capacities is estimated to far exceed the levels of income and impact that could be generated under the monetary benefit-sharing conditions outlined in the MLS (8).
DSI
The Plant Treaty, Convention, and Nagoya Protocol explicitly tie ABS obligations to the use of physical genetic resources. These international agreements do not explicitly mention or discuss DSI or genetic sequence information. This fact is not entirely surprising as both the Convention (1993) and the Plant Treaty (2004) were negotiated before genomics and analysis of sequence information became central to biological research, innovation, and breeding.
The absence of explicit mention of DSI in these international agreements has different implications for different forms of commercial exploitation. For example, under the Plant Treaty benefit sharing is tied to the commercialization of Products. The SMTA defines “Product” as “PGRFA that incorporate”—as evidenced, for example, by pedigree or notation of gene insertion—“the Material or any of its genetic parts or components that are ready for commercialization, excluding commodities and other products used for food, feed and processing.” On the face of it, this would appear to include only material PGRFA products, not information products, and to this extent DSI appears to be clearly outside the scope of the Plant Treaty.
It could be argued, however, that a different assessment could apply to the use of DSI as a tool to develop material PGRFA products, recalling that the SMTA regulates PGRFA products that incorporate material received from the MLS but not the method of incorporation. In particular, the Plant Treaty and the SMTA are silent on the question of how DNA from the original material is copied and how the copies are ultimately incorporated into the genome of the product. For example, nothing in the Plant Treaty or its SMTA specifies that DNA has to be copied solely through the biological processes of mitosis and meiosis. Moreover, nothing specifies that the information content of DNA sequences must always be carried by DNA throughout the development process. In this case, the silence of the Plant Treaty on the matter of DSI may be construed not to exclude other mechanisms of replicating the information content of DNA. Hence, sequencing a genome derived from PGRFA accessed under an SMTA, storing it in a database, using it to synthesize artificial DNA, and editing that into a genome could arguably fall within scope of the Plant Treaty/SMTA. We recognize that this is not the model of application that was in the minds of negotiators of the SMTA, but in light of recent technological and policy-related developments this interpretation could gain ground/adherents as being, retrospectively, logical and evidence-based.
The current lack of agreement or clarity on benefit-sharing obligations for access to and use of DSI is frustrating to providers of PGR and those who believe it opens the door to exploitation of countries’ genetic resources without the benefit sharing that has been negotiated (9). As such, free access to DSI is seen as a way to circumvent the international agreements, to the detriment mainly of biodiversity-rich countries. Critics see the current “dematerialization” of genetic resources as a potential new form of exploitation.
The value of DSI for expediting the breeding and development of improved varieties, as well as its applications in identification, characterization, and conservation of PGR, is well known in the scientific community. It is important for the international scientific community to monitor international discussion of DSI and clarify obligations, as there are proposals under discussion in both the Convention and the Plant Treaty to tie use of digital sequence information to benefit sharing obligations.
Conclusion: Best Practices and a Pathway Forward
The landscape for compliance with the international ABS treaties is clearly complex and, particularly with respect to genetic sequence information, subject to change. Despite this, there are practices that those in the plant genomics and breeding communities can take to adhere to the tenets of these international agreements.
-
1)
It is critical for users of PGR to ensure that they have legitimate, well-documented access to the genetic resources, are aware of its provenance, and understand clearly whether the PGR is subject to the Plant Treaty’s SMTA or to other applicable requirements pursuant to national or subnational laws implementing the Convention, the Nagoya Protocol, and/or existing intellectual property agreements (Fig. 2). Similarly, when accessing or using genetic sequence data, it is important to identify the benefits that flow to others from such use, including nonmonetary benefits as outlined in the Plant Treaty or the Nagoya Protocol Annex. Recent research has illustrated the complex flow of information derived from use of genetic sequence data across high-, middle-, and low-income countries (10). Better documentation of how benefits are shared across the plant research and breeding communities will help the public and policymakers understand current practices, as well as the broad value of such research, not only to users but also to those enabling access in the countries providing the PGR (11).
-
2)
Several prominent scientific journals have expressed support for efforts to document the use of PGR and track the impacts of that use by encouraging researchers to publish Digital Object Identifiers (DOI) issued by the Plant Treaty for any PGRFA used in research publications. Not only will this facilitate the accurate identification and provenance of genetic materials but it will contribute to the interoperability of digital data and information derived from the use of those materials (12, 13). In one specific example, the journals Molecular Ecology and Molecular Ecology Resources revised their Data Accessibility Statement in late 2020 to incorporate the requirements and goals of the Nagoya Protocol (14). The statement reads: “Molecular Ecology and Molecular Ecology Resources require, as a condition for publication, that the research described in the publication complies with relevant national laws implementing the Convention on Biological Diversity and Nagoya Protocol agreements. Authors will be required to make an affirmative statement during the submission process as to compliance with national laws, if applicable. Molecular Ecology and Molecular Ecology Resources also encourage authors to disclose benefits generated commensurate with the Nagoya Protocol.”
-
3)
These initial efforts are intended to help make benefit-sharing considerations a regular part of research output. In addition, routine reporting about nonmonetary benefit sharing in scientific journals has the potential to offer a standardized way of promoting compliance with the principles of the Nagoya Protocol, even when researchers or the resources they use are from countries that are not parties to the Convention or Protocol. Organizations such as the DivSeek International Network are also exploring mechanisms for establishing norms of benefit sharing in the plant genetics research community (15). Other scientific groups, including the CGIAR (16) and the DSI Scientific Network (17), have come together to provide direct input into the Convention’s deliberations about multilateral benefit sharing, while at the same time ensuring continued open access to DSI in support of both science and biodiversity conservation.
-
4)
There are other important efforts underway to establish recognition about the inherent interests that Indigenous peoples and local communities have over knowledge and data that come from their lands, territories, and waters. As part of the localcontexts.org initiative, researchers are being encouraged to make use of traditional knowledge (TK) and biocultural (BC) labels. TK labels are designed to be customized by Indigenous peoples to reflect ongoing relationships and authority including proper use, guidelines for action, or responsible stewardship, while BC labels are digital markers that focus on accurate provenance, transparency, and integrity in research engagements around Indigenous data (18).
Collectively, these efforts are shifting how plant scientists think about and document the benefits that flow from the use of PGR in research and are important in establishing best practices going forward. While there remains ambiguity about the scope and application of many provisions of the international agreements discussed here, users should adhere to best practices, as understood at the time they access PGR.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We benefitted from discussions among participants in the June 2020 DivSeek Workshop “Community Based Benefit Sharing Norms for Agricultural Genomics Researchers,” as well as from constructive comments provided by two anonymous reviewers and by Loren Rieseberg on an earlier draft of this paper.
Author contributions
E.M., R.S.H., M.H., and S.M. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
*See, e.g., https://www.canada.ca/en/environment-climate-change/corporate/international-affairs/partnerships-organizations/plant-genetic-resources-food-agriculture.html. (“The following Canadian federal gene bank collections holding public domain PGRFA under the management and control of Canada are included within the MLS: Plant Gene Resources of Canada, Saskatoon, Saskatchewan; Canadian Clonal Genebank, Harrow, Ontario; and Canadian Potato Genetic Resources, Fredericton, New Brunswick. These PGRFA are available under the terms of the Standard Material Transfer Agreement.”)
†These gene banks include Centre for Pacific Crops and Trees of the South Pacific Community; International Cocoa Genebank; Mutant Germplasm Repository of the Food and Agriculture Organization (FAO)/International Atomic Energy Agency Joint Division; International Coconut Genebanks for the South Pacific and for Africa and India Ocean; Tropical Agricultural Research and Higher Education Centre; World Agroforestry Centre (ICRAF); International Rice Research Institute (IRRI); International Potato Center (CIP); International Livestock Research Institute (ILRI); International Institute of Tropical Agriculture (IITA); the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT); the International Centre for Agricultural Research in the Dry Areas; the International Maize and Wheat Improvement Center (CIMMYT); International Center for Tropical Agriculture (CIAT); Bioversity International; and Africa Rice Centre.
Data, Materials, and Software Availability
There are no data underlying this work.
Supporting Information
References
- 1.McCouch S., et al. , Agriculture: Feeding the future. Nature 499, 23–24 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Kloppenburg J. R., First the Seed: The Political Economy of Plant Biotechnology (Cambridge University Press, New York, 1988), pp. 1492–2000. [Google Scholar]
- 3.Noriega I. L., Halewood M., Risk-Mitigation for Projects That Rely on Genetic Resources from Multiple Sources: A Project Planner’s Decision-Making Tool (Alliance of Biodiversity International and CIAT/EUCLEG, 2021), p. 31. [Google Scholar]
- 4.Marden E., International agreements may impact genomic technologies. Nat. Plants 4, 2–4 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Moore G., Tymowski W., Explanatory Guide to the International Treaty on Plant Genetic Resources for Food and Agriculture (IUCN, 2005). [Google Scholar]
- 6.Joint Capacity Building Programme, “Decision-making tool for national implementation of the Plant Treaty’s multilateral system of access and benefit-sharing” (Bioversity International, Rome, 2018).
- 7.Glowka L., Burhenne-Guilmin F., Synge H., McNeely J. A., Gundling L., “A guide to the Convention on Biological Diversity, Environmental Policy and Law Paper No. 30” (IUCN, 1994).
- 8.Noriega I. L., et al. , CGIAR Operations under the Plant Treaty Framework. Crop Sci. 59, 819–832. (2019). [Google Scholar]
- 9.Nehring R., Digitising biopiracy? The global governance of plant genetic resources in the age of digital sequencing information. Third World Q. 43, 1970–1987 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholz A. H., et al. , Myth-busting the provider-user relationship for digital sequence information. Gigascience 10, 85 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marden E., et al. , Sharing and reporting benefits from biodiversity research. Mol. Ecol. 30, 1103–1107 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Alercia A., López F. M., Sackville Hamilton N. R., Marsella M., “Digital object identifiers for food crops—Descriptors and guidelines of the global information system” (Food and Agriculture Organization of the United Nations, Rome, 2018).
- 13.Weise S., Lohwasser U., Oppermann M., Document or lose it-on the importance of information management for genetic resources conservation in genebanks. Plants 9, 1050 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiley, Molecular Ecology author guidelines. https://onlinelibrary.wiley.com/page/journal/1365294x/homepage/forauthors.html#Ed_policy. Accessed 19 September 2022.
- 15.Divseek International Network, International policy and training. https://divseekintl.org/working-groups/policies/. Accessed 19 September 2022.
- 16.CGIAR, Potential implications of the use of digital sequence information on genetic resources for the three objectives of the Convention on Biological Diversity. A submission from CGIAR to the Secretary of the Convention on Biological Diversity. https://www.cbd.int/abs/DSI-views/CGIAR-DSI-en.pdf. Accessed 19 September 2022.
- 17.Scholz A. H., et al. , Multilateral benefit-sharing from digital sequence information will support both science and biodiversity conservation. Nat. Commun. 13, 1086 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liggins L., Hudson M., Anderson J., Creating space for Indigenous perspectives on access and benefit-sharing: Encouraging researcher use of the Local Contexts Notices. Mol. Ecol. 30, 2477–2482 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
There are no data underlying this work.