Abstract
Breast cancer is one of the most common cancers and causes several complications in females. Currently, MRI is a necessary method for preoperative studies in patients with breast cancer. A high frequency of breast MRI can lead to an increase in the number of incidental extramammary findings. Moreover, it can provide accurate preoperative workup; therefore, the prognosis of patients can be improved. Herein, we provide several extramammary findings, including the mediastinum, lung, upper abdomen, bone, and soft tissue, correlating with US, chest CT, liver MRI, PET-CT, and bone scan.
Keywords: Breast, Magnetic Resonance Imaging, Breast Cancer
Abstract
유방암은 여성에서 가장 흔한 암이며, 많은 합병증을 발생시킨다고 알려져 있다. 오늘날 자기공명영상촬영(이하 MRI)은 유방암 수술 전 검사에서 필수적인 방법이다. 유방 MRI의 사용 빈도가 높아지면서 우연히 발견되는 유방 외 소견이 증가하고 있다. 이에 본 임상 화보에서는 유방 MRI 촬영에서 발견된 종격동, 폐, 상복부, 뼈 및 연조직 등의 다양한 유방 외 소견을 초음파(ultrasonography), 흉부 컴퓨터단층촬영(이하 CT), 간 MRI, 양전자방출단층촬영/컴퓨터단층촬영 스캔(PET/CT), 뼈 스캔(bone scan) 등과 비교하여 알아보고자 한다.
INTRODUCTION
Breast cancer is one of the most common cancer and cause many complications in female (1,2). MRI is a powerful tool in breast imaging (3,4,5), and rapid increased of performance (6). It has been widely used for assessment of lesion extent in patients with known breast cancer, monitoring of the response to neoadjuvant chemotherapy, characterization of equivocal findings at conventional mammography and ultrasonography (USG), screening in high risk female, and examination of patients after breast surgery such as augmentation or reconstruction surgery (3).
Usually MR device with a field of strength of 1.5T or greater was performed (3,7). The patients lies prone with both breasts hanging into the surface coil. Imaging between days 7–14 of the menstrual cycle is recommended to reduce background parenchymal enhancement.
Because breast MRI protocol is not standardized, breast MRI is a variety of protocol (7). When the field of view (FOV) has extent of other anatomical structures such as mediastinum, lung, skeletal, spleen, and liver, breast MRI can incidentally detect extramammary findings (8). As the use of breast MRI increases, the number of incidental extramammary findings may increase. These results are important because they can provide an accurate preoperative work-up, so the prognosis of patients will more improved. The purpose of this article is to introduce various incidental benign and malignant extramammary findings, such as mediastinum, lung, epigastric, bone, and soft tissue, found on breast MRI in breast cancer patients and to emphasize the need to pay attention to these findings.
MRI PROTOCOL AND FOV OF BREAST MRI
Breast MRI were performed using a 3T MRI unit (Achieva, Philips Medical Systems, Best, the Netherlands). The following sequences were evaluated: T2-weighted spectral selection attenuated inversion recovery (SPAIR) sagittal sequence (repetition time [TR] 3805 ms, echo time [TE] 70 ms, FOV 200 mm × 200 mm × 90 mm, matrix 400 × 392 × 30 [slice], voxel 0.5 × 0.5 × 3 (slice thickness), recon voxel 0.417 × 0.417), diffusion-weighted images (DWI) b-value 1000 (FOV 320 mm × 320 mm × 192 mm, voxel 2.5 × 2.5 × 5 [slice thickness], matrix 400 × 392 × 35 [slice], recon voxel 1.25 × 1.25 [RL × AP]), T1-weighted turbo spin echo (TSE) axial sequence (FOV 320 mm × 320 mm × 192 mm, voxel 0.94 × 1.05 × 5 [slice thickness], matrix 400 × 392 × 35 (slices), recon voxel 0.5 × 0.5), before and five times after intravenous administration of gadovist (7.5 mL) or uniray prefilled (15 mL). eTHRIVE 6 dynamic sequence (TE 2.5, TR 5.0, FOV 320 mm × 320 mm ×180 mm, voxel 0.73 × 0.8 × 1.5, matrix 440 × 400 × 120 [slice], recon voxel 0.62 × 0.62 × 1.5). Contrast material was injected with a 6s delay into the dorsal metacarpal vein with 18G or 20G needle at a flow rate of 1.5 mL/s followed by a flush of 25 mL saline solution.
In this study, FOV about breast MRI included part of the mediastinum, lung, upper abdomen, bone, and soft tissue.
EXTRAMAMMARY FINDINGS IN MEDIASTINUM
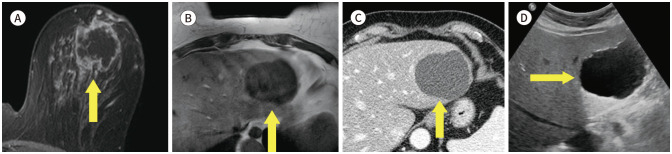
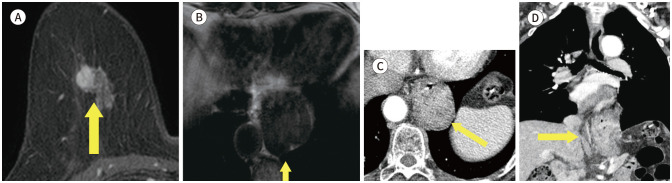
Usually most of mediastinal lesions are asymptomatic and can find incidentally (9). Alduk et al. (10) analysed that the extra mammary finding rate of mediastinal is 15.9% (22/138). In adult, most of mediastinum mass finding is primary thymic masses (Figs. 1, 2), thyroid masses and lymphomas (9). The most important tool for evaluation of mediastinal mass is CT. But MRI also help to diagnosis of cystic mass and assessment of relationships with the surrounding structures (9).
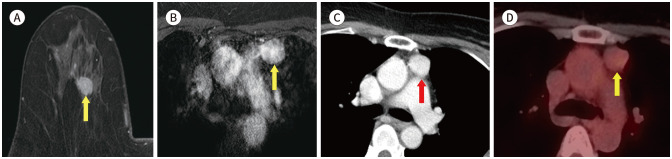
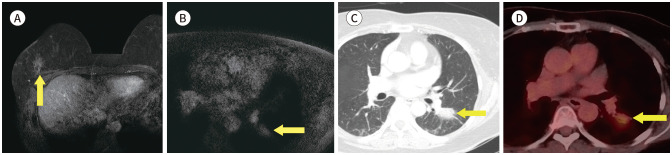
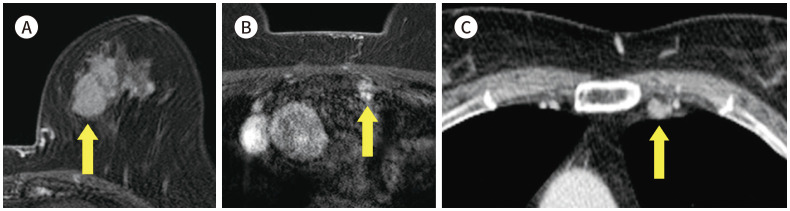
Fig. 1. 56-year-old female with IDC in right breast and thymoma.
A. CE T1-weighted-MRI shows a 1.4 cm in size IDC in the right breast (arrow).
B. CE T1-weighted-MRI shows an enhancing mass in the anterior mediastinum (arrow).
C. In the axial chest CT, there is an enhancing nodule at the same location as the MRI (arrow).
D. PET/CT shows a round-shape mass with fluorodeoxyglucose uptake (max SUV 2.3) in the same location as the MRI (arrow). The mass was diagnosed as a thymoma after excision and biopsy.
CE = contrast-enhanced, IDC = invasive ductal carcinoma, max SUV = maximum standardized uptake value
Fig. 2. 48-year-old female with DCIS in left breast and thymic cyst.
A. CE T1-weighted-MRI shows a 1.2 cm in size DCIS in the left breast (arrow).
B. In axial chest CT, there is a low attenuated non-enhancing nodule at the same location as the MRI (arrow).
C. PET/CT shows a round-shape nodule with no fluorodeoxyglucose uptake in the same location as the MRI (arrow).
D. CE T1-weighted-MRI shows 1.3 cm in size non-enhancing nodule in the anterior mediastinum (arrow).
CE = contrast-enhanced, DCIS = ductal carcinoma in situ
Cardiac hypertrophy (Fig. 3) is an abnormal finding that is easily detected on a chest X-ray, and can be confirmed through the cardiothoracic ratio (11). Cardiothoracic ratio is calculated as the ratio between the maximum transverse diameter of the heart and the maximum width of the chest above the costophrenic angles measured at the inner edge of the rib. Cardiac hypertrophy is defined as a cardiothoracic ratio > 0.5. This can be easily detected in other radiological tests (12).
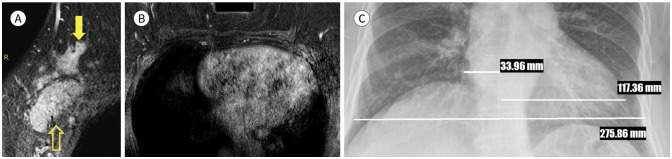
Fig. 3. 72-year-old female with adenocarcinomas of unknown primary site in left breast and cardiomegaly.
A. CE T1-weighted-MRI shows a biopsy-proven adenocarcinoma in the right axilla (blank arrow) and an enhancing nodule in the right breast of approximately 3.2 cm in size (arrow).
B. CE T1-weighted-MRI shows an enlarged heart in the middle mediastinum.
C. On posteroanterior chest X-ray, the cardiothoracic ratio is 0.55.
CE = contrast-enhanced
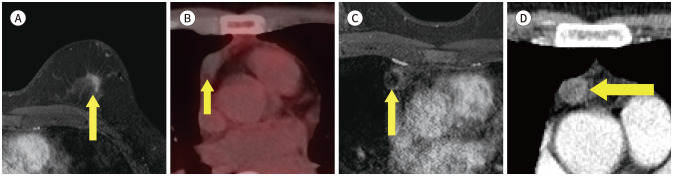
A incidence of aberrant right subclavian artery (Fig. 4) has been reported 0.16% to 2% (13). We found one case of the aberrant right subclavian artery on breast MRI. It was further evaluated on CT scan.
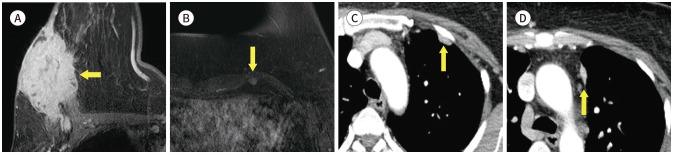
Fig. 4. 74-year-old female with DCIS in right breast and aberrant right subclavian artery.
A. Contrast-enhanced T1-weighted-MRI shows a 1 cm in size DCIS in the right breast (arrow).
B. T1-weighted-MRI shows a vascular structure (arrow) arising from the aortic arch after left subclavian artery running posterior to esophagus.
C-E. In the serial axial and reformatted coronal chest CT, there is an aberrant right subclavian artery (arrows) running posterior aspect to esophagus (blank arrow).
DCIS = ductal carcinoma in situ
EXTRAMAMMARY FINDINGS IN LUNG
The breast cancer commonly spreads to the lung and lymph nodes (14). Casey et al. (15) found that breast cancer patients with a solitary pulmonary nodule had primary lung cancer (52%) (Fig. 5) and lung metastasis (43%) (Fig. 6). Alduk et al. (10) founds that the prevalence of incidental extra mammary finding on breast MRI is lung (24/138; 17.4%) and pleural cavity (15/138; 10.9%), including pleural metastasis (Fig. 7). One case is atelectasis (Fig. 8) that resembles a pleural metastasis. The Lymph node metastasis (Fig. 9) is 20% (5/25) of malignant findings (10). Those finding is basic assessment of treatment plan for breast cancer.
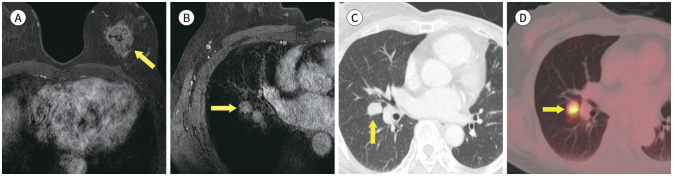
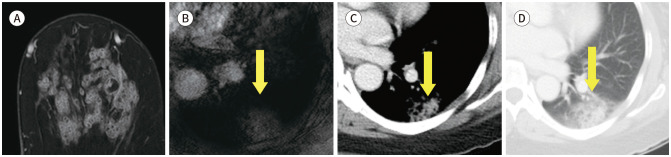
Fig. 5. 61-year-old female with DCIS in right breast and synchronous primary lung cancer.
A. CE T1-weighted-MRI shows a 3.5 cm DCIS in the right breast (arrow).
B. CE T1-weighted-MRI shows a 2.8 cm in size enhancing nodule in the left lung (arrow).
C. Axial chest CT also shows an enhancing nodule in left lower lobe (arrow). It was diagnosed as an adenocarcinoma by percutaneous transthoracic needle biopsy.
D. PET/CT shows an increased fluorodeoxyglucose uptake (max SUV 4.3) in left lower lobe of the lung (arrow).
CE = contrast-enhanced, DCIS = ductal carcinoma in situ, max SUV = maximum standardized uptake value
Fig. 6. 62-year-old female with IDC in left breast and lung metastasis.
A. CE T1-weighted-MRI shows a 3.7 cm IDC in left upper center of the left breast (arrow).
B. CE T1-weighted-MRI shows a 1.6 cm in size enhancing nodule in the right lung (arrow).
C. Axial chest CT also shows an enhancing nodule in the right middle lobe (arrow). It was diagnosed as a metastatic cancer by percutaneous transthoracic needle biopsy.
D. PET/CT shows an increased fluorodeoxyglucose uptake (max SUV 8.43) in the right lung (arrow).
CE = contrast-enhanced, IDC = invasive ductal carcinoma, max SUV = maximum standardized uptake value
Fig. 7. 50-year-old female with IDC in right breast and pleural metastasis.
A. CE T1-weighted-MRI shows an IDC with skin invasion (arrow) in right breast of approximately 7 cm in size.
B. CE T1-weighted-MRI shows small enhancing nodule on the left anterior pleural surface (arrow).
C, D. Axial CE CT image shows multiple pleural metastases (arrows).
CE = contrast-enhanced, IDC = invasive ductal carcinoma
Fig. 8. 63-year-old female with IDC in both breast and atelectasis mimicking pleural metastasis.
A, B. CE T1-weighted-MRI shows two IDCs, one on each breast (arrows), of approximately 1.7 cm and 1 cm.
C, D. CE T1-weighted-MRI shows an enhancing lesion in right superior diaphragmatic area of approximately 1.3 cm in size, mimicking pleural metastasis on the MRI. However, this lesion is correlated with right middle lobe atelectasis on the axial chest CT (arrows).
CE = contrast-enhanced, IDC = invasive ductal carcinoma
Fig. 9. 64-year-old female with IDC in right breast and internal mammary lymph node metastasis.
A. CE T1-weighted-MRI shows a 3.3 cm in size IDC in the left breast (arrow).
B. CE T1-weighted-MRI shows a 7 mm size enhancing nodule in the left internal mammary chain (arrow).
C. In the CE axial chest CT, there is an enhancing nodule at the same location as the MRI (arrow). The nodule was diagnosed as a metastatic lymph node after operation.
CE = contrast-enhanced, IDC = invasive ductal carcinoma
Incidental extra mammary findings on breast MRI include benign finding like pneumonia (Fig. 10), pleural effusion, tuberculosis sequelae (Fig. 11) and pulmonary hamartoma (Fig. 12). There is no criteria of staging of breast cancer, but some of benign findings required extra treatment like antibiotics against pneumonia, percutaneous catheter drainage of pleural effusion or anti tuberculosis drugs against active tuberculosis.
Fig. 10. 54-year-old female with IDC in left breast and pneumonia.
A. CE T1-weighted-MRI shows an IDC in the left breast.
B. CE T1-weighted-MRI shows a 2.5 cm extent non-enhancing mass in the left lung parenchyma (arrow).
C, D. In the axial chest CT, there is a consolidation and ground glass opacity in the left lower lobe superior segment (arrows). Patient showed elevated white blood cell count and was treated with antibiotics.
CE = contrast-enhanced, IDC = invasive ductal carcinoma
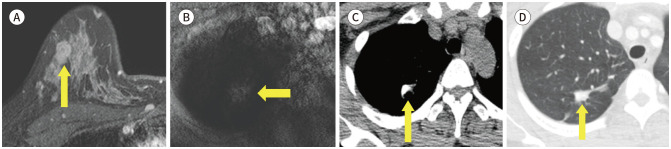
Fig. 11. 43-year-old female with IDC in right breast and tuberculosis sequelae.
A. CE T1-weighted-MRI shows a 1.5 cm in size IDC in the right breast (arrow).
B. T1-weighted-MRI shows an irregular mass in the right upper lobe (arrow).
C, D. In pre-contrast axial chest CT, there is a calcified nodule at the same location as the MRI (arrow). Also, in the lung window setting, there is a band-like opacity around the calcified nodule (arrow). Considering the patient’s history and radiologic findings, it was presumed to be tuberculosis sequelae.
CE = contrast-enhanced, IDC = invasive ductal carcinoma
Fig. 12. 44-year-old female with IDC in right breast and pulmonary hamartoma.
A. CE T1-weighted-MRI shows 1.1 cm IDC in the right breast (arrow).
B. CE T1-weighted-MRI shows a 0.8 cm in size well-circumscribed solitary pulmonary nodule in the right lung (arrow).
C, D. Axial chest CT also shows a non-enhancing nodule with a fatty component in the right lower lobe (arrows).
CE = contrast-enhanced, IDC = invasive ductal carcinoma
EXTRAMAMMARY FINDINGS IN UPPER ABDOMEN
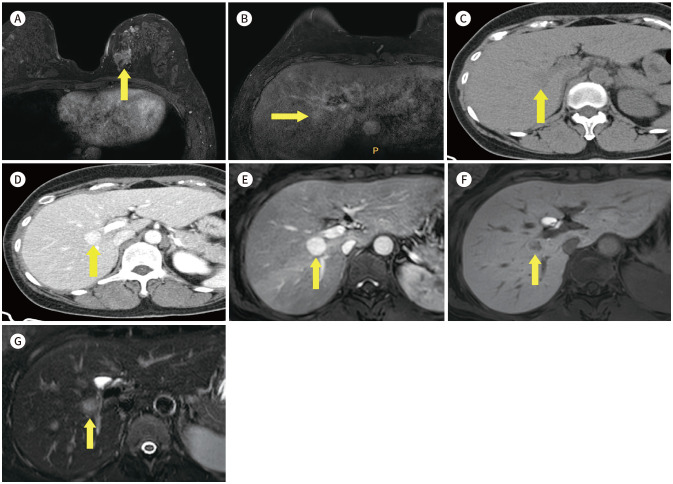
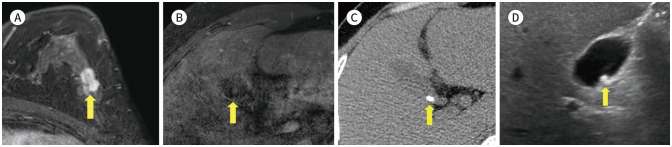
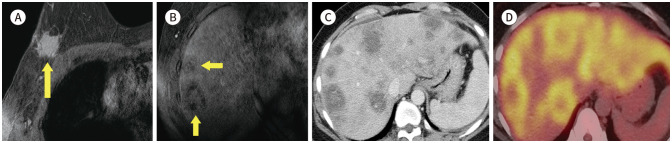
The most common extra mammary findings is liver (16,17). Iodice et al. (17) reported that the half of non-significant extra mammary finding is liver cysts (51%, 210/414) (Fig. 13). We found another benign finding, such as focal nodular hyperplasia (Fig. 14), liver cirrhosis, ascites (Fig. 15), gallstone (Fig. 16) and hiatal hernia (Fig. 17). This findings are not necessary to prompt further evaluation. However, 8% (5/66) of significant extra mammary finding was liver metastasis (Fig. 18) (17). Half of metastatic breast cancer was known liver metastasis (18). This finding is important to set up the treatment plan and needs to further evaluation.
Fig. 13. 79-year-old female with IDC in right breast and hepatic cyst.
A. CE T1-weighted-MRI shows a 3.1 cm in size IDC in the left breast (arrow).
B. T1-weighted-MRI shows a 5.7 cm in size hypointense lesion in the left lateral segment of the liver (arrow).
C. In the CE axial CT, there is a non-enhancing low-attenuated lesion at the same location as the MRI (arrow).
D. On ultrasonography, there is anechoic lesion with posterior acoustic enhancement in the left lateral segment of liver (arrow).
CE = contrast-enhanced, IDC = invasive ductal carcinoma
Fig. 14. 51-year-old female with IDC in right breast and focal nodular hyperplasia.
A. CE T1-weighted-MRI shows a 2.9 cm in size IDC in the left breast (arrow).
B. T1-weighted-MRI shows a 1.7 cm in size enhancing lesion in the liver S8 (arrow).
C, D. In the axial chest CT, there is an enhancing low-attenuated lesion at the same location as the MRI (arrows).
E, F. CE T1-weighted liver MRI in the arterial phase reveals strong enhancement of the lesion and (F) wash-out in hepatobiliary phase (arrows).
G. Axial T2W MRI shows a well-circumscribed T2 iso to hyperintense lesion (arrow) in the liver S8 with a central T2 hyperintense scar.
CE = contrast-enhanced, IDC = invasive ductal carcinoma
Fig. 15. 41-year-old female with IDC in left breast and liver cirrhosis with ascites.
A. CE T1-weighted-MRI shows a 5.2 cm IDC in the left breast (arrow).
B. CE T1-weighted-MRI shows nodular contour of the liver (red arrows) and ascites (yellow arrows), which suggests liver cirrhosis.
C. Axial CE CT imaging also shows a nodular contour of the liver and ascites, which is well correlated with the previous breast MRI.
CE = contrast-enhanced, IDC = invasive ductal carcinoma
Fig. 16. 49-year-old female with IDC in left breast and gallstone.
A. CE T1-weighted-MRI shows a 1.5 cm IDC in the left breast (arrow).
B. CE T1-weighted-MRI shows a hypointense nodular lesion in the gallbladder (arrow), which suggests a GB stone.
C, D. The axial non-enhanced CT image (C) and abdominal ultrasonography (D) also show a high density/echogenic nodular lesion (arrows) in the gallbladder, which is well correlated with the previous breast MRI.
CE = contrast-enhanced, IDC = invasive ductal carcinoma
Fig. 17. 78-year-old female with invasive lobular breast cancer in right breast and hiatus hernia.
A. CE T1-weighted-MRI shows a 2.4 cm in size invasive lobular breast cancer in the right breast (arrow).
B. T1-weighted-MRI shows a hypointense mass-like lesion of 5.7 cm in size in the para-esophageal area (arrow).
C, D. CE axial and coronal reformatted CT shows herniation of abdominal contents through the esophageal hiatus (arrows).
CE = contrast-enhanced
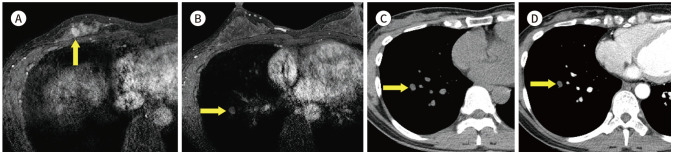
Fig. 18. 45-year-old female who underwent excision biopsy for right breast mass, which was proven IDC and hepatic metastasis.
A. CE T1-weighted-MRI shows an approximately 2.5 cm post-op hematoma in the right breast (arrow).
B. CE T1-weighted-MRI shows multiple ill-defined hypointense nodules in the liver (arrows).
C. Axial CT image shows correlating multiple ill-defined lesions in the liver, which suggests liver metastasis.
D. PET/CT shows diffuse and heterogeneous fluorodeoxyglucose uptake in both lobes of the liver.
CE = contrast-enhanced, IDC = invasive ductal carcinoma
EXTRAMAMMARY FINDINGS IN BONE & SOFT TISSUE
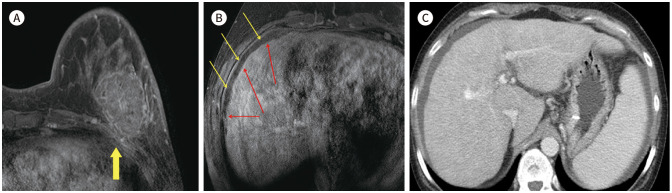
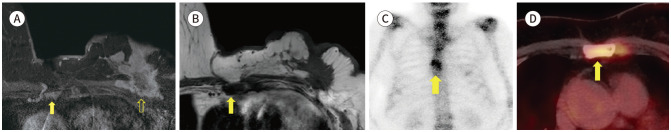
MRI is known the high positive predictive tool for defection of bone and soft tissue lesion. Studies reported that bony findings on breast MRI is 3.1% (26/828) to 21% (66/308) (4). The Bone metastasis (Fig. 19) is highly suggested that combined abnormality of multiple lesion and soft tissue masses. But multiple bony lesions are confused bone metastasis with bone fracture (Fig. 20). It needs to further evaluation like bone scan or PET, because of very different condition to make a treatment plan (8). Skin or chest wall invasion are also important to stage of breast cancer. There is one case of representing breast cancer with skin and muscle invasion (Fig. 21).
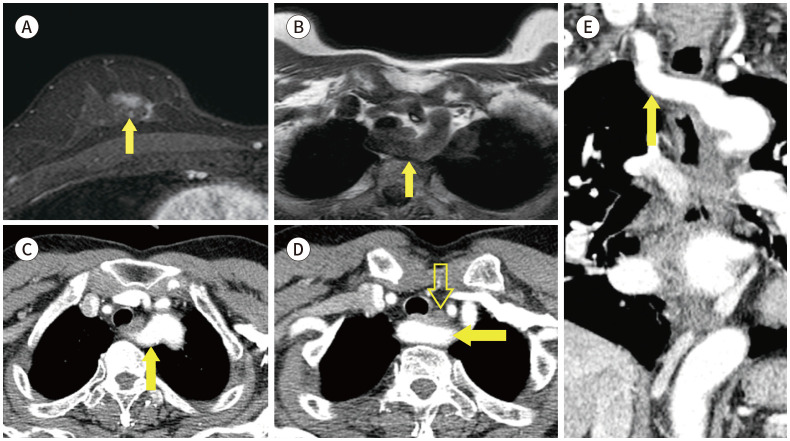
Fig. 19. 76-year-old female with IDC in left breast and bone metastasis.
A. Contrast-enhanced T1-weighted-MRI shows a more than 5 cm in size IDC in the left breast (blank arrow) and enhancing lesions in the sternum (arrow).
B. T1-weighted-MRI shows hypointense lesions in the sternum (arrow), which suggest bone metastasis.
C, D. Whole body bone scan and PET/CT shows focal uptakes in sternum (arrows).
IDC = invasive ductal carcinoma
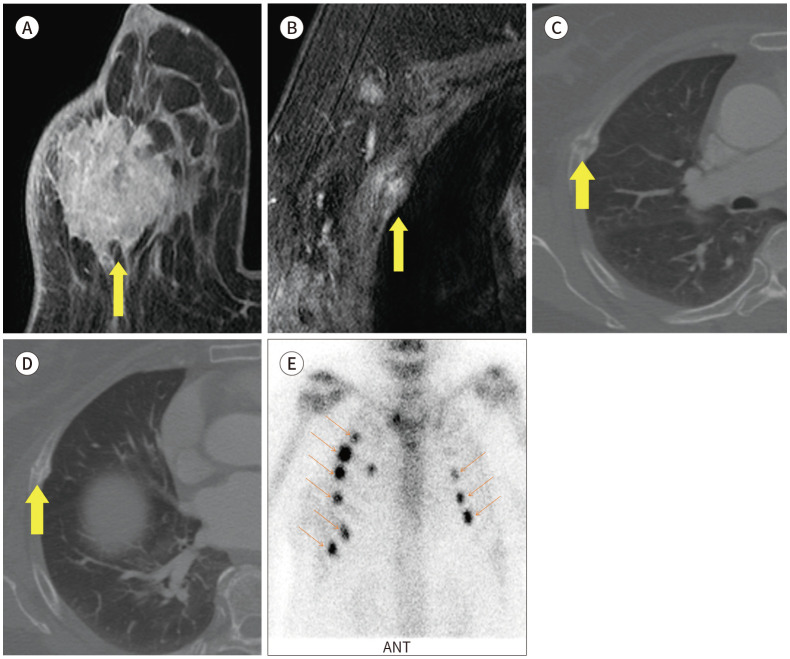
Fig. 20. 73-year-old female with IDC in right breast and rib fracture mimicking bone metastasis.
A. CE T1-weighted-MRI shows a 4.2 cm extent IDC in the right breast with skin invasion (arrow).
B. CE T1-weighted-MRI shows a focal enhancing lesion in right rib, which is suggestive of bone metastasis on the MRI (arrow).
C, D. Axial CE CT image also shows multiple rib fractures with callus formation (arrows).
E. Bone scan also shows multiple rib fractures in both sides (arrows).
CE = contrast-enhanced, IDC = invasive ductal carcinoma
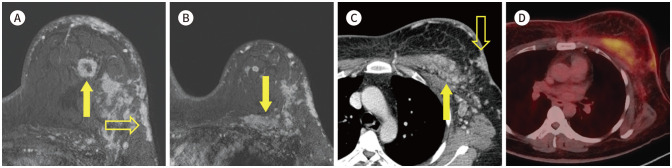
Fig. 21. 45-year-old female with IDC in left beast and pectoralis muscle metastasis.
A. CE T1-weighted-MRI shows multiple IDCs (arrow) with skin invasion (blank arrow) in the left breast.
B. CE T1-weighted-MRI shows enlargement of the pectoralis muscle with enhancement (arrow).
C. In the CE axial CT, there is metastasis in the left pectoralis muscle (arrow) and skin invasion (blank arrow).
D. PET/CT shows increased FDG uptake in the left pectoralis muscle, which is suggestive of metastasis.
CE = contrast-enhanced, IDC = invasive ductal carcinoma
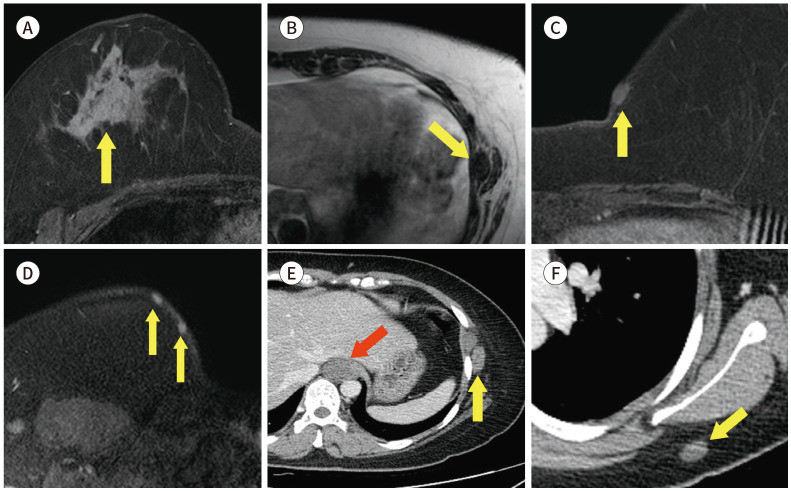
Some reports announced the relationship with breast cancer and neurofibromatosis. Breast cancer patients of under 50 years of age have more risk of neurofibromatosis (Fig. 22) (19). MRI is not required to diagnosis of neurofibromatosis, but it help to identify asymptomatic tumours (20).
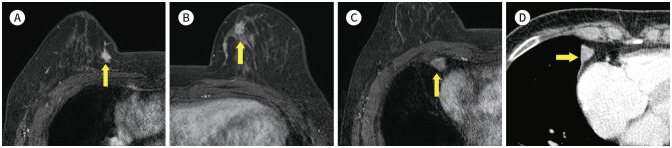
Fig. 22. 35-year-old female with IDC in right breast with history of neurofibromatosis.
A. CE T1-weighted-MRI shows a 7 cm in size IDC in the right breast (arrow).
B. T1-weighted-MRI shows a 1.6 cm in size nodule in the left lateral chest wall (arrow).
C, D. CE T1-weighted-MRI shows several small nodules in the subcutaneous layer (arrows).
E, F. In the CE axial CT, there is a 2.4 cm in size hardly-enhancing nodule in the paraaortic area (red arrow) and small nodules in the soft tissue (yellow arrows).
CE = contrast-enhanced, IDC = invasive ductal carcinoma
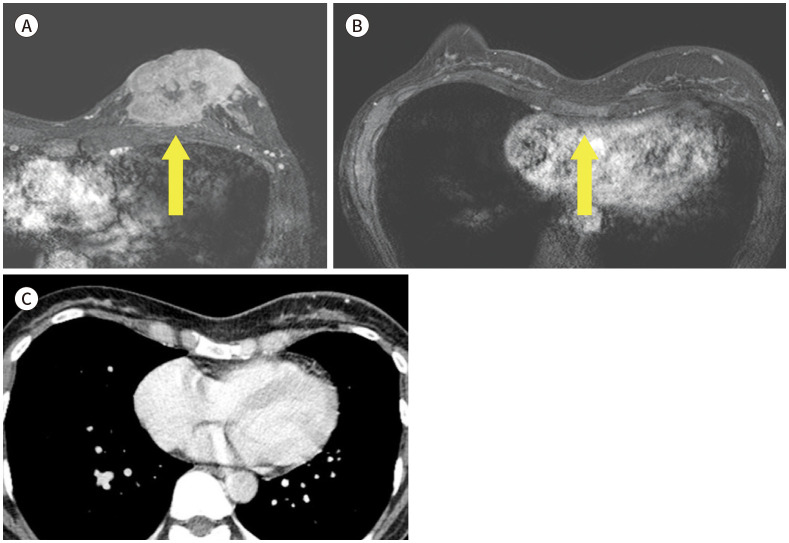
One of common chest wall malformations is pectus excavatum. Those patients should initially be evaluated morphology of thorax wall, tracheal bronchus and heart. CT and MRI is tool that can measure the degree of severity of pectus excavatum (Fig. 23) (21).
Fig. 23. 45-year-old female with IDC in left beast and pectus excavatum.
A. CE T1-weighted-MRI shows a 7 cm in size IDC with skin invasion in the left breast (arrow).
B. CE T1-weighted-MRI shows concave depression of the sternum and chest wall deformity (arrow).
C. With the CE CT, the Haller index was calculated as 3.06, compatible with pectus excavatum.
CE = contrast-enhanced, IDC = invasive ductal carcinoma
CONCLUSION
Few studies of the extramammary finding of breast MRIs have been reported. Morakkabati-Spitz et al. (22) analysed the prevalence of incidental findings on breast MRI. 9% of the patients with a history of breast cancer and 81% of patients on preoperative staging had incidental findings. Extramammary findings found in these patients have a high probability of malignancy (4,22). Therefore, findings found incidentally on breast MRI may be helpful in treating cancer or developing a treatment plan. In conclusion, it is the responsibility of the radiologist to observe these findings carefully and not to ignore them.
Footnotes
- Conceptualization, all authors.
- data curation, all authors.
- funding acquisition, K.S.Y.
- investigation, all authors.
- project administration, K.S.Y.
- resources, K.S.Y.
- supervision, K.S.Y.
- visualization, all authors.
- writing—original draft, all authors.
- writing—review & editing, all authors.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding: This work was supprted by the Soonchunhyang University Research Fund and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2018R1C1B5041484).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17:43–46. doi: 10.7314/apjcp.2016.17.s3.43. [DOI] [PubMed] [Google Scholar]
- 3.Leithner D, Wengert GJ, Helbich TH, Thakur S, Ochoa-Albiztegui RE, Morris EA, et al. Clinical role of breast MRI now and going forward. Clin Radiol. 2018;73:700–714. doi: 10.1016/j.crad.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y, Ibidapo O, Toth HK, Moy L. Delineating extramammary findings at breast MR imaging. Radiographics. 2017;37:10–31. doi: 10.1148/rg.2017160051. [DOI] [PubMed] [Google Scholar]
- 5.Yang SM, Kim SH, Kang BJ, Song BJ. Extramammary findings on breast MRI: prevalence and imaging characteristics favoring malignancy detection: a retrospective analysis. World J Surg Oncol. 2016;14:119. doi: 10.1186/s12957-016-0865-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassett LW, Dhaliwal SG, Eradat J, Khan O, Farria DF, Brenner RJ, et al. National trends and practices in breast MRI. AJR Am J Roentgenol. 2008;191:332–339. doi: 10.2214/AJR.07.3207. [DOI] [PubMed] [Google Scholar]
- 7.Yun BL, Kim SM, Jang M, Kang BJ, Cho N, Kim SH, et al. Current practices in breast magnetic resonance imaging: a survey involving the Korean Society of Breast Imaging. Investig Magn Reson Imaging. 2017;21:233–241. [Google Scholar]
- 8.Suh HJ, Choi JS, Ko K. Extra-mammary findings detected on breast magnetic resonance imaging: a pictorial essay. Korean J Radiol. 2014;15:423–429. doi: 10.3348/kjr.2014.15.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laurent F, Latrabe V, Lecesne R, Zennaro H, Airaud JY, Rauturier JF, et al. Mediastinal masses: diagnostic approach. Eur Radiol. 1998;8:1148–1159. doi: 10.1007/s003300050525. [DOI] [PubMed] [Google Scholar]
- 10.Alduk AM, Prutki M, Stern-Padovan R. Incidental extra-mammary findings in breast MRI. Clin Radiol. 2015;70:523–527. doi: 10.1016/j.crad.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Brakohiapa EKK, Botwe BO, Sarkodie BD, Ofori EK, Coleman J. Radiographic determination of cardiomegaly using cardiothoracic ratio and transverse cardiac diameter: can one size fit all? Part one. Pan Afr Med J. 2017;27:201. doi: 10.11604/pamj.2017.27.201.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alghamdi SS, Abdelaziz I, Albadri M, Alyanbaawi S, Aljondi R, Tajaldeen A. Study of cardiomegaly using chest x-ray. J Radiat Res Appl Sci. 2020;13:460–467. [Google Scholar]
- 13.Choi Y, Chung SB, Kim MS. Prevalence and anatomy of aberrant right subclavian artery evaluated by computed tomographic angiography at a single institution in Korea. J Korean Neurosurg Soc. 2019;62:175–182. doi: 10.3340/jkns.2018.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang EY, Johnson W, Karamlou K, Khaki A, Komanapalli C, Walts D, et al. The evaluation and treatment implications of isolated pulmonary nodules in patients with a recent history of breast cancer. Am J Surg. 2006;191:641–645. doi: 10.1016/j.amjsurg.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Casey JJ, Stempel BG, Scanlon EF, Fry WA. The solitary pulmonary nodule in the patient with breast cancer. Surgery. 1984;96:801–805. [PubMed] [Google Scholar]
- 16.Rinaldi P, Costantini M, Belli P, Giuliani M, Bufi E, Fubelli R, et al. Extra-mammary findings in breast MRI. Eur Radiol. 2011;21:2268–2276. doi: 10.1007/s00330-011-2183-6. [DOI] [PubMed] [Google Scholar]
- 17.Iodice D, Di Donato O, Liccardo I, Lamanna L, Segreto S, Salvatore M, et al. Prevalence of extramammary findings on breast MRI: a large retrospective single-centre study. Radiol Med. 2013;118:1109–1118. doi: 10.1007/s11547-013-0937-8. [DOI] [PubMed] [Google Scholar]
- 18.Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: long-term survival after curative resection. Surgery. 2000;127:383–389. doi: 10.1067/msy.2000.103883. [DOI] [PubMed] [Google Scholar]
- 19.Howell SJ, Hockenhull K, Salih Z, Evans DG. Increased risk of breast cancer in neurofibromatosis type 1: current insights. Breast Cancer (Dove Med Press) 2017;9:531–536. doi: 10.2147/BCTT.S111397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferner RE, Huson SM, Thomas N, Moss C, Willshaw H, Evans DG, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81–88. doi: 10.1136/jmg.2006.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abid I, Ewais MM, Marranca J, Jaroszewski DE. Pectus excavatum: a review of diagnosis and current treatment options. J Am Osteopath Assoc. 2017;117:106–113. doi: 10.7556/jaoa.2017.021. [DOI] [PubMed] [Google Scholar]
- 22.Morakkabati-Spitz N, Sondermann E, Schmiedel A, Leutner C, Riehm K, Schmutzler R, et al. Prevalence and type of incidental extramammary findings in MRI of the breast. Rofo. 2003;175:199–202. doi: 10.1055/s-2003-37227. [DOI] [PubMed] [Google Scholar]