Abstract
Introduction
Hypogonadism is prevalent during opioid treatment, and low testosterone concentrations are associated with cardiovascular disease. The effect of testosterone replacement therapy (TRT) on the coagulation system in men with hypogonadism is not clarified. We investigate the effects of TRT on the tissue factor (TF) and contact activation pathways of coagulation in opioid-treated men.
Materials and methods
This was a double-blinded, placebo-controlled study in 37 men with total testosterone < 12 nmol/L randomized to 24 weeks of testosterone injections (n = 17) or placebo (n = 20). Variables of the coagulation system were analysed at baseline and after 24 weeks. Measurements included the TF pathway (endogenous thrombin potential (ETP) and peak thrombin), the contact activation pathway (endogenous kallikrein potential (EKP) and peak kallikrein), coagulation factors (FVII, FX, prothrombin, and FXII), and inhibitors (tissue factor pathway inhibitor (TFPI), protein C, protein S, antithrombin, and C1 esterase inhibitor (C1inh)). Between-group differences at 24 weeks were determined with analysis of covariance. Within-group changes in TRT and placebo were analysed with paired t-test.
Results
Between-group differences at 24 weeks were observed for ETP (P = 0.036), FVII (P = 0.044), FX (P = 0.015), prothrombin (P = 0.003), protein C (P = 0.004), and protein S (P = 0.038). Within the TRT group, ETP, peak thrombin, FVII, FX, prothrombin, TFPI, protein C, FXII, and C1inh decreased and protein S increased (all P < 0.05). Within the placebo group, coagulation outcomes were unchanged.
Conclusion
TRT affects the coagulation system in an anticoagulant direction through suppressed TF pathway in men with opioid-induced hypogonadism.
Keywords: testosterone, thrombin generation, blood coagulation, hormone therapy, hypogonadism
Introduction
Opioid medications are widely used to treat chronic non-cancer pain (1). Male hypogonadism, characterized by low concentrations of testosterone and luteinizing hormone (LH), is one of the most well-described hormonal adverse effects of opioid treatment (2). The risk of hypogonadism ranges from 19 to 86% in men treated with opioids, depending on the treatment duration, the dosage of opioids, and the applied threshold concentration for total testosterone used to define hypogonadism (3). Low testosterone is associated with a non-beneficial cardiovascular risk profile and increased mortality (4, 5). The cardiovascular safety of testosterone replacement therapy (TRT) in men with reduced testosterone concentrations is, however, controversial (6). TRT improves body composition by increasing the lean body mass and decreasing the total fat mass, resulting in a potential beneficial cardiovascular profile (7, 8, 9). TRT, however, may increase cardiovascular risk by lowering subcutaneous fat, concentrations of adiponectin, and HDL cholesterol (10, 11), and studies have demonstrated increased risk of cardiovascular disease (CVD) in men receiving TRT (12, 13, 14, 15). Other studies, however, reported neutral or beneficial effects of TRT on CVD risk (16, 17, 18), but most studies were small and inconclusive. Randomized controlled trials and long-term data on TRT and cardiovascular safety are requested, as recently concluded in a meta-analysis (19).
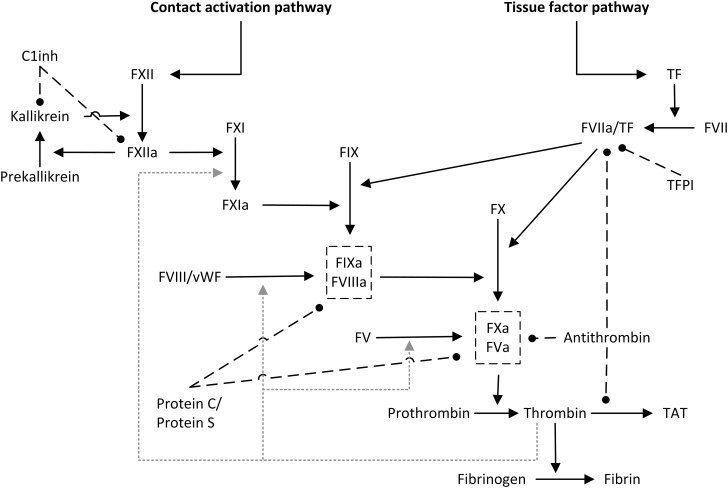
CVD can be caused by atherosclerosis and thrombosis, and alterations in the coagulation system (Fig. 1) contribute significantly to the pathogenesis of thrombosis. It remains unclear whether TRT induces a pro- or anticoagulant state. Reportedly, physiological doses of testosterone to elderly men with hypogonadism induce no significant change in the coagulation system in terms of tissue factor (TF)-induced thrombin generation (20), whereas another study in men without hypogonadism points towards a TRT-induced suppression of coagulation (21). In line with this, reduced thrombin generation, and thus a potential anticoagulant effect of TRT, is reported in men with hypogonadism due to Klinefelter syndrome (22). High doses of testosterone administrated to men without hypogonadism augment the activation of the coagulation system (23). More extreme doses of androgens, as can be seen during anabolic androgenic steroid (AAS) abuse, induce a procoagulant effect related to TF-induced thrombin generation (24). Another study on AAS abuse demonstrated reduced contact-induced kallikrein generation in terms of peak kallikrein concentration and endogenous kallikrein potential (EKP) in current abusers compared with former abusers and controls (25). Conversely, TRT of transgender men (born women) is not associated with a procoagulant state (26) and TRT given to castrated male rats normalizes the castration-induced increase in prothrombin and coagulation factor VII (FVII) concentrations (27). Earlier studies report reduced concentrations of prothrombin in TRT-treated rats (28, 29).
Figure 1.
Coagulation system. TF, tissue factor; F, coagulation factor (e.g. FVII); TFPI, tissue factor pathway inhibitor; C1inh, C1 esterase inhibitor; TAT, thrombin–antithrombin complexes; vWF, von Willebrand factor. The letter ‘a’ denotes activated enzymes. Solid lines indicate activation, dashed lines inhibition, and dashed grey lines indicate thrombin-induced feedback activation.
In the present randomized, double-blinded, placebo-controlled intervention study, we investigate in detail the effect of physiological doses of testosterone on the capacity of the TF and contact activation pathways of coagulation and related coagulation factors and inhibitors in opioid-treated men.
Materials and methods
Participants and study design
This study is a continuation of a previous study described elsewhere (7). In short, the study is a single-centre, randomized, double-blinded, placebo-controlled study conducted from August 2016 to August 2019 at Odense University Hospital, Denmark. Forty-one men aged >18 years, treated with opioids for non-malignant pain disease, were randomized to receive TRT (testosterone undecanoate (Nebido), 1000 mg i.m.) or identically looking placebo injections. The participants received injections at time of randomization and at 6 and 18 weeks. Inclusion criteria were opioid treatment for at least 3 months at a dosage corresponding to at least 50 mg morphine/day, at least two measures of total testosterone < 12 nmol/L measured in the fasting state between 08:00 and 10:00 in the morning, and LH and prolactin levels within the reference interval. We excluded men wishing to conceive during the trial period, subjected to previous or current testosterone treatment, treated with 5α-reductase inhibitors, oral glucocorticoid steroids, or anticoagulants. We also excluded men with haematocrit > 53%, previous or ongoing malignant disease, prostate-specific antigen > 3 ng/dL, abnormal routine blood samples (thyroid-stimulating hormone, ionized calcium, haemoglobin, liver, and kidney function), untreated ischemic heart or respiratory disease, and alcohol or drug abuse.
For various reasons, three participants dropped out during the intervention period (7), and for one participant the blood sampling was incomplete, leaving 17 and 20 participants in the TRT and placebo groups, respectively. The subjects’ characteristics before and 6 months after TRT or placebo are presented in Table 1, showing that body weight, lean body mass, and concentrations of testosterone increase after TRT, whereas the total fat mass is reduced, as previously reported (7).
Table 1.
Clinical characteristics at baseline and after 24 weeks in men treated with placebo and testosterone replacement therapy.
| Placebo | Testosterone replacement therapy | |||||
|---|---|---|---|---|---|---|
| Variable | Baseline | 24 weeks | P | Baseline | 24 weeks | P |
| N | 20 | 20 | 17 | 17 | ||
| Age (years) | 53 ± 11 | 54 ± 7 | ||||
| Weight (kg) | 98.7 ± 15.5 | 99.7 ± 15.5 | 0.37 | 100.9 ± 13.2 | 104.4 ± 14.1 | <0.01 |
| BMI (kg/m2) | 32.0 ± 4.1 | 32.3 ± 4.0 | 0.56 | 31.3 ± 3.8 | 32.5 ± 4.3 | <0.01 |
| Total fat mass (kg) | 33.2 ± 6.1 | 33.8 ± 6.5 | 0.26 | 33.0 ± 7.5 | 31.6 ± 7.8 | <0.01 |
| Fat percentage (%) | 34.9 ± 2.8 | 35.5 ± 3.4 | 0.24 | 33.8 ± 5.1 | 31.6 ± 4.4 | <0.01 |
| Lean body mass (kg) | 59.7 ± 9.3 | 59.3 ± 9.8 | 0.57 | 61.5 ± 7.7 | 65.2 ± 7.3 | <0.01 |
| Total testosterone (nmol/L)a | 7.4 (5.1; 9.6) | 7.7 (5.2; 8.5) | 0.46 | 6.8 (5.2; 9.5) | 19.4 (14.3; 26.8) | <0.01 |
| Sex hormone-binding globulin (nmol/L)a | 32.2 (24.5; 44.5) | 31.5 (20.7; 51.2) | 0.48 | 41.9 (24.5; 51.7) | 36.9 (24.6; 45.9) | <0.01 |
| Bioavailable testosterone (nmol/L)a | 3.5 (2.1; 4.4) | 3.0 (1.9; 4.1) | 0.40 | 2.7 (2.0; 3.8) | 8.6 (8.1; 12.4) | <0.01 |
| Total cholesterol (mmol/L) | 4.8 ± 1.0 | 4.8 ± 1.2 | 0.71 | 4.5 ± 0.9 | 4.3 ± 0.9 | 0.30 |
| LDL (mmol/L) | 2.8 ± 1.0 | 2.7 ± 1.0 | 0.38 | 2.6 ± 0.8 | 2.5 ± 0.7 | 0.41 |
| Triglycerides (mmol/L)a | 1.6 (1.1; 2.9) | 1.8 (1.2; 3.1) | 0.53 | 1.5 (1.0; 2.2) | 1.8 (1.0; 2.3) | 0.60 |
Data presented as mean ± s.d. were compared at baseline and 24 weeks with a paired t-test. Subjects’ characteristics were previously reported in Glintborg et al (7). aData presented as median (25 and 75 percentiles) were compared with a Wilcoxon test. Significant differences are highlighted in bold. BMI, body mass index; LDL, low-density lipoprotein cholesterol.
Ethics
The study was planned in May 2015 and approved by The Regional Scientific Ethical Committee of Southern Denmark (S-20150004) and the Danish Health and Medicines Agency. Written informed consent was obtained from all participants. The trial was declared in www.clinicaltrials.gov (NCT02433730).
Blood sampling
The collection and handling of blood specimens followed the G41 guideline from Clinical and Laboratory Standards Institute (CLSI) (30) with specific focus on the recommendations for collection, transport, and processing of blood specimens for testing plasma-based coagulation assays, as detailed in the H21-A5 guideline from CLSI (31). In brief, fasting blood was collected from an antecubital vein in evacuated 2.7 mL tubes containing 0.109 mol/L sodium citrate (Vacutainer 9NC, Becton Dickinson, Plymouth, UK). Samples were obtained at baseline and after 6 month (24 week) intervention. Platelet-poor plasma was collected after centrifugation for 20 min at 2000 × g . The citrate-stabilized plasma specimens were stored at −80°C in tightly capped cryotubes (Sarstedt, VWR-Bie & Berntsen, Søborg, Denmark). Before analysis, the samples were thawed for 5 min at 37°C, kept at room temperature, and analysed within 1 h.
Biochemical analyses
Coagulation assays were performed at the Unit for Thrombosis Research, Hospital of South West Jutland, Esbjerg, Denmark.
Thrombin and kallikrein generation
TF-induced thrombin generation was analysed by the calibrated automated thrombin generation assay (Thrombinoscope BV, Maastrict, the Netherlands) using 5 pM TF and the Fluoroskan Ascent microplate fluorometer (Thermo Fisher Scientific) (32). In brief, thrombin was generated by mixing 80 µL plasma with 20 µL fluorogenic substrate-calcium chloride and 20 µL activating reagent with a final concentration of 5 pM TF and 4 µM phospholipids. The Thrombinoscope BV software was used for the calculation of various measures of thrombin generation: the lag time of the thrombin formation process (lag time, min), the time until the peak thrombin concentration was reached (time to peak, min), the peak thrombin concentration (peak, nmol/L), and the endogenous thrombin potential (ETP, nmol/L × min), representing the total amount of thrombin formed.
Contact-induced kallikrein generation was determined as described previously (33). In brief, undiluted citrate plasma was activated with silica (Sigma) diluted in activated partial thromboplastin time reagents (Triolab, Brøndby, Denmark), and FXII-dependent kallikrein generation was measured with the fluorogenic substrate Boc-Leu-Lys-Arg-AMC (Bachem, Bubendorf, Switzerland). Affinity-purified human kallikrein (Enzyme Research Laboratories, Swansea, UK) was used as a calibrator. Fluorescence was read using the Fluoroskan Ascent microplate fluorometer. The Thrombinoscope software was used to generate kallikrein formation curves. The curves display kallikrein generation lag time (lag time, min), peak kallikrein generation (peak, nmol/L), and area under the curve (EKP, nmol/L × min), representing the total amount of kallikrein formed.
Coagulation factors and inhibitors
The plasma protein concentration of coagulation factor XII (FXII) was determined with enzyme-linked immunosorbent assay (ELISA), as described previously (34). Plasma coagulation factor X (FX), FVII, and prothrombin were determined by clotting assays employing the ACLTOP350 Coagulation Analyzer, the HemosIL RecombiPlasTin 2G, and FX-, FVII-, and prothrombin-deficient plasmas. Analyzer, calibrators, and factor diluents were all from Instrumentation Laboratory, ILS Denmark, Lillerød, Denmark. Plasma antithrombin was determined by an automated chromogenic assay (STA-Stachrom AT III, Stago, Asnieres-sur-Seine, Paris, France). Plasma protein C activity was determined by an automated chromogenic assay (Protein C, HemosIL, Instrumentation Laboratory). Plasma-free protein S antigen was determined using an immune-turbidimetric assay kit (Free Protein S, HemosIL, Instrumentation Laboratory). The assays were performed on the ACLTOP350 Coagulation Analyzer. Plasma tissue factor pathway inhibitor (TFPI) antigen was determined using an ELISA (Human TFPI, Quantikine ELISA, R&D Systems Inc) using a microplate reader (Sunrise, Tecan Trading AG, Basle, Switzerland). The protein concentration of C1 esterase inhibitor (C1inh) was determined using N antiserum against human C1inh, buffers, and reagents, employing the BN II analyzer (all from Siemens Healthcare Diagnostics).
Statistical analysis
Variables of the haemostatic system as outcome measures were not defined in the original study (7), which was planned in 2015, and the power of the study was determined by the effect of TRT on lean body mass. Likewise, no studies examined the effect of TRT on variables of the haemostatic system in opioid-induced hypogonadism, and a power calculation was not performed. Thus, the present study should be regarded as a pilot study. However, in a post hoc power calculation, we selected ETP as the main outcome variable. The number of patients included in the study was sufficient to detect a 200 nmol/L × min difference in ETP between TRT and placebo groups, at a significance level of 5%, a power of 80%, and a standard deviation (s.d.) of 225 nmol/L × min. This difference in ETP is comparable to the difference observed in a previous study of TRT-treated men with Klinefelter syndrome (22). The present analysis is an efficacy analysis with the aim to determine biological effects of TRT, and data were analysed according to a per-protocol analysis on completers only. We did not include an intention-to-treat (ITT) analysis, because the coagulation variables were only measured in study completers.
Baseline values were compared between the TRT and placebo groups using an unpaired t-test. Between-group differences (TRT vs placebo) at 24 weeks were determined with an analysis of covariance, adjusted for baseline values, due to the randomized study design where the focus was on the size of treatment effects. Within-group changes (in TRT and placebo groups, respectively) were analysed with a paired t-test. TFPI was logarithmically transformed to meet the normal distribution assumption. Results are presented as mean (or geometric mean for TFPI) values and s.d.. A significance level of 0.05 was used throughout the study. The Statistical Package for Social Sciences (SPSS) program version 28 (IBM) was used for all statistical analyses.
Results
Measures of thrombin generation, kallikrein generation, coagulation factors, and inhibitors at baseline and after 24 weeks of placebo and TRT are shown in Table 2 and Fig. 2. No significant between-group differences were detected in baseline values.
Table 2.
Coagulation factors and inhibitors at baseline and after 24 weeks in men treated with placebo and testosterone replacement therapy.
| Placebo | Testosterone replacement therapy | |||||
|---|---|---|---|---|---|---|
| Variable | Baseline | 24 weeks | P | Baseline | 24 weeks | P |
| N | 20 | 20 | 17 | 17 | ||
| Coagulation factor VII (%) | 126 ± 23 | 120 ± 28 | 0.13 | 118 ± 21 | 101 ± 21 | <0.001 |
| Coagulation factor X (%) | 119 ± 20 | 118 ± 19 | 0.87 | 119 ± 19 | 110 ± 13 | <0.01 |
| Prothrombin (%) | 111 ± 14 | 109 ± 12 | 0.22 | 106 ± 12 | 97 ± 9 | <0.01 |
| Protein C (%) | 128 ± 29 | 127 ± 31 | 0.70 | 120 ± 26 | 107 ± 20 | <0.01 |
| Free protein S (%) | 111 ± 14 | 110 ± 16 | 0.53 | 107 ± 19 | 112 ± 16 | <0.01 |
| Tissue factor pathway inhibitor (ng/mL)a | 31.1 ± 1.25 | 30.1 ± 1.27 | 0.33 | 30.1 ± 1.25 | 28.3 ± 1.26 | <0.05 |
| Antithrombin (%) | 100 ± 10 | 100 ± 8 | 0.97 | 94 ± 12 | 91 ± 13 | 0.08 |
| Coagulation factor XII (%) | 134 ± 29 | 131 ± 29 | 0.47 | 136 ± 28 | 123 ± 27 | <0.01 |
| C1 esterase inhibitor (%) | 88 ± 20 | 83 ± 21 | 0.10 | 91 ± 26 | 84 ± 25 | <0.05 |
Data presented as mean ± s.d. were compared at baseline and 24 weeks with a paired t-test. Significant differences are highlighted in bold.aTissue factor pathway inhibitors were logarithmically transformed before analysis (geometric mean ± s.d.).
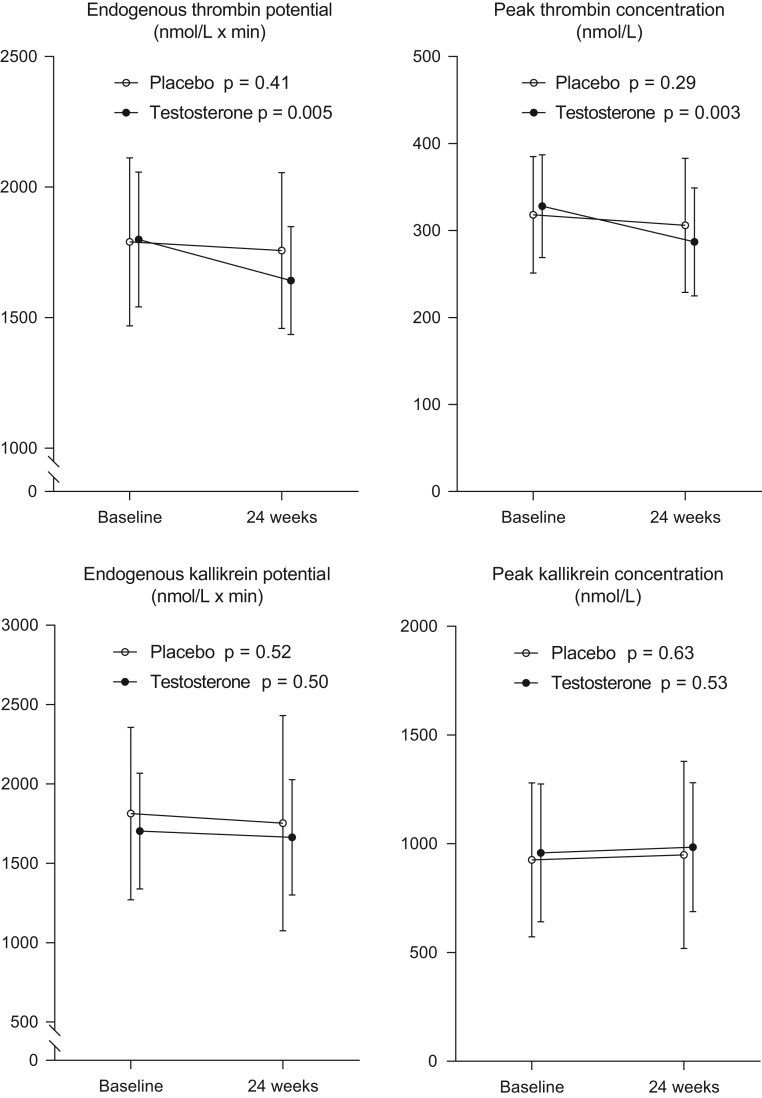
Figure 2.
Measures of generation of thrombin and kallikrein generation. Endogenous thrombin potential, peak thrombin concentration, endogenous kallikrein potential, and peak kallikrein concentration at baseline and after 24 weeks of testosterone replacement therapy or placebo. Results are presented as mean ± s.d.
In the TF pathway of coagulation, we observed between-group differences at 24 weeks for ETP (P = 0.036), FVII (P = 0.044), FX (P = 0.015), prothrombin (P = 0.003), protein C (P = 0.004), and protein S (P = 0.038). No between-group differences at 24 weeks were observed for peak thrombin concentration (P = 0.10), TFPI (P = 0.41), or antithrombin (P = 0.053).
Within the TRT group, ETP decreased (1642 ± 207 vs 1799 ± 258 nmol/L × min, P = 0.005) and peak thrombin concentration decreased (287 ± 62 vs 328 ± 59 nmol/L, P = 0.003) (Fig. 2). Within the placebo group, ETP and peak thrombin concentration were not significantly different (1757 ± 299 vs 1790 ± 322 nmol/L × min, P = 0.41 and 306 ± 77 vs 318 ± 67 nmol/L, P = 0.29, respectively) (Fig. 2). Furthermore, TRT was associated with significant decreases from baseline to 24 weeks in plasma levels of FVII, FX, and prothrombin and in the coagulation inhibitors TFPI and protein C. Free protein S increased significantly (Table 2).
The variables of the contact activation pathway (EKP (P = 0.92), peak kallikrein concentration (P = 0.96), FXII (P = 0.051), and C1inh (P = 0.64)) were comparable between TRT and placebo at 24 weeks. Within the TRT group, EKP and peak kallikrein concentration were not significantly different (1664 ± 364 vs 1703 ± 365 nmol/L × min, P = 0.50 and 984 ± 297 nmol/L vs 958 ± 317 nmol/L, P = 0.53, respectively). In the placebo group, no significant changes were found after 24 weeks in EKP (1753 ± 678 vs 1813 ± 543 nmol/L × min, P = 0.52) or in peak kallikrein concentration (949 ± 430 vs 926 ± 354 nmol/L, P = 0.63) (Fig. 2). We found significantly reduced concentrations of FXII and C1inh from baseline to 24 weeks in the TRT group but not in the placebo group (Table 2).
Discussion
This study represents the largest randomized, placebo-controlled study addressing the effect of physiological doses of testosterone on the coagulation system in men with hypogonadism. We present a potential anticoagulant effect of TRT by demonstrating decreased TF-induced thrombin generation, decreased activity of the vitamin K-dependent coagulation factors, and protein C and increased protein concentration of free protein S. TRT compared to placebo did not affect the contact system proteins FXII and C1inh and did not translate into changes in the kallikrein generation capacity of the contact system. Thus, our findings support that TRT reduces the coagulation potential of the TF pathway of coagulation.
The influence of TRT on measures of thrombin generation was addressed in a previous controlled study, where no changes in peak thrombin concentration or ETP were found after 52 weeks of testosterone undecanoate injections in 13 elderly men with low baseline testosterone concentrations (20). The modest study population, a mean age of 69 years, and the age-related baseline low testosterone levels may explain these discrepancies. The 37 men included in the present study had a mean age of 53 years and medication-induced decreased testosterone concentrations. Furthermore, the duration between the latest injection of testosterone and blood sampling was 12 weeks in the former study (20) and 6 weeks in the present study, indicating that the effect of TRT could decline in 12 weeks. This could point towards an acute and not persistent effect of testosterone on thrombin generation.
The thrombin generation method applied in the present study involves the TF pathway of coagulation encompassing FVII, FX, and prothrombin, and FX and prothrombin are here the major determinants of thrombin generation (35). Accordingly, we demonstrate that TRT is associated with a significant decrease in clotting activities of FX and prothrombin, resulting in a decrease in ETP. A study of transgender men receiving TRT demonstrated decreased prothrombin concentrations in line with our findings (26). The decrease in FVII and prothrombin induced by TRT is in accordance with previous findings in animal studies (27, 28, 29, 36, 37, 38). The influence of TRT on FX has not been addressed in other studies.
Studying the effect of TRT on the inhibitory components of the TF pathway, we demonstrate that TRT is associated with a decrease in TFPI and protein C and an increase in protein S and has no significant effect on antithrombin. However, only protein C and protein S were significantly different from the placebo group. Corresponding to our findings, previous studies report a reduction in protein C (23), an increase in free protein S (26), and no effect on TFPI and antithrombin (20, 22) after testosterone therapy.
The mechanism behind the decreased thrombin formation through the TF pathway in the testosterone-treated men is not elucidated in the present study. Speculatively, a direct effect of testosterone through the androgen receptor on liver protein synthesis is possible, as we demonstrate decreased levels of liver-derived coagulation factors and inhibitors among testosterone-treated men. In fact, only the functional level of the vitamin K-dependent coagulation factors and inhibitors were reduced by TRT compared with placebo, indicating an effect of testosterone supplementation on vitamin K metabolism. A previous animal study has suggested that androgens may affect the components of the vitamin K cycle, resulting in reduced synthesis of the vitamin K-dependent coagulation factors and inhibitors (29). Regarding free protein S, more than half of plasma protein S circulates in complex with C4b-binding protein, and studies have indicated that TRT reduces the concentration of this plasma protein (23). Thus, a TRT-induced altered balance between free and total protein S may increase concentrations of free protein S.
This is the first study to address the potential effect of TRT on the capacity of the contact activation pathway of coagulation. Although we observed a decrease in FXII, in line with previous findings (21), and in C1inh, the turnover of the contact activation pathway was not affected by TRT as no overall changes in the kallikrein generation capacity was observed, indicating an equivalent testosterone-induced reduction in the plasma concentrations of the two proteins.
The clinical impact of the observed effects on thrombin generation remains to be elucidated. Recently, increased measures of thrombin generation were associated with a procoagulant state and increased risk of deep venous thrombosis, thrombosis recurrence, as well as acute ischemic stroke (39). Hence, our documented reduction in ETP could indicate an antithrombotic effect of testosterone. TRT may affect various organs and processes, and TRT has a positive impact on body composition with reduced fat mass and fat percentage and increased lean body mass (8, 9, 10), as confirmed in this study population (Table 1). Excessive erythrocytosis (40) and elevated hematocrit values, however, occur in up to 40% of patients receiving androgen treatment (41), leading to increased blood viscosity (42), which potentially augments blood-flow resistance and hence causes a prothrombotic effect. It is tempting to speculate that the presently observed decrease in thrombin generation potential and the beneficial effects on body composition counterbalance the prothrombotic increase in blood viscosity induced by TRT.
The strength of the present study is the randomized, double-blinded, and placebo-controlled design. The study is considered a pilot study as the power calculation of the original study was based on lean body mass; hence, the present study might be underpowered to study other outcomes. The relatively low number of study participants increases the risk of type II errors. Thus, we cannot exclude an effect of TRT on all liver-derived coagulation proteins and not only the vitamin K-dependent proteins. We did not perform an ITT analysis, but only three study participants were lost to follow-up, and therefore we do not expect a significant difference between ITT and per-protocol analyses. The study participants had relative hypogonadism with testosterone concentrations < 12 nmol/L; hence the observed effect on thrombin generation is expected to be greater in men with absolute hypogonadism.
In conclusion, TRT has a suppressive effect on the TF pathway of coagulation as we demonstrate a significant reduction in ETP and in the functional levels of coagulation factors and inhibitors and an increase in free protein S in men with opioid-induced decreased testosterone concentrations.
Declaration of interest
None declared.
Funding
The Karola Jørgensens Forskningsfond provided financial grant for analysis of data. Bayer provided Nebido® and placebo and the Novo Nordisk Foundation provided financial support, but they were otherwise not involved in the study planning or interpretation of results. The authors would also like to thank the Institute of Clinical Research – University of Southern Denmark for support.
Authors contribution statement
The authors’ contributions to the present study were as follows: DG, MSA, JBG, and JJS: conceptualization and methodology; MB, EMB, and JJS: data analysis and data interpretation; MB: drafting of the manuscript. All authors were involved in the design of the study, critical revision of the manuscript, and approval of the final manuscript.
Acknowledgements
The authors thank the technical staff, Anette Larsen, Kathrine Overgaard, and Lars Nielsen for plasma analysis.
References
- 1.De Sola H, Dueñas M, Salazar A, Ortega-Jiménez P, Failde I. Prevalence of therapeutic use of opioids in chronic non-cancer pain patients and associated factors: a systematic review and meta-analysis. Frontiers in Pharmacology 202011 564412 ( 10.3389/fphar.2020.564412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali K, Raphael J, Khan S, Labib M, Duarte R. The effects of opioids on the endocrine system: an overview. Postgraduate Medical Journal 201692677–681 ( 10.1136/postgradmedj-2016-134299) [DOI] [PubMed] [Google Scholar]
- 3.Coluzzi F, Billeci D, Maggi M, Corona G. Testosterone deficiency in non-cancer opioid-treated patients. Journal of Endocrinological Investigation 2018411377–1388 ( 10.1007/s40618-018-0964-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. Journal of Sexual Medicine 2018151260–1271. ( 10.1016/j.jsxm.2018.06.012) [DOI] [PubMed] [Google Scholar]
- 5.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. European Journal of Endocrinology 2013169725–733. ( 10.1530/EJE-13-0321) [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Medicine 201311 108. ( 10.1186/1741-7015-11-108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glintborg D, Vaegter HB, Christensen LL, Bendix E, Graven-Nielsen T, Andersen PG, Andersen M. Testosterone replacement therapy of opioid-induced male hypogonadism improved body composition but not pain perception: a double-blind, randomized, and placebo-controlled trial. European Journal of Endocrinology 2020182539–548. ( 10.1530/EJE-19-0979) [DOI] [PubMed] [Google Scholar]
- 8.Frederiksen L, Højlund K, Hougaard DM, Brixen K, Andersen M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age 201234145–156. ( 10.1007/s11357-011-9213-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnussen LV, Glintborg D, Hermann P, Hougaard DM, Højlund K, Andersen M. Effect of testosterone on insulin sensitivity, oxidative metabolism and body composition in aging men with type 2 diabetes on metformin monotherapy. Diabetes, Obesity and Metabolism 201618980–989. ( 10.1111/dom.12701) [DOI] [PubMed] [Google Scholar]
- 10.Magnussen LV, Andersen PE, Diaz A, Ostojic J, Højlund K, Hougaard DM, Christensen AN, Nielsen TL, Andersen M. MR spectroscopy of hepatic fat and adiponectin and leptin levels during testosterone therapy in type 2 diabetes: a randomized, double-blinded, placebo-controlled trial. European Journal of Endocrinology 2017177157–168. ( 10.1530/EJE-17-0071) [DOI] [PubMed] [Google Scholar]
- 11.Frederiksen L, Højlund K, Hougaard DM, Mosbech TH, Larsen R, Flyvbjerg A, Frystyk J, Brixen K, Andersen M. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. European Journal of Endocrinology 2012166469–476. ( 10.1530/EJE-11-0565) [DOI] [PubMed] [Google Scholar]
- 12.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang Aet al. Adverse events associated with testosterone administration. New England Journal of Medicine 2010363109–122. ( 10.1056/NEJMoa1000485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF, Hoover RN. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One 20149 e85805. ( 10.1371/journal.pone.0085805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clinical Endocrinology 201685436–443. ( 10.1111/cen.13084) [DOI] [PubMed] [Google Scholar]
- 15.Walker RF, Zakai NA, MacLehose RF, Cowan LT, Adam TJ, Alonso A, Lutsey PL. Association of testosterone therapy with risk of venous thromboembolism among men with and without hypogonadism. JAMA Internal Medicine 2020180190–197. ( 10.1001/jamainternmed.2019.5135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander GC, Iyer G, Lucas E, Lin D, Singh S. Cardiovascular risks of exogenous testosterone use among men: a systematic review and meta-analysis. American Journal of Medicine 2017130293–305. ( 10.1016/j.amjmed.2016.09.017) [DOI] [PubMed] [Google Scholar]
- 17.Elliott J, Kelly SE, Millar AC, Peterson J, Chen L, Johnston A, Kotb A, Skidmore B, Bai Z, Mamdani Met al. Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open 20177 e015284. ( 10.1136/bmjopen-2016-015284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houghton DE, Alsawas M, Barrioneuvo P, Tello M, Farah W, Beuschel B, Prokop LJ, Layton JB, Murad MH, Moll S. Testosterone therapy and venous thromboembolism: a systematic review and meta-analysis. Thrombosis Research 201817294–103. ( 10.1016/j.thromres.2018.10.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson J, Cruickshank M, Quinton R, Aucott L, Aceves-Martins M, Gillies K, Bhasin S, Snyder PJ, Ellenberg SS, Grossmann Met al. Adverse cardiovascular events and mortality in men during testosterone treatment: an individual patient and aggregate data meta-analysis. Lancet. Healthy Longevity 20223e381–e393. ( 10.1016/S2666-7568(2200096-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agledahl I, Brodin E, Svartberg J, Hansen JB. Impact of long-term testosterone treatment on plasma levels of free TFPI and TF-induced thrombin generation ex vivo in elderly men with low testosterone levels. Thrombosis and Haemostasis 2009102945–950. ( 10.1160/TH09-02-0090) [DOI] [PubMed] [Google Scholar]
- 21.Zitzmann M, Junker R, Kamischke A, Nieschlag E. Contraceptive steroids influence the hemostatic activation state in healthy men. Journal of Andrology 200223503–511. [PubMed] [Google Scholar]
- 22.Chang S, Biltoft D, Skakkebæk A, Fedder J, Bojesen A, Bor MV, Gravholt CH, Münster AB. Testosterone treatment and association with thrombin generation and coagulation inhibition in Klinefelter syndrome: a cross-sectional study. Thrombosis Research 2019182175–181. ( 10.1016/j.thromres.2019.08.011) [DOI] [PubMed] [Google Scholar]
- 23.Anderson RA, Ludlam CA, Wu FC. Haemostatic effects of supraphysiological levels of testosterone in normal men. Thrombosis and Haemostasis 199574693–697. ( 10.1055/s-0038-1649799) [DOI] [PubMed] [Google Scholar]
- 24.Chang S, Rasmussen JJ, Frandsen MN, Schou M, Johansen ML, Faber J, Münster AB, Sidelmann JJ, Kistorp C. Procoagulant State in current and former anabolic androgenic steroid abusers. Thrombosis and Haemostasis 2018118647–653. ( 10.1055/s-0038-1636540) [DOI] [PubMed] [Google Scholar]
- 25.Sidelmann JJ, Gram JB, Palarasah Y, Rasmussen JJ, Kistorp C. Effect of anabolic-androgenic steroid abuse on the contact activation system. Thrombosis and Haemostasis 20211211268–1273. ( 10.1055/a-1346-3384) [DOI] [PubMed] [Google Scholar]
- 26.Scheres LJJ, Selier NLD, Nota NM, van Diemen JJK, Cannegieter SC, den Heijer M. Effect of gender-affirming hormone use on coagulation profiles in transmen and transwomen. Journal of Thrombosis and Haemostasis 2021191029–1037. ( 10.1111/jth.15256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alqahtani SA, Alhawiti NM. Administration of testosterone improves the prothrombotic and antifibrinolytic parameters associated with its deficiency in an orchidectiomized rat model. Platelets 201930624–630. ( 10.1080/09537104.2018.1499886) [DOI] [PubMed] [Google Scholar]
- 28.Matschiner JT, Willingham AK. Influence of sex hormones on vitamin K deficiency and epoxidation of vitamin K in the rat. Journal of Nutrition 1974104660–665. ( 10.1093/jn/104.6.660) [DOI] [PubMed] [Google Scholar]
- 29.Uchida K, Shike T, Kakushi H, Takase H, Nomura Y, Harauchi T, Yoshizaki T. Effect of sex hormones on hypoprothrombinemia induced by N-methyltetrazolethiol in rats. Thrombosis Research 198539741–750. ( 10.1016/0049-3848(8590258-0) [DOI] [PubMed] [Google Scholar]
- 30.CLSI. GP41 Collection of Diagnostic Venous Blood Specimens, 7th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA,2017. (available at: https://clsi.org/standards/products/general-laboratory/documents/gp41/) [Google Scholar]
- 31.CLSI. H21-A5 Collection, Transport, and Processing of Blood Specimens for Testing Plasma-Based Coagulation Assays and Molecular Hemostasis Assays; Approved Guideline, 5thed.Clinical and Laboratory Standards Institute: Wayne, PA, USA,2008. (available at: https://clsi.org/standards/products/hematology/documents/h21/) [Google Scholar]
- 32.Hemker HC, Al Dieri R, De Smedt E, Béguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thrombosis and Haemostasis 200696553–561. ( 10.1160/TH06-07-0408) [DOI] [PubMed] [Google Scholar]
- 33.Biltoft D, Sidelmann JJ, Olsen LF, Palarasah Y, Gram J. Calibrated kallikrein generation in human plasma. Clinical Biochemistry 2016491188–1194. ( 10.1016/j.clinbiochem.2016.06.011) [DOI] [PubMed] [Google Scholar]
- 34.Madsen DE, Sidelmann JJ, Overgaard K, Koch C, Gram JB. ELISA for determination of total coagulation factor XII concentration in human plasma. Journal of Immunological Methods 201339432–39. ( 10.1016/j.jim.2013.04.012) [DOI] [PubMed] [Google Scholar]
- 35.Duchemin J, Pan-Petesch B, Arnaud B, Blouch MT, Abgrall JF. Influence of coagulation factors and tissue factor concentration on the thrombin generation test in plasma. Thrombosis and Haemostasis 200899767–773. ( 10.1160/TH07-09-0581) [DOI] [PubMed] [Google Scholar]
- 36.Alhawiti NM, Alqahtani SA. Chronic testosterone administration improves cardiac contractility and has a beneficial effect on the haemostatic system by enhancing fibrinolytic activity and inducing hypocoagulation in healthy rats. Archives of Physiology and Biochemistry 2019125311–320. ( 10.1080/13813455.2018.1458244) [DOI] [PubMed] [Google Scholar]
- 37.Raheja KL, Reber EF, Anderson DL, Bert MH. Effects of testosterone propionate and dicumarol on plasma prothrombin rate and serum cholesterol level in capons and orchiectomized rats. General and Comparative Endocrinology 197116385–390. ( 10.1016/0016-6480(7190051-7) [DOI] [PubMed] [Google Scholar]
- 38.Nishino Y.. Hormonal control of prothrombin synthesis in rat liver microsomes, with special reference to the role of estradiol, testosterone and prolactin. In Archives of Toxicology: Supplement 2 pp 397–402. Eds Chambers PL, Günzel P. Berlin, Heidelberg, Germany: Springer, 1979. ( 10.1007/978-3-642-67265-1_45) [DOI] [PubMed] [Google Scholar]
- 39.Binder NB, Depasse F, Mueller J, Wissel T, Schwers S, Germer M, Hermes B, Turecek PL. Clinical use of thrombin generation assays. Journal of Thrombosis and Haemostasis 2021192918–2929. ( 10.1111/jth.15538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 20181031715–1744. ( 10.1210/jc.2018-00229) [DOI] [PubMed] [Google Scholar]
- 41.Abildgaard J, Petersen JH, Bang AK, Aksglaede L, Christiansen P, Juul A, Jørgensen N. Long-term testosterone undecanoate treatment in the elderly testosterone deficient male: an observational cohort study. Andrology 202210322–332. ( 10.1111/andr.13124) [DOI] [PubMed] [Google Scholar]
- 42.Byrnes JR, Wolberg AS. Red blood cells in thrombosis. Blood 20171301795–1799. ( 10.1182/blood-2017-03-745349) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a