Abstract
Objective
Thyroid nodule ultrasound characteristics are used as an indication for fine-needle aspiration cytology, usually as the basis for Thyroid Imaging Reporting and Data System (TIRADS) score calculation. Few studies on interobserver variation are available, all of which are based on analysis of preselected still ultrasound images and often lack surgical confirmation.
Methods
After the blinded online evaluation of video recordings of the ultrasound examinations of 47 consecutive malignant and 76 consecutive benign thyroid lesions, 7 experts from 7 thyroid centers answered 17 TIRADS-related questions. Surgical histology was the reference standard. Interobserver variations of each ultrasound characteristic were compared using Gwet’s AC1 inter-rater coefficients; higher values mean better concordance, the maximum being 1.0.
Results
On a scale from 0.0 to 1.0, the Gwet’s AC1 values were 0.34, 0.53, 0.72, and 0.79 for the four most important features in decision-making, i.e. irregular margins, microcalcifications, echogenicity, and extrathyroidal extension, respectively. The concordance in the discrimination between mildly/moderately and very hypoechogenic nodules was 0.17. The smaller the nodule size the better the agreement in echogenicity, and the larger the nodule size the better the agreement on the presence of microcalcifications. Extrathyroidal extension was correctly identified in just 45.8% of the cases.
Conclusions
Examination of video recordings, closely simulating the real-world situation, revealed substantial interobserver variation in the interpretation of each of the four most important ultrasound characteristics. In view of the importance for the management of thyroid nodules, unambiguous and widely accepted definitions of each nodule characteristic are warranted, although it remains to be investigated whether this diminishes observer variation.
Keywords: thyroid nodule, cytology, thyroid cancer, TIRADS, ultrasound
Introduction
For more than three decades, the cornerstones in the clinical management of patients with thyroid nodules have been ultrasound (US) and fine-needle aspiration cytology (FNA) (1, 2, 3). Robust evidence demonstrates that the risk of malignancy (primarily papillary cancer) in thyroid lesions is significantly correlated to the presence of specific US features, which include hypoechogenicity, microcalcifications, taller than wide shape, irregular margins, and extrathyroidal extension (ETE) (4, 5, 6). Several US thyroid nodule risk-classification systems have been proposed by scientific societies (6, 7, 8, 9, 10, 11). These thyroid nodule image reporting and data systems (TIRADS) aim at providing indications for FNA, based on the combined results of the TIRADS malignancy risk scores and nodule size. All of these scoring systems include at least four out of the five suspicious characteristics but are clearly not congruent (12); they handle different microcalcifications, taller-than-wide and taller-than-long shape, and do not define the extent of irregularities which is required to consider a nodule border irregular.
Judgment of the US characteristics of a thyroid nodule can vary widely by the observer (13). The ranges regarding the reported frequency of suspicious characteristics in thyroid cancers vary: 20–100% for hypoechogenicity (4, 6, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23), 14–41% for very hypoechoic nodules (6, 19, 20), 13–56% for microcalcifications (14, 18, 19, 20, 21, 22, 23), and 13–48% for irregular margins (6, 14, 19, 20, 21). Such between-study differences in the prevalence of US characteristics may be explained by differences in (i) the ratio of benign and malignant cases in the study cohort, (ii) the prevalence of follicular or medullary cancer in the given series, due to the ambiguous US features that may be presented by these less frequent types of cancer, (iii) histopathological confirmation and interpretation, (iv) iodine intake, (v) a bias toward smaller and therefore easier to analyze lesions, (vi) the US equipment used, or (vii) image interpretation.
The aim of the current study was to analyze video recordings, rather than still images of histologically verified lesions, in order to determine the inter- and intra-observer variations of nodule characteristics in predicting thyroid cancer. To this end, seven highly experienced investigators from as many centers participated in the analysis. Instead of the traditional kappa-values and percentage agreement, the more novel statistical analyses, Gwet’s agreement coefficient (Gwet’s AC1) was applied (24).
Materials and methods
Patients and video records
Between January 2014 and December 2016, the US examinations of 16,407 consecutive patients were video-recorded using a pre-specified protocol (see Supplementary text, see section on supplementary materials given at the end of this article) and archived at the Thyroid Clinic of the Bugat Pal Hospital (Gyöngyös, Hungary) as part of the institutional routine record keeping. A Philips CX 50 US machine equipped with a 12-5 MHz linear transducer was used for thyroid US. Statistical power calculations have shown that a minimum of 102 cases, including at least 39 malignant cases, were required. We added 20% to the calculated number to ensure ample power. In total, 709 cases had surgery for nodular goiter. From this chronological list of patients, the starting point was chosen at random, and the US video records of 47 and 76 patients with malignant and benign final histology, respectively, were used. The indication for surgery was based on cytology in 79 patients (Bethesda IV in 19 patients, Bethesda V in 32 patients, and Bethesda VI in 28 patients), symptoms and/or signs of compression caused by the goiter in 35 cases, an autonomously functioning nodule causing hyperthyroidism in 5 patients, and patients’ wish in 4 cases. Final diagnoses were, in all cases, obtained by histological examination of the surgical samples. Relevant patient data appear in Table 1.
Table 1.
Postoperative thyroid nodule histology and tumor stage.
| Histology | n | Tumor statusa | Tumor stagea | Male/female | Mean age (range) |
|---|---|---|---|---|---|
| Benign | 76 | n/a | n/a | 18/58 | 51.1 (24–75) |
| No nodule | 4 | n/a | n/a | 0/4 | 49.3 (38–60) |
| Hyperplastic nodule | 43 | n/a | n/a | 10/33 | 54.8 (30–75) |
| Adenoma | 29 | n/a | n/a | 8/21 | 45.9 (24–67) |
| Malignant | 47 | T1 n = 25 T2 n = 8 T3 n = 2 T4 n = 11 n.a. = 1 |
13/34 | 44.6 (18–89) | |
| Papillary carcinoma | 37 | T1 n = 22 T2 n = 7 T3 n = 1 T4 n = 7 |
Stage 1 n = 33 Stage 2 n = 2 Stage 3 n = 1 Stage 4 n = 1 |
11/26 | 42.5 (18–67) |
| Follicular carcinoma | 3 | T1 n = 2 T3 n = 1 |
Stage 1 n = 3 | 0/3 | 51 (21–53) |
| Poorly differentiated cancer | 1 | T1 n = 1 | Stage 1 n = 1 | 0/1 | 42 |
| Anaplastic carcinoma | 2 | T4 n = 2 | Stage 4 n = 2 | 2/0 | 69.5 (57–82) |
| Medullary carcinoma | 2 | T2 n = 1 T4 n = 1 |
Stage 2I n = 1 Stage 4V n = 1 |
0/2 | 65.5 (32–89) |
| B-cell lymphoma | 1 | n/a | Stage 2 n = 1 | 0/1 | 49 |
| Parathyroid carcinoma | 1 | T4 4 n = 1 | Stage 4 n = 1 | 0/1 | 54 |
| Total | 123 |
aTNM classification of malignant tumors (50).
n/a, not applicable.
Representative parts of each US video recording were presented to seven investigators (see later), who were blinded to the outcome data. The video recordings are available for the reader (http://thyrosite.com/case_studies/section08/consecutively_operated.php).
Written consent has been obtained from each patient after a full explanation of the purpose and nature of all procedures used. The study was approved by the Research Ethics Committee of Bugat Hospital, Gyöngyös, Hungary.
Evaluation phase
The expert evaluations were performed online using a website developed for this purpose. Seven investigators, from different thyroid centers in four European countries, with at least 15 years of experience in thyroid US (SB, AF, LJ, GK, EP, KR, and GR) analyzed the US video recordings of the 123 histologically verified thyroid lesions. The investigators were aware that all lesions had been surgically removed but blinded to the final histopathology and the benign-to-malignant ratio of the series of nodules under examination (25). In order to reproduce a setting similar to the real world, a short summary of the pre-US clinical data, including thyroid hormone and antibody levels, was provided to the investigators.
During the training phase, 1 month before the study started, ten nodules from ten patients (not included in the study of the 123 cases) were analyzed by the seven investigators. The aim was for the investigators to become acquainted with the study methodology and resolve any questions before the launch of the study case series. The steering committee (LH, TS, and EVN) resolved any issue raised by the US investigators. No further communication among investigators or with the steering committee was allowed.
The 123 cases were presented one by one, in random order, to each investigator. The transducer orientation above the upper, middle, or lower as well as the medial or lateral lobe region was indicated. A still image of the whole gland was also included and the position of the nodule to be studied was shown. The videos (median duration 43 s, range 20 to 73 s) allowed slow-motion assessment, repeat evaluation, and image-freezing, without time constraints. After the analysis of each video, investigators answered the questions in the electronic Case Report Form (CRF). The questions pertained to various US features, including four widely accepted suspicious US characteristics in relation to nodule echogenicity, microcalcifications, irregular borders, and ETE (Supplementary Table 1). To simulate a real-life evaluation, investigators could not modify their answers or re-review the video recordings once the ‘patient completed’ button had been activated. Four weeks were allowed for the completion of the 123 cases.
Eight weeks after completing the evaluation of the 123 nodules (first run), the investigators were requested to repeat the full analysis (second run), which all accepted. The video recordings were presented in a revised computer-generated random order, different from the first run.
Statistical analysis
We used Gwet’s Agreement Coefficient (AC1) values to analyze inter- and intra-observer concordance and reliability (24). For comparability with other studies, we also calculated the traditional (Cohen’s and Fleiss’) Kappa values. However, Kappa counting provides some ‘meaningless’ values (26). Others also found that interrater variability is more accurately described by percentage agreement than Kappa, if raters are well trained and little guessing is likely to exist during the evaluation process (27). Therefore, Gwet’s AC1 was considered to be the appropriate statistical method for agreement studies. In contrast to Kappa, the Gwet’s AC1 provides a more realistic estimate for the chance effect, is more stable against marginal probabilities, and can handle ordinal scales well (28). We used the following categories for the description of the degree of agreement: poor, fair, moderate, good, and very good, corresponding to Gwet’s AC1 values ≤0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1.00, respectively (29).
For testing the influence of nodule size (large, middle, or small) on the agreement among investigators, tertiles of each of the histologically proven entities of (i) hyperplastic nodules, (ii) adenomas, and (iii) papillary carcinomas, each containing one-third of the respective pathology, were created. The answers by the investigators to the following five questions were analyzed separately in each size group: presence of microcalcifications; irregular margins; ETE; iso-, hyper-, or hypoechogenic appearance; and if hypoechogenic whether minimally, moderately, or very hypoechogenic. Chi-square tests were used for comparisons.
While testing the suspicious characteristics for predicting malignancy, the sum of yes and no answers was compared to the final histopathology. Sensitivities, specificities, and the 95% confidence intervals (95% CI) were calculated by the package ‘epiR’ in R version 1.0–2 (30).
Results
Microcalcifications and punctate echogenic foci
Moderate (AC1 = 0.53) and fair (AC1 = 0.39) interobserver agreements were found for microcalcifications (CRF question 5) and for punctate echogenic foci (CRF question 4), respectively (Table 2). The percentage of nodules in which punctate echogenic foci were deemed to be present by the investigators ranged from 39.4 to 93.5%, while the range for unequivocal microcalcifications was 9.3 to 50.4% (Table 3).
Table 2.
The concordance between the seven investigators in the judgment of ultrasound characteristics, listed according to Gwet’s AC1, starting with the lowest concordance. The numbers in the characteristic/property/feature column identify the corresponding question answered by the investigators for each nodule. For comparison purposes, kappa is also shown as earlier studies used kappa values.
| Characteristic/property/feature | No. of nodules analyzed | Interobserver mean (95% CI) | Intraobserver mean (95% CI) | ||
|---|---|---|---|---|---|
| Gwet’s AC1 value | Fleiss kappa | Gwet’s AC1 value | Cohen’s kappa | ||
| 6. Uncertain hyperechogenic spots | 123 | 0.12 (0.05–0.19) | 0.05 (0.00–0.10) | 0.48 (0.42–0.54) | 0.48 (0.42–0.53) |
| 13/B. Mild/moderately vs very hypoechogenic nodulea | 39 | 0.17 (0.06–0.27) | 0.07 (−0.03–0.16) | 0.63 (0.56–0.70) | 0.57 (0.49–0.64) |
| 12/B. Does a partially cystic nodule have an eccentric solid part?b | 9 | 0.28 (−0.16–0.72) | 0.26 (−0.09–0.60) | 0.78 (0.70–0.86) | 0.71 (0.61–0.81) |
| 14. Irregular margins | 123 | 0.34 (0.26–0.42) | 0.18 (0.14–0.24) | 0.62 (0.57–0.67) | 0.51 (0.47–0.56) |
| 4. Punctate echogenic foci | 123 | 0.39 (0.29–0.49) | 0.27 (0.21–0.33) | 0.68 (0.63–0.72) | 0.52 (0.48–0.57) |
| 3. Back wall cystic figures | 123 | 0.48 (0.38–0.57) | 0.11 (0.07–0.16) | 0.74 (0.70–0.78) | 0.49 (0.43–0.55) |
| 5. Microcalcification | 123 | 0.53 (0.43–0.63) | 0.29 (0.22–0.36) | 0.73 (0.69–0.78) | 0.59 (0.53–0.65) |
| 2. Comet-tail artifact | 123 | 0.62 (0.53–0.70) | 0.23 (0.15–0.30) | 0.78 (0.74–0.81) | 0.48 (0.42–0.54) |
| 12/A Is a nodule partially cystic? | 123 | 0.63 (0.54–0.72) | 0.50 (0.41–0.59) | 0.77 (0.73–0.81) | 0.71 (0.66–0.76) |
| 13. Echogenicity of a nodule | 123 | 0.72 (0.68–0.76) | 0.24 (0.19–0.29) | 0.81 (0.79–0.84) | 0.53 (0.49–0.58) |
| 13/A hyper/isoechogenic vs hypoechogenic nodulec | 74 | 0.73 (0.63–0.83) | 0.43 (0.29–0.57) | 0.79 (0.75–0.83) | 0.67 (0.61–0.74) |
| 12. Partially cystic nodule | 123 | 0.79 (0.73–0.85) | 0.40 (0.34–0.48) | 0.86 (0.83–0.88) | 0.67 (0.62–0.72) |
| 15. Extrathyroidal extension | 123 | 0.79 (0.73–0.85) | 0.28 (0.15–0.41) | 0.87 (0.85–0.90) | 0.56 (0.48–0.64) |
| 7. Coarse calcification | 123 | 0.80 (0.73–0.87) | 0.46 (0.34–0.57) | 0.87 (0.85–0.90) | 0.65 (0.59–0.71) |
| 11. Solid vs cystic nodule | 123 | 0.84 (0.79–0.89) | 0.50 (0.44–0.58) | 0.90 (0.87–0.92) | 0.66 (0.61–0.70) |
| 8. Central intranodular coarse calcification | 123 | 0.86 (0.81–0.91) | 0.40 (0.28–0.52) | 0.92 (0.89–0.94) | 0.62 (0.55–0.69) |
| 10. Peripheral (rim) calcification | 123 | 0.92 (0.89–0.95) | 0.21 (0.13–0.29) | 0.95 (0.94–0.96) | 0.45 (0.38–0.52) |
| 1. Nodule or not nodule | 123 | 0.94 (0.91–0.97) | 0.12 (0.02–0.22) | 0.97 (0.96–0.98) | 0.62 (0.47–0.77) |
| 9. Isolated macrocalcification occupying the entire nodule | 123 | 0.98 (0.97–0.99) | −0.01 (−0.02–0.00) | 0.98 (0.97–0.99) | 0.26 (0.09–0.43) |
aCalculation 13/B was performed for responses to Question 13 which found any degree of hypoechogenicity; bCalculation 12/B was performed for ‘yes’ responses to Question 12; cCalculation 13/A was performed using the respective responses to Question 13.
Table 3.
The percentage of the nodules examined (n = 123) in which the seven investigators deemed the given characteristic to be present (all values are given in %; mean, s.d., minimum, and maximum of the individual % values of the seven investigators).
| Feature | Answer | Mean | s.d. | Minimum | Maximum |
|---|---|---|---|---|---|
| Punctate echogenic foci | |||||
| Present | 53 | 19.1 | 39.4 | 93.5 | |
| Probably present | 33.3 | 14.3 | 6.1 | 48.0 | |
| Probably absent | 8.7 | 6.5 | 0.4 | 18.3 | |
| Absent | 5.1 | 4.6 | 0 | 13.8 | |
| Microcalcification | |||||
| Present | 27.1 | 14.4 | 9.3 | 50.4 | |
| Absent | 72.9 | 14.4 | 49.6 | 90.7 | |
| Extrathyroidal extension | |||||
| Present | 12.8 | 5.9 | 4.1 | 19.9 | |
| Absent | 87.2 | 5.9 | 80.1 | 95.9 | |
| Margins | |||||
| Smooth | 45.2 | 16.9 | 22 | 63.4 | |
| Ill-defined | 26.1 | 11.2 | 7.7 | 39.8 | |
| Irregular | 20.8 | 8.9 | 11.4 | 34.1 | |
| Cannot be determined | 7.8 | 9.1 | 0 | 18.3 | |
| Echogenicity | |||||
| Iso-/hyperechogenic | 24.2 | 12.9 | 8.1 | 41.1 | |
| Mildly/moderately hypoechogenic | 43.7 | 12.8 | 28.5 | 60.2 | |
| Very hypoechogenic | 21.5 | 11.6 | 7.7 | 37.4 | |
| Anechoic | 2.7 | 1.5 | 0.8 | 5.3 | |
| Cannot be determined | 7.8 | 11.1 | 0 | 28.0 | |
| Echogenicity (iso-/hyperechogenic and hypoechogenic nodules only) | |||||
| Iso-/hyperechogenic | 26.9 | 13.3 | 8.2 | 41.9 | |
| Hypoechogenic | 73.1 | 13.3 | 58.1 | 91.8 | |
| Comet-tail artifact | |||||
| Present | 18.0 | 7.4 | 7.3 | 27.2 | |
| Absent | 72.6 | 13.7 | 46.7 | 84.1 | |
| Uncertain | 9.3 | 9.7 | 0 | 27.2 | |
| Back-wall cystic figure | |||||
| Present | 21.1 | 10.9 | 3.7 | 35.8 | |
| Absent | 70.4 | 12.6 | 51.6 | 90.7 | |
| Uncertain | 8.5 | 8.0 | 0 | 24.0 | |
| Macrocalcification | |||||
| Present | 17.8 | 7.0 | 11.4 | 27.2 | |
| Absent | 79.6 | 6.5 | 70.7 | 87.8 | |
| Uncertain | 2.7 | 2.1 | 0 | 6.5 | |
| Is the lesion a nodule? | |||||
| Yes | 96.5 | 4.0 | 87.8 | 99.6 | |
| No | 3.5 | 4.0 | 0.4 | 12.2 | |
Echogenicity of the nodule
Good (AC1 = 0.72) and very good (AC1 = 0.81) interobserver and intraobserver agreements, respectively, were found for overall general. Both the interobserver (AC1 = 0.73) and intraobserver agreements (AC1 = 0.67) proved to be good in the distinction between iso/hyperechogenic vs hypoechogenic nodules. On the other hand, the agreements were poor (AC1 = 0.17) and good (AC1 = 0.63) for the interobserver and intraobserver variation, respectively, in the differentiation between minimally/moderately and very hypoechogenic nodules (CRF question 13) (Table 2).
The percentage of nodules which were deemed to be iso/hyperechogenic by the investigators ranged from 8.1 to 41.1%. For the level of hypoechogenicity, 28.5 to 60.2% and 7.7 to 37.4% of the nodules were found to be mildly/moderately or very hypoechogenic, respectively.
Margins of the nodule
Fair (AC1 = 0.34) and good (AC1 = 0.62) agreements were found for interobserver and intraobserver variations, respectively (CRF question 14) (Table 2). The percentage of nodules in which irregular margins were deemed to be present by the investigators ranged from 11.4 to 34.1% (Table 3).
Extrathyroidal extension
Regarding the presence of ETE, good (AC1 = 0.79) and very good (AC1 = 0.87) agreements were found for interobserver and intraobserver variation, respectively (CRF question 15) (Table 2). The percentage of nodules in which the investigators deemed ETE to be present ranged from 4.1 to 19.9% (Table 3).
A biological standard, namely pathology, exists for this US characteristic. Thus, we compared pathology results with US findings. ETE was correctly identified by US in only 45.8% of the cases. We analyzed the sensitivity of detecting ETE in relation to nodule size tertile; the values for small nodules (maximal diameter <17 mm), middle-size nodules (diameter between 17 and 29 mm), and large nodules (maximal diameter ≥30 mm) were 78.6, 44.6, and 31.7%, respectively ( = 0.0001).
The presence/absence of a nodule: was there a nodule at all?
This is another characteristic for which pathology defines the biological standard. The investigators provided a correct answer in 4 of 28 (14.3%) cases in which no nodule was found on histopathology. However, both the interobserver (AC1 = 0.94) and intraobserver agreements proved to be very good (AC1 = 0.97) for the presence/absence of a nodule (CRF question 1) (Table 2).
Other characteristics
The interobserver agreement was good (AC1 = 0.80) in various subtypes of calcifications (CRF question 7 to 10) and in the composition of nodules (CRF question 11). The agreement was good (AC1 = 0.62) and moderate (AC1 = 0.48) in the judgment of comet-tail artifacts (CRF question 2) and back wall cystic figures (CRF question 3), respectively.
The influence of the size of the nodule on the interobserver agreement
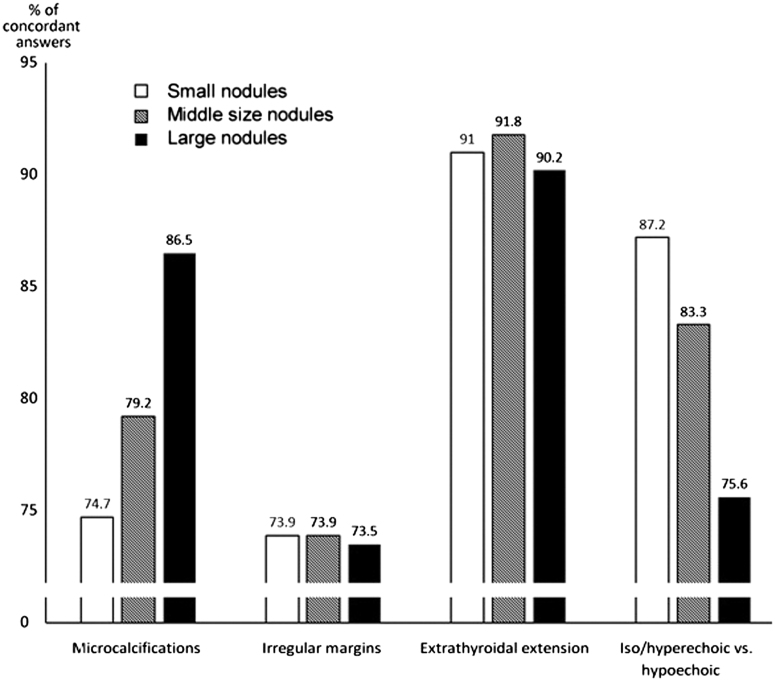
For microcalcifications, the larger the nodule size the better the agreement (P = 0.004, chi-square = 11.0). When differentiating between iso/hyperechogenicity and hypoechogenicity, agreement was better for the small nodules (P = 0.005, chi-square = 10.5). In contrast, size did not influence interobserver agreement in the discrimination between minimally/moderately and very hypoechogenic nodules, irregular margins, or ETE (Fig. 1).
Figure 1.
The influence of nodule size on interobserver agreement. Nodules are grouped according to nodule size tertiles (small nodules with a maximal diameter <17 mm, middle-size nodules 17–29 mm, and large nodules ≥30 mm).
The diagnostic value of suspicious characteristics in predicting thyroid cancer
The 47 malignant and 76 benign cases resulted in 329 and 532 answers, respectively, from the seven investigators. The diagnostic sensitivity of microcalcifications in predicting malignancy was 42.2% (139 out of 329 answers), while the specificity was 82.9% (441 out of 532 answers). For ‘very hypoechogenic’ echogenicity and all degrees of hypoechogenicity, respectively, the diagnostic sensitivity in predicting malignancy was 37.4% (123 out of 329 answers) and 80.2% (264/329), while the specificity was 87.8% (467 out of 532 answers) and 46.6% (248 out of 532 answers), respectively. The diagnostic sensitivity of irregular margins in predicting malignancy proved to be 37.4% (123 out of 329 answers), while the specificity was 88.3% (470 out of 532 answers).
Eight cases showed ETE, while 115 cases did not, which resulted in 56 (ETE present) and 805 (ETE absent) answers from the seven investigators. The diagnostic sensitivity of ETE in predicting malignancy was 26.8% (15 out of 56 answers), while the specificity was 95.9% (772 out of 805 answers) (Table 4).
Table 4.
The diagnostic sensitivity and specificity of suspicious ultrasound characteristics for predicting thyroid cancer. Comparison with the previously published data (25, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49).
| Sensitivity (%) | Specificity (%) | |||
|---|---|---|---|---|
| Present study | Literature – median (range) | Present study | Literature – median (range) | |
| Hypoechoic | 80.2% (264/329) | 65.4% (20.0–100.0) | 46.6% (248/532) | 64.6% (43.4–92.0) |
| Very hypoechoic | 37.4% (123/329) | 17.4% (14.2–41.4) | 87.8% (467/532) | 97.1% (92.2–97.1) |
| Microcalcifications | 42.2% (139/329) | 36.9% (12.5–55.8) | 82.9% (441/532) | 91.6% (12.1–98.0) |
| Irregular margins | 37.4% (123/329) | 44.2% (13.0–48.3) | 88.3% (470/532) | 90.0% (69.1–98.4) |
| Extrathyroidal extension | 26.7% (88/329) | 20.8% (one study) | 95.9% (510/532) | 97.5% (one study) |
Discussion
This is the first study using US video recordings of consecutively operated patients for comparison of the evaluations of nodule characteristics. Employing highly experienced investigators the diagnostic value of individual US features was analyzed. Our study design minimized factors which might have caused bias in other studies. Thus, large and difficult-to-examine nodules were not excluded, the investigators did not have a common educational background, and the use of real-time videos rather than one or a few preselected still images simulated the real-world situation. Furthermore, we used an international and diverse group of investigators, because in single-institution studies, investigators are likely to interpret US signs more uniformly.
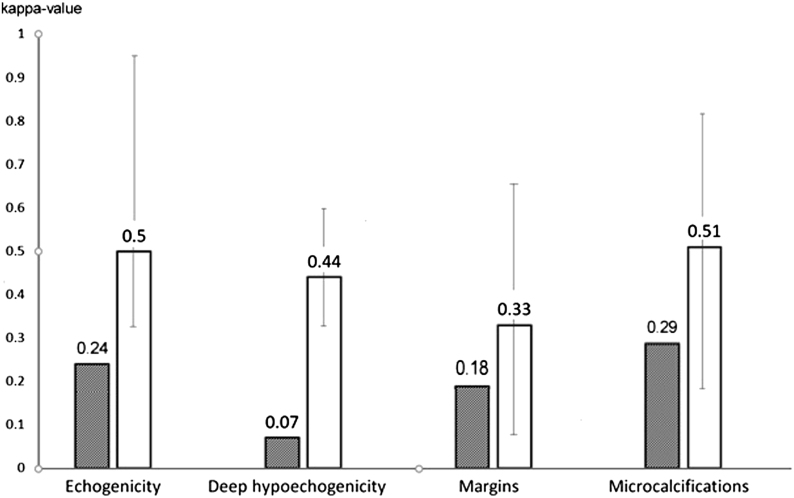
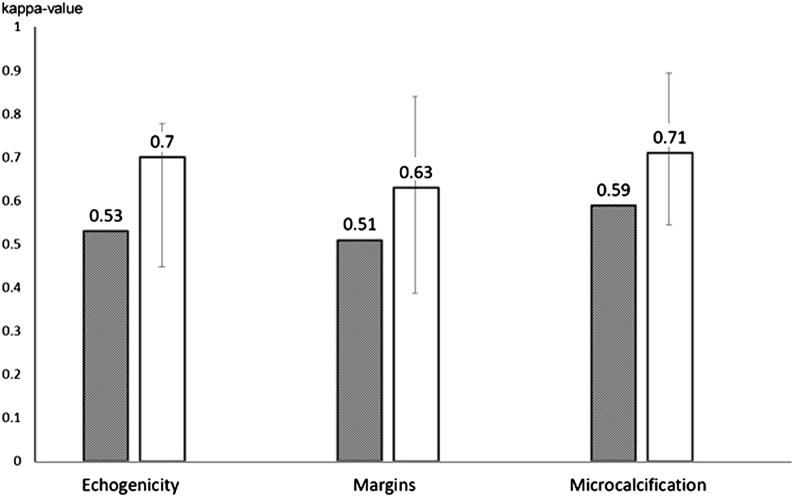
Based on our findings, interobserver agreement was insufficient for the evaluation of nodule margins and moderate for microcalcifications, a clear difference compared to previous studies which found better agreement (19, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49) using still images (Fig. 2), while intraobserver variation was comparable (Fig. 3). This is explained almost exclusively by the difference in observer-dependent interpretation of nodule characteristics; while microcalcification and taller-than-wide shape have clear definitions, this is less true for nodule margins. Neither is there a well-defined reference for echogenicity; indeed, we have found poor interobserver agreement for the distinction between minimally/moderately and very hypoechoic nodules. On the other hand, good interobserver agreement has been found for the distinction between iso/hyperechoic and hypoechoic nodules, as well as for ETE.
Figure 2.
Interobserver variation in the interpretation of certain ultrasound characteristics. For comparison purposes, we calculated Kappa-s, as earlier studies used Kappa values (4, 6, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23). Gray bars: present study; white bars: median of literature data. Error bars represent ranges.
Figure 3.
Intraobserver variation in the interpretation of certain ultrasound characteristics. For comparison purposes, we calculated Kappa-s, as earlier studies used Kappa values (31, 32, 42). Gray bars: present study; white bars: median of literature data. Error bars represent ranges.
Only ETE and presence/absence of a real nodule have a biological standard, namely pathology. However, significant interobserver variability is also described for the pathology assessment of ETE (50). Judgment of other characteristics relies on consensual interpretation of US images (7, 8, 9, 10, 11). The guidelines do not specify the number of protrusions or the extent that a protrusion must exceed in order to describe the margins as lobulated or spiculated. For nodule echogenicity, either the ‘normal thyroid’ or the strap muscles are used as reference. To increase confusion, it is unspecified if the muscle as a whole or only the muscle section with low adipose tissue content should be considered as reference tissue. The surrounding ‘reference’ thyroid tissue may be hypoechoic itself due to autoimmune thyroid disease or aging. Finally, there is a lack of clarity as to which combination of the three US features of ETE (discontinuous capsule, abutting, and bulging contours) offers the best combination of sensitivity and specificity for diagnosing ETE.
The size of the nodule was found to have a significant effect on interobserver variation. The larger the nodule the better the agreement for microcalcifications, while the smaller the nodule the better the agreement in discriminating between iso/hyperechogenic and hypoechogenic lesions. Nodule size was without effect on the evaluation of the borders of the nodule and ETE.
Our data on diagnostic sensitivity and specificity of microcalcifications, irregular margins, and nodule echogenicity are in agreement with those previously published in the literature (4, 6, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23). Although three TIRADS scoring systems use US signs of ETE for predicting thyroid cancers irrespective of the real ETE (7, 8, 9), there is only one publication which evaluated the diagnostic sensitivity and specificity of US signs of ETE in this context (19). Similarly to Hoang and coworkers (19), we found that compared with other suspicious characteristics, US signs of ETE have a limited role in confirming malignancy while the lack of these signs provides excellent assurance for excluding malignancy.
The very high misclassification rate in ETE, when compared to histology, suggests that US might be a suitable tool for this purpose only in nodules ≤17 mm in maximal diameter. This raises a serious concern about the use of preoperative US for postoperative staging, as suggested by the current TNM classification (51).
Interobserver agreement for a given characteristic may be influenced by the number of choices offered to the examiner, especially if no universally accepted definition is available for each nodule characteristic, or the examiner is less trained. However, if there is one single ‘correct’ choice among the offered ones, and the examiner is in the possession of the widely accepted definition of the characteristics (choices), this effect ought to be less dependent on the number of choices offered and the ‘correct’ one easier selected. This adds support for the need of improving the consistency of the US lexicons and the terminology herein.
Interestingly, investigators described nodules in cases where histopathology failed to reveal a true nodule, and there was a good interobserver agreement in these cases. Disregarding the unlikely chance that the histopathologist missed a nodule, we conclude that there is an inherent weakness in that US, at no variance with any other imaging technique, may produce an identical visual image of a nodule in the absence of a true nodule. While we cannot offer a sound explanation of this, it is clearly worthy of further exploration.
Despite adequate power and surgical confirmation of all nodules studied, a limitation of our work is the relatively low number of patients. Moreover, taller-than-wide shape was not included in the analysis as a nodule characteristic. The reason being that we deemed it superfluous to test the US diameter measurement capability of expert US users. Two patients with thyroid malignancy other than thyroid cancer were also among the studied nodules, as by definition, consecutive cases were included. Strengths of our study include only evaluating surgically removed thyroid nodules, the US investigator team consisting of highly skilled physicians with extensive US experience, and the use of videos rather than still pictures thereby resembling the real-world situation. While the participation of experienced investigators might have positively affected sensitivity and specificity, the true extent and direction of this influence for the interpretation of our data remains unclarified and awaits testing in a number of different settings.
In conclusion, examination of video recordings, a condition close to the real-world situation, revealed substantial interobserver variation in the interpretation of each of the four important US characteristics of thyroid nodules. This variation was dependent on nodule size for microcalcifications and nodule echogenicity. The international establishment of uniformly accepted US definitions for nodule characteristics used by TIRADS is much needed. An international TIRADS accompanied by the development of a manual including an atlas of the images corresponding to standardization of the definitions for each sign used in TIRADS is warranted. When available, it remains to be proven whether teaching and implementing this instrument achieves a substantial improvement of the agreement in thyroid US reporting, and how this influences the use of FNA.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contribution statement
T S – study design, preparation of the 123 video records involved in the study, steering, first and last versions of the manuscript. L H – study design, steering, manuscript preparation, final manuscript approval. S B – evaluation of the cases, approval of the manuscript. A F – evaluation of the cases, approval of the manuscript. L J – evaluation of the cases, approval of the manuscript. G L K – evaluation of the cases, approval of the manuscript. E P – evaluation of the cases, manuscript preparation. K R – evaluation of cases, first version of the manuscript. G R – evaluation of cases, approval of the manuscript. Z K – development of the eCRF and the online evaluation system, statistical analysis. E V N – study design, steering, manuscript preparation, final manuscript approval.
References
- 1.Hegedüs L.Clinical practice. The thyroid nodule. New England Journal of Medicine 20043511764–1771. ( 10.1056/NEJMcp031436) [DOI] [PubMed] [Google Scholar]
- 2.Burman KD, Wartofsky L. Thyroid nodules. New England Journal of Medicine 20163741294–1295. ( 10.1056/NEJMc1600493) [DOI] [PubMed] [Google Scholar]
- 3.Todsen T, Bennedbaek FN, Kiss K, Hegedüs L. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules. Head and Neck 2021431009–1013. ( 10.1002/hed.26598) [DOI] [PubMed] [Google Scholar]
- 4.Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, Park JS, Yoo RE, Baek JH, Baek SMet al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology consensus statement and recommendations. Korean Journal of Radiology 2021222094–2123. ( 10.3348/kjr.2021.0713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo B, Li J, Qian L, Cui L, Chen Wet al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine 202070256–279. ( 10.1007/s12020-020-02441-y) [DOI] [PubMed] [Google Scholar]
- 6.Grani G, Sponziello M, Pecce V, Ramundo V, Durante C. Contemporary thyroid nodule evaluation and management. Journal of Clinical Endocrinology and Metabolism 20201052869–2883. ( 10.1210/clinem/dgaa322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger Met al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016261–133. ( 10.1089/thy.2015.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, Paschke R, Valcavi R, Vitti P. & AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules – 2016 update. Endocrine Practice 201622622–639. ( 10.4158/EP161208.GL) [DOI] [PubMed] [Google Scholar]
- 9.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates MCet al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. Journal of the American College of Radiology 201714587–595. ( 10.1016/j.jacr.2017.01.046) [DOI] [PubMed] [Google Scholar]
- 10.Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. European Thyroid Journal 20176225–237. ( 10.1159/000478927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, Lim HK, Moon WJ, Na DG, Park JSet al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean Journal of Radiology 201617370–395. ( 10.3348/kjr.2016.17.3.370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoang JK, Asadollahi S, Durante C, Hegedüs L, Papini E, Tessler FN. An international survey on utilization of five thyroid nodule risk stratification systems: a needs assessment with future implications. Thyroid 202232675–681. ( 10.1089/thy.2021.0558) [DOI] [PubMed] [Google Scholar]
- 13.Jarløv AE, Nygård B, Hegedüs L, Karstrup S, Hansen JM. Observer variation in ultrasound assessment of the thyroid gland. British Journal of Radiology 199366625–627. ( 10.1259/0007-1285-66-787-625) [DOI] [PubMed] [Google Scholar]
- 14.Jabar ASS, Koteshwara P, Andrade J. Diagnostic reliability of the thyroid imaging reporting and data system (TI-RADS) in routine practice. Polish Journal of Radiology 201984e274–e280. ( 10.5114/pjr.2019.86823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, Jung HK, Choi JS, Kim BM, Kim EK. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 2011260892–899. ( 10.1148/radiol.11110206) [DOI] [PubMed] [Google Scholar]
- 16.Chandramohan A, Khurana A, Pushpa BT, Manipadam MT, Naik D, Thomas N, Abraham D, Paul MJ. Is TIRADS a practical and accurate system for use in daily clinical practice? Indian Journal of Radiology and Imaging 201626145–152. ( 10.4103/0971-3026.178367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivas MN, Amogh VN, Gautam MS, Prathyusha IS, Vikram NR, Retnam MK, Balakrishna BV, Kudva N. A prospective study to evaluate the reliability of thyroid imaging reporting and data system in differentiation between benign and malignant thyroid lesions. Journal of Clinical Imaging Science 20166 5. ( 10.4103/2156-7514.177551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skowrońska A, Milczarek-Banach J, Wiechno W, Chudziński W, Żach M, Mazurkiewicz M, Miśkiewicz P, Bednarczuk T. Accuracy of the European Thyroid Imaging Reporting and Data System (EU-TIRADS) in the valuation of thyroid nodule malignancy in reference to the post-surgery histological results. Polish Journal of Radiology 201883e579–e586. ( 10.5114/pjr.2018.81556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoang JK, Middleton WD, Farjat AE, Teefey SA, Abinanti N, Boschini FJ, Bronner AJ, Dahiya N, Hertzberg BS, Newman JRet al. Interobserver variability of sonographic features used in the American College of Radiology thyroid imaging reporting and data system. American Journal of Roentgenology 2018211162–167. ( 10.2214/AJR.17.19192) [DOI] [PubMed] [Google Scholar]
- 20.Yoon JH, Han K, Kim EK, Moon HJ, Kwak JY. Diagnosis and management of small thyroid nodules: a comparative study with six guidelines for thyroid nodules. Radiology 2017283560–569. ( 10.1148/radiol.2016160641) [DOI] [PubMed] [Google Scholar]
- 21.Na DG, Baek JH, Sung JY, Kim JH, Kim JK, Choi YJ, Seo H. Thyroid imaging reporting and data system risk stratification of thyroid nodules: categorization based on solidity and echogenicity. Thyroid 201626562–572. ( 10.1089/thy.2015.0460) [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Gabriel H, Nemcek AA, Nayar R, Du H, Nikolaidis P. Subcentimeter thyroid nodules: utility of sonographic characterization and ultrasound-guided needle biopsy. American Journal of Roentgenology 2011197W1123–W1128. ( 10.2214/AJR.10.5684) [DOI] [PubMed] [Google Scholar]
- 23.Cappelli C, Castellano M, Pirola I, Gandossi E, De Martino E, Cumetti D, Agosti B, Rosei EA. Thyroid nodule shape suggests malignancy. European Journal of Endocrinology 200615527–31. ( 10.1530/eje.1.02177) [DOI] [PubMed] [Google Scholar]
- 24.Gwet KL.Computing inter-rater reliability and its variance in the presence of high agreement. British Journal of Mathematical and Statistical Psychology 20086129–48. ( 10.1348/000711006X126600) [DOI] [PubMed] [Google Scholar]
- 25.Solymosi T, Hegedüs L, Bonnema SJ, Frasoldati A, Jambor L, Kovacs GL, Papini E, Rucz K, Russ G, Karanyi Zet al. Ultrasound-based indications for thyroid fine-needle aspiration: outcome of a TIRADS-based approach versus operators’ expertise. European Thyroid Journal 202110416–424. ( 10.1159/000511183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zec S, Soriani N, Comoretto R, Baldi I. High agreement and high prevalence: the paradox of Cohen’s kappa. Open Nursing Journal 201711211–218. ( 10.2174/1874434601711010211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McHugh ML.Interrater reliability: the kappa statistic. Biochemia Medica 201222276–282. ( 10.11613/BM.2012.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wongpakaran N, Wongpakaran T, Wedding D, Gwet KL. A comparison of Cohen’s Kappa and Gwet’s AC1 when calculating inter-rater reliability coefficients: a study conducted with personality disorder samples. BMC Medical Research Methodology 201313 61. ( 10.1186/1471-2288-13-61) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman DG.. Sample Size In Practical Statistics for Medical Research, pp. 455–460. Ed DG Altman. London: Chapman & Hall. [Google Scholar]
- 30.Stevenson M, Sergeant E, Nunes T, Heuer C, Marshall J, Sanchez J, Thornton R, Reiczigel J, Robison-Cox J, Sebastiani Pet al. epiR: tools for the analysis of epidemiological data. Indianapolis, IN, USA:The R Foundation,2019. (available at: https://CRAN.R-project.org/package=epiR) [Google Scholar]
- 31.Park CS, Kim SH, Jung SL, Kang BJ, Kim JY, Choi JJ, Sung MS, Yim HW, Jeong SH. Observer variability in the sonographic evaluation of thyroid nodules. Journal of Clinical Ultrasound 201038287–293. ( 10.1002/jcu.20689) [DOI] [PubMed] [Google Scholar]
- 32.Choi SH, Kim EK, Kwak JY, Kim MJ, Son EJ. Interobserver and intraobserver variations in ultrasound assessment of thyroid nodules. Thyroid 201020167–172. ( 10.1089/thy.2008.0354) [DOI] [PubMed] [Google Scholar]
- 33.Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, Kim J, Kim HS, Byun JS, Lee DHet al. Benign and malignant thyroid nodules: US differentiation – multicenter retrospective study. Radiology 2008247762–770. ( 10.1148/radiol.2473070944) [DOI] [PubMed] [Google Scholar]
- 34.Sahli ZT, Sharma AK, Canner JK, Karipineni F, Ali O, Kawamoto S, Hang JF, Mathur A, Ali SZ, Zeiger MAet al. TIRADS interobserver variability among indeterminate thyroid nodules: a single-institution study. Journal of Ultrasound in Medicine 2019381807–1813. ( 10.1002/jum.14870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grani G, Lamartina L, Cantisani V, Maranghi M, Lucia P, Durante C. Interobserver agreement of various thyroid imaging reporting and data systems. Endocrine Connections 201871–7. ( 10.1530/EC-17-0336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SH, Park CS, Jung SL, Kang BJ, Kim JY, Choi JJ, Kim YI, Oh JK, Oh JS, Kim Het al. Observer variability and the performance between faculties and residents: US criteria for benign and malignant thyroid nodules. Korean Journal of Radiology 201011149–155. ( 10.3348/kjr.2010.11.2.149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koltin D, O’Gorman CS, Murphy A, Ngan B, Daneman A, Navarro OM, García C, Atenafu EG, Wasserman JD, Hamilton Jet al. Pediatric thyroid nodules: ultrasonographic characteristics and inter-observer variability in prediction of malignancy. Journal of Pediatric Endocrinology and Metabolism 201629789–794. ( 10.1515/jpem-2015-0242) [DOI] [PubMed] [Google Scholar]
- 38.Lim-Dunham JE, Erdem Toslak I, Alsabban K, Aziz A, Martin B, Okur G, Longo KC. Ultrasound risk stratification for malignancy using the 2015 American Thyroid Association management guidelines for children with thyroid nodules and differentiated thyroid cancer. Pediatric Radiology 201747429–436. ( 10.1007/s00247-017-3780-6) [DOI] [PubMed] [Google Scholar]
- 39.Norlén O, Popadich A, Kruijff S, Gill AJ, Sarkis LM, Delbridge L, Sywak M, Sidhu S. Bethesda III thyroid nodules: the role of ultrasound in clinical decision making. Annals of Surgical Oncology 2014213528–3533. ( 10.1245/s10434-014-3749-8) [DOI] [PubMed] [Google Scholar]
- 40.Kim HG, Kwak JY, Kim EK, Choi SH, Moon HJ. Man to man training: can it help improve the diagnostic performances and interobserver variabilities of thyroid ultrasonography in residents? European Journal of Radiology 201281e352–e356. ( 10.1016/j.ejrad.2011.11.011) [DOI] [PubMed] [Google Scholar]
- 41.Wienke JR, Chong WK, Fielding JR, Zou KH, Mittelstaedt CA. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. Journal of Ultrasound in Medicine 2003221027–1031. ( 10.7863/jum.2003.22.10.1027) [DOI] [PubMed] [Google Scholar]
- 42.Persichetti A, Di Stasio E, Coccaro C, Graziano F, Bianchini A, Di Donna V, Corsello S, Valle D, Bizzarri G, Frasoldati Aet al. Inter- and intraobserver agreement in the assessment of thyroid nodule ultrasound features and classification systems: a blinded multicenter study. Thyroid 202030237–242. ( 10.1089/thy.2019.0360) [DOI] [PubMed] [Google Scholar]
- 43.Pang Z, Margolis M, Menezes RJ, Maan H, Ghai S. Diagnostic performance of 2015 American Thyroid Association guidelines and inter-observer variability in assigning risk category. European Journal of Radiology Open 20196122–127. ( 10.1016/j.ejro.2019.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoang JK, Middleton WD, Tessler FN. Update on ACR TI-RADS: successes, challenges, and future directions, from the AJR special series on radiology reporting and data systems. American Journal of Roentgenology 2021216570–578. ( 10.2214/AJR.20.24608) [DOI] [PubMed] [Google Scholar]
- 45.Seifert P, Görges R, Zimny M, Kreissl MC, Schenke S. Interobserver agreement and efficacy of consensus reading in Kwak-, EU-, and ACR-thyroid imaging recording and data systems and ATA guidelines for the ultrasound risk stratification of thyroid nodules. Endocrine 202067143–154. ( 10.1007/s12020-019-02134-1) [DOI] [PubMed] [Google Scholar]
- 46.Özel A Türkyılmaz Mut D Ağrıdağ Üçpınar B Özdal Sayer A Yanç U von Bodelschwingh B & Gemalmaz A. Interobserver variability of ultrasound features based on American College of Radiology thyroid imaging reporting and data system lexicon in American College of Radiology Thyroid Imaging Reporting and Data System: a single-center study with radiologists and radiology residents. Ultrasound Quarterly 202137324–328. ( 10.1097/RUQ.0000000000000512) [DOI] [PubMed] [Google Scholar]
- 47.Liu H, Ma AL, Zhou YS, Yang DH, Ruan JL, Liu XD, Luo BM. Variability in the interpretation of grey-scale ultrasound features in assessing thyroid nodules: a systematic review and meta-analysis. European Journal of Radiology 2020129 109050. ( 10.1016/j.ejrad.2020.109050) [DOI] [PubMed] [Google Scholar]
- 48.Dobruch-Sobczak K, Migda B, Krauze A, Mlosek K, Słapa RZ, Wareluk P, Bakuła-Zalewska E, Adamczewski Z, Lewiński A, Jakubowski Wet al. Prospective analysis of inter-observer and intra-observer variability in multi ultrasound descriptor assessment of thyroid nodules. Journal of Ultrasonography 201919198–206. ( 10.15557/JoU.2019.0030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itani M, Assaker R, Moshiri M, Dubinsky TJ, Dighe MK. Inter-observer variability in the American College of Radiology thyroid imaging reporting and data system: in-depth analysis and areas for improvement. Ultrasound in Medicine and Biology 201945461–470. ( 10.1016/j.ultrasmedbio.2018.09.026) [DOI] [PubMed] [Google Scholar]
- 50.Turk AT, Asa SL, Baloch ZW, Faquin WC, Fellegara G, Ghossein RA, Giordano TJ, LiVolsi VA, Lloyd R, Mete Oet al. Interobserver variability in the histopathologic assessment of extrathyroidal extension of well differentiated thyroid carcinoma supports the new American Joint Committee on cancer eighth edition criteria for tumor staging. Thyroid 201929619–624. ( 10.1089/thy.2018.0286) [DOI] [PubMed] [Google Scholar]
- 51.Tuttle M Morris L Haugen B Shah J Sosa J Rohren E Subramaniam R Hunt J & Perrier ND. Thyroid-differentiated and anaplastic carcinoma. In AJCC Cancer Staging Manual, ch 73, 8th ed. Eds MB Amin, SB Edge, F Greene, D Byrd, RK Brookland, MK Washington, CC Compton, KR Hess, DC Sullivan, JM Jessup et al. New York, NY, USA: Springer International Publishing, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a