Abstract
Background
Polycystic ovary syndrome (PCOS) is an androgen disorder and ovarian dysfunction disease in women of reproductive age. The cell death of granulosa cells (GCs) plays an important role in the development of PCOS. However, the mechanism of GC death is still unclear.
Methods
In the current study, NEDD4L was found to be elevated in PCOS GEO (Gene Expression Omnibus) databases and mouse models. The cell viability was analyzed by CCK-8 and FDA staining. The expression of ferroptosis markers was assessed by ELISA and immunofluorescence. The direct interaction of GPX4 and NEDD4L was verified by co-immunoprecipitation assay.
Result
Functionally, results from CCK-8 and FDA staining demonstrated that NEDD4L inhibited the cell viability of KGN cells and NEDD4L increased the levels of iron, malonyldialdehyde, and reactive oxygen species and decreased glutathione levels. Moreover, the cell death of KGN induced by NEDD4L was blocked by ferroptosis inhibitor, suggesting that NEDD4L regulates KGN cell ferroptosis. Mechanistically, NEDD4L directly interacts with GPX4 and promotes GPX4 ubiquitination and degradation.
Conclusion
Taken together, our study indicated that NEDD4L facilitates GC ferroptosis by promoting GPX4 ubiquitination and degradation and contributes to the development of PCOS.
Keywords: PCOS, ferroptosis, NEDD4L, GPX4, ubiquitination
Introduction
Polycystic ovary syndrome (PCOS) is an androgen disorder and ovarian dysfunction disease in women of reproductive age. PCOS is responsible for more than 90% of adult and adolescent hyperandrogenism (1, 2). The pathogenesis of PCOS remains controversial. Recently, more and more studies have suggested that functional ovarian hyperandrogenemia is the main inducement of PCOS (3), which is exacerbated by insulin-resistant hyperinsulinemia (4, 5). Insulin-resistant hyperinsulinism and androgen alter the function of granulosa cells (GCs) (6, 7). GCs play a key role in the maturation and ovulation of oocytes. Recently, studies have reported that oxidative stress and oxidative stress-induced cell death in GCs are associated with the pathogenesis of PCOS (8, 9). However, the underlying mechanism of GC death remains unclear.
Ferroptosis is a recently identified new form of programmed cell death that is characterized by reactive oxygen species (ROS) generation and iron overload (10, 11). Ferroptosis has different biochemical, morphological, and genetic properties from other forms of programmed cell death, such as necroptosis and apoptosis (12, 13). Ferroptosis can be considered as a kind of cell death that is triggered by the imbalance between the antioxidant system and oxidative stress (12, 14). There are two major pathways that contribute to the development of ferroptosis: the enzyme-regulated pathway and transporter-dependent pathway. The enzyme-regulated pathway activates ferroptosis by inhibiting glutathione peroxidase 4 (GPX4) and promoting lipid peroxidation (15). The transporter-dependent pathway activated ferroptosis by inhibiting SLC7A11 (16). As we know, oxidative stress and oxidative stress-induced cell death in GCs are associated with the pathogenesis of PCOS (9, 17). However, whether oxidative stress-mediated GC ferroptosis is involved in the development of PCOS is still unclear.
Given the role of ubiquitination in the development of PCOS and the regulation of ferroptosis, we investigated whether ubiquitination contributes to the development of PCOS by regulating GS ferroptosis. In the current study, we found that neural precursor cell-expressed developmentally down-regulated 4-like (NEDD4L), a member of the Nedd4 family, was increased in PCOS mice. Here, we confirmed that NEDD4L facilitates the ferroptosis of GCs. Moreover, NEDD4L facilitates ubiquitination-mediated degradation of GPX4.
Methods
Animals
Adult female C57BL/6J mice were purchased from Cyagen Bioscience (Santa Clara, CA, USA). Prior to the experiment, the animals were allowed to adapt to the environment for 1 week. For this study, all procedures were approved by the Ethics Committee of Shanghai Seventh People’s Hospital (item number: 2021-AR-059). Adult female C57BL/6J mice were housed withaccess to food and water ad libitum. The PCOS mouse model was established as described previously (18). In brief, female C57BL/6J mice (4 weeks old) were subcutaneously injected with DHEA (6 mg/0.1kg body weight) daily for 20 days.
KGN cell culture
Human ovarian cancer GC line KGN cells were purchased from the ATCC and cultured in DMEM/F-12 culture medium (Gibco) containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. For in vitro cell model, KGN cells were treated with dihydrotestosterone (DHT) 500 nM for 24 h.
Cell counting kit-8 assay
KGN cell viability was analyzed by Cell counting kit-8 (CCK-8) assay (Abcam) according to the instructions. In brief, 5 ×104 KGN cells with different pretreatments were inoculated on 96-well plates for 24 h. Then, 10 µL CCK-8 solution was added to each well and incubated at 37°C with 5% CO2 for additional 4 h, and then the absorbance of each well was taken by using a microplate reader at optical density 450 nm.
Fluorescein diacetate staining
KGN cells with different pretreatments were treated with 20 μL of fluorescein diacetate (FDA) reagent (5 mg/mL; Sigma) for 20 min, and then KGN cells images were captured by a fluorescence microscope (Olympus IX71).
Fe2+ concentration
The concentration of iron (Fe2+) in KGN cells was detected by an iron assay kit (Abcam ab83366) according to the instructions.
Lipid ROS assay
The level of lipid ROS in KGN cells was assessed by using C11-BODIPY probe assay kit (Invitrogen) according to the instructions. Briefly, 5 ×104 KGN cells were seeded in 96-well plates and cultured with 1 μM C11-BODIPY probe for 30 min, and then the level of lipid ROS was read by flow cytometer.
MDA and GSH content
The KGN cell malonyldialdehyde (MDA) content was assessed by a lipid peroxidation assay kit (Sigma, MAK085) according to the standard protocol. The content of GSH in KGN cells was assessed by a glutathione assay kit (Sigma, CS0260) according to the instructions.
Transient transfection of NEDD4L or si-NEDD4L
To overexpress NEDD4L, lentivirus production of NEDD4L was purchased from GeneChem (Shanghai, China) and infected KGN cells according to the instructions. To knockdown NEDD4L, si-NEDD4L was transfected into KGN cells by using Lipofectamine 3000. Briefly, KGN cells were transfected with 20 nM of si-NEDD4L (AGUCAUAAAUCUCGAGUCA) or si-control RNA (CAACUUGAGCAACUUAUUU).
Quantitative real-time PCR
Total RNA of KGN cell or PCOS mouse tissue was isolated by using the Trizol reagent (Invitrogen). Then, the reverse transcription-PCR was performed by using the iScript cDNA synthesis kit (Bio-Rad), and the real-time quantitative PCR was performed by using PrimeScript RT Master Mix kit (TaKaRa) in accordance with the following protocol: 95°C for 5 min, 40 cycles of 95°C for 10 s, and 55 °C for 20 s. The fold changes of target genes were analyzed by the 2–ΔΔCt method, and GAPDH was used as an internal control.
Western blotting analysis
Total protein of KGN cells and PCOS mice was extracted by using Minute™ total protein extraction kit (Invent, Eden Prairie, MN, USA), and the concentration of protein was detected by BCA (bicinchoninic acid) protein assay kit (Abcam). The primary antibodies were SKP2 antibody (1:500, Abcam, ab183039), NEDD4L antibody (1:500, Abcam, ab168349), GPX4 antibody (1:500, Abcam, ab134953), UB antibody, and anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (1:5000, Abcam, ab9485). The membranes were washed with TBST (TBS with Tween-20) for three times after incubation with primary antibodies for 2 h. Thereafter, membranes were incubated with goat anti-rabbit IgG H&L (HRP) (1:5000, ab205718) for 1 h at 37°C. The signals were visualized using enhanced chemiluminescence (Beyotime, Shanghai, China).
Co-immunoprecipitation
KGN cells were lyzed by NP-40 buffer, and the lysate was coated with anti-NEDD4L or anti-GPX4 for 4 h, and then Protein A/G beads were added to the immunoprecipitation complex and rotated at 4°C overnight. The next day, the Protein A/G beads were washed by PBS for three times. The immunoprecipitate complex was detected by western blotting with anti-NEDD4L or anti-GPX4.
Statistical analysis
All experimental data are displayed as mean ±SD. The statistical analyses were performed by SPSS 20.0. The significance between two groups was evaluated by the Student’s t-test, and more than two groups was determined by one-way ANOVA followed by the Tukey–Kramer multiple comparison test, and P < 0.05 was considered statistically significant.
Results
NEDD4L was upregulated in PCOS mice
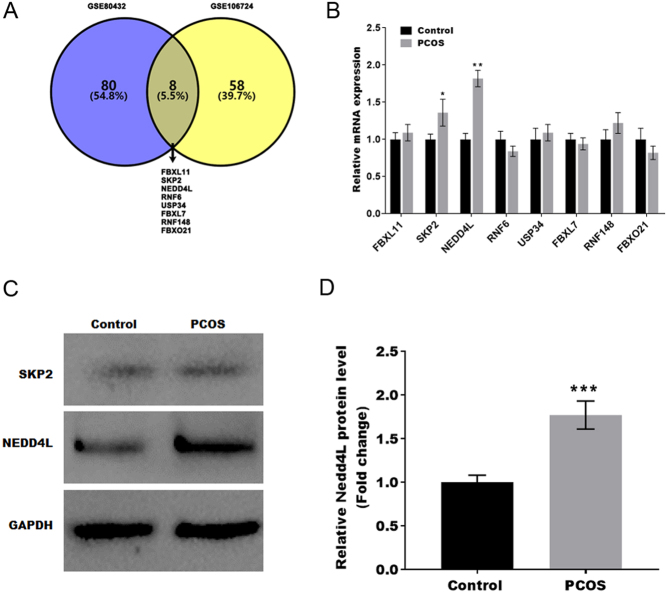
To investigate whether ubiquitination genes are involved in the occurrence of PCOS, the differentially expressed ubiquitination genes in PCOS were obtained from GSE80432 and GSE106724. Of these, 88 differentially expressed ubiquitination genes in GSE80432 and 66 in GSE42952 with significant changes ( P < 0.05) were filtered out, and the common ubiquitination genes were obtained by Venn diagram analysis. Figure 1A shows that eight common ubiquitination genes were identified (FBXL11, SKP2, NEDD4L, RNF6, USP34, FBXL7, RNF148, and FBXO21). The expression of these eight genes was assessed in PCOS mice using qPCR analysis. As shown in Fig. 1B, the mRNA expression of SKP2 and NEDD4L was markedly increased in PCOS mice compared with control mice. Similar to the qPCR results, the data from western blotting indicated that NEDD4L, but not SKP2, was significantly increased in PCOS mice. These results suggest that NEDD4L may contribute to the development of PCOS (Fig. 1C and D).
Figure 1.
NEDD4L contributed to the development of PCOS. (A) Venn diagram of differentially expressed ubiquitination genes in the datasets GSE80432 and GSE106724. (B) The mRNA level of eight genes in the PCOS mouse model was assessed by qRT-PCR (n = 5) *P < 0.05, **P < 0.01. (C and D) The protein level of NEDD4L in PCOS mice was assessed by western blot. ***P < 0.001.
NEDD4L overexpression promoted granulosa cells ferroptosis
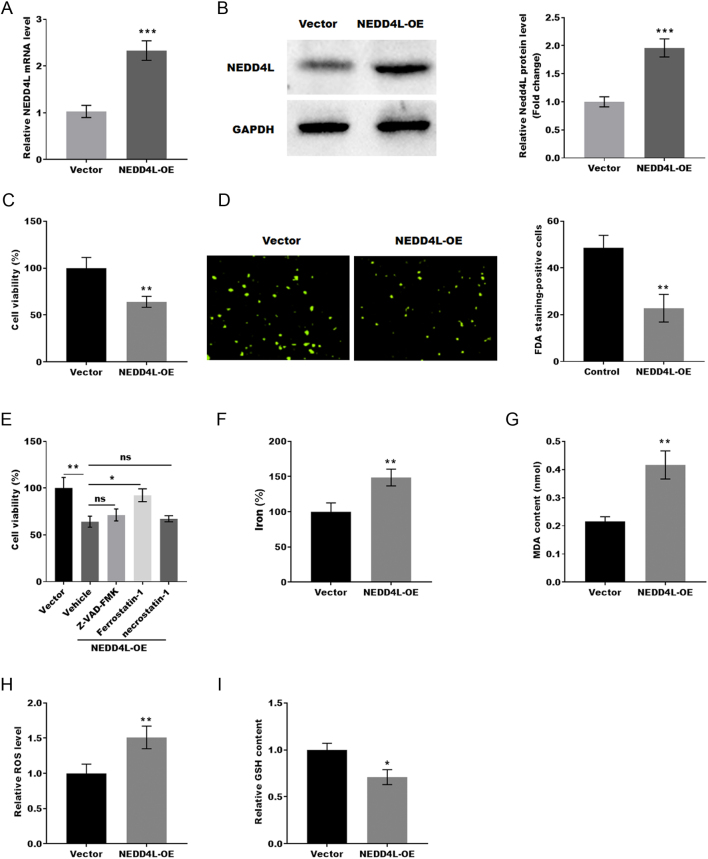
Previous research has demonstrated that the function of GCs is crucial to the development of PCOS. Therefore, the function of NEDD4L in GCs was evaluated. To clarify its role, NEDD4L was overexpressed in KGN cells. qRT-PCR and western blotting were used to confirm the effectiveness of NEDD4L-OE (Fig. 2A and B). The CCK-8 assay and FDA staining were used to determine the cell viability of KGN cell. As shown in Figs. 2C and D, KGN cell viability was obviously lower in the NEDD4L-OE group compared to the control group. Further evidence for this conclusion was confirmed by FDA staining.
Figure 2.
NEDD4L overexpression promoted granulosa cell ferroptosis. (A and B) qRT-PCR and western blot analysis of NEDD4L mRNA and protein levels in GCs with and without NEDD4L overexpression (n = 3). ***P < 0.001. (C) The GC viability was assessed by the CCK-8 assay (n = 3). **P < 0.01. (D and E) The GC viability was assessed by FDA (n = 3). **P < 0.01. (E) The cell viability of NEDD4L-OE GCs was determined using the CCK-8 assay in the presence or absence of ZVAD-FMK (5 M), necrostatin-1 (10 M), or Fer-1 (2 M).0.05. **P < 0.01; ns, not significant. (F–I) The level of Fe2+, the content of MDA, the level of ROS, and the content of GSH in NEDD4L-OE GCs was analyzed by ELISA (n = 3) *P < 0.05, **P < 0.01.
Multiple modes of programmed cell death are related to cell viability, including cell apoptosis, cell necroptosis, and cell ferroptosis. Therefore, the cell viability of NEDD4L-OE KGN cells treated with necrostatin-1 (a specific inhibitor of necroptosis), ZVAD-FMK (a specific inhibitor of apoptosis), and ferrostatin-1 (a specific inhibitor of ferroptosis) was further analyzed by the CCK-8 assay. As shown in Fig. 2E, our data revealed that ferrostatin-1, but not necrostatin-1 or ZVAD-FMK, significantly reversed the decrease of cell viability caused by NEDD4L-OE. To further confirm the function of NEDD4L in GC ferroptosis, the ferroptosis signaling was assessed in NEDD4L-OE and control KGN cells. Figure 2F, G and H showed that NEDD4L-OE significantly increased the level of Fe2+, MDA content, and ROS level in KGN cells. In addition, the GSH level in KGN cells was significantly decreased by NEDD4L-OE (Fig. 2I). These data indicated that NEDD4L-OE facilitated GCs' ferroptosis.
NEDD4L knockdown inhibited GC ferroptosis
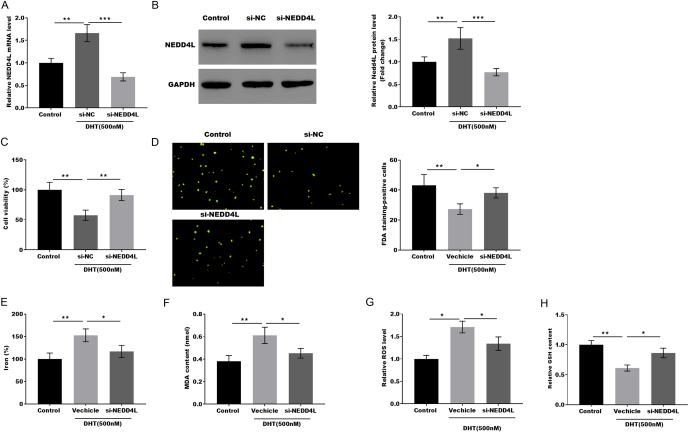
In a DHT-induced PCOS cell model, NEDD4L was knocked down by si-NEDD4L, and the expression of NEDD4L was confirmed by qRT-PCR and western blotting. As shown in Fig. 3A and B, NEDD4L expression was promoted by 500 nM DHT, whereas the effect was blocked by NEDD4L knockdown. KGN cell viability was inhibited by DHT treatment, while these effects were restored by NEDD4L knockdown (Fig. 3C). Moreover, FDA staining results showed that NEDD4L knockdown restored the KGN cell viability that had been decreased by DHT treatment (Fig. 3D). In addition, the levels of iron, MDA content, and ROS were markedly increased by DHT treatment, while these effects were restored by NEDD4L knockdown (Fig. 3E, F, and G). Moreover, the GSH content was significantly decreased by DHT treatment, while the effect was restored by NEDD4L knockdown (Fig. 3H).
Figure 3.
NEDD4L knockdown alleviated granulosa cell ferroptosis. (A and B) The mRNA and protein level of NEDD4L in DHT-induced GCs was analyzed by qRT-PCR and western blot with or without si-NEDD4L treatment (n = 3). **P < 0.01, ***P < 0.001. (C) The cell viability of DHT-induced GCs was analyzed by the CCK-8 assay after treatment with or without si-NEDD4L (n = 3). **P < 0.01. (D) The cell viability of DHT-induced GCs was analyzed by FDA staining after treatment with or without si-NEDD4L (n = 3). *P < 0.05, **P < 0.01. (E–H) the level of Fe2+, the content of MDA, the level of ROS, and the content of GSH in DHT-induced GCs were assessed by the ELISA assay after treatment with or without si-NEDD4L (n = 3). *P < 0.05, **P < 0.01.
GPX4 is the direct target of NEDD4L
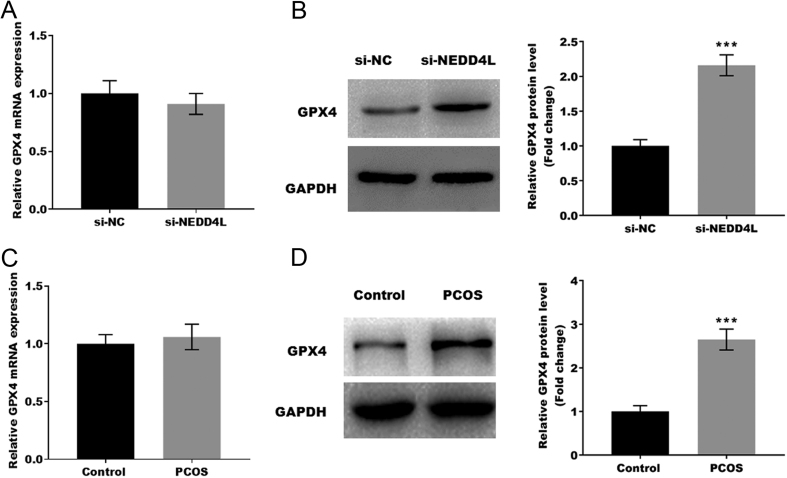
Previous studies have shown that dysregulated ubiquitination contributes to the development of diseases by regulating cell ferroptosis. Therefore, the target of NEDD4L was predicted by ubibrowser (http://ubibrowser.bio-it.cn/ubibrowser/) (Supplementary Fig. 1, see section on supplementary materials given at the end of this article), and we found that GPX4, a key gene of ferroptosis, is the target of NEDD4L. Next, the mRNA and protein expression of GPX4 were analyzed in the NEDD4L knockdown KGN cell. As shown in Fig. 4A and B, NEDD4L knockdown did not promote GPX4 mRNA expression but promoted GPX4 protein expression. The GPX4 expression in the PCOS mouse model was further verified by qRT-PCR and western blotting. Figure 4C and D showed that GPX4 protein expression was markedly increased in the PCOS model compared with control mice, but the mRNA expression did not increase.
Figure 4.
GPX4 is the direct target of NEDD4L. (A and B) The mRNA and protein level of GPX4 in NEDD4L knockdown GCs was assessed by qRT-PCR and western-blot. **P < 0.01. (C and D) The mRNA and protein level of GPX4 in the PCOS mouse model were assessed by qRT-PCR and western-blot. ***P < 0.001.
NEDD4L facilitated the ferroptosis of GCs by promoting the ubiquitin-mediated proteasome degradation of GPX4
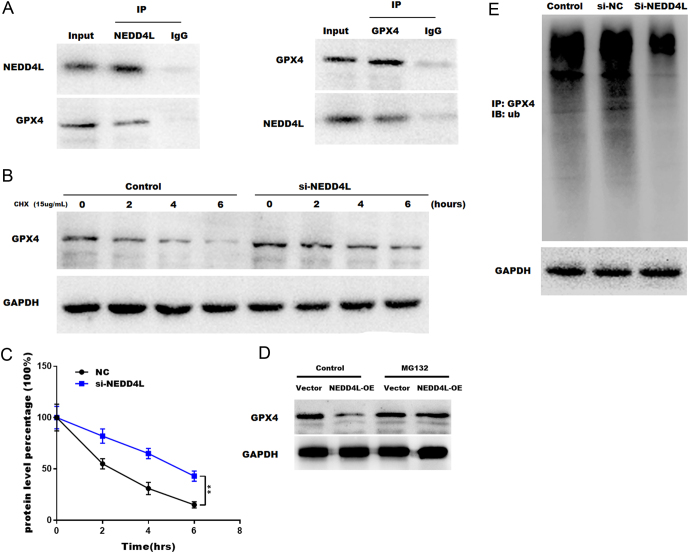
To further confirm that GPX4 is the target of NEDD4L, reciprocal co-immunoprecipitation (Co-IP) experiments were conducted to verify that NEDD4L directly interacts with GPX4. Figure 5A shows that a positive GPX4 signal was detected in the protein complex pulled down by an anti-NEDD4L-specific antibody. Besides, NEDD4L signals were also detected in the protein complex pulled down by the anti-GPX-specific antibody. Moreover, a cycloheximide experiment was conducted to analyze GPX4 protein stability in NEDD4L-KO (knock-out) KGN cells. As shown in Fig. 5B and C, GPX4 protein stability was markedly increased in NEDD4L-KO KGN cells. To further verify that NEDDL4 regulates GPX4 through ubiquitin proteasome, NEDD4L-OE KGN cells were treated with proteasome inhibitors MG132 (20 μM). Figure 5D shows that MG132 restored GPX4 expression levels in NEDD4L-OE KGN cells, suggesting that the ubiquitin proteasome pathway contribute to the degradation of GPX4. Next, the ubiquitination level of GPX4 was detected through IP (immunoprecipitation) with anti-GPX4 antibody and IB (immunoblotting) with anti-ubiquitin antibody. Figure 5E showed that NEDD4L-KO significantly decreased the ubiquitination of GPX4. Taken together, our data suggest that NEDD4L directly interacts with GPX4 and facilitates its degradation.
Figure 5.
NEDD4L accelerated the ferroptosis of GCs by promoting the ubiquitin-mediated proteasome degradation of GPX4. (A) NEDD4L interacts with GPX4 directly. The protein lysis from GCs was immunoprecipitated with IgG or antibodies against NEDD4L or GXP4 and then IB with NEDD4L and GXP4 (n = 3). (B and C) GPX4 protein stability is regulated by NEDD4L. GCs with or without NEDD4L knockdown were treated with 15 µg/mL cycloheximide (CHX) for various times, as indicated by the GPX4 protein stability assessed by western blot (n = 3). **P < 0.01. (D) NEDD4L regulates GPX4 stability through proteasome-mediated ubiquitination and degradation pathway. GC cells with or without NEDD4L-OE were treated with MG132 (20 μM) and then analyzed by western blotting. (E) Cell lysates were extracted from scramble and si-NEDD4L-infected GCs and immunoprecipitated with anti-GPX4 antibody and then analyzed by western blot using anti-ubiquitin antibody (n = 3).
Discussion
PCOS is an androgen disorder and ovarian dysfunction disease in women of reproductive age. Oxidative stress and oxidative stress-induced cell death in GCs are associated with the pathogenesis of PCOS (20). Ferroptosis is a kind of programmed cell death characterized by iron overload and ROS generation. However, whether ferroptosis contributes to the development of PCOS is still unclear. In the current study, we verified that NEDD4L facilitates GC ferroptosis by promoting GPX4 ubiquitination and degradation, as evidenced by the following: (i) NEDD4L was upregulated in the PCOS mice; (ii) NEDD4L overexpression promoted GC ferroptosis; (iii) NEDD4L knockdown inhibited GC ferroptosis; (iv) GPX4 is the direct target of NEDD4L; (v) NEDD4L facilitated the ferroptosis of GCs by promoting the ubiquitin-mediated proteasome degradation of GPX4.
NEDD4L, a member of the HECT (homologous to E6AP C terminus) family of E3 ligases, has been implicated in the regulation of various diseases by regulating the ubiquitination of substrates. Peng Gao et al. reported that Nedd4l promotes type I interferon production in response to virus by catalyzing ubiquitination of the cysteine in TRAF3 (21). Deping Kong et al. reported that the D2/DP1 axis reduces age-related Th1 activation and consequent hypertensive response by increasing NEDD4L-mediated T-bet degradation via ubiquitination (22). In recent years, accumulating evidence has indicated that NEDD4L contributes to the development of various diseases by regulating cell proliferation. However, the function of NEDDL4 in cell proliferation is multifaceted. Some studies indicate that NEDD4L inhibited cell proliferation. Yi Zeng et al. reported that Nedd4L overexpression inactivated transforming growth factor-β/Smad2/3 signaling pathway and depressed cell apoptosis (23). Suyoun Chung et al. found that NEDD4 promotes keloid development by increasing fibroblast proliferation and invasion (24). While some studies indicated that NEDD4L promotes cell proliferation. Xuming Wang et al. found that NEDD4L significantly suppressed cell proliferation, migration, and invasion abilities of non-small cell lung cancer cell (25). Fengbo Zhao et al. demonstrated that knockdown of Nedd4L could significantly promote the proliferation of HCC cells by CCK-8 and colony formation assays in vitro (26). These studies suggest that NEDD4L inhibits cell proliferation. In the current study, we found that NEDD4L was significantly increased in PCOS mice and cell models. An in vitro study showed that NEDD4L inhibits GC proliferation by promoting the ferroptosis of GCs. Consistent with our results, Rui Liu et al. found that ESR1 and NEDD4L functioned together after radiation treatment and finally induced ferroptosis in breast cancer cells (27). This work, as well as other studies, further confirms that the regulation of NEDD4L on cell proliferation is a complex and diverse process.
In PCOS, follicles have a relative abnormal function of GCs and/or degenerating GCs, indicating abnormal GC proliferation or death (28). GCs regulate the destiny of the follicle by delivering nutrients and growth factors to the oocyte (29). Therefore, both GC cell proliferation and apoptosis are related to the development of PCOS. Ferroptosis is a form of cell death different from apoptosis and necroptosis, characterized by the imbalance between oxidation and antioxidant, which is also the key cause of the development of PCOS (30). Lingzhi Zhang et al. reported that transferrin receptor-mediated ROS promotes KGN cell ferroptosis by regulating NOX1/PINK1/ACSL4 signaling (31). Dan Zhang et al. reported that circ-RHBG suppresses PCOS cell ferroptosis through the circ-RHBG/miR-515-5p/SLC7A11 axis in PCOS (32). These studies suggest that ferroptosis contributes to the development of PCOS. In the current study, we found that NEDD4L mediated GC ferroptosis. Combining these studies with our experimental results, we speculate that NEDD4L may contribute to the development of PCOS by promoting the GC ferroptosis. However, the mechanism is still unclear. As we know, NEDD4L is a HECT type E3 ligase and regulates protein expression of target genes through ubiquitination. Peng Gao et al. reported that Nedd4L promotes antiviral innate immunity by catalyzing K29-linked cysteine ubiquitination of TRAF3 (21). Laura Novellasdemunt et al. reported that NEDD4 and NEDD4L regulate Wnt signaling and intestinal stem cell priming by degrading the LGR5 receptor (33). In the current study, we found that GPX4 is the direct target of NEDD4L, and in vitro data showed that NEDD4L promoted GPX4 ubiquitination and degradation in GCs. Taken together, our data suggest that NEDD4L facilitated the ferroptosis of GCs by promoting the ubiquitin-mediated proteasome degradation of GPX4.
Supplementary Material
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships.
Funding
This work was supported by the health care project of Shanghai Pudong New Area Science and Technology Development Fund for Livelihood Research (PKJ2021-Y05) and scientific research project of Shanghai Municipal Health Commission (202140279).
Ethical approval
Animal experimentation followed European Union Directive 2010/63/EU under license 2021-AR-059 granted by the Ethics Committee of Shanghai Seventh People’s Hospital.
Authors’ contribution statement
LL and HT designed the study. XJ, YH, and HL performed the laboratory assays and analyzed the data. LL led the writing of the manuscript. All authors contributed substantially to the drafts.
Acknowledgements
The authors thank the funders of this project; Health care project of Shanghai Pudong New Area Science and Technology Development Fund for Livelihood Research (PKJ2021-Y05) and scientific research project of Shanghai Municipal Health Commission (202140279).
References
- 1.Walters KA, Bertoldo MJ, Handelsman DJ. Evidence from animal models on the pathogenesis of PCOS. Best Practice and Research. Clinical Endocrinology and Metabolism 201832271–281. ( 10.1016/j.beem.2018.03.008) [DOI] [PubMed] [Google Scholar]
- 2.Azziz R.Polycystic ovary syndrome. Obstetrics and Gynecology 2018132321–336. ( 10.1097/AOG.0000000000002698) [DOI] [PubMed] [Google Scholar]
- 3.Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocrine Reviews 201637467–520. ( 10.1210/er.2015-1104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calcaterra V, Verduci E, Cena H, Magenes VC, Todisco CF, Tenuta E, Gregorio C, De Giuseppe R, Bosetti A, Di Profio Eet al. Polycystic ovary syndrome in insulin-resistant adolescents with obesity: the role of nutrition therapy and food supplements as a strategy to protect fertility. Nutrients 2021131848. ( 10.3390/nu13061848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moghetti P.Insulin resistance and polycystic ovary syndrome. Current Pharmaceutical Design 2016225526–5534. ( 10.2174/1381612822666160720155855) [DOI] [PubMed] [Google Scholar]
- 6.Sakumoto T, Tokunaga Y, Tanaka H, Nohara M, Motegi E, Shinkawa T, Nakaza A, Higashi M. Insulin resistance/hyperinsulinemia and reproductive disorders in infertile women. Reproductive Medicine and Biology 20109185–190. ( 10.1007/s12522-010-0062-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Human Reproduction Update 200814367–378. ( 10.1093/humupd/dmn015) [DOI] [PubMed] [Google Scholar]
- 8.Avila J, Gonzalez-Fernandez R, Rotoli D, Hernandez J, Palumbo A. Oxidative stress in granulosa-lutein cells from in vitro fertilization patients. Reproductive Sciences 2016231656–1661. ( 10.1177/1933719116674077) [DOI] [PubMed] [Google Scholar]
- 9.Taheri M, Hayati Roudbari N, Amidi F, Parivar K. The protective effect of sulforaphane against oxidative stress in granulosa cells of patients with polycystic ovary syndrome (PCOS) through activation of AMPK/AKT/NRF2 signaling pathway. Reproductive Biology 202121 100563. ( 10.1016/j.repbio.2021.100563) [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy 2021172054–2081. ( 10.1080/15548627.2020.1810918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death and Disease 202011 88. ( 10.1038/s41419-020-2298-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends in Cell Biology 201626165–176. ( 10.1016/j.tcb.2015.10.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Hu S, Bian Y, Yao J, Wang D, Liu X, Guo Z, Zhang S, Peng L. Targeting cell death: pyroptosis, ferroptosis, apoptosis and necroptosis in osteoarthritis. Frontiers in Cell and Developmental Biology 20219 789948. ( 10.3389/fcell.2021.789948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G, Liu Y, Zhao X, Qian L, Liu Pet al. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discovery 20217 193. ( 10.1038/s41420-021-00579-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radical Biology and Medicine 2019133144–152. ( 10.1016/j.freeradbiomed.2018.09.014) [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Yu C, Kang R, Kroemer G, Tang D. Cellular degradation systems in ferroptosis. Cell Death and Differentiation 2021281135–1148. ( 10.1038/s41418-020-00728-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Li N, Zeng Z, Tang L, Zhao S, Zhou F, Zhou L, Xia W, Zhu C, Rao M. Humanin regulates oxidative stress in the ovaries of polycystic ovary syndrome patients via the Keap1/Nrf2 pathway. Molecular Human Reproduction 202127gaaa081. ( 10.1093/molehr/gaaa081) [DOI] [PubMed] [Google Scholar]
- 18.Aragno M, Mastrocola R, Brignardello E, Catalano M, Robino G, Manti R, Parola M, Danni O, Boccuzzi G. Dehydroepiandrosterone modulates nuclear factor-kappaB activation in hippocampus of diabetic rats. Endocrinology 20021433250–3258. ( 10.1210/en.2002-220182) [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Wang Y, Li Z, Qin J, Wang P. Regulation of ferroptosis pathway by ubiquitination. Frontiers in Cell and Developmental Biology 20219 699304. ( 10.3389/fcell.2021.699304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammadi M.Oxidative stress and polycystic ovary syndrome: a brief review. International Journal of Preventive Medicine 201910 86. ( 10.4103/ijpvm.IJPVM_576_17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao P, Ma X, Yuan M, Yi Y, Liu G, Wen M, Jiang W, Ji R, Zhu L, Tang Zet al. E3 ligase Nedd4l promotes antiviral innate immunity by catalyzing K29-linked cysteine ubiquitination of TRAF3. Nature Communications 202112 1194. ( 10.1038/s41467-021-21456-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong D, Wan Q, Li J, Zuo S, Liu G, Liu Q, Wang C, Bai P, Duan SZ, Zhou Bet al. DP1 Activation reverses age-related hypertension via NEDD4L-mediated T-bet degradation in T cells. Circulation 2020141655–666. ( 10.1161/CIRCULATIONAHA.119.042532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng Y, Feng Z, Liao Y, Yang M, Bai Y, He Z. Diminution of microRNA-98 alleviates renal fibrosis in diabetic nephropathy by elevating Nedd4L and inactivating TGF-beta/Smad2/3 pathway. Cell Cycle 2020193406–3418. ( 10.1080/15384101.2020.1838780) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Chung S, Nakashima M, Zembutsu H, Nakamura Y. Possible involvement of NEDD4 in keloid formation; its critical role in fibroblast proliferation and collagen production. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences 201187563–573. ( 10.2183/pjab.87.563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Duan J, Fu W, Yin Z, Sheng J, Lei Z, Wang H. Decreased expression of NEDD4L contributes to NSCLC progression and metastasis. Biochemical and Biophysical Research Communications 2019513398–404. ( 10.1016/j.bbrc.2019.04.001) [DOI] [PubMed] [Google Scholar]
- 26.Zhao F, Gong X, Liu A, Lv X, Hu B, Zhang H. Downregulation of Nedd4L predicts poor prognosis, promotes tumor growth and inhibits MAPK/ERK signal pathway in hepatocellular carcinoma. Biochemical and Biophysical Research Communications 20184951136–1143. ( 10.1016/j.bbrc.2017.11.139) [DOI] [PubMed] [Google Scholar]
- 27.Liu R, Liu L, Bian Y, Zhang S, Wang Y, Chen H, Jiang X, Li G, Chen Q, Xue Cet al. The dual regulation effects of ESR1/NEDD4L on SLC7A11 in breast cancer under ionizing radiation. Frontiers in Cell and Developmental Biology 20219 772380. ( 10.3389/fcell.2021.772380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxton DW, Farquhar CM, Rae T, Beard RW, Anderson MC, Wadsworth J. Accuracy of ultrasound measurements of female pelvic organs. British Journal of Obstetrics and Gynaecology 199097695–699. ( 10.1111/j.1471-0528.1990.tb16241.x) [DOI] [PubMed] [Google Scholar]
- 29.Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. Journal of Reproduction and Development 20125844–50. ( 10.1262/jrd.2011-012) [DOI] [PubMed] [Google Scholar]
- 30.Dubey P, Reddy S, Boyd S, Bracamontes C, Sanchez S, Chattopadhyay M, Dwivedi A. Effect of nutritional supplementation on oxidative stress and hormonal and lipid profiles in PCOS-affected females. Nutrients 2021132938. ( 10.3390/nu13092938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Wang F, Li D, Yan Y, Wang H. Transferrin receptor-mediated reactive oxygen species promotes ferroptosis of KGN cells via regulating NADPH oxidase 1/PTEN induced kinase 1/acyl-CoA synthetase long chain family member 4 signaling. Bioengineered 2021124983–4994. ( 10.1080/21655979.2021.1956403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang D, Yi S, Cai B, Wang Z, Chen M, Zheng Z, Zhou C. Involvement of ferroptosis in the granulosa cells proliferation of PCOS through the circRHBG/miR-515/SLC7A11 axis. Annals of Translational Medicine 20219 1348. ( 10.21037/atm-21-4174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novellasdemunt L, Kucharska A, Jamieson C, Prange-Barczynska M, Baulies A, Antas P, van der Vaart J, Gehart H, Maurice MM, Li VS. NEDD4 and NEDD4L regulate Wnt signalling and intestinal stem cell priming by degrading LGR5 receptor. EMBO Journal 202039 e102771. ( 10.15252/embj.2019102771) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a