Abstract
Objectives
Preclinically, curcumin has been shown to protect against glucocorticoid-induced insulin resistance. We evaluated the effect of curcumin administered with prednisolone in healthy overweight or obese men.
Methods
In a double-blind, parallel-group trial, 24 overweight/obese non-diabetic men were randomised to one of three intervention groups (A) prednisolone placebo+curcumin placebo, (B) prednisolone (50 mg/day)+curcumin placebo or (C) prednisolone and curcumin (400 mg/day). Curcumin or curcumin placebo treatment started 1 day prior to 10-day prednisolone or prednisolone placebo treatment. The primary endpoint was change in prednisolone-induced insulin resistance assessed by homeostatic model assessment of insulin resistance (HOMA2-IR). Other endpoints included anthropometric measurements, magnetic resonance spectroscopy-assessed hepatic fat content, blood pressure, circulating metabolic markers and continuous glucose monitoring measures.
Results
Baseline characteristics (mean ± s.d): age 44.2 ± 13.7 years, BMI 30.1 ± 3.5 kg/m2, HbAlc 33.3 ± 3.2 mmol/mol, HOMA2-IR 1.10 ± 0.45 and fasting plasma glucose 5.2 ± 0.4 mmol/L. Prednisolone significantly increased HOMA2-IR (estimated treatment difference 0.36 (95% CI 0.16; 0.57)). Co-treatment with curcumin had no effect on HOMA2-IR (estimated treatment difference 0.08 (95% CI −0.13; 0.39)). Prednisolone increased HbAlc, insulin, C-peptide, glucagon, blood pressure, mean interstitial glucose, time spent in hyperglycaemia and glucose variability, but no protective effect of curcumin on any of these measures was observed.
Conclusions
In this double-blind, placebo-controlled parallel-group study involving 24 overweight or obese men randomised to one of three treatment arms, curcumin treatment had no protective effect on prednisolone-induced insulin resistance or other glucometabolic perturbations.
Keywords: prednisolone, glucose, insulin resistance, continuous glucose monitoring, curcumin
Introduction
Prednisolone, a corticosteroid-based drug, is used in a wide range of diseases due to its anti-inflammatory and immunosuppressant effects (1, 2, 3, 4). Glucocorticoids affect various human tissues, and it has been shown that expression of 2% of our genome is regulated by glucocorticoids (5). Prednisolone is known to induce insulin resistance, increased hepatic glucose production, hyperglycaemia and secondary diabetes. Even lean, healthy individuals can experience significant glucometabolic perturbations during short-term (<1 month) treatment with glucocorticoid-based drugs (6, 7, 8, 9, 10, 11, 12). Also, hepatic steatosis is a well-known side effect of prednisolone (7, 13, 14, 15, 16). Besse and colleagues showed that obese individuals are particularly prone to the adverse glucometabolic effects of dexamethasone, another synthetic glucocorticoid, exhibiting decreased whole-body glucose disposal compared to lean individuals during a hyperglycaemic clamp (17).
Some countries have national guidelines regarding screening for and treatment of corticosteroid-induced diabetes, some have not. In some guidelines (e.g. the Danish (https://endocrinology.dk/nbv/diabetes-melitus/behandling-og-kontrol-af-type-2-diabetes/) and English (https://abcd.care/sites/abcd.care/files/site_uploads/JBDS_Guidelines_Archive/JBDS_08_Steroids_DM_Guideline_FINAL_28052021_Archive.pdf) guidelines), it is stated that the evidence in the area is sparse, and that the guidelines are built on empirical data. The American Diabetes Association mentions glucometabolic side effects of glucocorticoid drugs in their Standards of Medical Care in Diabetes but recommends no specific screening, prevention or treatment regimens (18, 18). Thus, validated preventive measures and treatment options for prednisolone-induced glucometabolic derangements are lacking, but, nevertheless, highly needed.
Interestingly, curcumin, a phenolic compound extracted from the turmeric root, and curcumin analogues have been shown to ameliorate glucocorticoid-induced ‘dysmetabolism’ in rodents (19, 20), and several preclinical studies have shown promising effects of curcumin with regard to prevention of glucocorticoid-induced steatosis (19, 21, 22, 23, 24, 25, 26). A few clinical studies have demonstrated that curcumin treatment can decrease homeostatic model assessment of insulin resistance (HOMA-IR) in dysmetabolic patients (27, 28, 29). Many different targets have been proposed to be responsible for the metabolic effects of curcumin, including modulation of transcription factors, inflammatory mediators, cytokines, protein kinases and various cellular pathways (30).
Here, we employed a randomised, double-blind, parallel-group clinical trial design to investigate the protective effect of curcumin on prednisolone-induced insulin resistance and other relevant glucometabolic derangements in 24 overweight/obese men.
Methods
Trial design, approvals, and ethics
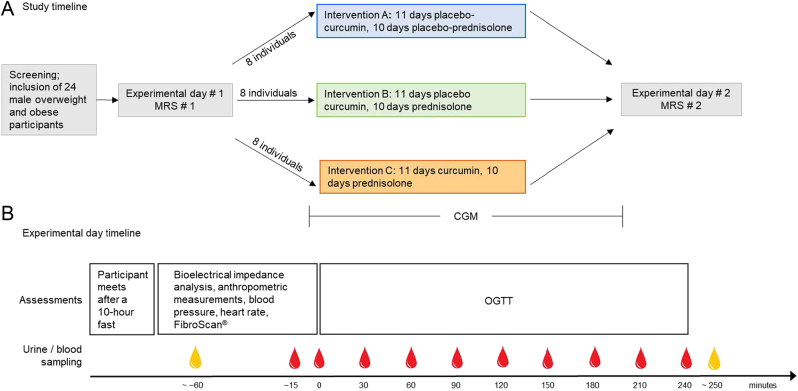
The study was a single-centre, randomised, double-blind, placebo-controlled, double-dummy, three-arm, parallel-group study conducted from December 2019 to June 2021 at Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark, in accordance with the Declaration of Helsinki (2013 edition) and Good Clinical Practice guidelines. The study was designed to assess the efficacy and safety of curcumin treatment for the prevention of prednisolone-induced hepatic insulin resistance assessed by HOMA2-IR (primary endpoint) in overweight/obese non-diabetic subjects. Study design and timeline are shown in Fig. 1. The study was approved by the Scientific–Ethical Committee of the Capital Region of Denmark (ID no. H-19017550) and the Danish Data Protection Agency (P-2019-657) and registered at https://www.clinicaltrials.gov/ (registration no. NCT04315350). Written informed consent was obtained from all participants.
Figure 1.
Timeline of the study (A) and timeline of experimental days #1 and #2 (B). The 11-day curcumin (400 mg per day)/curcumin placebo treatment was started 1 day prior to the 10-day prednisolone (50 mg per day)/prednisolone placebo treatment. Red drops indicate blood sampling, yellow drops indicate urine sampling. CGM, continuous glucose monitoring; MRS, magnetic resonance spectroscopy; OGTT, oral glucose tolerance test.
Eligibility criteria
Key inclusion criteria included male sex, age above 18 years, BMI above 24.9 kg/m2 and otherwise good health. Key exclusion criteria included use of glucose-lowering, lipid-lowering and/or steatogenic drugs, known viral, inherited and/or alcoholic liver disease, alcohol abuse and any condition that would interfere with trial participation and/or participant's safety. Detailed eligibility criteria can be found in Supplementary methods.
Treatment and randomisation
After obtaining oral and written informed consent and a screening visit at which fulfilment of eligibility criteria were secured, participants were randomised (using https://www.random.org/ by a person not involved with any other study procedures) 1:1:1 to one of the following three interventions: (A) prednisolone placebo + curcumin placebo, (B) prednisolone + curcumin placebo or (C) prednisolone + curcumin. The 11-day curcumin/curcumin placebo treatment was started 1 day before prednisolone/prednisolone placebo treatment (Fig. 1). Curcumin was administered as tablets containing Meriva® (Indena S.p.A. Milan, Italy) 1000 mg twice daily, corresponding to a daily dose of 400 mg curcumin. Curcumin and curcumin placebo tablets were produced and sponsored by Indena S.p.A , and placebo tablets contained, apart from curcumin, the same ingredients as the curcumin tablets (Meriva®), which in the following will be referred to as curcumin. Meriva® is a phospholipid formulation containing the three main curcuminoids found in the turmeric root and has been shown to have enhanced pH stability and bioavailability as compared to unformulated curcumin (31). The manufacturer was not involved in the design of the study or analysis of data. The Hospital Pharmacy of the Capital Region of Denmark was responsible for the production and delivery of prednisolone (50 mg) and prednisolone placebo, which were administered once daily as encapsulated tablets for optimal blinding. Compliance was deemed acceptable if more than 75% of dosages were taken.
Participants reported missed dosages, concomitant medication and adverse events. Participants and everybody involved in the study procedures remained blinded to treatment allocation until the last study procedure and initial data analysis were finalised.
Experimental procedures
After inclusion, participants attended baseline magnetic resonance spectroscopy (MRS) for assessment of hepatic fat content, subcutaneous adipose tissue volume (SAT) and visceral adipose tissue volume (VAT) and experimental day #1, where they were randomised to one of the interventions mentioned above. After the intervention, the participants underwent another MRS and experimental day #2. Participants wore a continuous glucose monitor (CGM) on the upper arm throughout the treatment period and were blinded for the measurements (Fig. 1, panel A). The participant fasted for 10 h prior to each experimental day. On experimental days, bioelectrical impedance analysis, body weight, BMI, hip circumference, and waist circumference were assessed. The urinary bladder was emptied, and a urine sample was collected. Transient elastography (FibroScan®), resting heart rate and blood pressure were assessed with participants in a supine position. A cannula was inserted in a cubital vein for blood sampling with the forearm wrapped in a heating pad for arterialisation of the venous blood. An oral glucose tolerance test (OGTT) was performed (75 g glucose dissolved in 300 mL tap water ingested over 5 min, starting at time point 0 min). Blood samples were collected at time points −15, 0, 30, 60, 90, 120, 150, 180, 210 and 240 min, and hereafter, urine sampling was repeated (Fig. 1, panel B). A sensor for CGM was applied to the upper arm >24 h prior to the start of prednisolone or prednisolone placebo and was removed before MRS #2 or the night before experimental day #2, whichever came first. A detailed description can be found in Supplementary methods.
For plasma glucose measurements, blood was collected in fluoride tubes and centrifuged immediately at 7400 g for 45 s at room temperature. For the analysis of free fatty acids, insulin and C-peptide in serum, blood was sampled into tubes containing serum clot activator and left to coagulate for at least 10 min at room temperature before centrifugation at 2900 g and 4°C for 15 min. Insulin and C-peptide samples were stored at −80°C until analysis, and free fatty acid samples were stored at −20°C until analysis. For plasma glucagon, blood was sampled into chilled tubes containing EDTA. The samples were immediately cooled on ice and centrifuged at 2900 g and 4°C for 15 min. The samples were stored at −20°C until analysis.
More details about experimental procedures can be found in Supplementary methods.
Analyses
The main analyses applied in the study are described in the following sections (details about the remaining analyses can be found in Supplementary methods). Plasma glucose was measured bedside using the glucose oxidase method (YSI model 2900D, Yellow Springs, OH, USA). For serum insulin and C-peptide concentrations, a two-sided chemical luminescence immunoassay (Siemens Atellica IM analyser, Erlangen, Germany) was used. Plasma glucagon was measured using a C-terminally directed antiserum (code no 4305) measuring glucagon of pancreatic origin as described earlier (32). For analysis of free fatty acids, an enzymatic colorimetric method was used (manual ELISA (NEFA-HR (2)), ACS-ACOD, Wako Chemicals, Neuss, Germany).
Calculations and statistical analysis
The primary endpoint was HOMA2-IR calculated from plasma glucose and serum C-peptide in the fasted state, as the mean of the −15 min and the 0 min values (The HOMA2 Calculator© version 2.2.3, The University of Oxford 2004–2021) (https://www.dtu.ox.ac.uk/homacalculator/). Detailed calculations of study power, oral glucose insulin sensitivity (OGIS), insulinogenic index, disposition index, area under the curve (AUC) and incremental AUC (iAUC) for parameters measured during OGTT as well as calculations regarding CGM data are provided in Supplementary methods. A sample size of seven participants in each group was needed to detect a curcumin-induced 50% amelioration of prednisolone-induced insulin resistance with 80% power and assuming a s.d. similar to Hansen and colleagues (8). A constrained linear mixed model with inherent baseline adjustment (33) was used to calculate the effect of interventions, including visit, treatment and their interaction as fixed factors. CGM data were compared between treatments using Welch two-sample t test. P values < 0.05 are considered statistically significant.
Statistical analyses were made with R version 4.0.3. Graphs were made with GraphPad Prism v. 9.0.0.
Results
We screened 30 participants after informed consent was given. Six were ineligible for participation. At baseline, the participants had the following characteristics (mean ± s.d.): age 44.2 ± 13.7 years, BMI 30.1 ± 3.5 kg/m2, HbA1c 33.3 ± 3.2 mmol/mol, HOMA2-IR 1.10 ± 0.45, fasting plasma glucose 5.2 ± 0.4 mmol/L, hepatic fat content 2.7 ± 2.6 % and waist circumference 105.7 ± 9.6 cm (Table 1). All participants exhibited self-reported compliance above 75%, with mean compliance of 98% regarding ingested dosages. All included participants completed the study. Eleven adverse events were reported in Group A, nine in Group B and five in Group C. No serious adverse events were reported. One participant in Group B experienced epistaxis and terminated treatment as per investigator’s instructions after 8 days of prednisolone treatment and was invited for experimental day #2, the day after the last dose.
Table 1.
Baseline characteristics of the 24 included participants in the three intervention groups.
| Group A (n = 8) prednisolone placebo + curcumin placebo (mean ± s.d.) | Group B (n = 8) prednisolone + curcumin placebo (mean ± s.d.) | Group C (n = 8) prednisolone + curcumin (mean ± s.d.) | |
|---|---|---|---|
| Age (years) | 41.6 ± 9.8 | 44.0 ± 13.5 | 47.0 ± 17.8 |
| BMI (kg/m2) | 30.4 ± 4.3 | 30.3 ± 3.3 | 29.3 ± 3.2 |
| HOMA2-IR | 1.17 ± 0.50 | 1.2 ± 0.53 | 0.94 ± 0.31 |
| Fasting plasma glucose (mmol/L) | 5.15 ± 0.42 | 5.37 ± 0.41 | 4.99 ± 0.41 |
| Hepatic fat content (%) | 3.1 ± 2.5 | 3.1 ± 3.0 | 2.4 ± 2.3 |
| HbA1c (mmol/mol) | 34.4 ± 2.3 | 32.0 ± 3.4 | 33.4 ± 3.7 |
HbA1c, glycated haemoglobin A1c; HOMA2-IR, homeostatic model assessment of insulin resistance. HbA1c, glycated haemoglobin A1c; HOMA2-IR, homeostatic model assessment of insulin resistance.
HOMA2-IR
HOMA2-IR increased by 0.28 (95% CI 0.14; 0.44) from baseline to end-of-treatment (EOT) in Group B receiving prednisolone and curcumin placebo, thus resulting in an estimated treatment difference (ETD) of 0.36 (95% CI 0.16; 0.57) compared to Group A receiving placebo only.
However, no effect of co-treatment with curcumin on the adverse effect of prednisolone on HOMA2-IR was seen when comparing Group C receiving prednisolone and curcumin with Group B receiving prednisolone and curcumin placebo (ETD 0.08 (95% CI −0.13; 0.39)) (Fig. 2).
Figure 2.
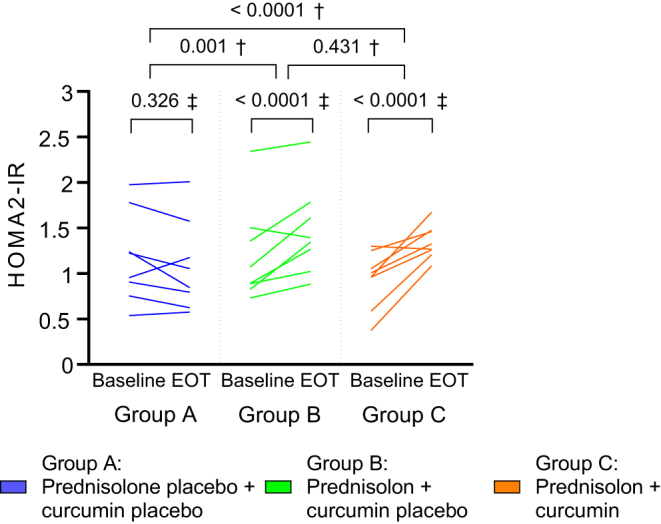
Individual changes in homeostatic model assessment for insulin resistance (HOMA2-IR) from baseline to end-of-treatment (EOT) in the three intervention arms. †P value for treatment difference (between-group comparison); ‡P value for change from baseline to EOT (within-group comparison). P values are from constrained linear mixed model.
CGM
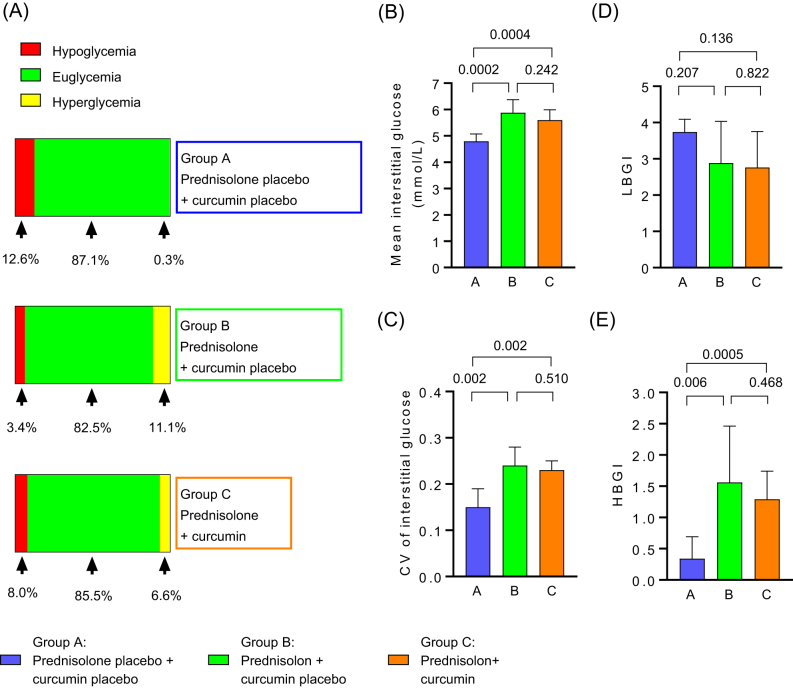
Prednisolone increased the percentage of time spent in hyperglycaemia, mean interstitial glucose concentrations, coefficient of variance (CV) of interstitial glucose and high blood glucose index when compared to placebo (Group B vs A). Curcumin did not affect these glycaemic adverse effects of prednisolone (Group C vs B). We found no effect of any intervention on the percentage of time spent in hypoglycaemia or euglycaemia, or on low blood glucose index when compared to placebo (Group B vs A) (Fig. 3, Table 3).
Figure 3.
Data from continuous glucose monitoring (CGM). Data collection started at 8:00 h first day on prednisolone/prednisolone placebo and terminated at the night before experimental day #2 or right before magnetic resonance spectroscopy #2, whichever came first. Hyperglycaemia is defined as blood glucose > 7.8 mmol/L and hypoglycaemia is defined as blood glucose < 4 mmol/L. Distribution of glycaemic ranges in the three intervention groups (A); mean interstitial glucose (B); glycaemic variability of interstitial glucose (coefficient of variance (CV)) (C); low blood glucose index (LBGI) (D); high blood glucose index (HBGI) (E). P values in B–E refer to intra-group differences calculated using Welch two-sample t-test comparing groups individually. For details about statistical analysis, please refer to Table 3.
Table 3.
Data from continuous glucose monitoring (CGM).
| Mean ± s.d. in Group A receiving prednisolon placebo/curcumin placeboa | Mean ± s.d. in Group B receiving prednisolone/curcumin placeboa | Mean ± s.d. in Group C receiving prednisolone/ curcumina | Difference between Group B and Group A, mean value (CI)b | P value (B vs A) | Difference between Group C and Group A mean value (CI)b | P value (C vs A) | Difference between Group C and Group B mean value (CI)b | P value (C vs B) | |
|---|---|---|---|---|---|---|---|---|---|
| Percentage of time spent in euglycaemia (4−7.8 mmol/L) (%) | 87.1 ± 13.0 | 82.5 ± 13.2 | 85.5 ± 6.9 | 4.6 (−19.3; 10.1) | 0.511 | −1.6 (−14.1; 10.8) | 0.772 | 2.9 (−8.7; 13.6) | 0.587 |
| Percentage of time spent in hypoglycaemia (<4 mmol/L) (%) | 12.6 ± 12.5 | 3.4 ± 6.9 | 8.0 ± 8.1 | −6.2 (−18.2; 5.9) | 0.276 | −4.6 (−16.9; 7.7) | 0.426 | 1.6 (−6.5; 9.7] | 0.682 |
| Percentage of time spent in hyperglycaemia (>7.8 mmol/L) (%) | 0.3 ± 0.6 | 11.1 ± 9.1 | 6.6 ± 4.5 | 10.6 (3.1; 18.4) | 0.012 | 6.2 (2.5; 10.0) | 0.006 | −4.5 (−12.5; 3.5) | 0.237 |
| Mean interstitial glucose (mmol/L) | 4.8 ± 0.3 | 5.9 ± 0.5 | 5.6 ± 0.4 | 1.1 (0.6; 1.5) | 0.0002 | 0.8 (0.44; 1.19) | 0.0004 | −0.3 (−0.7; 0.2) | 0.242 |
| CV of interstitial glucose | 0.15 ± 0.04 | 0.24 ± 0.04 | 0.23 ± 0.02 | 0.08 (0.04; 0.13) | 0.002 | 0.07 (0.03; 0.11) | 0.002 | −0.01 (−0.05; 0.03) | 0.510 |
| LBGI | 3.7 ± 1.3 | 2.9 ± 1.1 | 2.8 ± 1.0 | −0.9 (−2.3; 0.5) | 0.207 | −1.0 (−2.4; 0.4) | 0.136 | 0.1 (−1.0; 1.3) | 0.822 |
| HBGI | 0.3 ± 0.4 | 1.6 ± 0.9 | 1.3 ± 0.5 | 1.2 (0.4; 2.0) | 0.006 | 1.0 (0.5; 1.4) | 0.0005 | −0.3 (−1.1; 0.5) | 0.468 |
aParameters calculated from data recorded from 8:00 h at the day of prednisolone/prednisolone placebo treatment initiation until second magnetic resonance spectroscopy or the night before experimental day #2 whichever came first. bWelch two-sample t test. CV, coefficient of variance; HBGI, high blood glucose index; LBGI, low blood glucose index.
CV, coefficient of variance; HBGI, high blood glucose index; LBGI, low blood glucose index.
HbA1c and glucometabolic measures from OGTT
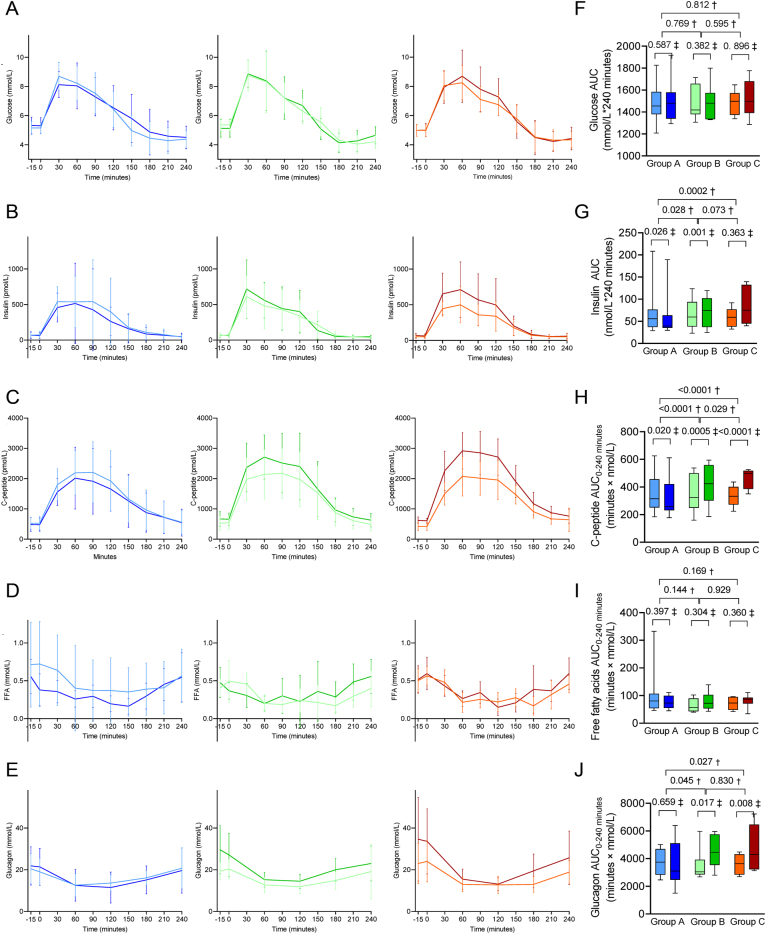
Administration of prednisolone increased HbA1c, insulinogenic index, and disposition index compared to placebo (Group B vs A), and adjuvant curcumin treatment did not affect these prednisolone-induced glucometabolic perturbations (Group C vs B) (Table 2). OGIS (Table 2), circulating concentrations of glucose and free fatty acids in the fasted state and during OGTT (as assessed by AUC and iAUC, respectively) were not significantly affected by any intervention (Table 2, Fig. 4). Serum insulin in the fasted state was not affected by prednisolone, but the insulin response to the OGTT was greater in Group B receiving prednisolone and curcumin placebo vs Group A receiving placebo only. Curcumin had no significant effect on this (Group C vs B) (Table 2, Fig. 4). C-peptide was also significantly increased by prednisolone both during fasting and in response to the OGTT. Co-treatment with curcumin further increased C-peptide levels compared to prednisolone treatment alone (Group C vs B) (Table 2, Fig. 4). Prednisolone increased plasma glucagon concentrations in the fasted state as well as during the OGTT (Group B vs A), and no effect of curcumin on this was observed (Group C vs B) (Table 2, Fig. 4).
Table 2.
Intervention effect on glucometabolic measures, FibroScan® measurements, parameters from MRI, anthropometric measurements, heart rate and blood pressure and biochemical parameters.
| Baseline outcome estimate (95% CI)a | Δ treatment A prednisolon placebo/curcumin placebo, estimate (95% CI)b | Δ treatment B prednisolone/curcumin placebo, estimate (95% CI)b | Δ treatment C prednisolone/curcumin, estimate (95% CI)b | ETD (B vs A), estimate (95% CI)c | P value (B vs A) | ETD (C vs A) estimate (95% CI)c | P value (C vs A) | ETD (C vs B) estimate (95% CI)c | P value (C vs B) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Glucometabolic parameters | ||||||||||
| OGIS | 415 (393; 438) | 3.0 (26; 32) | 3.2 (−25.2; 31.8) | −13.9 (−42.3; 14.6) | 0 (−38; 39) | 0.988 | −17 (−55;22) | 0.382 | −17 (−56; 21) | 0.374 |
| Insulinogenic index (pmol/mmol, %) | 39.7 (32.5; 48.5) | −18.9 (−32.1; −3.1) | 7.7 (−9.8; 28.7) | 31.3 (9.9; 56.9) | 32.7 (3.3; 70.6) | 0.028 | 61.9 (25.9; 108.0) | 0.0004 | 21.9 (−5.2; 56.7) | 0.118 |
| Disposition index (-, %) | 16319 (13695; 19447) | −17 (−32; 0.2) | 9 (−10; 33) | 26 (4; 53) | 32 (0.4; 74) | 0.046 | 52 (16; 101) | 0.003 | 15 (−12; 52) | 0.297 |
| HbA1c (mmol/mol)d | 33.3 (31.9; 34.6) | −0.8 (−1.6; 0.0) | 0.9 (0.0; 1.7) | 1.0 (0.1; 1.8) | 1.7 (0.5; 2.8) | 0.005 | 1.8 (0.6; 2.9) | 0.003 | 0.1 (−1.0; 1.2) | 0.860 |
| Glucose | ||||||||||
| Fasting (mmol/L)d | 5.2 (5.0; 4.3) | 0.2 (−0.2; 0.5) | −0.2 (−0.5; 0.2) | −0.1 (−0.4; 0.2) | −0.3 (−0.8; 0.2) | 0.207 | −0.3 (−0.7; 0.2) | 0.282 | 0.0 (−0.4; 0.5) | 0.848 |
| AUC (minutes × mmol/L) | 1481 (1421; 1541) | 24 (−64; 112) | 6 (−82; 94) | 39 (−50; 127) | −18 (−142; 106) | 0.769 | 15 (−109; 139) | 0.812 | 33 (−91; 157) | 0.595 |
| iAUC (minutes × mmol/L) | 240 (188; 291) | −13 (104; 78) | 55 (−36; 146) | 54 (−37; 145) | 68 (−60; 196) | 0.293 | 67 (−61; 195) | 0.299 | 1 (−129; 127) | 0.989 |
| Insulin | ||||||||||
| Fasting (pmol/L, %)d | 55 (45; 67) | −6 (−23; 15) | 5 (−14; 27) | 24 (2; 51) | 11 (−15; 45) | 0.428 | 32 (1; 73) | 0.044 | 19 (−9; 55) | 0.207 |
| AUC (minutes × nmol/L, %) | 58.5 (47.3; 72.0) | −18 (−31; −2) | 8 (−9; 28) | 34 (13; 50) | 31 (3; 67) | 0.028 | 64 (29; 108) | 0.0002 | 25 (−2; 58) | 0.073 |
| iAUC (minutes × nmol/L, %) | 44.6 (35.7; 55.7) | −21 (−34; −5) | 10 (−8; 32) | 36 (13; 63) | 39 (8; 80) | 0.014 | 72 (33;122) | 0.0001 | 24 (−5; 60) | 0.106 |
| C-peptide | ||||||||||
| Fasting (pmol L, %)d | 459 (390; 539) | −7 (−20; 7) | 31 (14; 52) | 45 (23; 68) | 41 (17; 71) | 0.0006 | 56 (29; 89) | < 0.0001 | 10 (−9; 33) | 0.299 |
| AUC (minutes × nmol/L, %) | 327.8 (285.3; 376.5) | −11 (−19; −2) | 20 (9; 32) | 39 (27; 54) | 35 (18; 54) | < 0.0001 | 57 (37; 79) | < 0.0001 | 16 (2; 33) | 0.029 |
| iAUC (minutes × nmol/L, %) | 213.9 (187.9; 248.8) | 13 (−21; −3) | 14 (3; 27) | 36 (22; 52) | 31 (12; 52) | 0.001 | 56 (33; 81) | < 0.0001 | 19 (2; 39) | 0.026 |
| Glucagon | ||||||||||
| Fasting (mmol / L, %)d | 20 (17; 23) | 9 (−7; 29) | 33 (13; 56) | 38 (17; 63) | 22 (−3; 53) | 0.095 | 27 (5; 29) | 0.045 | 4 (−17; 31) | 0.727 |
| AUC (minutes × mmol/L) | 3629 (3257; 4000) | −174 (−963; 615) | 970 (181; 1759) | 1090 (301; 1879) | 1144 (29; 2260) | 0.045 | 1264 (149; 2379) | 0.027 | 120 (−996; 1235) | 0.830 |
| iAUC (minutes × mmol/L) | −1401 (−1928; −875) | −767 (−1733; 199) | −930 (−1895; 36) | −1337 (−2303; −371) | −163 (−1520; 1195) | 0.810 | −570 (−1928; 788) | 0.402 | −407 (−1765; 950) | 0.549 |
| Free fatty acids | ||||||||||
| Fasting (mmol/L, %)d | 0.51 (0.42; 0.61) | −10 (−27; 12) | −9 (27; 12) | −15 (−31; 6) | 1 (−24; 33) | 0.969 | −6 (−29; 25) | 0.685 | −6 (−29; 24) | 0.657 |
| AUC (minutes × mmol/L, %) | 72 (59; 87) | −9 (−26; 13) | 12 (−10; 38) | 10 (−11; 27) | 22 (−7; 61) | 0.144 | 21 (−8; 29) | 0.169 | −1 (−25; 30) | 0.929 |
| iAUC (minutes × mmol/L) | −54 (−69; −40) | 25 (4; 47) | 25 (3; 46) | 4 (−18; 25) | −1 (−31; 29) | 0.966 | −22 (−52; 8) | 0.150 | 21 (−9; 51) | 0.162 |
| Anthropometric measurements | ||||||||||
| Body weight (kg) | 95.7 (92.0; 99.3) | 0.2 (−0.7; 1.0) | 0.3 (−0.6; 1.1) | 0.3 (−0.6; 1.2) | 0.1 (−1.1; 1.3) | 0.878 | 0.1 (−1.1; 1.4) | 0.823 | 0.0 (−1.2; 1.3) | 0.945 |
| BMI (kg/m2, %) | 29.83 (28.46; 31.27) | 0.24 (−0.69; 1.18) | 0.28 (−0.66; 0.12) | 0.29 (−0.54; 0.12) | 0.04 (−1.28; 1.37) | 0.954 | 0.06 (−1.26; 1.01) | 0.933 | 0.02 (−1.01; 1.3) | 0.979 |
| Waist circumference (cm) | 106.0 (102.1; 110.0) | 0.2 (−1.3; 1.7) | 0.6 (−0.8; 2.1) | 1.0 (−0.8; 2.7) | 0.5 (−1.6; 2.6) | 0.639 | 0.8 (−1.5; 3.0) | 0.480 | 0.3 (−1.9; 2.5) | 0.785 |
| Waist-hip-ratio | 1.04 (1.01; 1.06) | 0.01 (−0.01; 0.02) | 0.00 (−0.01; 0.02) | −0.01 (−0.03; 0.00) | −0.01 (−0.03; 0.01) | 0.508 | −0.02 (−0.04; 0.00) | 0.054 | −0.01 (−0.04; 0.01) | 0.181 |
| FibroScan® | ||||||||||
| CAP (dB) | 251 (211;291) | 14 (−20;49) | 35 (1; 70) | 2 (−36; 33) | 21 (−19; 61) | 0.292 | −16 (−56; 24) | 0.419 | −37 (−77; 3) | 0.067 |
| Liver stiffness (kPa) | 3.79 (3.38; 4.20) | 0.38 (0.35; 1.11) | 0.4 (−0.3; 1.1) | −0.02 (0.75; 0.71) | 0.00 (1.00; 1.00) | 0.997 | −0.40 (−1.40; 0.60) | 0.427 | −0.40 (−1.40; 0.60) | 0.425 |
| Magnetic resonance imaging | ||||||||||
| HFC (%) | 2.8 (1.8; 3.9) | −0.3 (−1.4; 0.8) | −0.1 (−1.2; 1.0) | 0.1 (−1.0; 1.2) | 0.2 (−1.3; 1.7) | 0.794 | 0.4 (−1.1; 1.9) | 0.578 | 0.2 (−1.3; 1.8) | 0.781 |
| SAT (cm3) | 274 (234; 313) | −1 (−8; 7) | 0.7 (−7.1; 8.4) | −4 (−12; 3) | 1 (−8; 11) | 0.780 | −4 (−13; 5) | 0.390 | −5 (−14; 4) | 0.269 |
| VAT (cm3) | 203 (151; 254) | 1 (−7; 8) | 3.9 (−4.1; 12.0) | −4 (−11; 4) | 3 (−8; 14) | 0.552 | −5 (−15; 6) | 0.380 | −8 (−19; 3) | 0.153 |
| Blood pressure and heart rate | ||||||||||
| Systolic blood pressure (mmHg) | 125 (120; 129) | −4 (−9; 1) | 2.1 (−2.9; 7.1) | 8 (3;13) | 6 (−1; 13) | 0.083 | 12 (5; 19) | 0.002 | 6 (−1; 12) | 0.117 |
| Diastolic blood pressure (mmHg) | 78 (75; 82) | −5 (−8; −2) | 1 (−1; 4) | 5 (3; 8) | 6 (3; 10) | 0.002 | 11 (7; 15) | < 0.0001 | 4 (0; 8) | 0.035 |
| Heart rate (b.p.m) | 62.4 (59.3; 65.4) | 3.5 (−1.4; 8.5) | −1.0 (−6.0; 3.9) | −6.0 (−10.9; −1.0) | −4.6 (−11.1; 1.9) | 0.157 | −9.5 (−16.0; −3.0) | 0.005 | −4.9 (−11.4; 1.6) | 0.135 |
| Biochemistry | ||||||||||
| High-density lipoprotein (mmol/L)d | 1.06 (0.99;1.13) | 0.06 (−0.05; 0.18) | 0.32 (0.21; 0.43) | 0.30 (0.18; 0.41) | 0.26 (0.10; 0.42) | 0.002 | 0.23 (0.08; 0.39) | 0.005 | −0.03 (−0.18; 0.13) | 0.726 |
| Triglyceride (mmol/L)d | 1.4 (0.9; 1.9) | −0.1 (−0.5; 0.3) | 0.1 (−0.4; 0.3) | 0.3 (−0.1; 0.6) | 0.0 (−0.4; 0.5) | 0.849 | 0.4 (−0.9; 0.8) | 0.111 | 0.3 (0.1; 0.8) | 0.159 |
| Bilirubin (µmol/L)d | 12.3 (9.3; 15.3) | 0.6 (−2.4; 3.5) | −0.3 (−3.3; 2.7) | 0.4 (−2.6; 3.3) | −0.9 (5.0; 3.3) | 0.683 | −0.2 (−4.4; 4.0) | 0.923 | 0.7 (−3.5; 4.8) | 0.755 |
| Albumin (g/L)d | 40.0 (39.1; 40.8) | 0.2 (−0.8; 1.1) | −2.8 (−3.8; −1.9) | −2.1 (−3.0; −1.2) | −3.0 (−4.3; −1.7) | < 0.0001 | −2.2 (−3.6; −0.9) | 0.001 | 0.8 (−0.6; 2.1) | 0.252 |
| Alkalic phosphatase (U/L)d | 70.9 (63.4; 78.4) | −1.5 (−5.1; 2.1) | 12.3 (−15.9; −8.7) | −8.2 (−11.8; −4.7) | −10.8 (−15.6; −6.1) | < 0.0001 | −6.8 (−11.5; −2.0) | 0.006 | 4.1 (−0.7; 8.6) | 0.092 |
| Alanine transaminase (U/L)d | 28.96 (25.61; 32.31) | 0.181 (−4.11; 4.47) | −0.92 (−5.21; 3.37) | 0.867 (−3.42; 5.16) | −1.104 (−7.17; 4.96) | 0.715 | 0.686 (−5.38; 6.75) | 0.821 | −1.79 (−7.85; 4.27) | 0.555 |
| Aspartate transaminase (U/L)d | 22.13 (19.61; 24.65) | −0.79 (−4.30; 2.71) | −7.88 (−11.39; −4.38) | −7.07 (−10.58; −3.57) | −7.09 (−11.47; −2.71) | 0.002 | −6.28 (−10.66; −1.90) | 0.006 | 0.81 (−3.57; 5.19) | 0.712 |
| LDH (U/L)d | 177 (167; 186) | −7 (−18; 5) | −8.8 (−19.6; 2.0) | −25 (−36; −13) | −2 (−17; 13) | 0.803 | −18 (−34; −2) | 0.027 | −16 (−31; −1) | 0.040 |
| GGT (U/L, %)d | 22.0 (17.8; 27.3) | −1.3 (−16.1; 15.9) | 23.8 (5.3; 45.5) | 14.5 (−2.6; 24.6) | 25.4 (0.2; 56.9) | 0.048 | 16.1 (−7.3; 45.2) | 0.188 | −7.5 (−26.1; 15.8) | 0.489 |
| Urea synthesis (mmol/minute)d | 0.342 (0.295; 0.390) | 0.007 (−0.084; 0.097) | −0.0 (−0.1; 0.1) | 0.010 (−0.084; 0.103) | −0.007 (−0.142; 0.127) | 0.912 | 0.003 (−0.126; 0.133) | 0.959 | 0.011 (−0.126; 0.148) | 0.875 |
| CRP (mg/L)d | 1.414 (1.004; 1.824) | 0.226 (−0.173; 0.624) | −0.632 (−1.031; −0.234) | −0.887 (−1.286; −0.489) | −0.858 (−1.321; −0.396) | 0.001 | −1.113 (−1.567; −0.650) | < 0.0001 | −0.255 (−0.717; 0.208) | 0.273 |
| Creatine kinase (U/L)d | 164 (126; 202) | −7 (−39; 25) | −71.8 (−102.1; −41.5) | −72 (−104; 41) | −65 (−103; −26) | 0.002 | −65 (−105; −26) | 0.002 | −1 (−39; 38) | 0.972 |
aBaseline outcome is mean for normally distributed outcomes/geometric mean for skewed outcomes. bΔ is estimated mean difference/relative difference in geometric means. cETD is baseline adjusted mean difference/relative difference in geometric means. All results are based on the constrained linear mixed model. The following outcomes were log-transformed prior to analysis due to skewness: insulinogenic index, disposition index, fasting levels of insulin, C-peptide, glucagon and free fatty acids, body mass index (BMI), gamma glutamyltransferase (GGT), area under the curve (AUC) of insulin, C-peptide, free fatty acids, incremental AUC (iAUC) of insulin and C-peptide. dConcentrations in the fasted state.
ALT, alanine transaminase; AST, aspartate transaminase; CAP, controlled attenuation parameter; CRP, C-reactive protein; ETD; estimated treatment difference; HbA1c, glycated haemoglobin A1c; HFC, hepatic fat content; HOMA2-IR, homeostatic model assessment of insulin resistance; LDH, lactate dehydrogenase; OGIS, oral glucose insulin sensitivity index; SAT, subcutaneous adipose tissue volume; VAT, visceral adipose tissue volume.
Figure 4.
Values (mean and SD) of glucose (A), insulin (B), C-peptide (C), free fatty acids (FFA) (D) and glucagon (E) measured during oral glucose tolerance test (OGTT) at baseline (light colours) and at end-of-treatment (EOT) (dark colours). The boxplots show median and IQR values for area under the curve (AUC) of the parameters measured during the OGTT (glucose (F), insulin (G), C-peptide (H), free fatty acids (I) and glucagon (J) at baseline (light colours) and at end-of-treatment (EOT) (dark colours)). Group A (blue): prednisolone placebo + curcumin placebo; Group B (green), prednisolone + curcumin placebo; Group C (red), prednisolone + curcumin. †P value for treatment difference (between-group comparisons), ‡P value for change from baseline to EOT (within-group comparisons) calculated using the constrained linear mixed model. Further statistical results can be found in Table 2.
Anthropometric measurements, bioelectrical impedance analysis, FibroScan® measures, hepatic fat content, SAT and VAT
We found no significant effect of prednisolone compared to placebo (Group B vs A) on anthropometrics, FibroScan® measures, or MRS-assessed hepatic fat content, SAT or VAT (Table 2) or bioelectrical impedance analysis (Supplementary Table 1, see section on supplementary materials given at the end of this article).
Blood pressure and heart rate
Systolic blood pressure was not changed by prednisolone compared to placebo (Group B vs A), and when adding curcumin as a co-treatment, a significant difference in systolic blood pressure was seen compared to the group receiving prednisolone placebo and curcumin placebo (Group C vs A), but not compared to the group receiving prednisolone and curcumin placebo (Group C vs B); thus, curcumin co-treatment had no statistically significant effect. Prednisolone increased diastolic blood pressure vs placebo (Group B vs A), and co-treatment with curcumin caused a significant increase compared to the group not receiving curcumin (Group C vs B); thus, curcumin added to the effects of prednisolone on blood pressure. Heart rate remained unchanged (Table 2).
Lipids, liver parameters and C-reactive protein
High-density lipoprotein and gamma glutamyltransferase (GGT) were increased, whereas albumin, alkalic phosphatase, aspartate aminotransferase, C-reactive protein (CRP), and creatine kinase were lowered by prednisolone when compared to placebo (Group B vs A), and curcumin did not affect these changes (Group C vs B) (Table 2). Triglycerides, bilirubin, alanine transaminase and urea production were not significantly affected by prednisolone. Lactate dehydrogenase (LDH) was not significantly affected by prednisolone treatment alone (Group B vs A), but co-treatment with curcumin resulted in a significant decrease in LDH (Group C vs B) and also when compared to the group receiving placebo (Group C vs A) (Table 2).
Discussion
We report that administration of the phenol curcumin, previously reported to protect against glucocorticoid-induced hepatic steatosis and ‘dysmetabolism’ in rodents and reduce HOMA-IR in dysmetabolic humans, did not ameliorate HOMA2-IR-assessed insulin resistance induced by a 10-day prednisolone treatment course (50 mg per day). Also, co-treatment with curcumin did not protect against prednisolone-induced increases in HbA1c, CGM measures, insulinogenic index, disposition index and circulating levels of insulin, C-peptide and glucagon.
In a mouse study, curcumin and dexamethasone treatment resulted in a greater plasma glucose-lowering response to an insulin tolerance test compared to mice treated with only dexamethasone, suggesting that curcumin decreases insulin resistance (20). The mechanisms behind curcumin-mediated amelioration of insulin resistance are unclear, but in vitro evidence suggests that curcumin increases glucose uptake in muscle cells via increased GLUT4 translocation and suppresses glycogenolysis and gluconeogenesis in hepatocytes (as reviewed by Den Hartogh and colleagues (34). In line with this, preclinical studies also indicate that curcumin counteracts glucocorticoid-induced gluconeogenesis (20, 35). We did not measure gluconeogenesis, but results from CGM showed increased time above range, mean interstitial glucose, coefficient of variance and high blood glucose index which could – at least partly – be explained by increased gluconeogenesis.
Curcumin has been shown to have advantageous effects on HOMA-IR, Quantitative Insulin Sensitivity Check Index, fasting glucose and insulin levels in several clinical studies in individuals with impaired glucose tolerance, obesity, type 2 diabetes and non-alcoholic fatty liver disease, respectively, not caused by glucocorticoids (28, 29, 36, 37). Such favourable effects were not seen in the present study investigating the effect of curcumin on prednisolone-induced glucometabolic perturbations. However, the individuals in our study were in general less dysmetabolic (before and after our prednisolone intervention) than individuals included in previous studies, which might partly explain lack of effect. In a mouse study, Tian and colleagues found that curcumin ameliorated dexamethasone-induced insulin resistance (20), but no previous human studies have investigated the effect of curcumin on glucocorticoid-induced insulin resistance. In another human study, half the dose of the same curcumin formulation (previously shown to be absorbed to the circulation after oral administration (31)) as used in the present study was administered for eight weeks. The study showed reduction in steatosis in people with non-alcoholic fatty liver disease (38). Another clinical study investigating a lower dose of unformulated curcuminoids administered for a 3-month period showed significant reduction of HOMA-IR in patients with overweight or obesity and type 2 diabetes (29). Based on these studies, we evaluated that the chosen dose of curcumin (400 mg/day) was sufficient for the purpose of the present study, but, nevertheless, we cannot exclude that (1) a higher dose of curcumin and/or a longer treatment duration may counteract high-dose prednisolone-induced increase in HOMA-IR, and (2) the dose used in the present study may be sufficient to protect from low-dose glucocorticoid-induced adverse metabolic effects.
In line with what has been found in other trials (14, 41), prednisolone increased circulating insulin and C-peptide concentrations during OGTT. co-administration of curcumin potentiated this increase, without affecting insulin resistance, which is in line with data indicating that curcumin increases insulin secretion (40, 41, 42). The insulin-promoting effect of curcumin may be via direct effects on pancreatic beta cells as several studies reported insulinotropic effects of curcumin in in vitro experiments performed on beta cell lines and isolated pancreatic islets (reviewed by Den Hartogh and colleagues(34). Curcumin may also cause beta cell hypertrophy and proteasome inhibition which could positively affect insulin secretion (40).
Circulating glucagon concentrations are known to increase as a result of glucocorticoid treatment (9, 43). A study in mice indicated that chronic mild stress increased corticosterone concentration accompanied by increased circulating glucagon levels, which could be ameliorated by concomitant curcumin treatment (44). The effect of curcumin on circulating glucagon concentrations is uncertain and the present results indicate that curcumin does not affect prednisolone-induced hyperglucagonaemia in humans. A previous study by our group in obese individuals showed no effects of curcumin on circulating glucagon concentrations after 6 weeks treatment (45).
Long-term use of glucocorticoid treatment is known to induce steatosis (46), but short-term use has not been found to change hepatic fat content in healthy young men (10). In the present study, we found no effect of a 10-day prednisolone treatment course (50 mg per day) on hepatic fat content in overweight/obese non-diabetic individuals, and, accordingly, no beneficial effects of curcumin treatment were observed.
We observed a significant prednisolone-induced increase in diastolic blood pressure, and co-treatment with curcumin further increased diastolic blood pressure. The increase in blood pressure could be due to prednisolone’s mineralocorticoid effects (47). To our knowledge, curcumin has not previously been found to increase blood pressure under normal physiological conditions, as also reported in a meta-analysis (48). However, with concomitant prednisolone treatment, we speculate that curcumin may increase the level of prednisolone in the active form in the distal nephron due to curcumin-induced inhibition of the enzyme 11-beta-hydroxysteroid dehydrogenase-2 (49), resulting in increased diastolic blood pressure.
Strengths of the present study include the use of high-dose curcumin formulated with lecithin, which is known to have a high bioavailability (31, 50) and to be well-tolerated in humans (51, 52). The three-armed design with two placebo treatments and the use of both OGTT, HbA1c and CGM, provide a broad overview of prednisolone’s and curcumin’s glucometabolic effects. By enrolling overweight/obese otherwise healthy individuals, we augmented the probability of detectable prednisolone-induced adverse glucometabolic effects and, thus, relevant curcumin treatment targets. Limitations of our study include a small sample size, a sample size calculation based on suboptimal data and that P values were not adjusted for multiple testing, as we did not predefine secondary and exploratory endpoints. Therefore, results for other endpoints than our primary endpoint should be interpreted with caution.
In conclusion, the present study did not show beneficial effects of curcumin co-administered with a 10-day prednisolone treatment course (50 mg/day), suggesting curcumin to be ineffective in mitigating prednisolone-induced changes in insulin resistance as estimated by HOMA2-IR and other glucometabolic parameters. However, our study is limited by a small sample size inferring that a larger study with a longer treatment period and/or a higher dose of curcumin is warranted to fully clarify if curcumin has potential to ameliorate glucocorticoid-induced insulin resistance in humans.
Supplementary Material
Declaration of interest
All authors declare that there are no conflicts of interests associated with this manuscript.
Funding
The authors thank Indena S.p.A. Milan, Italy, for sponsoring curcumin and curcumin placebo, and Beckett Foundation and Tømmerhandler Vilhelm Bangs Foundation for providing funding for the study.
Data availability statement
Data are available on request by contacting corresponding author.
Author contribution statement
PHH, JIB, KBH, TV and FKK contributed to the study design. PHH, JIB, KBH, KRC, JJH, JS, EC, TV and FKK analysed and interpreted data. PHH and KRC included participants and acquired data. JF, JIB and PHH performed the statistical analyses. PHH and FKK wrote the manuscript. All authors revised the manuscript and approved the final version for submission.
Acknowledgements
The authors thank all participants and colleagues in Center for Clinical Metabolic Research and Steno Diabetes Center Copenhagen, who contributed with help and expertise. Also, acknowledgement to Giorgia Centorame, who helped during experimental days. The authors also thank Indena S.p.A. Milan, Italy, for sponsoring curcumin and curcumin placebo, and Beckett Foundation and Tømmerhandler Vilhelm Bangs Foundation for providing funding for the study.
References
- 1.Greaves M & Gatti S. The use of glucocorticoids in dermatology. Journal of Dermatological Treatment 19991083–91. ( 10.3109/09546639909056008) [DOI] [Google Scholar]
- 2.Triadafilopoulos G. Glucocorticoid therapy for gastrointestinal diseases. Expert Opinion on Drug Safety 201413563–572. ( 10.1517/14740338.2014.904852) [DOI] [PubMed] [Google Scholar]
- 3.van der Goes MC Jacobs JW & Bijlsma JW. The value of glucocorticoid co-therapy in different rheumatic diseases--positive and adverse effects. Arthritis Research and Therapy 201416(Supplement 2) S2. ( 10.1186/ar4686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willox JC Corr J Shaw J Richardson M Calman KC & Drennan M. Prednisolone as an appetite stimulant in patients with cancer. British Medical Journal 1984288 27. ( 10.1136/bmj.288.6410.27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy TE Pauli F Sprouse RO Neff NF Newberry KM Garabedian MJ & Myers RM. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Research 2009192163–2171. ( 10.1101/gr.097022.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Raalte DH Ouwens DM & Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? European Journal of Clinical Investigation 20093981–93. ( 10.1111/j.1365-2362.2008.02067.x) [DOI] [PubMed] [Google Scholar]
- 7.Nielsen MF Caumo A Chandramouli V Schumann WC Cobelli C Landau BR Vilstrup H Razzo RA & Schmitz O. Impaired basal glucose effectiveness but unaltered fasting glucose release and gluconeogenesis during short-term hypercortisolemia in healthy subjects. American Journal of Physiology-Endocrinology and Metabolism 2004286E102–E110. ( 10.1152/ajpendo.00566.2002) [DOI] [PubMed] [Google Scholar]
- 8.Hansen KB Vilsbøll T Bagger JI Holst JJ & Knop FK. Reduced glucose tolerance and insulin resistance induced by steroid treatment, relative physical inactivity, and HighCalorie diet impairs the incretin effect in healthy subjects. Journal of Clinical Endocrinology and Metabolism 2010953309–3317. ( 10.1210/jc.2010-0119) [DOI] [PubMed] [Google Scholar]
- 9.Hansen KB Vilsbøll T Bagger JI Holst JJ & Knop FK. Increased postprandial GIP and glucagon responses, but unaltered GLP-1 response after intervention with steroid hormone, relative physical inactivity, and high-calorie diet in healthy subjects. Journal of Clinical Endocrinology and Metabolism 201196447–453. ( 10.1210/jc.2010-1605) [DOI] [PubMed] [Google Scholar]
- 10.Raalte van DH Brands M Zijl van der NJ Muskiet MH Pouwels PJW Ackermans MT Sauerwein HP Serlie MJ & Diamant M. Low-dose glucocorticoid treatment affects multiple aspects of intermediary metabolism in healthy humans: a randomised controlled trial. Diabetologia 2011542103–2112. ( 10.1007/s00125-011-2174-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raalte van DH Nofrate V Bunck MC Iersel van T Schaap JE Nässander UK Heine RJ Mari A Dokter WHA & Diamant M. Acute and 2-week exposure to prednisolone impair different aspects of beta-cell function in healthy men. European Journal of Endocrinology 2010162729–735. ( 10.1530/EJE-09-1034) [DOI] [PubMed] [Google Scholar]
- 12.Hollingdal M Juhl CB Dall R Sturis J Veldhuis JD Schmitz O & Pørksen N. Glucocorticoid induced insulin resistance impairs basal but not glucose entrained highfrequency insulin pulsatility in humans. Diabetologia 20024549–55. ( 10.1007/s125-002-8244-y) [DOI] [PubMed] [Google Scholar]
- 13.Woods CP Hazlehurst JM & Tomlinson JW. Glucocorticoids and non-alcoholic fatty liver disease. Journal of Steroid Biochemistry and Molecular Biology 201515494–103. ( 10.1016/j.jsbmb.2015.07.020) [DOI] [PubMed] [Google Scholar]
- 14.Zhou PZ Zhu YM Zou GH Sun YX xiu XL Huang X & Zhang QH. Relationship between glucocorticoids and insulin resistance in healthy individuals. Medical Science Monitor 2016221887–1894. ( 10.12659/MSM.895251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicod N Giusti V Besse C & Tappy L. Metabolic adaptations to DexamethasoneInduced insulin resistance in healthy volunteers. Obesity Research 200311625–631. ( 10.1038/oby.2003.90) [DOI] [PubMed] [Google Scholar]
- 16.Kautzky-Willer A Thomaseth K Clodi M Ludvik B Waldhäusl W Prager R & Pacini G. β-cell activity and hepatic insulin extraction following dexamethasone administration in healthy subjects. Metabolism: Clinical and Experimental 199645486–491. ( 10.1016/S0026-0495(9690224-3) [DOI] [PubMed] [Google Scholar]
- 17.Besse C Nicod N & Tapy L. Changes in insulin secretion and glucose metabolism induced by dexamethasone in lean and obese females. Obesity Research 200513306–311. ( 10.1038/oby.2005.41) [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Standards of medical Care in diabetes—2022. Clinical Diabetes 20224010–38. ( 10.2337/cd22-as01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L Pan Y Peng K Wang Z Li J Li D Tong C Wang Y & Liang G. Inhibition of 11βHSD1 by LG13 improves glucose metabolism in type 2 diabetic mice. Journal of Molecular Endocrinology 201555119–131. ( 10.1530/JME-14-0268) [DOI] [PubMed] [Google Scholar]
- 20.Tian L Zeng K Shao W Yang BB Fantus IG Weng J & Jin T. Short-term curcumin gavage sensitizes insulin signaling in dexamethasone-treated C57BL/6 mice. Journal of Nutrition 20151452300–2307. ( 10.3945/jn.115.216853) [DOI] [PubMed] [Google Scholar]
- 21.Maithilikarpagaselvi N Sridhar MG Swaminathan RP Sripradha R & Badhe B. Curcumin inhibits hyperlipidemia and hepatic fat accumulation in high-fructose-fed male Wistar rats. Pharmaceutical Biology 2016542857–2863. ( 10.1080/13880209.2016.1187179) [DOI] [PubMed] [Google Scholar]
- 22.Hasan ST Zingg JM Kwan P Noble T Smith D & Meydani M. Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis 201423240–51. ( 10.1016/j.atherosclerosis.2013.10.016) [DOI] [PubMed] [Google Scholar]
- 23.Ding L Li J Song B Xiao X Zhang B Qi M Huang W Yang L & Wang Z. Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicology and Applied Pharmacology 201630499–109. ( 10.1016/j.taap.2016.05.011) [DOI] [PubMed] [Google Scholar]
- 24.Feng WW, Kuang SY, Tu C, Ma ZJ, Pang JY, Wang YH, Zang QC, Liu TS, Zhao YL, Xiao XH, et al. Natural products berberine and curcumin exhibited better ameliorative effects on rats with non-alcohol fatty liver disease than lovastatin. Biomedicine and Pharmacotherapy 201899325–333. ( 10.1016/j.biopha.2018.01.071) [DOI] [PubMed] [Google Scholar]
- 25.Liu Y Cheng F Luo Y Zhan Z Hu P Ren H Tang H & Peng M. Pegylated curcumin derivative attenuates hepatic steatosis via CREB/PPAR-γ/CD36 pathway. BioMed Research International 20172017 8234507. ( 10.1155/2017/8234507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao W Yu Z Chiang Y Yang Y Chai T Foltz W Lu H Fantus IG & Jin T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One 20127 e28784. ( 10.1371/journal.pone.0028784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuengsamarn S Rattanamongkolgul S Luechapudiporn R Phisalaphong C & Jirawatnotai S. Curcumin extract for prevention of Type 2 diabetes. Diabetes Care 2012352121–2127. ( 10.2337/dc12-0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cicero AFG Sahebkar A Fogacci F Bove M Giovannini M & Borghi C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: a double-blind, placebo-controlled clinical trial. European Journal of Nutrition 202059477–483. ( 10.1007/s00394-019-01916-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Na LX Li Y Pan HZ Zhou XL Sun DJ Meng M Li XX & Sun CH. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a doubleblind, placebo-controlled trial. Molecular Nutrition and Food Research 2013571569–1577. ( 10.1002/mnfr.201200131) [DOI] [PubMed] [Google Scholar]
- 30.Kotha RR & Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 201924. ( 10.3390/molecules24162930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuomo J Appendino G Dern AS Schneider E KcKinnon TP Brown MJ Togni S & Dixon BM. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. Journal of Natural Products 201174664–669. ( 10.1021/np1007262) [DOI] [PubMed] [Google Scholar]
- 32.Orskov C Jeppesen J Madsbad S & Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. Journal of Clinical Investigation 199187415–423. ( 10.1172/JCI115012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzmaurice GM Laird NM & Ware JH. Applied Longitudinal Analysis 2nd ed.New Jersey: John Wiley&Sons; 2011. [Google Scholar]
- 34.Den Hartogh DJ Gabriel A & Tsiani E. Antidiabetic properties of curcumin I: evidence from in vitro studies. Nutrients 202012. ( 10.3390/nu12010118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim T Davis J Zhang AJ He X & Mathews ST. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochemical and Biophysical Research Communications 2009388377–382. ( 10.1016/j.bbrc.2009.08.018) [DOI] [PubMed] [Google Scholar]
- 36.Jazayeri-Tehrani SA Rezayat SM Mansouri S Qorbani M Alavian SM DaneshiMaskooni M & Hosseinzadeh-Attar MJ. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutrition and Metabolism 2019168. ( 10.1186/s12986-019-0331-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navekar R Rafraf M Ghaffari A Asghari-Jafarabadi M & Khoshbaten M. Turmeric supplementation improves serum glucose indices and leptin levels in patients with nonalcoholic fatty liver diseases. Journal of the American College of Nutrition 201736261–267. ( 10.1080/07315724.2016.1267597) [DOI] [PubMed] [Google Scholar]
- 38.Panahi Y Kianpour P Mohtashami R Jafari R Simental-Mendía LE & Sahebkar A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Research (Stuttg) 201767244–251. ( 10.1055/s-0043-100019) [DOI] [PubMed] [Google Scholar]
- 39.Abdelmannan D Tahboub R Genuth S & Ismail-Beigi F. Effect of dexamethasone on oral glucose tolerance in healthy adults. Endocrine Practice 201016770–777. ( 10.4158/EP09373.OR) [DOI] [PubMed] [Google Scholar]
- 40.Weisberg S Leibel R & Tortoriello DV. Proteasome inhibitors, including curcumin, improve pancreatic β-cell function and insulin sensitivity in diabetic mice. Nutrition and Diabetes 20166 e205. ( 10.1038/nutd.2016.13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semwal DK Kumar A Aswal S Chauhan A & Semwal RB. Protective and therapeutic effects of natural products against diabetes mellitus via regenerating pancreatic β-cells and restoring their dysfunction. Phytotherapy Research 2021351218–1229. ( 10.1002/ptr.6885) [DOI] [PubMed] [Google Scholar]
- 42.Wickenberg J Ingemansson SL & Hlebowicz J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutrition Journal 20109 43. ( 10.1186/1475-2891-9-43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolthers T Hamberg O Grøfte T & Vilstrup H. Effects of budesonide and prednisolone on hepatic kinetics for urea synthesis. Journal of Hepatology 200033549–554. ( 10.1034/j.1600-0641.2000.033004549.x) [DOI] [PubMed] [Google Scholar]
- 44.Shen JD Wei Y Li YJ Qiao JY & Li YC. Curcumin reverses the depressive-like behavior and insulin resistance induced by chronic mild stress. Metabolic Brain Disease 2017321163–1172. ( 10.1007/s11011-017-0017-1) [DOI] [PubMed] [Google Scholar]
- 45.Hellmann PH Bagger JI Carlander KR Forman JL Chabanova E Svenningsen JS Holst JJ Gillum MP Vilsbøll T & Knop FK. The effect of curcumin on hepatic fat content in individuals with obesity. Diabetes, Obesity and Metabolism 2022242192–2202. ( 10.1111/dom.14804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahimi L Rajpal A & Ismail-Beigi F. Glucocorticoid-induced fatty liver disease. Diabetes, Metabolic Syndrome and Obesity : Targets and Therapy 2020131133–1145. ( 10.2147/DMSO.S247379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torpy DJ Mullen N Ilias I & Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion: a series of 58 cases. Annals of the New York Academy of Sciences 2002970134–144. ( 10.1111/j.1749-6632.2002.tb04419.x) [DOI] [PubMed] [Google Scholar]
- 48.Hadi A Pourmasoumi M Ghaedi E & Sahebkar A. The effect of curcumin/Turmeric on blood pressure modulation: a systematic review and meta-analysis. Pharmacological Research 2019150 104505. ( 10.1016/j.phrs.2019.104505) [DOI] [PubMed] [Google Scholar]
- 49.Lin H Hu GX Guo J Ge Y Liang G Lian QQ Chu Y Yan X Huang P & Ge RS. Mono-carbonyl curcumin analogues as 11β-hydroxysteroid dehydrogenase 1 inhibitors. Bioorganic and Medicinal Chemistry Letters 2013234362–4366. ( 10.1016/j.bmcl.2013.05.080) [DOI] [PubMed] [Google Scholar]
- 50.Marczylo TH Verschoyle RD Cooke DN Morazzoni P Steward WP & Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemotherapy and Pharmacology 200760171–177. ( 10.1007/s00280-006-0355-x) [DOI] [PubMed] [Google Scholar]
- 51.Lao CD Ruffin MT Normolle D Heath DD Murray SI Bailey JM Boggs ME Crowell J Rock CL & Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complementary and Alternative Medicine 20066 10. ( 10.1186/1472-6882-6-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chainani-Wu N Madden E Lozada-Nur F & Silverman S. High-dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. Journal of the American Academy of Dermatology 201266752–760. ( 10.1016/j.jaad.2011.04.022) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request by contacting corresponding author.



 This work is licensed under a
This work is licensed under a