Abstract
Atrial fibrillation (AF) is the most prevalent arrhythmia worldwide. The presence of AF is associated with increased risk of systemic thromboembolism, but with the uptake of oral anticoagulant (OAC) and implementation of a holistic and integrated care management, this risk is substantially reduced. The diagnosis of AF requires a 30-s-long electrocardiographic (ECG) trace, irrespective of the presence of symptoms, which may represent the main indication for an ECG tracing. However, almost half patients are asymptomatic at the time of incidental AF diagnosis, with similar risk of stroke of those with clinical AF. This has led to a crucial role of screening for AF, to increase the diagnosis of population at risk of clinical events. The aim of this review is to give a comprehensive overview about the epidemiology of asymptomatic AF, the different screening technologies, the yield of diagnosis in asymptomatic population, and the benefit derived from screening in terms of reduction of clinical adverse events, such as stroke, cardiovascular, and all-cause death. We aim to underline the importance of implementing AF screening programmes and reporting about the debate between scientific societies’ clinical guidelines recommendations and the concerns expressed by the regulatory authorities, which still do not recommend population-wide screening. This review summarizes data on the ongoing trials specifically designed to investigate the benefit of screening in terms of risk of adverse events which will further elucidate the importance of screening in reducing risk of outcomes and influence and inform clinical practice in the next future.
Keywords: Atrial fibrillation, Screening strategies, Review, Stroke
Introduction
Atrial fibrillation (AF) is one of the most prevalent cardiovascular conditions, being present in more than 59 million people worldwide.1 The presence of AF is associated with an increased risk of thromboembolic and clinical events, which has been mitigated by the broad use of oral anticoagulant (OAC), and by the implementation of holistic integrated care management.2–4 Patients with AF may suffer of AF-related symptoms, including palpitations, chest pain, shortness of breath, and fatigue, and in these occasions, AF diagnosis could be documented through a 30-second electrocardiography (ECG) tracing.2 However, the diagnosis of clinical AF does not necessarily requires a symptomatic presentation; indeed, according to the 2020 European Society of Cardiology (ESC) guidelines for the management of AF, clinical AF is defined by ECG documentation (12-lead ECG recording or a single-lead ECG tracing of ≥30 seconds) irrespective of the presence of specific symptoms.2
Diagnosis of AF is crucial to implement strategies to reduce thromboembolic risk, particularly treatment with OAC. Many patients have no symptoms, and unrecognized AF becomes overt only when complications occur. The true proportion of patients with asymptomatic AF (AAF) is unknown; a meta-analysis including 81 462 patients provided a pooled estimate of 26%,5 even though other studies reported higher prevalence, up to 45%.6 The rationale for implementing screening for AF is based on data revealing that patients with AAF have similar risk of stroke compared to that of patients with clinical AF7 and that at least half of AAF patients might be eligible for anticoagulation.8
Current AF guidelines recommend opportunistic screening for AF;2 however, recently, several studies have focused on the potential role of the active screening. Indeed, the eHealth-based Bavarian Alternative Detection of Atrial Fibrillation (eBRAVE-AF) trial9 showed a two-fold increase in the detection of AF requiring OAC, by using a smart device compared to usual screening.
The aim of this review is to provide a comprehensive summary of the evidence regarding epidemiology of AAF, impact of screening on its detection, the techniques and methods used to perform such screening, the association with risk of stroke and other adverse outcomes, and impact of OAC prescription according to AAF detection.
Epidemiology of asymptomatic atrial fibrillation and risk of outcomes
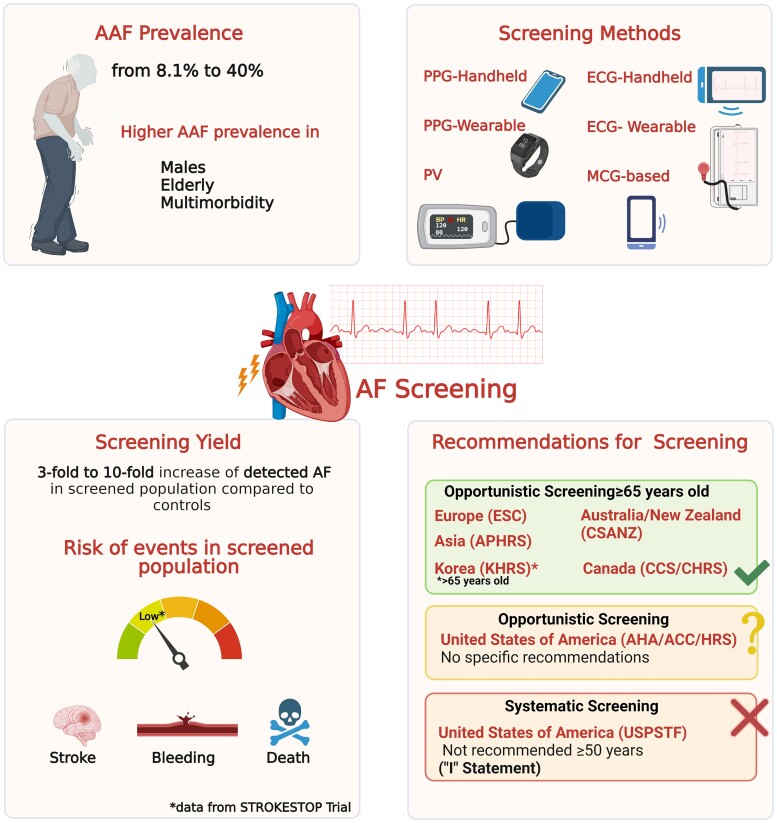
The analysis of the actual burden of AAF is challenging, and its prevalence may be largely underestimated. AAF is often diagnosed in specific clinical settings, including pre-surgical assessment, screening programmes, workup of cryptogenic strokes, and implantable devices interrogations.10–14 Previous studies reported a prevalence of AAF ranging from 10% to 40% depending on several factors (e.g. study design, population studied, patient’s risk profile, geographical differences, etc.).
The pathophysiological mechanisms underlying the different clinical manifestations in AF patients (i.e. asymptomatic vs. symptomatic) are not yet completely understood. A higher prevalence of AAF has been reported among males and elderly and in patients with a high burden of comorbidities. However, short AF episodes may also be detected in patients who have a relatively low burden of comorbidity and are in the early phase of the disease.15
The development of AF-related symptoms is largely due to haemodynamic impairment and fast, irregular ventricular response. However, other factors—such as younger age, female sex, and hypertension—may be associated with the development of symptoms in AF patients, as suggested by the Canadian Registry of Atrial Fibrillation (CARAF) study.16 More recently, contemporary AF registries confirmed that almost one-third of AF patients are asymptomatic or present mild symptoms. In the Prevention of Thromboembolic Events—European Registry in Atrial Fibrillation (PREFER in AF) study, 8.1% of patients were classified as asymptomatic (EHRA I), and 37.9% were classified as having mild symptoms (EHRA II). Of note, the study found that there was a significantly lower proportion of females in the asymptomatic group (22.8% vs. 41.2%) compared to symptomatic patients.17 In the EURObservational Research Programme-Atrial Fibrillation (EORP-AF) General Pilot Registry, approximately 40% of the AF cases enrolled were asymptomatic (EHRA I).18 Male sex, older age, previous myocardial infarction, and limited physical activity were significantly associated with AAF; interestingly, permanent AF was three-fold more common among asymptomatic patients.18 Similarly, the Global Anticoagulant Registry in the Field-Atrial Fibrillation (GARFIELD-AF) recently confirmed these findings, reporting that at presentation, 25.4% of patients were asymptomatic, with a higher prevalence of AAF among elderly and males.7
From a clinical perspective, it is crucial to determine if AAF patients have lower rates of adverse outcomes compared to symptomatic AF patients. A recent systematic review and meta-analysis,5 including more than 80 000 patients, found that patients with AAF and symptomatic AF had the same thromboembolic risk and there were no differences in the risk of stroke and other major outcomes. Hence, the thromboembolic risk prevention should not be limited to symptomatic clinical AF.7,19,20 In a recent paper published by Wallenhorst and colleagues, reporting data about more than 22 000 with incident AF, matched to non-AF controls, ambulatory AAF patients had similar risk of stroke than ambulatory patients with symptomatic AF as well as hospitalized AF patients, both having AF as primary and secondary diagnosis.21 Conversely, risk of all-cause death was significantly higher in hospitalized patients, particularly those having AF as a secondary diagnosis, while it was similar between ambulatory symptomatic and asymptomatic ones.21 Considering the high prevalence of AAF, underdiagnosis and undertreatment of these patients may expose them to a higher risk of adverse cardiovascular events. Therefore, it appears clear how the role of screening is increasingly important to identify AAF patients, especially in high-risk populations.22
Appropriate early management of AAF patients has yet to be fully investigated and particularly in terms of rhythm or rate control management. The ongoing Comparison Study of Drugs for Symptom Control and Complication Prevention of Atrial Fibrillation (CODE-AF) prospective registry interestingly found that rhythm control was associated with a significant reduction in adverse cardiovascular events in AAF patients; these findings were confirmed also in patients with high thromboembolic risk (CHA2DS2-VASc score ≥3).23 Recently, an Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4) subanalysis24 evaluated the effect of early rhythm control therapy in asymptomatic patients (EHRA I) compared to symptomatic patients. The results showed that the clinical benefit of early systematic rhythm control was similar between asymptomatic and symptomatic patients, suggesting that a rhythm control strategy may be beneficial also in AAF patients.
Strategies for atrial fibrillation screening
The need for an earlier AF detection and implementation of an integrated care management approach raises the urgency regarding the use of mobile health (mHealth) devices in cardiology that could facilitate detection and management of AF.25–27 Traditional classification of mHealth devices identifies three groups, based on different technologies, namely, devices using photoplethysmography (PPG), pulse variability (PV), or ECG traces, based on mechanocardiography (MCG). All these mHealth solutions are also supported by systems which allow healthcare professionals’ referral. However, the overall accuracy varies widely,28,29 depending on the technology, the type of the devices (handheld vs. wearable), the study population (hospitalized vs. general population), and the AF detection monitoring method (intermittent vs. continuous monitoring).30,31
PPG devices implement an optical method, which identifies variations in peak-to-peak intervals and the pulse morphology of the illuminated microvascular blood to detect AF. Handled PPG-based devices like FibriCheck,32 CardioRhythm,33 and Preventicus9,34 typically use the smartphone camera flashlight as light source, with variable duration, and the accuracy of detecting AF is unaffected by the length of the recording.29 The most promising mHealth devices are PPG-based wearable technology35 which includes smartwatches (such as the Huawei Watch GT, Apple Watch, or Amazfit Health Band), wristbands (e.g. Samsung Simband, Fitbit devices), armbands, finger-bands, and earlobe sensor devices. To date, the sensitivity (Sn) and specificity (Sp) of validated wearable PPG devices vary from 67.7% to 100% and 60.7% to 100%, respectively.36
Those variations are the result of various reference tests and monitoring durations (Table 1).
Table 1.
Studies investigating atrial fibrillation screening strategies
| Study | Design | N | F (%) | Agea | Main characteristics | Country | Devices | Monitoring time | % AF | Sn (%) | Sp (%) | Reference test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPG-handheld | ||||||||||||
| Yan et al.33 | PSA | 217 | 28.6 | 70.3 | Hospitalized | China | CardioRhythm/smartphone camera | 20 s × 3 s.t. | 34.6 | 95 | 96 | 12-l-ECG |
| Verbrugge et al.32 | PSA | 12 328 | 42 | 49 | General | Belgium | FibriCheck/smartphone camera | 7 d | 0.01 | — | — | — |
| Brasier et al.34 | PSA | 592 | 45.3 | 78 | Hospitalized | Germany/Switzerland | Preventicus/smartphone camera | 5 m s.t. | 41.9 | 91.5 | 99.6 | s-l-ECG |
| Rizas et al.9 | RCT | 5551 | 31 | n.a. | >65 y.o. | Germany | Preventicus/smartphone camera | 6 m | 1.33 | 89.9 | 99.1 | 12-l-ECG Holter |
| PPG-wearable | ||||||||||||
| Guo et al.26 | PSA | 187 912 | 13.3 | 34.7 | Amb. | China | HonorBand, Huawei Watch GT/wristband and wristwatch | 60 s every 10 m for 14 d | 87 | n.a. | n.a. | 12-l-ECG; 24-h-Holter |
| Zhang et al.37 | PSA | 361 | 49.3 | 50 | Amb. | China | HonorBand, Huawei Watch GT/wristband and wristwatch | 60–45 s every 10 m for 14 d | 8.6 | 100 | 100 | 12-l-ECG |
| Perez et al.38 | mPSA | 419 297 | 42 | 41 | General | USA | Apple Watch/wristwatch (PPG) | 117 d | 0.52 | — | — | — |
| Chen et al.39 | PR | 401 | 49.1 | n.a. | Hosp./Amb. | China | Amazfit/Wristband | 3 m | 37 | 88 | 96.4 | 12-l-ECG |
| Nemati et al.40 | RSA | 46 | n.a. | n.a. | Hospitalized | USA | Samsung Simband/wristwatch | 3.5–8.5 m | 33 | 97 | 94 | s-l-ECG |
| Lubitz et al.41 | PSA | 1057 | n.a | n.a | ≥22 y.o. | USA | Fitbit | 122 d | 32.2 | 67.6 | 98.4 | ECG patch monitor |
| PV-based | ||||||||||||
| Marazzi et al.42 | PSA | 503 | 45.7 | 67 | Hypertension | Italy | Microlife (1) and Omron (2)/sphygmomanometer | 3 s.t. | 20 | (1) 92 (2) 100 | (1) 97 (2) 94 | 12-l-ECG |
| ECG-handheld | ||||||||||||
| Boriani et al.22 | PSA | 2814 | 55.5 | 66 | General | Italy | MyDiagnostick/stick | 1 m s.t. | 2.0 | 98.2 | 23.6 | 12-l-ECG |
| Battipaglia et al.43 | PSA | 855 | n.a. | n.a. | General | UK | MyDiagnostik/stick | 15 s s.t. | 0.8 | 100 | 100 | e.d. |
| Desteghe et al.44 | PTA | 445 | 58.9 | 72.2 | Hospitalized | Belgium | MyDiagnostick/stick | 1 m s.t. | 11.9–36 | 60.5–81.8 | 93.3–96.1 | 12-l-ECG |
| Kaasenbrood et al.45 | PSA | 3269 | 51 | 69.4 | General | Netherlands | MyDiagnostick/stick | 1 m s.t. | 3.7 | 96 | 100 | e.d. |
| Rivezzi et al.46 | PSA | 1820 | 53.4 | ≥65 yo | ≥65 yo | Italy | MyDiagnostick/stick | 1 m s.t. | 5.5 | 94 | 100 | 12-l-ECG |
| Tavernier et al.47 | PSA | 214 | 61.7 | 84 | Hospitalized | Belgium | MyDiagnostick/stick | 1 m s.t. | 33 | 88 | 97 | 12-l-ECG |
| Chan et al.48 | PSA | 1013 | 53.2 | 68.4 | ≥65 yo | China | KardiaMobile/plate | 30 s s.t. | 2.8 | 71.4 | 99.4 | e.d. |
| Chan et al.49 | PSA | 2052 | 54.2 | 67.8 | ≥65 yo | China | KardiaMobile/plate | 30 s s.t. | 1.2 | 66.7 | 99.5 | 12-l-ECG |
| Chan et al.50 | PTA | 13 122 | 71.5 | 64.7 | General | China | KardiaMobile/plate | 30 s s.t. | 1.8 | 98 | 29.2 | e.d. |
| Desteghe et al.44 | PTA | 445 | 58.9 | 72.2 | Hospitalized | Belgium | KardiaMobile/plate | 30 s s.t. | 11.9–36 | 36.8–72.7 | 96.1–98.1 | 12-l-ECG |
| Orchard et al.51 | RCT | 3103 | 36 | 75.1 | ≥65 yo | Australia | KardiaMobile/plate | 30 s s.t. | 1.2 | 97 | 92 | 12-l-ECG |
| Lowres et al.52 | PSA | 1000 | 56 | 76 | ≥65 yo | Australia | KardiaMobile/plate | 30–60 s s.t. | 6.7 | 98.5 | 91.4 | 12-l-ECG |
| Soni et al.53 | PSA | 2074 | 52.2 | 33.7% ≥66 yo | General | India | KardiaMobile/plate | 30 s 2–3 t./5 d | 1.6 | 38 | n.a. | e.d. |
| Zaprutko et al.54 | PSA | 525 | 68.2 | 73.7 | ≥65 yo | Poland | KardiaMobile/plate | 30 s s.t. | 2.3 | 100 | 98.7 | e.d. |
| ECG-wearable | ||||||||||||
| Lown et al.55 | mPSA | 418 | n.a. | n.a. | ≥65 yo | UK | Polar-H7/chest belt | 45 s | 19.6 | 96.3 | 98.2 | 12-l-ECG |
| Sabar et al.56 | PSA | 750 | 51 | n.a. | High AF risk | UK | Rhythm Pad/patch | 10 s | 10 | 95.4 | 98.8 | 12-l-ECG |
| Lin et al.57 | PSA | 30 | n.a. | n.a. | Hospitalized | China | Medi-Trace 200/wireless record | 6 m | 67 | 94.6 | n.a. | 12-l-ECG |
| Steinhubl et al.58 | PR | 2659 | 38.6 | 72.4 | High AF risk | USA | Zio XT/patch | 4 m | 3.9 | n.a. | n.a. | e.d. |
| Rizas et al.9 | RCT | 5551 | 31 | n.a. | >65 yo | Germany | CardioMem/ECG loop | 6 m | 1.33 | 91 | n.a. | 12-l-ECG Holter |
| MCG-based | ||||||||||||
| Jaakkola et al.59 | CC | 300 | 44 | 74.8 | General | Finland | MCG tech. | 3 m s.t. | n.a. | 95.3 | 96 | Tele-ECG |
Mean age reported except where specified; AF, atrial fibrillation; Amb, ambulatory; CC, case-control; d, days; ECG, electrocardiography; ECV, electrical cardioversion; e.d., expert diagnosis; f, female; m, minutes; mPSA, multileft prospective single arm; n.a., not available; Mon., monitoring; PPG, photoplethysmography; PSA, prospective single arm; PR, prospective randomized; PTA, prospective two arms; pts, patients; PV, pulse variability; Ref. reference; RFs, risk factors; RSA, retrospective single arm; s, seconds; s-l-ECG, single-lead ECG; Sn, sensitivity; Sp, specificity; s.t., single time; tech, technology; y.o., years old; 12-l-ECG, 12 leads ECG.; MCG, mechanocardiography.
The PV technology uses the variance of heartbeats detected by the arm cuff during at least three blood pressure measurements. Microlife BP and OMRON are the most extensively investigated sphygmomanometers and should be considered as a first-step AF screening strategy for hypertensive patients managed in dedicated clinics.42
The ECG-based devices may transmit and monitor an ECG trace, allowing a direct AF diagnosis.2 Depending on the devices, they can record a single, 3-, 6-, or even 12-lead ECG trace for of at least 30 seconds. The most well known ECG-based handheld devices are MyDiagnostick and KardiaMobile, with similar Sn/Sp.36
The wearable ECG-based devices can be chest belts (e.g. Polar-H7, Zio XT) with short electrodes like the Rhythm Pad and smartwatches, with all similar accuracy.36 The MCG-based devices, consisting of the accelerometers and gyroscopes placed in smartphones, have lesser evidence in screening scenarios (Sn 67%; Sp 99%).59
Combinations of various technologies appear the most useful in AF detection. Photoplethysmography signals appear to be more useful in general setting, due to the frequent coupling with smartphones and the consequent ubiquitous presence, and the ECG could be more effective in specific settings (e.g. post-stroke patients) thanks to their diagnostic accuracy. The Apple Heart Study38 and the Fitbit Heart Study41 utilized both PPG and ECG technologies; similarly, the eBRAVE-AF study,9 comparing conventional and digital AF screening, showed that the most effective AF screening strategy was the digital one utilizing a combination of smartphone-based PPG signals validated by an external ECG loop recorder. Lastly, the most proficient systems to integrate AF screening with AF treatment and follow-up seem to be the mHealth tools that arrange an effective interaction between medical professionals and patients, making smoother and complete the chain of screening AF, as clearly shown in the Mobile Atrial Fibrillation Apps (mAFA) II trial.26,60,61
In summary, various mHealth tools are used in AF screening, and these are broadly regarded as reliable, thus being useful in the early detection of AF; moreover, the variety of tools available makes it easier to tailor the AF screening strategies even in populations difficult to reach. In any case, a standard ECG tracing recording AF ≥30 seconds is needed to make diagnosis.2 In an EHRA consensus paper, the authors underlined how a significant gap in evidence exists regarding very short (<30 seconds) tachyarrhythmia episodes resembling AF, hence not fulfilling diagnostic criteria.62 There is not a specified strategy for such situations, but we can consider that keeping monitoring could be reasonable to identify more structured arrhythmias.
Screening yield and risk for stroke and adverse outcomes
The increasing interest on AAF and the technological progresses described above, despite variable accuracy,63 led to a significant development in the field of AF screening. Several studies explored so far the yield of new diagnosis of AF throughout screening programmes.
Table 2 summarizes the studies which explored screening AF strategies in asymptomatic population. In the eBRAVE-AF trial,9 the screening yield was found to be two- to three-fold gain in detection rate of AF requiring OAC compared to conventional screening. Other studies reported the screening yield of AF: in the Belgian Heart Rhythm Week programme,73 participants were screened for AF with single-lead ECG. The study showed that the AF prevalence was higher in males and in those aged ≥ 65. The most important aspect is that more than two-thirds of patients with AF had a CHA2DS2-VASc score ≥ 2, being eligible for OAC. A patient-level meta-analysis74 confirmed that people with screen-detected AF have a high risk of stroke as they were above age 65 and more than two-thirds have an additional stroke risk factor other than age/sex. Moreover, it was found that the number needed to screen to identify one new treatable AF was inversely associated to increasing age. Conversely, the percentage of OAC prescription increased with age. Atrial fibrillation screening yield may also depend on risk stratification of screened populations. The STROKESTOP II study72 used a 125 ng/L cut-off of N-terminal B-type natriuretic peptide for 75- to 76-year-old patients, to identify high-risk patients that were offered to prolong the screening and were found to have a higher rate of detected AF; in the lower-risk group, consistently, the study found a lower rate of detected AF. Nonetheless, two cluster randomized trials with single timepoint screening in patients ≥65 years failed to demonstrate a higher rate of AF detection. The VITAL-AF71 study did not find differences between systematic screening and usual care arms in primary care patients; however, older age (≥85 years old) was found at higher incidence of new AF. Moreover, general characteristics of the patients included showed a very high risk of AF, and the rate of AF diagnosis was significantly higher in the control arm, compared to previous studies.71 Also, in the Detecting and Diagnosing Atrial Fibrillation (D2AF)67 study, which tested an opportunistic screening approach in the context of primary care patients, no difference was found between the two arms of the study, even though in this case, the results were strongly affected by a low uptake of patients to the screening programme (∼45% of the total assigned to screening). All these results underline how the identification of the targeted population performing the screening campaign and its success are essential to obtain a significant yield of screening.
Table 2.
Studies about atrial fibrillation screening strategies and risk of adverse outcomes
| Study | Year | ToS | Location | Screened population | n | Modality | Screening period | Screening Yield—AF detection | Summary screening yield | FU | Outcomes | Results | Summary outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STROKESTOP64,65 | 2015, 2021 | RCT | Sweden | 75–76 | Screened (n = 7173) (For follow-up n = 13 979) | 12-ECG + handheld ECG for 2 weeks | 14 days | 3.0% | Intermittent ECGs increased new AF detection four-fold | 5 years | Combined endpoint of ischaemic or haemorrhagic stroke, SE, hospitalization for bleeding, or death from any cause | HR: 0.96, 95% CI 0.92–1.00, P = 0.045 | Subjects randomized to screening had a lower risk of the composite endpoint throughout the follow-up observation |
| Control (n = 14 381) (For Follow-up n = 13 996) | Usual care | 14 days | — | ||||||||||
| REHEARSE-AF66 | 2017 | RCT | The UK | >65 with CHA2DS2-VASc > 2 with no AF and no OAC or pacing | Screening (n = 501) | 30 s single-lead handheld ECG twice weekly | 1 year | 3.7% | Almost four-fold increase in the diagnosis of AF in the screened population (HR 3.9, 95% CI 1.4–10.4, P = 0.007) | 1 year | Stroke/TIA/SE | HR: 0.61 (95% CI 0.22–1.69; P = 0.34 | No significant difference was found between the two groups |
| No screening (n = 500) | — | — | 1.0% | ||||||||||
| D2AF67 | 2020 | Cluster RCT | The Netherlands | ≥65 with no AF in primary care | Screening (n = 8874) | Single-lead handheld ECG in primary care | 1 y | 1.62% | Opportunistic screening for atrial fibrillation in primary care patients did not increase the detection rate of AF | No FU | |||
| Usual care (n = 9102) | Usual care | 1 year | 1.53% | ||||||||||
| mSToPS58,68 | 2018, 2021 | NRCT | The USA | ≥75 or males ≥55/females ≥65 with one risk factor/comorbidity | Immediate Screening (n = 1366) | Single-lead patch monitor for up to 14 days (screened period) | 1 year | 6.7 per 100 person-years | Volunteer monitored individuals, compared with nonmonitored matched controls, had higher rates of AF diagnosis | 3 years | Combined endpoint of ischaemic stroke, systemic embolism, myocardial infarction all-cause death | 8.4 vs. 13.8 per 100 person-years HR: 0.53, 95% CI: 0.40–0.78, P < 0.01 | Subjects assigned to screening have a lower risk of the combined endpoint |
| Delayed screening (n = 1293) | Single-lead patch monitor for up to 14 days (unscreened period) | 1 year | |||||||||||
| Unmonitored (n = 3476) | — | 1 y | 2.6 per 100 person-years | ||||||||||
| LOOP Study69 | 2021 | RCT | Denmark | 70–90 without AF, with at least one additional stroke risk factor | Screened (n = 1501) | ILR monitoring | 4 years | 8.04 per 100 person-years | Three times increase in detection of atrial fibrillation and concomitant anticoagulation in the screened population (HR: 3,17 (95% CI 2,81–3,59, P < 0.00001) | 6 years | Stroke or systemic arterial embolism | 0.88 vs. 1.09 events per 100 person-years HR: 0.80 (95% CI 0.61–1.05]; P = 0.11 | No significant decrease in the risk of stroke or systemic arterial embolism |
| Control (n = 4503) | 4 years | 2.48 per 100 person-years | |||||||||||
| SCREEN-AF70 | 2021 | RCT | Canada/Germany | ≥75 without know AF | Screened (n = 434) | 2-week ambulatory cECG patch monitor + automated home BP monitor with an AF screening algorithm to be used twice daily during monitoring periods | 6 months | 5.3% | 10-fold increase of the AF detection in the screened population | No FU | |||
| Control (n = 422) | standard care | 6 months | 0.5% | ||||||||||
| VITAL-AF71 | 2022 | Cluster RCT | The USA | General Population without AF | Screened (n = 15 393) | Handheld single-lead ECG | 1 year | 1.72% | Screening for AF using a single-lead ECG at primary care visits did not affect new AF diagnoses among all individuals aged 65 years or older compared with usual care | No FU | |||
| Control (n = 15 322) | Usual care | 1 year | 1.59% | ||||||||||
| eBRAVE-AF9 | 2022 | RCT | Germany | 50–90 with a CHA2DS2-VASc score of ≥1 (men) or ≥2 (women), not known to have paroxysmal or persistent AF and not treated with OAC | Screened (n = 2860) | Digital screening | 6 months | 1.33% | Digital screening increases the detection rate of AF requiring OAC compared to routine symptom-based screening (OR 2.12, 95% CI, 1.19–3.76; P = 0.010) and AF Detection (OR 1.90, 95% CI 1.16–3.11, P = 0.011) | 1 year | Stroke; Thromboembolic events; Major bleeding Death | Stroke: OR 1.03 (95% CI 0.45–2.33, P = 0.950 Thromboembolic events: OR 2.07 (95% CI 0.72–5.98, P = 0.177) Major bleeding: OR 1.01 (95% CI 0.49–2.09, P = 983) Death: OR 1.88 (95% CI 0.47–7.54, P = 0.371) | No statistical risk decrease for any of the secondary outcomes between the two groups |
| Control (n = 2691) | Routine symptom-based screening | 6 months | 0.63% | ||||||||||
| STROKESTOP II72 | 2020 | RCT | Sweden | 75–76 | Screened with NT-proBNP ≥ 125 (n = 3766) | Handheld ECG monitoring intermittent for 2 weeks | 14 days | 4.4% | The screening procedure resulted in an absolute increase in AF prevalence from 8.1% to 10.5% among participants. All new AF diagnosed in the screening group were 2.6%, (95% CI 2.2–3.0%) | See Table 3. | |||
| Screened with NTproBNP < 125 (n = 3766) | Handheld ECG monitoring | One-time | 0.04% | ||||||||||
| Control (n = 14 356) | Usual care (for follow-up) | — | — | ||||||||||
Legend: AF, atrial fibrillation; CI, confidence interval; ECG, electrocardiogram; FU, follow-up; HR, hazard ratio; ILR, implanted loop recorded; OAC, oral anticoagulant; OR, odds ratio; NT-proBNP, N-terminal pro-B-terminal natriuretic peptide; NRCT, non-randomized clinical trial; RCT, randomized clinical trial; ToS, type of study.
So far, the research in this field showed that the AF screening is effective in detecting AF; however, assuming that patients with detected AF and moderate-high risk of stroke would be prescribed with OAC, whether the AF screening is also effective in reducing the risk of adverse events at follow-up remains unclear. Recently, several studies investigated the screening methods for AF and analysed the adverse outcomes at follow-up. The Remote Heart Rhythm Sampling Using the AliveCor Heart Monitor to Screen for Atrial Fibrillation (REHEARSE-AF)66 study was one of the first to explore adverse outcomes. In 2017, this study randomized over 65-year-old patients with CHA2DS2-VASc ≥ 2 and no OAC prescription, to single-lead handheld ECG screening with the AliveCor Kardia monitor twice a week or standard care. The results showed a four-fold increase in AF detection in the active arm; however, there was no difference in the reduction of clinical events in the two groups at 1 year. The study had several limitations, including the relatively short length of follow-up, and the low rate of clinical events. In the LOOP Study,69 patients ≥70-year-old were randomized to receive implantable loop recorder (ILR) monitoring or usual care; despite the introduction of OAC in those eligible, there was no significant difference in the risk of primary outcome of stroke/systemic embolism between the two groups. Afterwards, the STROKESTOP64,65 study showed a lower risk of the primary composite outcome of stroke (ischaemic/haemorrhagic), systemic embolism, hospitalization for bleeding, and all-cause death in patients actively screened for AF compared to those allocated to usual care, even though survival curves start to diverge only after 4 years of follow-up {with a hazard ratio [HR] of 0.96 [95% confidence interval (CI) 0.92–1.00]}. Notwithstanding, the ‘as-treated’ analysis, comparing subjects who actually participated to the screening programme to those randomized to control group, excluding those randomized but which never showed up (n = 6814, 48.7% of those invited), found that undergoing the screening programme was associated with a consistent reduction in the risk of the composite outcome (HR 0.76, 95% CI 0.67–0.85), which emerged since the very beginning of follow-up observation. Furthermore, the mSToPS,58,68 using a patch system for AF screening, showed a prominent risk reduction of composite of death, stroke, systemic embolism, and myocardial infarction; however, this analysis was a post-hoc non-randomized comparison, with significant limitation of comparability between the propensity-matched controls and volunteer study group. Also, even in the control arm, after the initial phase of the study, screening patch was offered in the case they volunteered. These aspects need to be taken in mind when considering the follow-up phase results. Recently, a systematic review and meta-analysis collecting data about 35 386 subjects coming from 5 different screening studies showed that screening strategies were associated to a reduction in the risk of stroke (relative risk 0.91, 95% CI 0.84–0.99). Notwithstanding, the sequential trial analysis underlined how the number of patients needed to prove the benefit of screening in reducing the risk of thromboembolic events would slightly exceed the 100 000 subjects, in the light of the very low incidence of adverse events, underlining the need for new studies.75
The pre-mAFA trial26 implemented an mHealth technology both for the AF screening and then for the management of general population-based patients with AF.27 In this trial, at least 14-day monitoring with wristband has been proposed for a high-risk population (CHA2DS2-VASc ≥ 2). Subsequently, screened AF patients were cluster randomized to receive the structured care pathway (mAFA intervention) or the usual care in the mAFA II trial.27 This study showed a significant reduction of the primary composite outcome of stroke, thromboembolism, death and rehospitalization. However, mAFA II trial also includes inpatients and outpatients with symptomatic AF and those results cannot be translated to all the AAF.
Currently, four randomized trials are ongoing to further examine the effectiveness of screening strategies in reducing stroke occurrence. All these studies (Table 3) were specifically designed and powered to investigate the risk of stroke and other adverse outcomes. Indeed, Screening for Atrial Fibrillation with ECG to Reduce stroke (SAFER) trial,76 the five years follow-up of STROKESTOP II,77 the ReducinG stroke by screening for UndiAgnosed atRial fibrillation in elderly inDividuals (GUARD-AF) (NCT04126486), and the HEARTLINE (NCT04276441) will generate data on more than 200 thousand patients, likely providing definitive evidence regarding the implementation of screening strategies to reduce the occurrence of stroke and other adverse outcomes in AAF subjects. Furthermore, the European Union’s Horizon 2020 programme recently funded a project, the Digital, Risk-Based Screening for Atrial Fibrillation in the European Community (AFFECT-EU, http://affect-eu.eu), which aims to develop a specific algorithm to implement a risk-based screening approach, identifying those populations at higher risk of AF in which develop specific screening programmes, and also includes a progressive patient-level data meta-analysis of all randomized studies.78
Table 3.
Ongoing randomized studies about atrial fibrillation screening strategies and risk of adverse outcomes
| Ongoing studies | ||||||
|---|---|---|---|---|---|---|
| Study | Year | Study design | N | Follow-up | Outcomes | Trial registration |
| SAFER76 | 2017 | ≥70-year-old subjects from a primary care unit network randomized to receive screening through a single-lead handheld ECG four times daily for 3 weeks; the study comprises two feasibility phases and one large interventional trial | 126 000 | 5 years | Ischaemic and haemorrhagic stroke | ISRCTN: ISRCTN72104369 |
| GUARD-AF | 2019 | ≥70-year-old subjects from a primary care unit network randomized to receive screening through an ECG skin patch with no AF and no OAC | 52 000 | 2 years | Stroke leading to hospitalization and bleeding leading to hospitalization | ClinicalTrials.gov: NCT04126486 |
| HEARTLINE | 2020 | ≥65-year-old subjects randomized to receive screening through a smart watch device and a healthy heart engagement program | 150 000 | 3 years | Composite of cerebrovascular events and all-cause death | ClinicalTrials.gov: NCT04276441 |
| STROKESTOP II | 2017 | 75–76-year-old Stockholm region inhabitants, randomized to receive screening procedure or usual care; subjects randomized to screening were assigned to handheld ECG monitoring either intermittent for 2 weeks or one-stop screening according to NT-proBNP levels | 6868 | 5 years | Primary outcome: stroke or systemic embolism; secondary outcome: bleeding stroke, systemic embolism, or all-cause death | ClinicalTrials.gov: NCT02743416 |
ECG, electrocardiography; OAC, oral anticoagulant; NT-proBNP, N-terminal pro-B-terminal natriuretic peptide.
Current guideline recommendations
The screening for AF aims at early arrhythmia detection, possibly leading to a better prevention of thromboembolic events. Despite the increasing interest in this field promoted by new AF screening tools/devices, not all international guidelines recommend AF screening. Indeed, recommendations in each guideline can be conditioned by different regional epidemiological features of AF and availability of new mHealth devices. Similarly, the perception of AF screening utility by the treating physicians may vary according to patient symptoms, being underused in asymptomatic patients.
Overall, European approach to AF screening appears substantially different as compared to United States. In the latest ESC guidelines,2 the section on AF screening is extensively represented, discussing in detail all digital devices for AF screening and indicating the most reliable strategies. Recommendations are stratified by patient age, with a Class I recommendation (level of evidence B) for opportunistic screening in patients ≥65 years and Class IIa (level of evidence B) for systematic screening in individuals aged ≥75. In the recent European Heart Rhythm Association (EHRA) practical guide on how to use digital devices to detect and manage arrhythmias,79 the proposed organization of AF screening depends mainly on its opportunistic/systematic nature. Age (<65, 65–75, ≥ 75 years), number of comorbidities (0, 1, ≥ 2), digital literacy (a continuum from limited to complete), and use of PPG vs. ECG devices drive the choice between these two different screening types. In patients aged ≥65 years, PPG devices (confirmed by an ECG) are proposed in the opportunistic setting for less comorbid patients with limited digital skills. On the other hand, ECG devices are proposed in systematic AF screening setting for patients aged ≥75 years, those with multimorbidity, and fully digital skilled. For younger, non-comorbid, and symptomatic patients, ECG devices are recommended. No screening is suggested for asymptomatic, non-comorbid, young patients.
Also, the National Institute for Health and Care Excellence (NICE) guidelines support the opportunistic screening using WatchBP Home during blood pressure measurement by primary care professionals,80 even though the UK National Screening Committee recommends against screening for AF.81
The 2018 Heart Foundation and Cardiac Society of Australia and New Zealand (CSANZ) guidelines82 include a recommendation for opportunistic screening in the clinic or community in people ≥65 years, suggesting pulse palpation and single-lead handheld ECG devices as screening strategie. On the same side, also the 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society (CCS/CHRS) guidelines recommend opportunistic screening for patients ≥65 years by pulse-based screening (pulse palpation, BP monitors, plethysmograph) or single-lead ECG devices.83 Similarly, the Asia Pacific Heart Rhythm Society (APHRS)84 suggests an opportunistic screening in people aged ≥65 years and a systematic screening in people aged ≥75 years with high-risk factors for AF development (e.g. post-stroke patients). The 2018 Korean Heart Rhythm Society (KHRS) AF guidelines85 recommend the opportunistic screening for >65 years by pulse taking or ECG strip (Class I, level of evidence B); systematic screening may also be considered in patients >75 years or at high stroke risk (Class IIa, level of evidence B). On the contrary, the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) AF guidelines86 make no specific recommendation for AF screening. The subsequent AHA/ACC/HRS focused update published on 201987 introduced a possible role for screening of silent AF with a remote ECG acquisition by smartwatches or handheld ECG devices.
Recently, the United States Preventive Services Task Force (USPSTF)88 published the 2022 updated version of their document dedicated to AF screening, still highlighting their concern about the lack of effectiveness of an AF screening structured pathway as compared to usual care opposed to some risk of adverse events, as anxiety, excessive testing, and overtreatment. Thus, given that current evidence is deemed insufficient to assess the balance of benefits and harms of screening for AF, the agency has expressed against the implementation of screening in asymptomatic adults aged ≥50 years.
Summary and discussion
In this review about AF screening strategies, we presented an extensive overview of evidence, which allows us to make some important assumptions: (i) AAF is common among the overall population of AF patients, not differing significantly from symptomatic AF in terms of thromboembolic risk and of occurrence of stroke and other adverse outcomes (Figure 1); (ii) nowadays the technological advances and the widespread diffusion of mobile health/wearables devices allows to implement AF screening strategies which are more suitable to each specific setting, with all screening methods recognizing super-imposable performances in terms of sensitivity and specificity; (iii) epidemiological evidence underline how, irrespective of the method, screening strategies provide a significant yield of AF diagnosis, identifying large proportions of patients with AAF worth to be prescribed with OAC due to a high thromboembolic risk; (iv) the studies published so far, even though with some limitations due to low sample size and other several bias, seem to suggest that implementation of screening strategies to identify AAF, with a subsequent prescription of OAC, appears to reduce the risk of outcomes over follow-up, even though further studies properly powered are still needed to fully clarify these observations; and (v) most of the current clinical guidelines recommend the implementation of opportunistic/systematic screening strategies based on chronological age and baseline thromboembolic risk; nonetheless health agencies currently do not recommend the large scale implementation of AF screening due to the lack of solid evidence regarding the reduction of stroke and other adverse outcomes.
Figure 1.
AAF, asymptomatic atrial fibrillation; AF, Atrial Fibrillation; APHRS, Asia Pacific Heart Rhythm Society; AHA/ACC/HRS, American Heart Association/American College of Cardiology/ Heart Rhythm Society; CSANZ: Cardiac Society of Australia and New Zealand; CCS/CHRS, Canadian Cardiovascular Society/Canadian Heart Rhythm Society; ECG, electrocardiography; PPG, photoplethysmography; PV, pulse variability; MCG, mechanocardiography; KHRS, Korean Heart Rhythm Society; USPSTF, United States Preventive Services Task Force. (Created with Biorender.com).
Our review suggests how screening can identify from 3 to 10 times more AF. This variability is directly linked to inter- and intra-individual variability, related to the presence of mild subjective symptoms, as well as the potential temporal distance between AF and symptoms onset. Maximization of the yield of screening could depend on the duration of the monitoring, as reported by a recent position paper of EHRA,62 which suggests a monitoring time lasting 2 weeks or longer. The different screening yield of AF screening could be due to the different screened population. Most of the studies were conducted in population aged ≥ 65 years old, others targeted to an older population (≥75 years old) and at higher risk to develop AF.65,72 Notwithstanding these differences, the detection of AF allows to identify patients who need OAC. Two large Canadian studies confirmed the need for AF screening, pointing out the existence of a consistent proportion of patients with unknown AF who need to be prescribed with appropriate anticoagulant treatment.89,90 Similarly, stronger data were shown in the eBRAVE-AF trial9 demonstrating two-fold increase in the AF detection in screened population requiring OAC [odds ratio (OR) 2.12, 95% CI, 1.19–3.76]. If the large diffusion of wearables can represent a positive factor, the increasing request for clinical referral related to the consumer-led screening still poses questions and represents a critical issue in terms of appropriateness, privacy, and risk of excessive medicalization of patients for which it is substantially useless. New care and management pathways are needed to manage those subjects and avoid mass request for inappropriate medical checks.91
It can be possible that mass screening for AF puts patients at greater risk of overdiagnosis, anxiety, misinterpretation of the ECG, and, eventually, unnecessary additional tests. This could be a reason to refuse the enrolment in a screening programme, reflecting the failure of some trials in demonstrating the benefit of AF screening. Even the STROKESTOP study, which showed a reduction of adverse events in the screened population compared to controls in secondary analysis, was affected by the declination of half of participants invited to screen.64,65 Also, the results coming from the mSToPS, again coming from a secondary analysis, are reassuring in terms of adverse events reduction in patients undergoing screening.
Furthermore, LOOP Study did not show any significant difference in the reduction of stroke or systemic arterial embolism; this was probably due to the ability of ILR, used as screening method, to pick up very short episode of AF, whereas in STROKESTOP, intermittent ECG were more likely to identify individual with clinically meaningful AF. Moreover, in the same study, there was a higher-than-expected rate of AF detection in the control group. Moreover, LOOP Study was also affected by a higher rate of early discontinuation of ILR monitoring.13 These data underline an important aspect of the research studies involving this topic so far, which were mostly underpowered and methodologically not suitable to verify the studies hypotheses, as already pointed out.75
Other relevant aspects of performing AF screening are related to the identification of specific subjects’ subsets in which the procedure is implemented. A subgroup analysis of LOOP trial suggested that screening reduced the risk of stroke and systemic embolism only in patients with the highest systolic blood pressure, underlining the idea that screening strategies could be targeted towards specific populations with a higher likelihood of presenting AF with a higher risk of stroke and other clinical outcomes, and, in general, underlies the need to better identify the population worth to screen.69 In this regard, stratification of the population, by biomarkers (e.g. NT-pro-BNP in STROKESTOP II72) or by one of the validated scores, such as C2HEST risk score,92 could identify people at risk to develop AF and, therefore, the best candidates for active screening. The importance of this aspect emerges also from the fact that European Union, by funding the AFFECT-EU project,78 has considered relevant to invest a considerable amount of funds to develop a more refined screening strategy, which could allow a higher yield of screening and the identification of patients at an even higher risk of adverse outcomes.
The ‘debate’ between scientific societies’ clinical guidelines and the USPSTF again strengthens the need for further research. Several guidelines worldwide generally agree on recommending an opportunistic screening strategy for patients ≥65 years old,2,82–85 with some of them also suggesting systematic screening in those over 75 years old.2,84,85 If from a scientific point of view appears justified to suggest the implementation of screening strategies (which are indeed recommended with an overall low level of evidence), it may be understandable that from a regulatory point of view, stronger evidence is required.
Also, data coming from cost-effectiveness analyses are reassuring regarding the implementation of screening studies which generate significant yield of diagnosis and reduction of events at reasonable willingness-to-pay thresholds.93–96 However, these studies weren’t designed and powered to detect differences in clinical outcomes but rely mostly on statistical modelling.93–96 Recently, a cost-effectiveness analysis derived from the follow-up phase of STROKESTOP study demonstrated an improvement in cost-effectiveness which also increases with a progressively higher participation to the screening programme.97 Moreover, a study based on UK population shows how implementation of a screening strategy in targeted population at risk of AF would substantially reduce healthcare costs of AF-related stroke.98
The ongoing studies (e.g. SAFER,76 GUARD-AF, HEARTLINE, and the 5-year follow-up of STROKESTOP II), which will be specifically powered to detect differences in incidence of stroke and other adverse clinical outcomes, will certainly provide consistent evidence able to confirm and hopefully extend the current evidence which strongly point out to a beneficial effect of the screening strategies, informing the guidelines’ authors more properly and strengthening the recommendations in the next years.
Learning points
In this comprehensive narrative review, we summarized all the major evidence regarding the relevance, feasibility, and effectiveness of AF screening strategies in both identifying new AAF patients worth to be treated and reducing the occurrence of stroke and major adverse events (Table 4). This issue remains one of the most interesting in the field of AF clinical research. Epidemiological data indicate how AAF represents a big healthcare issue, with high prevalence of patients unaware of the increased risk of developing major adverse events. Screening strategies are feasible and effective, with high yields of diagnosis and large widespread among the general population. While caution is still needed for the consumer-led screening, it is clear how their implementation can identify patients requiring treatment. Moreover, performing AF screening do not recognize clear contraindications, while some caution is needed regarding possible adverse effects such stress/anxiety or overdiagnosis/overtreatment.88 The main point of debate is still related to their effectiveness in reducing risk of stroke and other adverse events. Despite some methodological and contextual limitations of the studies performed so far, the evidence strongly suggests that AF screening strategies are associated with a significant reduction of adverse outcomes. Future studies will hopefully elucidate more strongly and solidly, even for the regulatory authorities, the actual impact of screening strategies, influencing the clinical practice in the next decades.
Table 4.
Learning points: the ‘6 Ws’ about AF screening strategies
| Learning points: the ‘6 Ws’ about the AF screening strategies | |
|---|---|
| Why to screen for AF? | AAF is highly prevalent (8–40%) and patients with AAF has the same risk to develop adverse events compared to those symptomatic |
| Where to screen? | Structured programmes in targeted high-risk population irrespective of their clinical setting |
| What to use to screen? | PPG, PV, and MCG technologies or ECG trace |
| Who we need to screen? | a. Opportunistic screening in patients ≥65 (Class I)* |
| b. Systematic screening in individuals aged ≥75 (Class IIa)* | |
| What do to if screening if positive? | a. Check the need of confirmation with 30 s ECG trace (if non-ECG technologies were used) |
| b. Assess thromboembolic risk (CHA2DS2VASc score) | |
| c. Decide the need to start oral anticoagulation | |
| d. Optimize treatment of AF through implementation of ABC Pathway for Integrated Care | |
| What to do if a AAF patient has no need for anticoagulation? | Regular follow-up and re-assessment of thromboembolic risk over time |
AAF, asymptomatic atrial fibrillation; ECG, electrocardiography; PPG, photoplethysmography; PV, pulse variability; MCG, mechanocardiography.*From 2020 ESC AF guidelines.
Contributor Information
Bernadette Corica, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart and Chest Hospital, 6 West Derby Street, Liverpool L7 8TX, UK; Department of Translational and Precision Medicine, Sapienza University of Rome, Viale del Policlinico 155, Rome 00161, Italy.
Niccolò Bonini, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart and Chest Hospital, 6 West Derby Street, Liverpool L7 8TX, UK; Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Policlinico di Modena, via Giuseppe Campi 287, Modena 41125, Italy; Clinical and Experimental Medicine PhD Program, University of Modena and Reggio Emilia, via Giuseppe Campi 287, Modena 41125, Italy.
Jacopo Francesco Imberti, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart and Chest Hospital, 6 West Derby Street, Liverpool L7 8TX, UK; Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Policlinico di Modena, via Giuseppe Campi 287, Modena 41125, Italy; Clinical and Experimental Medicine PhD Program, University of Modena and Reggio Emilia, via Giuseppe Campi 287, Modena 41125, Italy.
Giulio Francesco Romiti, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart and Chest Hospital, 6 West Derby Street, Liverpool L7 8TX, UK; Department of Translational and Precision Medicine, Sapienza University of Rome, Viale del Policlinico 155, Rome 00161, Italy.
Marco Vitolo, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart and Chest Hospital, 6 West Derby Street, Liverpool L7 8TX, UK; Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Policlinico di Modena, via Giuseppe Campi 287, Modena 41125, Italy; Clinical and Experimental Medicine PhD Program, University of Modena and Reggio Emilia, via Giuseppe Campi 287, Modena 41125, Italy.
Lisa Attanasio, Centro Medico, Corso S. Gottardo 6 e/13, Chiasso 6830, Switzerland.
Stefania Basili, Department of Translational and Precision Medicine, Sapienza University of Rome, Viale del Policlinico 155, Rome 00161, Italy.
Ben Freedman, The Heart Research Institute, Charles Perkins Centre, Faculty of Medicine and Health, University of Sydney, Sydney, NSW 2006, Australia.
Tatjana S Potpara, School of Medicine, University of Belgrade, dr Subotica 8, Belgrade 11000, Serbia; Cardiology Clinic, University Clinical Centre of Serbia, Visegradska 26, Belgrade 11000, Serbia.
Giuseppe Boriani, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Policlinico di Modena, via Giuseppe Campi 287, Modena 41125, Italy.
Gregory Y H Lip, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart and Chest Hospital, 6 West Derby Street, Liverpool L7 8TX, UK; Danish Center for Clinical Health Services Research, Alboorg University, Søndre Skovvej 15, Aalborg 9000, Denmark.
Marco Proietti, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart and Chest Hospital, 6 West Derby Street, Liverpool L7 8TX, UK; Division of Subacute Care, IRCCS Istituti Clinici Scientifici Maugeri, Via Camaldoli 64, Milan 20138, Italy; Department of Clinical Sciences and Community Health, University of Milan, Via della Commenda 19, Milan 20122, Italy.
Lead author biography
 Marco Proietti is Consultant and Researcher at the IRCCS Istituti Clinici Scientifici Maugeri in Milan, Italy, and Honorary Senior Research Fellow at ‘Liverpool Centre for Cardiovascular Science’ at University of Liverpool, Liverpool John Moores University, and Liverpool Heart and Chest Hospital, Liverpool, United Kingdom. His research interests are related to epidemiology and clinical management of AF. He studies clinical factors and concomitant diseases associated with AF in relation to OAC therapy and risk of adverse events. Furthermore, he gained significant experience in Geriatric Medicine, with particular interest for the issues of frailty, multimorbidity, and polypharmacy.
Marco Proietti is Consultant and Researcher at the IRCCS Istituti Clinici Scientifici Maugeri in Milan, Italy, and Honorary Senior Research Fellow at ‘Liverpool Centre for Cardiovascular Science’ at University of Liverpool, Liverpool John Moores University, and Liverpool Heart and Chest Hospital, Liverpool, United Kingdom. His research interests are related to epidemiology and clinical management of AF. He studies clinical factors and concomitant diseases associated with AF in relation to OAC therapy and risk of adverse events. Furthermore, he gained significant experience in Geriatric Medicine, with particular interest for the issues of frailty, multimorbidity, and polypharmacy.
Data availability
No new data were generated in support of the article.
Author contributions
M.P. contributed to the conception and the design of the work. B.C. and M.P. wrote the first draft and finalized the final version of the manuscript. N.B., J.F.I., G.F.R., M.V., and L.A. contributed to draft the manuscript; S.B., B.F., T.S.P., G.B., and G.Y.H.L. critically revised the manuscript and gave relevant intellectual contributions. All authors approved the last version of the manuscript.
Funding
None declared.
References
- 1. Kornej J, Benjamin EJ, Magnani JW. Atrial fibrillation: global burdens and global opportunities. Heart 2021;107:516–518. [DOI] [PubMed] [Google Scholar]
- 2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 3. Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D, Gumprecht J, Kozieł M, Yang PS, Guo Y, Lip GYH, Proietti M. Adherence to the ‘atrial fibrillation better care’ pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb Haemost 2022;122:406–414. [DOI] [PubMed] [Google Scholar]
- 4. Proietti M, Vitolo M, Harrison SL, Lane DA, Fauchier L, Marin F, Nabauer M, Potpara TS, Dan GA, Boriani G, Lip GYH; ESC-EHRA EORP-AF Long-Term General Registry Investigators . Impact of clinical phenotypes on management and outcomes in European atrial fibrillation patients: a report from the ESC-EHRA EURObservational Research Programme in AF (EORP-AF) General Long-Term Registry. BMC Med 2021;19:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sgreccia D, Manicardi M, Malavasi VL, Vitolo M, Valenti AC, Proietti M, Lip GYH, Boriani G. Comparing outcomes in asymptomatic and symptomatic atrial fibrillation: a systematic review and meta-analysis of 81,462 patients. J Clin Med 2021;10:3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer M, Potpara T, Dan GA, Kalarus Z, Diemberger I, Tavazzi L, Maggioni AP, Lip GYH; EORP-AF Long-Term General Registry Investigators; Steering Committee (National Coordinators) . Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: a report from the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) Long-Term General Registry. Europace 2018;20:747–757. [DOI] [PubMed] [Google Scholar]
- 7. Gibbs H, Freedman B, Rosenqvist M, Virdone S, Al Mahmeed W, Ambrosio G, Camm AJ, Jacobson B, Jerjes-Sanchez C, Kayani G, Oto A, Panchenko E, Ragy H, Kakkar AK; GARFIELD-AF Investigators . Clinical outcomes in asymptomatic and symptomatic atrial fibrillation presentations in GARFIELD-AF: implications for AF screening. Am J Med 2021;134:893–901.e11. [DOI] [PubMed] [Google Scholar]
- 8. Turakhia MP, Shafrin J, Bognar K, Trocio J, Abdulsattar Y, Wiederkehr D, Goldman DP. Estimated prevalence of undiagnosed atrial fibrillation in the United States. PLoS One 2018;13:e0195088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rizas KD, Freyer L, Sappler N, von Stülpnagel L, Spielbichler P, Krasniqi A, Schreinlechner M, Wenner FN, Theurl F, Behroz A, Eiffener E, Klemm MP, Schneidewind A, Zens M, Dolejsi T, Mansmann U, Massberg S, Bauer A. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat Med 2022;28:1823–1830. [DOI] [PubMed] [Google Scholar]
- 10. Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O'Donnell M, Laupacis A, Côté R, Sharma M, Blakely JA, Shuaib A, Hachinski V, Coutts SB, Sahlas DJ, Teal P, Yip S, Spence JD, Buck B, Verreault S, Casaubon LK, Penn A, Selchen D, Jin A, Howse D, Mehdiratta M, Boyle K, Aviv R, Kapral MK, Mamdani M; EMBRACE Investigators and Coordinators . Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–2477. [DOI] [PubMed] [Google Scholar]
- 11. Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res 2020;127:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Albini A, Malavasi VL, Vitolo M, Imberti JF, Marietta M, Lip GYH, Boriani G. Long-term outcomes of postoperative atrial fibrillation following non cardiac surgery: a systematic review and metanalysis. Eur J Intern Med 2021;85:27–33. [DOI] [PubMed] [Google Scholar]
- 13. Boriani G, Vitolo M, Imberti JF, Potpara TS, Lip GYH. What do we do about atrial high rate episodes? Eur Heart J Suppl 2020;22:O42–O52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boriani G, Vitolo M. Atrial fibrillation in patients with cardiac implantable electronic devices: new perspectives with important clinical implications. Kardiol Pol 2019;77:1119–1120. [DOI] [PubMed] [Google Scholar]
- 15. Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol 2000;4:369–382. [DOI] [PubMed] [Google Scholar]
- 16. Kerr C, Boone J, Connolly S, Greene M, Klein G, Sheldon R, Talajic M. Follow-up of atrial fibrillation: the initial experience of the Canadian Registry of Atrial Fibrillation. Eur Heart J 1996;17:48–51. [DOI] [PubMed] [Google Scholar]
- 17. Bakhai A, Darius H, de Caterina R, Smart A, Le Heuzey JY, Schilling RJ, Zamorano JL, Shah M, Bramlage P, Kirchhof P. Characteristics and outcomes of atrial fibrillation patients with or without specific symptoms: results from the PREFER in AF registry. Eur Heart J Qual Care Clin Outcomes 2016;2:299–305. [DOI] [PubMed] [Google Scholar]
- 18. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH, Sinagra G, Petrescu L, Tavazzi L, Maggioni AP, Lip GY. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF pilot general registry. Am J Med 2015;128:509–518.e2. [DOI] [PubMed] [Google Scholar]
- 19. Potpara TS, Polovina MM, Marinkovic JM, Lip GYH. A comparison of clinical characteristics and long-term prognosis in asymptomatic and symptomatic patients with first-diagnosed atrial fibrillation: the Belgrade Atrial Fibrillation Study. Int J Cardiol 2013;168:4744–4749. [DOI] [PubMed] [Google Scholar]
- 20. Thind M, Holmes DJN, Badri M, Pieper KS, Singh A, Blanco RG, Steinberg BA, Fonarow GC, Gersh BJ, Mahaffey KW, Peterson ED, Reiffel JA, Piccini JP, Kowey PR; ORBIT-AF Investigators and Patients . Embolic and other adverse outcomes in symptomatic versus asymptomatic patients with atrial fibrillation (from the ORBIT-AF registry). Am J Cardiol 2018;122:1677–1683. [DOI] [PubMed] [Google Scholar]
- 21. Wallenhorst C, Martinez C, Freedman B. Risk of ischemic stroke in asymptomatic atrial fibrillation incidentally detected in primary care compared with other clinical presentations. Thromb Haemost 2022;122:277–285. [DOI] [PubMed] [Google Scholar]
- 22. Boriani G, Palmisano P, Malavasi VL, Fantecchi E, Vitolo M, Bonini N, Imberti JF, Valenti AC, Schnabel RB, Freedman B. Clinical factors associated with atrial fibrillation detection on single-time point screening using a hand-held single-lead ECG device. J Clin Med 2021;10:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim JY, Park HS, Park HW, Choi EK, Park JK, Kim JB, Kang KW, Shim J, Joung B, Park KM. Clinical outcomes of rhythm control strategies for asymptomatic atrial fibrillation according to the quality-of-life score: the CODE-AF (Comparison Study of Drugs for Symptom Control and Complication Prevention of Atrial Fibrillation) registry. J Am Heart Assoc 2022;11:25956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Gessler N, Goette A, Haegeli LM, Heidbuchel H, Kautzner J, Ng GA, Schnabel RB, Suling A, Szumowski L, Themistoclakis S, Vardas P, van Gelder IC, Wegscheider K, Kirchhof P. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J 2022;43:1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mairesse GH, Moran P, van Gelder IC, Elsner C, Rosenqvist M, Mant J, Banerjee A, Gorenek B, Brachmann J, Varma N, de Lima G Glotz, Kalman J, Claes N, Lobban T, Lane D, Lip GYH, Boriani G; ESC Scientific Document Group . Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). Europace 2017;19:1589–1623. [DOI] [PubMed] [Google Scholar]
- 26. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, Yan L, Xing Y, Shi H, Li S, Liu Y, Liu F, Feng M, Chen Y, Lip GYH; MAFA II Investigators . Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–2375. [DOI] [PubMed] [Google Scholar]
- 27. Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, Wen J, Xing Y, Wu F, Xia Y, Liu T, Wu F, Liang Z, Liu F, Zhao Y, Li R, Li X, Zhang L, Guo J, Burnside G, Chen Y, Lip GYH; mAF-App II Trial Investigators . Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol 2020;75:1523–1534. [DOI] [PubMed] [Google Scholar]
- 28. Lane DA, McMahon N, Gibson J, Weldon JC, Farkowski MM, Lenarczyk R, Watkins CL, Dilaveris P, Caiani EG, Potpara TS. Mobile health applications for managing atrial fibrillation for healthcare professionals and patients: a systematic review. Europace 2020;22:1567–1578; euaa269. [DOI] [PubMed] [Google Scholar]
- 29. Hermans ANLL, Gawalko M, Dohmen L, van der Velden RMJJ, Betz K, Duncker D, Verhaert DVM, Heidbuchel H, Svennberg E, Neubeck L, Eckstein J, Lane DA, Lip GYH, Crijns HJGM, Sanders P, Hendriks JM, Pluymaekers NAHA, Linz D. Mobile health solutions for atrial fibrillation detection and management: a systematic review. Clin Res Cardiol 2022;111:479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Sullivan JW, Grigg S, Crawford W, Turakhia MP, Perez M, Ingelsson E, Wheeler MT, Ioannidis JPA, Ashley EA. Accuracy of smartphone camera applications for detecting atrial fibrillation: a systematic review and meta-analysis. JAMA Netw Open 2020;3:e202064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boriani G, Schnabel RB, Healey JS, Lopes RD, Verbiest-van Gurp N, Lobban T, Camm JA, Freedman B. Consumer-led screening for atrial fibrillation using consumer-facing wearables, devices and apps: a survey of health care professionals by AF-SCREEN international collaboration. Eur J Intern Med 2020;82:97–104. [DOI] [PubMed] [Google Scholar]
- 32. Verbrugge FH, Proesmans T, Vijgen J, Mullens W, Rivero-Ayerza M, van Herendael H, Vandervoort P, Nuyens D. Atrial fibrillation screening with photo-plethysmography through a smartphone camera. Europace 2019;21:1167–1175. [DOI] [PubMed] [Google Scholar]
- 33. Yan BP, Lai WHS, Chan CKY, Chan SCH, Chan LH, Lam KM, Lau HW, Ng CM, Tai LY, Yip KW, To OTL, Freedman B, Poh YC, Poh MZ. Contact-free screening of atrial fibrillation by a smartphone using facial pulsatile photoplethysmographic signals. J Am Heart Assoc 2018;7:e008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brasier N, Raichle CJ, Dörr M, Becke A, Nohturfft V, Weber S, Bulacher F, Salomon L, Noah T, Birkemeyer R, Eckstein J. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). Europace 2019;21:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gill S, Bunting KV, Sartini C, Cardoso VR, Ghoreishi N, Uh HW, Williams JA, Suzart-Woischnik K, Banerjee A, Asselbergs FW, Eijkemans M, Gkoutos GV, Kotecha D. Smartphone detection of atrial fibrillation using photoplethysmography: a systematic review and meta-analysis. Heart 2022;108:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonini N, Vitolo M, Imberti JF, Proietti M, Romiti GF, Boriani G, Johnsen S Paaske, Guo Y, Lip GYH. Mobile health technology in atrial fibrillation. Expert Rev Med Devices 2022;19:327–340. [DOI] [PubMed] [Google Scholar]
- 37. Zhang H, Zhang J, Li HB, Chen YX, Yang B, Guo YT, Chen YD. Validation of single centre pre-mobile atrial fibrillation apps for continuous monitoring of atrial fibrillation in a real-world setting: pilot cohort study. J Med Internet Res 2019;21:e14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia MP; Apple Heart Study Investigators . Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen E, Jiang J, Su R, Gao M, Zhu S, Zhou J, Huo Y. A new smart wristband equipped with an artificial intelligence algorithm to detect atrial fibrillation. Heart Rhythm 2020;17:847–853. [DOI] [PubMed] [Google Scholar]
- 40. Nemati S, Ghassemi MM, Ambai V, Isakadze N, Levantsevych O, Shah A, Clifford GD. Monitoring and detecting atrial fibrillation using wearable technology. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2016. p. 3394–3397. IEEE. [DOI] [PubMed] [Google Scholar]
- 41. Lubitz SA, Faranesh AZ, Selvaggi C, Atlas SJ, McManus DD, Singer DE, Pagoto S, McConnell MV, Pantelopoulos A, Foulkes AS. Detection of atrial fibrillation in a large population using wearable devices: the Fitbit Heart Study. Circulation 2022;146:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marazzi G, Iellamo F, Volterrani M, Lombardo M, Pelliccia F, Righi D, Grieco F, Cacciotti L, Iaia L, Caminiti G, Rosano G. Comparison of Microlife BP A200 Plus and Omron M6 blood pressure monitors to detect atrial fibrillation in hypertensive patients. Adv Ther 2011;29:64–70. [DOI] [PubMed] [Google Scholar]
- 43. Battipaglia I, Gilbert K, Hogarth AJ, Tayebjee MH. Screening for atrial fibrillation in the community using a novel ECG recorder. J Atr Fibrillation 2016;9:1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Desteghe L, Raymaekers Z, Lutin M, Vijgen J, Dilling-Boer D, Koopman P, Schurmans J, Vanduynhoven P, Dendale P, Heidbuchel H. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 2017;19:29–39. [DOI] [PubMed] [Google Scholar]
- 45. Kaasenbrood F, Hollander M, Rutten FH, Gerhards LJ, Hoes AW, Tieleman RG. Yield of screening for atrial fibrillation in primary care with a hand-held, single-lead electrocardiogram device during influenza vaccination. Europace 2016;18:1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rivezzi F, Vio R, Bilato C, Pagliani L, Pasquetto G, Saccà S, Verlato R, Migliore F, Iliceto S, Bossone V, Bertaglia E. Screening of unknown atrial fibrillation through handheld device in the elderly. J Geriatr Cardiol 2020;17:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tavernier R, Wolf M, Kataria V, Phlips T, Huys R, Taghji P, Louw R, Hoeyweghen RV, Vandekerckhove Y, Knecht S, Duytschaever M. Screening for atrial fibrillation in hospitalised geriatric patients. Heart 2018;104:588–593. [DOI] [PubMed] [Google Scholar]
- 48. Chan PH, Wong CK, Poh YC, Pun L, Leung WWC, Wong YF, Wong MMY, Poh MZ, Chu DWS, Siu CW . Diagnostic performance of a smartphone‐based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016;5:e003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chan PH, Wong CK, Pun L, Wong YF, Wong MM, Chu DW, Siu CW. Head-to-Head comparison of the AliveCor heart monitor and Microlife WatchBP Office AFIB for atrial fibrillation screening in a primary care setting. Circulation 2017;135:110–112. [DOI] [PubMed] [Google Scholar]
- 50. Chan N, Choy C. Screening for atrial fibrillation in 13 122 Hong Kong citizens with smartphone electrocardiogram. Heart 2017;103:24–31. [DOI] [PubMed] [Google Scholar]
- 51. Orchard J, Li J, Freedman B, Webster R, Salkeld G, Hespe C, Gallagher R, Patel A, Kamel B, Neubeck L, Lowres N. Atrial fibrillation screen, management, and guideline-recommended therapy in the rural primary care setting: a cross-sectional study and cost-effectiveness analysis of eHealth tools to support all stages of screening. J Am Heart Assoc 2020;9:e017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, Wallenhorst C, Lau JK, Brieger DB, Sy RW, Freedman SB. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. Thromb Haemost 2014;111:1167–1176. [DOI] [PubMed] [Google Scholar]
- 53. Soni A, Karna S, Fahey N, Sanghai S, Patel H, Raithatha S, Thanvi S, Nimbalkar S, Freedman B, Allison J, McManus DD. Age-and-sex stratified prevalence of atrial fibrillation in rural western India: results of SMART-India, a population-based screening study. Int J Cardiol 2019;280:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zaprutko T, Zaprutko J, Baszko A, Sawicka D, Szałek A, Dymecka M, Telec W, Kopciuch D, Ratajczak P, Michalak M, Rafał D, Szyszka A, Nowakowska E. Feasibility of atrial fibrillation screening with Mobile health technologies at pharmacies. J Cardiovasc Pharmacol Ther 2020;25:142–151. [DOI] [PubMed] [Google Scholar]
- 55. Lown M, Yue AM, Shah BN, Corbett SJ, Lewith G, Stuart B, Garrard J, Brown M, Little P, Moore M. Screening for atrial fibrillation using economical and accurate technology (from the SAFETY study). Am J Cardiol 2018;122:1339–1344. [DOI] [PubMed] [Google Scholar]
- 56. Sabar MI, Ara F, Henderson A, Ahmed O, Potter C, John I, Mitchell ARJ, Yáñez-Muñoz RJ, Kaba RA. A study to assess a novel automated electrocardiogram technology in screening for atrial fibrillation. Pacing Clin Electrophysiol 2019;42:1383–1389. [DOI] [PubMed] [Google Scholar]
- 57. Lin C-T, Chang K-C, Lin C-L, Chiang C-C, Lu S-W, Chang S-S, Lin BS, Liang HY, Chen RJ, Lee YT, Ko LW. An intelligent telecardiology system using a wearable and wireless ECG to detect atrial fibrillation. IEEE Trans Inf Technol Biomed 2010;14:726–733. [DOI] [PubMed] [Google Scholar]
- 58. Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS, Carter C, Baca-Motes K, Felicione E, Sarich T, Topol EJ. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018;320:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jaakkola J, Jaakkola S, Lahdenoja O, Hurnanen T, Koivisto T, Pänkäälä M, Knuutila T, Kiviniemi TO, Vasankari T, Airaksinen KEJ. Mobile phone detection of atrial fibrillation with mechanocardiography: the MODE-AF study (mobile phone detection of atrial fibrillation). Circulation 2018;137:1524–1527. [DOI] [PubMed] [Google Scholar]
- 60. Guo Y, Lane DA, Wang L, Chen Y, Lip GYH, Eckstein J. Mobile health (mHealth) technology for improved screening, patient involvement and optimising integrated care in atrial fibrillation: the mAFA (mAF-app) II randomised trial. Int J Clin Pract 2019;73:e13352. [DOI] [PubMed] [Google Scholar]
- 61. Guo Y, Wang H, Zhang H, Liu T, Li L, Liu L, Chen M, Chen Y, Lip GYH. Photoplethysmography-based machine learning approaches for atrial fibrillation prediction: a report from the huawei heart study. JACC Asia 2021;1:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kalarus Z, Mairesse GH, Sokal A, Boriani G, Średniawa B, Arroyo RC, Wachter R, Frommeyer G, Traykov V, Dagres N, Lip GYH, Boersma L, Peichl P, Dobrev D, Bulava A, Blomström-Lundqvist C, de Groot NMS, Schnabel R, Heinzel F, Van Gelder IC, Carbuccichio C, Shah D, Eckardt L. Searching for atrial fibrillation: looking harder, looking longer, and in increasingly sophisticated ways. An EHRA position paper’. Europace 2023;25:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Giebel GD, Gissel C. Accuracy of mHealth devices for atrial fibrillation screening: systematic review. JMIR Mhealth Uhealth 2019;7:e13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021;398:1498–1506. [DOI] [PubMed] [Google Scholar]
- 65. Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131:2176–2184. [DOI] [PubMed] [Google Scholar]
- 66. Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, Gravenor MB. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation the REHEARSE-AF study. Circulation 2017;136:1784–1794. [DOI] [PubMed] [Google Scholar]
- 67. Uittenbogaart SB, Verbiest-Van Gurp N, Lucassen WAM, Winkens B, Nielen M, Erkens PMG, Knottnerus JA, van Weert HCPM, Stoffers HEJH. Opportunistic screening versus usual care for detection of atrial fibrillation in primary care: cluster randomised controlled trial. BMJ 2020;370:m3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Steinhubl SR, Waalen J, Sanyal A, Edwards AM, Ariniello LM, Ebner GS, Baca-Motes K, Zambon RA, Sarich T, Topol EJ. Three year clinical outcomes in a nationwide, observational, siteless clinical trial of atrial fibrillation screening—mHealth Screening to Prevent Strokes (mSToPS). PLoS One 2021;16:e0258276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Svendsen JH, Diederichsen SZ, Højberg S, Krieger DW, Graff C, Kronborg C, Olesen MS, Nielsen JB, Holst AG, Brandes A, Haugan KJ, Køber L. Implantable loop recorder detection of atrial fibrillation to prevent stroke (the LOOP Study): a randomised controlled trial. Lancet 2021;398:1507–1516. [DOI] [PubMed] [Google Scholar]
- 70. Gladstone DJ, Wachter R, Schmalstieg-Bahr K, Quinn FR, Hummers E, Ivers N, Marsden T, Thornton A, Djuric A, Suerbaum J, von Grünhagen D, McIntyre WF, Benz AP, Wong JA, Merali F, Henein S, Nichol C, Connolly SJ, Healey JS; SCREEN-AF investigators and coordinators . Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol 2021;6:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lubitz SA, Atlas SJ, Ashburner JM, Lipsanopoulos ATT, Borowsky LH, Guan W, Khurshid S, Ellinor PT, Chang Y, McManus DD, Singer DE. Screening for atrial fibrillation in older adults at primary care visits: VITAL-AF randomized controlled trial. Circulation 2022;145:946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kemp Gudmundsdottir K, Fredriksson T, Svennberg E, Al-Khalili F, Friberg L, Frykman V, Hijazi Z, Rosenqvist M, Engdahl J. Stepwise mass screening for atrial fibrillation using N-terminal B-type natriuretic peptide: the STROKESTOP II study. Europace 2020;22:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Proietti M, Mairesse GH, Goethals P, Scavee C, Vijgen J, Blankoff I, Vandekerckhove Y, Lip GY; Belgian Heart Rhythm Week Investigators . A population screening programme for atrial fibrillation: a report from the Belgian Heart Rhythm Week screening programme. Europace 2016;18:1779–1786. [DOI] [PubMed] [Google Scholar]
- 74. Lowres N, Olivier J, Chao TF, Chen SA, Chen Y, Diederichsen A, Fitzmaurice DA, Gomez-Doblas JJ, Harbison J, Healey JS, Hobbs FDR, Kaasenbrood F, Keen W, Lee VW, Lindholt JS, Lip GYH, Mairesse GH, Mant J, Martin JW, Martín-Rioboó E, McManus DD, Muñiz J, Münzel T, Nakamya J, Neubeck L, Orchard JJ, de Torres LÁ Pérula, Proietti M, Quinn FR, Roalfe AK, Sandhu RK, Schnabel RB, Smyth B, Soni A, Tieleman R, Wang J, Wild PS, Yan BP, Freedman B. Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single time screening: a multicountry patient-level meta-analysis of 141,220 screened individuals. PLoS Med 2019;16:e1002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McIntyre WF, Diederichsen SZ, Freedman B, Schnabel RB, Svennberg E, Healey JS. Screening for atrial fibrillation to prevent stroke: a meta-analysis. Eur Heart J Open 2022;2:oeac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Williams K, Modi RN, Dymond A, Hoare S, Powell A, Burt J, Edwards D, Lund J, Johnson R, Lobban T, Lown M, Sweeting MJ, Thom H, Kaptoge S, Fusco F, Morris S, Lip G, Armstrong N, Cowie MR, Fitzmaurice DA, Freedman B, Griffin SJ, Sutton S, Hobbs FR, McManus RJ, Mant J; The Safer Authorship Group . Cluster randomised controlled trial of screening for atrial fibrillation in people aged 70 years and over to reduce stroke: protocol for the pilot study for the SAFER trial. BMJ Open 2022;12:e065066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Engdahl J, Svennberg E, Friberg L, Al-Khalili F, Frykman V, Gudmundsdottir KK, Fredriksson T, Rosenqvist M. Stepwise mass screening for atrial fibrillation using N-terminal pro b-type natriuretic peptide: the STROKESTOP II study design. Europace 2017;19:297–302. [DOI] [PubMed] [Google Scholar]
- 78. Engler D, Heidbuchel H, Schnabel RB. Digital, risk-based screening for atrial fibrillation in the European community—the AFFECT-EU project funded by the European Union. Eur Heart J 2021;42:2625–2627. [DOI] [PubMed] [Google Scholar]
- 79. Svennberg E, Tjong F, Goette A, Akoum N, di Biase L, Bordachar P, Boriani G, Burri H, Conte G, Deharo JC, Deneke T, Drossart I, Duncker D, Han JK, Heidbuchel H, Jais P, de Oliviera Figueiredo MJ, Linz D, Lip GYH, Malaczynska-Rajpold K, Márquez M, Ploem C, Soejima K, Stiles MK, Wierda E, Vernooy K, Leclercq C, Meyer C, Pisani C, Pak HN, Gupta D, Pürerfellner H, Crijns HJGM, Chavez EA, Willems S, Waldmann V, Dekker L, Wan E, Kavoor P, Turagam MK, Sinner M. How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace 2022;24:979–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Willits I, Keltie K, Craig J, Sims A. WatchBP Home A for opportunistically detecting atrial fibrillation during diagnosis and monitoring of hypertension: a NICE medical technology guidance. Appl Health Econ Health Policy 2014;12:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Atrial Fibrillation—UK National Screening Committee (UK NSC)—GOV.UK . https://view-health-screening-recommendations.service.gov.uk/atrial-fibrillation/(26 November 2022).
- 82. Brieger D, Amerena J, Attia J, Bajorek B, Chan KH, Connell C, Freedman B, Ferguson C, Hall T, Haqqani H, Hendriks J, Hespe C, Hung J, Kalman JM, Sanders P, Worthington J, Yan TD, Zwar N. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ 2018;27:1209–1266. [DOI] [PubMed] [Google Scholar]
- 83. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, Cox JL, Dorian P, Gladstone DJ, Healey JS, Khairy P, Leblanc K, McMurtry MS, Mitchell LB, Nair GM, Nattel S, Parkash R, Pilote L, Sandhu RK, Sarrazin JF, Sharma M, Skanes AC, Talajic M, Tsang TSM, Verma A, Verma S, Whitlock R, Wyse DG, Macle L; Members of the Secondary Panel . The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol 2020;36:1847–1948. [DOI] [PubMed] [Google Scholar]
- 84. Chan NY, Orchard J, Agbayani MJ, Boddington D, Chao TF, Johar S, John B, Joung B, Krishinan S, Krittayaphong R, Kurokawa S, Lau CP, Lim TW, Linh PT, Long VH, Naik A, Okumura Y, Sasano T, Yan B, Raharjo SB, Hanafy DA, Yuniadi Y, Nwe N, Awan ZA, Huang H, Freedman B. 2021 Asia Pacific Heart Rhythm Society (APHRS) practice guidance on atrial fibrillation screening. J Arrhythm 2022;38:31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Joung B, Lee JM, Lee KH, Kim TH, Choi EK, Lim WH, Kang KW, Shim J, Lim HE, Park J, Lee SR, Lee YS, Kim JB; KHRS Atrial Fibrillation Guideline Working Group . 2018 Korean guideline of atrial fibrillation management. Korean Circ J 2018;48:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the society of thoracic surgeons. Circulation 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 88. Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Epling JW Jr, Kubik M, Li L, Ogedegbe G, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for atrial fibrillation: US preventive services task force recommendation statement. JAMA 2022;327:360–367. [DOI] [PubMed] [Google Scholar]
- 89. Godin R, Yeung C, Baranchuk A, Guerra P, Healey JS. Screening for atrial fibrillation using a mobile, single-lead electrocardiogram in Canadian primary care clinics. Can J Cardiol 2019;35:840–845. [DOI] [PubMed] [Google Scholar]
- 90. Andrade JG, Godin R, Nault I. Large-scale implementation of a pragmatic atrial fibrillation screening program in Canadian community practice. Pacing Clin Electrophysiol 2020;43:768–769. [DOI] [PubMed] [Google Scholar]
- 91. Brandes A, Stavrakis S, Freedman B, Antoniou S, Boriani G, Camm AJ, Chow CK, Ding E, Engdahl J, Gibson MM, Golovchiner G, Glotzer T, Guo Y, Healey JS, Hills MT, Johnson L, Lip GYH, Lobban T, Macfarlane PW, Marcus GM, McManus DD, Neubeck L, Orchard J, Perez MV, Schnabel RB, Smyth B, Steinhubl S, Turakhia MP. Consumer-led screening for atrial fibrillation: frontier review of the AF-SCREEN International Collaboration. Circulation 2022;146:1461–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Li YG, Pastori D, Farcomeni A, Yang PS, Jang E, Joung B, Wang YT, Guo YT, Lip GYH. A simple clinical risk score (C2HEST) for predicting incident atrial fibrillation in Asian subjects: derivation in 471,446 Chinese subjects, with internal validation and external application in 451,199 Korean subjects. Chest 2019;155:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Proietti M, Farcomeni A, Goethals P, Scavee C, Vijgen J, Blankoff I, Vandekerckhove Y, Lip GY, Mairesse GH; Belgian Heart Rhythm Week Investigators . Cost-effectiveness and screening performance of ECG handheld machine in a population screening programme: the Belgian Heart Rhythm week screening programme. Eur J Prev Cardiol 2019;26:964–972. [DOI] [PubMed] [Google Scholar]
- 94. Jacobs MS, Kaasenbrood F, Postma MJ, van Hulst M, Tieleman RG. Cost-effectiveness of screening for atrial fibrillation in primary care with a handheld, single-lead electrocardiogram device in the Netherlands. EP Europace 2018;20:12–18. [DOI] [PubMed] [Google Scholar]
- 95. Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al-Khalili F, Friberg L, Frykman-Kull V, Levin LÅ. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace 2015;17:1023–1029. [DOI] [PubMed] [Google Scholar]
- 96. Welton NJ, McAleenan A, Thom HHZ, Davies P, Hollingworth W, Higgins JPT, Okoli G, Sterne JA, Feder G, Eaton D, Hingorani A, Fawsitt C, Lobban T, Bryden P, Richards A, Sofat R. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess 2017;21:1–236. [DOI] [PubMed] [Google Scholar]
- 97. Lyth J, Svennberg E, Bernfort L, Aronsson M, Frykman V, Al-Khalili F, Friberg L, Rosenqvist M, Engdahl J, Levin LÅ. Cost-effectiveness of population screening for atrial fibrillation: the STROKESTOP study. Eur Heart J 2023;21:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Burdett P, Lip GYH. Targeted vs. full population screening costs for incident atrial fibrillation and AF-related stroke for a healthy population aged 65 years in the United Kingdom. Eur Heart J Qual Care Clin Outcomes 2022;8:892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated in support of the article.