Abstract
Traditionally, studies of the neurocognitive correlates of obesity have computed a central tendency across trials of a task to estimate the functional abilities of individual members of obese and non-obese groups. This computation assumes that the correlate is stable over time—a questionable assumption when individuals are impulsive, periodically inattentive, and capable of overcompensation following awareness of failure. The present investigation departs from the tradition by focusing on the second moment, or variability, in brain activation during a simple selective attention task. It compared 124 non-obese and 80 obese teenaged girls on the across-trial average amplitude and inter-trial variability (ITV) of a sensitive biomarker of attention, the P300 event-related electroencephalographic potential. It found that P300 ITV outperformed P300 average amplitude in differentiating the groups. Further, it found that the elevated P300 ITV among obese teenagers was associated with other indicators of impulsivity and inattention as well as slower reaction times and a trend toward more variable reaction times. Future studies should investigate the value of P300 ITV as an objective and sensitive endpoint for cognitive training focused on improving the attention skills of obese children.
Keywords: Obesity, adolescent, attention, cognitive, intraindividual variability, brain activation, P300, electroencephalography
1. Introduction
Many explanations have been offered for the high prevalence of pediatric obesity in the United States (Fryar et al., 2020). The research literature (McAllister et al., 2009) names several possibilities including cultural and economic differences, marketing practices, and poor parental monitoring among other contributors. It also offers explanations that focus more directly on obese children and the intrinsic cognitive factors that influence their diet choices and eating patterns.
The list of cognitive factors on which obese children differ from normal weight children includes cognitive control, attention, and motor skills (Liang et al., 2014; Reinert et al., 2013). Their decrements in these domains are consistent with associations shown in clinical and epidemiological studies between an elevated body mass and attention deficit hyperactivity disorder [ADHD; (Cortese et al., 2016)] as well as conduct disorder (Pauli-Pott et al., 2014; Pine et al., 1997) in which executive cognitive functions (ECF) are primarily affected. The theory connecting ECF to obesity is obvious: an impulsive and inattentive child is less likely to monitor internal and external cues to satiety, monitor meal intervals and tolerate delays, and devote limited capacity attentional resources to a consideration of the adverse effects of weight gain on future physical and mental health.
The present study continued this tradition of research on cognitive skill differences. However, it departs from the tradition by focusing exclusively on the capacity of obese children to attend and maintain attention. This focus is justified by three reasons. First, attention is an overarching skill that regulates or influences most other ECF skills [e.g., working memory (Oberauer, 2019) and cognitive control (Matzke et al., 2017)]. Second, attention is potentially more malleable (Vantieghem et al., 2018) and may be a better target for a cognitive training or remediation approach in future studies. The third and most compelling reason is the prevailing argument (Burke et al., 2011) in the obesity literature asserting that poor self-monitoring of behavior (i.e., inattention) is the primary cognitive deficit promoting overeating, weight gain, and obesity treatment failure. According to this argument, deficits in the separate but overlapping domain of response inhibition are less prevalent and only become relevant (i.e., challenged) when there is a recognition of overeating or obesity as a problem and a motivation to change. The detection and remediation of deficits in attention, problem recognition, and motivation to change have been named as the most formidable challenges in the effective treatment of pediatric obesity (Brennan et al., 2012).
A more significant departure involves the adoption of a different theory of measurement of compromised attention. The present study recognizes that inattention is not a constant state of being among obese children. Their cognitive failures, if present, are likely episodic and infrequent. Accordingly, this study did not rely upon the conventional measurement approach of averaging behavior or brain activation over time or trials of a task. Instead, it adopted a more innovative approach involving the measurement of within-person variability over time. This approach borrows ideas from the ADHD literature wherein studies have reliably demonstrated (Kofler et al., 2013) that within-person reaction time variability outperforms mean reaction time in differentiating groups.
The study was also innovative in focusing on brain activation variability versus reaction time variability. Variability in brain activation has rarely been explored for technical reasons. It requires a metric that has excellent signal-to-noise characteristics on individual trials. The metric must also exhibit good temporal resolution and minimal hysteresis. Accordingly, functional resonance imaging is not appropriate for the task. However, electroencephalography does meet these requirements. In prior studies of adults, we have examined the intra-individual variability (IIV) in the amplitude of an electrocortical orienting response (Donchin, 1981), the P300 event-related electroencephalographic potential (ERP), and shown that it differentiates control groups from groups affected by the presence of an elevated body mass (Bauer, 2018a), antisocial personality disorder (Bauer, 2021), and alcohol (Bauer & Hesselbrock, 2022) and drug (Bauer, 2022) abuse. In all cases, the analyses showed that P300 amplitude IIV had greater sensitivity than mean P300 amplitude in demonstrating neural decrements among affected adults. The elevation in IIV was interpreted as evidence of a diagnostically-nonspecific and episodic dysregulation of P300 generators as well as an instability in brain function affecting both self-monitoring and environmental monitoring (Bauer, 2022).
The goal of the present study was to show that a similar episodic decrement in the maintenance of brain activation is present among children, aged 14–19 years, with an obese body mass. It predicted greater P300 IIV among children with a body mass index percentile (BMIP) greater than or equal to 95% in comparison to children with a BMIP less than 95%. Furthermore, it predicted that P300 IIV would positively correlate with other known indicators of inattention and impulsivity including ADHD and impulsivity symptoms as well as poor task performance.
2. Methods
2.1. Subject Recruitment & Assessment Procedures
The recruitment strategy was designed to maximize success in enrolling female adolescents who possess a propensity toward impulsivity or an externalizing disorder, of which excess adiposity may be an example. Adolescents were recruited indirectly by contacting substance-dependent parents through newspaper advertisements, and presentations at substance abuse treatment programs and support groups. We also recruited adolescents directly with print and radio ads [e.g., “Are you a risk taker?”] and presentations to high school classes, YWCA/YMCA, police athletic leagues, and high school counselors. These recruitment efforts were supplemented with a separate effort that targeted overweight or obese children.
One parent of each prospective participant was invited to telephone a research assistant for screening. The telephone screen was brief and pertinent to eligibility. The questions asked about the age and gender of the prospect, the presence/absence of major medical and psychiatric conditions or disorders, height and weight, and treatment history. Prospects who passed this initial screen were invited to visit the Health Center on a subsequent day for further evaluation.
On the occasion of the in-person visit, the prospect and a parent/guardian were asked to review and sign informed consent and HIPAA agreements. In addition, they signed a medical release form authorizing a request to the prospect’s pediatrician for previous height and weight data. Afterward, the parent/guardian and adolescent girl were escorted to different rooms and interviewed separately. The interview with the parent/guardian was brief and focused on the completion of a medical history questionnaire for the child as well as the child’s family history of obesity, diabetes, and psychiatric disorders. It was primarily used to identify child medical issues that would complicate the interpretation of the neurophysiolocal and behavioral data and prompt exclusion. It also inquired about history of head injury, genetic disorders (e.g., Prader-Willi Syndrome), FAS/FAE (i.e., level of alcohol use during the pregnancy), seizures and serious neurological diseases (e.g., HIV), hypothyroidism, and cardiovascular or renal disease. The parent/guardian was paid $200 for his/her time and effort.
The adolescent girl was a participant in a more extensive assessment. The first step in the assessment was an attempt to detect recent nicotine, alcohol, and other drug use. For this purpose, a saliva sample was collected and analyzed for cannabinoids, cocaine, opiates, PCP, amphetamine, methamphetamine, and prescription drug use during the previous 2–5 days. The saliva test was complemented by a toxicological test of a hair sample (PDT-90™, Psychemedics Inc., Cambridge, MA). Breath samples were also collected and tested for recent tobacco (CO level) and alcohol use. The Timeline-Follow-back procedure (TLFB) was used to elicit self-reports of the quantity and frequency of alcohol, nicotine, and other substance use for the previous 8 weeks. The TLFB used a calendar format to provide temporal cues to assist in recall. The next step in the assessment established the presence or absence, and full symptom counts, of the most common adolescent mental disorders. It employed DSM-IV–TR diagnostic criteria (American_Psychiatric_Association, 2000). A few assessments of psychological variables were added. These included the Barratt Impulsiveness Scale [BIS-11; (Stanford et al., 2009)], Borderline Symptom List [BSL; (Bohus et al., 2009)], and Toronto Alexithymia Scale [TAS; (Bagby et al., 1986)].
Upon completion of the substance use/abuse, medical, and mental health assessments, the data were assembled and presented to a supervisor for interim evaluation. The supervisor attended to urgent clinical issues (e.g., suicidality, child abuse) that emerged during the assessment. A decision to retain or reject a participant from the data set was made at a later time. Participants who reported no past or current pregnancy, psychosis, or major medical disorders that would complicate body weight (e.g., HIV, thyroid disease) or evoked electroencephalographic responses (e.g., seizure disorder, heart disease, hearing loss, uncorrected visual impairment) will be eligible. They were paid $150 for time and effort at the end of the day. Ineligible subjects and their parents/guardians were paid the same amount as eligible subjects for their good faith efforts.
2.2. Adiposity Measurement Procedures
BMI was calculated from measured height and weight. It was converted to an age- and gender-corrected BMI percentile score using growth charts published by the Centers for Disease Control. Triceps skinfold thickness was be recorded to the nearest 0.5 mm (Lange calipers, QuickMedical, Issaquah, WA) by research assistants following a standard protocol. It was measured at the middle of the left and right arm triceps muscles and averaged.
The change over time in BMIP was calculated as the difference between BMIP on the study day and the BMIP (from height and weight data) recorded by the adolescent’s pediatrician during an office exam occurring during the previous 2–4 years. This difference was divided by the interval in years between the study day and the date of the office exam.
2.3. Neurophysiological and Cognitive Task Procedures
After lunch, the neurophysiological and cognitive task battery were administered. Prior to the administration of the battery, a 64-channel electrode cap (Neuroscan/Compumedics) was placed on the girl’s scalp for the later recording of resting EEG power spectral density, event related EEG potentials, and event related time-frequency oscillations. The ground electrode was placed on the forehead. Electrodes placed on the earlobes were the reference. Two electrodes, used for recording eye movements and eyeblinks, were placed above and below the left eye. Interelectrode impedances will be less than 10 kilohms.
After the application of the electrodes, the child completed a simple task (Bauer, 1997) that required attention to, and discrimination of, rarely occurring events that did or did not require a motor response. The task involved the presentation of three visual stimuli--the letters “X” (p=0.1), “C” (p=0.1), and “T” (p=0.8)—at a visual angle of 7.15 deg on a computer screen. They were presented in a Bernoulli series presented for 200 ms each at a rate of one stimulus every 1.5–1.8 s. Three hundred stimuli were presented in total. The participant was instructed to press a key whenever the “X” was presented. She was instructed to ignore all other stimuli.
At the conclusion of the task battery, the participant was dismissed.
2.4. Data collection and reduction
During the task, EEG and eye movement channels were sampled at a rate of 500Hz, digitized and stored for off-line reduction and analysis. Also, markers identifying stimulus and button press onsets were stored. Data reduction used batch processing routines that allowed all data files to be filtered, edited, and summarized identically.
The first step in data reduction was to create a montage that extracted and retained EEG signals recorded from Fz, Cz, Pz, and facial eye movement electrodes. The signals were submitted to a digital filter with a bandpass of 0.5–30 Hz, no phase shift, and a roll-off of 24 db. The next step was to segment the signals into epochs spanning a period of 200 ms preceding and 600 ms following the onsets of the rare target, rare non-target, and frequent stimuli. Subsequently, epochs containing a voltage deviation greater than 50 μV or less than −50 μV in any of the 4 channels were discarded. In addition, epochs containing omission or commission errors were also discarded. Also, some epochs surrounding frequent non-target stimuli were randomly discarded to achieve an approximate balance in the number of retained epochs across rare target, rare non-target, and frequent non-target stimuli. The succeeding steps included the removal of residual eye movement artifact using the method (Semlitsch et al., 1986) implemented in Neuroscan 4.5 software, a compensatory recalculation of the difference between the active EEG channels and the reference channel, an offset to align all epochs to the mean voltage during the pre-stimulus period, and the calculation of the mean voltage as well as the standard deviation in voltage at each sampling point. These steps were performed separately for epochs surrounding the three types of stimuli.
To create summary values suitable for statistical analysis, a routine was applied that calculated the averages of the mean voltages and the average of the standard deviations in voltage over of period of 30 ms preceding and 30 ms following the P300 peak (the maximum voltage) which occurred between 270 and 500 ms (Figure 1). The result was a single estimate of P300 average amplitude at the Fz, Cz, and Pz sites for each stimulus type.
Figure 1.
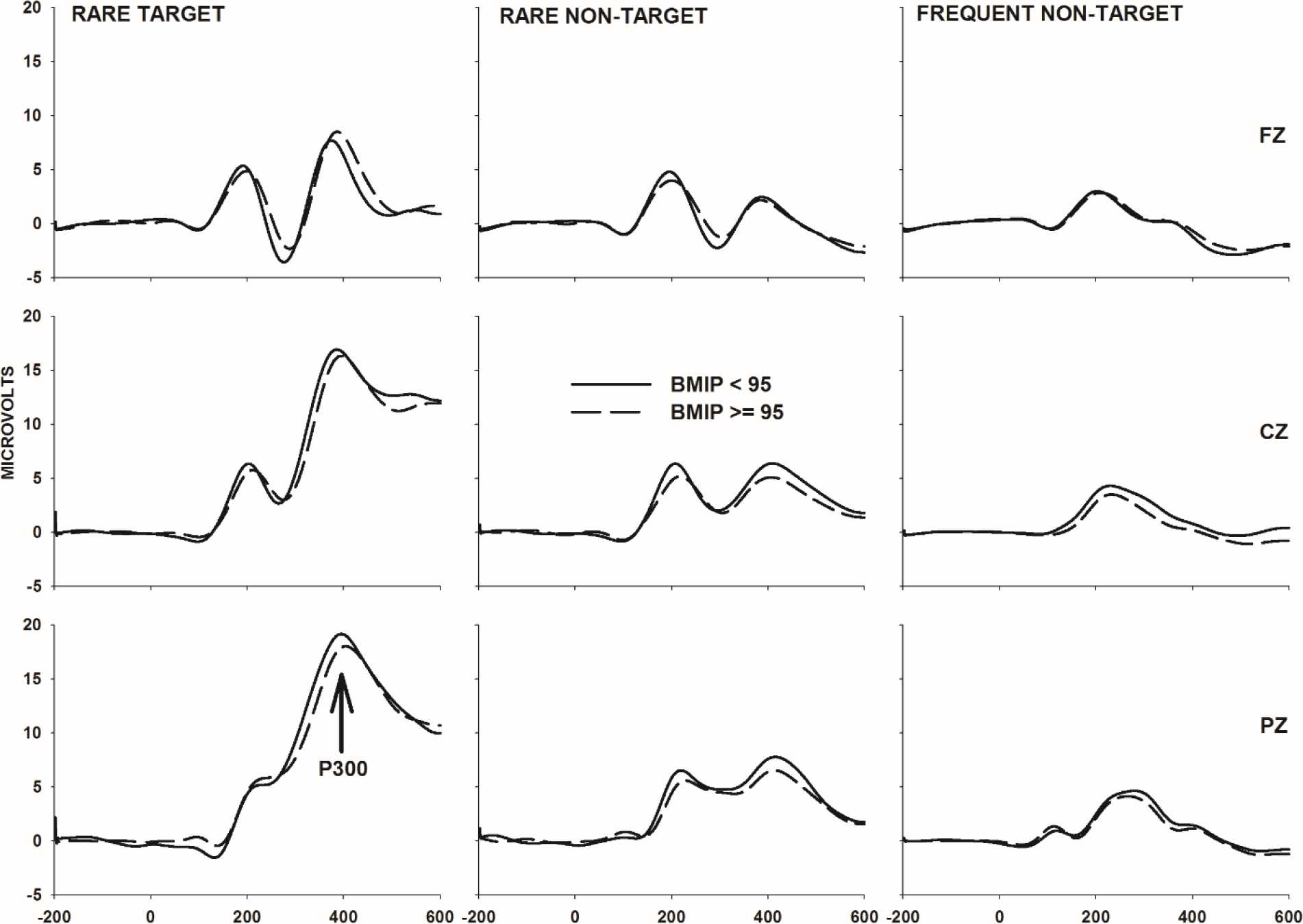
Rare target, rare non-target, and frequent non-target ERP waveforms at Fz, Cz, and Pz sites sorted by group. The X-axis spans a period of 200 ms preceding to 600 ms following stimulus onset
An additional routine was applied to the standard deviation data. To compensate for the possibility that amplifier noise or uncorrected eye movement artifacts remained in the rare target and rare non-target ERPs, IIV in P300 amplitude was calculated as the ratio of the P300 standard deviations in these ERPs divided by the P300 standard deviation over the same interval in the frequent non-target ERP.
The task performance data were summarized in a straightforward manner. Routines calculated the average and standard deviation of reaction times for responses emitted during target trials. They also calculated the proportion of non-target trials with a commission error and the proportion of target trials with a correct response.
2.5. Data analysis
In addition to the medical history criteria described above, participants were excluded for other reasons, including fewer than 15 artifact-free EEG epochs (n=63) in any trial category, a 30% or greater omission or commission error rate (n=12), or equipment failure (n=23). A total of 124 non-obese and 80 obese adolescents met all criteria for inclusion in the present analysis.
The analyses of differences between these groups in their demographic, psychiatric, and psychological characteristics used t-tests (unequal variance assumed) for continuous variables and Pearson’s chi-square test for dichotomous variables. The analysis of task performance, P300 amplitude, and P300 amplitude ITV differences used a different strategy. It began with a 2 (BMIP ≥ 95 vs. BMIP < 95 group) by 2 (white non-Hispanic vs. black or Hispanic) MANOVA with age as a covariate. The MANOVA was employed as an attempt to control for artifacts associated with the conduct of multiple independent tests. Univariate tests of main or interaction effects were performed if---and only if—the multivariate test for the effect was statistically significant (p<0.05).
The final set of analyses was designed to ask if the sensitivity of P300 ITV to group differences in BMIP can also be demonstrated when the distribution of body mass index percentiles is not arbitrarily segmented. In addition, these correlational analyses with age and race/ethnicity as covariates asked if P300 ITV is related to the background variables that best differentiated the BMIP<95 and BMIP≥95 groups.
3. Results
3.1. Background characteristics
Table 1 shows that the obese and non-obese groups differed significantly and expectedly in triceps skinfold thickness as well as BMI z-score. There were also statistically significant differences in racial composition and ethnicity. The obese group contained fewer non-white (32.5% vs. 68.5%; X2=25.4, 1 df, p<0.01) and more Hispanic members (36.3% vs. 19.4%; X2=7.2, 1 df, p<0.01) than the non-obese group. The groups were similar in age (190.5 months vs. 190.8 months).
Table 1.
Background characteristics [M(SD) or %(n)].
| BMIP<95 (n=124) |
BMIP≥95 (n=80) |
Test statistic (equal variances not assumed) | |
|---|---|---|---|
| Age, months | 190.8(15.0) | 190.5(14.5) | t=0.15, p=0.87 |
| Race, %(n) white | 68.5(85) | 32.5(26) | X2=25.4, p<0.01 |
| Hispanic, %(n) | 19.4(24) | 36.3(29) | X2=7.21, p<0.01 |
| BMI Z-score | 0.53(0.79) | 2.15(0.26) | t=−20.97, p<0.01 |
| BMIP change per yr | −0.28(0.59) | 0.39(0.71) | t=−1.40, p=0.16 |
| Triceps skinfold thickness, mm | 27.00(8.21) | 44.66(7.60) | t=−15.64, p<0.01 |
| ADHD Diagnosis, %(n) | 1.6(2) | 2.5(2) | -- |
| Conduct Disorder, %(n) | 1.6(2) | 3.8(3) | -- |
| ADHD Hyperactivity, symptoms/total symptoms | 0.06(0.21) | 0.13(0.26) | t=−1.78, p=0.07 |
| ADHD Impulsiveness, symptoms/total symptoms | 0.04(0.17) | 0.14(0.29) | t=−2.64, p<0.01 |
| ADHD Inattention, symptoms/total symptoms | 0.08(0.17) | 0.17(0.32) | t=−2.09, p<0.04 |
| Borderline Symptom List | 9.50(11.94) | 12.86(12.24) | t=−1.93, p<0.05 |
| Depressive Disorder—single or multiple episode, %(n) | 16.9(21) | 31.3(25) | X2=5.70, p<0.02 |
| BIS Attention Scale | 15.62(4.41) | 16.37(3.82) | t=−1.26, p=0.21 |
| BIS Motor Scale | 20.06(3.29) | 20.59(3.10) | t=−1.15, p=0.24 |
| BIS Nonplanning Scale | 23.61(5.30) | 25.48(5.65) | t=−2.35, p<0.02 |
| TAS Difficulty Describing Feelings | 13.12(2.46) | 13.52(2.47) | t=−1.14, p=0.25 |
| TAS Difficulty Identifying Emotions | 17.16(3.83) | 17.38(3.96) | t=−0.40, p=0.68 |
| TAS Externally Oriented Thinking | 23.65(2.77) | 23.89(2.75) | t=−0.61, p=0.53 |
| Nicotine use, cigarettes/week | 0.04(0.40) | 1.41(10.25) | t=−1.19, p=0.23 |
| Alcohol use, standard drinks/week | 0.48(1.58) | 0.31(1.14) | t=0.88, p=0.37 |
| Cannabis use, episodes/week | 0.23(1.34) | 0.38(1.67) | t=−0.70, p=0.48 |
A number of other predicted differences were detected. In comparison to their unaffected peers, the obese group reported more ADHD symptoms in the impulsiveness (0.14 vs. 0.04; t=−2.64, df=114.03, p<0.01) and inattention (0.17 vs. 0.08; t=−2.09, df=130.60, p<0.04) categories and a trend in this direction for the hyperactivity category. They also reported more non-planning impulsiveness (25.4 vs. 23.6; t=−2.35, df=158.30, p<0.02) on the Barratt Impulsiveness Scale and more symptoms of borderline personality on the Borderline Symptom List scale (12.8 vs. 9.5; t=−1.93, df=165.69, p<0.05). The final noteworthy finding was a higher prevalence of depressive disorder (31.3% vs. 16.9%; X2=5.70, 1 df, p<0.02). The remaining dependent variables, including substance use and alexithymia indicators, did not distinguish obese and non-obese participants.
3.2. ERP and task performance data
The MANCOVA of ERP and task performance data revealed a significant main effect of BMIP [Pillai’s Trace=0.16, F(18,182)=1.98, p=0.012, ήp2=0.16]. The main effect of race/ethnicity and the interaction of BMIP and race/ethnicity were not statistically significant. Univariate ANOVAs (Table 1) were performed to clarify the significant BMIP main effect detected by the multivariate analysis.
In comparison to their normal or overweight weight peers, obese adolescents showed task performance decrements. They responded on fewer target trial [0.95 vs. 0.98; F(1,199)=7.21, p=0.008, ήp2=0.035] and also responded more slowly [406 vs. 384 ms; F(1,199)=6.17, p=0.014, ήp2=0.030]. In addition, they demonstrated more variability over trials in their response times [SD of RT: 102.63 vs. 87.37; F(1,199)=7.55, p=0.007, ήp2=0.037].
Univariate analyses of the ERP data demonstrated no differences between the groups in P300 averaged amplitude. However, there was a difference in P300 ITV. More specifically, adolescents with a BMIP≥95 demonstrated greater P300 ITV than their peers in the control group. The elevation was statistically significant only at Cz [1.34 vs. 1.18; F(1,199)=6.42, p=0.012, ήp2=0.031] and Pz [1.27 vs. 1.17; F(1,199)=3.76, p=0.050, ήp2=0.019] electrode sites and only during the processing of target stimuli. It is worthy of note that this group difference in ITV cannot be easily ascribed to a difference in the reliability of the ITV estimates: the groups did not significantly differ in the number of target trials over which ITV was calculated [25.3 vs. 25.7; F(1,199)=0.25, p=0.612, ήp2=0.001].
A final set of analyses used simple regression, with age and race/ethnicity as covariates, to examine the within-person associations of P300 ITV at the Cz electrode site (Table 2) with all task performance indices, ADHD and BSL symptoms, BIS-11 scores, and BMI-Z-score and BMIP change. The partial correlations were computed across all participants. The analyses revealed significant positive correlations with BMI-Z score (r=0.14, p=0.04) and BMIP change (r=0.19, p=0.006), ADHD Impulsiveness (r=0.15, p=0.02) and Hyperactivity (r=0.14, p=0.03) symptoms, and the BIS-11 Motor Impulsivity subscale (r=0.14, p=0.03) score.
Table 2.
ERP and task performance data [Age- and race/ethnic-adjusted M(SE)].
| BMIP<95 (n=124) |
BMIP≥95 (n=80) |
Test statistic | |
|---|---|---|---|
| Task performance | |||
| Correct responses, proportion* | 0.98(0.01) | 0.95(0.01) | F=7.21, p=0.008 |
| Reaction time, ms* | 384(5.58) | 406(6.89) | F=6.17, p=0.014 |
| Commission errors, proportion | 0.003(0.01) | 0.003(0.01) | F=0.85, p=0.356 |
| SD of reaction time* | 87.37(3.49) | 102.63(4.31) | F=7.55, p=0.007 |
| Target stimulus ERP | |||
| # ERP epochs processed | 25.77(0.56) | 25.32(0.69) | F=0.25, p=0.612 |
| Amplitude @ Fz, μV | 8.18(0.91) | 7.13(1.12) | F=0.51, p=0.473 |
| Amplitude @ Cz, μV | 18.28(0.91) | 16.66(1.13) | F=1.23, p=0.268 |
| Amplitude @ Pz, μV | 20.34(0.80) | 19.34(0.99) | F=0.60, p=0.437 |
| Target/Standard Ratio @ Fz | 1.24(0.05) | 1.35(0.06) | F=2.07, p=0.152 |
| Target/Standard Ratio @ Cz* | 1.18(0.04) | 1.34(0.05) | F=6.42, p=0.012 |
| Target/Standard Ratio @ Pz* | 1.17(0.03) | 1.27(0.03) | F=3.76, p=0.054 |
| Rare non-target stimulus ERP | |||
| # ERP epochs processed | 20.52(0.53) | 20.85(0.66) | F=0.14, p=0.702 |
| Amplitude @ Fz, μV | 2.11(0.71) | 2.75(0.88) | F=0.31, p=0.574 |
| Amplitude @ Cz, μV | 7.05(0.66) | 6.65(0.81) | F=0.14, p=0.705 |
| Amplitude @ Pz, μV | 9.25(0.58) | 8.79(0.72) | F=0.24, p=0.622 |
| Novel/Standard Ratio @ Fz | 1.22(0.04) | 1.23(0.05) | F=0.05, p=0.815 |
| Novel/Standard Ratio @ Cz | 1.18(0.03) | 1.18(0.04) | F=0.01, p=0.938 |
| Novel/Standard Ratio @ Pz | 1.12(0.03) | 1.08(0.04) | F=0.74, p=0.388 |
4. Discussion
Previous research has documented the presence of statistically significant but subtle differences between obese and non-obese adolescents in cognitive task and structural and functional magnetic resonance imaging findings. Researchers have described subtle reductions in orbitofrontal cortical thickness (Yau et al., 2014), altered activation of brain reward systems (Letra et al., 2017), and executive cognitive function deficits (Reinert et al., 2013) among other findings. Studies of simple cognitive abilities, such as the ability to direct and sustain attention, have rarely been investigated among obese children using P300 event related potentials and other sensitive biomarkers. Yet, as we described previously, the recruitment and maintenence of attention may be the sine qua non underlying self-monitoring behaviors (Burke et al., 2011) that prevent or reduce obesity.
The present study used a biomarker to show that a subtle disruption in the maintenance of attention is detectable among obese adolescents. It is noteworthy that the disruption was evident in a participant sample that was not complicated by diabetes or a high prevalence of ADHD or conduct disorder although their symptom levels of these psychiatric disorders were elevated (Table 1). More significantly, the biomarker that differentiated the groups was not the conventional choice in ERP research--P300 amplitude computed as an average across time and trials. Instead, it was a more subtle neurophysiological disruption detectable on some but not all trials and indicated by an elevation in the inter-trial variability of P300 amplitude.
This interpretation of the present findings is informed by a similar literature that examines inter-trial variability in reaction time (MacDonald et al., 2009) within which there has long been a suggestion that there are not consistent stimulus monitoring behavior differences between, for example, ADHD-positive and ADHD-negative groups (Kofler et al., 2013): reaction times during stimulus monitoring tasks are highly variable and more variable among children with ADHD than their unaffected peers. In fact, it may be this asymmetric elevation in variability that underlies and promotes differences between the groups in mean reaction time. Given the association between pediatric obesity and ADHD symptoms documented presently and elsewhere, one could speculate that obese adolescents are similarly prone to brief episodes of behavioral and neural dysfunction and do not possess an impairment that is consistently evident.
Other findings from our previous studies of adult participants further buttress these hypotheses. For example, we (Bauer, 2018a) have previously shown that overweight or obese adults demonstrate more variability over trials in the amplitude of cortical readiness potentials in comparison to normal weight adults. Here, we demonstrate a similar finding in obese children. In addition, we have demonstrated enhanced inter-trial variability in P300 amplitude among adults with features suggestive of heightened impulsivity and inattention such as drug abuse (Bauer, 2018b) and antisocial personality disorder (Bauer, 2021). Another recent study suggests that elevated P300 ITV is a stable trait: it correlates with a genetic polymorphism within the GRM8 region associated with substance dependence (Bauer & Covault, 2020).
We would be remiss to not acknowledge a few limitations of the present investigation. An obvious limitation is the cross-sectional design. A prospective design that demonstrates covariation of body mass and P300 ITV over time would support a stronger causal inference. Another limitation is the sampling strategy which was biased toward the recruitment of adolescents who were impulsive, rule breaking, and obese. An analysis of a randomly-drawn and unrestricted sample of obese or at-risk-for-overweight adolescents may yield different results.
A separate question may be raised by experts in ERP research methods: is the elevation in P300 ITV among obese adolescents simply a reflection of undetected and uncorrected artifacts? In support of this argument, one might note that P300 ITV in the target response was positively correlated with both ADHD Hyperactivity symptoms and Barratt Impulsiveness Scale motor impulsivity ratings. Yet, an explanation based on artifact looses credence when one considers that ITV was computed as a ratio of the P300 standard deviations in the rare target and frequent non-target responses. Furthermore, the same group difference (obese > non-obese) was not detected in the ratio of the standard deviations of the rare non-target and frequent non-target responses. An artifact would not be expected to show this level of selectivity—it would be expected to appear in all responses equivalently. The aggressive voltage deviation and eye movement correction algorithms used during preprocessing of the data add further weight to the counter-argument and discount artifacts as an explanation for the group difference in P300 ITV.
In sum, the present findings suggest a novel path forward in our understanding of the neurocognitive factors that contribute to pediatric obesity. They show that variability in brain activation is a more sensitive indicator of obesity risk than the average level of brain activation. Inter-trial variability in brain activation is also not behaviorally silent: it is positively correlated with reaction time and, to a lesser degree (p=0.07), with variability in reaction time. Future studies should investigate the value of P300 ITV as an objective and sensitive endpoint for cognitive training focused on improving the attention and maintenance-of-attention skills of obese children.
Table 3.
Partial correlations, adjusting for age and race/ethnicity, of selected inattention and impulsivity indicators with target stimulus P300 ITV at Cz.
| BMI-for-age Z score | rp=0.145, p=0.040 |
| BMI change/yr | rp=0.198, p=0.006 |
| Hit rate | rp=0.023, p=0.749 |
| Reaction time | rp=0.176, p=0.012 |
| SD of reaction time | rp=0.126, p=0.073 |
| ADHD Hyperactivity | rp=0.146, p=0.038 |
| ADHD Impulsivity | rp=0.156, p=0.026 |
| ADHD Inattention | rp=0.118, p=0.093 |
| BIS-11 Motor Impulsivity | rp=0.147, p=0.037 |
| BIS-11 Attention Impulsivity | rp=0.055, p=0.437 |
| BIS-11 Nonplanning Impulsivity | rp=0.081, p=0.251 |
| Borderline Symptom List | rp=0.074, p=0.293 |
Acknowledgement
This research was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (P50AA027055).
Footnotes
Declaration of competing interest
The author has no competing interest.
Data availability
Data will be made available on request
References
- American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders, DSM-IV (4th edition, text revision). Washington DC: American Psychiatric Press. 10.1176/appi.books.9780890420249.dsm-iv-tr [DOI] [Google Scholar]
- Bagby RM, Taylor GJ, & Ryan D (1986). Toronto Alexithymia Scale: relationship with personality and psychopathology measures. Psychother Psychosom, 45(4), 207–215. http://www.ncbi.nlm.nih.gov/pubmed/3588819 [DOI] [PubMed] [Google Scholar]
- Bauer LO (2018a). Inter-trial variability in brain activity as an indicator of synergistic effects of HIV-1 and drug abuse. Drug and Alcohol Dependence, 191, 300–308. 10.1016/j.drugalcdep.2018.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO (2018b). HIV/AIDS and an overweight body mass are associated with excessive intra-individual variability in response preparation. Journal of Neurovirology, 24(5), 577–586. 10.1007/S13365-018-0644-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO (2021). Temporal instability in brain activation: a novel paradigm for evaluating the maintenance of attention among substance dependent patients. Psychopharmacology, 238(10), 2937–2946. 10.1007/S00213-021-05909-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO (2022). Inter-trial variability in postural control and brain activation: Effects of previous opiate abuse. Biological Psychology, 174. 10.1016/J.BIOPSYCHO.2022.108424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, & Covault JM (2020). GRM8 genotype is associated with externalizing disorders and greater inter-trial variability in brain activation during a response inhibition task. Clinical Neurophysiology, 131, 1180–1186. 10.1016/j.clinph.2020.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, & Hesselbrock VM (2022). Signal in the noise: Altered brain activation among adolescent alcohol users detected via the analysis of intra-individual variability1. Psychopharmacology. 10.1007/S00213-022-06234-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohus M, Kleindienst N, Limberger MF, Stieglitz RD, Domsalla M, Chapman AL, Steil R, Philipsen A, & Wolf M (2009). The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology, 42(1), 32–39. 10.1159/000173701 [DOI] [PubMed] [Google Scholar]
- Brennan L, Walkley J, & Wilks R (2012). Parent- and adolescent-reported barriers to participation in an adolescent overweight and obesity intervention. Obesity (Silver Spring, Md.), 20(6), 1319–1324. 10.1038/OBY.2011.358 [DOI] [PubMed] [Google Scholar]
- Burke LE, Wang J, & Sevick MA (2011). Self-monitoring in weight loss: a systematic review of the literature. Journal of the American Dietetic Association, 111(1), 92–102. 10.1016/J.JADA.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Peñalver C, Rohde LA, & Faraone SV (2016). Association Between ADHD and Obesity: A Systematic Review and Meta-Analysis. The American Journal of Psychiatry, 173(1), 34–43. 10.1176/APPI.AJP.2015.15020266 [DOI] [PubMed] [Google Scholar]
- Donchin E (1981). Presidential address, 1980. Surprise!...Surprise? Psychophysiology, 18(5), 493–513. 10.1111/J.1469-8986.1981.TB01815.X [DOI] [PubMed] [Google Scholar]
- Fryar CD, Carroll MD, & Afful J (2020). Prevalence of Overweight, Obesity, and Severe Obesity Among Children and Adolescents Aged 2–19 Years: United States. National Center for Health Statistics. https://www.cdc.gov/nchs/products/index.htm. [Google Scholar]
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, & Kolomeyer EG (2013). Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin Psychol Rev, 33(6), 795–811. [DOI] [PubMed] [Google Scholar]
- Letra L, Pereira D, & Castelo-Branco M (2017). Functional Neuroimaging in Obesity Research. Advances in Neurobiology, 19, 239–248. 10.1007/978-3-319-63260-5_10 [DOI] [PubMed] [Google Scholar]
- Liang J, Matheson BE, Kaye WH, & Boutelle KN (2014). Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes (Lond), 38(4), 494–506. 10.1038/ijo.2013.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SW, Li SC, & Backman L (2009). Neural underpinnings of within-person variability in cognitive functioning. Psychol Aging, 24(4), 792–808. 10.1037/a0017798 [DOI] [PubMed] [Google Scholar]
- Matzke D, Hughes M, Badcock JC, Michie P, & Heathcote A (2017). Failures of cognitive control or attention? The case of stop-signal deficits in schizophrenia. Attention, Perception, and Psychophysics, 79(4), 1078–1086. 10.3758/S13414-017-1287-8/TABLES/2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M, Benca RM, Biggio J, Boggiano MM, Eisenmann JC, Elobeid M, Fontaine KR, Gluckman P, Hanlon EC, Katzmarzyk P, Pietrobelli A, Redden DT, Ruden DM, Wang C, … Allison DB (2009). Ten Putative Contributors to the Obesity Epidemic. Critical Reviews in Food Science and Nutrition, 49(10), 868. 10.1080/10408390903372599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberauer K (2019). Working memory and attention - A conceptual analysis and review. Journal of Cognition, 2(1), 1–23. 10.5334/JOC.58/METRICS/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli-Pott U, Neidhard J, Heinzel-Gutenbrunner M, & Becker K (2014). On the link between attention deficit/hyperactivity disorder and obesity: do comorbid oppositional defiant and conduct disorder matter? Eur Child Adolesc Psychiatry, 23(7), 531–537. 10.1007/s00787-013-0489-4 [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Brook J, & Coplan JD (1997). Psychiatric symptoms in adolescence as predictors of obesity in early adulthood: a longitudinal study. Am J Public Health, 87(8), 1303–1310. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9279265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert KR, Po’e EK, & Barkin SL (2013). The relationship between executive function and obesity in children and adolescents: a systematic literature review. J Obes, 2013, 820956. 10.1155/2013/820956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, & Presslich O (1986). A Solution for Reliable and Valid Reduction of Ocular Artifacts, Applied to the P300 ERP. Psychophysiology, 23(6), 695–703. 10.1111/j.1469-8986.1986.tb00696.x [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, & Patton JH (2009). Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences, 47, 385–395. 10.1016/j.paid.2009.04.008 [DOI] [Google Scholar]
- Vantieghem S, Bautmans I, Guchtenaere A. De, Tanghe A, & Provyn S (2018). Improved cognitive functioning in obese adolescents after a 30-week inpatient weight loss program. Pediatric Research, 84(2), 267–271. 10.1038/S41390-018-0047-3 [DOI] [PubMed] [Google Scholar]
- Yau PL, Kang EH, Javier DC, & Convit A (2014). Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring), 22(8), 1865–1871. 10.1002/oby.20801 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request


