Abstract
Canine obesity negatively influences health and well-being, but can be managed by altering diet composition and caloric intake. Restricted feeding, dietary intervention, and consequent weight loss may be used to improve health and modify gastrointestinal microbiota. In this study, we aimed to determine the effects of restricted feeding of specially formulated foods on weight loss, body composition, voluntary physical activity, serum hormones and oxidative stress markers, and fecal metabolites and microbiota populations of obese dogs. Twenty-four obese dogs [body weight (BW) = 15.2 ± 1.7 kg; body condition score (BCS) = 8.7 ± 0.4; muscle condition score (MCS) = 3.5 ± 0.3; age = 7.2 ± 1.6 yr] were used in a 24-wk study. A control (OR) food was fed during a 4-wk baseline to identify intake needed to maintain BW. After baseline, dogs were allotted to one of two diets: OR or test (FT), and then fed to lose 1.5% BW/wk. Food intake, BW, BCS, and MCS were measured, blood and fecal samples were collected, DEXA scans were performed, and voluntary physical activity was measured over time. Microbiota data were evaluated using QIIME2 and change from baseline data from other measures were evaluated using the Mixed Models procedure of SAS, with P < 0.05 being significant. Restricted feeding led to reduced BW, BCS, fat mass, and blood cholesterol, triglyceride, glucose, and leptin concentrations, and increased MCS and lean body mass percentage. Blood cholesterol reduction was greater in dogs fed FT vs. OR. Fecal metabolites and bacterial alpha-diversity were affected by diet and weight loss. Dogs fed FT had greater reductions in fecal short-chain fatty acid, branched-chain fatty acid, and ammonia concentrations than those fed OR. Dogs fed OR had a higher alpha-diversity than those fed FT. Weight loss increased alpha-diversity (weeks 16, 20, and 24 > weeks 0 and 4). Beta-diversity showed separation between dietary groups and between week 0 and all other time points after week 8. Weight loss increased fecal Allobaculum and Ruminococcus torques. Weight loss also increased fecal Bifidobacterium, Faecalibaculum, and Parasutterella, but were greater in dogs fed OR. Weight loss decreased fecal Collinsella, Turicibacter, Blautia, Ruminococcus gnavus, Faecalibacterium, and Peptoclostridium, but were greater in dogs fed OR. In summary, restricted feeding promoted safe weight and fat loss, reduced blood lipid and leptin concentrations, and altered fecal microbiota of obese dogs.
Keywords: canine microbiome, canine obesity, 16S rRNA gene sequencing
In this study, we aimed to determine the effects of restricted feeding of specially formulated foods on weight loss, body composition, voluntary physical activity, serum hormones and oxidative stress markers, and fecal metabolites and microbiota populations of obese dogs. Restricted feeding, with high-protein, low-starch kibble diets, was shown to promote safe weight and fat loss, reduce blood lipid and leptin concentrations, and alter fecal microbiota.
Introduction
In 2021, 44% of dog owners in the United States believed that their pet was overweight or obese (APOP, 2021). In contrast, veterinarian reports indicate that more than half of these dogs were overweight or obese (Courcier et al., 2010; APOP, 2018). Alarmingly, the problem is becoming more prevalent, with a survey indicating that the affected dog population increased by 58% between 2007 and 2017 (Banfield Pet Hospital., 2017). Obesity is uncommon early in life, peaks in the middle years, and then declines later in life (Lund et al. 2005, 2006; Pegram et al., 2021), most likely due to the onset of chronic diseases of old age. Breed (e.g., Labrador Retrievers, Golden Retrievers, Pugs, Springer Spaniels, Cocker Spaniels, and Beagles have increased rates), sex (e.g., greater in females), and neuter status (e.g., greater in neutered) are known to predispose dogs to obesity (Colliard et al., 2006; Lund et al., 2006; Pegram et al., 2021). Because of the chronic inflammatory condition that is caused by obesity, this metabolic condition and syndrome is associated with a shorter life span, a lower quality of life, and a higher incidence of osteoarthritis, diabetes mellitus, renal disease, and neoplasia (Kealy et al., 2002; Cottam et al., 2004; German, 2006; Lund et al., 2006; German et al., 2012; Raffan, 2013; Gabbay et al., 2015; Yam et al., 2016; Salt et al., 2019). Many professional veterinary health organizations now recognize obesity as a disease due to the negative impacts it has on pet health and quality of life (Day, 2017; Ward et al., 2019).
One method of treating obesity is calorie restriction; however, restricting calories without changing the concentration of macronutrients, particularly protein, can result in muscle mass loss (Shepherd, 2021). Furthermore, dietary energy restriction causes hunger, which leads to increased begging and scavenging activities, potentially leading to owner noncompliance or complete withdrawal from the weight loss program. When compared with diets supplemented with fiber or protein alone, diets formulated with high protein and fiber concentrations have been shown to improve satiety in dogs (German et al., 2010). As a result, when it comes to weight loss programs for dogs, high-protein, high-fiber diets are typically regarded as the best option (Blanchard et al., 2004; German et al., 2007; Floerchinger et al., 2015; André et al., 2017; Kieler et al., 2017; Salas-Mani et al., 2018; Sanchez et al., 2020; Phungviwatnikul et al., 2022). Dietary restriction and subsequent weight loss have been shown to increase life expectancy, decrease serum leptin, insulin, glucose, and triglyceride concentrations, and increase physical activity levels in dogs (Kealy et al., 2002; Jeusette et al., 2005; German et al., 2009; Warren et al., 2011; Bastien et al., 2015; Floerchinger et al., 2015; Phungviwatnikul et al., 2022).
Furthermore, there has been interest in researching the role that gut microbiota play in canine obesity. In previous studies, obesity has been linked to changes in the gut microbiota, with decreased biodiversity being reported (Handl et al., 2013; Park et al., 2015; Kieler et al., 2017; Forster et al., 2018; Salas-Mani et al., 2018; Sanchez et al., 2020; Phungviwatnikul et al., 2022). While the gut microbiota is linked with obesity, it is unclear if and/or how they contribute to obesity development. Proposed mechanisms include the production of fermentative products and other bioactive molecules that may result in increased dietary energy harvest (Turnbaugh et al., 2006), changes in lipid metabolism and fat storage regulation (Bäckhed et al. 2004, 2007; Ghazalpour et al., 2016), altered satiety (Arora et al., 2011), and increased systemic low-grade inflammation (Schwartz, 2000; Cani et al., 2007, 2012; de Lartigue et al., 2011; Tehrani et al., 2012). A couple of studies have analyzed the fecal microbiota of obese dogs before and after weight loss, reporting an increase in bacterial biodiversity and decrease in the Firmicutes/Bacteroidetes ratio after weight loss (Salas-Mani et al., 2018; Sanchez et al., 2020), but more research is necessary.
The purpose of this study was to determine the effects of restricted feeding of specially formulated diets (high-protein, low-starch) on weight loss, body composition, voluntary physical activity, blood metabolite profiles, serum markers of oxidative stress, and fecal microbiota populations and metabolites of obese dogs. We hypothesized that closely monitoring BW and adjusting dietary intake would result in consistent weight loss, fat loss, and lean mass preservation. Furthermore, we hypothesized that losing weight would increase voluntary physical activity levels, lower blood lipid concentrations, and reduce serum oxidative stress marker concentrations. Lastly, diet restriction and weight loss were expected to beneficially alter the fecal microbiota community and metabolite concentrations [e.g., increased short-chain fatty acid (SCFA) and decreased protein catabolite concentrations].
Materials and Methods
All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee prior to experimentation (IACUC #20225) and were performed in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Experimental design
Twenty-four obese adult spayed female Beagle dogs [body weight (BW) = 15.2 ± 1.7 kg; body condition score (BCS) = 8.7 ± 0.4; muscle condition score (MCS) = 3.5 ± 0.3; age = 7.2 ± 1.6 yr] were used in a longitudinal weight-loss study. The 28-wk experiment consisted of a 4-wk baseline phase, followed by a 24-wk weight loss phase. All dogs were considered healthy, except for being obese. Complete blood count and serum metabolite profiles were within normal reference ranges and a physical exam by a veterinarian confirmed health at baseline. Dogs had not received any medications that could affect blood parameters and gut microbiota for at least 4 wk before and during the experiment. The maintenance energy requirement (MER) of all dogs was obtained during a 4-wk baseline phase, where animals were fed the control diet. A control diet (OR; Orijen Original, Champion Petfoods LP, Edmonton, Canada) was formulated to meet all of the Association of American Feed Control Officials (AAFCO, 2020) nutrient profiles for adult dogs at maintenance. After the baseline phase (week 0), dogs were allotted to one of two diets [OR or Fit & Trim (FT; Champion Petfoods LP)] and fed at a rate to lose approximately 1.5% BW per wk as recommended by the American Animal Hospital Association weight management guidelines for dogs and cats (Brooks et al., 2014). To achieve weight loss, dogs initially received 70% to 80% of the food required to maintain BW during the baseline phase and then energy intake was adjusted weekly based on the level of weight loss. Food intake was measured daily. BW, BCS (9-point scale) according to Laflamme (1997), and MCS according to WSAVA (2011) were measured weekly prior to being fed their meal. So that statistics could be conducted on MCS data, the following MCS scale was used: 1 = severe muscle loss, 2 = moderate muscle loss, 3 = mild muscle loss, 4 = normal muscle mass. Once dogs met their target BW (TBW), food intake was adjusted to maintain BW.
Dogs were housed individually in pens (1.22 m wide × 1.85 m long) in a temperature-controlled room under a 12 h light:12 h dark cycle in the Veterinary Medicine Basic Sciences Building at the University of Illinois. Dogs had free access to fresh water and were fed once a day (0800 to 0900 hours) throughout the study. Food offerings and refusals were measured daily to calculate intake.
Dogs were allowed outside of their pens a couple of days a week for socialization with other dogs in compatible groups and humans. Pens were cleaned daily and dogs were bathed every 2 wk.
Blood samples were collected at baseline (week 0) and 6, 12, 18, and 24 wk during caloric restriction of the test diets for serum chemistry, hematology, oxidative stress marker [malondialdehyde (MDA); superoxide dismutase (SOD)], and leptin measurement. Fecal samples were collected at baseline (week 0) and 4, 8, 12, 16, 20, and 24 wk during caloric restriction of the test diets for fecal microbiota and metabolite analyses. At baseline (week 0), weeks 8, 16, and 24, accelerometers were used to measure voluntary physical activity. At baseline (week 0), weeks 6, 12, 18, and 24, dual-energy x-ray absorptiometry (DEXA) scans were performed to estimate body fat, lean, and bone mass.
Chemical analysis of diets and calculations
Diet subsamples were collected once a month. Diet samples were ground through a 2-mm screen using a Wiley Mill (model 4, Thomas Scientific, Swedesboro, NJ). Dry matter (DM) and ash contents were analyzed according to the Association of Official Analytical Chemists (AOAC, 2006; DM: method 934.01; ash: method 942.05), with organic matter (OM) calculated. Fat content was measured using acid hydrolysis and extraction methods of the American Association of Cereal Chemists (AACC, 1983) and Budde (1952). Crude protein (CP) content was calculated from Leco total nitrogen values (TruMac N, Leco Corporation, St. Joseph, MI; AOAC, 2006). Gross energy was measured using an oxygen bomb calorimeter (model 6200, Parr Instruments, Moline, IL). Total dietary fiber (TDF), soluble dietary fiber, and insoluble dietary fiber concentrations were determined according to Prosky et al. (1985). The nitrogen-free extract (NFE) content of foods was calculated as described by equation (1). Metabolizable energy (ME, in kcal “as is”) content was calculated using predictive equations for ME (equation 2; NRC, 2006).
| (1) |
| (2) |
Metabolic body weight (MBW) and resting metabolic rate (RMR) were calculated using equations (3) and (4), respectively (NRC, 2006).
| (3) |
| (4) |
Complete blood count, serum chemistry profile, blood hormones, and oxidative stress markers
Fasted (at least 12 h) blood samples were collected via jugular puncture. Samples were immediately transferred to vacutainer serum tubes containing a clot activator and gel for serum separation (BD Vacutainer SST Tubes—367988; Becton, Dickinson, and Co., Franklin Lakes, NJ) for serum chemistry profile, oxidative stress marker, and leptin analyses. Serum tubes were centrifuged at 1,000 × g for 15 min at 4 °C for serum collection. Whole blood tubes containing K2EDTA additive (BD Microtainer Tubes-363706, Becton, Dickinson, and Co.) were used for complete blood count. Serum chemistry profile and complete blood count was analyzed using a Hitachi 911 clinical chemistry analyzer (Roche Diagnostics, Indianapolis, IN) at the University of Illinois Veterinary Medicine Diagnostics Laboratory. Concentrations of MDA (MBS9713416; MyBioSource, San Diego, CA), SOD (MBS2707828; MyBioSource), and leptin (EZCL-31K; Millipore Sigma, Burlington, MA) were measured using commercial canine-specific enzyme-linked immunosorbent assay kits.
Body composition
Body composition was evaluated by DEXA (Hologic X-ray Bone Densitometer QDR 4500 Elite Acclaim Series; Hologic Inc., Waltham, MA) at the University of Illinois Veterinary Teaching Hospital. On the mornings of DEXA scans, dogs were transported to the Small Animal Clinic according to the university’s transportation guidelines. Dogs were sedated by an intramuscular injection of a combination of Torbugesic (butorphanol tartrate; 0.2 mg/kg BW; Zoetis, Parsippany-Troy Hills, NJ) and Dexdomitor (dexmedetomidine; 0.02 mg/kg BW; Zoetis) and positioned in sternal recumbency. The four legs, trunk, and head of each dog were scanned individually, and measurements of fat, lean, and bone mineral content were taken in each body region. Body fat percentage was calculated for each part and the entire body. After DEXA was complete, an intramuscular injection of the reversal agent for dexmedetomidine, Antisedan (atipamezole HCl; 0.2 mg/kg BW; Zoetis), was given. Dogs were anesthetized for approximately 20 to 30 min and were monitored by lab personnel at all times until they awakened.
Voluntary physical activity
Voluntary physical activity was measured using an accelerometers (Actical devices; Mini Mitter, Bend, OR) and analyzed by computer software (Mini Mitter). During activity monitoring periods, Actical devices were attached to collars worn around the neck for four consecutive days. Mean activity was presented in activity counts per epoch (epoch length = 0.25 min), with light hour (0700 to 1900 hours) and dark hour (1900 to 0700 hours) activity counts also being measured.
Fecal sample collection
One fresh fecal sample from each dog was collected at each time point within 15 min of defecation for measurement of pH, DM, metabolite concentrations [SCFA, branched-chain fatty acids (BCFA), ammonia, phenols, and indoles] and microbiota composition. Fecal pH was measured immediately using a pH meter (Accumet AP1001 Portable pH Meter; Fisher Scientific, Waltham, MA) equipped with an electrode (InLab Surface pH Electrodes 51343157; Mettler Toledo, Columbus, OH). Four aliquots were collected for further analyses: 1) for DM determination, the aliquot was dried at 105 °C for 2 d; 2) for SCFA, BCFA, and ammonia measurements, the aliquot was mixed with 2 N hydrochloric acid in a 1:1 (weight:weight) ratio and stored at –20 °C until analyses; 3) for phenol and indole measurements, the aliquot was stored at –20 °C until analyses; and 4) for microbial samples, the aliquots were frozen on dry ice, and stored at −80 °C until analyses.
Fecal scores
All fecal samples during the collection phase were scored according to the following 5-point scale: 1 = hard, dry pellets, small hard mass; 2 = hard formed, dry stool, remains firm and soft; 3 = soft, formed and moist stool, retains shape; 4 = soft, unformed stool, assumes shape of container; 5 = watery, liquid that can be poured.
Fecal chemical analyses
Fecal samples were analyzed according to procedures of the Association of Official Analytical Chemists (AOAC, 2006) for DM using a 105 °C oven. Fecal SCFA and BCFA concentrations were determined by gas chromatography according to Erwin et al. (1961) using a gas chromatograph (Hewlett-Packard 5890A series II, Palo Alto, CA) and a glass column (180 cm × 4 mm i.d.) packed with 10% SP-1200/1% H3PO4 on 80/100 + mesh Chromosorb WAW (Supelco Inc., Bellefonte, PA). Nitrogen was the carrier with a flow rate of 75 mL/min. Oven, detector, and injector temperatures were 125 °C, 175 °C, and 180 °C, respectively. Fecal ammonia concentrations were determined according to the method of Chaney and Marbach (1962). Fecal phenol and indole concentrations were determined using gas chromatography according to the methods described by Flickinger et al. (2003).
Fecal microbiota populations
Total DNA from fecal samples was extracted using DNeasy PowerLyzer PowerSoil Kit (Qiagen, Carlsbad, CA). The concentration of extracted DNA was quantified using a Qubit 3.0 Fluorometer (Life Technologies, Grand Island, NY). 16S rRNA gene amplicons were generated using a Fluidigm Access Array (Fluidigm Corporation, South San Francisco, CA) in combination with Roche High Fidelity Fast Start Kit (Roche, Indianapolis, IN). The primers 515F (5ʹ-GTGCCAGCMGCCGCGGTAA-3ʹ) and 806R (5ʹ-GGACTACHVGGGTWTCTAAT-3ʹ) target a 252-bp fragment of the V4 region of the 16S rRNA gene that was used for amplification (primers synthesized by IDT Corp., Coralville, IA) according to Caporaso et al. (2012). CS1 forward tag and CS2 reverse tag were added according to the Fluidigm protocol. The quality of the amplicons was assessed using a Fragment Analyzer (Advanced Analytics, Ames, IA) to confirm amplicon regions and sizes. A DNA pool was generated by combining equimolar amounts of the amplicons from each sample. The pooled samples were then size selected on a 2% agarose E-gel (Life Technologies, Grand Island, NY) and extracted using a Qiagen gel purification kit (Qiagen). Cleaned size-selected pooled products were run on an Agilent Bioanalyzer to confirm the appropriate profile and average size. Illumina sequencing was performed on a MiSeq using v3 reagents (Illumina Inc., San Diego, CA) at the Roy J. Carver Biotechnology Center at the University of Illinois.
Bioinformatics and statistical analyses for assessing fecal microbial communities
Forward reads were trimmed using FASTX-Toolkit (version 0.0.14), and sequences were analyzed using QIIME 2.0, version 2021.4 (Caporaso et al., 2010) and DADA2 pipeline for quality control (Callahan et al., 2016). High-quality (quality value ≥ 20) sequence data derived from the sequencing process were demultiplexed. Samples were assigned to taxonomic groups with the Silva database (Silva 138 99% OUT from 515F/806R region of sequences, with the QIIME 2 classifier trained on the 515F/806R V4 region of 16S) (Bokulich et al., 2018; Robeson et al., 2021). A total of 5,963,956 reads were obtained, with an average of 35,499 reads (range = 18,479 to 71,102) per sample. The dataset was rarified to 18,479 reads for analysis of diversity and species richness. Principal coordinates analysis was performed using both weighted and unweighted unique fraction metric (UniFrac) distances (Lozupone and Knight, 2005).
Statistical analyses
All data were analyzed using SAS (version 9.4; SAS Institute, Cary, NC) using the Mixed Models procedure with dog being the random effect. The study was conducted as a repeated measures design, testing the effects of treatment, time, and treatment × time interactions. Change from baseline differences were determined using a Fisher-protected LSD with a Tukey adjustment to control for experiment-wise error. Data normality was checked using the univariate procedure and Shapiro–Wilk statistic, with log transformation being used when normal distribution was lacking. If after the logarithmic transformation of the data, the data did not reach normality, the data were analyzed using the npar1way procedure and Wilcoxon statistic. Data were reported as means, with P < 0.05 considered significant. Linear discriminant analysis (LDA) effect size (Segata et al., 2011) was used to evaluate the QIIME-generated taxonomic tables and to identify genera that were enriched at week 0 vs. 24 and enriched in different dietary groups.
Results
Chemical analysis of diets
Analyzed dietary chemical composition is provided in Table 1. Diets had similar DM, ash, CP, TDF, soluble dietary fiber, insoluble dietary fiber, and NFE concentrations on a dry matter basis (DMB). Acid-hydrolyzed fat (16% vs. 21%, DMB), GE (5.3 vs. 5.6 kcal/g, DMB), and ME (4.6 vs. 4.9 kcal/g, DMB) were lower in the FT diet. When based on caloric density (g/Mcal), the FT diet contained greater amounts of CP and TDF and lower acid-hydrolyzed fat than the OR diet.
Table 1.
Analyzed chemical composition of the experimental diets
| Item | Fit & Trim1 | Original2 |
|---|---|---|
| Dry matter, % | 89.3 | 90.0 |
| Dry matter basis | ||
| Organic matter, % | 90.4 | 91.0 |
| Ash, % | 9.6 | 9.0 |
| Crude protein, % | 45.5 | 43.7 |
| Acid-hydrolyzed fat, % | 16.3 | 20.8 |
| Total dietary fiber, % | 15.7 | 14.6 |
| Insoluble fiber, % | 12.5 | 11.9 |
| Soluble fiber, % | 3.3 | 2.7 |
| Nitrogen-free extract3, % | 12.8 | 11.9 |
| Gross energy, kcal/g4, % | 5.29 | 5.59 |
| Metabolizable energy (ME)NRC5, kcal/g | 4.64 | 4.94 |
| MEMA6, kcal/g | 3.43 | 3.71 |
| Crude protein intake (g)/caloric intake (Mcal)NRC | 98.1 | 88.5 |
| Crude protein intake (g)/calorie intake (Mcal)MA | 132.7 | 117.8 |
| Acid-hydrolyzed fat intake (g)/caloric intake (Mcal)NRC | 35.1 | 42.1 |
| Total dietary fiber intake (g)/caloric intake (Mcal)NRC | 33.8 | 29.6 |
| Insoluble fiber intake (g)/caloric intake (Mcal)NRC | 26.9 | 24.1 |
| Soluble fiber intake (g)/caloric intake (Mcal)NRC | 7.1 | 5.5 |
1Fit & Trim diet (Orijen Fit & Trim; Champion Petfoods LP, Edmonton, Canada): Fresh chicken meat, fresh cage-free eggs, fresh whole herring, fresh turkey meat, fresh chicken liver, fresh whole flounder, fresh whole mackerel, fresh whole Pacific hake, fresh turkey liver, fresh chicken heart, chicken (dehydrated), turkey (dehydrated), whole mackerel (dehydrated), whole sardine (dehydrated), whole herring (dehydrated), Alaskan pollock (dehydrated), lentil fiber, whole red lentils, whole green lentils, fresh whole green peas, fresh whole chickpeas, fresh whole yellow peas, whole pinto beans, whole navy beans, chicken cartilage (dehydrated), fresh turkey heart, apple fiber, dried algae (source of DHA and EPA), pumpkin (dehydrated), butternut squash (dehydrated), carrots (dehydrated), chicken liver (freeze-dried), turkey liver (freeze-dried), fresh whole pumpkin, fresh whole butternut squash, fresh whole zucchini, fresh whole parsnips, fresh carrots, fresh whole Red Delicious apples, fresh whole Bartlett pears, fresh kale, fresh spinach, fresh beet greens, fresh turnip greens, brown kelp, whole cranberries, whole blueberries, whole Saskatoon berries, chicory root, turmeric root, milk thistle, burdock root, lavender, marshmallow root, rosehips.
2Original diet (Orijen Original): Fresh chicken meat, fresh turkey meat, fresh cage-free eggs, fresh chicken liver, fresh whole herring, fresh whole flounder, fresh turkey liver, fresh chicken necks, fresh chicken heart, fresh turkey heart, chicken (dehydrated), turkey (dehydrated), whole mackerel (dehydrated), whole sardine (dehydrated), whole herring (dehydrated), whole red lentils, whole green lentils, whole green peas, lentil fiber, whole chickpeas, whole yellow peas, whole pinto beans, whole navy beans, herring oil, chicken fat, chicken cartilage, chicken liver (freeze-dried), turkey liver (freeze-dried), fresh whole pumpkin, fresh whole butternut squash, fresh whole zucchini, fresh whole parsnips, fresh carrots, fresh whole Red Delicious apples, fresh whole Bartlett pears, fresh kale, fresh spinach, fresh beet greens, fresh turnip greens, brown kelp, whole cranberries, whole blueberries, whole Saskatoon berries, chicory root, turmeric root, milk thistle, burdock root, lavender, marshmallow root, rosehips.
3Nitrogen-free extract = 100 − (ash + crude protein + acid-hydrolyzed fat + total dietary fiber).
4Measured by bomb calorimetry.
5
6MEMA (kcal/g) = (3.5 × crude protein) + (8.5 × crude fat) + (3.5 × nitrogen-free extract).
Food intake, caloric intake, BW, BCS, body composition, and voluntary physical activity
All baseline (week 0) data were analyzed between groups. At baseline, the only difference between treatments was caloric intake and the factors used to estimate total daily energy needs (Supplementary Table 1). No other outcomes differed between groups at baseline (Supplementary Tables 1–8). Throughout the study, average weight loss percentage was 1.65 to 1.68 ± 0.06% per week. By the end of the study, dogs lost approximately 40% of their baseline BW (Supplementary Table 9).
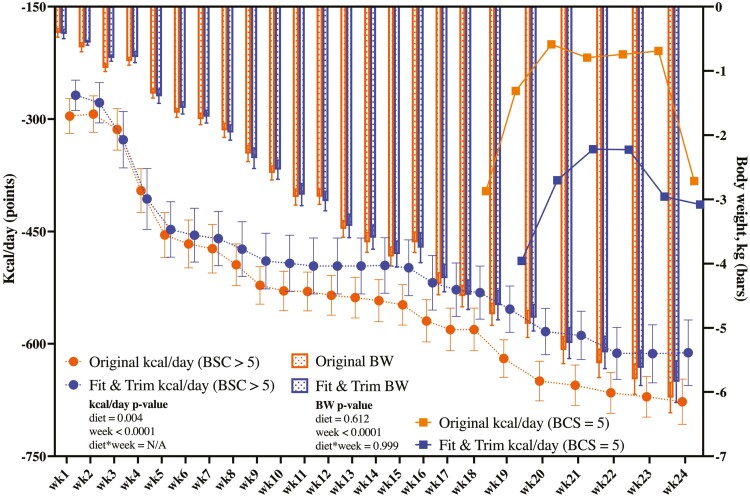
Figure 1, Supplementary Tables 9 and 10 represent change from baseline food, nutrient, and caloric intake data. Diet- and time-related differences were noted, but no diet × time interactions were significant. At baseline, obese animals consumed 919.27 ± 110.59 kcal/d or 60.51 ± 4.17 kcal/kg BW (1.70 ± 0.12 × RMR or 119.33 ± 8.43 × MBW) to maintain BW. At that intake level, 10 ± 1.2 g of protein was consumed per kg MBW. To initiate weight loss in week 1, caloric intake needed to be reduced by 268.34 ± 69.39 kcal/d or 16.91 ± 4.34 kcal/kg BW (1.21 ± 0.11 × RMR or × 84.94 ± 7.89 × kg MBW) for the FT group and 295.39 ± 79.00 kcal/d or 17.65 ± 4.61 kcal/kg BW (1.21 ± 0.08 × RMR or 84.29 ± 5.65 × kg MBW) for the OR group. This reduced protein intake to 8.4 ± 0.79 g of protein/MBW for the FT group and 6.4 ± 0.43 g of protein/MBW for the OR group during week 1. Caloric intake was continually adjusted to maintain a mean weight loss of 1.5% per week. By the end of the weight loss program, dogs were consuming 53.59 ± 8.83 kcal × kg MBW or 0.77 ± 0.13 × RMR, and 4.0 to 5.5 g of protein/kg MBW to maintain the weight loss at a ratio of 1.5% per week. In dogs consuming the OR diet, the level of caloric restriction to maintain weight loss was greater (P = 0.0004) than those fed the FT diet (Figure 1). As expected, restricted feeding and weight loss reduced BW, BCS, total body mass, fat, lean and bone mass, and the lean:fat ratio (Table 2; Supplementary Table 9), but there were no differences between diets. MCS increased in both groups with restricted feeding and weight loss, but it was more pronounced in the FT group (P = 0.0341, Supplementary Table 9). Restricted feeding and weight loss did not affect voluntary physical activity levels (Supplementary Table 11).
Figure 1.
Change from baseline caloric intake (kcal/d) and body weight (kg) data of obese dogs during weight loss. Data are presented as least square means ± SEM.
Table 2.
Change from baseline body composition measures of obese dogs during weight loss
| Measurements | △Week 0 to 6 | △Week 0 to 12 | △Week 0 to 18 | △Week 0 to 24 | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FT1 | OR1 | FT | OR | FT | OR | FT | OR | SEM2 | Diet | Week | Diet × week | |
| Body mass (kg) | −1.51 | −1.41 | −3.00 | −2.90 | −4.26 | −4.33 | −5.64 | −5.82 | 0.176 | 0.974 | <0.0001 | 0.412 |
| Fat mass (kg) | −1.08 | −0.96 | −2.14 | −2.02 | −3.18 | −3.14 | −4.09 | −4.11 | 0.145 | 0.724 | <0.0001 | 0.692 |
| LBM3 (kg) | −0.41 | −0.45 | −0.83 | −0.87 | −1.04 | −1.16 | −1.50 | −1.67 | 0.105 | 0.560 | <0.0001 | 0.654 |
| BMC3 (g) | −13.4 | −5.12 | −21.3 | −15.1 | −33.3 | −30.1 | −48.1 | −41.6 | 4.430 | 0.266 | <0.0001 | 0.820 |
| LBM + BMC (kg) | −0.42 | −0.45 | −0.85 | −0.89 | −1.08 | −1.19 | −1.55 | −1.71 | 0.104 | 0.558 | <0.0001 | 0.641 |
| BMC (%) | 0.11 | 0.13 | 0.29 | 0.29 | 0.43 | 0.42 | 0.60 | 0.65 | 0.039 | 0.688 | <0.0001 | 0.733 |
| Fat (%) | −3.17 | −2.38 | −7.23 | −5.80 | −12.9 | −11.2 | −18.3 | −16.0 | 0.883 | 0.148 | <0.0001 | 0.563 |
| Lean (%) | 3.07 | 2.25 | 6.94 | 5.51 | 12.5 | 10.7 | 17.7 | 15.3 | 0.888 | 0.144 | <0.0001 | 0.564 |
| LBM + BMC (%) | 3.17 | 2.37 | 7.23 | 5.79 | 12.9 | 11.1 | 18.3 | 16.0 | 0.883 | 0.144 | <0.0001 | 0.564 |
| Lean:fat ratio | 0.16 | 0.12 | 0.44 | 0.32 | 0.95 | 0.73 | 1.66 | 1.22 | 0.116 | 0.181 | <0.0001 | 0.194 |
1FT, Fit & Trim (Champion Petfoods LP); OR, Orijen Original (Champion Petfoods LP).
2SEM, pooled standard error of the means.
3BMC, bone mineral content; LBM, lean body mass.
Serum metabolites, complete blood count, and blood hormones and oxidative stress markers
Restricted feeding and weight loss affected many serum metabolites, blood cell counts, and blood hormone and oxidative stress marker concentrations (Tables 3 and 4; Figure 2). Serum creatinine, calcium, total bilirubin, total cholesterol, total protein, albumin, globulin, sodium, chloride, total alkaline phosphatase (ALP), corticosteroid isozyme of ALP (CALP), gamma-glutamyltransferase, creatine phosphokinase, and anion gap were impacted by restricted feeding and weight loss over time (Table 3). Serum blood urea nitrogen (P = 0.0016), phosphorus (P = 0.0349), triglycerides (P = 0.0198), albumin (P = 0.0018), total ALP (P = 0.0237), CALP (P = 000147), and creatine phosphokinase (P = 0.0136) decreased in both dietary groups with restricted feeding and weight loss, but its reduction was more (P < 0.05) pronounced in the OR group than FT group. Total bilirubin increased (P = 0.0061) in both dietary groups, but the increase was more (P < 0.05) pronounced in the OR group than FT group. The albumin/globulin ratio increased in the OR group, but decreased in the FT group (P = 0.0002).
Table 3.
Change from baseline serum metabolite concentrations of obese dogs during weight loss
| ΔWeek 0 to 6 | ΔWeek 0 to 12 | ΔWeek 0 to 18 | ΔWeek 0 to 24 | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | FT1 | OR1 | FT | OR | FT | OR | FT | OR | SEM2 | diet | week |
| --- mg/dL --- | |||||||||||
| Creatinine | 0.07 | -0.03 | 0.15 | 0.03 | 0.25 | 0.07 | 0.15 | 0.03 | 0.07 | 0.0814 | 0.0237 |
| BUN3 | −3.67 | −5.00 | −3.08 | −5.58 | 0.67 | −4.08 | −2.33 | −5.92 | 1.91 | 0.0016 | 0.0642 |
| Calcium | 0.15 | 0.24 | −0.45 | −0.29 | −0.67 | −0.39 | −0.73 | −0.56 | 0.09 | 0.1239 | <0.0001 |
| Phosphorus | −0.53 | −0.78 | −0.35 | −0.41 | −0.21 | −0.81 | −0.41 | −0.83 | 0.27 | 0.0349 | 0.7919 |
| Glucose | −4.17 | −3.42 | −5.33 | −7.33 | −7.83 | −8.58 | −7.17 | −7.83 | 3.10 | 0.8618 | 0.0890 |
| Total bilirubin | −0.02 | 0.03 | 0.00 | 0.03 | 0.02 | 0.08 | 0.06 | 0.08 | 0.01 | 0.0061 | <0.0001 |
| Total cholesterol | −36.75 | −19.92 | −43.17 | −34.75 | −63.08 | −57.17 | −65.17 | −77.58 | 14.36 | 0.3993 | <0.0001 |
| Triglycerides | −17.42 | −39.25 | −26.33 | −40.08 | −21.08 | −38.92 | −25.58 | −45.92 | 10.44 | 0.0198 | 0.7855 |
| Bicarbonate | 0.83 | −0.75 | 0.00 | −0.92 | 0.08 | −0.50 | −0.25 | −1.00 | 0.79 | 0.3690 | 0.3491 |
| --- g/dL --- | |||||||||||
| Total protein | 0.26 | 0.04 | −0.12 | −0.12 | −0.35 | −0.51 | −0.18 | −0.47 | 0.20 | 0.9853 | <0.0001 |
| Albumin | −0.13 | −2.56 | −0.14 | −2.55 | −0.37 | −2.66 | −0.35 | −2.70 | 1.81 | 0.0018 | 0.0086 |
| Globulin | 0.14 | 0.05 | 0.03 | −0.12 | −0.23 | −0.40 | −0.08 | −0.33 | 0.08 | 0.0622 | <0.0001 |
| --- mmol/L --- | |||||||||||
| Sodium | 1.33 | 0.92 | −0.17 | 0.08 | −0.75 | 0.67 | −0.42 | 0.75 | 0.58 | 0.4388 | 0.0152 |
| Potassium | 0.09 | −0.05 | 0.10 | −0.07 | 0.08 | −0.14 | 0.04 | −0.14 | 0.08 | 0.0684 | 0.4338 |
| Chloride | 1.83 | 1.50 | 1.00 | 1.08 | 1.25 | 2.08 | 1.33 | 2.92 | 0.60 | 0.4494 | 0.0244 |
| U/L | |||||||||||
| Total ALP3 | −8.50 | −16.17 | −14.25 | −31.00 | −18.92 | −41.33 | −20.83 | −48.58 | 8.90 | 0.0237 | 0.0065 |
| CALP3 | 0.00 | −8.25 | −3.67 | −17.58 | −6.58 | −27.17 | −7.00 | −32.08 | 7.23 | 0.0147 | 0.0284 |
| ALT3 | −4.25 | 24.08 | −9.17 | −0.42 | 0.25 | −6.42 | −10.33 | −10.25 | 10.97 | 0.9357 | 0.2050 |
| GGT3 | 1.42 | 0.42 | −0.08 | 0.42 | 1.00 | 0.83 | 1.00 | 1.00 | 0.75 | 0.7078 | 0.0014 |
| CPK3 | 0.42 | −7.75 | −21.92 | −37.67 | −9.75 | −39.92 | −10.00 | −39.33 | 9.85 | 0.0136 | 0.0233 |
| A/G3 ratio | −0.09 | −0.03 | −0.07 | 0.04 | −0.02 | 0.15 | −0.09 | 0.08 | 0.04 | 0.0002 | 0.2099 |
| Na/K ratio | −0.58 | 0.58 | −1.00 | 0.58 | −0.75 | 1.25 | −0.58 | 1.17 | 0.69 | 0.0619 | 0.5991 |
| Anion gap | −0.33 | 0.25 | −0.25 | −0.17 | −1.08 | −1.08 | −0.67 | −1.42 | 0.39 | 0.9589 | 0.0017 |
1FT, Fit & Trim (Champion Petfoods LP); OR, Orijen Original (Champion Petfoods LP).
2SEM, pooled standard error of the means.
3BUN, blood urea nitrogen; total ALP, total alkaline phosphatase; CALP, corticosteroid isoenzyme of ALP; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; CPK, creatine phosphokinase; A/G, albumin/globulin.
Table 4.
Change from baseline complete blood cell counts of obese dogs during weight loss
| ΔWeek 0 to 6 | ΔWeek 0 to 12 | ΔWeek 0 to 18 | ΔWeek 0 to 24 | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | FT1 | OR1 | FT | OR | FT | OR | FT | OR | SEM2 | Diet | Week |
| 106/μl | |||||||||||
| RBC3 | 0.17 | 0.55 | 0.23 | 0.66 | 0.24 | 0.44 | 0.16 | 0.30 | 0.17 | 0.188 | 0.073 |
| /μL | |||||||||||
| Reticulocyte count | 3671.60 | −3331.08 | −9937.92 | −10282.00 | 1578.42 | −7295.00 | −7130.08 | −15964.00 | 5873.55 | 0.398 | <0.0001 |
| fl | |||||||||||
| Platelets | 95.00 | 41.83 | −14.42 | −53.50 | −4.17 | −33.33 | 2.50 | −49.83 | 24.12 | 0.013 | 0.0002 |
| MCV3 | 0.23 | −1.07 | 0.08 | −1.25 | 0.18 | −0.74 | −0.36 | −1.17 | 0.21 | 0.0003 | 0.002 |
| pg | |||||||||||
| MCH3 | 0.34 | −0.13 | −0.13 | −0.53 | 0.04 | −0.29 | 0.01 | −0.35 | 0.11 | 0.005 | <0.0001 |
| g/dL | |||||||||||
| MCHC3 | 0.42 | 0.36 | −0.23 | −0.15 | −0.03 | −0.03 | 0.18 | 0.05 | 0.13 | 0.839 | <0.0001 |
| Hemoglobin | 0.67 | 1.16 | 0.45 | 1.10 | 0.59 | 0.78 | 0.42 | 0.43 | 0.38 | 0.631 | 0.652 |
| 103/μl | |||||||||||
| Mean platelet volume | −0.03 | −0.35 | −0.28 | −0.56 | −0.34 | −0.72 | −0.66 | −0.82 | 0.10 | 0.049 | <0.0001 |
| WBC3 count | 0.42 | 0.44 | −0.85 | −0.84 | −1.51 | −1.38 | −1.90 | −2.07 | 0.33 | 0.663 | <0.0001 |
| Neutrophils | 0.19 | 0.54 | −0.97 | −0.65 | −1.32 | −1.00 | −1.40 | −1.61 | 0.34 | 0.131 | <0.0001 |
| Lymphocytes | 0.09 | −0.08 | 0.02 | −0.24 | −0.18 | −0.32 | −0.36 | −0.53 | 0.11 | 0.026 | 0.002 |
| Monocytes | 0.05 | 0.02 | −0.06 | −0.04 | −0.10 | −0.11 | −0.09 | −0.12 | 0.05 | 0.457 | 0.0001 |
| Eosinophils | 0.02 | 0.01 | 0.04 | 0.01 | −0.03 | −0.05 | −0.05 | −0.06 | 0.03 | 0.424 | 0.004 |
| % | |||||||||||
| Reticulocyte count | 0.00 | −0.11 | −0.16 | 0.04 | 0.00 | −0.16 | −0.13 | −0.27 | 0.13 | 0.410 | 0.090 |
| Hematocrit | 1.39 | 2.98 | 1.63 | 3.57 | 1.81 | 2.44 | 0.98 | 1.25 | 1.08 | 0.623 | 0.471 |
| Neutrophils | −0.78 | 3.28 | −2.35 | 0.34 | −1.21 | 1.19 | −0.72 | 0.56 | 1.13 | 0.034 | 0.110 |
| Lymphocytes | 0.84 | −2.81 | 2.88 | −0.95 | 2.14 | −0.42 | 0.62 | −2.32 | 1.27 | 0.0002 | 0.333 |
| Monocytes | −0.11 | −0.03 | −0.43 | 0.13 | −0.61 | 0.09 | −0.31 | −0.32 | 0.64 | 0.921 | 0.851 |
| Eosinophils | 0.99 | 0.03 | 0.68 | 0.42 | 0.03 | −0.25 | −0.26 | −0.16 | 0.55 | 0.379 | 0.257 |
| Basophils | 0.03 | 0.06 | 0.08 | 0.16 | 0.28 | 0.11 | −0.04 | −0.01 | 0.08 | 0.912 | 0.008 |
1FT, Fit & Trim (Champion Petfoods LP); OR, Orijen Original (Champion Petfoods LP).
2SEM, pooled standard error of the means.
3RBC, red blood cells; MCV, mean cell volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cells.
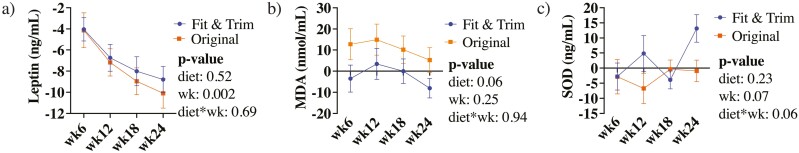
Figure 2.
Change from baseline blood (a) leptin, (b) malondialdehyde (MDA), and (c) superoxide dismutase (SOD) concentrations of obese dogs during weight loss.
Reticulocyte count, platelet count, mean cell volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, basophil %, mean platelet volume, and white blood cell (WBC), neutrophil, lymphocyte, monocyte, and eosinophil counts were impacted by restricted feeding and weight loss over time (Table 4). Blood lymphocyte % (P = 0.0002), mean corpuscular hemoglobin (P = 0.005), mean cell volume (P = 0.0003), and platelet counts (P = 0.013) increased in the FT group, but decreased in the OR group. Changes in blood neutrophil percentage were the opposite, being decreased in the FT group and increasing in the OR group (P = 0.034). Lymphocyte counts decreased (P = 0.026) in both dietary groups, but its reduction was more pronounced in the FT group. As expected, serum leptin concentrations decreased (P = 0.002) in both dietary groups with restricted feeding and weight loss, but differences due to diet were not observed (Figure 2). Serum MDA and SOD concentrations were highly variable and not different over time or due to diet (Figure 2).
Fecal characteristics and fermentative metabolites
Fecal acetate, total phenol, and total phenol and indole concentrations were decreased (P < 0.05) by restricted feeding and weight loss over time (Table 5). Fecal DM increased in both dietary groups, but its reduction was more pronounced in the FT group than the OR group (P = 0.008). Total SCFA, acetate, and propionate concentrations decreased in both dietary groups, but their reduction was more pronounced in the FT group than the OR group (P ≤ 0.002). Fecal butyrate, total BCFA, isobutyrate, isovalerate, valerate, and ammonia concentrations increased in the OR group, but were decreased in FT group (P ≤ 0.0001).
Table 5.
Change from baseline fecal characteristics and metabolites of obese dogs during weight loss
| △Week 0 to 4 | △ Week 0 to 8 | △ Week 0 to 12 | △ Week 0 to 16 | △Week 0 to 20 | △ Week 0 to 24 | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | FT1 | OR1 | FT | OR | FT | OR | FT | OR | FT | OR | FT | OR | SEM2 | Diet | Week |
| Fecal score3 | −0.167 | −0.042 | −0.083 | 0.000 | −0.083 | 0.000 | −0.042 | 0.167 | −0.042 | 0.167 | −0.083 | 0.125 | 0.150 | 0.318 | 0.743 |
| Fecal pH | 0.278 | 0.021 | −0.129 | 0.293 | 0.114 | 0.230 | 0.066 | 0.493 | −0.010 | 0.458 | 0.031 | 0.270 | 0.345 | 0.310 | 0.997 |
| Fecal DM4 | 3.17 | 0.549 | 4.03 | −0.610 | 2.33 | −0.039 | 2.62 | −0.413 | 2.97 | −0.047 | 1.92 | 0.833 | 0.878 | 0.008 | 0.806 |
| umole/g (DMB) | |||||||||||||||
| Total SCFA4 | −174 | −118 | −149 | −43.4 | −194 | −78.0 | −173 | −90.6 | −206 | −141 | −219 | −157 | 31.5 | <0.0001 | 0.088 |
| Acetate | −106 | −62.7 | −88.6 | −32.0 | −113 | −63.0 | −96.4 | −70.0 | −123 | −95.8 | −133 | −108 | 18.9 | 0.002 | 0.038 |
| Propionate | −55.1 | −50.4 | −52.9 | −18.9 | −67.1 | −26.1 | −64.6 | −35.2 | −72.0 | −47.6 | −81.2 | −57.5 | 14.6 | 0.002 | 0.212 |
| Butyrate | −13.22 | −5.08 | −7.36 | 7.51 | −13.92 | 11.1 | −11.63 | 14.5 | −11.07 | 2.53 | −4.78 | 8.47 | 5.71 | <0.0001 | 0.543 |
| Total BCFA4 | −7.55 | −1.24 | −6.95 | 5.08 | −8.79 | 3.15 | −8.71 | 3.88 | −6.72 | 0.108 | −6.64 | 3.60 | 2.83 | <0.0001 | 0.961 |
| Isobutyrate | −2.94 | −1.53 | −2.79 | 3.52 | −3.65 | 0.078 | −3.10 | 0.945 | −2.85 | −1.07 | −2.35 | 0.346 | 1.47 | <0.0001 | 0.850 |
| Isovalerate | −1.20 | 0.265 | −1.21 | 1.29 | −1.79 | 2.27 | −2.56 | 1.96 | −1.04 | 0.679 | −1.35 | 2.25 | 1.26 | 0.0001 | 0.988 |
| Valerate | −3.41 | 0.033 | −2.95 | 0.273 | −3.35 | 0.801 | −3.06 | 0.975 | −2.83 | 0.499 | −2.94 | 1.007 | 1.35 | <0.0001 | 0.215 |
| Ammonia | −9.40 | 4.50 | −22.9 | 15.2 | −22.5 | 4.94 | −18.2 | 13.8 | −6.51 | 23.4 | −4.94 | 9.05 | 8.31 | <0.0001 | 0.369 |
| Total P/I4 | −1.04 | −1.66 | −2.07 | −1.87 | −3.79 | −2.75 | −4.23 | −3.24 | −3.92 | −2.97 | −4.39 | −2.98 | 0.839 | 0.530 | <0.0001 |
| Total phenols | −0.94 | −1.83 | −1.98 | −2.09 | −3.42 | −2.91 | −3.73 | −3.12 | −3.64 | −3.17 | −3.94 | −3.34 | 0.597 | 0.372 | 0.003 |
| Phenol | 0.038 | −0.200 | −0.046 | −0.142 | −0.110 | −0.143 | −0.528 | −0.276 | −0.263 | −0.155 | −0.439 | −0.312 | 0.144 | 0.804 | 0.268 |
| Total indoles | −0.094 | 0.167 | −0.086 | 0.218 | −0.368 | 0.159 | −0.505 | −0.113 | −0.280 | 0.206 | −0.452 | 0.363 | 0.317 | 0.088 | 0.781 |
| Indole | 0.027 | 0.218 | 0.075 | 0.310 | −0.198 | 0.323 | −0.350 | 0.063 | −0.118 | 0.387 | −0.303 | 0.534 | 0.321 | 0.130 | 0.894 |
1FT, Fit & Trim (Champion Petfoods LP); OR, Orijen Original (Champion Petfoods LP).
2SEM, pooled standard error of the means.
3Fecal score: 1 = hard, dry pellets, small hard mass; 2 = hard formed, dry stool, remains firm and soft; 3 = soft, formed and moist stool, retains shape; 4 = soft, unformed stool, assumes shape of container; 5 = watery, liquid that can be poured.
4DM = dry matter; SCFA = acetate + propionate + butyrate; BCFA = valerate + isovalerate + isobutyrate; P/I = phenols + indoles.
Fecal microbiota
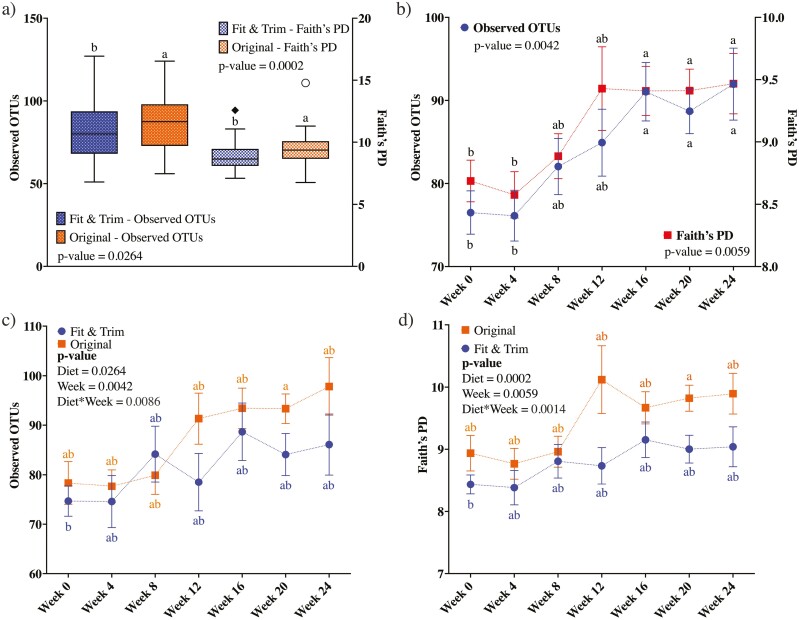
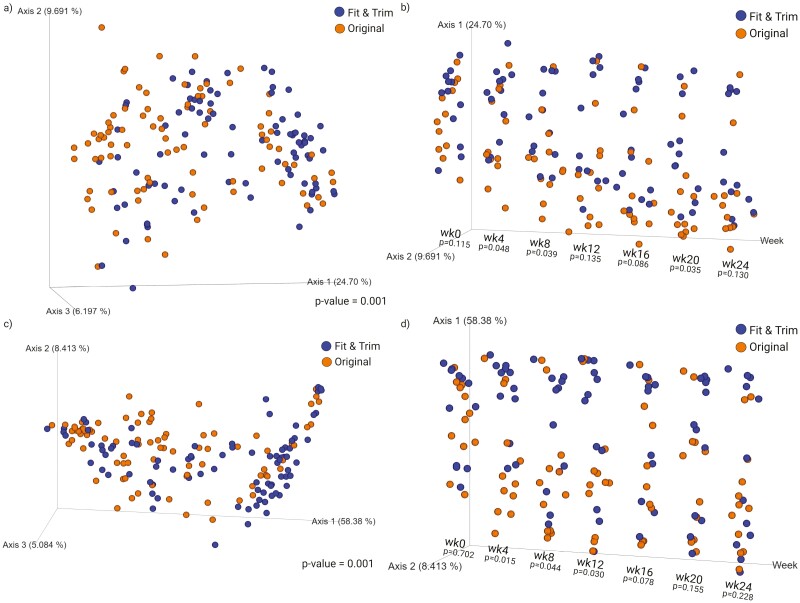
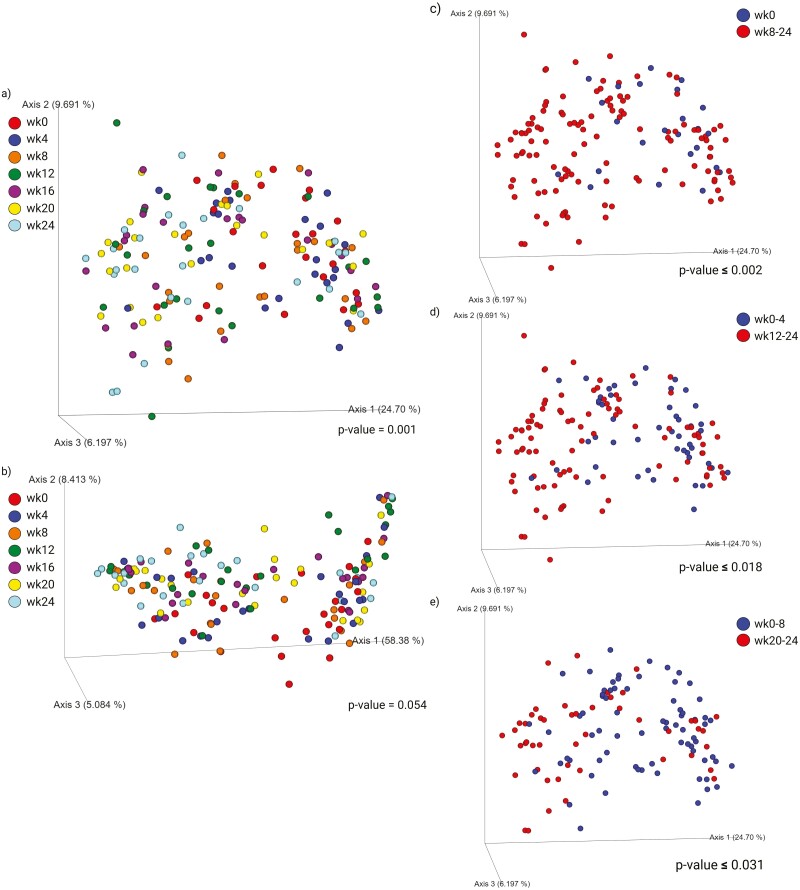
Fecal bacterial alpha-diversity is represented by observed OTU and Faith’s phylogenetic diversity (PD; Figure 3). When considering the data from all time points, dogs in the OR group had higher species richness than dogs in the FT group (observed OTU: P = 0.0264; Faith’s PD: P = 0.0002, Figure 3a). Fecal bacterial alpha-diversity was not different at baseline, but increased (P < 0.01) over time with restricted feeding and weight loss (Figure 3b). The treatment-week interaction shows that species richness increased over time, but at a higher (P < 0.01) level in dogs in the OR group than the FT group (Figure 3c and d). Fecal bacterial beta-diversity is represented by principal coordinates analysis plots of weighted and unweighted UniFrac distances of fecal microbial communities (Figures 4 and 5). UniFrac distances, both weighted and unweighted, suggested a separation of bacterial populations between the dietary groups (P = 0.001, Figure 4a and c). Separation was observed over time with restricted feeding and weight loss too (P ≤ 0.048, Figure 4b; P ≤ 0.03, Figure 4d). Unweighted UniFrac distances suggested sample clustering due to time (P = 0.001, Figure 5a), but weighted UniFrac distances were unable to show sample clustering due to time (P = 0.054, Figure 5b). Unweighted UniFrac distances indicated that baseline populations were distinct from all other wk, with the exception for week 4 (P ≤ 0.002, Figure 5c). Weeks 0 and 4 samples also clustered separately from those at weeks 12 to 24 (P ≤ 0.018, Figure 5d), while samples from week 0 to 8 clustered separately from those at weeks 20 to 24 (P ≤ 0.031, Figure 5e).
Figure 3.
Alpha-diversity measures of fecal samples collected from obese dogs during weight loss. (a) Alpha-diversity measures by dietary group throughout the study. (b) Alpha-diversity measures by time point throughout the study. (c) Observed OTU by dietary group in each time-point throughout the study. (d) Faith’s phylogenetic diversity (PD) by dietary group in each time point throughout the study. At baseline (week 0), all animals were fed the same diet (Original diet). Alpha-diversity, represented by observed OTU and Faith’s PD, suggested that species richness was higher in dogs fed the Original diet than those fed the Fit & Trim diet. Additionally, caloric restriction and weight loss increased species richness.
Figure 4.
Beta-diversity measures of fecal samples collected from obese dogs during weight loss. Principal coordinates analysis plots of unweighted (a–b) and weighted (c–d) unique fraction metric distances of fecal microbial communities were performed on the 97% operational taxonomic unit abundance matrix. At baseline (week 0), all animals were fed the same diet (Original diet). Weighted and unweighted unique fraction metric distances suggested a separation of bacterial populations between dietary groups.
Figure 5.
Beta-diversity measures of fecal samples collected from obese dogs during weight loss. Principal coordinates analysis plots of unweighted (a) and weighted (b) unique fraction metric distances of fecal microbial communities were performed on the 97% operational taxonomic unit abundance matrix. At baseline (week 0), all animals were fed the same diet (Original diet). Unweighted unique fraction metric distances suggested that baseline (week 0) samples clustered separately from samples from all other weeks except for week 4 (c); weeks 0 to 4 samples clustered separately from weeks 16 to 24 (d); weeks 0 to 8 samples clustered separately from week 24 samples (e).
The relative abundance of one bacterial phyla and two bacterial genera were altered due to restricted feeding and weight loss (Table 6). The relative abundance of fecal Actinobacteriota was decreased (P = 0.01), while the relative abundances of fecal Allobaculum and Ruminococcus Torques were increased (P ≤ 0.03) by restricted feeding and weight loss over time. The relative abundances of 15 bacterial genera were different among dietary groups. The relative abundances of fecal Bifidobacterium, Dubosiella, Faecalibaculum, and Parasutterella increased in both dietary groups, but the increase was more pronounced in the OR group than FT group (P ≤ 0.006). Conversely, the relative abundances of fecal Collinsella, Turicibacter, unclassified Lachnospiraceae, Blautia, Ruminococcus gnavus, uncultured Lachnospiraceae, Faecalibacterium, and Peptoclostridium decreased in both dietary groups, but the decrease was more pronounced in the OR group than FT group (P < 0.05). The relative abundances of fecal Alloprevotella and Escherichia-Shigella increased in the FT group, but decreased in the OR group (P ≤ 0.01). Finally, the relative abundance of uncultured Erysipelotrichaceae increased in the OR group, but decreased in the FT group (P < 0.0001).
Table 6.
Change from baseline fecal bacterial phyla and genera (relative abundance, %) of obese dogs during weight loss
| Phyla | Genus | △Week 0 to 4 | △Week 0 to 8 | △Week 0 to 12 | △Week 0 to 16 | △Week 0 to 20 | △Week 0 to 24 | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FT1 | OR1 | FT | OR | FT | OR | FT | OR | FT | OR | FT | OR | SEM2 | Diet | Week | ||
| Actinobacteriota | −0.55 | −3.30 | −3.80 | −3.77 | −0.62 | −1.95 | 0.28 | 2.10 | 0.04 | −1.43 | −1.05 | −2.55 | 1.66 | 0.62 | 0.01 | |
| Bifidobacterium | −0.25 | 4.18 | 2.74 | 4.91 | 1.01 | 3.12 | 1.36 | 2.94 | 1.21 | 4.42 | 2.01 | 3.78 | 1.42 | 0.006 | 0.757 | |
| Collinsella | −0.29 | −0.28 | 0.97 | −0.74 | −1.15 | −0.39 | −0.38 | −0.80 | 0.00 | −0.72 | −0.59 | −0.80 | 0.55 | 0.007 | 0.814 | |
| Bacteroidota | 0.55 | 6.80 | 4.07 | −0.75 | 0.30 | 0.87 | −0.79 | −3.36 | 1.11 | 2.94 | 4.70 | 2.06 | 2.46 | 0.89 | 0.17 | |
| Firmicutes | 0.89 | −9.34 | −0.95 | 3.40 | 1.45 | −0.48 | 3.28 | 10.24 | 0.28 | −1.94 | −3.00 | −0.97 | 4.92 | 0.95 | 0.35 | |
| Alloprevotella | 0.57 | −0.80 | −0.16 | −0.70 | 0.78 | −0.39 | −0.04 | −0.26 | −0.08 | −0.16 | −0.29 | −0.07 | 0.46 | 0.005 | 0.919 | |
| Allobaculum | −0.65 | 1.25 | 2.33 | 2.25 | 4.02 | 6.74 | 6.19 | 12.04 | 10.75 | 11.86 | 16.15 | 13.62 | 3.70 | 0.362 | 0.009 | |
| Dubosiella | 0.23 | 1.00 | 0.17 | 1.54 | 0.28 | 0.92 | 0.21 | 1.27 | 0.46 | 1.19 | 1.26 | 1.71 | 0.44 | <0.0001 | 0.675 | |
| Faecalibaculum | 0.15 | 0.42 | 0.17 | 0.25 | 0.24 | 0.42 | 0.21 | 0.76 | 1.24 | 0.80 | 1.17 | 3.13 | 0.52 | 0.0003 | 0.090 | |
| Turicibacter | −0.01 | 0.19 | 0.04 | −0.17 | −0.19 | −0.05 | 0.22 | −0.07 | −0.44 | −0.44 | 0.23 | −0.13 | 0.33 | 0.049 | 0.402 | |
| uncultured Erysipelotrichaceae | −4.08 | 7.50 | 3.19 | 12.08 | 1.04 | 11.22 | −1.79 | 6.70 | −2.02 | 4.47 | −0.92 | 7.26 | 3.13 | <0.0001 | 0.347 | |
| unclassified Lachnospiraceae | −0.13 | −0.82 | −0.28 | −0.93 | −0.64 | −0.87 | −0.20 | −0.75 | 0.20 | −0.83 | −0.10 | −0.80 | 0.30 | <0.0001 | 0.795 | |
| Blautia | −0.40 | −3.13 | −0.13 | −4.39 | −3.53 | −4.45 | −0.66 | −5.05 | 0.26 | −4.93 | −2.77 | −4.91 | 1.38 | <0.0001 | 0.463 | |
| Ruminococcus gnavus | 0.45 | −1.46 | −0.09 | −2.13 | −1.81 | −2.99 | −0.72 | −3.06 | −1.28 | −2.71 | −2.03 | −2.46 | −3.06 | 0.002 | 0.142 | |
| Ruminococcus torques | 0.20 | 0.46 | 0.69 | 0.78 | 0.83 | 1.16 | 0.93 | 1.04 | 1.59 | 1.62 | 0.95 | 1.30 | 0.39 | 0.289 | 0.030 | |
| uncultured Lachnospiraceae | 0.64 | −0.45 | 0.31 | −0.62 | −0.55 | −0.34 | −0.22 | −0.53 | −0.02 | −0.54 | −0.62 | −0.64 | 0.33 | 0.001 | 0.415 | |
| Faecalibacterium | −0.35 | −0.67 | −0.06 | −0.53 | −0.31 | −0.47 | 0.22 | −0.33 | 0.37 | −0.49 | 0.07 | −0.49 | 0.30 | 0.027 | 0.592 | |
| Peptoclostridium | 1.32 | −5.41 | −1.02 | −6.36 | −5.24 | −7.16 | −3.56 | −6.18 | −1.17 | −7.06 | −4.38 | −7.41 | 1.65 | <0.0001 | 0.339 | |
| Fusobacteriota | 2.01 | 5.35 | 3.20 | 2.96 | 2.18 | 0.74 | −2.48 | −7.21 | 2.13 | 2.05 | 1.08 | 2.31 | 3.28 | 0.87 | 0.12 | |
| Proteobacteria | −2.89 | 0.51 | −2.59 | −1.86 | −3.32 | 0.82 | −0.28 | −1.79 | −3.57 | −1.60 | −1.74 | −0.83 | 1.76 | 0.40 | 0.76 | |
| Parasutterella | −0.31 | 0.53 | 0.15 | 1.05 | 0.67 | 0.81 | −0.03 | 0.51 | −0.09 | 0.71 | 0.17 | 1.37 | 0.37 | 0.0004 | 0.094 | |
| Escherichia−Shigella | 1.78 | −0.18 | 1.44 | 0.29 | 0.23 | −0.08 | 0.54 | −0.36 | 0.02 | −0.14 | 0.23 | −0.36 | 0.50 | 0.010 | 0.421 | |
| Firmicutes/Bacteroidota ratio | −8.77 | −3.44 | −4.55 | −0.77 | −4.05 | −0.92 | 6.52 | −0.91 | −9.06 | −4.18 | −12.58 | −2.55 | 5.77 | 0.3428 | 0.3428 | |
1FT, Fit & Trim (Champion Petfoods); OR, Orijen Original (Champion Petfoods LP).
2SEM, pooled standard error of the means.
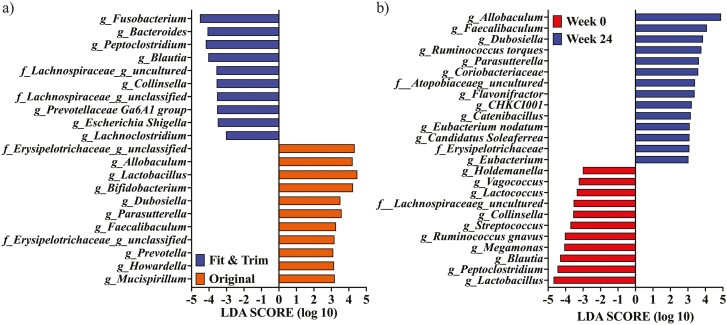
LDA effect size analysis identified taxa enriched in dogs fed the OR vs. FT diets and in dogs at baseline vs. week 24 (Figure 6). When all time points were considered, these analyses showed that Fusobacterium, Bacteroides, Peptoclostridium, Blautia, uncultured Lachnospiraceae, Collinsella, unclassified Lachnospiraceae, Prevotellaceae group Ga6A1, Escherichia Shigella, and Lachnoclostridium were enriched in dogs fed the FT diet (P < 0.05). In contrast, uncultured Erysipelotrichaceae, Allobaculum, Lactobacillus, Bifidobacterium, Dubosiella, Parasutterella, Faecalibaculum, Prevotella, Howardella, and Mucispirillum were enriched in dogs fed the OR diet when all time points were considered (P < 0.05). Holdemanella, Vagococcus, Lactococcus, uncultured Lachnospiraceae, Collinsella, Streptococcus, Ruminococcus gnavus, Megamonas, Blautia, Peptoclostridium, and Lactobacillus were enriched in dog feces at week 0 (P < 0.05). In contrast, Allobaculum, Faecalibaculum, Dubosiella, Ruminococcus torques, Parasutterella, Coriobacteriaceae, uncultured Atopobiaceae, Flavonifractor, CHKC1001, Catenibacillus, Eubacterium nodatum, Candidatus Soleaferrea, unclassifeid Erysipelotrichaceae, and Eubacterium were enriched in dog feces at week 24 (P < 0.05).
Figure 6.
Linear discriminant analysis effect size analysis of obese dogs during weight loss. (a) Analysis identified fecal bacterial genera enriched in obese dogs fed the Fit & Trim diet compared with those fed the Original diet [across all time points; linear discriminant analysis (LDA) score ≥ 3]. (b) Analysis identified fecal bacterial genera enriched in samples at weeks 0 vs. 24. At baseline (week 0), all animals were fed the same diet (Original diet).
Discussion
The first step in a weight-loss program is to diagnose obesity, which is most commonly done with a subjective BCS system. In this study, a 9-point BCS system and a subjective assessment of MCS (WSAVA, 2011) were used. Additionally, the BCS was confirmed by using DEXA to estimate the body fat of the animals (45% to 46%) at baseline. This level of body fat would place animals in a BCS category of nine based on existing BCS scale-body fat relationships (Brooks et al., 2014). Caloric restriction and transition to a specially formulated diet are common weight-loss recommendations (German et al., 2015). To estimate daily energy requirements for weight loss, it is currently recommended to use the calculation based on TBW (70 kcal/kg0.75 × TBW). To estimate TBW, one must assume that each BCS point (9-point scale) above the ideal BW/target represents 10% of the current overweight BW (Brooks et al., 2014; Flanagan et al., 2017; Shepherd, 2021). Instead of using the TBW as described (BCS 9% to 40% above the TBW), we used 10% above the TBW to avoid drastic weight loss during the first few week of the weight loss program and to achieve a controlled and consistent weight loss of 1.5% per week. Using this reduction, the animals in the present study lost 2.6% to 2.9% of their initial BW during the first week. The daily energy intake creating this level of weight loss was 84.61 ± 6.72 kcal/kg0.75 BW or 1.21 ± 0.10 × RMR (based on equation 2, NRC, 2006). By the end of the weight-loss program (weeks 20 to 24), animals that were still in the weight-loss program were consuming 53.77 ± 8.25 kcal/kg0.75 BW or 0.77 ± 0.12 × RMR. As a result, using a high-protein, low-starch diet, it was possible to lose weight at a controlled and consistent rate of ≥ 1.6% per week while avoiding drastic caloric restriction.
The amount of caloric restriction required to achieve weight loss varies greatly among dogs, and it has been shown that nutrient intakes can become deficient during restricted feeding (Linder et al., 2012, 2013; German et al., 2015; Gaylord et al., 2018). In the present study, CP intake for both diets (88 to 98 g/Mcal) were above the minimum protein needed in the diet to meet NRC recommended allowances (79 g/Mcal when fed at 60% of RER for ideal BW; NRC, 2006; Brooks et al., 2014). One of the complications that must be avoided during weight loss is the loss of lean mass, which is impacted by intake of dietary protein (Diez et al., 2002). Compared with some commercial dog foods, the CP content (88 to 98 g/Mcal) of the high-protein, low-starch diet fed in this study was in the same range as many veterinary/prescription weight loss diets (82 to 115 g/Mcal; Shepherd, 2021). The acid-hydrolyzed fat content of FT diet (35 g/Mcal) was in the range of veterinary/prescription weight loss diets (22 to 37g/Mcal); however, the OR diet was in the range of adult maintenance dog diets (36 to 44 g/Mcal) (Shepherd, 2021). The TDF content of weight management/light diets can vary greatly (6 to 38g/Mcal; Shepherd, 2021), with test diets being toward the higher end of this range (30 to 34 g/Mcal). Because MCS, body lean percentage, and lean:fat ratio all increased in dogs during the weight loss program, these macronutrient inclusion levels were suitable to support weight loss while preserving lean body tissue.
As expected, restricted feeding of both diets and consequent weight loss led to changes in clinical biochemistry. Similar to the previous studies (German et al., 2009; Linder et al., 2013; Salas-Mani et al., 2018; Phungviwatnikul et al., 2022), WBC (neutrophils, lymphocytes, monocytes, and eosinophils), albumin, globulin, cholesterol, glucose, ALP, and leptin had progressive reductions due to weight loss in the current study. Serum triglycerides were decreased in the first few weeks and remained below baseline throughout the weight-loss program. Additionally, serum creatinine increased due to weight loss. Although a few serum chemistry measures were outside of the reference ranges, all dogs remained healthy during their period of weight loss and did not demonstrate any signs of deficiency. While some biochemical parameters changed as a result of weight loss, the vast majority of results remained within the reference interval, and most abnormalities were only mild. This appears to imply that such changes are not clinically significant (German et al., 2009; Linder et al., 2013). Moreover, it has been shown that changes in these biochemical parameters are unlikely to be associated with observed changes in nutritional status (Linder et al., 2013). Additionally, many amino acids and B vitamin requirements are directly related to energy metabolism (King et al., 2006; NRC, 2006); as energy intake decreases during weight loss, so may the requirement for some essential nutrients (German et al., 2015). In the present study, a more pronounced reduction in serum cholesterol concentrations was observed in animals consuming the FT diet. This response may have been due to the greater reduction in fat intake (g/MBW) that resulted from restricted feeding of a diet with lower fat content.
Restricted feeding and weight loss impacted the fecal microbiota population in the present study, with alpha-diversity increasing (weeks 0 to 4 < week 16 to 24), beta-diversity separating due to time (samples from week 0 to 8 clustered separately from week 20 to 24), and many fecal microbial taxa shifting over time. Although they were not affected by diet, the relative abundances of Actinobacteria decreased and Allobaculum and Ruminococcus torques increased during restricted feeding and weight loss. Previous studies have reported an increased relative abundance of Allobaculum with weight loss (Salas-Mani et al., 2018; Phungviwatnikul et al., 2022). Increased alpha-diversity due to weight loss and significant clustering of microbial communities before and after weight loss has been reported in dogs (Sanchez et al., 2020). Our LDA identified fecal Lactobacillus (α = 0.05, LDA score > 4.0) and Allobaculum (α = 0.05, LDA score > 4.5) as being bacterial taxa with some of the largest and consistent changes before and after weight loss, which agrees with the results from a previous study (Salas-Mani et al., 2018).
The experimental design of the existing study made it impossible to separate the effects of weight loss from restricted feeding and diet composition. However, the primary reasons for the bacterial shifts may still be hypothesized. First, restricted feeding of any diet will reduce the substrate load reaching the large intestine, and consequently impacting the fecal microbial populations. In the current study, animals fed the OR diet required a greater caloric restriction to lose weight than those fed the FT diet. Plus, the FT diet contained higher TDF (34 vs. 30 g/Mcal), insoluble dietary fiber (27 vs. 24 g/Mcal), soluble dietary fiber (7 vs. 5g/Mcal), and CP (98 vs. 88 g/Mcal) concentrations compared with the OR diet, all of which may explain some of the differences in fecal microbiota and metabolites between dietary groups. In a previous study, Firmicutes, Clostridium hiranonis, Clostridium perfringens, and Ruminococcus gnavus were more abundant in dogs fed a high-protein, low-starch diet (53% CP and 12% carbohydrate, DMB) diet than those fed a low-protein, high-carbohydrate diet (Li et al., 2017). Although there was less disparity in protein and carbohydrate concentrations between the diets tested in the present study, Ruminococcus gnavus were more abundant in dogs fed FT than those fed the OR diet.
In the current study, the relative abundances of a few beneficial fecal microbial taxa, including Bifidobacterium, Faecalibaculum, and Parasutterella, were increased with restricted feeding and weight loss in all dogs, something that has been reported previously (Suzuki et al., 1979; Teixeira et al., 2018; Ju et al., 2019; Mao et al., 2019; Aljahdali et al., 2020). Several fecal microbial taxa were decreased with restricted feeding and weight loss in dogs fed both diets, including Collinsella, Peptoclostridium, and a few SCFA-producing bacteria (Turicibacter, Blautia, Ruminococcus, and Faecalibacterium; Suchodolski et al., 2012), but these reductions were greater in dogs fed the OR diet. Data from a previous study conducted in our lab were in agreement with the current study, with relative abundance increases in fecal Bifidobacterium and Parasutterella and relative abundance decreases in Ruminococcus gnavus and Peptoclostridium being observed with weight loss in dogs fed a high-protein, high-fiber diet (Phungviwatnikul et al., 2022). Comparable results have also been observed in cats after weight loss, with increases in Actinobacteria being noted, which was primarily driven by increases in Bifdobacterium and Collinsella (Pallotto et al., 2018). Human obesity studies have also measured changes in fecal microbiota. Fecal Faecalibacterium and Blautia are known to be differentially abundant in obese patients with different metabolic abnormalities. Moreoever, a negative relationship has been observed between Parasutterella and serum cholesterol concentrations, while a positive relationship is known to exist between fecal Blautia, BW, and serum cholesterol concentration (Zeng et al., 2019).
In general, dogs fed high-protein, low-starch diets in the current study had decreased fecal SCFA and phenol concentrations as a result of the weight loss program; this response likely occurred because restricted feeding reduces the amount of substrate reaching the large intestine and available for bacterial fermentation. This lower substrate load most likely contributed to the reduction in SCFA-producing bacteria (Turicibacter, Blautia, Ruminococcus, and Faecalibacterium) with restricted feeding and weight loss. Despite the fact that the FT diet had more CP and TDF than the OR diet, dogs fed that diet had lower fecal concentrations of butyrate, ammonia, and BCFA. This response could be due to differences in diet digestibility, with the FT diet possibly having a higher digestibility than the OR diet. Because nutrient digestibility analyses were not performed in the current study, however, this is speculation.
In summary, restricted feeding of high-protein, low-starch diets as part of a weight-loss program reduced BW, fat mass, and circulating cholesterol and leptin concentrations. Along with these metabolic changes, restricted feeding and weight loss increased bacterial alpha-diversity and lead to significant shifts in the fecal microbial communities that were distinct before and after the weight-loss program. More specifically, restricted feeding and weight loss lead to reductions in fecal SCFA and phenol concentrations and relative abundance of fecal Actinobacteriota. In contrast, restricted feeding and weight loss lead to increased relative abundances of fecal Allobaculum and Ruminococcus torques. Diet-specific differences were noted in fecal metabolite concentrations and microbiota populations, which were likely due to differences in ingredient profile, nutrient concentrations, and/or nutrient digestibilities that altered substrate loads reaching the large intestine and available for fermentation.
Supplementary Material
Acknowledgment
The funding for this study was provided by Champion Petfoods LP, Edmonton, Canada.
Glossary
Abbreviations
- AAFCO
Association of American Feed Control Officials
- ALP
total alkaline phosphatase
- BCFA
branched-chain fatty acids
- BCS
body condition score
- BW
body weight
- CALP
corticosteroid isozyme of alkaline phosphatase
- CP
crude protein
- DEXA
dual-energy x-ray absorptiometry
- DM
dry matter
- DMB
dry matter basis
- FT
fit & trim diet
- MBW
metabolic body weight
- MCS
muscle condition score
- MDA
malondialdehyde
- ME
metabolizable energy
- MER
maintenance energy requirement
- NFE
nitrogen-free extract
- OM
organic matter
- OR
control diet
- OTU
operational taxonomic unit
- RMR
resting metabolic rate
- SCFA
short-chain fatty acids
- SOD
superoxide dismutase
- TBW
target body weight
- TDF
total dietary fiber
- UniFrac
unique fraction metric
- WBC
white blood cells
Contributor Information
Patrícia M Oba, Department of Animal Sciences, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
Janelle Kelly, Champion Petfoods LP, Edmonton, Canada.
Darcia Kostiuk, Champion Petfoods LP, Edmonton, Canada.
Kelly S Swanson, Department of Animal Sciences, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA; Department of Veterinary Clinical Medicine, College of Veterinary Medicine, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA; Division of Nutritional Sciences, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
Conflict of Interest Statement
D. K. and J.K. are employed by Champion Petfoods LP. All other authors have no conflicts of interest.
References
- AACC. 1983. Approved methods. 8th ed. (AACC, editor). St Paul, MN: American Association of Cereal Chemists [Google Scholar]
- Aljahdali, N., Gadonna-Widehem P., Anton P. M., and Carbonero F.. . 2020. Gut microbiota modulation by dietary barley malt melanoidins. Nutrients. 12:241. doi: 10.3390/nu12010241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- André, A., Leriche I., Chaix G., Thorin C., Burger M., and Nguyen P.. . 2017. Recovery of insulin sensitivity and optimal body composition after rapid weight loss in obese dogs fed a high-protein medium-carbohydrate diet. J. Anim. Physiol. Anim. Nutr. 101:21–30. doi: 10.1111/jpn.12744 [DOI] [PubMed] [Google Scholar]
- AOAC. 2006. Official methods of analysis of AOAC International. 17th ed. Arlington, VA, USA: Association of Official Analysis Chemists International. [Google Scholar]
- APOP. 2018. 2018 Pet obesity prevalence and pet food survey results. Assoc. Pet Obes.Prev. Available from https://petobesityprevention.org/2018
- APOP. 2021. 2021 U.S. Pet; Food, Nutrition and Weight Management Survey Results. Assoc. Pet Obes. Prev. Available from https://petobesityprevention.org/2021 [Google Scholar]
- Arora, T., Sharma R., and Frost G.. . 2011. Propionate. Anti-obesity and satiety enhancing factor? Appetite. 56:511–515. doi: 10.1016/j.appet.2011.01.016 [DOI] [PubMed] [Google Scholar]
- Association of American Feed Control Officials (AAFCO). 2020. Official publication 2020. Oxford, IN: AAFCO. [Google Scholar]
- Bäckhed, F., Ding H., Wang T., Hooper L. V., Gou Y. K., Nagy A., Semenkovich C. F., and Gordon J. I.. . 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U.S.A. 101:15718–15723. doi: 10.1073/pnas.0407076101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed, F., Manchester J. K., Semenkovich C. F., and Gordon J. I.. . 2007. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. U.S.A. 104:979–984. doi: 10.1073/pnas.0605374104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BanfieldPetHospital. 2017. State of pet health 2017 report. Banf. Pet Hosp. Available from https://www.banfield.com/en/pet-health/State-of-pet-health
- Bastien, B. C., Patil A., and Satyaraj E.. . 2015. The impact of weight loss on circulating cytokines in Beagle dogs. Vet. Immunol. Immunopathol. doi: 10.1016/j.vetimm.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Blanchard, G. G., Nguyen P., Gayet C., Leriche I., Siliart B., and Paragon B.-M.. . 2004. Rapid weight loss with a high-protein low-energy diet allows the recovery of ideal body composition and insulin sensitivity in obese dogs. J. Nutr. 134:2148S–2150S. doi: 10.1093/jn/134.8.2148s [DOI] [PubMed] [Google Scholar]
- Bokulich, N. A., Kaehler B. D., Rideout J. R., Dillon M., Bolyen E., Knight R., Huttley G. A., and Caporaso J. G.. . 2018. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:90. doi: 10.1186/s40168-018-0470-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, D., Churchill J., Fein K., Linder D., Michel K. E., Tudor K., Ward E., and Witzel A.; American Animal Hospital Association. 2014. 2014 AAHA weight management guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 50:1–11. doi: 10.5326/JAAHA-MS-6331 [DOI] [PubMed] [Google Scholar]
- Budde, E. F. 1952. The determination of fat in baked biscuit type of dog foods. J. AOAC Int. 35:799–805. doi: 10.1093/jaoac/35.3.799 [DOI] [Google Scholar]
- Callahan, B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J. A., and Holmes S. P.. . 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 13:581–583. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani, P. D., Amar J., Iglesias M. A., Poggi M., Knauf C., Bastelica D., Neyrinck A. M., Fava F., Tuohy K. M., Chabo C., . et al. 2007. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 56:1761–1772. doi: 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- Cani, P. D., Osto M., Geurts L., and Everard A.. . 2012. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 3:279–288. doi: 10.4161/gmic.19625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., . et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 7:335–336. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J. G., Lauber C. L., Walters W. A., Berg-Lyons D., Huntley J., Fierer N., Owens S. M., Betley J., Fraser L., Bauer M., . et al. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6:1621–1624. doi: 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney, A. L., and Marbach E. P.. . 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132. doi: 10.1093/clinchem/8.2.130 [DOI] [PubMed] [Google Scholar]
- Colliard, L., Ancel J., Benet J.-J., Paragon B.-M., and Blanchard G.. . 2006. Risk factors for obesity in dogs in France. J. Nutr. 136:1951S–1954S. doi: 10.1093/jn/136.7.1951S [DOI] [PubMed] [Google Scholar]
- Cottam, D. R., Mattar S. G., Barinas-Mitchell E., Eid G., Kuller L., Kelley D. E., and Schauer P. R.. . 2004. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes. Surg. 14:589–600. doi: 10.1381/096089204323093345 [DOI] [PubMed] [Google Scholar]
- Courcier, E. A., Thomson R. M., Mellor D. J., and Yam P. S.. . 2010. An epidemiological study of environmental factors associated with canine obesity. J. Small Anim. Pract. 51:362–367. doi: 10.1111/j.1748-5827.2010.00933.x [DOI] [PubMed] [Google Scholar]
- Day, M. J. 2017. One health approach to preventing obesity in people and their pets. J. Comp. Pathol. 156:293–295. doi: 10.1016/j.jcpa.2017.03.009 [DOI] [PubMed] [Google Scholar]
- Diez, M., Nguyen P., Jeusette I., Devois C., Istasse L., and Biourge V.. . 2002. Weight loss in obese dogs: evaluation of a high-protein, low-carbohydrate diet. J. Nutr. 132:1685S–1687S. doi: 10.1093/jn/132.6.1685S [DOI] [PubMed] [Google Scholar]
- Erwin, E. S. S., Marco G. J. J., and Emery E. M. M.. . 1961. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. doi: 10.3168/jds.S0022-0302(61)89956-6 [DOI] [Google Scholar]
- Flanagan, J., Bissot T., Hours M. A., Moreno B., Feugier A., and German A. J.. . 2017. Success of a weight loss plan for overweight dogs: the results of an international weight loss study. PLoS One doi: 10.1371/journal.pone.0184199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flickinger, E. A., Schreijen E. M. W. C., Patil A. R., Hussein H. S., Grieshop C. M., Merchen N. R., and Fahey G. C.. . 2003. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 81:2008–2018. doi: 10.2527/2003.8182008x [DOI] [PubMed] [Google Scholar]
- Floerchinger, A. M., Jackson M. I., Jewell D. E., MacLeay J. M., Paetau-Robinson I., and Hahn K. A.. . 2015. Effect of feeding a weight loss food beyond a caloric restriction period on body composition and resistance to weight gain in dogs. J. Am. Vet. Med. Assoc. 247:375–384. doi: 10.2460/javma.247.4.375 [DOI] [PubMed] [Google Scholar]
- Forster, G. M., Stockman J., Noyes N., Heuberger A. L., Broeckling C. D., Bantle C. M., and Ryan E. P.. . 2018. A comparative study of serum biochemistry, metabolome and microbiome parameters of clinically healthy, normal weight, overweight, and obese companion dogs. Top. Companion Anim. Med. 33:126–135. doi: 10.1053/j.tcam.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Gabbay, E., Slotki I., and Shavit L.. . 2015. Weighing the evidence: obesity, metabolic syndrome, and the risk of chronic kidney disease. BMC Nephrol. 16:1–4. doi: 10.1186/s12882-015-0137-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord, L., Remillard R., and Saker K.. . 2018. Risk of nutritional deficiencies for dogs on a weight loss plan. J. Small Anim. Pract. 59:695–703. doi: 10.1111/jsap.12913. [DOI] [PubMed] [Google Scholar]
- German, A. J. 2006. The growing problem of obesity in dogs and cats. J. Nutr. 136:1940S–1946S. doi: 10.1093/jn/136.7.1940S [DOI] [PubMed] [Google Scholar]
- German, A. J. J., Hervera M., Hunter L., Holden S. L. L., Morris P. J. J., Biourge V., and Trayhurn P.. . 2009. Improvement in insulin resistance and reduction in plasma inflammatory adipokines after weight loss in obese dogs. Domest Anim. Endocrinol. 37:214–226. doi: 10.1016/j.domaniend.2009.07.001 [DOI] [PubMed] [Google Scholar]
- German, A. J., Holden S. L., Bissot T., Hackett R. M., and Biourge V.. . 2007. Dietary energy restriction and successful weight loss in obese client-owned dogs. J. Vet. Intern. Med. 21:1174–1180. doi: 10.1892/06-280.1 [DOI] [PubMed] [Google Scholar]
- German, A. J., Holden S. L., Bissot T., Morris P. J., and Biourge V.. . 2010. A high protein high fibre diet improves weight loss in obese dogs. Vet. J. 183:294–297. doi: 10.1016/j.tvjl.2008.12.004 [DOI] [PubMed] [Google Scholar]
- German, A. J., Holden S. L., Serisier S., Queau Y., and Biourge V.. . 2015. Assessing the adequacy of essential nutrient intake in obese dogs undergoing energy restriction for weight loss: a cohort study. BMC Vet. Res. 11:1–11. doi: 10.1186/s12917-015-0570-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- German, A. J., Holden S. L., Wiseman-Orr M. L., Reid J., Nolan A. M., Biourge V., Morris P. J., and Scott E. M.. . 2012. Quality of life is reduced in obese dogs but improves after successful weight loss. Vet. J. 192:428–434. doi: 10.1016/j.tvjl.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Ghazalpour, A., Cespedes I., Bennett B. J., and Allayee H.. . 2016. Expanding role of gut microbiota in lipid metabolism. Curr. Opin. Lipidol. 27:141–147. doi: 10.1097/MOL.0000000000000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handl, S., German A. J., Holden S. L., Dowd S. E., Steiner J. M., Heilmann R. M., Grant R. W., Swanson K. S., and Suchodolski J. S.. . 2013. Faecal microbiota in lean and obese dogs. FEMS Microbiol. Ecol. 84:332–343. doi: 10.1111/1574-6941.12067 [DOI] [PubMed] [Google Scholar]
- Jeusette, I. C., Lhoest E. T., Istasse L. P., and Diez M. O.. . 2005. Influence of obesity on plasma lipid and lipoprotein concentrations in dogs. Am. J. Vet. Res. 66:81–86. doi: 10.2460/ajvr.2005.66.81 [DOI] [PubMed] [Google Scholar]
- Ju, T., Kong J. Y., Stothard P., and Willing B. P.. . 2019. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. 13:1520–1534. doi: 10.1038/s41396-019-0364-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealy, R. D., Lawler D. F., Ballam J. M., Mantz S. L., Biery D. N., Greeley E. H., Lust G., Segre M., Smith G. K., and Stowe H. D.. . 2002. Effects of diet restriction on life span and age-related changes in dogs. J. Am. Vet. Med. Assoc. 220:1315–1320. doi: 10.2460/javma.2002.220.1315. [DOI] [PubMed] [Google Scholar]
- Kieler, I. N., Kamal S. S., Vitger A. D., Nielsen D. S., Lauridsen C., Bjornvad C. R., Shamzir Kamal S., Vitger A. D., Nielsen D. S., Lauridsen C., . et al. 2017. Gut microbiota composition may relate to weight loss rate in obese pet dogs. Vet. Med. Sci. 3:252–262. doi: 10.1002/VMS3.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, J. C., Cousins R. J., Shils M., Shike M., Ross A. C., and Caballero B.. . 2006. Modern nutrition in health and disease. (King J. C., Cousins R. J., Shils M., Shike M., Ross A. C., and Caballero B., editors.). Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Laflamme, D. P. 1997. Development and validation of a body condition score system for dogs: a clinical tool. Canine Pract. 25:10–15. [Google Scholar]
- de Lartigue, G., de La Serre C. B., and Raybould H. E.. . 2011. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol. Behav. 105:100–105. doi: 10.1016/j.physbeh.2011.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q., Lauber C. L., Czarnecki-Maulden G., Pan Y., and Hannah S. S.. . 2017. Effects of the dietary protein and carbohydrate ratio on gut microbiomes in dogs of different body conditions. J. C. Clemente, editor. MBio. 8. doi: 10.1128/mBio.01703-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder, D. E., Freeman L. M., Holden S. L., Biourge V., and German A. J.. . 2013. Status of selected nutrients in obese dogs undergoing caloric restriction. BMC Vet. Res. 9:219. doi: 10.1186/1746-6148-9-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder, D. E., Freeman L. M., Morris P., German A. J., Biourge V., Heinze C., and Alexander L.. . 2012. Theoretical evaluation of risk for nutritional deficiency with caloric restriction in dogs. Vet. Q. 32:123–129. doi: 10.1080/01652176.2012.733079 [DOI] [PubMed] [Google Scholar]
- Lozupone, C., and Knight R.. . 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, E. M., Armstrong P. J., Kirk C. A., and Klausner J. S.. . 2005. Prevalence and risk factors for obesity in adult cats from private US veterinary practices. J. Appl. Res. Vet. Med. 3:88–96. [Google Scholar]
- Lund, E. M., Armstrong P. J., Kirk C. A., and Klausner J. S.. . 2006. Prevalence and risk factors for obesity in adult dogs from private US veterinary practices. Int. J. Appl. Res. Vet. Med. 4:177–186. [Google Scholar]
- Mao, G., Li S., Orfila C., Shen X., Zhou S., Linhardt R. J., Ye X., and Chen S.. . 2019. Depolymerized RG-I-enriched pectin from citrus segment membranes modulates gut microbiota, increases SCFA production, and promotes the growth of Bifidobacterium spp., Lactobacillus spp. and Faecalibaculum spp. Food Funct. 10:7828–7843. doi: 10.1039/c9fo01534e [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council). 2006. Nutrient requirements of dogs and cats. Washington, USA: National Academies Press. [Google Scholar]
- Pallotto, M. R., de Godoy M. R. C., Holscher H. D., Buff P. R., and Swanson K. S.. . 2018. Effects of weight loss with a moderate-protein, high-fiber diet on body composition, voluntary physical activity, and fecal microbiota of obese cats. Am. J. Vet. Res. 79:181–190. doi: 10.2460/ajvr.79.2.181 [DOI] [PubMed] [Google Scholar]
- Park, H.-J., Lee S.-E., Kim H.-B., Isaacson R. E., Seo K.-W., and Song K.-H.. . 2015. Association of obesity with serum leptin, adiponectin, and serotonin and gut microflora in beagle dogs. J. Vet. Intern. Med. 29:43–50. doi: 10.1111/jvim.12455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram, C., Raffan E., White E., Ashworth A. H., Brodbelt D. C., Church D. B., and O’Neill D. G.. . 2021. Frequency, breed predisposition and demographic risk factors for overweight status in dogs in the UK. J. Small Anim. Pract. 62:521–530. doi: 10.1111/jsap.13325 [DOI] [PubMed] [Google Scholar]
- Phungviwatnikul, T., Lee A. H., Belchik S. E., Suchodolski J. S., and Swanson K. S.. . 2022. Weight loss and high-protein, high-fiber diet consumption impact blood metabolite profiles, body composition, voluntary physical activity, fecal microbiota, and fecal metabolites of adult dogs. J. Anim. Sci. 100:skab379. doi: 10.1093/jas/skab379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosky, L., Asp N. -G., Furda I., DeVries J. W., Schweizer T. F., and Harland B. F.. . 1985. Determination of total dietary fiber in foods and food products: collaborative study. Journal-Association Off. Anal. Chem. 68:677–679. doi: 10.1093/jaoac/68.4.677 [DOI] [PubMed] [Google Scholar]
- Raffan, E. 2013. The big problem: battling companion animal obesity. Vet. Rec. 173:287–291. doi: 10.1136/vr.f5815 [DOI] [PubMed] [Google Scholar]
- Robeson, M. S.II, O’Rourke D. R., Kaehler B. D., Ziemski M., Dillon M. R., Foster J. T., and Bokulich N. A.. . 2021. RESCRIPt: Reproducible sequence taxonomy reference database management. PLoS Comput. Biol. 17:e1009581. doi: 10.1371/journal.pcbi.1009581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Mani, A., Jeusette I., Castillo I., Manuelian C. L., Lionnet C., Iraculis N., Sanchez N., Fernández S., Vilaseca L., and Torre C.. . 2018. Fecal microbiota composition changes after a BW loss diet in Beagle dogs. J. Anim. Sci. 96:3102–3111. doi: 10.1093/jas/sky193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt, C., Morris P. J., Wilson D., Lund E. M., and German A. J.. . 2019. Association between life span and body condition in neutered client-owned dogs. J. Vet. Intern. Med. 33:89–99. doi: 10.1111/jvim.15367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, S. B., Pilla R., Sarawichitr B., Gramenzi A., Marsilio F., Steiner J. M., Lidbury J. A., Woods G. R. T., German A. J., and Suchodolski J. S.. . 2020. Fecal microbiota in client-owned obese dogs changes after weight loss with a high-fiber-high-protein diet. PeerJ. 8:e9706. doi: 10.7717/peerj.9706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, G. J. 2000. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition. 16:866–873. doi: 10.1016/s0899-9007(00)00464-0 [DOI] [PubMed] [Google Scholar]
- Segata, N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W. S., and Huttenhower C.. . 2011. Metagenomic biomarker discovery and explanation. Genome Biol. 12:R601–R618. doi: 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd, M. 2021. Canine and Feline Obesity Management. Vet. Clin. North Am. Small Anim. Pract. 51:653–667. doi: 10.1016/j.cvsm.2021.01.005 [DOI] [PubMed] [Google Scholar]
- Suchodolski, J. S., Markel M. E., Garcia-Mazcorro J. F., Unterer S., Heilmann R. M., Dowd S. E., Kachroo P., Ivanov I., Minamoto Y., Dillman E. M., . et al. 2012. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. M. M. Heimesaat, editor. PLoS One. 7:e51907. doi: 10.1371/journal.pone.0051907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, K., Benno Y., Mitsuoka T., Takebe S., Kobashi K., and Hase J.. . 1979. Urease-producing species of intestinal anaerobes and their activities. Appl. Environ. Microbiol. 37:379–382. doi: 10.1128/aem.37.3.379-382.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani, A. B., Nezami B. G., Gewirtz A., and Srinivasan S.. . 2012. Obesity and its associated disease: a role for microbiota? Neurogastroenterol. Motil. 24:305–311. doi: 10.1111/j.1365-2982.2012.01895.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira, C., Prykhodko O., Alminger M., Fåk Hållenius F., and Nyman M.. . 2018. Barley products of different fiber composition selectively change microbiota composition in rats. Mol. Nutr. Food Res. 62:1701023. doi: 10.1002/mnfr.201701023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, E., German A. J., and Churchill J. A.. . 2019. The global pet obesity initiative position statement. 5. Available from https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwi1y_eMipr6AhUrj4kEHThCDxMQFnoECAoQAQ&url=https%3A%2F%2Fstatic1.squarespace.com%2Fstatic%2F597c71d3e58c621d06830e3f%2Ft%2F5da311c5519bf62664dac512%2F1570968005938%2FGlobal%2Bpet%2Bob
- Warren, B. S., Wakshlag J. J., Maley M., Farrell T. J., Struble A. M., Panasevich M. R., and Wells M. T.. . 2011. Use of pedometers to measure the relationship of dog walking to body condition score in obese and non-obese dogs. Br. J. Nutr. 106:S85–S89. doi: 10.1017/S0007114511001814 [DOI] [PubMed] [Google Scholar]
- WSAVA, L. F., Becvarova I., Cave N., MacKay C., Nguyen P., Rama B., Takashima G., Tiffin R., Tsjimoto H., and van Beukelen P.. . 2011. WSAVA nutritional assessment guidelines. J. Small Anim. Pract. 52:385–396. doi: 10.1111/j.1748-5827.2011.01079.x [DOI] [PubMed] [Google Scholar]
- Yam, P. S., Butowski C. F., Chitty J. L., Naughton G., Wiseman-Orr M. L., Parkin T., and Reid J.. . 2016. Impact of canine overweight and obesity on health-related quality of life. Prev. Vet. Med. doi: 10.1016/j.prevetmed.2016.03.013 [DOI] [PubMed] [Google Scholar]
- Zeng, Q., Li D., He Y., Yinhu L., Yang Z., Zhao X., Liu Y., Wang Y., Sun J., Feng X., . et al. 2019. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 9:13424. doi: 10.1038/s41598-019-49462-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.