Abstract
Introduction
The antibody-drug conjugate trastuzumab deruxtecan (T-DXd) has led to a change in the clinical management of breast cancer. Nausea and vomiting are the most common adverse events of T-DXd, which cannot be completely alleviated by standard prophylactic regimens. Olanzapine is particularly effective in preventing delayed nausea caused by chemotherapy. In this study, we will evaluate the efficacy of olanzapine in managing persistent nausea and vomiting during T-DXd treatment.
Methods and analysis
The ERICA study is a multicentre, placebo-controlled, double-blind, randomised phase II study with the aim to evaluate the antiemetic effects of the prophylactic olanzapine (5 mg orally, on days 1–6) or placebo combined with a 1,5-hydroxytryptamine-3 (5-HT3)–receptor antagonist and dexamethasone in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer undergoing T-DXd treatment. For a period of 22 days from the day of T-DXd treatment, patients will document their experience in an electronic symptom diary daily during observational periods. The primary endpoint is the complete response rate, defined as no vomiting and no rescue medications during the ‘delayed phase’ of 24–120 hours post-T-DXd administration. In addition, we define 120–504 hour as the ‘persistent phase’ and 0–504 hours as the ‘overall phase’ for secondary endpoint analysis. We have estimated that a total sample size of at least 156 patients is needed to allow a power of 80% at a one-sided significance level of 20% in this study. The target sample size is set to 166 to account for possible case exclusions.
Ethics and dissemination
The study protocol is approved by the West Japan Oncology Group protocol review committee and the SHOWA University Clinical Research Review Board. The study results will be presented at international conferences and published in a peer-reviewed journal.
Trial registration number
jRCTs031210410.
Keywords: CHEMOTHERAPY, Breast tumours, Toxicity, Adverse events
STRENGTHS AND LIMITATIONS OF THIS STUDY.
A ‘persistent phase (120–504 hours)’ has been newly defined to evaluate persistent symptoms induced by trastuzumab deruxtecan.
Electronic patient-reported outcome is used to record patient symptoms.
A limitation of this study is that symptom progression in the second cycle or later remains unknown.
Introduction
Trastuzumab deruxtecan (T-DXd) is an antibody drug conjugate (ADC) composed of a human epidermal growth factor receptor 2 (HER2)-targeting antibody, a cleavable tetrapeptide-based linker and a DNA topoisomerase I inhibitor payload.1 2 Its high drug-to-antibody ratio and a bystander killing effect achieve a strong antitumour effect.1
T-DXd has been approved by US Food and Drug Administration (FDA), European Medicines Agency (EMA) and Pharmaceuticals and Medical Devices Agency for the treatment of HER2-positive unresectable or metastatic breast cancer that progressed after two or more anti-HER2-based chemotherapies and/or received one line of anti-HER2-based chemotherapy in a metastatic setting, or developed recurrent disease during or within 6 months of completing adjuvant or neoadjuvant therapy based on the results of the DESTINY-Breast013 and DESTINY-Breast03 trials.4 Furthermore, T-DXd has been approved by FDA and EMA for the treatment of patients with HER2-low (immunohistochemistry (IHC) 1+or IHC 2+/in situ hybridisation (ISH)-) metastatic breast cancer who have received one or two chemotherapies in a metastatic setting or developed recurrent disease during or within 6 months of completing adjuvant or neoadjuvant chemotherapy based on the results of DESTINY-Breast04 trial.5 The ADC T-DXd has led to changes in the clinical management of previously treated HER2-positive/low metastatic breast cancer.
Although T-DXd is highly effective in many patients with breast cancer, its toxicity remains challenging. Nausea and vomiting are the most common adverse events for T-DXd. In the DESTINY-Breast013 and DESTINY-Breast03 trials,4 nausea of any grade was reported in 77.7% and 75.9% of patients and vomiting was reported in 45.7% and 49.0% of patients, respectively. Since T-DXd is classified as a moderately emetogenic agent in guidelines,6 7 antiemetic therapy consisting of 5-HT3 receptor antagonist (5-HT3RA) and dexamethasone is the standard recommended regimen. However, persistent or refractory nausea and vomiting are not rare in clinical settings under prophylaxis with standard recommended regimens. T-DXd achieves a longer half-life (about 6 days at a dose of 5.4 mg/kg) than typical small-molecule inhibitors by combining payload with antibody.8 We speculate that the persistent nausea and vomiting induced by T-DXd may be attributed to its long half-life.
Olanzapine treatment effectively alleviates refractory nausea and vomiting by targeting multiple receptors, including dopaminergic D1, D2, D3 and D4 receptors; serotonergic 5-HT2A, 5-HT2C, 5-HT3 and 5-HT6 receptors; the adrenergic α1 receptor; the histamine H1 receptor; and several muscarinic receptor subtypes.9–12 A randomised controlled trial13 and network meta-analysis14 indicated that olanzapine is more effective than a neurokinin-1 (NK1) receptor antagonist in preventing delayed nausea in combination with 5-HT3RA and dexamethasone.
In previous studies, olanzapine was found to be equally effective at 5 and 10 mg for the prophylaxis of chemotherapy-induced nausea and vomiting, but 5 mg was associated with a lower incidence of adverse events than 10 mg.15 16 Furthermore, olanzapine at 5 mg has been shown to reduce the risk of delayed nausea and vomiting in Japanese patients undergoing highly emetogenic chemotherapy.12 Moreover, olanzapine was prophylactically administered for 4–8 days in previous studies.11 12 17 In the current study, we administer 5 mg olanzapine for 6 days, taking into account the half-life of T-DXd and olanzapine. In the ERICA study, we evaluate the efficacy of olanzapine in managing persistent nausea and vomiting during T-DXd treatment.
Precise assessment is essential in the management of nausea and vomiting. There are validated self-assessment scales for evaluating nausea and vomiting. The US National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) has been used in several clinical trials.18 The recall period for the PRO-CTCAE is the past 7 days. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ C-30) is one of the comprehensive scales that could evaluate nausea and vomiting.19 The recall period of EORTC QLQ-C30 items is the past 7 days. Other measures are also too voluminous to answer every day for 22 days.20 Accordingly, we adopted 4-point categorical scales for daily and quick assessment of nausea and vomiting. Furthermore, an electronic symptom diary is employed for assessment of symptom severity and duration as PROs.
Methods and analysis
Study design
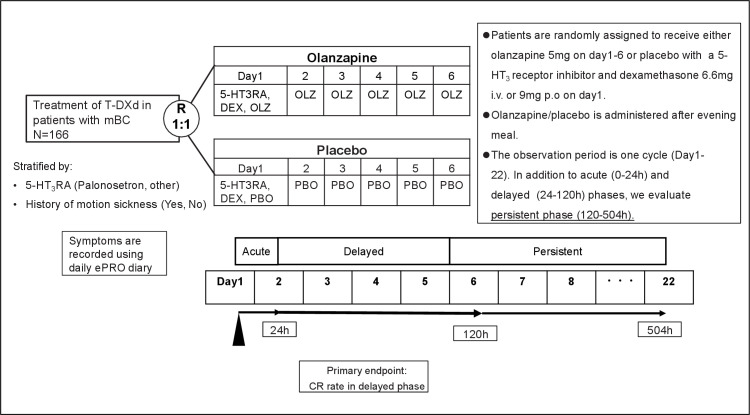
This study is a multicentre, placebo-controlled, double-blind, randomised phase II study that aims to evaluate the anti-emetic effect of prophylactic olanzapine combined with 5-HT3RA and dexamethasone compared with placebo combined with 5-HT3RA, and dexamethasone for nausea and vomiting induced by T-DXd treatment in patients with HER2-positive metastatic breast cancer (figure 1).
Figure 1.
ERICA study scheme. The flow chart summarises the design of the study. DEX, dexamethasone; ePRO, electronic patient-reported outcome; h, hours.; 5-HT3RA, 1,5-hydroxytryptamine-3 receptor antagonist; i.v., intravenous; mBC, metastatic breast cancer; OLZ, olanzapine; PBO, placebo; p.o., per oral; R, randomisation; T-DXd, trastuzumab deruxtecan.
Patients
This study is ongoing at 43 hospitals of the West Japan Oncology Group (WJOG). The Key inclusion and exclusion criteria are as follows:
Inclusion criteria
At least 20 and up to 75 years of age at the time of consent.
Histologically confirmed HER2-positive (IHC3+or ISH+) unresectable or distant metastatic breast cancer.
Patients have an indication for and are scheduled to start T-DXd treatment.
No symptomatic brain metastases.
No ascites or pleural effusion requiring puncture therapy.
No gastrointestinal obstruction such as gastric pyloric stenosis or intestinal obstruction.
Eastern Cooperative Oncology Group Performance Status of 0–2.
-
No severe organ damage and meet the following criteria based on blood tests within 2 weeks prior to registration:
White cell count: ≥3000/mm3.
Neutrophil count: ≥1500/mm3.
Haemoglobin: ≥80 g/L.
Platelet count: ≥80×109/L.
Aspartate aminotransferase: ≤5×upper limit normal (ULN).
Alanine aminotransferase: ≤5×ULN.
Total bilirubin: ≤3×ULN.
Serum creatinine: ≤1.5×ULN.
Hemoglobin A1c (NGSP) less than 6.5% at enrolment and not receiving insulin or antidiabetic drugs.
No active hepatitis B infection.
Patients able to access the electronic symptom diary (electronic PRO, ePRO) and accurately record their diary.
Exclusion criteria
A history of allergic reactions to the medications used in this study.
Nausea and vomiting requiring treatment with antiemetic agents at the time of enrolment.
Started strong opioid analgesics within 48 hours before enrolment (dose modifications for drugs that have already been in use are permitted).
A history of unstable angina pectoris, myocardial infarction, cerebral haemorrhage or cerebral infarction within 6 months before enrolment.
Concurrent or a history of interstitial lung disease.
Pregnant women, lactating women, women who may be currently pregnant or patients who do not intend to use contraception.
Patients whose enrolment in the study is judged as difficult due to a clinically confirmed psychiatric condition.
Patients who are regularly taking the drugs listed below*.
Local infections or active systemic infections that require treatment.
Other conditions that make the patient ineligible based on investigators’ judgement.
Concurrent treatment with another antipsychotic agent, such as phenothiazine or butyrophenone, and antidepressants such as selective serotonin reuptake inhibitors (SSRIs) or serotonin noradrenalin reuptake inhibitors (SNRIs) is not allowed.* In addition, continuous therapy with second-generation antihistamines, benzodiazepines, dopamine antagonists and systemic steroids is not allowed* because it could have antiemetic effects. Taking these medicines as needed is allowed.
Study treatment
All patients receive a 5-HT3RA, type and dose chosen by the site investigator, with dexamethasone intravenously at a dose of 6.6 mg or orally at a dose of 8 mg on day 1. In addition, patients receive olanzapine (5 mg per day orally) or matching placebo from days 1 to 6. Based on the previous study,12 we recommend that patients take olanzapine/placebo after their evening meal. Patients are allowed to take rescue medication throughout the study period for nausea or vomiting, if necessary. The investigators may/can select rescue treatment from antiemetic agents other than olanzapine, 5-HT3RA, steroids, NK1 receptor antagonists, serotonin and dopamine antagonists, SSRI and SNRI.
Randomisation and masking
Randomisation is centrally performed at 1:1 by random allocation modules of electronic data capture. The minimisation method with a random component is applied for randomisation. As stratification factors, we use 5-HT3RA (palonosetron, other) and a history of motion sickness (Yes, No) based on previous studies.21 22
We prepared olanzapine and a matching placebo with no identification code printed. The study drugs are packaged in press-through package sheets, and 12 tablets are further packaged in an aluminium pillow for one patient. We affix the study-specific drug numbers to the aluminium pillows. The patients, investigators, pharmacists and clinical research coordinators (CRCs) are blinded. On enrolment, allocation results are presented with the drug number, and the pharmacist dispenses the drug with that number.
Efficacy and safety assessment
The observational period of this study is 504 hours post-T-DXd administration. In addition to an ‘acute’ (0–24 hours post-T-DXd administration) and ‘delayed’ phase (24–120 hours), we define 120–504 hours as the ‘persistent phase’ and 0–504 hours as the ‘overall phase’.
The primary endpoint is a complete response (CR) rate defined as no emetic episodes and no use of rescue medication during the delayed phase (24–120 hours post-T-DXd administration), as reported in the electronic symptom diary. The secondary endpoints include (1) CR rate during the acute, acute and delayed, persistent, and overall phase, (2) complete control (no emetic episodes, no rescue medication and mild nausea of 0 or 1/4) during the acute, delayed, acute and delayed, persistent and overall phase, (3) total control (no emetic episodes, no rescue medication and no nausea) during the acute, delayed, acute and delayed, persistent, and overall phase, (4) no nausea during the acute, delayed, acute and delayed, persistent, and overall phase, (5) CR per day, (6) no nausea per day, (7) quality of life assessed by EORTC QLQ C-30,19 23 (8) other symptoms, including diarrhoea, constipation, abdominal pain, bloating, decreased appetite, fatigue and insomnia assessed by PRO-CTCAE,24 25 and (9) safety.
Concerning safety, investigators evaluate and grade treatment-associated toxic effects according to the National Cancer Institute CTCAE version 4.
Electronic patient-reported outcome
Patients document their experience in an electronic symptom diary every day during observational periods. Vomiting is rated on a 4-point categorical scale of none, once, twice or three times or more, and nausea is rated on a 4-point categorical scale of none, mild, moderate or severe. Whether or not olanzapine/placebo is taken is recorded for days 1–6. The use of rescue medication is also recorded daily by patients. EORTC QLQ C-3019 23 is assessed before T-DXd and on day 8. The presence and severity of diarrhoea, constipation, abdominal pain, bloating, decreased appetite, fatigue and insomnia are assessed using PRO-CTCAE before T-DXd administration and on days 8, 15 and 22. Automatic email alerts are sent for missed ePRO entries, and diary entries are locked if the allowance period is exceeded.
Site investigators and CRCs are trained on the purpose and use of ePRO and on how to instruct patients.
Study schedule
Figure 2 provides the schedule for enrolment and assessment. We define the date of T-DXd administration as day 1. The patient assesses the symptoms using a daily electronic symptom diary from day 1 to day 22. Medical interviews and physical examinations are conducted on days 1, 8 and 22. Laboratory studies are also performed on these days. Blood is collected for the biomarker study on days 1 and 8 (details are provided in the Biomarker study section).
Figure 2.
Schedule of the ERICA study. The calendar shows the schedule for patient enrolment and assessment. T-DXd, trastuzumab deruxtecan.
Biomarker study
The neuropeptides ghrelin and leptin promote and suppress appetite, respectively, thereby, participating in a network regulating appetite.26–28 The association of ghrelin and leptin blood levels with nausea, vomiting and anorexia in patients with cancer undergoing anticancer pharmacotherapy is unknown. The effects of olanzapine on ghrelin have been investigated in patients with schizophrenia and in patients with cancer with cachexia, with inconsistent results.29–32 Therefore, this biomarker test aims to explore the association of ghrelin and leptin blood levels with nausea and anorexia. To this end, blood samples are collected before T-DXd administration on day 1 and day 8, and plasma is separated via centrifugation. Leptin and ghrelin levels are measured using ELISA kits.
Statistical analysis
A preliminary survey conducted at nine WJOG-affiliated centres revealed that among 44 patients with breast cancer treated with T-DXd, 14 (32%) had a CR of 0–120 hours. All patients received antiemetic prophylaxis. Based on previous studies,11 12 33–35 we expected late CR rates of 35% in the control group and 50% in the study group. We set the significance level at 20% (one sided) and the power at 80%. ERICA is a phase II study to explore the efficacy of the olanzapine-containing regimen, and the study results are urgently needed. Therefore, we have adopted a larger significance level than that used for conventional sample size calculation. Our Monte Carlo simulation showed that, assuming 78 subjects in each group, 80 092 of 100 000 simulation data sets were significant based on the Fisher’s exact test under this significance level, which implies that the power was 80.092%. We note that assuming 77 subjects in each group, the power was 79.979%. Therefore, 78 subjects per group (ie, 156 subjects in total) are needed in this study. Accounting for possible case exclusion, the target sample size is set to 166.
Efficacy endpoints will be analysed for the full analysis set (FAS) and per protocol set (PPS), with the PPS analysis being the primary analysis; FAS and PPS analyses will be based on the allocation group, while the safety analysis will be based on the actual treatment group. Safety endpoints will be analysed in the safety analysis population. Patient background will be analysed for FAS and PPS. Other items will be analysed for PPS.
Data management
The data centre of the WJOG conducts registration, randomisation, data collection, cleaning and central monitoring using an electronic data capturing system, E-DMS online. The ePRO data are collected directly from patients through electronic devices such as patients’ smart-phones. Therefore, the ePRO is the source of original data, and the investigators at each study site store individual records for each patient as the ‘data source’, including a copy of informed consent, medical records, laboratory data and other records or notes. The data management centre oversees the intrastudy data-sharing process. The protocol review committee and independent safety monitoring committee assess the protocol amendments, serious adverse event reports and monitoring reports, and then provide necessary recommendations to investigators. The WJOG conducts an audit as necessary for this study.
Patient and public involvement
Patients and the public are not involved in the design of this study.
Ethics and dissemination
All patients will be required to provide written informed consent (online supplemental file 1). This study will be performed in accordance with ethical standards in the 1964 Declaration of Helsinki and later amendments. The study protocol is approved by the WJOG protocol review committee and SHOWA University Clinical Research Review Board. The trial has been registered at the Japan Registry of Clinical Trials, https://jrct.niph.go.jp/latest-detail/jRCTs031210410. The study results will be presented at international conferences and published in a peer-reviewed journal.
bmjopen-2022-070304supp001.pdf (755.5KB, pdf)
Supplementary Material
Acknowledgments
We thank study participants, their families and investigators in this study. We also thank members in the data centre at WJOG.
Footnotes
Contributors: HS is the principal investigator. HS and JT were involved in conception of the study. HS, JT, TY, CKI, KM, TI, DT and TT were involved in developing the study design. YC played a primary role in designing statistical analysis. HS, JT and DT developed the data management plan. HS, JT, YO, KN, HI, KW, SM, KM, TI and TT are carrying out recruitment and data collection. HS wrote the first draft of the manuscript. All authors have read, revised the manuscript critically, approved the paper and meet the criteria for authorship established by the International Committee of Medical Journals Editors.
Funding: This study is funded by Daiichi Sankyo. In March 2019, AstraZeneca entered into a global development and commercialisation collaboration agreement with Daiichi Sankyo for trastuzumab deruxtecan (T-DXd; DS-8201).
Competing interests: All authors have completed the ICMJE uniform disclosure form and declare that this research is supported by Daiichi Sankyo. HS has received speaker’s honorarium and medical writing support from Daiichi Sankyo as well as research funding from Eisai. JT has received consulting/advisory fees, conference support, speaker’s honoraria, research funding, and other fees from Daiichi Sankyo, research funding and speaker’s honoraria from Eli Lilly, research funding from Sant Joan de Déu Research Foundation, and consulting/advisory fees from AstraZeneca. YO has received speaker’s honoraria from Chugai and Daiichi Sankyo. KM has received speaker’s honoraria from Daiichi Sankyo, Chugai, Kyowa-Kirin and MSD, and advisory fees from Daiichi Sankyo and contracts for registration trials from MSD, Chugai, Eisai, Daiichi Sankyo, Eli Lilly, ICON Japan. KN, TY, SM, TI and YC have no conflicts of interest to disclose. HI has received speaker’s honoraria from Pfizer Japan, Eisai, Chugai Pharmaceutical, Kyowa Kirin, JMS, Taiho and Daiichi Sankyo. KW has received speaker’s honoraria from Chugai, Eli Lilly, Nippon-Kayaku, Kyowa Kirin, Novartis, Taiho, Eisai, Pfizer, Shionogi, Daiichi Sankyo and AstraZeneca. CMI has received research funding from Otsuka and Eli Lilly, in addition to speaker’s honorarium from Taiho. DT is an employee of Daiichi Sankyo. TT has received speaker’s honoraria from Daiichi Sankyo, Chugai, Eisai, Eli Lilly, and Celltrion Healthcare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016;107:1039–46. 10.1111/cas.12966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trail PA, Dubowchik GM, Lowinger TB. Antibody drug conjugates for treatment of breast cancer: novel targets and diverse approaches in ADC design. Pharmacol Ther 2018;181:126–42. 10.1016/j.pharmthera.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 3.Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2020;382:610–21. 10.1056/NEJMoa1914510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortés J, Kim S-B, Chung W-P, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med 2022;386:1143–54. 10.1056/NEJMoa2115022 [DOI] [PubMed] [Google Scholar]
- 5.Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 2022;387:9–20. 10.1056/NEJMoa2203690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCCN clinical practice guidelines in oncology (NCCN guidelines®) antiemesis version 2.2022. March 23, 2022.
- 7.Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: ASCO guideline update. J Clin Oncol 2020;38:2782–97. 10.1200/JCO.20.01296 [DOI] [PubMed] [Google Scholar]
- 8.Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol 2017;18:1512–22. 10.1016/S1470-2045(17)30604-6 [DOI] [PubMed] [Google Scholar]
- 9.Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996;14:87–96. 10.1016/0893-133X(94)00129-N [DOI] [PubMed] [Google Scholar]
- 10.Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2014;722:180–6. 10.1016/j.ejphar.2013.08.048 [DOI] [PubMed] [Google Scholar]
- 11.Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 2016;375:134–42. 10.1056/NEJMoa1515725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto H, Abe M, Tokuyama O, et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:242–9. 10.1016/S1470-2045(19)30678-3 [DOI] [PubMed] [Google Scholar]
- 13.Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011;9:188–95. 10.1016/j.suponc.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Zhang Y, Chen G, et al. Olanzapine-based triple regimens versus neurokinin-1 receptor antagonist-based triple regimens in preventing chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy: a network meta-analysis. Oncologist 2018;23:603–16. 10.1634/theoncologist.2017-0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow R, Navari RM, Terry B, et al. Olanzapine 5 Mg vs 10 Mg for the prophylaxis of chemotherapy-induced nausea and vomiting: a network meta-analysis. Support Care Cancer 2022;30:1015–8. 10.1007/s00520-021-06606-x [DOI] [PubMed] [Google Scholar]
- 16.Yanai T, Iwasa S, Hashimoto H, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 Mg or 5 Mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol 2018;23:382–8. 10.1007/s10147-017-1200-4 [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Wang L, Wang H, et al. Effectiveness of olanzapine combined with ondansetron in prevention of chemotherapy-induced nausea and vomiting of non-small cell lung cancer. Cell Biochem Biophys 2015;72:471–3. 10.1007/s12013-014-0489-0 [DOI] [PubMed] [Google Scholar]
- 18.Basch E, Reeve BB, Mitchell SA, et al. Development of the National cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014;106:dju244. 10.1093/jnci/dju244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 20.Okuyama T, Wang XS, Akechi T, et al. Japanese version of the MD anderson symptom inventory: a validation study. J Pain Symptom Manage 2003;26:1093–104. 10.1016/j.jpainsymman.2003.05.003 [DOI] [PubMed] [Google Scholar]
- 21.Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 2009;10:115–24. 10.1016/S1470-2045(08)70313-9 [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto K, Takahashi M, Sato K, et al. A double-blind, randomized, multicenter phase 3 study of palonosetron vs granisetron combined with dexamethasone and fosaprepitant to prevent chemotherapy-induced nausea and vomiting in patients with breast cancer receiving anthracycline and cyclophosphamide. Cancer Med 2020;9:3319–27. 10.1002/cam4.2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi K, Takeda F, Teramukai S, et al. A cross-validation of the European organization for research and treatment of cancer QLQ-C30 (EORTC QLQ-C30) for Japanese with lung cancer. Eur J Cancer 1998;34:810–5. 10.1016/s0959-8049(97)00395-x [DOI] [PubMed] [Google Scholar]
- 24.Miyaji T, Iioka Y, Kuroda Y, et al. Japanese translation and linguistic validation of the US national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Patient Rep Outcomes 2017;1:8. 10.1186/s41687-017-0012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaguchi T, Azuma K, Sano M, et al. The japanese version of the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE): psychometric validation and discordance between clinician and patient assessments of adverse events. J Patient Rep Outcomes 2017;2:2. 10.1186/s41687-017-0022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–60. 10.1038/45230 [DOI] [PubMed] [Google Scholar]
- 27.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–70. 10.1038/27376 [DOI] [PubMed] [Google Scholar]
- 28.Wolf I, Sadetzki S, Kanety H, et al. Adiponectin, ghrelin, and leptin in cancer cachexia in breast and colon cancer patients. Cancer 2006;106:966–73. 10.1002/cncr.21690 [DOI] [PubMed] [Google Scholar]
- 29.Murashita M, Kusumi I, Inoue T, et al. Olanzapine increases plasma ghrelin level in patients with schizophrenia. Psychoneuroendocrinology 2005;30:106–10. 10.1016/j.psyneuen.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 30.Hosojima H, Togo T, Odawara T, et al. Early effects of olanzapine on serum levels of ghrelin, adiponectin and leptin in patients with schizophrenia. J Psychopharmacol 2006;20:75–9. 10.1177/0269881105056647 [DOI] [PubMed] [Google Scholar]
- 31.Naing A, Dalal S, Abdelrahim M, et al. Olanzapine for cachexia in patients with advanced cancer: an exploratory study of effects on weight and metabolic cytokines. Support Care Cancer 2015;23:2649–54. 10.1007/s00520-015-2625-9 [DOI] [PubMed] [Google Scholar]
- 32.Lu M-L, Wang T-N, Lin T-Y, et al. Differential effects of olanzapine and clozapine on plasma levels of adipocytokines and total ghrelin. Prog Neuropsychopharmacol Biol Psychiatry 2015;58:47–50. 10.1016/j.pnpbp.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay S, Kwatra G, Alice K P, et al. Role of olanzapine in chemotherapy-induced nausea and vomiting on platinum-based chemotherapy patients: a randomized controlled study. Support Care Cancer 2017;25:145–54. 10.1007/s00520-016-3386-9 [DOI] [PubMed] [Google Scholar]
- 34.Tienchaiananda P, Nipondhkit W, Maneenil K, et al. A randomized, double-blind, placebo-controlled study evaluating the efficacy of combination olanzapine, ondansetron and dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving doxorubicin plus cyclophosphamide. Ann Palliat Med 2019;8:372–80. 10.21037/apm.2019.08.04 [DOI] [PubMed] [Google Scholar]
- 35.Tan L, Liu J, Liu X, et al. Clinical research of olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res 2009;28:131. 10.1186/1756-9966-28-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-070304supp001.pdf (755.5KB, pdf)