Abstract
Introduction
Recent studies suggest that the urban advantage of lower neonatal mortality in urban compared with rural areas may be reversing, but methodological challenges include misclassification of neonatal deaths and stillbirths, and oversimplification of the variation in urban environments. We address these challenges and assess the association between urban residence and neonatal/perinatal mortality in Tanzania.
Methods
The Tanzania Demographic and Health Survey (DHS) 2015–2016 was used to assess birth outcomes for 8915 pregnancies among 6156 women of reproductive age, by urban or rural categorisation in the DHS and based on satellite imagery. The coordinates of 527 DHS clusters were spatially overlaid with the 2015 Global Human Settlement Layer, showing the degree of urbanisation based on built environment and population density. A three-category urbanicity measure (core urban, semi-urban and rural) was defined and compared with the binary DHS measure. Travel time to the nearest hospital was modelled using least-cost path algorithm for each cluster. Bivariate and multilevel multivariable logistic regression models were constructed to explore associations between urbanicity and neonatal/perinatal deaths.
Results
Both neonatal and perinatal mortality rates were highest in core urban and lowest in rural clusters. Bivariate models showed higher odds of neonatal death (OR=1.85; 95% CI 1.12 to 3.08) and perinatal death (OR=1.60; 95% CI 1.12 to 2.30) in core urban compared with rural clusters. In multivariable models, these associations had the same direction and size, but were no longer statistically significant. Travel time to the nearest hospital was not associated with neonatal or perinatal mortality.
Conclusion
Addressing high rates of neonatal and perinatal mortality in densely populated urban areas is critical for Tanzania to meet national and global reduction targets. Urban populations are diverse, and certain neighbourhoods or subgroups may be disproportionately affected by poor birth outcomes. Research must capture, understand and minimise risks specific to urban settings.
Keywords: maternal health, child health, cross-sectional survey
WHAT IS ALREADY KNOWN ON THIS TOPIC
The urban advantage, suggesting better health outcomes in urban compared with rural populations, has been questioned, both for adult and child mortality.
An analysis using Demographic and Health Survey data in Tanzania in 2015–2016 showed a twofold higher risk of neonatal mortality in urban compared with rural areas.
A reversal of the urban advantage in neonatal survival might be occurring in other sub-Saharan African countries.
WHAT THIS STUDY ADDS
Our work suggests that the categorisation of locations as urban or rural on the 2015–2016 Demographic and Health Survey in Tanzania may be both simplistic and inaccurate.
Risks of neonatal and perinatal mortality are highest in core, densely populated urban areas in mainland Tanzania, and lowest in rural areas.
Travel time to nearest public hospital was not associated with neonatal or perinatal mortality in mainland Tanzania.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Urbanicity as an exposure variable follows a spectrum which needs to be better measured and understood.
More research is urgently needed to understand the neonatal and perinatal mortality in core urban areas to guide specific actions.
Known risk factors such as anaemia and young maternal age continue to play a role in neonatal and perinatal mortality and must be urgently addressed.
Introduction
Africa is the most rapidly urbanising continent, with its population expected to double by 2050 and two-thirds of this growth will be in urban areas.1 Health status and outcomes have generally been described as better in urban compared with rural areas, likely due to a variety of factors, including better infrastructure and improved access to healthcare.2 However, this phenomenon is not universal and shows signs of reversal.3 4 A recent study of Demographic and Health Surveys (DHS) data collected between 1992 and 2018 in 53 low-income and middle-income countries found that the urban advantage in adult mortality has diminished while an urban advantage continues to be observed among children under-5 years of age.5
In sub-Saharan Africa (SSA), neonatal mortality has historically been higher in rural areas compared with urban ones6 and is posited to be related to a combination of socioeconomic factors (maternal education, nutrition, care affordability) and care accessibility (more births occurring in health facilities, shorter distance and travel time to health facilities). With rapid reductions in under-5 mortality, the proportions of under-5 deaths have concentrated in infancy, specifically during the neonatal period.7 Recent population surveys have shown that in SSA, the urban advantage in neonatal mortality rate (NMR) might be waning. The most extreme example is Tanzania where urban neonatal mortality (40/1000 live births) is twice the level in rural areas (20/1000 live births) and this difference persists even when some confounders are adjusted for (2015–2016 DHS). Within the neonatal period, the disparity between urban and rural areas is highest among deaths on days 1–7 after birth.6 The potential drivers of this observed higher urban neonatal mortality are not well understood; several hypotheses have been proposed and multiple factors could be at play such as limited access to clean water and sanitation, variable quality of maternal and newborn healthcare and poor air quality, all highly prevalent in urban settings in general and informal settlements in particular.6 Further, extreme inequality is present within urban areas, despite improved resources and infrastructure.8
There is no agreement on a definition of the exposure of urbanicity, but satellite derived data sets offer an opportunity to use more objective and continuous measures which quantify the degree of urbanisation at high spatial resolution as a combination of built environment and population density. This is derived independently from national administrative boundaries or designations. Previous studies found that satellite derived urbanicity measures strongly align with administrative data but may fail to capture some rural areas.9 By using satellite derived data, multiple categories of ‘urban’ can be derived to validate the observed pattern of higher neonatal mortality in urban areas. It would also allow for an exploration of potential misclassification bias when using DHS-based classification, that is, usually based on country administrative regions or population thresholds to define urban or rural in some countries compared with satellite derived classification based on high resolution population density, built-up areas and other land use and cover classes. A second issue for research to understand in the association between neonatal mortality and urbanicity is the potential misclassification between stillbirths and neonatal deaths due to challenges with establishing whether there were signs of life after birth.10 The combination of omission of stillbirths, potential misclassification of birth outcomes (neonatal deaths and stillbirths), misclassification or oversimplification of urbanicity and residual confounding may mask the true direction and strength of the association between urbanicity and neonatal mortality.
With a view to address these limitations affecting our previous work,6 we aim to more accurately estimate the direction and strength of the association between urban residence and neonatal mortality in mainland Tanzania. We address the limitations by reducing misclassification of exposure by using geospatial techniques to reclassify urban/rural areas and use a more granular measure of urbanicity (three categories), and by reducing misclassification of outcome (neonatal deaths reported as stillbirth) by also examining perinatal mortality (stillbirths and early neonatal deaths combined).
Methods
Overview
We start by identifying recent births occurring to women of reproductive age sampled on the Tanzania DHS in 2015–2016. Based on the coordinates of the household clusters where women live, we created an alternative urbanicity using satellite imagery in lieu of the binary residence variable provided by the DHS. Confounders were retrieved from the DHS or generated through geospatial modelling. We then used bivariate and multilevel multivariable logistic regression models to assess the strength of the association between urban residence and (1) neonatal mortality and (2) perinatal mortality.
Data sources and measures
We used the most recent DHS conducted in Tanzania in 2015–2016. DHS are cross-sectional nationally representative household surveys which use standard model questionnaires which countries can adapt. DHS respondents are women of reproductive age (15–49 years), and in several countries men are also interviewed. The surveys include questions on household and individual characteristics, fertility, maternal and child health, mortality, among others. The survey sampling design was based on a two-stage strategy, the first stage involved selection of sampling points (clusters, based on the 2012 Tanzanian census enumeration areas (EAs)) and the second selection of households within clusters. The stratification allowed estimation of certain indicators for 25 regions in mainland Tanzania. Each EA typically contains 20–30 households randomly selected to be surveyed from about 100–300 households per cluster. To reduce the disclosure risk, the cluster is first assigned the coordinates of the EA centre and then geomasked by displacing the Global Positioning System coordinates. Urban clusters were displaced by up to 2 km while rural clusters were displaced by up to 5 km, with a further 1% randomly selected rural clusters displaced by up to 10 km.11
Population
Our study population included women aged 15–49 years at the time of the DHS who lived in sampled households and agreed to participate in the survey. We analysed all live births and stillbirths occurring in the 5 years prior to the survey reported by participating women who had a permanent address in mainland Tanzania.
Outcome variables
The main outcome of this study was neonatal death. While neonatal deaths are usually defined as deaths between birth and day 28, we also included deaths reported on day 29. This is due to the coding of the response in the DHS data set and to remain consistent with the cut-off that the DHS report used.12 We defined NMR as the number of neonatal deaths per 1000 live births. We further assessed early (within the first 7 days of life, within which we separated deaths on day of birth) and late (8–29 days inclusive) NMR. The secondary outcome was perinatal death, defined as a combination of stillbirths (defined as deaths of babies at or after 7 months of pregnancy and before birth in line with the WHO recommended definition of late gestation stillbirth for international comparisons) and early neonatal deaths. Perinatal mortality rate was expressed as the number of stillbirths and early neonatal deaths per 1000 pregnancies of gestational age 7 or more months, including live births. We extracted stillbirths from the DHS contraceptive calendar based on DHS guidance.13 14
Main exposure
Our primary explanatory variable of interest was residence (urban or rural) based on DHS designation and urbanicity (core urban, semi-urban and rural) derived from satellite imagery. As an alternative to the DHS urban and rural classifications, we derived three classes of the urban continuum (urbanicity)—rural, semi-urban and core urban based on satellite imagery. We used the 2015 Global Human Settlement Layer-settlement model (GHS-SMOD)15 16 to classify the location of DHS clusters into different degrees of urbanicity, namely core urban, semi-urban and areas in transition and rural areas. Details on how these classes were generated are provided in online supplemental file 1.
bmjgh-2022-011253supp001.pdf (761.9KB, pdf)
Modelling travel time to hospitals
Given that short distances in urban areas can obscure long travel times,17 we also included a consideration for accessibility of emergency obstetrical healthcare during pregnancy and childbirth generally provided only in hospitals as a potential explanation (effect moderator) between urbanicity and neonatal mortality. A proxy of geographical accessibility to hospital was not available in the DHS and was thus modelled independently for each cluster. It was proxied by the time taken to travel between a DHS cluster and the nearest public hospital, based on a least-cost path algorithm implemented in a Geographical Information System via WHO AccessMod 5 software (alpha V.5.7.8)18 widely used across healthcare applications in SSA.19 The detailed steps undertaken to compute travel time are provided in online supplemental file 1.
Confounder variables
Potential confounders related to both neonatal/perinatal mortality and urbanicity were identified based on the literature. We relied on confounders available in the DHS capturing the lived environment of the woman (geographical zone), household characteristics, socioeconomic characteristics of the woman and variables capturing information about the pregnancy and health-seeking behaviour during index pregnancy and childbirth. Some of the variables were only available for live births and others still only for the most recent live birth in the 5-year period (online supplemental file 2).
bmjgh-2022-011253supp002.pdf (32.3KB, pdf)
Data analysis
We conducted the analysis in three steps. First, we explored the correspondence between the DHS characterisation of clusters as urban or rural in comparison to the three categories based on GHS-SMOD. We also describe the distribution of mean travel time to the nearest public hospital among the study population for both the DHS and GHS-SMOD urban–rural classifications. Second, we described characteristics of the sample and calculated neonatal and perinatal mortality rates, and the distribution of age at death using both DHS and GHS-MOD categorisations. Third, we tested bivariate and multivariable associations between the GHS-MOD urbanicity measure and neonatal/perinatal mortality. The main hypothesis was that there is an association between urbanicity and neonatal/perinatal mortality. Due to inconsistent availability of key variables, we ran four separate multivariable models. The first three models had neonatal mortality as an outcome and were conducted: (1) among all live births, (2) among the most recent live births and (3) among most recent live births with newborn’s birth weight and antenatal care (ANC) history available. The fourth model included all births, and the outcome was perinatal mortality.
To assess the effect of urbanicity on neonatal/perinatal mortality, our model building strategy aimed to adjust for confounding, not to overparameterise and to account for any multilevel effects. The selection of variables into adjusted regression models followed previously used approaches.20–22 First, based on previous research, we identified all potential confounders (variables that influence both mortality and residence). For each confounder, we ran a bivariate regression to estimate the crude association between each potential confounder and both outcomes (neonatal and perinatal mortality). Only confounders significant at p value<0.20 were incorporated into the subsequent multivariable regression analysis step.
In the multivariable multilevel logistic regression model, we added urbanicity as the first variable, followed by one confounder at a time, starting with the confounder with the lowest p value in the bivariate model. Confounders were only retained in the model if they met two criteria; (1) having a p value<0.05, and (2) effects on the adjusted OR of the confounders already selected (ie, confounders causing at least a 10% change in the effect size of variables were retained even if not significant at p<0.05). Confounders not meeting these criteria were not retained in the final models except for the geographical zone, which was included a priori to capture the lived environment.
Further, we accounted for the multistage sampling design and nesting structure in the DHS data through multilevel hierarchical modelling regardless of the significance.23 24 This strategy accounts for contextual factors which are not captured in the fixed variables. We included random intercepts that vary across households and clusters. The household-level random intercept captured the effect of latent household-specific covariates that cause some households to be more similar than others. Cluster-level unobserved characteristics, such as cultural norms, were captured by the cluster random-effect. The choice of cluster and household level random effects was informed by intracommunity correlation coefficient tested at the zonal, cluster and household level. Therefore, all four multilevel logistic regression models contained fixed effects and random effects with three levels, clusters at level 1, households at level 2 and individuals (woman–baby dyads) at level 3. We considered variables to be highly correlated if they had a coefficient of over 0.80 based on Pearson correlation coefficient.
Analyses were conducted in Stata/SE V.15. In all analyses, we adjusted for survey design (svyset with clusters, individual sampling weights and stratification). There was no missingness in the urbanicity measure, the main outcomes or other key confounders. There was substantial missingness in the birth weight variable, largely because women reported that their newborns were not weighed.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination of this research.
Results
Geographical classification of clusters
Mainland Tanzania DHS 2015–2016 contained 527 clusters. Based on the GHS-SMOD urbanicity measure, 61 (11.6%) were core urban, 224 (42.5%) semi-urban and 242 (45.9%) rural. The comparison of DHS and GHS-SMOD classification of clusters is shown in table 1. All the core urban clusters were correctly identified as urban by DHS. However, there were discrepancies in the other two classes. Among the 224 semi-urban clusters, 138 were reported by DHS as rural and 86 as urban, while among the 242 GHS-SMOD rural clusters, 226 were identified by DHS as rural while 16 were identified as urban. It is expected that the semi-urban classes contain a mixture of urban and rural cells. However, 16 rural clusters were misclassified by the DHS as urban although 13 of these clusters (81%) had the majority of the pixels within their buffers as very low-density rural pixels and 9 of these clusters (56%) had maximum values of either 1 or 2. Therefore, these 16 clusters had a very high likelihood of being truly rural.
Table 1.
DHS Tanzania 2015–2016 mainland clusters based on DHS versus GHS-SMOD classification
| GHS-SMOD urbanicity classes | Total | ||||
| Core urban | Semi-urban | Rural | |||
| DHS residence | Rural | 0 | 138 | 226 | 364 |
| Urban | 61 | 86 | 16 | 163 | |
| Total | 61 | 224 | 242 | 527 | |
DHS, Demographic and Health Surveys; GHS-SMOD, Global Human Settlement Layer-settlement model.
Travel time to nearest hospital
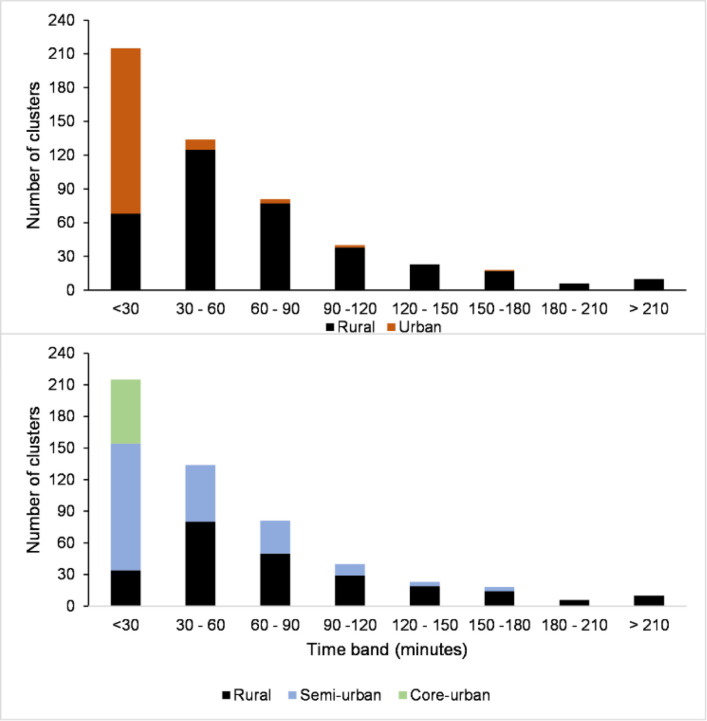
The average travel time from each cluster to the nearest public hospital was 63 min, with large subnational variations at high spatial resolution. At cluster level, modelled travel time estimates ranged between 0 and 418 min (7 hours). Among the 527 included clusters, 349 (66%) were within a 1-hour catchment of the nearest public hospital, while 23% (121 clusters) were within 2–3 hours (online supplemental file 1). Stratification by urbanicity showed that the DHS rural and urban classes had an average of 14 and 78 min of travel time to the nearest hospital, respectively. In the three new urbanicity classes, the average travel time was 89 min in rural clusters, 41 min in semi-urban clusters and 4 min in core urban clusters. Majority of semi-urban and core urban clusters were within 30 min of the nearest public hospital (figure 1).
Figure 1.
Distribution of 527 clusters in mainland Tanzania by travel time to nearest public hospital in minutes by the Demographic and Health Surveys (top panel) and Global Human Settlement Layer-settlement model (bottom panel) urban classification of clusters.
Description of the sample
The analysis data set contained 8915 pregnancies of 7 or more months among 6156 unique women: 3765 women contributed one pregnancy, 2042 women contributed two pregnancies, 330 women contributed three pregnancies and 19 women contributed four pregnancies. Among these 8915 pregnancies, 8739 resulted in live births and 176 in stillbirths. Among the live births, 217 neonatal deaths were reported (180 early neonatal and 37 late neonatal). A total of 356 perinatal deaths (stillbirths+early neonatal deaths) were reported. Table 2 shows the distribution of the outcome variables and the characteristics of the analysis subgroups based on availability of variables, for Tanzania mainland overall and by GHS-MOD urbanicity categories. More than half of all births in the sample occurred in core urban or semi-urban areas.
Table 2.
Characteristics of pregnancies and births in analysis, overall and by urbanicity class
| Numbers of observations | Total | Core urban | Semi-urban | Rural | |||||
| Live births in last 5 years | 8739 | 692 | 3597 | 4450 | |||||
| Most recent live births in last 5 years | 6099 | 560 | 2544 | 2995 | |||||
| Total births in last 5 years (live births and stillbirths) | 8915 | 707 | 3671 | 4537 | |||||
| Neonatal deaths within last 5 years | 217 | 26 | 94 | 97 | |||||
| Early neonatal deaths within last 5 years | 180 | 25 | 72 | 83 | |||||
| Stillbirths within last 5 years | 176 | 15 | 74 | 87 | |||||
| Perinatal deaths in last 5 years (early neonatal deaths and stillbirths) | 356 | 40 | 146 | 170 | |||||
| Part A: All births (n=8915) | Total | Core urban | Semi-urban | Rural | |||||
| n | Column % | n | Column % | n | Column % | n | Column % | ||
| Urbanicity class | Core urban | 707 | 11.7 | ||||||
| Semi-urban | 3671 | 39.5 | |||||||
| Rural | 4537 | 48.8 | |||||||
| Geographical zone | Western | 979 | 12.4 | 50 | 4.1 | 322 | 9.9 | 607 | 16.5 |
| Lake | 2929 | 32.7 | 123 | 16.3 | 1535 | 41.9 | 1271 | 29.2 | |
| Northern | 770 | 9.5 | 108 | 16.4 | 312 | 9.4 | 350 | 8.0 | |
| Central | 1010 | 11.4 | 0 | 0.0 | 247 | 7.4 | 763 | 17.3 | |
| Southwest highlands | 1149 | 9.9 | 35 | 4.6 | 504 | 9.1 | 610 | 11.8 | |
| Southern highlands | 734 | 5.5 | 5 | 0.4 | 299 | 6.1 | 430 | 6.3 | |
| Southern | 418 | 4.1 | 5 | 0.6 | 148 | 3.9 | 265 | 5.1 | |
| Eastern | 926 | 14.5 | 381 | 57.6 | 304 | 12.3 | 241 | 5.8 | |
| Household wealth quintile | Poorest | 2355 | 24.7 | 3 | 0.6 | 595 | 14.7 | 1757 | 38.5 |
| Poorer | 1978 | 21.5 | 2 | 0.3 | 728 | 20.4 | 1248 | 27.6 | |
| Middle | 1766 | 19.5 | 13 | 1.7 | 830 | 23.4 | 923 | 20.5 | |
| Richer | 1594 | 18.4 | 148 | 22.0 | 913 | 25.3 | 533 | 11.9 | |
| Richest | 1222 | 15.9 | 541 | 75.4 | 605 | 16.2 | 76 | 1.5 | |
| Maternal education and literacy | No education | 1899 | 21.0 | 42 | 6.5 | 658 | 17.4 | 1199 | 27.3 |
| Primary education/illiterate | 988 | 10.6 | 45 | 6.6 | 418 | 10.9 | 525 | 11.4 | |
| Primary education/literate | 4921 | 55.3 | 375 | 53.5 | 2051 | 56.8 | 2498 | 54.6 | |
| Secondary or higher | 1107 | 13.1 | 248 | 33.4 | 544 | 14.9 | 315 | 6.7 | |
| Marital status | Married or cohabiting | 7388 | 82.5 | 551 | 78.1 | 2939 | 79.8 | 3898 | 85.6 |
| Not married or cohabiting | 1527 | 17.5 | 156 | 21.9 | 732 | 20.2 | 639 | 14.4 | |
| Maternal age group (in years) | <20 | 1555 | 17.8 | 90 | 13.4 | 662 | 18.3 | 803 | 18.5 |
| 20–29 | 4417 | 49.5 | 417 | 58.7 | 1802 | 48.3 | 2198 | 48.3 | |
| 30–49 | 2943 | 32.7 | 200 | 27.9 | 1207 | 33.4 | 1536 | 33.2 | |
| Maternal decision-making about health | Self (fully or partly) | 6743 | 75.6 | 581 | 81.8 | 2809 | 77.2 | 3353 | 72.8 |
| Others | 2172 | 24.4 | 126 | 18.2 | 862 | 22.8 | 1184 | 27.2 | |
| Maternal relocation (fewer than 5 years lived in current residence) | Yes | 2275 | 25.8 | 432 | 37.7 | 2608 | 27.9 | 3600 | 21.3 |
| No | 6640 | 74.2 | 275 | 62.3 | 1063 | 72.1 | 937 | 78.7 | |
| Maternal anaemia at survey | Yes | 4000 | 45.3 | 311 | 45.1 | 1635 | 44.9 | 2054 | 45.3 |
| No | 4915 | 54.7 | 396 | 54.9 | 2036 | 55.1 | 2483 | 54.7 | |
| Maternal mobile ownership | Yes | 3958 | 46.0 | 600 | 84.8 | 1842 | 50.7 | 1516 | 32.9 |
| No | 4957 | 54.0 | 107 | 15.2 | 1829 | 49.3 | 3021 | 67.1 | |
| Ownership of health insurance | Yes | 670 | 7.5 | 73 | 9.2 | 319 | 9.1 | 278 | 7.5 |
| No | 8245 | 92.5 | 634 | 90.8 | 3352 | 90.9 | 4259 | 92.5 | |
| Travel to nearest hospital (hours) | Less than 2 hours | 7444 | 87.8 | 707 | 100.0 | 3474 | 97.3 | 3429 | 77.1 |
| Two hours or more | 1471 | 12.2 | 0 | 0 | 197 | 2.7 | 1108 | 22.9 | |
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| Travel to nearest hospital (minutes) | 59.6 | 3.6 | 4.7 | 0.50 | 38.1 | 2.40 | 90.0 | 6.30 | |
| Household size (mean number of members) | 7.2 | 0.14 | 5.8 | 0.20 | 7.1 | 0.17 | 7.6 | 0.26 | |
| Part B: Additional variables available for all live births (n=8739) | |||||||||
| n | Column % | n | Column % | n | Column % | n | Column % | ||
| Mode of delivery | Vaginal | 8263 | 94.1 | 589 | 84.8 | 3398 | 94.2 | 4276 | 96.2 |
| Caesarean | 476 | 5.9 | 103 | 15.2 | 199 | 5.8 | 174 | 3.8 | |
| Multiple birth | Yes | 297 | 3.6 | 28 | 4.8 | 129 | 3.3 | 140 | 3.6 |
| No | 8442 | 96.4 | 664 | 95.2 | 3468 | 96.7 | 4310 | 96.4 | |
| Birth order and preceding birth interval (months) | First child | 2094 | 24.7 | 244 | 35.0 | 883 | 24.7 | 967 | 22.2 |
| Second/third; <24 | 568 | 6.3 | 40 | 5.9 | 233 | 6.2 | 295 | 6.6 | |
| Second/third; 24+ | 2345 | 27.8 | 275 | 40.4 | 1011 | 28.3 | 1059 | 24.4 | |
| Fourth+; <24 | 712 | 7.7 | 18 | 2.2 | 262 | 7.4 | 432 | 9.2 | |
| Fourth+; 24+ | 3020 | 33.5 | 115 | 16.5 | 1208 | 33.4 | 1697 | 37.6 | |
| Sex of child | Male | 4438 | 49.2 | 370 | 46.8 | 1826 | 51.0 | 2242 | 50.0 |
| Female | 4301 | 50.8 | 322 | 53.2 | 1771 | 49.0 | 2208 | 50.0 | |
| Pregnancy wanted at the time | Yes | 6092 | 69.4 | 481 | 69.3 | 2419 | 66.9 | 3192 | 71.6 |
| No | 2647 | 30.6 | 211 | 30.7 | 1178 | 33.1 | 1258 | 28.4 | |
| Place of birth | Home | 3336 | 37.5 | 48 | 6.6 | 1166 | 31.9 | 2122 | 49.4 |
| Lower-level facility | 2750 | 30.6 | 157 | 25.1 | 1174 | 32.1 | 1419 | 30.7 | |
| Hospital | 2653 | 31.9 | 487 | 68.3 | 1257 | 36.0 | 909 | 19.9 | |
| Part C: Additional variables available for most recent live births (n=6099)** | |||||||||
| Antenatal care during pregnancy | No ANC | 124 | 2.0 | 11 | 1.7 | 34 | 1.4 | 79 | 2.7 |
| 1–3 visits | 2981 | 47.3 | 160 | 26.9 | 1283 | 49.3 | 1538 | 51.5 | |
| 4 or more visits | 2994 | 50.7 | 389 | 71.4 | 1227 | 49.3 | 1378 | 45.8 | |
| Child weighed at birth | Yes | 4050 | 67.5 | 537 | 96.1 | 1842 | 73.2 | 1671 | 54.3 |
| No | 2049 | 32.5 | 23 | 3.9 | 702 | 26.8 | 1324 | 45.7 | |
| Part D: Additional variable available for most recent live births whose birth weight was taken (n=4050)** | |||||||||
| Child’s birth weight category (in grams) | Low (<2500 g) | 248 | 6.2 | 40 | 7.3 | 109 | 6.0 | 99 | 5.8 |
| Normal (2500–4000 g) | 3547 | 87.7 | 473 | 88.7 | 1609 | 87.0 | 1465 | 88.0 | |
| Macrosomia (>4000 g) | 255 | 6.1 | 24 | 4.0 | 124 | 7.0 | 107 | 6.2 | |
*Child’s birth weight is available for all live births but we restricted to most recent live births to improve accuracy of recall and flow of analysis subsamples.
ANC, antenatal care.
Neonatal and perinatal mortality
Table 3 presents the neonatal and perinatal mortality rates by DHS residence, GHS-SMOD urbanicity classification and for mainland Tanzania overall. The comparison shows that mortality estimates for rural areas did not differ between DHS and GHS-SMOD classifications. The perinatal and neonatal mortality rates in the new urbanicity class of semi-urban were similar to levels in rural categories of both DHS and GHS-SMOD. Within the GHS-SMOD classification, core urban areas reached the highest perinatal (56.4/1000 pregnancies) and neonatal mortality rates (39.8/1000 live births); these were significantly higher than those observed in semi-urban and rural areas.
Table 3.
Neonatal and perinatal mortality rates by DHS urban/rural residence and GHS-SMOD urbanicity categories, with 95% CI in mainland Tanzania
| DHS residence | Overall (Tanzania mainland) | Urban | Rural | P value |
| Perinatal mortality (per 1000 pregnancies of 7 months and more) | 39.1 (34.8 to 43.9) | 46.9 (38.3 to 57.3) | 36.2 (31.4 to 41.7) | 0.0387 |
| Neonatal mortality (per 1000 live births) | 25.1 (21.3 to 29.6) | 38.6 (30.2 to 49.3) | 20.1 (16.2 to 24.9) | <0.001 |
| GHS-SMOD urbanicity class | Core urban | Semi-urban | Rural | P value |
| Perinatal mortality (per 1000 pregnancies of 7 months and more) | 56.4 (41.5 to 76.2) | 37.9 (31.2 to 46.0) | 35.9 (30.6 to 42.2) | 0.0277 |
| Neonatal mortality (per 1000 live births) | 39.8 (26.3 to 59.9) | 24.8 (19.6 to 31.4) | 21.9 (16.8 to 28.5) | 0.0371 |
DHS, Demographic and Health Surveys; GHS-SMOD, Global Human Settlement Layer-settlement model.
Further details of neonatal and perinatal mortality are shown in online supplemental file 3. Briefly, among the 217 neonatal deaths, the distribution of timing of death was significantly different by urbanicity. In core urban clusters, more than 95% of neonatal deaths occurred in the first week of life (predominantly on days 2–7), compared with 19% in semi-urban and 14% in rural areas. However, within the early neonatal period, semi-urban and rural areas had a higher percentage of deaths on day of birth compared with core urban areas. The mean age at death was 4.1 days; this was shortest in the core urban category of clusters (2.9 days) compared with semi-urban (5.1) and rural (3.6). Among the 73 most recent neonatal deaths of babies born in facilities, we looked at whether the death occurred before or after discharge from the facility. Two-fifths of neonatal deaths in core urban and rural clusters occurred after discharge; this was much higher (73%) in semi-urban areas, a significant difference despite the small sample size; but corresponding with the results on distribution of time of death. We also examined the ratio of stillbirths to early neonatal deaths which is a proxy for misclassification between the two outcomes and stillbirth data quality. The ideal ratio should be around 1.2 with much lower or higher values indicating possible under-reporting or misclassification.25 Overall, among all areas in Tanzania the ratio was 0.85 indicating a small degree of under-reporting. However, when examined according to urbanicity status, core urban areas had the most under-reporting or misclassification of stillbirths with a ratio of 0.52 compared with semi-urban and rural areas which had reasonably good ratios just below 1.
bmjgh-2022-011253supp003.pdf (33.6KB, pdf)
Bivariate analysis
Bivariate analysis examining the association of variables with neonatal death and perinatal death is shown in table 4. Compared with GHS-SMOD rural class, the odds of death were not higher in semi-urban areas, but were significantly higher in core urban areas (OR=1.85, 95% CI 1.12 to 3.08) for neonatal death and 1.60 (95% CI 1.2 to 2.3) for perinatal death. Compared with the Lake zone, only Southern and Eastern zones had significantly different (higher) neonatal and perinatal mortality. Women from richer households and more educated women had higher odds of reporting neonatal mortality compared with women from poorer households and without formal education. Age and maternal anaemia were associated with both neonatal and perinatal death. Among live births, the crude odds of neonatal death was higher for caesarean mode of delivery, multiple births, primiparous mothers, male newborns, hospital births and lack of ANC during pregnancy. Among newborns who were weighed, both low birth weight and macrosomia were associated with higher odds of neonatal mortality compared with normal birth weight.
Table 4.
Bivariate associations with neonatal death and perinatal death
| Neonatal death (n=217) | Perinatal death (n=393) | ||||||
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| n=8739 (all live births) | n=8915 (all births) | ||||||
| Urbanicity class | Core urban | 1.85 | 1.12 to 3.08 | 0.017 | 1.6 | 1.12 to 2.3 | 0.011 |
| Semi-urban | 1.13 | 0.8 to 1.62 | 0.476 | 1.06 | 0.82 to 1.37 | 0.67 | |
| Rural | Ref | Ref | |||||
| Geographical zone | Western | 1.06 | 0.58 to 1.96 | 0.837 | 0.88 | 0.58 to 1.32 | 0.532 |
| Lake | Ref | Ref | |||||
| Northern | 0.93 | 0.47 to 1.84 | 0.838 | 0.88 | 0.51 to 1.51 | 0.65 | |
| Central | 1.32 | 0.71 to 2.47 | 0.385 | 1.05 | 0.67 to 1.64 | 0.837 | |
| Southwest highlands | 1.55 | 0.84 to 2.84 | 0.158 | 1.06 | 0.69 to 1.63 | 0.797 | |
| Southern highlands | 1.5 | 0.77 to 2.91 | 0.23 | 1.06 | 0.68 to 1.67 | 0.788 | |
| Southern | 2.19 | 1.13 to 4.25 | 0.021 | 2.11 | 1.34 to 3.14 | 0.001 | |
| Eastern | 2.01 | 1.18 to 3.43 | 0.01 | 1.46 | 1 to 2.13 | 0.048 | |
| Household wealth quintile | Poorest | Ref | Ref | ||||
| Poorer | 1.52 | 0.92 to 2.51 | 0.103 | 1.24 | 0.84 to 1.82 | 0.287 | |
| Middle | 1.11 | 0.66 to 1.86 | 0.702 | 1.38 | 0.97 to 1.98 | 0.076 | |
| Richer | 1.81 | 1.08 to 3.04 | 0.024 | 1.43 | 0.97 to 2.11 | 0.068 | |
| Richest | 2 | 1.16 to 3.43 | 0.012 | 1.42 | 0.94 to 2.12 | 0.092 | |
| Maternal education and literacy | No education | Ref | Ref | ||||
| Primary education/illiterate | 2 | 1.05 to 3.81 | 0.036 | 1.34 | 0.84 to 2.15 | 0.215 | |
| Primary education/literate | 2.34 | 1.46 to 3.73 | <0.001 | 1.52 | 1.09 to 2.12 | 0.014 | |
| Secondary or higher | 2.25 | 1.25 to 4.05 | 0.007 | 1.23 | 0.8 to 1.9 | 0.352 | |
| Marital status | Married or cohabiting | Ref | Ref | ||||
| Not married or cohabiting | 1.24 | 0.87 to 1.78 | 0.234 | 1.23 | 0.91 to 1.66 | 0.177 | |
| Maternal age group (in years) | <20 | 1.53 | 1.02 to 2.3 | 0.041 | 1.5 | 1.08 to 2.1 | 0.017 |
| 20–29 | Ref | Ref | |||||
| 30–49 | 1.08 | 0.71 to 1.65 | 0.704 | 1.09 | 0.81 to 1.44 | 0.571 | |
| Maternal decision-making about health | Self (fully or partly) | Ref | Ref | ||||
| Others | 1.02 | 0.68 to 1.54 | 0.918 | 1.15 | 0.85 to 1.56 | 0.366 | |
| Maternal relocation (<5 years lived in current residence) | Yes | 1.15 | 0.78 to 1.7 | 0.468 | 1.07 | 0.8 to 1.43 | 0.663 |
| No | Ref | Ref | |||||
| Maternal anaemia at survey | Yes | 1.36 | 1 to 1.84 | 0.049 | 1.35 | 1.07 to 1.7 | 0.011 |
| No | Ref | Ref | |||||
| Maternal mobile ownership | Yes | 1.24 | 0.9 to 1.72 | 0.187 | 1.06 | 0.83 to 1.35 | 0.632 |
| No | Ref | Ref | |||||
| Ownership of health insurance | Yes | 1.41 | 0.78 to 2.53 | 0.258 | 1.15 | 0.68 to 1.94 | 0.591 |
| No | Ref | Ref | |||||
| Travel to nearest hospital (hours) | 0.92 | 0.75 to 1.13 | 0.425 | 0.97 | 0.86 to 1.1 | 0.675 | |
| Number of household members | 0.9 | 0.83 to 0.97 | 0.005 | 0.92 | 0.87 to 0.97 | 0.002 | |
| Mode of delivery | Vaginal | Ref | |||||
| Caesarean | 2.22 | 1.3 to 3.79 | 0.003 | ||||
| Multiple birth | Yes | 5.4 | 3.08 to 9.45 | <0.001 | |||
| No | Ref | ||||||
| Birth order and preceding birth interval (months) | First child | 2.1 | 1.38 to 3.16 | <0.001 | |||
| Second/third; <24 | 1.25 | 0.57 to 2.77 | 0.575 | ||||
| Second/third; 24+ | Ref | ||||||
| Fourth+; <24 | 1.72 | 0.96 to 3.05 | 0.066 | ||||
| Fourth+; 24+ | 1.22 | 0.76 to 1.96 | 0.403 | ||||
| Sex of child | Male | 1.41 | 1.02 to 1.95 | 0.04 | |||
| Female | Ref | ||||||
| Pregnancy wanted at the time | Yes | Ref | |||||
| No | 0.78 | 0.55 to 1.13 | 0.187 | ||||
| Place of birth | Home | Ref | |||||
| Lower-level facility | 1.3 | 0.87 to 1.93 | 0.198 | ||||
| Hospital | 1.76 | 1.19 to 2.59 | 0.005 | ||||
| n=6099 (most recent live births) | |||||||
| Antenatal care during pregnancy | No ANC | 3.32 | 1.14 to 9.64 | 0.028 | |||
| 1–3 visits | 1.13 | 0.73 to 1.76 | 0.587 | ||||
| 4 or more visits | Ref | ||||||
| Child weighed at birth | Yes | 0.76 | 0.48 to 1.22 | 0.255 | |||
| No | Ref | ||||||
| n=4050 (most recent live births with weight available) | |||||||
| Child's birth weight category (in grams) | Low (<2500 g) | 5.34 | 2.62 to 10.88 | <0.001 | |||
| Normal (2500–4000 g) | Ref | ||||||
| Macrosomia (>4000 g) | 2.77 | 1.12 to 6.88 | 0.028 | ||||
Grey shading—variable not available for all observations.
ANC, antenatal care.
Multivariable analysis
Table 5 shows the results of the four multivariable models. Overall, these models show that the adjusted odds of neonatal death in core urban areas was between 26% and 136% higher, and in semi-urban areas 26%–77% higher compared with rural areas. The adjusted odds of perinatal death in core urban areas were 71% higher and in semi-urban areas 8% higher compared with rural areas. The direction of association was consistent across the four models, but in none of them was it significant at the p<0.05 level.
Table 5.
Multivariable associations with neonatal death and perinatal death (four models)
| Model | 1 | 2 | 3 | 4 | ||||||||
| Outcome | Neonatal death (n=217) | Neonatal death (n=97) | Neonatal death (n=60) | Perinatal death (n=356) | ||||||||
| Sample | All live births (n=8739) | Most recent live births (n=6099) | Most recent live births with birth weight (n=4050) | All births (n=8915) | ||||||||
| aOR | 95% CI | P value | aOR | 95% CI | P value | aOR | 95% CI | P value | aOR | 95% CI | P value | |
| Urbanicity class | ||||||||||||
| Core urban | 1.26 | 0.57 to 2.79 | 0.568 | 1.52 | 0.3 to 7.75 | 0.615 | 2.36 | 0.69 to 8.04 | 0.17 | 1.71 | 0.89 to 3.27 | 0.106 |
| Semi-urban | 1.26 | 0.79 to 2.01 | 0.323 | 1.77 | 0.71 to 4.4 | 0.22 | 1.66 | 0.74 to 3.77 | 0.222 | 1.08 | 0.77 to 1.53 | 0.653 |
| Rural | Ref | Ref | Ref | Ref | ||||||||
| Geographical zone | ||||||||||||
| Western | 1.51 | 0.71 to 3.21 | 0.287 | 0.82 | 0.19 to 3.6 | 0.789 | 1.5 | 0.39 to 5.75 | 0.557 | 1.04 | 0.61 to 1.75 | 0.895 |
| Lake | Ref | Ref | Ref | Ref | ||||||||
| Northern | 0.78 | 0.33 to 1.82 | 0.566 | 0.64 | 0.15 to 2.72 | 0.542 | 1.43 | 0.42 to 4.83 | 0.566 | 0.74 | 0.41 to 1.34 | 0.322 |
| Central | 1.41 | 0.67 to 3.01 | 0.362 | 1.12 | 0.29 to 4.3 | 0.874 | 2.62 | 0.82 to 8.4 | 0.105 | 1.06 | 0.63 to 1.8 | 0.826 |
| Southwest highlands | 1.4 | 0.65 to 3.06 | 0.39 | 0.83 | 0.2 to 3.42 | 0.799 | 1.04 | 0.27 to 4.02 | 0.95 | 0.96 | 0.55 to 1.67 | 0.876 |
| Southern highlands | 1.41 | 0.59 to 3.39 | 0.439 | 0.61 | 0.12 to 3.05 | 0.549 | 0.86 | 0.2 to 3.57 | 0.831 | 1.02 | 0.53 to 1.95 | 0.95 |
| Southern | 2.2 | 0.88 to 5.51 | 0.094 | 1.81 | 0.38 to 8.68 | 0.455 | 0.83 | 0.18 to 3.89 | 0.817 | 2.21 | 1.2 to 4.07 | 0.011 |
| Eastern | 1.57 | 0.75 to 3.28 | 0.227 | 1.08 | 0.28 to 4.13 | 0.915 | 1.49 | 0.49 to 4.53 | 0.481 | 1.16 | 0.7 to 1.94 | 0.564 |
| Household wealth quintile | ||||||||||||
| Poorest | Ref | Ref | Ref | |||||||||
| Poorer | 1.76 | 0.5 to 6.17 | 0.378 | 1.3 | 0.4 to 4.26 | 0.667 | 1.21 | 0.76 to 1.91 | 0.417 | |||
| Middle | 2.29 | 0.65 to 8.07 | 0.196 | 1.97 | 0.62 to 6.27 | 0.253 | 1.45 | 0.91 to 2.31 | 0.118 | |||
| Richer | 1.33 | 0.33 to 5.32 | 0.685 | 1.53 | 0.47 to 5.08 | 0.481 | 1.24 | 0.75 to 2.05 | 0.411 | |||
| Richest | 1.37 | 0.26 to 7.34 | 0.71 | 1.22 | 0.31 to 4.81 | 0.779 | 0.93 | 0.49 to 1.75 | 0.818 | |||
| Maternal education and literacy | ||||||||||||
| No education | Ref | Ref | Ref | |||||||||
| Primary education/illiterate | 1.81 | 0.8 to 4.1 | 0.154 | 4.33 | 0.99 to 19.02 | 0.052 | 3.91 | 1.06 to 14.48 | 0.041 | |||
| Primary education/literate | 1.95 | 1.06 to 3.6 | 0.032 | 2.91 | 0.9 to 9.46 | 0.075 | 3.84 | 1.26 to 11.78 | 0.018 | |||
| Secondary or higher | 1.43 | 0.63 to 3.25 | 0.393 | 1.69 | 0.36 to 8.01 | 0.506 | 4.06 | 1.03 to 16.01 | 0.045 | |||
| Maternal age group (in years) | ||||||||||||
| <20 | 1.44 | 0.8 to 2.61 | 0.224 | 3.69 | 1.37 to 9.95 | 0.01 | 1.76 | 0.67 to 4.66 | 0.253 | 1.76 | 1.23 to 2.5 | 0.002 |
| 20–29 | Ref | Ref | Ref | Ref | ||||||||
| 30–49 | 1.23 | 0.73 to 2.1 | 0.436 | 3.4 | 1.46 to 7.96 | 0.005 | 1.18 | 0.6 to 2.35 | 0.63 | 1.27 | 0.92 to 1.75 | 0.149 |
| Maternal anaemia at survey | ||||||||||||
| Yes | 1.48 | 0.99 to 2.21 | 0.058 | 1.44 | 1.07 to 1.92 | 0.015 | ||||||
| No | Ref | Ref | ||||||||||
| Maternal mobile ownership | ||||||||||||
| Yes | 0.65 | 0.34 to 1.21 | 0.174 | |||||||||
| No | Ref | |||||||||||
| No. of household members | 0.89 | 0.83 to 0.96 | 0.002 | 0.79 | 0.68 to 0.92 | 0.002 | 0.8 | 0.7 to 0.9 | <0.001 | 0.92 | 0.88 to 0.96 | <0.001 |
| Mode of delivery | ||||||||||||
| Vaginal | Ref | Ref | ||||||||||
| Caesarean | 1.7 | 0.87 to 3.36 | 0.123 | 2.39 | 0.71 to 8.07 | 0.161 | ||||||
| Multiple birth | ||||||||||||
| Yes | 14.97 | 7.25 to 30.89 | <0.001 | 11.15 | 1.98 to 62.67 | 0.006 | ||||||
| No | Ref | Ref | ||||||||||
| Birth order and preceding birth interval (months) | ||||||||||||
| First child | 2.15 | 1.21 to 3.85 | 0.01 | 0.43 | 0.18 to 1.05 | 0.064 | ||||||
| Second/third; <24 | 0.57 | 0.24 to 1.37 | 0.207 | 0.29 | 0.04 to 2.2 | 0.23 | ||||||
| Second/third; 24+ | Ref | Ref | ||||||||||
| Fourth+; <24 | 2.11 | 0.97 to 4.58 | 0.061 | 3.48 | 1.07 to 11.24 | 0.037 | ||||||
| Fourth+; 24+ | 1.18 | 0.64 to 2.16 | 0.595 | 1.87 | 0.85 to 4.12 | 0.119 | ||||||
| Sex of child | ||||||||||||
| Male | 1.91 | 1.32 to 2.75 | 0.001 | 2.32 | 1.32 to 4.06 | 0.003 | ||||||
| Female | Ref | Ref | ||||||||||
| Place of birth | ||||||||||||
| Home | Ref | Ref | Ref | |||||||||
| Lower-level facility | 1.03 | 0.63 to 1.69 | 0.898 | 1.37 | 0.51 to 3.67 | 0.527 | 6.08 | 0.54 to 68.34 | 0.144 | |||
| Hospital | 1.25 | 0.74 to 2.12 | 0.41 | 2.09 | 0.75 to 5.84 | 0.159 | 8.2 | 0.73 to 91.92 | 0.088 | |||
| Pregnancy wanted at the time | ||||||||||||
| Yes | Ref | Ref | ||||||||||
| No | 0.56 | 0.26 to 1.22 | 0.144 | 0.77 | 0.42 to 1.42 | 0.408 | ||||||
| Antenatal care during pregnancy | ||||||||||||
| No ANC | 25.65 | 4.02 to 163.33 | 0.001 | 3.13 | 0.33 to 29.65 | 0.319 | ||||||
| 1–3 visits | 1.36 | 0.66 to 2.79 | 0.406 | 1.87 | 1.08 to 3.26 | 0.026 | ||||||
| 4 or more visits | Ref | Ref | ||||||||||
| Child's birth weight category | ||||||||||||
| Low (<2500 g) | 9.69 | 4.45 to 21.11 | <0.001 | |||||||||
| Normal (2500–4000 g) | Ref | |||||||||||
| Macrosomia (>4000 g) | 3.79 | 1.62 to 8.89 | 0.002 | |||||||||
| Random effects | ||||||||||||
| Household and cluster (variance and SE) | 5.32 (0.88) | 11.62 (3.08) | 2.04 (0.77)* | 3.71 (0.52) | ||||||||
Variable not included.
Variable not available for all observations.
*Model 3 frandom effects included clusters only as due to small sample size of outcomes, the model with both cluster and household did not converge.
ANC, antenatal care ; aOR, adjusted OR.
Discussion
We found a consistent pattern of higher odds of neonatal and perinatal death with increasing levels of urbanicity in mainland Tanzania, which was particularly pronounced in densely populated core urban areas. The category of semi-urban areas had levels of neonatal and perinatal mortality similar to rural areas. However, the multivariable associations were not significant at the p<0.05 level, most likely due to a small sample size of neonatal and perinatal deaths. Taken together with previous studies,6 these findings bolster our confidence in the evidence showing an association between higher levels of urbanicity and higher neonatal and perinatal mortality.
In terms of the exposure, satellite imagery-based urbanicity categories captured the meaning of urbanicity more accurately than the DHS urban/rural residence. The most important cause of misclassification between the two methods was that some clusters considered urban by DHS were rural according to GHS-SMOD. Much of the existing research frames urban areas as a monolith, but urban areas are not homogenous, and most studies are not able to differentiate between peri-urban and suburban areas, areas of informal settlements, urban slums or affluent parts of cities and ignore variations within a single city. There is no uniform definition of an urban area. The DHS relies on the country’s definition of urban/rural which is variable between countries and across time. Statistical offices across countries use population thresholds of a settlement or a combination of population size and the proportion of residents employed in agriculture to define an urban area.26 Specifically in Tanzania, the definition of urban areas is based on all regional and district headquarters and wards with urban characteristics.27 Urban wards have above a specified population density and/or a certain percentage of residents in non-agricultural occupations. Consequently, many studies rely on categorisations of urbanicity based on national administrative definitions that are not always an accurate reflection of reality. This is due partly to (1) lack of use of standard criteria, (2) lack of re-evaluation and recategorisation of areas over time and (3) the possible political influence on the categorisation (eg, redefining an area as urban may trigger different requirements regarding government allocation of resources or infrastructure).28 29
We discuss several findings from our study to expound potential mechanisms underlying this association between urbanicity and neonatal and perinatal mortality. A recent paper on the reasons for loss of urban mortality advantage among adults (15–49 year olds) from multiple DHS surveys using the urban/rural stratification noted that rapid expansion of population in slums has led to premature mortality linked to overcrowding, poverty, road traffic accidents, lack of sanitation and the double burden of malnutrition leading to non-communicable diseases in this population.5 On the other hand, they described an urban advantage in child survival, which they attributed in part to better access to healthcare, better infrastructure, greater economic opportunities and other factors such as lower fertility levels and longer birth intervals. Causes of stillbirths and deaths in the neonatal period are a combination of the various factors affecting the health of adults in general and pregnant women in particular (eg, maternal nutrition, exposure to infections such as sexually-transmitted infections and malaria, occupational hazards, exposure to heat and pollution), as well as that of children (access to healthcare and the quality of that care, particularly at time of labour and birth).
Multiple causal pathways for the effect of urban residence on neonatal survival have been proposed, including individual health-seeking behaviour/accessibility of care, obstetrical risk factors, quality of care during pregnancy, childbirth and the postnatal period, as well as broader issues related to socioeconomic determinants, urban living conditions and urbanisation processes.6 Our multivariable models included variables capturing all these four dimensions. While some of these were significantly associated with neonatal and perinatal mortality, their inclusion did not completely explain the association between urbanicity and neonatal/perinatal mortality. We highlight several findings which could inform future analyses to explore the causal pathways in more depth.
Issues linked to access to care and care quality in urban areas are numerous. The use of ANC and facility-based childbirth care in large cities in Africa is near-universal (>94% in Dar es Salaam), but characterised by high levels of private sector use and inconsistent receipt of evidence-based interventions.30 The analysis of 22 large African cities also showed variable levels of essential care elements (>99% of babies born in health facilities were weighed but only half of babies initiated breast feeding within an hour of birth) and high levels of early discharge from health facilities following both vaginal and caesarean section births in Dar es Salaam. Further, literature shows that poor women, especially those living in informal settlements, might also receive poorer quality of care, encounter stigmatising attitudes and disrespectful care in health facilities.31–33 Our additional analysis on the timing of deaths showed that a comparatively low percentage of neonatal deaths in core urban areas occurred on the day of birth compared with semi-urban and rural areas. Interpretation is difficult, but one explanation may be better access to emergency obstetrical care including neonatal resuscitation in core urban compared with rural areas.34 Some resuscitated babies may still die a few days later because of underlying conditions due to complications of preterm birth, infections and late complications from asphyxia.
The high degree of under-reporting of stillbirths in the core urban area points to potential misclassification of stillbirths as neonatal deaths or general under-reporting of stillbirths in these contexts. Misclassification in household surveys has been reported in several studies.10 35–37 That this pattern appears largely confined to urban areas in our study warrants further investigation. We would expect better differentiation between these outcomes in an urban setting where higher quality services and more skilled personnel are available. Another contributing factor could be the impact of recent training on neonatal resuscitation in several health facilities in these areas which may be improving the survival of babies thus leading to fewer stillbirths. The three models looking at neonatal mortality showed a consistent association between higher levels of education and higher neonatal mortality. This is unlikely to be a result of confounding by older maternal age (which is linked to poorer perinatal survival38) because age was included in the multivariable models. One possible explanation is that the extent of under-reporting of neonatal deaths is higher among women with no education because of stigma,39 thus artificially increasing the odds of mortality among those with higher levels of education.
Higher number of household members was consistently and significantly associated with lower adjusted odds of neonatal and perinatal mortality. We estimate that for every additional household member, the odds of neonatal and perinatal mortality declined by approximately 10%. This points to the importance of familial support including advocating and enabling timely care-seeking (eg, by recognising danger signs, providing childcare during woman’s absence or assisting during travel), help within the household and with enabling positive behaviours such as self-care and breast feeding.40 The availability of such support is likely lower for women residing in urban areas. In addition, we identified several known biological risk factors which are linked to increased neonatal and perinatal mortality in the absence of accessible, high-quality care. These include young and older maternal age, maternal anaemia, male sex of newborn, multiplicity, first birth and birth after a short birth interval. It is possible that the manner in which these known and yet unknown risk factors operate is different in densely populated urban settings compared with rural areas. While the sample size available on the DHS did not allow us to test for interactions, we note that improving access to good quality care both during pregnancy and at the time of birth is essential for preventing neonatal and perinatal deaths.
Strengths and limitations
Our in-depth analysis of the association between urban residence and neonatal and perinatal mortality addressed several critical limitations of previous studies. We were able to more accurately classify the gradient of urbanicity41 based on data that incorporates satellite imagery, built environment and population density, rather than on administrative delineation. By disaggregating urbanicity to core urban and semi-urban we more accurately captured the variation in human settlement on a continuum and exposed any dose-response associations. However, our indicator of urbanicity has limitations, including grouping affluent parts together with slums or informal settlements in core urban areas. The alternative could have been to use a composite measure combining wealth quintile and urbanicity to construct a fourth category referring to slums and informal settlements—proxied by the poorest quintiles living in core urban areas slums.42 However, sample size constraints of the main outcomes made this approach unfeasible.
By including the two outcomes of neonatal mortality and perinatal mortality, we addressed some misclassifications between stillbirth and neonatal deaths. However, our data indicate that neonatal and perinatal deaths are under-reported in these survey self-reports, in view of the implausible higher neonatal mortality in better educated and more wealthy groups. Many key variables on pregnancy and birth, such as place and mode of birth, were not available for pregnancies resulting in stillbirths.43 Also, other key confounders were not available, meaning that none of the four models were theoretically complete.
Limitations also exist in several other variables. Travel time was based on the nearest public hospital, whereas in reality, women often bypass the nearest facility.44 45 Further, we made assumptions about travel speed, which may not hold true in all places and might have a larger margin of error within cities due to, for example, variability in traffic and weather, and waiting time.46 However, this was necessary due to lack of observational data.47 The exact location of the household of residence for each woman is obscured by provision of one cluster location and by cluster displacement in DHS due to reasons of anonymity. We tried to ameliorate this by including some cluster level variables which would tell us about the lived environment of the ‘neighbourhood’. Additionally, we did not have a variable accounting for daily mobility of people in and out of urban areas during the day due to lack of such data.
Our Model 3 did not have fixed effects for households, instead additional key variables (birth weight and number of ANC visits) captured some differences between the households that were being captured by the random effect. The data excludes babies born to women who subsequently died themselves. Finally, even though the DHS is a nationally representative survey and the number of women interviewed had increased in recent years, the sample size of neonatal deaths and stillbirths was relatively small. The limited sample size could be one reason why we did not detect a significant association between urbanicity and mortality in the multivariable results.
Conclusion
In our advanced analysis which improved the accuracy of the exposure variable (urbanicity), reduced reporting bias in outcome (by adding stillbirths) and adjusted for confounding and clustering more completely, we found moderate evidence of higher neonatal and perinatal mortality in semi-urban and particularly in core urban areas compared with rural areas in mainland Tanzania. The effect seemed to follow a dose-response pattern with increasing extent of urbanicity. This is consistent with earlier findings, and might extend to other countries with slower neonatal mortality declines in urban areas. Our multivariable analysis aimed to provide an in-depth understanding of the mechanisms of this association, however, we appreciate that many questions are still unanswered due to the data limitations. Therefore, we call for collection and analysis of more granular primary data to disentangle the contribution of pregnancy factors, living conditions and quality of care in birthing facilities.
Addressing the high rates of mortality in urban areas is also critical for Tanzania to meet the Sustainable Development Goals target on reduction of NMR to less than 16/1000 live births by 2030. Focusing solely or predominantly on rural areas is unlikely to tackle the high and largely preventable neonatal and perinatal mortality identified in urban areas, whether in the core, densely populated urban centres and particularly informal settlements or the growing semi-urban areas around Tanzania’s main and secondary cities and towns. In order to appropriately target interventions, we must rely on more up-to-date, accurate and granular capture of urbanicity, which is possible through using innovative satellite technologies and spatial epidemiology approaches. We call for better data allowing disaggregation’s into neighbourhoods of slums and informal settlements to ascertain whether across communities the ‘urban’ category is masking heterogeneities.
Acknowledgments
We would like to thank the Demographic and Health Survey (DHS) team, the survey enumerators and the women who contributed information about their lives. We also acknowledge discussions with Dr Ulrika Baker (UNICEF Tanzania) and with Cameron Taylor on DHS measurement of urban/rural residence.
Footnotes
Handling editor: Seye Abimbola
Twitter: @Pete_M_M, @lenkabenova, @alichristou
Contributors: Conceptualisation: PMM, LB, CH, JP. Investigation: PMM, LB, JP, AS, AC, AP, CH. Data curation: PMM, JP, LB. Formal analysis: PMM, LB. Visualisation: PMM, LB, AS. Writing—Original draft preparation: PMM, LB, JP, AS, AC, CH. Writing—Review and editing: PMM, LB, JP, AS, AC, AP, CH.
Funding: LB was funded in part by the Research Foundation – Flanders (Fonds Wetenschappelijk Onderzoek) as part of her Senior Postdoctoral Fellowship (award number 1234820N). PMM was supported by ITM’s EWI People Program. PMM also acknowledges the Royal Society Newton International Fellowship (NIF/R1/201418) and the Wellcome Trust support to the Kenya Major Overseas Programme (#203077). AC is funded by the Research Foundation – Flanders (Fonds Wetenschappelijk Onderzoek) as part of a Junior Postdoctoral Fellowship (award number 1294322N).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographical or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Data may be obtained from a third party and are not publicly available. DHS datasets are available from the DHS programme upon request at https://dhsprogram.com/data/available-datasets.cfm. The health facility database used to compute travel time in Tanzania is available at https://doi.org/10.6084/m9.figshare.c.4399445 while the urbanicity surfaces can be accessed at 10.2905/42E8BE89-54FF-464E-BE7B-BF9E64DA5218
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The DHS received government permission and followed ethical practices including informed consent and assurance of confidentiality. Permission to study this data set for secondary data analysis was approved by the DHS Program. We did not require separate ethics approval to analyse these secondary data sets. Participants gave informed consent to participate in the study before taking part.
References
- 1.Saghir J, Santoro J. Urbanization in Sub-Saharan Africa. Washington, DC: CSIC.ORG, 2018. Available: http://thegreentimes.co.za/wp-content/uploads/2019/03/Urbanization-in-Sub-Saharan-Africa.pdf [Google Scholar]
- 2.Knudsen C, Moreno E, Arimah B, et al. The value of sustainable urbanization: United Nations human settlements programme. 2020. Available: https://unhabitat.org/World%20Cities%20Report%202020
- 3.Matthews Z, Channon A, Neal S, et al. Examining the “urban advantage” in maternal health care in developing countries. PLOS Med 2010;7:e1000327. 10.1371/journal.pmed.1000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinchoff J, Mills CW, Balk D. Urbanization and health: the effects of the built environment on chronic disease risk factors among women in Tanzania. PLOS ONE 2020;15:e0241810. 10.1371/journal.pone.0241810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menashe-Oren A, Masquelier B. The shifting rural-urban gap in mortality over the life course in low- and middle-income countries. Popul Stud (Camb) 2022;76:37–61. 10.1080/00324728.2021.2020326 [DOI] [PubMed] [Google Scholar]
- 6.Norris M, Klabbers G, Pembe AB, et al. A growing disadvantage of being born in an urban area? analysing urban-rural disparities in neonatal mortality in 21 African countries with a focus on Tanzania. BMJ Glob Health 2022;7:e007544. 10.1136/bmjgh-2021-007544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hug L, You D, Blencowe H, et al. Global, regional, and national estimates and trends in stillbirths from 2000 to 2019: a systematic assessment. Lancet 2021;398:772–85. 10.1016/S0140-6736(21)01112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United Nations Department of Economic and Social Affairs . World social report: United Nations department of economic and social affairs. 2020. 10.18356/7f5d0efc-en
- 9.Dorélien AM, Balk D, Todd M. What is urban? Comparing a satellite view with the demographic and health surveys. Popul Dev Rev 2013;39:413–39. 10.1111/j.1728-4457.2013.00610.x [DOI] [Google Scholar]
- 10.Blencowe H, Bottecchia M, Kwesiga D, et al. Stillbirth outcome capture and classification in population-based surveys: EN-INDEPTH study. Popul Health Metr 2021;19:13. 10.1186/s12963-020-00239-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgert CR, Colston J, Roy T, et al. Geographic displacement procedure and georeferenced data release policy for the demographic and health surveys. DHS spatial analysis reports no.7. Calverton, Maryland, USA: ICF International, 2013. [Google Scholar]
- 12.Ministry of Health CDGE, Children - Mo HTM, Ministry of Health - Mo HZ, et al . Tanzania demographic and health survey and malaria indicator survey 2015-2016. Dar es Salaam, Tanzania: MoHCDGEC, MoH, NBS, OCGS, and ICF, 2016. Available: http://dhsprogram.com/pubs/pdf/FR321/FR321.pdf [Google Scholar]
- 13.The DHS Program Code Library . DHS indicators – stata – code to compute perinatal mortality 2021. 2021. Available: https://github.com/DHSProgram/DHS-Indicators-Stata/blob/master/Chap08_CM/CM_PMR.do
- 14.The DHS Program . DHS contraceptive calendar tutorial. n.d. Available: https://dhsprogram.com/data/Calendar-Tutorial/index.cfm
- 15.Pesaresi M, Florczyk A, Schiavina M, et al. GHS settlement grid, updated and refined REGIO model 2014 in application to GHS-BUILT R2018A and GHS-POP R2019A, multitemporal (1975-1990-2000-2015), R2019A 2019. 2019. 10.2905/42E8BE89-54FF-464E-BE7B-BF9E64DA5218 [DOI]
- 16.Florczyk A, Corbane C, Ehrlich D, et al. GHSL data package 2019, EUR 29788 EN, ISBN 978-92-76-13186-1. Luxembourg: Publications Office of the European Union, 2019. 10.2760/290498 [DOI] [Google Scholar]
- 17.Banke-Thomas A, Wong KLM, Collins L, et al. An assessment of geographical access and factors influencing travel time to emergency obstetric care in the urban state of Lagos, Nigeria. Health Policy Plan 2021;36:1384–96. 10.1093/heapol/czab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray N, Ebener S. AccessMod 3.0: computing geographic coverage and accessibility to health care services using anisotropic movement of patients. Int J Health Geogr 2008;7:63. 10.1186/1476-072X-7-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouma PO, Maina J, Thuranira PN, et al. Access to emergency hospital care provided by the public sector in sub-saharan africa in 2015: a geocoded inventory and spatial analysis. Lancet Glob Health 2018;6:e342–50. 10.1016/S2214-109X(17)30488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okwaraji YB, Mulholland K, Schellenberg JRMA, et al. The association between travel time to health facilities and childhood vaccine coverage in rural Ethiopia. A community based cross sectional study. BMC Public Health 2012;12:476. 10.1186/1471-2458-12-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anichukwu OI, Asamoah BO. The impact of maternal health care utilisation on routine immunisation coverage of children in Nigeria: a cross-sectional study. BMJ Open 2019;9:e026324. 10.1136/bmjopen-2018-026324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph NK, Macharia PM, Ouma PO, et al. Spatial access inequities and childhood immunisation uptake in Kenya. BMC Public Health 2020;20:1407. 10.1186/s12889-020-09486-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruktanonchai CW, Ruktanonchai NW, Nove A, et al. Equality in maternal and newborn health: modelling geographic disparities in utilisation of care in five East African countries. PLoS ONE 2016;11:e0162006. 10.1371/journal.pone.0162006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masters SH, Burstein R, Amofah G, et al. Travel time to maternity care and its effect on utilization in rural Ghana: a multilevel analysis. Social Science & Medicine 2013;93:147–54. 10.1016/j.socscimed.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 25.Bradley SEK, William W, Trevor N. C. Contraceptive use and perinatal mortality in the DHS: an assessment of the quality and consistency of calendars and histories. DHS methodological reports no.17. Rockville, Maryland, USA: ICF International, 2015. [Google Scholar]
- 26.Rutstein SO, Staveteig S, Winter R, et al. Urban child poverty, health, and survival in low- and middle-income countries Rockville. Rockville, Maryland, USA: ICF International, 2016. Available: http://dhsprogram.com/pubs/pdf/CR40/CR40.pdf [Google Scholar]
- 27.Fish TD, Bradley J, Trinadh D, et al. Predicting geospatial covariates: proxies for mapping urban-related indicators. DHS spatial analysis reports no.19. Rockville, Maryland, USA, 2020. Available: https://www.dhsprogram.com/pubs/pdf/SAR19/SAR19.pdf [Google Scholar]
- 28.Lewis Dijkstra L, Ellen Hamilton E, Lall S, et al. How do we define cities, towns, and rural areas? Washington, DC: World Bank Group, 2020. Available: https://blogsworldbankorg/sustainablecities/how-do-we-define-cities-towns-and-rural-areas [Google Scholar]
- 29.Balk D, Leyk S, Jones B, et al. Understanding urbanization: a study of census and satellite-derived urban classes in the United States, 1990-2010. PLOS ONE 2018;13:e0208487. 10.1371/journal.pone.0208487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong KL, Banke-Thomas A, Sholkamy H, et al. Tale of 22 cities: utilisation patterns and content of maternal care in large African cities. BMJ Glob Health 2022;7:e007803. 10.1136/bmjgh-2021-007803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNab S, Scudder E, Syed U, et al. Maternal and newborn health for the urban poor: the need for a new mental model and implementation strategies to accelerate progress. Global Health 2022;18:46. 10.1186/s12992-022-00830-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayou YT, Mashalla YS, Thupayagale-Tshweneagae G. The adequacy of antenatal care services among slum residents in Addis ababa, Ethiopia. BMC Pregnancy Childbirth 2016;16:142. 10.1186/s12884-016-0930-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jolly SP, Rahman M, Afsana K, et al. Evaluation of maternal health service indicators in urban slum of Bangladesh. PLOS ONE 2016;11:e0162825. 10.1371/journal.pone.0162825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Msemo G, Massawe A, Mmbando D, et al. Newborn mortality and fresh stillbirth rates in Tanzania after helping babies breathe training. Pediatrics 2013;131:e353–60. 10.1542/peds.2012-1795 [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Kalter HD, Chu Y, et al. Understanding misclassification between neonatal deaths and stillbirths: empirical evidence from malawi. PLOS ONE 2016;11:e0168743. 10.1371/journal.pone.0168743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helleringer S, Liu L, Chu Y, et al. Biases in survey estimates of neonatal mortality: results from a validation study in urban areas of Guinea-Bissau. Demography 2020;57:1705–26. 10.1007/s13524-020-00911-6 [DOI] [PubMed] [Google Scholar]
- 37.Woods CR, Davis DW, Duncan SD, et al. Variation in classification of live birth with newborn period death versus fetal death at the local level may impact reported infant mortality rate. BMC Pediatr 2014;14:108. 10.1186/1471-2431-14-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One 2017;12:e0186287. 10.1371/journal.pone.0186287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haws RA, Mashasi I, Mrisho M, et al. These are not good things for other people to know: how rural Tanzanian women’s experiences of pregnancy loss and early neonatal death may impact survey data quality. Soc Sci Med 2010;71:1764–72. 10.1016/j.socscimed.2010.03.051 [DOI] [PubMed] [Google Scholar]
- 40.Ogallo W, Speakman S, Akinwande V, et al. Identifying factors associated with neonatal mortality in Sub-Saharan Africa using machine learning. AMIA Annu Symp Proc 2020;2020:963–72. [PMC free article] [PubMed] [Google Scholar]
- 41.Dorélien A, Balk D, Todd M. What is urban? Comparing a satellite view with the demographic and health surveys. Popul Dev Rev 2013;39:413–39. 10.1111/j.1728-4457.2013.00610.x [DOI] [Google Scholar]
- 42.Assaf S, Riese S, Sauter S. Urban poverty and child health indicators in six african countries with DHS data. DHS analytical studies. no 81. Rockville, MD, USA: ICF, 2022. [Google Scholar]
- 43.Christou A, Dibley MJ, Raynes-Greenow C. Beyond counting stillbirths to understanding their determinants in low- and middle-income countries: a systematic assessment of stillbirth data availability in household surveys. Trop Med Int Health 2017;22:294–311. 10.1111/tmi.12828 [DOI] [PubMed] [Google Scholar]
- 44.Bezu S, Binyaruka P, Mæstad O, et al. Pay-for-performance reduces bypassing of health facilities: evidence from Tanzania. Soc Sci Med 2021;268:113551. 10.1016/j.socscimed.2020.113551 [DOI] [PubMed] [Google Scholar]
- 45.Clarke-Deelder E, Afriyie DO, Nseluke M, et al. Health care seeking in modern urban LMIC settings: evidence from Lusaka, Zambia. BMC Public Health 2022;22:1205. 10.1186/s12889-022-13549-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banke-Thomas A, Macharia PM, Makanga PT, et al. Leveraging big data for improving the estimation of close to reality travel time to obstetric emergency services in urban low- and middle-income settings. Front Public Health 2022;10:931401. 10.3389/fpubh.2022.931401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macharia PM, Ray N, Giorgi E, et al. Defining service catchment areas in low-resource settings. BMJ Glob Health 2021;6:e006381. 10.1136/bmjgh-2021-006381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2022-011253supp001.pdf (761.9KB, pdf)
bmjgh-2022-011253supp002.pdf (32.3KB, pdf)
bmjgh-2022-011253supp003.pdf (33.6KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Data may be obtained from a third party and are not publicly available. DHS datasets are available from the DHS programme upon request at https://dhsprogram.com/data/available-datasets.cfm. The health facility database used to compute travel time in Tanzania is available at https://doi.org/10.6084/m9.figshare.c.4399445 while the urbanicity surfaces can be accessed at 10.2905/42E8BE89-54FF-464E-BE7B-BF9E64DA5218