Abstract
Objectives
The aim of this systematic overview of reviews was to synthesise available evidence on inequalities in infectious disease based on three dimensions of inequalities; inclusion health groups, protected characteristics and socioeconomic inequalities.
Methods
We searched MEDLINE, Embase, Web of Science and OpenGrey databases in November 2021. We included reviews published from the year 2000 which examined inequalities in the incidence, prevalence or consequences of infectious diseases based on the dimensions of interest. Our search focused on tuberculosis, HIV, sexually transmitted infections, hepatitis C, vaccination and antimicrobial resistance. However, we also included eligible reviews of any other infectious diseases. We appraised the quality of reviews using the Assessment of Multiple Systematic Reviews V.2 (AMSTAR2) checklist. We conducted a narrative data synthesis.
Results
We included 108 reviews in our synthesis covering all the dimensions of inequalities for most of the infectious disease topics of interest, however the quality and volume of review evidence and consistency of their findings varied. The existing literature reviews provide strong evidence that people in inclusion health groups and lower socioeconomic status are consistently at higher risk of infectious diseases, antimicrobial resistance and incomplete/delayed vaccination. In the protected characteristics dimension, ethnicity, and sexual orientation are important factors contributing to inequalities across the various infectious disease topics included in this overview of reviews.
Conclusion
We identified many reviews that provide evidence of various types of health inequalities in different infectious diseases, vaccination, and antimicrobial resistance. We also highlight areas where reviews may be lacking. The commonalities in the associations and their directions suggest it might be worth targeting interventions for some high risk-groups that may have benefits across multiple infectious disease outcomes rather than operating purely in infectious disease siloes.
Keywords: Public health, INFECTIOUS DISEASES, Epidemiology
Strengths and limitations of this study
The protocol used for this systematic overview of reviews was predesigned and registered in advance.
We had wide inclusion criteria including various dimensions of inequalities across several key infectious diseases, providing a broad overview of inequalities in infectious diseases, especially those relevant to high-income countries.
This overview focused on tuberculosis, HIV, sexually transmitted infections, hepatitis C, vaccination and antimicrobial resistance, however, we also included evidence from other infectious diseases except COVID-19.
We used Assessment of Multiple Systematic Reviews V.2 (AMSTAR2) to assess the methodological quality of each of the included reviews, however, some of the included reviews are not systematic reviews for which AMSTAR2 was designed.
Because this is an overview of reviews, we are unable to incorporate evidence within primary studies that have not been synthesised in reviews, which means there may be evidence we are missing.
Introduction
The WHO regards experiencing the highest possible standard of health as a fundamental human right of every individual regardless of personal or social circumstances.1 Nevertheless, avoidable inequalities exist in the prevalence of diseases and illnesses, general health status and access to healthcare between various social groups.2 A complex interaction between structural (e.g. income and wealth distribution) and individual-level (eg, health behaviours and living conditions) determinants of health contributes to the increased vulnerability to poorer health among particular social groups.3 4
Infectious diseases pose a significant health burden with substantial health inequalities globally.5 In the UK, infectious diseases constitute 7% of deaths alongside 4% of lost life years.6 The economic burden of infectious diseases in the UK is estimated to be around £30 billion per year.6 Although infectious diseases impose substantial, negative health and economic consequences within populations, many infectious diseases are vaccine-preventable and avoidable through adequate control measures.7 However, some groups remain under-vaccinated8 and other control measures may be difficult or impossible to implement for some, depending on circumstances.9 Traditionally, policymakers often target infectious diseases individually, but it is known that specific groups are often at higher risks regardless of specific infectious diseases.10–14 In efforts to tackle the observed disparities and to reduce the burden of infectious diseases, a strategic approach that tackles infectious diseases among high-risk groups is required.15 To inform the development of needs-tailored public health policies and initiatives to achieve this goal, a comprehensive synthesis of evidence is required, highlighting the inequalities in infectious diseases according to varying personal and social characteristics.
This project was commissioned by Public Health England (PHE) to gain an overview of the available evidence on health inequalities relating to key infectious disease topics in the UK from a population perspective. PHE had specific interest in three dimensions of inequalities; inclusion health groups (socially excluded and vulnerable populations), protected characteristics and socioeconomic inequalities. The infectious disease topics of interest were tuberculosis (TB), HIV, sexually transmitted infections (STIs), hepatitis C virus (HCV), vaccination and antimicrobial resistance (AMR). Therefore, the aim of this systematic overview of reviews was to describe the existing literature, relevant to the UK, relating to inequalities in the prevalence/incidence of key infectious disease topics as specified by PHE.
Methods
We conducted a systematic overview of reviews, preregistered in PROSPERO, an international prospective register of systematic reviews (2020 CRD42020220203 https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020220203).
Patient and public involvement
This study had no direct patient or public engagement.
Search strategy and study selection
We developed a search strategy using synonyms and Medical Subject Headings (MeSH) terms for inequalities, inclusion health groups, protected characteristics and socioeconomic factors which were combined with synonyms and MeSH terms for infectious diseases and synonyms for reviews (online supplemental file 1). We searched electronic databases from inception to November 2021; MEDLINE, Embase, Web of Science and to identify relevant grey literature, we searched OpenGrey database (http://www.opengrey.eu/) and contacted experts in our network.
bmjopen-2022-067429supp001.pdf (86.5KB, pdf)
We exported citations into EndNote, removed duplicates and then exported them into a web-tool, Rayyan (https://www.rayyan.ai/) to facilitate citation screening. Articles were screened against predefined eligibility criteria (table 1). Titles and abstracts were screened by a single reviewer and 10% were double screened by a second reviewer. Full texts of the potentially relevant articles were obtained and screened independently by two reviewers. Discrepancies were resolved by discussion.
Table 1.
Eligibility criteria
| Inclusion criteria | Exclusion criteria |
|
Population: Review including studies from the UK population or other high-income countries. Exposure:
Outcome: Inequalities relating to incidence, prevalence and consequences of infectious diseases. Despite specific interest in TB, HIV, STIs, HCV, immunisation, and AMR, we included reviews relating to any infectious diseases, except reviews focused on COVID-19. Types of reviews: Any literature review which reports all the following (a) explicit objectives, (b) clear search strategies and (c) eligibility criteria. Publication date: Published from the year 2000 onwards. Language: No language restrictions. |
We excluded reviews of qualitative studies and articles that are not systematic reviews as defined above. We excluded review protocols, but we searched the titles to check if the findings had been published. We excluded reviews on COVID-19, as advised by PHE, to avoid overlap with other reviews. We also excluded articles focused on travel-related infections. Reviews which excluded the UK in their eligibility criteria or had not included populations relevant to the UK population (e.g. papers where all results were from low-income countries) were excluded. |
AMR, antimicrobial resistance; HCV, hepatitis C virus; PHE, Public Health England; STIs, sexually transmitted infections; TB, tuberculosis.
Data extraction
We designed and piloted a data extraction form in Microsoft Excel to extract information including: First author’s last name, publication year, corresponding author’s country, review methodology, inclusion and exclusion criteria, infectious disease(s), population(s) included, dimension(s) of inequality, outcomes, conclusions and strengths and limitations. Data extraction was performed by one reviewer and checked by another.
Quality assessment
Two reviewers independently assessed review quality using the Assessment of Multiple Systematic Reviews V.2 (AMSTAR2) checklist.16 Disagreements were resolved by discussion. Due to the multidimensional nature of this overview of reviews, inclusion of various types of reviews with diverse aims and outcomes, we did not perform an overall rating of confidence for each review. To provide a sense of overall quality of evidence, we calculated the proportion of reviews which fulfilled each AMSTAR2 item.
Data synthesis
We tabulated the dimension of inequalities against the infectious disease topic to create an evidence matrix which was used to highlight areas where reviews already exist and where there may be gaps. Data were synthesised narratively based on the dimensions of inequalities.
Results
Figure 1 shows the study selection. We retrieved 14 713 citations from the electronic database searches and 11 135 titles and abstracts were screened after the removal of duplicates. One of the experts we contacted sent an article which highlighted UK-based evidence for several inclusion health groups, but did not fulfil other criteria for inclusion.17 After examining 437 full-text articles against the eligibility criteria, 108 were included in our synthesis.
Figure 1.
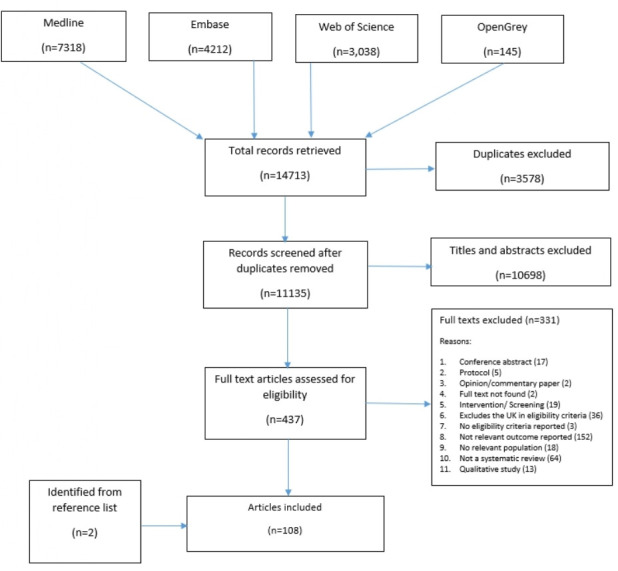
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for study selection.
Characteristics of included reviews are summarised in online supplemental file 2. Included reviews were published between 2005 and 2020 with 95% published after 2010. Fifty-eight (54%) included meta-analysis while the remaining studies used narrative/descriptive synthesis approaches. The reviews covered the three dimensions of inequalities across various infectious disease topics. A summarised version of the evidence matrix, showing how many reviews were identified for each cell is presented in figure 2. The full evidence matrix is presented in online supplemental file 3.
Figure 2.
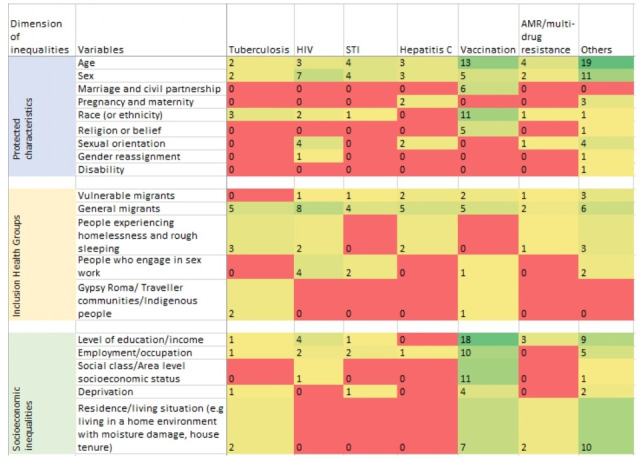
Matrix showing the number of reviews identified for each dimension of inequality and infectious disease topic. Colour ranges from red which indicates where no review was identified, up to green for a maximum number of reviews (19). AMR, antimicrobial resistance; STIs, sexually transmitted infections.
bmjopen-2022-067429supp002.pdf (89.8KB, pdf)
bmjopen-2022-067429supp003.pdf (96.7KB, pdf)
Methodological quality of included reviews
Assessment of the methodological quality of each included review is presented in online supplemental file 4 and the proportion of included reviews that met each AMSTAR2 criteria are presented in figure 3. Many reviews fulfilled criteria such as including components of PICO (population intervention, comparator group and outcome) in their research questions and inclusion criteria (87%), performing duplicate study selection (55%), discussing heterogeneity (70%) and disclosure of conflicts of interest (83%). Only a few of the reviews (19%) clearly indicated that the review methods were established a priori and 37% performed risk of bias assessment using satisfactory techniques. Although only 24% of the included reviews were judged to have comprehensive literature search, 59% were classed as ‘partial yes’ for literature search often due to lack of grey literature searches. Justification of the exclusions for each excluded study were not presented and none reported funding sources for included studies. These two criteria are more common among Cochrane reviews of interventions and are generally omitted from most published non-Cochrane reviews.
Figure 3.
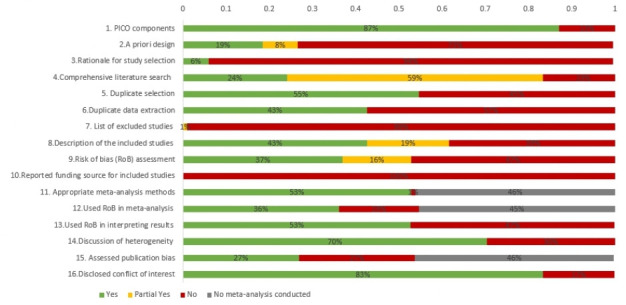
Assessment of Multiple Systematic Reviews V.2 results for included reviews. PICO, population, intervention, comparator group and outcome.
bmjopen-2022-067429supp004.pdf (129.1KB, pdf)
Evidence relating to inclusion health groups
Of the 108 included reviews, 43 reported on inclusion health groups. The evidence was generally consistent across these groups showing that people who belong to inclusion health groups are often at higher risk of infectious diseases, AMR and under-vaccination. For example, many reviews reported on general migrants,14 18–41 and vulnerable migrants such as asylum seekers, refugees and trafficked sex workers.14 19 22 28 34 42 43 The reviews often reported that migrants have a higher risk of infectious diseases than the host population, though the magnitude of the association may vary for different geographical regions and different infectious diseases. For example, in the UK, 35% of people with chronic HCV are migrants despite being just 9% of the general population.20 Other reviews showed that HIV and STIs were more prevalent among migrants.31 32 The prevalence of HIV-TB co-infection was higher among immigrants compared with nationals in various countries including England and Wales although the immigrant group reported slightly better survival/lower mortality which authors commented may be due to the possible healthy migrant effect.37 In another review, migrants were reported to be at higher risk of TB death.26 Evidence from the UK showed an increasing number of migrants contracted HIV after they arrived in the UK between 2002 and 2011 suggesting that the higher prevalence of infectious diseases among migrants is not limited to pre-migration infection.27 A meta-analysis showed that refugees were more likely to have chronic hepatitis B virus (HBV) compared with general migrants (OR 1.42, 95% CI 1.01 to 1.99).34 Some reviews reported no clear evidence that immigrant sex workers had higher risk of HIV and STIs compared with non-migrant sex workers.40 41 However, one reported that trafficked sex workers were at a higher risk of HIV and STIs compared with female sex workers in general.43 The prevalence of Helicobacter pylori among immigrants varied according to continent of origin and the prevalence is higher among migrants compared with their children.29
Several reviews reported lower vaccination rates or delayed/incomplete vaccination among migrants and refugees in Europe.25 28 30 36 However, the association may vary depending on the type of migrant group. For example, authors have reported that the uptake of vaccination among refugees was lower compared with asylum seekers.19 In a meta-analysis, migrants had increased odds of multidrug-resistant (MDR)-TB incidence compared with non-migrants (OR 3.91, 95% CI 2.98 to 5.14).38 In two other reviews, AMR carriage and infection were reported to be more prevalent among migrants in Europe.21 42
Three reviews examined Gypsy Roma and Traveller communities.8 44 45 One showed that Roma and Irish Travellers in the UK were often under-vaccinated.8 Another reported that Roma in Barcelona had a TB incidence 5.3 times greater than Spain’s national TB incidence.45 Higher prevalence of HIV has also been reported among Iranian, Roma and Peruvian Indigenous populations compared with the general population.44 We did not identify any reviews that examined the association between being from Gypsy Roma or Traveller communities and AMR.
We identified eight reviews which examined the association of homelessness with infectious diseases. They all reported a higher risk of various infectious diseases or AMR among people experiencing homelessness compared with those who were not homeless.26 35 46–51 We did not identify any reviews that examined the association between vaccination and homelessness. Eight reviews explored infectious disease risks among those engaging in sex work compared with the general population.39 43 52–57 The evidence suggests higher risks of various infectious diseases, such as HBV, hepatitis D virus (HDV), HIV and human papillomavirus (HPV), among sex workers.39 52–54 56 57 A review which examined factors associated with HBV vaccination among men who have sex with men (MSM) found mixed evidence relating to sexual risk-taking including involvement in sex trade.55 We did not identify any reviews exploring the association of being a sex worker with AMR.
Evidence relating to protected characteristics
Seventy-four reviews reported on protected characteristics, however, our synthesis only found clear evidence for inequalities by ethnicity and sexual orientation. Inequalities in infectious diseases relating to race and ethnicity were explored in 19 reviews.26 30 33 36 49 51 55 58–69 The available evidence suggests a higher rate of various infectious diseases such as TB, HIV and STIs and under-vaccination in people who belong to an ethnic minority group. For example, a meta-analysis found that recent transmission of TB was associated with being of ethnic minority (OR 3.03, 95% CI 2.21 to 4.16).49 A meta-analysis indicated that on average young black women were less likely to initiate HPV vaccination than young white women (combined OR 0.89, 95% CI 0.82 to 0.97).61 In a meta-analysis of studies from Europe, children from parents of ethnic minorities (compared with the majority) were less likely to be vaccinated for measles, mumps and rubella (MMR) (OR 0.89, 95% CI 0.86 to 0.93 in a fixed effect model).67 However, the effect disappeared in the random effects model (OR 1.03, 95% CI 0.79 to 1.34), probably due to heterogeneity between studies.67 Seasonal influenza vaccine uptake among older people was associated with being white (combined OR 1.30, 95% CI 1.14 to 1.49).30 Only one review on race and AMR was identified and it reported that people from some black ethnic groups in the USA and Europe, and Aboriginal ethnic groups living in Canada and Australia are less likely to have AMR-Neisseria gonorrhoea (AMR-NG) than the white majority population.58 Ten included reviews examined the association of sexual orientation with infectious disease topics, mostly focused on MSM.24 51 53 54 56 58 70–73 In a review, MSM were found to be at risk of acquiring HIV post-migration.71 However, in a network meta-analysis the highest risk of advanced HIV disease among people living with HIV was found in those with heterosexual contact compared with MSM as well as injection drug use.70 Some reviews examined disparities of HIV in MSM but did not compare risk between MSM and other populations.33 64 Other infectious diseases such as HBV, HCV and HDV are also higher in MSM than in the general population.24 54 72 AMR-NG was reported to be more common among MSM than heterosexual men in England and Wales (OR 5.47, 95% CI 3.99 to 7.48).58 We did not identify any reviews which assessed the association of sexual orientation with vaccination.
From the reviews identified, inequalities in infectious disease topics based on other protected characteristics, such as age and sex are mixed and for other protected characteristics the synthesised evidence is scant and inconclusive. The association of infectious diseases with age has been reported in various reviews.21 22 30 36 49 51 55–60 62 63 65 67 69 74–103 However, the association varied. Infectious diseases such as HIV, STIs and TB have been reported to be associated with younger age in some reviews,49 58 76 79 88 while HCV and hepatitis E virus were associated with older age.22 82 95 Seasonal influenza vaccine uptake was higher in older age groups.30 65 69 100 A review reported that many studies found an association between HBV vaccination and younger age.55 Suboptimal vaccination compliance was associated with mother’s younger age.36 63 On the other hand, another review reported that HPV vaccine intention and initiation were positively associated with younger parent’s age.62 Four included reviews examined the association of AMR with age.21 58 102 103 In one review, MDR-TB was associated with being younger than 65 years (pooled OR 2.53, 95% CI 1.74 to 4.83)21 while another review reported that AMR-NG was more common in those 25 years or older than in younger adults.58 Overall, age group classifications often varied between reviews which made it difficult to identify a clear pattern.
Twenty-eight reviews explored inequalities based on sex,21 39 40 46 48 49 51 53 58 65 67 74 78 82 84 85 87 89 90 93 96 100 104–109 but the findings varied depending on specific infectious disease. For example, TB transmission was reported to be associated with being male48 49 while the prevalence of chlamydia was slightly higher in women than in men.78 Other reviews reported that seasonal influenza vaccine uptake is often higher in elderly men compared with elderly women, but the differences are not statistically significant in multivariate regression analysis.65 A meta-analysis of studies from Europe showed that male patients were more likely to have MDR-TB (OR 1.38, 95% CI 1.16 to 1.65).21
Six reviews described the influence of being married or in civil partnerships on vaccination.25 30 65 67 69 96 Generally, those who were married had higher vaccination uptake although some studies found no association or higher uptake among those who were never married. No included review examined inequalities in infectious disease prevalence or AMR based on marital status. Only four included reviews reported the prevalence of infectious diseases (HBV, HCV, latent or acute toxoplasma infection) in pregnant women compared with the general population and the findings were mixed.22 24 56 110 We found no reviews that examined the association of pregnancy with vaccination uptake or risk of AMR.
Six reviews reported on inequalities relating to religion and meningococcal disease, as well as vaccination.8 25 59 61 63 90 A recent meta-analysis of two studies showed that religious events attendance was significantly associated with a decreased risk of invasive meningococcal disease (OR 0.47 (95% CI, 0.28 to 0.79, p=0.0004).90 Meta-analyses showed no strong evidence between various vaccination and religion including frequency of attendance at a place of worship.25 61 Jewish Orthodox people in the UK and Belgium and Orthodox Protestants in The Netherlands were described as being under-vaccinated.8 We did not identify any studies on the association of religion with the risk of any of the key infectious diseases or AMR.
Two reviews examined the association between gender reassignment and the risk of infectious diseases (HIV and HBV).53 56 The prevalence of HIV was significantly higher among transgender female sex workers compared with biologically female sex workers (relative risk=4.02, 95% CI 1.60 to 10.11).53 However, in another review, transgender persons had lower prevalence of HBV compared with other groups such as sex workers, injection drug users, MSM and pregnant women.56 We did not identify any reviews examining the association of gender reassignment with other infectious diseases of interest, vaccination or AMR.
We did not identify any review that reported the association of disability with our key infectious diseases topics. However, we identified one review which reported that disability was associated with a higher incidence of listeriosis.111
Evidence relating to socioeconomic inequalities
Fifty reviews explored socioeconomic status. The evidence consistently shows that those with lower level of income, lower educational attainment, unemployment, higher area level deprivation, lower socioeconomic status or poor living situations are at higher risk of infectious diseases, AMR, and lower vaccine uptake. For example, many reviews highlighted that low income, poverty and unemployment were associated with various infectious diseases including, HIV, STIs, TB, HBV, and HCV among others.26 27 35 39 64 75 82 84 85 90 94 111–114
Level of education, income or occupation are often associated with vaccination uptake.19 25 30 36 55 60 61 63 65 67 69 100 115–119 Reviews have also reported an association of MDR-TB and AMR with lower level of income or education.47 120 121 Many reviews examined the association of infectious disease topics of interest with areal level socioeconomic status,25 30 36 59 60 63 65 69 100 116 119 122 deprivation61 65 66 75 78 111 119 123 or living situation.30 36 44 47 49 51 60 65 67 69 84 85 87 90 94 96 101 105 113 121 One meta-analysis showed significant association between neighbourhood deprivation and chlamydia infection (pooled OR 1.76, 95 % CI 1.15 to 2.71).78 In another meta-analysis, TB was associated with residing in an urban area (OR 1.52, 95% CI 1.35 to 1.72).49 Those living in overcrowded or poor housing conditions had higher risk of TB.44 AIDS mortality is significantly associated with lower socioeconomic status.122 Group A streptococcal infection, gastrointestinal infections and meningococcal disease were associated with poor living conditions.75 84 85 90 111 113
Two included reviews explored the association between AMR and areal level deprivation.47 121 Although the evidence is scant, the findings suggest that those living in deprived areas or poor living conditions may be at higher risk of AMR.
Discussion
This overview of reviews provides a broad synopsis of three dimensions of inequalities (inclusion health groups, protected characteristics and socioeconomic inequalities) across several infectious disease topics. We synthesised the existing evidence based on the dimension of inequalities. Of the three dimensions of inequalities assessed, the evidence relating to people in the inclusion health groups is the most consistent although the volume of evidence identified for each group varied. Most of the reviews identified under this dimension were on migration status, with a higher prevalence of infectious diseases, AMR and lower vaccine uptake among migrants compared with non-migrants. Vulnerable migrants (such as refugees, asylum seekers and trafficked persons) are at higher risk when compared with general migrants and the size of inequalities varied depending on the country of origin. Although few reviews were identified for the remaining inclusion health groups, the evidence suggests that homelessness is associated with risk of infectious diseases and AMR; Gypsy Roma/Traveller communities are often under-vaccinated and are also at greater risk of infectious diseases; and people who engage in sex work are at greater risk of some infectious diseases.
There is a plethora of evidence from reviews showing higher prevalence of infectious diseases and under-vaccination among minority ethnic groups. We also identified several reviews suggesting higher prevalence of infectious diseases and AMR among MSM. These suggest that ethnicity and sexual orientation are important protected characteristics and targeting or tailoring interventions for such groups may be beneficial to reduce inequalities in infectious diseases. It is important to note that there is inequality in access to vaccinations as shown in reviews included in this overview of reviews and beyond. Since many infectious diseases are vaccine preventable, identified inequalities in infectious diseases that we have noted in this overview of reviews, may also be related to inequalities in access to vaccination.
Many reviews examined the association with age and sex, however, the identified associations varied depending on the specific infectious disease or type of vaccination. In addition, for most of the reviews, the comparator age groups were often unclear. Therefore, we are not able to identify specific age groups with higher risk across various infectious disease topics. Other factors besides equity issues may contribute significantly to associations with age. For example, people in the most sexually active age groups are more likely to contract STIs whereas people of older ages, where immunity is weaker, are more likely to get infectious diseases associated with low immunity. Also, vaccinations are often offered at specific ages so it is expected that uptake would be higher among those groups that are targeted. However, it is important to highlight that we found evidence suggesting that childhood vaccination compliance is lower for those with younger mothers/parents.36 59 63 Based on this review, age and sex may be important for some infectious diseases but the group at higher risk may vary across diseases.
Reviews exploring marital status focused on vaccination, particularly seasonal influenza vaccine in older adults, tetanus vaccination among pregnant women and MMR vaccination in children. Reviews generally reported higher vaccination uptake among adults who are married and children whose parents are married. It is not possible to draw a conclusion regarding the association of religion, disability, transgenderism, and pregnancy with infectious diseases based on the findings of this review as the synthesised evidence is scant and often inconsistent. More evidence is therefore needed to be able to establish the presence and direction of any associations of these factors with infectious diseases.
Several reviews provide compelling evidence of higher risk of infectious diseases, AMR, and lower vaccination uptake among those with lower level of income, lower educational attainment, unemployment, higher area level deprivation, lower socioeconomic status and poor living situations. Although most of the evidence in this dimension is on vaccination, those of lower socioeconomic groups are often at higher risk from infectious diseases and should be targeted for intervention.
Strengths and limitations
The protocol used to guide the conduct of this review was designed a priori. We conducted a comprehensive literature search of four electronic databases with no language limits and searched for grey literature. Data extraction was checked by a second reviewer to improve accuracy and quality assessments were performed by two reviewers independently. Due to the time frame required for the work, we could not complete all the initial titles and abstract screening in duplicate, however, full texts of potentially relevant reviews were independently screened by two reviewers. Despite our best efforts, we acknowledge that some relevant reviews may have been missed in the study selection process. The lack of synthesised evidence observed in some areas does not necessarily mean a lack of evidence. This is because there may be primary studies in those areas which have not been synthesised in reviews and meta-analyses. Also, this project focused on specific infectious disease topics and we included specific search terms for those topics in the search strategy and included broad terms for infectious diseases. This allowed us to include other infectious diseases that were identified in our search to capture evidence of health inequalities from various infectious diseases (excluding COVID-19). Therefore, this is not intended to provide a comprehensive overview of reviews in those topics which are not the focus of this project. Furthermore, some underserved populations (such as people who inject drugs and prisoners) are not covered in this overview as these are beyond the scope of the work. Notwithstanding, we believe this provides a useful summary of available evidence relating to inequalities in infectious diseases relevant to high-income countries and highlights areas where evidence may be lacking or minimal.
The quality of the included reviews varied significantly as we included various types of reviews, and some were not necessarily systematic reviews for which AMSTAR2 is designed. Heterogeneity between studies was a limitation reported in many of the included reviews where meta-analyses were performed, therefore, pooled estimates should be interpreted with caution.
Conclusion
Overall, we provide evidence from many papers with accordant findings, of groups consistently at higher risk of infectious diseases, AMR and under-vaccination. Developing targeted interventions for high-risk groups rather than focusing on individual infections could contribute significantly to reducing infectious disease burden. This review also highlights important evidence gaps that should be considered when commissioning future evidence syntheses or primary studies.
Supplementary Material
Acknowledgments
We would like to acknowledge colleagues from the University of Warwick, Samantha Johnson who supported us in developing the search strategy and Dr Yen-Fu Chen who helped with language translation. We are also grateful to those who responded to our request for information. We also like to thank Dr Ines Campos-Matos from the UK Health Security Agency (UKHSA) and Office of Health Improvement and Disparities, Katy Sinka from the UKHSA and Dr Rebecca Wilkinson from Hampshire Hospitals Foundation Trust for their support and guidance through the project. AA and IG are funded by the National Institute for Health and Social Care Research (NIHR) Applied Research Collaboration (ARC) West Midlands, grant number NIHR200165. The views expressed are those of the author(s) and not necessarily those of the NIHR, the Department of Health and Social Care or Public Health England.
Footnotes
Twitter: @AbiAyorinde
Contributors: AA, IG, BB, NM and OOy contributed to the design of the review. AA, IG, IA, IZ, MS, EM, SSA, OOl and SR contributed to data collection, including selection of studies, data extraction and quality assessment. AAA, IG, BB, NM and OOy contributed to the data synthesis and interpretation of the data. AA wrote the first draft of the paper. All authors contributed to revising the draft and approved the final manuscript. AA is responsible for the overall content as the guarantor.
Funding: This work was funded by Public Health England. AA and IG are funded by the National Institute for Health and Social Care Research (NIHR) Applied Research Collaboration (ARC) West Midlands, grant number NIHR200165.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.World Health Organization . Human rights and health. 2017. Available: https://www.who.int/news-room/fact-sheets/detail/human-rights-and-health [Google Scholar]
- 2.Marmot M. Inequalities in health. N Engl J Med 2001;345:134–6. 10.1056/NEJM200107123450210 [DOI] [PubMed] [Google Scholar]
- 3.Marmot M, Wilkinson R. Social determinants of health Oxford2005. 10.1093/acprof:oso/9780198565895.001.0001 [DOI] [Google Scholar]
- 4.Graham H. Social determinants and their unequal distribution: Clarifying policy understandings. Milbank Q 2004;82:101–24. 10.1111/j.0887-378x.2004.00303.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn SC, Kumar S. Health inequalities and infectious disease epidemics: a challenge for global health security. Biosecur Bioterror 2014;12:263–73. 10.1089/bsp.2014.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.House of Parliament . UK trends in infectious disease. In: Technology (Elmsford, N.Y.). 2017: 545. [Google Scholar]
- 7.CDC centre for disease control and prevention. Vaccines and Preventable Diseases 2016. Available: https://www.cdc.gov/vaccines/vpd/index.html [Google Scholar]
- 8.Fournet N, Mollema L, Ruijs WL, et al. Under-vaccinated groups in Europe and their beliefs, attitudes and reasons for non-vaccination; two systematic reviews. BMC Public Health 2018;18:196. 10.1186/s12889-018-5103-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotheram S, Cooper J, Barr B, et al. How are inequalities generated in the management and consequences of gastrointestinal infections in the UK? an ethnographic study. Soc Sci Med 2021;282:114131. 10.1016/j.socscimed.2021.114131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes GJ, Gorton R. Inequalities in the incidence of infectious disease in the North East of England: a population-based study. Epidemiol Infect 2015;143:189–201. 10.1017/S0950268814000533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health England . Health protection report, weekly report. 2016. [Google Scholar]
- 12.Baker EH. Socioeconomic status, definition. In: The Wiley Blackwell Encyclopedia of Health, Illness, Behavior, and Society. 2014: 2210–4. [Google Scholar]
- 13.Public Health England . Public health matters. 2019. Available: https://publichealthmatters.blog.gov.uk/2019/03/22/tackling-tuberculosis-in-under-served-populations/ [Google Scholar]
- 14.Chernet A, Utzinger J, Sydow V, et al. Prevalence rates of six selected infectious diseases among African migrants and refugees: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2018;37:605–19. 10.1007/s10096-017-3126-1 [DOI] [PubMed] [Google Scholar]
- 15.Semenza JC, Giesecke J. Intervening to reduce inequalities in infections in Europe. Am J Public Health 2008;98:787–92. 10.2105/AJPH.2007.120329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens A. Putting high-risk under-served populations at the centre of joint efforts to eliminate hepatitis B and C, tuberculosis and HIV and halt the rise in sexually transmitted infections PHE infectious diseases strategy. 2020: 2025–2020.
- 18.Chan IHY, Kaushik N, Dobler CC. Post-migration follow-up of migrants identified to be at increased risk of developing tuberculosis at pre-migration screening: a systematic review and meta-analysis. Lancet Infect Dis 2017;17:770–9. 10.1016/S1473-3099(17)30194-9 [DOI] [PubMed] [Google Scholar]
- 19.De Vito E, Parente P, de Waure C, et al. A review of evidence on equitable delivery, access and utilization of immunization services for migrants and refugees in the WHO european region. In: WHO Regional Office for Europe WHO Health Evidence Network Synthesis Reports. 2017. [PubMed] [Google Scholar]
- 20.Falla AM, Ahmad AA, Duffell E, et al. Estimating the scale of chronic hepatitis C virus infection in the EU/EEA: a focus on migrants from anti-HCV endemic countries. BMC Infect Dis 2018;18:42. 10.1186/s12879-017-2908-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax 2006;61:158–63. 10.1136/thx.2005.045963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenaway C, Thu Ma A, Kloda LA, et al. The seroprevalence of hepatitis C antibodies in immigrants and refugees from intermediate and high endemic countries: a systematic review and meta-analysis. PLoS ONE 2015;10:e0141715. 10.1371/journal.pone.0141715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandgren A, Schepisi MS, Sotgiu G, et al. Tuberculosis transmission between foreign- and native-born populations in the EU/EEA: a systematic review. Eur Respir J 2014;43:1159–71. 10.1183/09031936.00117213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahné SJM, Veldhuijzen IK, Wiessing L, et al. Infection with hepatitis B and C virus in Europe: a systematic review of prevalence and cost-effectiveness of screening. BMC Infect Dis 2013;13:181. 10.1186/1471-2334-13-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain A, van Hoek AJ, Boccia D, et al. Lower vaccine uptake amongst older individuals living alone: a systematic review and meta-analysis of social determinants of vaccine uptake. Vaccine 2017;35:2315–28. 10.1016/j.vaccine.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 26.Kawatsu L Fau - Ishikawa N, Ishikawa I. Socio-economic factors that influence tuberculosis death among the youth and middle-aged population: a systematic review. 2014. [PubMed] [Google Scholar]
- 27.Kentikelenis A, Karanikolos M, Williams G, et al. How do economic crises affect migrants’ risk of infectious disease? A systematic-narrative review. Eur J Public Health 2015;25:937–44. 10.1093/eurpub/ckv151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mipatrini D, Stefanelli P, Severoni S, et al. Vaccinations in migrants and refugees: a challenge for European health systems. A systematic review of current scientific evidence. Pathog Glob Health 2017;111:59–68. 10.1080/20477724.2017.1281374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morais S, Costa AR, Ferro A, et al. Contemporary migration patterns in the prevalence of Helicobacter pylori infection: a systematic review. Helicobacter 2017;22. 10.1111/hel.12372 [DOI] [PubMed] [Google Scholar]
- 30.Okoli GN, Lam OLT, Racovitan F, et al. Seasonal influenza vaccination in older people: a systematic review and meta-analysis of the determining factors. PLoS One 2020;15:e0234702. 10.1371/journal.pone.0234702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platt L, Grenfell P, Fletcher A, et al. Systematic review examining differences in HIV, sexually transmitted infections and health-related harms between migrant and non-migrant female sex workers. Sex Transm Infect 2013;89:311–9. 10.1136/sextrans-2012-050491 [DOI] [PubMed] [Google Scholar]
- 32.Platt L, Jolley E, Rhodes T, et al. Factors mediating HIV risk among female sex workers in Europe: a systematic review and ecological analysis. BMJ Open 2013;3:e002836. 10.1136/bmjopen-2013-002836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prost A, Elford J, Imrie J, et al. Social, behavioural, and intervention research among people of sub-Saharan African origin living with HIV in the UK and Europe: literature review and recommendations for intervention. AIDS Behav 2008;12:170–94. 10.1007/s10461-007-9237-4 [DOI] [PubMed] [Google Scholar]
- 34.Rossi C, Shrier I, Marshall L, et al. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: a systematic review and meta-analysis [PLoS ONE [Electronic Resource]]. PLoS One 2012;7:e44611. 10.1371/journal.pone.0044611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suhrcke M, Stuckler D, Suk JE, et al. The impact of economic crises on communicable disease transmission and control: a systematic review of the evidence. PLoS ONE 2011;6:e20724. 10.1371/journal.pone.0020724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tauil M de C, Sato APS, Waldman EA. Factors associated with incomplete or delayed vaccination across countries: a systematic review. Vaccine 2016;34:2635–43. 10.1016/j.vaccine.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 37.Tavares AM, Fronteira I, Couto I, et al. Hiv and tuberculosis co-infection among migrants in Europe: a systematic review on the prevalence, incidence and mortality [PLoS ONE [Electronic Resource]]. PLoS One 2017;12:e0185526. 10.1371/journal.pone.0185526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson S, Kabir Z, Comiskey C. Effects of migration on tuberculosis epidemiological indicators in low and medium tuberculosis incidence countries: a systematic review. J Clin Tuberc Other Mycobact Dis 2021;23:100225. 10.1016/j.jctube.2021.100225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabapy AF, Shatat HZ, Abd El-Wahab EW. Attributes of HIV infection over decades (1982-2018): a systematic review and meta-analysis. Transbound Emerg Dis 2020;67:2372–88. 10.1111/tbed.13621 [DOI] [PubMed] [Google Scholar]
- 40.McBride B, Shannon K, Strathdee SA, et al. Structural determinants of HIV/STI prevalence, HIV/STI/sexual and reproductive health access, and condom use among immigrant sex workers globally. AIDS 2021;35:1461–77. 10.1097/QAD.0000000000002910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pega F-O, Govindaraj S, Tran NT. Health service use and health outcomes among international migrant workers compared with non-migrant workers: a systematic review and meta-analysis. PLoS One 2021;16:e0252651. 10.1371/journal.pone.0252651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nellums LB, Thompson H, Holmes A, et al. Antimicrobial resistance among migrants in europe: a systematic review and meta-analysis. Lancet Infect Dis 2018;18:796–811. 10.1016/S1473-3099(18)30219-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu YJ, Bruna S, McCarty C. Hiv risk among trafficked women: a systematic review of the global literature. AIDS Care 2021;33:1068–78. 10.1080/09540121.2020.1861178 [DOI] [PubMed] [Google Scholar]
- 44.Cormier M, Schwartzman K, N’Diaye DS, et al. Proximate determinants of tuberculosis in indigenous peoples worldwide: a systematic review. Lancet Glob Health 2019;7:e68–80. 10.1016/S2214-109X(18)30435-2 [DOI] [PubMed] [Google Scholar]
- 45.Tollefson D, Bloss E, Fanning A, et al. Burden of tuberculosis in Indigenous peoples globally: a systematic review. Int J Tuberc Lung Dis 2013;17:1139–50. 10.5588/ijtld.12.0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aldridge RW, Story A, Hwang SW, et al. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high-income countries: a systematic review and meta-analysis. Lancet 2018;391:241–50. 10.1016/S0140-6736(17)31869-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alividza V, Mariano V, Ahmad R, et al. Investigating the impact of poverty on colonization and infection with drug-resistant organisms in humans: a systematic review. Infect Dis Poverty 2018;7:76. 10.1186/s40249-018-0459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beijer U, Wolf A, Fazel S. Prevalence of tuberculosis, hepatitis C virus, and HIV in homeless people: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:859–70. 10.1016/S1473-3099(12)70177-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nava-Aguilera E, Andersson N, Harris E, et al. Risk factors associated with recent transmission of tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis 2009;13:17–26. [PubMed] [Google Scholar]
- 50.Arum C, Fraser H, Artenie AA, et al. Homelessness, unstable housing, and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Public Health 2021;6:e309–23. 10.1016/S2468-2667(21)00013-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ly TDA, Castaneda S, Hoang VT, et al. Vaccine-preventable diseases other than tuberculosis, and homelessness: A systematic review of the published literature, 1980 to 2020. Epidemiology [Preprint] 1980. 10.1101/2020.10.28.20220335 [DOI] [PubMed]
- 52.Oldenburg CE, Perez-Brumer AG, Reisner SL, et al. Transactional sex and the HIV epidemic among men who have sex with men (MSM): results from a systematic review and meta-analysis. AIDS Behav 2015;19:2177–83. 10.1007/s10461-015-1010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Operario D, Soma T, Underhill K. Sex work and HIV status among transgender women: systematic review and meta-analysis. J Acquir Immune Defic Syndr 2008;48:97–103. 10.1097/QAI.0b013e31816e3971 [DOI] [PubMed] [Google Scholar]
- 54.Stockdale AJ, Kreuels B, Henrion MYR, et al. The global prevalence of hepatitis D virus infection: systematic review and meta-analysis. J Hepatol 2020;73:523–32. 10.1016/j.jhep.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vet R, de Wit JB, Das E. Factors associated with hepatitis B vaccination among men who have sex with men: a systematic review of published research. Int J STD AIDS 2017;28:534–42. 10.1177/0956462415613726 [DOI] [PubMed] [Google Scholar]
- 56.Leumi S, Bigna JJ, Amougou MA, et al. Global burden of hepatitis B infection in people living with human immunodeficiency virus: a systematic review and meta-analysis. Clin Infect Dis 2020;71:2799–806. 10.1093/cid/ciz1170 [DOI] [PubMed] [Google Scholar]
- 57.Wu J, Ding C, Liu X, et al. Worldwide burden of genital human papillomavirus infection in female sex workers: a systematic review and meta-analysis. Int J Epidemiol 2021;50:527–37. 10.1093/ije/dyaa289 [DOI] [PubMed] [Google Scholar]
- 58.Abraha M, Egli-Gany D, Low N. Epidemiological, behavioural, and clinical factors associated with antimicrobial-resistant gonorrhoea: a review. F1000Res 2018;7:400. 10.12688/f1000research.13600.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Falagas ME, Zarkadoulia E. Factors associated with suboptimal compliance to vaccinations in children in developed countries: a systematic review. Curr Med Res Opin 2008;24:1719–41. 10.1185/03007990802085692 [DOI] [PubMed] [Google Scholar]
- 60.Fernández de Casadevante V, Gil Cuesta J, Cantarero-Arévalo L. Determinants in the uptake of the human papillomavirus vaccine: a systematic review based on European studies. Front Oncol 2015;5:141. 10.3389/fonc.2015.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fisher H, Trotter CL, Audrey S, et al. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int J Epidemiol 2013;42:896–908. 10.1093/ije/dyt049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radisic G, Chapman J, Flight I, et al. Factors associated with parents’ attitudes to the HPV vaccination of their adolescent sons: a systematic review. Prev Med 2017;95:26–37. 10.1016/j.ypmed.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 63.Malerba V, Costantino C, Napoli G, et al. Antimeningococcal and antipneumococcal vaccination determinants: a european systematic literature review. 2015. [PubMed] [Google Scholar]
- 64.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet 2012;380:341–8. 10.1016/S0140-6736(12)60899-X [DOI] [PubMed] [Google Scholar]
- 65.Nagata JM, Hernández-Ramos I, Kurup AS, et al. Social determinants of health and seasonal influenza vaccination in adults ≥65 years: a systematic review of qualitative and quantitative data. BMC Public Health 2013;13:388. 10.1186/1471-2458-13-388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Offer C, Lee A, Humphreys C. Tuberculosis in South Asian communities in the UK: a systematic review of the literature. J Public Health (Oxf) 2016;38:250–7. 10.1093/pubmed/fdv034 [DOI] [PubMed] [Google Scholar]
- 67.Tabacchi G, Costantino C, Napoli G, et al. Determinants of European parents’ decision on the vaccination of their children against measles, mumps and rubella: a systematic review and meta-analysis. Hum Vaccin Immunother 2016;12:1909–23. 10.1080/21645515.2016.1151990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wayal S, Aicken CRH, Griffiths C, et al. Understanding the burden of bacterial sexually transmitted infections and trichomonas vaginalis among black caribbeans in the united kingdom: findings from a systematic review. PLoS One 2018;13:e0208315. 10.1371/journal.pone.0208315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okoli GN, Reddy VK, Al-Yousif Y, et al. Sociodemographic and health-related determinants of seasonal influenza vaccination in pregnancy: a systematic review and meta-analysis of the evidence since 2000. Acta Obstet Gynecol Scand 2021;100:997–1009. 10.1111/aogs.14079 [DOI] [PubMed] [Google Scholar]
- 70.Chen Q, Zeng D, She Y, et al. Different transmission routes and the risk of advanced HIV disease: a systematic review and network meta-analysis of observational studies. EClinicalMedicine 2019;16:121–8. 10.1016/j.eclinm.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fakoya I, Álvarez-del Arco D, Woode-Owusu M, et al. A systematic review of post-migration acquisition of HIV among migrants from countries with generalised HIV epidemics living in Europe: mplications for effectively managing HIV prevention programmes and policy. BMC Public Health 2015;15:561. 10.1186/s12889-015-1852-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin F, Dore GJ, Matthews G, et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021;6:39–56. 10.1016/S2468-1253(20)30303-4 [DOI] [PubMed] [Google Scholar]
- 73.Oldenburg CE, Perez-Brumer AG, Reisner SL, et al. Global burden of HIV among men who engage in transactional sex: a systematic review and meta-analysis [PLoS ONE [Electronic Resource]]. PLoS One 2014;9:e103549. 10.1371/journal.pone.0103549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colledge S, Leung J, Grebely J, et al. Socio-demographic and ecological factors associated with anti-HCV prevalence in people who inject drugs: a systematic review. Drug Alcohol Depend 2020;209:107899. 10.1016/j.drugalcdep.2020.107899 [DOI] [PubMed] [Google Scholar]
- 75.Adams NL, Rose TC, Hawker J, et al. Relationship between socioeconomic status and gastrointestinal infections in developed countries: a systematic review and meta-analysis [PLoS ONE [Electronic Resource]]. PLoS One 2018;13:e0191633. 10.1371/journal.pone.0191633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Behera MR, Intarak R. Hiv risk among labor migrants: an in-depth study of the literature. Ind Jour of Publ Health Rese & Develop 2020;9:107. 10.5958/0976-5506.2018.01816.8 [DOI] [Google Scholar]
- 77.Bonten M M, Johnson JR, van den Biggelaar AHJ, et al. Epidemiology of escherichia coli bacteremia: A systematic literature review. 2021. [DOI] [PubMed] [Google Scholar]
- 78.Crichton J, Hickman M, Campbell R, et al. Socioeconomic factors and other sources of variation in the prevalence of genital Chlamydia infections: a systematic review and meta-analysis. BMC Public Health 2015;15:729. 10.1186/s12889-015-2069-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Denning DW, Kneale M, Sobel JD, et al. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis 2018;18:e339–47. 10.1016/S1473-3099(18)30103-8 [DOI] [PubMed] [Google Scholar]
- 80.Ghiasvand H, Higgs P, Noroozi M, et al. Social and demographical determinants of quality of life in people who live with HIV/AIDS infection: evidence from a meta-analysis. Biodemography and Social Biology 2020;65:57–72. 10.1080/19485565.2019.1587287 [DOI] [PubMed] [Google Scholar]
- 81.Hermann JS, Featherstone RM, Russell ML, et al. Immunization coverage of children in care of the child welfare system in high-income countries: a systematic review. Am J Prev Med 2019;56:e55–63. 10.1016/j.amepre.2018.07.026 [DOI] [PubMed] [Google Scholar]
- 82.Li P, Liu J, Li Y, et al. The global epidemiology of hepatitis E virus infection: a systematic review and meta-analysis. Liver Int 2020;40:1516–28. 10.1111/liv.14468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lindsay L, Wolter J, De Coster I, et al. A decade of norovirus disease risk among older adults in upper-middle and high income countries: a systematic review. BMC Infect Dis 2015;15:425. 10.1186/s12879-015-1168-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Possenti A, Manzano-Román R, Sánchez-Ovejero C, et al. Potential risk factors associated with human cystic echinococcosis: systematic review and meta-analysis. PLoS Negl Trop Dis 2016;10:e0005114. 10.1371/journal.pntd.0005114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Richterman A, Sainvilien DR, Eberly L, et al. Individual and household risk factors for symptomatic cholera infection. A Systematic Review and Meta-Analysis 2018. 10.1093/infdis/jiy444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rostami A, Riahi SM, Contopoulos-Ioannidis DG, et al. Acute toxoplasma infection in pregnant women worldwide: a systematic review and meta-analysis. PLoS Negl Trop Dis 2019;13:e0007807. 10.1371/journal.pntd.0007807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rostami A, Riahi SM, Holland CV, et al. Seroprevalence estimates for toxocariasis in people worldwide: a systematic review and meta-analysis. PLoS Negl Trop Dis 2019;13:e0007809. 10.1371/journal.pntd.0007809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schepisi MS, Motta I, Dore S, et al. Tuberculosis transmission among children and adolescents in schools and other congregate settings: a systematic review. New Microbiol 2019;41:282–90. [PubMed] [Google Scholar]
- 89.Schierhout G, McGregor S, Gessain A, et al. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. Lancet Infect Dis 2020;20:133–43. 10.1016/S1473-3099(19)30402-5 [DOI] [PubMed] [Google Scholar]
- 90.Spyromitrou-Xioufi P, Tsirigotaki M, Ladomenou F. Risk factors for meningococcal disease in children and adolescents: a systematic review and meta-analysis. Eur J Pediatr 2020;179:1017–27. 10.1007/s00431-020-03658-9 [DOI] [PubMed] [Google Scholar]
- 91.Strifler L, Morris SK, Dang V, et al. The health burden of invasive meningococcal disease: a systematic review. J Pediatric Infect Dis Soc 2016;5:417–30. 10.1093/jpids/piv065 [DOI] [PubMed] [Google Scholar]
- 92.Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther 2018;47:868–76. 10.1111/apt.14561 [DOI] [PubMed] [Google Scholar]
- 93.Badri M, Eslahi AV, Olfatifar M, et al. Keys to unlock the enigma of ocular toxocariasis: a systematic review and meta-analysis. Ocul Immunol Inflamm 2021;29:1265–76. 10.1080/09273948.2021.1875007 [DOI] [PubMed] [Google Scholar]
- 94.Dong S, Yang Y, Wang Y, et al. Prevalence of Cryptosporidium infection in the global population: a systematic review and meta-analysis. Acta Parasitol 2020;65:882–9. 10.2478/s11686-020-00230-1 [DOI] [PubMed] [Google Scholar]
- 95.Dugan E, Blach S, Biondi M, et al. Global prevalence of hepatitis C virus in women of childbearing age in 2019: a modelling study. Lancet Gastroenterol Hepatol 2021;6:169–84. 10.1016/S2468-1253(20)30359-9 [DOI] [PubMed] [Google Scholar]
- 96.Faria APV, da Silva TPR, Duarte CK, et al. Tetanus vaccination in pregnant women: a systematic review and meta-analysis of the global literature. Public Health 2021;196:43–51. 10.1016/j.puhe.2021.04.019 [DOI] [PubMed] [Google Scholar]
- 97.Lafond KE, Porter RM, Whaley MJ, et al. Global burden of influenza-associated lower respiratory tract infections and hospitalizations among adults: a systematic review and meta-analysis. PLoS Med 2021;18:e1003550. 10.1371/journal.pmed.1003550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Macina D, Evans KE. Bordetella pertussis in school-age children, adolescents and adults: a systematic review of epidemiology and mortality in Europe. Infect Dis Ther 2021;10:2071–118. 10.1007/s40121-021-00520-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mirzadeh M, Olfatifar M, Eslahi AV, et al. Global prevalence of Trichomonas vaginalis among female sex workers: a systematic review and meta-analysis. Parasitol Res 2021;120:2311–22. 10.1007/s00436-021-07216-6 [DOI] [PubMed] [Google Scholar]
- 100.Roller-Wirnsberger R, Lindner S, Kolosovski L, et al. The role of health determinants in the influenza vaccination uptake among older adults (65+): a scope review. Aging Clin Exp Res 2021;33:2123–32. 10.1007/s40520-021-01793-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rostami A, Riahi SM, Abdollahzadeh Sagha S, et al. Seroprevalence estimates of latent and acute Toxoplasma infections in HIV+ people-call for action in underprivileged communities. Microorganisms 2021;9:2034. 10.3390/microorganisms9102034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song W-M, Li Y-F, Liu Y-X, et al. Drug-resistant tuberculosis among children: a systematic review and meta-analysis. Front Public Health 2021;9:721817. 10.3389/fpubh.2021.721817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sultana ZZ, Hoque FU, Beyene J, et al. Hiv infection and multidrug resistant tuberculosis: a systematic review and meta-analysis. BMC Infect Dis 2021;21:51. 10.1186/s12879-020-05749-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eilami O, Nazari A, Dousti M, et al. Investigation of HIV/AIDS prevalence and associated risk factors among female sex workers from 2010 to 2017: a meta-analysis study. HIV AIDS (Auckl) 2019;11:105–17. 10.2147/HIV.S196085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fauroux B, Simões EAF, Checchia PA, et al. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther 2017;6:173–97. 10.1007/s40121-017-0151-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leung J, Peacock A, Colledge S, et al. A global meta-analysis of the prevalence of HIV, hepatitis C virus, and hepatitis B virus among people who inject drugs-do gender-based differences vary by country-level indicators? J Infect Dis 2019;220:78–90. 10.1093/infdis/jiz058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pulver A, Ramraj C, Ray JG, et al. A scoping review of female disadvantage in health care use among very young children of immigrant families. Soc Sci Med 2016;152:50–60. 10.1016/j.socscimed.2016.01.027 [DOI] [PubMed] [Google Scholar]
- 108.Gerwen OV, Muzny C, Austin E, et al. 784 Prevalence of stis and HIV in transgender women and men: A systematic review. Abstracts for the STI & HIV World Congress (Joint Meeting of the 23rd ISSTDR and 20th IUSTI), July 14–17, 2019, Vancouver, Canada; July 2019. 10.1136/sextrans-2019-sti.840 [DOI] [Google Scholar]
- 109.Whelan J, Abbing-Karahagopian V, Serino L, et al. Gonorrhoea: a systematic review of prevalence reporting globally. BMC Infect Dis 2021;21:1152. 10.1186/s12879-021-06381-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nourollahpour Shiadeh M, Esfandyari S, Ashrafmansouri M, et al. The prevalence of latent and acute toxoplasmosis in HIV-infected pregnant women: a systematic review and meta-analysis. Microb Pathog 2020;149:104549. 10.1016/j.micpath.2020.104549 [DOI] [PubMed] [Google Scholar]
- 111.Newman KL, Leon JS, Rebolledo PA, et al. The impact of socioeconomic status on foodborne illness in high-income countries: a systematic review. Epidemiol Infect 2015;143:2473–85. 10.1017/S0950268814003847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodrigo C, Rajapakse S. Hiv, poverty and women. Int Health 2010;2:9–16. 10.1016/j.inhe.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 113.Coffey PM, Ralph AP, Krause VL. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: a systematic review. PLoS Negl Trop Dis 2018;12:e0006577. 10.1371/journal.pntd.0006577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ding Y, Sun X, Xu Y, et al. Association between income and hepatitis B seroprevalence: a systematic review and meta-analysis. Hepat Mon 2020;20:10. 10.5812/hepatmon.104675 [DOI] [Google Scholar]
- 115.Larson HJ, Jarrett C, Eckersberger E, et al. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine 2014;32:2150–9. 10.1016/j.vaccine.2014.01.081 [DOI] [PubMed] [Google Scholar]
- 116.Arat A, Burström B, Östberg V, et al. Social inequities in vaccination coverage among infants and pre-school children in Europe and Australia-a systematic review. BMC Public Health 2019;19:290. 10.1186/s12889-019-6597-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Forshaw J, Gerver SM, Gill M, et al. The global effect of maternal education on complete childhood vaccination: a systematic review and meta-analysis. BMC Infect Dis 2017;17:801. 10.1186/s12879-017-2890-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bocquier A, Ward J, Raude J, et al. Socioeconomic differences in childhood vaccination in developed countries: a systematic review of quantitative studies. Expert Rev Vaccines 2017;16:1107–18. 10.1080/14760584.2017.1381020 [DOI] [PubMed] [Google Scholar]
- 119.Lucyk K, Simmonds KA, Lorenzetti DL, et al. The association between influenza vaccination and socioeconomic status in high income countries varies by the measure used: a systematic review. BMC Med Res Methodol 2019;19:153. 10.1186/s12874-019-0801-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Di Gennaro F, Pizzol D, Cebola B, et al. Social determinants of therapy failure and multi drug resistance among people with tuberculosis: a review. Tuberculosis 2017;103:44–51. 10.1016/j.tube.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 121.Najafizada M, Rahman A, Taufique Q, et al. Social determinants of multidrug-resistant tuberculosis: a scoping review and research gaps. Indian J Tuberc 2021;68:99–105. 10.1016/j.ijtb.2020.09.016 [DOI] [PubMed] [Google Scholar]
- 122.Salgado-Barreira Á, Estany-Gestal A, Figueiras A. Effect of socioeconomic status on mortality in urban areas: a systematic critical review. Cad Saude Publica 2014;30:1609–21. 10.1590/0102-311x00152513 [DOI] [PubMed] [Google Scholar]
- 123.Vukovic V, Lillini R, Lupi S, et al. Identifying people at risk for influenza with low vaccine uptake based on deprivation status: a systematic review. Eur J Public Health 2020;30:132–41. 10.1093/eurpub/cky264 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067429supp001.pdf (86.5KB, pdf)
bmjopen-2022-067429supp002.pdf (89.8KB, pdf)
bmjopen-2022-067429supp003.pdf (96.7KB, pdf)
bmjopen-2022-067429supp004.pdf (129.1KB, pdf)


