Abstract
Objectives
To determine the aetiological pathogens causing ear infections and their antimicrobial susceptibility patterns among patients with ear complaints at a tertiary hospital in Dar es Salaam.
Design
Hospital-based cross-sectional study.
Settings
Otorhinolaryngology clinic at Muhimbili National Hospital, Dar es Salaam, Tanzania.
Participants
Patients presenting with signs and symptoms of ear infection.
Main outcome measure
Bacteria and fungi isolated from ear swab specimens of patients presenting with signs and symptoms of ear infection; and antimicrobial susceptibility patterns of isolated bacteria.
Results
Two hundred and fifty-five participants were enrolled, with a median age of 31 years and an IQR of 15–49. Otitis externa was the predominant type of ear infection, accounting for 45.1%. We observed positive bacteria culture in 53.3% of study participants, in which 41% of isolates were obtained from patients with chronic suppurative otitis media. Moreover, Staphylococcus aureus (27.3%) and Pseudomonas aeruginosa (24.2%) were the most frequently isolated bacteria, while Candida spp, 12 (63.8%) and Aspergillus spp, 9 (36.2%) were the only isolated fungi. Furthermore, we report that 93% of isolated Enterobacterales were resistant to amoxicillin/clavulanic acid, and 73% were resistant to ceftazidime. In addition, we detected 34.4% extended-spectrum beta-lactamase-producing Enterobacterales (ESBL-PE) and 44.4% methicillin-resistance S. aureus (MRSA). We also found that 22% of the bacteria isolates were resistant to ciprofloxacin, a primary topical antibiotic used in managing ear infections.
Conclusions
The findings from this study reveal that the leading aetiological agent of ear infection is bacteria. Furthermore, our findings show a significant proportion of ESBL-PE and MRSA-causing ear infections. Hence, detecting multidrug-resistant bacteria is crucial to improving ear infection management.
Keywords: Microbiology, MYCOLOGY, BACTERIOLOGY
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study has some strengths, we report the common bacterial and fungi aetiology of ear infection in our study setting.
Notably, the study has revealed the antimicrobial susceptibility patterns that are useful in guiding the choice of empirical treatment in similar settings with limited resources and comparable geographical, demographic and social characteristics.
This study has some limitations; some fungal (moulds) isolates were not identified to species level.
Anaerobic culture was not performed.
Introduction
An ear infection is among the leading causes of deafness in many low/middle-income countries. Unfortunately, most patients with ear infections in resource-limited settings delay seeking medical attention; hence, usually present with complications.1 Bacteria are the leading pathogens of ear infection, whereby, Staphylococcus aureus, Pseudomonas aeruginosa, Proteus mirabilis and Klebsiella species are the dominant bacteria causing ear infection globally.1–6 In addition, Candida spp and Aspergillus spp are predominant fungal isolates responsible for ear infections.7–10 However, due to limited diagnostic opportunities, fungal ear infections are often undiagnosed, especially in resource-limited countries, including Tanzania.5 6
Most practitioners in our settings tend to treat ear infections empirically or adhere to the standard treatment guideline (STG) without considering laboratory investigation and antimicrobial susceptibility testing (AST) results. This has created a gap in managing most ear infections, which raises the risk of acquiring multidrug-resistant bacteria.11 12 When first-line antibiotics cannot treat diseases, more costly antibiotics must be used. This consequently affects patients’ treatment options, resulting in prolonged hospital stays and increased healthcare costs, which impacts families’ financial burden and quality of life.13 Furthermore, there needs to be more data on the effectiveness of empirical treatment in managing ear infections in Tanzania. However, experience based on the clinic’s patient return rate after initial treatment for ear infections, it appears that a considerable number of patients return to the clinic with the same problem. This suggests that relying solely on empirical treatment methods may not be effective in treating ear infections. Hence, this warrants further research to investigate the antimicrobial susceptibility patterns of bacteria isolated in ear infections to improve the outcome of ear infections following appropriate empirical treatment.
Aetiological studies of ear infections are essential to guide the choice of an effective antibiotic and monitor bacterial patterns and their varying antimicrobial susceptibilities. This is crucial for risk analysis, mitigation measures and logistical plans. Therefore, this study aimed to determine the aetiological pathogens and antimicrobial susceptibility patterns of bacteria-causing ear infections. The data obtained, if used, will strengthen the prevention and control measures and update the management and treatment options for ear infections. Also, the information will serve as a baseline for countrywide surveillance of antibiotic resistance.
Materials and methods
Study design and settings
We conducted a hospital-based cross-sectional study from March to July 2021 in the otorhinolaryngology clinic at Muhimbili National Hospital (MNH), Dar es Salaam, Tanzania. MNH is the leading national referral hospital, research centre and a university teaching hospital. It is the largest tertiary healthcare facility in Tanzania. The hospital has a capacity of 1500 beds, attending from 1000 to 1200 outpatients per week and admitting from 1000 to 1200 inpatients per week. The otorhinolaryngology department has inpatient and outpatient units; about 20–30 patients attend the outpatient clinic per day.
Study participants
The study included patients attending the otorhinolaryngology clinic with signs and symptoms of ear infection, such as accumulation of fluid in the middle ear, bulging of the eardrum, ear pain, ear itching, perforation of the eardrum and ear discharge (otorrhoea). We excluded patients with other hearing disorders unrelated to infection (congenital malformations, physical head injury) and those on regular check-ups.
Sample size and sampling procedure
The study sample size was estimated using a Kish Leslie formula (1965) for a cross-sectional study considering the prevalence of 62.1% reported previously by Mushi et al in a study conducted in a tertiary hospital in Mwanza city, Tanzania.3 The minimum sample size was 241 participants; considering the 5% non-response rate, we obtained a sample size of 255 participants.
Data collection
Data collection was conducted by two trained research assistants (RAs) and an ear, nose and throat (ENT) surgeon; briefly, a structured questionnaire was administered to the participants by two RAs. RAs used the questionnaire to collect demographic data (age, sex, marital status, occupation and education) and behavioural risk characteristics (swimming, frequent use of earphones, cotton buds, sharp objects and cigarette smoking). In addition, the participants’ clinical information, including the type of ear infection, use of antibiotics, nasal congestion or blockage, recurrent upper respiratory tract infection (URTI), and cerumen impaction, was also collected from the patient’s medical records and during a physical examination by ENT surgeon. In this study, chronic suppurative otitis media (CSOM) was diagnosed when there is persistent otorrhoea from the ear for at least 3–12 weeks despite appropriate medical treatment or when there is a persistent eardrum perforation with otorrhoea for more than 3 months. This chronicity of otorrhoea distinguishes CSOM from acute otitis media, a short-term middle ear infection with acute onset and rapid resolution.
Specimen collection
The ENT surgeon collected specimens with precaution to prevent contamination. The sterile swab was used to clear the oozing pus from the patient’s ear; another sterile swab was then used to collect fresh pus. The collected specimens were kept at room temperature in Stuart’s transport media before processing at central pathology laboratory.
Isolation and identification
On arrival in the laboratory, specimens were processed for culture and identification. Each specimen was inoculated on selective and non-selective media: chocolate agar (CA), sheep-blood agar, MacConkey agar (MCA) and Sabouraud dextrose agar (SDA). We used CA to isolate fastidious bacteria, such as Haemophilus influenzae and Streptococcus pneumoniae, the frequent aetiological agents of ear infection. MCA was used as a selective and differential medium for Gram-negative bacteria, and BA was used as a general-purpose medium. SDA was used for the isolation of fungal species. We incubated MCA in an aerobic environment and BA and CA in a 5% CO2 environment at 37°C for 18–24 hours.
Bacterial isolates were identified by interpreting colonial morphologies, microscopic examination (Gram stain) and biochemical tests. The catalase and coagulase tests were performed for Gram-positive bacteria, while Kligler iron agar, sulfur indole motility, citrate and urease tests were for gram-negative bacteria. Further, phenotypical identification and confirmation of Gram-negative bacterial isolates were performed by Analytical Profile Index tests, API 20E and API 20NE.
For fungal isolates, growth on the SDA plate was used preliminary to classify mould or yeast based on the colonial morphology and colour. A germ tube test was used to identify Candida albicans. In addition, lactophenol cotton blue was used for moulds to identify the conidial spore in Aspergillus spp.
Antimicrobial susceptibility testing
AST for bacterial isolates was performed using the Kirby-Bauer disc diffusion method on Mueller-Hinton agar (MHA), and MHA supplemented with 5% blood for S. pneumonia following the 2021 Clinical and Laboratory Standard Institute (CLSI) guidelines. Zones of inhibition were measured using a ruler in millimetres and interpreted as susceptible, resistant or intermediate according to the 2021 CLSI guideline.
The antibiotic discs used were as follows: Ciprofloxacin (5 µg), trimethoprim/sulfamethoxazole (1.25/23.75 µg), gentamycin (10 µg), clindamycin (2 µg), erythromycin (15 µg),) for Gram-positive bacteria. Ciprofloxacin (5 µg), trimethoprim/sulfamethoxazole (1.25/23.75 µg), gentamycin (10 µg), meropenem (10 µg), amoxicillin/clavulanic acid (20 µg), ceftriaxone (30 µg) and ceftazidime (30 µg) for Enterobacterales and Acinetobacter spp. Ciprofloxacin (5 µg), gentamycin (10 µg), meropenem (10 µg) and ceftazidime (30 µg) for Pseudomonas spp.
Standard methods were used to identify methicillin-resistance S. aureus (MRSA) using cefoxitin (30 µg) disc in which resistant isolates were considered MRSA positive. In addition, extended-spectrum beta-lactamase-producing Enterobacterales (ESBL-PE) screening was done using ceftazidime (30 µg) and cefotaxime (30 µg) antibiotic discs, and if resistant, ESBL-PE confirmation was done by the double-disc synergy method.14
Quality control
The reference organisms and reagents were clearly and uniquely labelled, dated and stored at optimal conditions. The room, incubator and refrigerator temperatures were monitored daily. The culture media were prepared following the manufacturer’s guidelines and internal standard operating procedures and tested for performance and sterility.
Data analysis
The data were analysed by using SPSS V.23 software. Continuous variables were summarised as the median and IQR, whereas percentages and proportions were used to describe categorical variables. The resistance rate was obtained by computing the number of bacteria that resisted a specific drug over a total number of isolated bacterial species. AST intermediate results were regarded as resistant.
Reporting guideline
This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cross-sectional studies, which provide a checklist for reporting observational studies. The checklist includes crucial elements that should be included in the report, such as the study design, participant selection, data collection and statistical analysis. The authors have carefully reviewed the checklist to ensure that they incorporated each relevant item into the study design and analysis. The authors used a standardised data collection tool to collect information on all study participants and employed appropriate statistical methods to analyse the data and draw conclusions.
Patient and public involvement
Patients and the public were not involved in this research’s design, conduct, reporting or dissemination plans.
Results
Participants’ demographic, clinical and risk behaviour characteristics
Two hundred and fifty-five participants were recruited; 52.5% (134/255) were males. The median age was 31 years (IQR: 15–49). Most participants (30.2%) were students, 32.9% had a college education and 15.7% were from outside Dar es Salaam region (table 1).
Table 1.
Sociodemographic characteristics of the study participants (N=255)
| Variables | Frequency (N) and percentage (%)/median (IQR) |
| Median age (years) | 31 (15–49) |
| Sex | |
| Male | 134 (52.5) |
| Female | 121 (47.5) |
| Occupation | |
| Self-employed | 56 (22.0) |
| Civil servants | 62 (24.3) |
| Retired | 49 (19.2) |
| Unemployed | 88 (33.5) |
| Education | |
| Primary | 75 (29.4) |
| Secondary | 59 (23.1) |
| College | 84 (32.9) |
| Illiterate | 37 (14.5) |
| Residence | |
| Within Dar es Salaam | 215 (84.3) |
| Outside Dar es Salaam | 40 (15.7) |
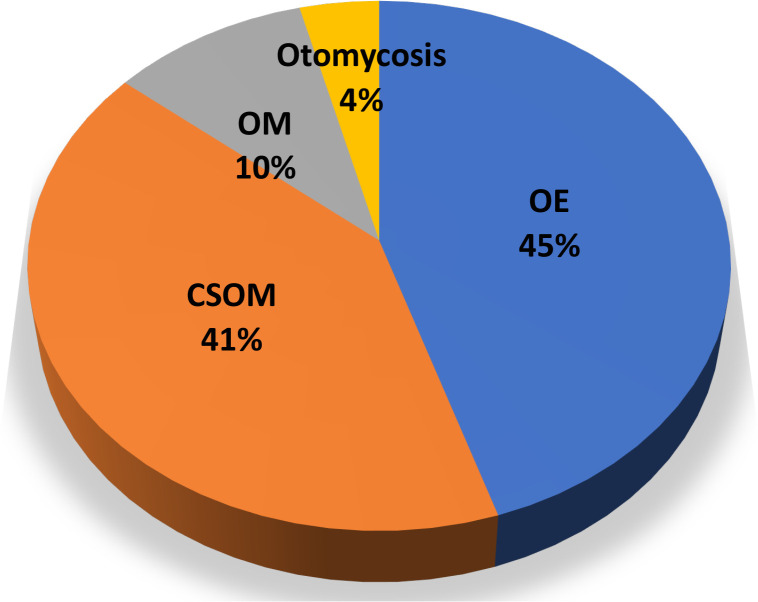
The median duration of ear infections was 210 days (IQR: 21–1095). Otitis externa (OE) was the most common type of ear infection, accounting for 45.1% (115/255), followed by CSOM (41.2%) (figure 1). Around 49% of the participants with ear infections had a history of antibiotic use, whereby ciprofloxacin eardrop was the most prescribed topical antibiotic. In addition, 33.3% of the study participants had nasal congestion/blockage/discharge, and 28.2% had recurrent URTI (table 2).
Figure 1.
Types of ear infection among study participants at MNH. The figure illustrates the distribution of ear infections among patients presenting with signs and symptoms of ear infection attending the otorhinolaryngology clinic at MNH (N=255). CSOM, chronic suppurative otitis media; MNH, Muhimbili National Hospital; OE, otitis externa; OM, otitis externa.
Table 2.
Baseline clinical and risk behavioural characteristics of the study participants (N=255)
| Patient characteristics | Frequency (N) and percentage (%)/median (IQR) (%) |
| Median duration of ear infection (days) | 210 (21–1095) |
| Nasal discharge/blockage | |
| Yes | 85 (33.3) |
| No | 170 (66.7) |
| Recurrent URTI | |
| Yes | 72 (28.2) |
| No | 183 (71.8) |
| Use of hearing aid | |
| Yes | 2 (0.8) |
| No | 253 (99.2) |
| Earphone use | |
| Yes | 41 (16.1) |
| No | 214 (83.9) |
| Swimming | |
| Yes | 8 (3.1) |
| No | 247 (96.9) |
| Cotton bud use | |
| Yes | 112 (43.9) |
| No | 143 (56.1) |
| Sharp object use | |
| Yes | 60 (23.5) |
| No | 195 (76.5) |
| Ear cleaning habit | |
| Yes | 119 (46.7) |
| No | 136 (53.3) |
| Cerumen impaction | |
| Yes | 45 (17.6) |
| No | 210 (82.4) |
URTI, upper respiratory tract infection.
Distribution of bacterial and fungal isolates causing ear infections
In this study, 136 out of 255 (53.3%) participants had a positive aerobic culture for either bacterial or fungal pathogen, whereby 10.3% (14/136) of participants had a polymicrobial infection (mixed growth of either two different bacteria or bacterial and fungal infection). A total of 150 isolates (bacteria and fungi) were identified, of which 87.3% (131/150) were bacteria. Of the isolated bacteria, Gram-negative, 71.0% (93/131) were predominant.
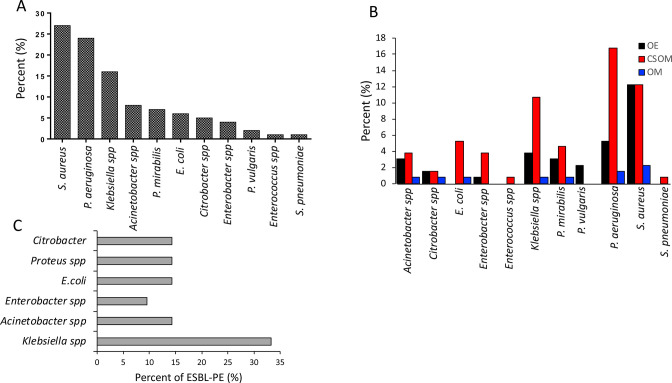
The predominant bacterial isolates were S. aureus, 27.5% (36/131), followed by P. aeruginosa, 24.4% (32/131) (figure 2A). On the other hand, Candida spp accounted for 63.2% (12/19) of the isolated fungi (data not shown). Moreover, 41% of isolates were obtained from CSOM patients. Further stratification of isolated pathogens by type of ear infection showed that S. aureus 16/131 (12.2%) was the most prevalent bacterium in OE patients, whereas P. aeruginosa 22/131 (16.8%) predominated in CSOM patients (figure 2B).
Figure 2.
(A–C) Distribution of bacterial isolates. The figure depicts the distribution of bacteria spp isolated among patients with ear infections attending the otorhinolaryngology clinic at MNH (n=131) (A). According to the type of ear infection (n=131), where OM (otitis media), OE (otitis externa) and CSOM (chronic suppurative otitis media) (B). Distribution of ESBL-producing bacteria among isolated gram-negative bacteria in patients attending the otorhinolaryngology clinic at MNH (n=61) (C). ESBL, extended-spectrum beta-lactamase-producing Enterobacterales; MNH, Muhimbili National Hospital.
In this study, 34.4% (21/61) of the Enterobacterales, excluding P. aeruginosa, were ESBL-PE; and Klebsiella spp was predominant, accounting for 33.3% (7/21) of the ESBL-PE isolates (figure 2C). On the other hand, 44.4% (16/36) of the S. aureus species were MRSA (data not shown).
Antimicrobial susceptibility pattern of bacterial isolates
Almost all (93%) isolated Enterobacterales were resistant to amoxicillin/clavulanic acid, more so Escherichia coli and Acinetobacter spp were 100% resistant. Also, 73% of isolated bacteria were resistant to ceftazidime (data not shown), whereby P. aeruginosa had the highest resistance rate of 75%. In addition, 43% of isolated bacteria were resistant to trimethoprim-sulfamethoxazole (data not shown), whereby E. coli was leading with a 75% resistance rate. Sulfamethoxazole-trimethoprim resistance rates ranged from 57% to 100% among ESBL producers, higher than 29%–100% among non-ESBL producers. Moreover, 14.6% (6/41) of the non-ESBL-PE bacteria were resistant to all the third-generation cephalosporins, and all non-ESBL-PE isolates were sensitive to meropenem. S. aureus had an 89% resistance rate to erythromycin. However, MRSA isolates were more resistant to sulfamethoxazole-trimethoprim (81%) and gentamicin (50%) than non-MRSA isolates 35% and 25% for sulfamethoxazole-trimethoprim and gentamicin, respectively. In this study, we report that resistance to ciprofloxacin, a primary topical antibiotic used to manage ear infections, is 22%. Most isolated bacteria had a low resistance rate against meropenem (4%) (table 3).
Table 3.
Antimicrobial resistance pattern for isolated bacteria
| Antibiotic | Bacteria isolates | |||||||
| Staphylococcus aureus (N=36) | Pseudomonas aeruginosa (N=32) | Klebsiella spp (N=20) |
Acinetobacter spp (N=10) |
Enterobacter spp (N=6) |
Escherichia coli (N=8) | Proteus spp (N=12) |
Citrobacter spp (N=5) |
|
| Amikacin | NA | 8 (25) | 4 (20) | 2 (20) | 3 (50) | 2 (25) | 1 (8) | 2 (40) |
| Sulfamethoxazole trimethoprim | 20 (56) | NA | 8 (40) | 1 (10) | 4 (66) | 6 (75) | 3 (25) | 4 (80) |
| Gentamicin | 13 (36) | 6 (19) | 9 (45) | 1 (10) | 1 (17) | 1 (13) | 7 (58) | 1 (20) |
| Ciprofloxacin | 11 (31) | 11 (34) | 1 (5) | 0 (0) | 2 (33) | 2 (25) | 1 (8) | 0 (0) |
| Amoxicillin/clavulanic acid | NA | NA | 18 (90) | 10 (100) | 5 (83) | 8 (100) | 11 (92) | 5 (100) |
| Ceftriaxone | NA | NA | 9 (45) | 5 (50) | 3 (50) | 5 (63) | 6 (50) | 3 (60) |
| Ceftazidime | NA | 24 (75) | 14 (70) | 7 (70) | 4 (66) | 5 (63) | 10 (83) | 4 (80) |
| Cefotaxime | NA | NA | 9 (45) | 8 (80) | 3 (50) | 5 (63) | 6 (50) | 5 (100) |
| Meropenem | NA | 2 (6) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 1 (8) | 0 (0) |
| Erythromycin | 32 (89) | NA | NA | NA | NA | NA | NA | NA |
| Clindamycin | 9 (25) | NA | NA | NA | NA | NA | NA | NA |
| Cefoxitin | 16 (44) | NA | NA | NA | NA | NA | NA | NA |
NA, not applicable.
Discussion
Understanding the aetiology of ear infections and resistance pattern is crucial in planning interventions and managing ear infections. The results indicate a substantial proportion of ear infections, with bacteria as the primary aetiological agent. Most isolated bacteria were resistant to third-generation cephalosporins, sulfamethoxazole-trimethoprim and amoxicillin/clavulanic acid. Gram-positive bacteria were highly resistant to erythromycin. The two antibiotics that worked the best were ciprofloxacin and meropenem. The results imply the need to review ear infection management and the selection of an efficient antibiotic.
The study found that many ear infections are of bacterial aetiology. The finding is similar to studies done in Tanzania by Kennedy et al in Morogoro,4 Zephania et al in Dar es Salaam,15 Martha et al in Mwanza3 and other studies in Kenya and India.16 17 Furthermore, we observed that S. aureus and P.s aeruginosa are ear infections’ leading bacterial aetiological agents, similar to previous studies in Tanzania, Nigeria, Angola, Kenya and India.3 17–19 In addition, this study found Candida spp and Aspergillus spp the fungal spp, causing ear infections consistent with previous findings in Tanzania and elsewhere (Nigeria, Iran, Ethiopia, Egypt and India).3–5 20–22 Nonetheless, the contribution of fungi aetiology in ear infections in this study was expected because many individuals had risk behaviours for fungal ear infections, including excessive use of eardrops containing antibiotics, regular cleaning of ears and swimming. Antibiotic overuse promotes the growth of fungi, and the regular ear cleaning habit removes cerumen and exposes ears to fungi colonisation and, subsequently, infection.23 24
The current study revealed a high proportion of MRSA (44.4%) and ESBL-PE (34.4%). In addition, our study showed Klebsiella spp (33.3%) as the dominant ESBL-PE. The higher proportion of MRSA and ESBL-PE coincides with studies done in Tanzania by Martha et al among patients with CSOM infection and another study in India.3 16 The greater inclination for self-prescribing and empirically prescribing antibiotics without considering laboratory culture and sensitivity may explain the higher proportion of ESBL and MRSA. Furthermore, an increased tendency for people to visit hospital facilities due to chronic ear infections can also explain the high incidence of ESBL and MRSA, which raises the danger of exposure to muiltidrug-resistant (MDR) bacteria. In addition, the tendency to use inanimate objects to remove earwax can be attributed to the increased proportion of ESBL and MRSA, as these inanimate objects are often found in environments that may be contaminated with ESBL-producing bacteria and MRSA.25
Almost all isolated bacteria (93%) were resistant to amoxicillin/clavulanic acid. Nearly three-quarters of Gram-negative bacteria were resistant to ceftazidime, and about half were resistant to trimethoprim-sulfamethoxazole. On the other hand, 89% of isolate Gram-positive were resistant to erythromycin. ESBL-PE and MRSA isolates were resistant to the most common antimicrobial agents compared with non-MRSA and non-ESBL-PE. The resistance patterns found in the current study are similar to those reported in other studies in Tanzania, Kenya, Ethiopia, India, Egypt and Romania.3 4 17 18 26–29 The frequent use of these antibiotics to treat various bacterial infections in our setting and the likelihood that most bacterial species have developed resistance to antimicrobial drugs over time may contribute to the observed resistance pattern.
In this study, most isolated bacteria were sensitive to meropenem and ciprofloxacin. Ciprofloxacin is a drug of choice for ear infections as per STGs in our setting. The fact that meropenem is infrequently used to treat ear infections may explain the high sensitivity rate. Surprisingly, we observed that ciprofloxacin is still effective despite being prescribed often in our setting for treating ear infections. There is no clinical rationale for why quinolones are still more effective in treating ear infections. However, these results assure that quinolones are still beneficial as first-line topical antibiotics for ear infections.
This study has some limitations. We were not able to identify the fungi isolates to species level. This is due to insufficient funding and the availability of resources. To mitigate this, all fungi isolates were stored appropriately for future testing. In addition, due to financial constraints and lack of equipment, it was impossible to isolate anaerobic bacteria from the collected pus specimen.
Conclusion
The results of this study indicate that bacteria are the most common cause of ear infections in our context. Furthermore, we report that many multidrug-resistant bacteria (ESBL-PE and MRSA) are implicated in causing ear infections. Therefore, antimicrobial susceptibility testing is crucial to guide clinicians on appropriately managing ear infections in our setting.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the Muhimbili National Hospital (MNH) administration and the Mwanza University, Tanzania, for their support. The authors are also grateful to all the study participants, the otorhinolaryngology clinic staff and CPL staff at MNH.
Footnotes
Contributors: AS, DK and WM contributed to the conceptualisation, data collection, and analysis study. AS, DK, WM, UK, AGM, AM, SM, AMM, MM, SEM, JM and MM were involved in manuscript preparation. JM and MM profoundly reviewed the manuscript. AS is the guarantor of the study. The corresponding author attests that all listed authors meet authorship criteria and that no one meeting the criteria has been omitted. The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Data availability statement
Data are available on reasonable request. All relevant data generated and analysed during this study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The ethical approval was obtained from the Muhimbili University of Health and Allied Sciences (MUHAS), Senate Research and Publication Committee. The reference number is DA.282/298/01.C/. Participants gave informed consent to participate in the study before taking part.
References
- 1.Schilder AGM, Chonmaitree T, Cripps AW, et al. Otitis media. Nat Rev Dis Primers 2016;2:16063. 10.1038/nrdp.2016.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson H, Wallis S, Coatesworth AP. Acute otitis media. Postgrad Med 2015;127:386–90. 10.1080/00325481.2015.1028872 [DOI] [PubMed] [Google Scholar]
- 3.Mushi MF, Mwalutende AE, Gilyoma JM, et al. Predictors of disease complications and treatment outcome among patients with chronic suppurative otitis media attending a tertiary hospital, Mwanza Tanzania. BMC Ear Nose Throat Disord 2016;16:1. 10.1186/s12901-015-0021-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mwambete KD, Eulambius M. High prevalence of antibiotic-resistant otitis media-associated bacterial flora of asymptomatic people living with HIV at Morogoro hospital, Tanzania. J Int Assoc Provid AIDS Care 2018;17:2325958218759761. 10.1177/2325958218759761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mushi MF, Buname G, Bader O, et al. Aspergillus fumigatus carrying TR34/L98H resistance allele causing complicated suppurative otitis media in Tanzania: call for improved diagnosis of fungi in sub-Saharan Africa. BMC Infect Dis 2016;16:464. 10.1186/s12879-016-1796-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minja BM, Machemba A. Prevalence of otitis media, hearing impairment and cerumen impaction among school children in rural and urban Dar ES Salaam, Tanzania. Int J Pediatr Otorhinolaryngol 1996;37:29–34. 10.1016/0165-5876(96)01363-8 [DOI] [PubMed] [Google Scholar]
- 7.Sinha A, Mohapatra LN. Otomycosis - A clinical and mycological study. Indian J Otolaryngol 1961;13:128–37. 10.1007/BF03047899 [DOI] [Google Scholar]
- 8.Vaidya K, Madhup SK, Shrestha BL, et al. Bacteriological and mycological profile of chronic suppurative otitis media among patients visiting dhulikhel Hospital. Ann Clin Chem & Lab Med 2015;1:37–41. 10.3126/acclm.v1i1.12314 [DOI] [Google Scholar]
- 9.Martin TJ, Kerschner JE, Flanary VA. Fungal causes of otitis externa and tympanostomy tube otorrhea. Int J Pediatr Otorhinolaryngol 2005;69:1503–8. 10.1016/j.ijporl.2005.04.012 [DOI] [PubMed] [Google Scholar]
- 10.Kumar A. Fungal spectrum in otomycosis patients. JK Sci 2005;7:152–5. [Google Scholar]
- 11.Hall JW, Bouchard J, Bookstaver PB, et al. The Mbeya antimicrobial stewardship team: implementing antimicrobial stewardship at a zonal-level hospital in southern Tanzania. Pharmacy (Basel) 2020;8:107. 10.3390/pharmacy8020107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seni J, Mapunjo SG, Wittenauer R, et al. Antimicrobial use across six referral hospitals in Tanzania: a point prevalence survey. BMJ Open 2020;10:e042819. 10.1136/bmjopen-2020-042819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NPS Medicinewise . Antibiotic resistance. Prev Control; 2010. 2005–6.Available: http://www.nps.org.au/__data/assets/pdf_file/0009/318879/NPSMW-antibiotic-resistance-the-facts.pdf
- 14.Testing S. M100 performance standards for antimicrobial. [Google Scholar]
- 15.Abraham ZS, Ntunaguzi D, Kahinga AA, et al. Prevalence and etiological agents for chronic suppurative otitis media in a tertiary hospital in Tanzania. BMC Res Notes 2019;12:429. 10.1186/s13104-019-4483-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavani K, Krishnamurthy S, K S S, et al. Chronic suppurative otitis media (CSOM): evaluation of fungal and aerobic bacterial agents and antibiotic sensitivity pattern of the bacterial isolates. IJMMTD 2020;5:214–7. 10.18231/j.ijmmtd.2019.049 [DOI] [Google Scholar]
- 17.Aduda DSO, Macharia IM, Mugwe P, et al. Bacteriology of chronic suppurative otitis media (CSOM) in children in Garissa district, Kenya: a point prevalence study. Int J Pediatr Otorhinolaryngol 2013;77:1107–11. 10.1016/j.ijporl.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 18.Kumar D, Agrawal A, Kumar S, et al. A study of the microbiological profile of CSOM in A tertiary care centre of north india. J Dent Med Sci 2019;18:20–4. 10.9790/0853-1805122024 [DOI] [Google Scholar]
- 19.Uddén F, Filipe M, Reimer Å, et al. Aerobic bacteria associated with chronic suppurative otitis media in Angola. Infect Dis Poverty 2018;7:42. 10.1186/s40249-018-0422-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia X, Liang Q, Chi F, et al. Otomycosis in Shanghai: aetiology, clinical features and therapy. Mycoses 2012;55:404–9. 10.1111/j.1439-0507.2011.02132.x [DOI] [PubMed] [Google Scholar]
- 21.Kiakojuri K, Armaki MT, Rajabnia R, et al. Outer ear infections in Iran: a review. Open Access Maced J Med Sci 2019;7:1233–40. 10.3889/oamjms.2019.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenna MA. Etiology and pathogenesis of chronic suppurative otitis media. Ann Otolaryngol 1988;97:2:16–7. 10.1177/00034894880970s205 [DOI] [Google Scholar]
- 23.Viswanatha B, Naseeruddin K. Fungal infections of the ear in immunocompromised host: a review. Mediterr J Hematol Infect Dis 2011;3:e2011003. 10.4084/MJHID.2011.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shuaib Kayode A, Kayode Rasaq A, Tayo I. A prospective analysis of otomycosis in a tertiary care hospital. Int J Trop Dis 2020;3:1–8. 10.23937/2643-461X/1710029 [DOI] [Google Scholar]
- 25.Endaylalu K, Abera B, Mulu W. Extended spectrum beta lactamase producing bacteria among outpatients with ear infection at felegehiwot referral hospital, North West Ethiopia. PLoS One 2020;15:e0238891. 10.1371/journal.pone.0238891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allam AAE, Tantawy AEE, Mohamed KAE, et al. Short communication: otitis externa in a tertiary care hospital in zagazig, Egypt: isolated pathogens and their antibiotic sensitivity patterns. Af J Clin Exp Micro 2019;21:60. 10.4314/ajcem.v21i1.8 [DOI] [Google Scholar]
- 27.Argaw-Denboba A, Abejew AA, Mekonnen AG. Antibiotic-resistant bacteria are major threats of otitis media in wollo area, northeastern Ethiopia: a ten-year retrospective analysis. Int J Microbiol 2016;2016:8724671. 10.1155/2016/8724671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anghelina F, Zlatian O, Ciolofan MS. Bacteriological profiles and antibiotic susceptibility patterns in complicated bacterial infections of the ears, nose and throat. RevChim 2019;70:4425–31. 10.37358/RC.19.12.7770 [DOI] [Google Scholar]
- 29.Seid A, Deribe F, Ali K, et al. Bacterial otitis media in all age group of patients seen at dessie referral hospital, north east Ethiopia. Egypt J Ear Nose Throat Allied Sci 2013;14:73–8. 10.1016/j.ejenta.2013.02.005 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request. All relevant data generated and analysed during this study are available from the corresponding author on reasonable request.