Abstract
Spatial navigation and memory are often seen as heavily intertwined at the cognitive and neural levels of analysis. We review models that hypothesize a central role for the medial temporal lobes, including the hippocampus, in both navigation and aspects of memory, particularly allocentric navigation and episodic memory. While these models have explanatory power in instances in which they overlap, they are limited in explaining functional and neuroanatomical differences. Focusing on human cognition, we explore the idea of navigation as a dynamically acquired skill and memory as an internally driven process, which may better account for the differences between the two. We also review network models of navigation and memory, which place a greater focus on connections rather than the functions of focal brain regions. These models, in turn, may have greater explanatory power for the differences between navigation and memory and the differing effects of brain lesions and age.
Spatial navigation and memory processing share some important commonalities. For example, if you want to take a short cut between two familiar paths on campus, you need to have some memory for how the unseen paths are oriented in terms of their spatial geometry. In this way, memory plays an important role in guiding our decisions during navigation. Accordingly, some models of navigation and memory have placed a heavy emphasis on their similar underlying cognitive processes and, by some accounts, their dependance on largely overlapping brain structures (for relevant reviews and theoretical proposals, see1, 2, 3, 4, 5, 6). The core idea behind these models is that some form of memory representation involving the directions and distances of locations relative to each other (and the navigator) is central to much of wayfinding. The medial temporal lobe has often been a focus of the commonalities between navigation and memory, with a particular focus on the hippocampus.
It is also increasingly clear that spatial navigation and memory involve substantial differences, both cognitively and in terms of the underlying brain structures and neural mechanisms that support them. While the commonalities between navigation and memory, such as those between allocentric navigation and episodic memory, are highly relevant to understanding the two, we will also review recent literature and models emphasizing their differences. Navigation can perhaps best be conceived as involving continuous sensory input, be it idiothetic (body-based, such as vestibular) or visual (including optic flow and landmarks), involving learning how to weight different cues and employ different heuristics depending on environmental demands. In contrast, episodic memory can be thought of as a largely internally driven process that, while often dependent on an initial cue, can function largely independently of external cues once initiated.
In this review, we will discuss current models of navigation and memory and the explanations they provide in terms of their functional similarities and differences. As part of these considerations, we will cover models that place a heavy emphasis on a role for the medial temporal lobes in both memory and navigation, particularly, episodic memory (i.e., event-memory) and allocentric navigation (i.e., wayfinding with reference to multiple external landmarks). We will also explore the idea that navigation and memory involve important differences. This will lead us into consideration of network models, which place greater weight on the connections between brain areas rather than the computational functions of a focal brain region, such as the hippocampus. We will also consider the explanatory power of focal and network models of navigation and memory in the context of brain lesions and aging, both of which are thought to affect navigation and memory performance.
Navigation as a dynamically acquired complex cognitive motor skill
In many situations, memory is undoubtedly a part of successfully navigating from one location to another. It is increasingly clear, however, that navigation has fundamental differences from memory. Navigation can be thought as involving two fundamental forms of sensory input: idiothetic and visual cues. Idiothetic cues involve changes in vestibular, somatosensory, proprioceptive, and motor efferent signals which are continuously present as we move our body through space. Visual cues, including optic flow (which provides additional idiothetic cues) and perception of external landmarks and boundaries provide input that, when integrated with internally generated body-based idiothetic cues, can be used to compute relative distances, directions, and track one’s location in space. As pointed out in other papers, this typically involves a dynamic comparison between idiothetic and visual cues which, in many cases, may involve little need for detailed memory about previous locations7, 8. Consider the simple task of finding something to eat. When searching for a restaurant, you have some idea about how far you have walked based on your body-senses (i.e., idiothetic cues); as you ambulate, you actively look for the restaurant you are searching for (i.e., beaconing to a landmark). More generally, navigation likely involves a continuum of demands on memory, with some forms of navigation placing little demand on memory (e.g., beaconing, Figure 1A) and others placing greater demands on memory (e.g., wayfinding, Figure 1A,B). A critical part of successfully navigating is knowing what sensory information (idiothetic vs. visual) and what forms of memory are most optimal to the situation at hand; in this way, navigation may better reflect a dynamically acquired skill rather than a map-like memory for a spatial environment.
Figure 1: Differences and similarities between navigation and memory.
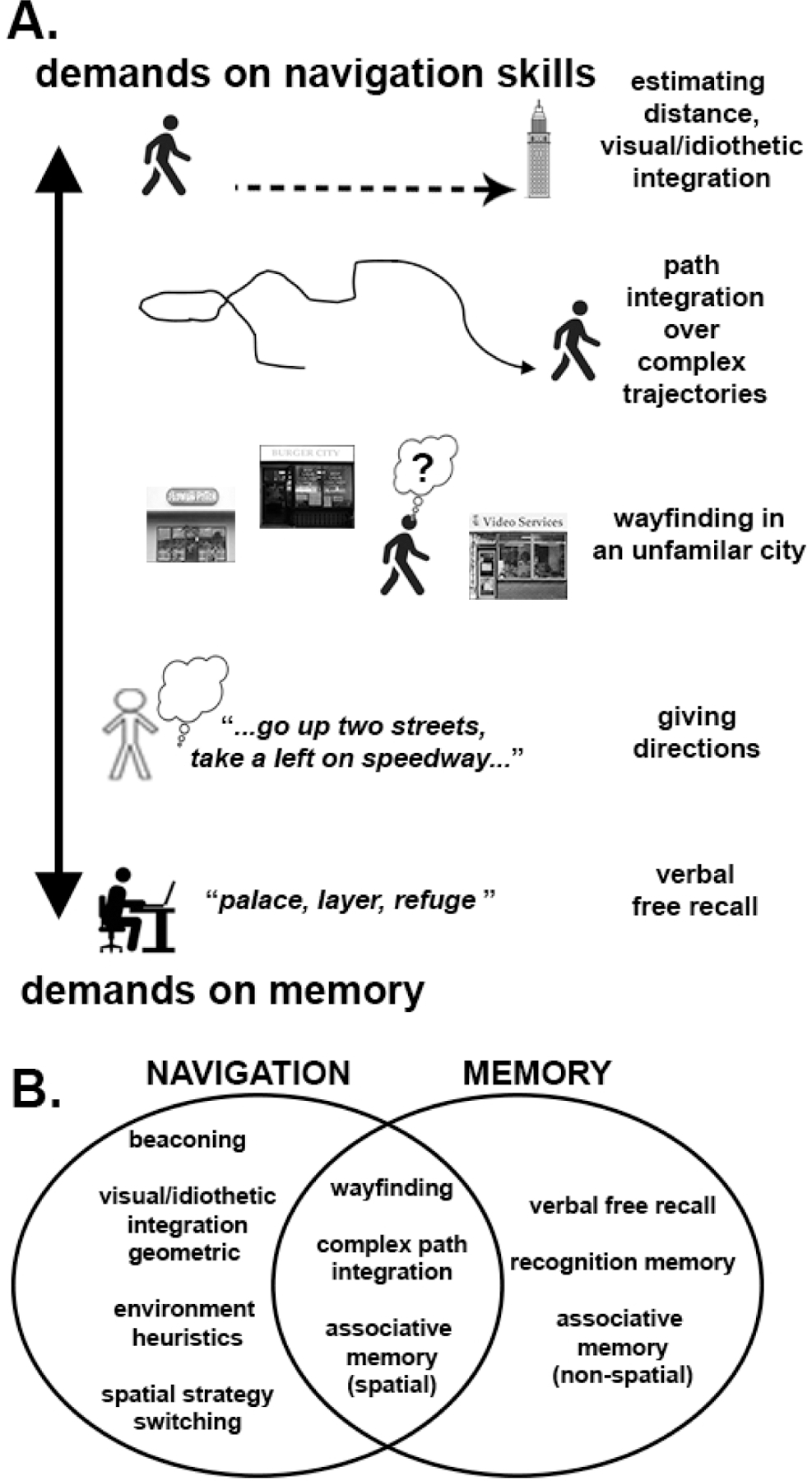
A. Different everyday tasks place different levels of demand on memory and navigation. Estimating distance from a beacon or integrating idiothetic (body-cues) and visual landmarks while walking involves little demand on memory but high demands on navigation skill. On the other end of the spectrum, verbal free recall places little demand on navigation skills but heavy demand on episodic and semantic memory, particularly temporal order.
B. Venn diagram of navigation and memory. There are areas of intersection involving navigation and memory, such as wayfinding, which typically involves remembering paths, and their geometry, and using these to find short cuts. Wayfinding thus involves relatively equal demands on memory and navigation. In contrast, there are many forms of navigation and memory that involve little overlap, consistent with the examples shown in A.
In two recent studies9, 10, the environment participants navigated previously (for example, when growing up) influenced how they navigated their current environment. In one large sample study involving millions of participants collected using the mobile app “Sea Hero Quest” (SHQ), those who reported growing up outside of a city consistently performed better on measures of wayfinding. Likewise, those who grew up in locations with higher “entropy” (curvier and less organized street grids) showed better navigation performance. Interestingly, better wayfinding performance on levels of SHQ with greater entropy correlated with growing up in less grid-like environments, suggesting that the environment one grows up in influences navigation9. The study also found a correlation (r=.1) between video game skill and SHQ performance. Therefore, it is important to validate these basic findings in real-world settings because of the lack of self-motion cues in desktop virtual reality may not fully capture real-world navigation11 and previous computer experience may benefit virtual navigation, a particular issue when studying older adults12.
The observation that navigational skills can be strongly affected by one’s home environment is supported by a recent study that compared participants living in Salt Lake City, Utah with Padua, Italy. Salt Lake City has a low entropy grid orientation with distal mountain landmarks surrounding the city) while Padua lacks a geometrical organization and instead requires learning paths based on proximal landmarks.10 Both groups of participants were matched in terms of age, education, and mental rotation abilities. Nonetheless, they showed significant differences in performance on a virtual version of the Morris Water Maze, which involves memory for a hidden target location, and the dual solution paradigm, which provides measures of taking short cuts and wayfinding13. Padua participants outperformed Utah participants at using proximal cues to navigate and taking short cuts, although interestingly, Salt Lake City participants did not show a greater likelihood to use distal cues. Padua participants also showed better performance at pointing to real-world familiar landmarks than those from Salt Lake City. These findings, similar to those obtained from the SHQ app, suggest that past experience strongly shapes current navigational ability.
Consistent with an influence of how past geographical experiences can affect navigation, an emerging perspective suggests that navigation might be better thought of as a complex cognitive-motor skill that can take years to develop and is often specific to the environment in which the navigator has the most experience (for a review and discussion of these ideas, see14). Take for example the Puluwat sailors of the south pacific who navigate, in some cases, thousands of miles of open ocean with no external aids. The Puluwat go through years of apprenticeship to master the ocean and wind currents, track star positions throughout the night, and learn heuristics for maintaining a constant bearing when exiting an island15. Contrast this with wilderness orienteering, which involves counting steps and memorizing unique combinations of mountain features and other local cues to track one’s bearing16. In the case of the Puluwat, navigation is finely tuned for the ocean environment (e.g., ocean and wind currents) while for wilderness orienteers, skills are developed and focused on land-based features. The idea of navigation as a skill is also consistent with the well-noted individual differences in navigation17, 18, which may stem in part from the different environments, conditions, and other factors that navigators experience throughout a lifetime. The idea of navigation as a skill may also help differentiate it from episodic memory, which (during retrieval) involves largely internally generated signals in response to verbal or non-verbal cues, rather than the continuous visual and vestibular updating typically characteristic of navigation.
Navigation is often conceptualized as involving decomposable systems comprised of egocentric and allocentric reference schemes. Egocentric navigation involves the use of objects or landmarks referenced to the navigator’s current position. This often involves retracing a well learned route or following a sequence of salient landmarks (e.g., take a left at the second stoplight and then turn right at the 7-Eleven). In contrast, allocentric navigation involves triangulating positions in reference to external landmarks rather than oneself (e.g., the student union on campus is located a certain distance and direction from other campus buildings). Allocentric navigation can allow for finding a target from a new location because the target is referenced to external landmarks, whose position stays constant, rather than the navigator, whose position changes continuously. The ideas of episodic / semantic memory and egocentric / allocentric navigation have played major roles in models focused on the similarities between navigation and memory, with models either hypothesizing converging brain circuits for episodic memory and allocentric navigation or for episodic memory / egocentric navigation and semantic memory / allocentric navigation1, 5, 19, 20, 21, ideas we explore critically in the neuroanatomical foundations section.
One of the difficulties in investigating allocentric vs. egocentric navigation relates to how we operationalize these terms and how easily one can be separated from the other. This relates to classically and frequently employed spatial navigation tasks such as the Morris Water Maze22. In these tasks, finding a hidden platform using distal cues is compared with finding the same platform when a brightly colored cue is placed above it22. The cue card, though, acts as a beacon, in which no spatial coordinates or spatial memory are required because it is visible from any location of the Morris Water Maze. The authors of some papers have pointed out that the appropriate control would be restarting from the same starting point23. Even if one observes a deficit for the so-called allocentric (repeated start location) vs. egocentric (repeated start location), it is not clear if this is due to impaired flexibility, difficulty, rotating an egocentric representation, or other task-related differences24, 25, 26. Nonetheless, despite some limitations in their explanatory power, egocentric/allocentric and episodic/semantic labels are often used to better isolate aspects of navigation and memory to specific neural systems and are terms we will consider in more detail as relates to underlying brain systems.
Declarative memory as a largely internally driven process
Memory is often divided broadly into declarative and non-declarative (e.g., procedural memory), with declarative memory further divided into episodic and semantic. Although navigation involves many procedural components, episodic and semantic memory have played significant roles in models of memory and navigation due to proposed commonalities with egocentric and allocentric navigation. Episodic memory, or memory for unique events in time and space, is strongly referenced to the self, allowing for mental time travel, termed autonoetic consciousness. Semantic memory, or memory for concepts or general facts, does not involve unique events nor strong anchoring in space and time27. Both episodic and semantic memory are often strongly verbal (sometimes referred to together as “declarative” memory28) and can be thought of as internally-driven. One example is verbal free recall, in which a participant studies a list of words and then recalls the words in any order. Computational models of memory suggest the encoding of the presented words occurs via internally generated associations, such as semantic associations and the spatiotemporal context29. Retrieval can operate almost completely independently of any external cues (other than “remember the list”). It also works perfectly well when stationary, somewhat in contrast to navigation, which benefits from path integration, the computational process of combining self-movement cues like optic flow and/or body-based idiothetic cues (Figure 1A)30.
There are important exceptions to the distinction between memory as largely internally driven and navigation as based largely on externally driven cues. As mentioned above, one form of memory involves non-declarative memory (“termed procedural memory”), which likely has significant overlaps with much of navigation. For example, a major component of memory likely involves procedural learning based on visuo-motor, visuo-tactile, and vestibulo-motor interactions, all of which play critical roles in way finding and whose impairment, in cases of cerebellar lesions, also lead to significant deficits in navigation31. In addition, verbal memory in everyday situations (rather than lab-based situations) may be more akin to navigation in terms of the continuous availability of externally generated cues. Nonetheless, such studies of memory in everyday situations (like recalling a visit to the museum32) emphasize the importance of internally-generated temporal context to both the success of encoding and retrieval of memories from the visit32. Finally, just as was discussed above for navigation33, episodic and semantic memories likely exist along a continuum and are often highly intertwined, even in lab-based situations. This is suggested by the high degree of overlap between the network of brain regions activated for recollective tasks (involving retrieving details associated with an encoded stimulus and thought to place greater demands on episodic memory) and conceptual processing (thought to place greater demands on semantic memory)34. Nonetheless, as will be discussed in more detail, meta-analyses comparing episodic/semantic tasks with allocentric/egocentric navigation tasks suggests only partial, and in some cases, no overlap between putative memory and navigation networks35.
Like navigation, verbal memory undoubtedly benefits from training although the manifestations are different than those for navigation training. Schema are a form of verbal semantic and non-verbal organization that helps improve memory by providing a scaffold on which to remember words, such as the method of loci36. While schema may share commonalities with those acquired through navigation (for a recent review, see3), there are also important differences between the schema employed for allocentric/egocentric navigation and episodic memory. Navigational schemas involve gradually learning the spatial structure of specific environments37 which may not always improve spatial memory. Consider for example the Borhost-Cates et al.10 study discussed above: those who grew up in a grid-like city had a disadvantage when trying to remember non-grid like environments while those navigating cities with non-regular paths had an advantage navigating. In contrast, both spatial (such as the method of loci) and temporal (autobiographical timelines) show equal benefits to verbal free recall38 and even emotionally-based schema may benefit memory36. It is unclear, however, how an autobiographical timeline or emotional-associational schema would benefit the more complex spatial representations often needed for successful navigation.
One way to view how training might differentially effect navigation compared to declarative memory is through the lens of individual traits, which may in turn interact with the ease of acquisition of spatial compared to verbal memory skills. A study by Malanchini et al. 202018 collected virtual navigation and neuropsychological data from a large group of monozygotic and di-zygotic twins, finding that both genetics and environment explained significant components of spatial navigation independent from other psychometric measures. Fan et al. 2021 collected a large sample (N=7,000) of participants who completed a survey about their self-assessed navigation, visual imagery for objects, visual imagery for space, and memory for events (i.e., episodic memory)39. The authors found that spatial navigation and memory ratings clustered separately based on a principal component (PCA) and partial least squares analysis. In addition, the authors found that episodic memory abilities tended to correlate with object imagery ratings while spatial navigation skills tended to relate to spatial imagery skills; for similar findings, see Malanchini et al. 2020. Together, these findings suggest that different traits may lead to different levels of performance at navigation and declarative memory (for a review, see40). For example, those with better spatial reasoning and quantitative skills may be better navigators while those with better visual imagery skills may have better episodic memories39. In addition, such traits may result in one individual being more amenable to training and development in one domain compared to another.
A recent paper by Waddington & Heisz 202341, who studied 150 orienteers (highly skilled wilderness navigators16), provides further evidence for the idea of navigation as a highly tuned cognitive-motor skill and as distinct from declarative memory. The orienteers studied had years of experience navigating in the wilderness as part of competitions using only a compass and map, often navigating off trail for miles. The questions specifically tasked what the authors hypothesized to be allocentric, egocentric, and procedural forms of navigation (with procedural navigation probably best related to the aforementioned beaconing). They were compared with a control group that exercised but did not participate in orienteering. The orienteers also answered questions about their episodic and semantic memory using the survey of autobiographical memory (SAM) used in the Fan et al. study39. The authors found that, depending on years of experience, orienteers endorsed greater use egocentric and allocentric strategies, with no difference between the two, significantly above what control participants endorsed. In contrast, there were no differences between controls and any of the orienteering groups for the SAM questionnaire. These findings support the idea that individual traits for highly skilled navigation and episodic memory likely develop independently, also supporting the idea that skilled navigators likely use a mixture of strategies involving egocentric and allocentric navigation to effectively find their way.
Navigation and memory: Neuroanatomical models that postulate emergence from computations in the medial temporal lobes
There are two broad perspectives when it comes to comparative models of memory and navigation centered within the hippocampus, which we term “focal brain models.” The first set of models we will consider postulates that allocentric navigation and episodic memory emerge from computations centered within the hippocampus4, 5, 19, 20. A central feature of these models is that allocentric navigation emerges via the computational properties of place cells, neurons in the hippocampus that fire at distinct spatial locations. Likewise, integration of hippocampal place and time cells (neurons that fire differently for different time durations) are conjectured to encode spatial and temporal dimensions of episodic memories, respectively4.
Much of the support for models that place a primary role for the hippocampus in allocentric navigation and episodic memory come from single cell recordings and lesion studies, both largely (although not exclusively) in rodents. As mentioned, place cells, first described in the rodent hippocampus42, may support aspects of allocentric navigation, but are also specific to a spatial environment. In this way, place cells provide a spatial code that can help partially differentiate a memory; hippocampal time cells may further help define episodic memories4. Hippocampal lesions disrupt the rodent’s ability to find a target based on distal landmarks compared to finding a beacon or finding the target from the same location22, 23, which is possibly supportive of an allocentric deficit (but see24, 25, 26) . Hippocampal lesions also affect temporal order memory in rodents, a critical part of episodic memories22, 43
In humans, the intersection between allocentric navigation, episodic memory, and hippocampal function is almost certainly more complicated. Two classically studied patients, H.M. and E.P., both experienced bilateral medial temporal lesions (due to resection and viral encephalitis, respectively), resulting in dense amnesia44, 45, 46, 47, 48, providing an early lead for the importance of the human hippocampus to episodic memory28, 46, 49, 50. Notably, the effects of hippocampal lesions in patients H.M. and E.P. were not exclusive to episodic memory, and included more subtle deficits in perception, working memory function, and semantic memory46, 48, 51, 52; for relevant reviews, see50, 53. Moreover, later histology work suggested that the bilateral surgical resection that H.M. received also damaged areas of his orbital frontal cortex and diencephalon, with much of his posterior medial temporal lobes (posterior hippocampus and posterior parahippocampal cortex) intact54. In this way, while both patients H.M. and E.P. provided support for the importance of the medial temporal lobes to episodic memory (and, to a lesser extent, other aspects of cognition), both patients involved incomplete lesions to the medial temporal lobes, with H.M., in particular, involving several lesions outside of the hippocampus.
With regard to navigation, while patient E.P. showed difficulty remembering recent navigational experiences, his ability to verbally navigate the neighborhood he grew up in was largely intact46, 47. H.M., despite his dense amnesia, drew accurate maps of the apartment he lived in decades following the insult to his brain, was able to navigate around town on his bicycle, and performed comparable to controls on some assays of allocentric navigation involving the Morris Water Maze45, 55. The findings for patient E.P. and H.M., although sometimes used to support the idea that recent memories are dependent on the hippocampus while remote memories are dependent on other brain regions28, would also be consistent with the idea that medial temporal lobe lesions have less of an effect on allocentric navigation than episodic memory.
Given that patients E.P. and H.M. were single case studies and involved idiosyncratic brain lesions, it is necessary to turn to other patients with medial temporal lobe lesions to understand the extent to which memory loss and navigation deficits are correlated. Some studies have reported that patients with lesions to the medial temporal lobes show deficits in virtual or real-world versions of the Morris Water Maze during navigation using distal cues56, 57 (i.e., allocentric navigation). The most robust deficits, however, are typically observed when comparing to the condition involving finding the bright cue card marking the target location (i.e., beaconing condition) and not when remembering from a repeated start location (i.e., egocentric navigation). In addition, consistent with other studies, the impairments in spatial memory following medial temporal lobe lesions are typically incomplete, suggesting some retained memory for the hidden location58, 59, 60. Patients with medial temporal lobe lesions and amnesia also draw overall geometrically accurate maps of neighborhoods they have lived in, even recently, although they are lacking some details61. Medial temporal lobe patients with amnesia can also use maps to successfully navigate despite little memory for the locations themselves62. These studies, however, involved patients with varying degrees of amnesia and medial temporal lobe lesions and it is possible that the partial (and in some cases, complete) preservation of navigation could have emerged due to partial amnesia.
In one study to address this issue in detail (Figure 2A,B), McAvan et al. (2022)63 tested a patient with dense amnesia, so profound that he was determined as having no episodic memory. The patient also had bilateral lesions to his medial temporal lobe due to multiple strokes. The patient was tested in an immersive virtual spatial navigation task in which he wore a head-mounted display and learned the locations of three objects in a room by walking to them. He then recalled the locations of the hidden targets in the room by walking to where he thought they were located and clicking a button on a hand-held controller. The patient performed comparably to a group of age-matched healthy controls and well-above permuted chance performance based on his memory for all of the targets63. The patient did not show a deficit in putative allocentric compared to egocentric navigation, which was assayed by comparing memory for the hidden target from a new (i.e., allocentric navigation) compared to a repeated (i.e., egocentric navigation) start point. These findings suggest that episodic memory and navigation deficits are not always correlated and suggest that allocentric navigation and episodic memory may be supported by at least partially independent brain systems.
Figure 2: Patient with dense amnesia and bilateral lesions to the medial temporal lobes shows navigation comparable to age-matched controls (McAvan et al. 2022).
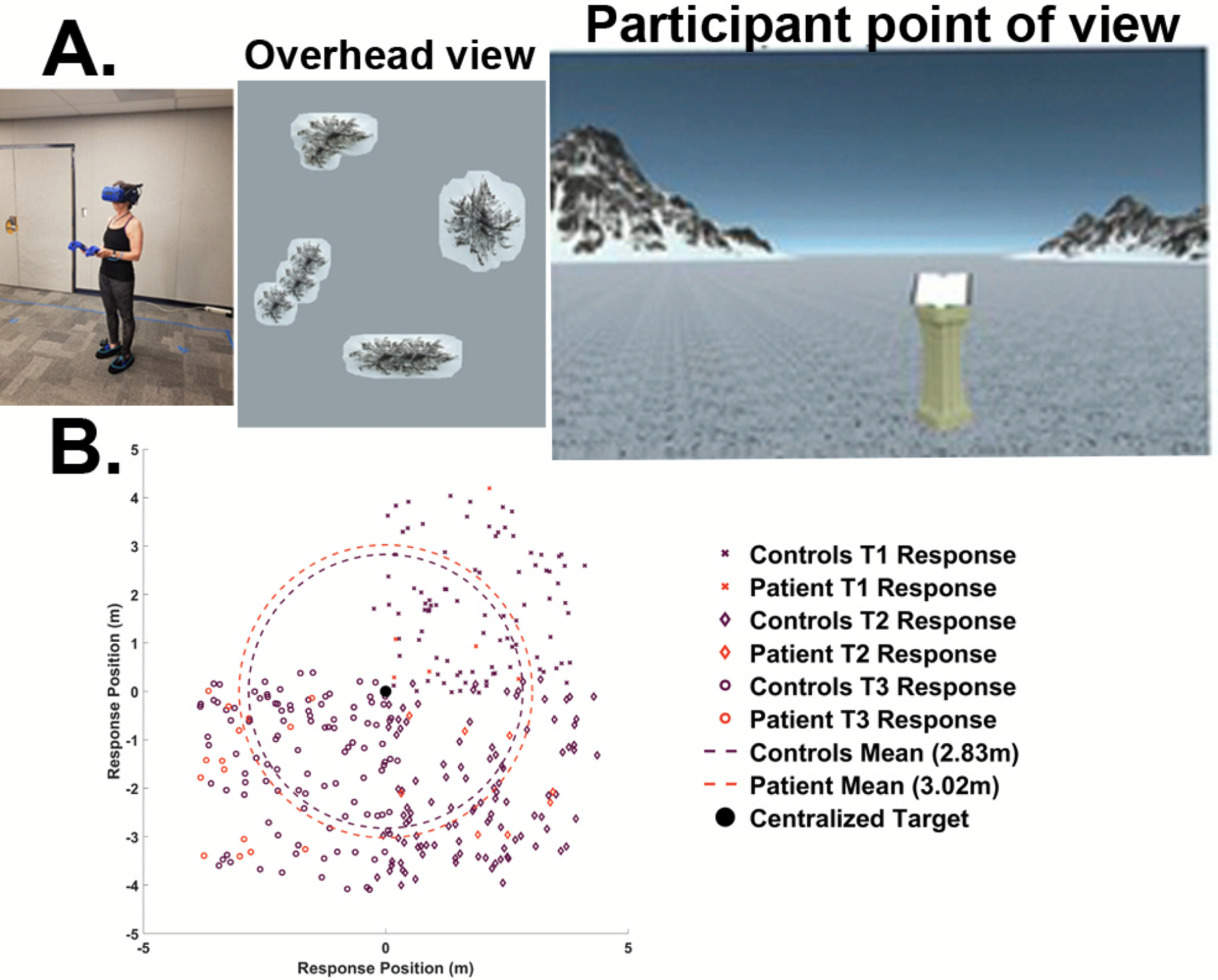
A. Participant wearing a wireless head-mounted display, allowing rich visual input and a full range of idiothetic (body-based) walking cues (left panel). Distal mountain cues required to find the locations of hidden targets in the environment (center panel). Overhead view of the environment was not seen by the participants. The view seen by participants in the study (right panel).
B. Placement error for all trials median centered for all three targets. The patient (red) and controls (black) showed statistically indistinguishable errors in target placement using the distal cues. Each shape represents a different target of three different possible targets; large circles indicate median error. Large black dot indicates centered target locations. Both the patient and controls were well above bootstrapped error predictions.
A second set of focal brain models stress the commonality between allocentric navigation and semantic memory based on the idea that they both involve some degree of generalization of egocentric navigation and episodic memory, respectively1, 64. According to these models, allocentric navigation and semantic memory are thought to depend on brain structures outside of the hippocampus, such as the entorhinal cortex65. These models also propose that spatiotemporal computations in the hippocampus provide a fundamental link between egocentric navigation and episodic memory1. A recent study reported egocentric vector cells in the human hippocampus66, providing some support for egocentric/episodic models (e.g., see1); on the surface, computing distances between concepts and distances in allocentric space has commonalities65 and is supported by empirical work67.
Additional empirical data provide some challenges to models postulating a functional equivalence between egocentric navigation and episodic memory and allocentric navigation and semantic memory. A large-sample meta-analysis of fMRI studies found many areas that did not overlap between episodic memory and egocentric navigation. Perhaps most problematic, a contrast of activation clusters for allocentric navigation and semantic memory showed no overlap at all35. In addition, a contrast of episodic memory > navigation revealed clusters of activation within the prefrontal cortex and hippocampus while a contrast of navigation > episodic memory revealed clusters of activation in more posterior regions, including retrosplenial cortex/precuneus (Figure 3). Additional theoretical considerations also pose some obstacles for these models. Semantic memory involves verbal associations that can be learned in artificial neural networks without any spatial metric68 and depends on a potentially problematic equivalence between cellular responses (grid-cells) and behavior (semantic memory)69, 70.
Figure 3: Little overlap between navigation and memory in an fMRI meta-analysis.
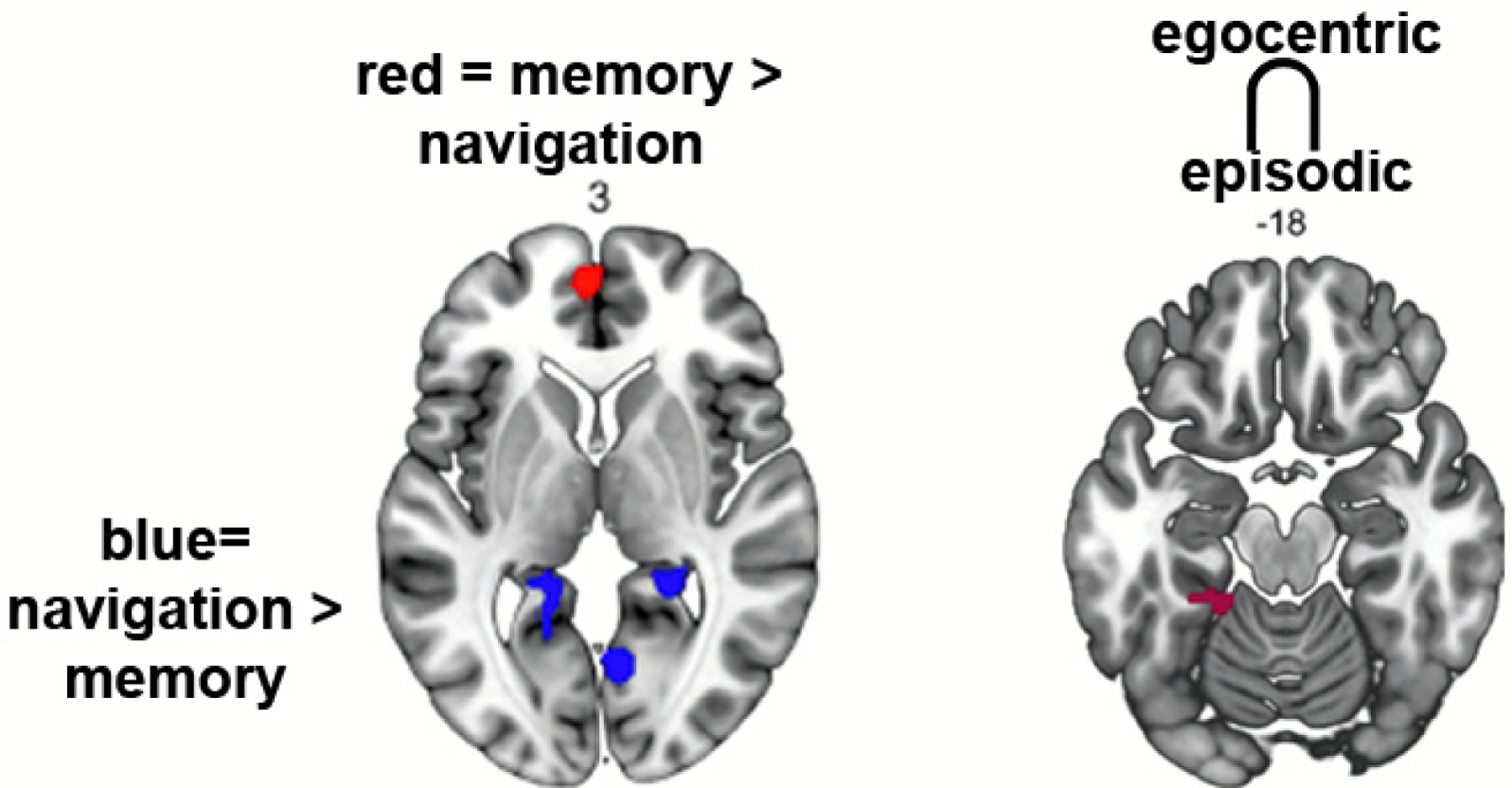
Meta-analysis from Teghil et al. 2021 using fMRI studies focused on navigation and memory. A comparison of navigation>memory yields greater activation in retrosplenial cortex and precuneus (blue clusters) while memory > navigation yields activation in prefrontal cortex (red clusters) and hippocampus (not shown). Intersection of episodic memory and egocentric navigation does not reveal hippocampal but posterior parahippocampal activation (right panel). Intersection of semantic and allocentric navigation reveal no common clusters (both not shown).
One way to reconcile lesion data and the Teghil et al. (2021)35 meta-analysis is that navigation and memory depend on at least partially dissociable brain structures. One intriguing possibility is that retrosplenial cortex, rather than the medial temporal lobe, may be an important “hub” for spatial navigation in humans (for reviews, see30, 71). This would explain some of the lack of overlap in the Teghil et al. (2021)35 meta-analysis for navigation vs. episodic memory and the lesion results explored so far. This also fits with the rich visual input coming from visual cortex to retrosplenial cortex, combined with potential memory related connections from the medial temporal lobes14, 30, 71. Interestingly, although rare, lesions to areas such as the retrosplenial cortex in humans result in profound disorientation and difficulty navigating 72, 73. Place cells are also observed in the retrosplenial cortex, which would support this idea that the retrosplenial cortex may contain similar computational machinery, at least with regard to navigation, as the hippocampus74. Retrosplenial cortex also contains head-direction responses, another important component to orientation and wayfinding75.
Emerging network perspectives on memory and navigation: Aggregate and non-aggregate network models
The field of cognitive neuroscience has largely focused on the computations within individual brain regions (i.e., based on the activity of principle cells and interneuron networks within focal gray matter). A somewhat complementary perspective to that discussed so far, network models emphasize the collective computations of distributed interactions across multiple brain areas, with a particular emphasis on connections between ensembles of neurons to coordinate such interactions76. Emerging evidence supports the importance of white matter and other polysynaptic connections as critical to such distributed interactions, providing a challenge to the focal brain models described above. For example, in the field of neurolinguistics, it is increasingly clear that white matter tracts, such as the left arcuate fasciculus, play a major role in language dysfunctions compared to classically considered regions like Broca’s area, which sits within inferior frontal gyrus, or Wernicke’s area, which spans superior temporal and middle temporal gyrus77. Likewise, in the domain of social neuroscience, there is emerging evidence that connectivity patterns, rather than specific brain areas such as the prefrontal cortex, play a pivotal role in social cognition78. Lesions to gray matter within individual brain regions can have “network” related effects insofar as they affect the connecting fibers, with computational modeling studies and theoretical considerations suggesting that these may provide better explanatory power for the effects of brain lesions on cognition than effects at single brain regions79, 80, 81.
Patients with developmental topological disorientation (DTD) provide additional empirical support for a focus on inter-regional connections in the context of navigation performance. DTD patients suffer from profound spatial orientation deficits that coincide with largely preserved function in other cognitive domains (including declarative memory). Symptoms can often include difficulties navigating and orienting in highly familiar environments, including homes and neighborhoods they have lived in for years82, 83, 84. Detailed analyses of structural and functional MRIs suggest that, compared to controls, DTD patients show no reductions in focal gray matter. Instead, one of the hallmarks appears to be impaired functional connectivity between hippocampus and prefrontal cortex83 and/or retrosplenial cortex and parts of the parahippocampal gyrus85. These unique patients suggest focusing on connectivity patterns may provide important explanatory power in understanding how memory and navigation differ.
Altered connectivity patterns may also better explain episodic memory impairments and amnesia. One study to address this issue employed a large group of patients with focal lesions to the hippocampus and compared structural MRI and functional connectivity analyses69. These analyses revealed that most of these patients showed impaired connectivity patterns as well as gray matter loss outside of the hippocampus, likely related to original brain insult. All associations of hippocampal volume loss with amnesia were fully mediated by connectivity changes. In other words, changes in functional connectivity explained relevant variance in amnesia, with hippocampal volume loss contributing only indirectly to amnesia via impaired network interactions. In a similar vein, a meta-analysis of patients with amnesia revealed that a substantial proportion of patients had lesions outside of the hippocampus. A subsequent network analysis based on presumed connectivity patterns from resting state data strongly pointed to aberrant connectivity rather than focal gray matter lesions as the best explanatory factor for impaired memory86.
The emerging field of network neuroscience, which emphasizes the importance of connections between brain regions and their potential for dynamic changes depending on behavior, may help to address the importance of connectivity patterns to navigation and memory30, 77, 87, 88, 89, 90. These models fall into two broad classes: aggregate models, which assume that the functions of a subnetwork can be localized to specific cognitive functions, and non-aggregate models that assume these functions emerge from the interactions between brain regions in a way that cannot be readily broken down into distinct or isolatable cognitive functions. A strength of focusing on networks is that they can deal with the variable effects of focal brain lesions on behavior by allowing for both some redundant processing and the idea that, via connections, some shifting of resources and function can occur over time79, 80. This in turn can more readily explain how navigation and memory can manifest as heavily interdependent, in some cases, but also distinct in others.
Aggregate models of memory and space
One relevant model that can potentially provide explanatory power for the similarities and differences between episodic memory and navigation is the posterior medial / anterior temporal (PMAT) model89. The model is based on both resting state and task-related fMRI data indicating that anterior hippocampus, perirhinal cortex, and other potentially connected areas like the ventral anterior temporal cortex and parts of orbitofrontal cortex, play a role in item processing. In contrast, posterior hippocampus, parahippocampal cortex, and retrosplenial cortex play a role in processing “context,” particularly spatial contextual details. According to this model, the hippocampus serves as a highly connected “hub” (along with ventral medial prefrontal cortex) to allow binding of item and (spatial) context in memory by combining information from anterior and posterior networks. The model is therefore well-equipped to explain findings from item recognition tasks (remembering whether you saw an item before) and contextual retrieval tasks (remembering that you saw a picture of the Niagra falls in the top part of the computer screen). More generally, though, the computations hypothesized to happen in the posterior network could help to account for the importance of areas like posterior parahippocampal cortex and retrosplenial cortex to navigation19, 35, 71. A potential limitation, however, is that the model postulates a fixed role for the anterior and posterior networks in distinct areas of memory. Specifically, task-related functional connectivity analyses suggest greater cross-network changes in connectivity during memory encoding and retrieval than the model can currently account for91, 92.
Another aggregate model that can seemingly account for putative similarities and differences between memory and navigation involves process specific alliances (PSAs)93. PSAs can be thought of as small coalitions of 2–3 brain areas that come online to perform specific computations for the task at hand. PSAs retain the idea that a single brain region may have a basic function (e.g., prefrontal cortex in cognitive control and hippocampus in episodic memory) but how the regions team up determines what the emergent behavior is. For example, prefrontal cortex and hippocampus could team up to allow for control over memory retrieval while hippocampus and retrosplenial cortex could team up to allow retrieval of spatial details. Note that PSAs are largely but not wholly determined by larger, more stable networks, like the resting state networks central to the PMAT model. A strength of the PSA model regarding our considerations about memory and navigation is that it can allow for differences to emerge depending on what areas the hippocampus is interacting with. A potential limitation is that the model still depends on single brain regions having clearly isolatable and unitary cognitive functions. It is also unclear how to falsify the potentially combinatorial number of PSAs that might be envisioned to occur during memory and navigation, to name only a few.
Non-aggregate network models of memory and navigation
Broadly speaking, non-aggregate models focus on an emergent cognitive state through interactions that cannot be reduced or distilled into separable cognitive processes or functions 25, 69, 87, 94. The central postulate of such non-aggregate models is that behavior can emerge through the dynamic interactions of multiple brain regions in a way that is not dependent on a function that can be assigned in isolation to a brain region. These models are equipped to deal with the notorious “levels” issue first pointed out by David Marr in that there is no clear theoretical path to go between explanations of cognition, algorithmic models, and mechanistic implementations69, 70. They also allow for functions to emerge via interactions with neural ensembles that may bridge multiple brain regions rather than those in isolation95. In this way, cognitive functions, like memory and navigation, can emerge dynamically and manifest via a potentially large number of connectivity pathways.
Non-aggregate network models help deal with the issue of the hippocampus potentially being involved in allocentric navigation and episodic memory by focusing instead on how different patterns of network interactions might contribute differently to both30, 96. Instead of focusing on individual brain regions or networks with specific cognitive functions, non-aggregate models focus on how multiple ensembles of neurons might dynamically configure based on task demands96. Non-aggregate models also help deal with the issue of reconciling which brain regions perform specific cognitive functions by postulating the importance of partially redundant neural ensembles across multiple brain regions30, 95, 96. Non-aggregate models also help account for the fact that lesions can occur outside of the hippocampus but still have consequences for memory or navigation, which would be dependent on which ensembles and functional interactions are affected80, 86.
Non-aggregate models have been applied most recently to the problem of allocentric navigaton25, 30. Such models postulates that the neural codes for visual, vestibular, somatosensory, and proprioceptive information are distributed across multiple brain regions. The interactions between distributed ensembles allow for emergent integrative functions like the approximate distance walked along a path. Memory information could play a role if mismatch is sufficient, which could be acquired by contributions from hippocampal communication and other brain regions with higher concentrations of memory-related cellular codes94. Much remains to be developed with these models, a key piece being how one might falsify models that potentially have infinite numbers of dynamic configurations. The circularity with non-aggregate models is potentially another issue that must be addressed.
Aging, spatial navigation, memory, and extra-hippocampal networks
Declines in spatial navigation abilities and episodic memory are well documented during normal aging and incipient Alzheimer’s disease. The hippocampus has often been considered as central to age-related differences in both allocentric navigation and episodic memory. In humans, this idea is supported in part by evidence for age-related structural and functional differences in the hippocampus and entorhinal cortex that seemingly coincide with changes in allocentric navigation and episodic memory function97, 98. For example, numerous studies in humans have shown that older adults perform worse than younger individuals when navigating the Morris Water Maze using a putative allocentric search strategy; for reviews, see99, 100. Likewise, age-related deficits in episodic memory have also been shown for tasks that are presumed to rely critically on intact hippocampal function, such as associative and source memory97.
In contrast to the classic perspective that age results in declines in allocentric navigation99, 100, there is now mounting evidence that allocentric navigation may show some degree of preservation with age101, 102, 103, 104, 105, 106. This idea is supported by a study by Zhong et al. (2017)102 in which spatial abilities were assessed in healthy young and older adult volunteers as they navigated a virtual Morris Water Maze. Substantial individual differences in spatial learning were evident in the older adults, supporting the idea that age does not affect navigation skills equally. Critically, age-related differences in navigation were only evident when comparing young and poor-performing older adults. High-performing older adults, by contrast, demonstrated spatial abilities that did not reliably differ from their younger counterparts, suggesting intact strategy use and selection (for a recent review of older adults with superior navigation skills, see107). Differences between high- and poor-performing older adults could not be accounted for by differences in age, education, or general cognitive abilities. Instead, the authors suggest that impaired spatial learning in the poor-performing older adults may have resulted from a failure to switch between navigation strategies during spatial learning.
Indeed, convergent findings from several studies suggest that navigation errors in older age stem, in part, from a failure to switch between appropriate navigation strategies102, 104, 105, 106, 108. The ability to dynamically select strategies based on environmental demands comports with our earlier arguments that navigation is a highly tuned cognitive motor skill rather than merely a memory function. As such, the ability to apply different navigation strategies, rather than rigid use of a single strategy (like an allocentric or beacon strategy100), may contribute to successful navigation in older age. In one study to support altered strategy switching in older age, Harris and Wolbers (2012)108 used a computerized version of the plus maze task to examine how older and younger adults switched from using putative allocentric vs. egocentric strategies. Young and older adults were placed in one of two opposing arms in a computerized plus-shaped maze and tasked with making a right or left turn at the junction. An allocentric (or place-based) navigational strategy was incentivized by placing a reward in a specific location (e.g., west arm). An egocentric strategy was incentivized by rewarding a specific locomotor response (e.g., left turn), regardless of heading direction. Both age groups successfully learned to navigate using allocentric and egocentric strategies; however, older adults were selectively impaired on blocks that required switching from an egocentric to allocentric strategy. This effect could not be wholly accounted for by preservation errors (i.e., failure to identify the change in reward contingencies). This selective egocentric-to-allocentric switching deficit was replicated in a larger town-like virtual environment in which older adults were less adept at switching between trials that required following familiar routes (egocentric) to trials that required taking a novel shortcut (allocentric)104. These findings suggest that age-related variance cannot be wholly accounted for by a failure to engage putative allocentric search strategies during navigation. Instead, they might emerge from inefficient switching between different strategies according to available cues and task demands (Figure 4A).
Figure 4: Strategy rigidity and decreases in precision accompany aging in some older adults.
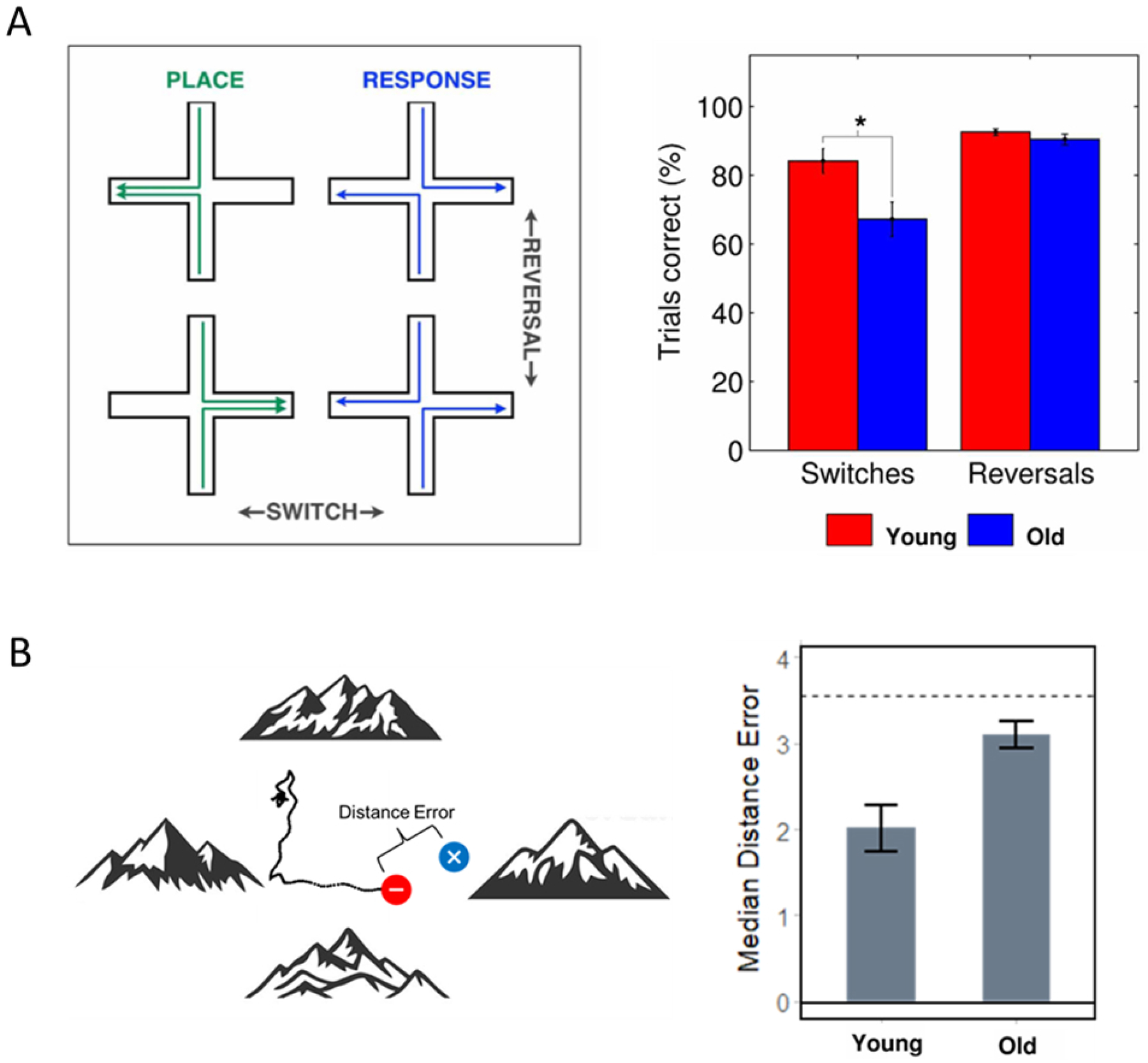
A. Study from Harris and Wolbers (2012). Older adults showed difficulty in switching from an egocentric (“response”) to allocentric (“place”) strategy but comparatively preserved egocentric and allocentric navigation (“reversals”).
B. Study from McAvan et al. (2021). Like in McAvan et al. (2022) described in Figure 2, distance error was measured by the distance from the remembered target location to the actual target (left panel). Bar shows mean distance error for young compared to older adults; younger adults showed lower distance error for both putative egocentric and allocentric navigation (averaged together in this bar graph).
Age related changes in strategy switching and optimization may be most apparent during navigation. In contrast, changes in the fidelity or precision of underlying memories may impact navigation and episodic memory. Consistent with this idea, there is mounting evidence that much of the age-related variance typically observed in spatial memory can be accounted for in part by the retrieval of noisier and/or lower fidelity mnemonic representations109, 110. In one recent study110, young and cognitively normal older adults studied objects presented at random locations on a background scene. At test, objects were placed in the center of the screen and subjects were tasked with remembering the original studied location. Mixture-modelling of the trial-wise distance errors between the remembered and true location of each object revealed that the spatial precision of item-location memories was significantly reduced in older relative to young adults. By comparison, young and older adults did not differ in their capacity to remember the coarse locations of studied objects, which was reliably above chance in both age groups (see also109).
Reduced spatial precision has also been observed in older adults when recalling the location of hidden objects with reference to global landmarks103, 111. For example, Schuck et al. (2015)111 reported greater distance error between the remembered and actual target location in older adults as they navigated a virtual arena. Importantly, performance was reliably greater than chance in both age groups, suggesting that spatial memory remained at least partially intact in older adults. This finding was recently replicated in younger and older adults navigating a virtual Morris Water Maze environment. Compared to their younger counterparts, older individuals showed evidence for reduced spatial precision (i.e., greater distance error) both when learning and subsequently retrieving the location of a hidden target (Figure 4B). This reduction in precision occurred for both putative egocentric navigation (repeated start point) and putative allocentric navigation (novel start point). Taken together, these results suggest that age-related variance in navigation performance might arise in part from a selective reduction in the precision of spatial memories, rather than the capacity to remember locations from an allocentric reference frame.
Reduced representational precision may have origins in computations in cortical gray matter, including the hippocampus50, 97 and angular gyrus112. Another possibility is that sensory representations in extrahippocampal nodes of the extended navigation network, such as the retrosplenial and parahippocampal cortices, become less distinctive or dedifferentiated in older age113, 114. Said another way, pattern separation mechanisms, the computational process whereby memories become more distinct, may be reduced with age, resulting in less differentiation (i.e., dedifferentiation) across a network of interconnected brain regions. In the domain of episodic memory, neural dedifferentiation at encoding has been shown to contribute to age-related variance in subsequent memory performance as well as age differences in the fidelity of retrieved memories and, consequently, memory accuracy115, 116 (for a review, see117). Reduced representational precision in regions such as the retrosplenial cortex and parahippocampal cortex may in turn affect downstream spatial representations in the hippocampus and entorhinal cortex115. One intriguing possibility is that dedifferentiated representations in these regions that accompany otherwise healthy aging interfere with the ability to encode features of an environment with sufficient detail to support accurate spatial recall and, consequently, successful navigation. This account may explain patterns of unstable or dedifferentiated hippocampal place codes in aged rats during navigation118 along with subsequent poor performance on navigation tasks like the Morris Water Maze119. While these network-based ideas are in their early stages of development and testing, they may provide novel explanatory power for age-related decline in both navigation and memory.
Conclusions and outstanding questions
Our review so far has focused on models of memory and navigation and how effectively they can account for both similarities and differences between the two. We have focused on two broad classes of models, focal brain region and network models. The medial temporal lobe is often at the center of focal brain region models, with the idea that the hippocampus in particular is responsible for both allocentric navigation and episodic memory. Other models postulate that different brain regions are responsible for egocentric navigation and episodic memory (i.e., hippocampus) and semantic memory and allocentric navigation (e.g., entorhinal cortex). While these models do a reasonable job explaining how some aspects of navigation and memory can emerge from a common set of brain regions and circuits, they are less effective at explaining the differences between the two, particularly navigation as a dynamically acquired complex skill and memory as an internally driven process. Network models appear to have greater explanatory power in accounting for both similarities and differences between episodic memory and navigation. These models also show promise in explaining the differing effects of brain lesions and aging on navigation and memory by focusing on connections. As network models (particularly non-aggregate models) are still under development, many questions remain about their viability.
Some remaining and important outstanding questions are generated by our considerations so far: if navigation and memory indeed depend on partially dissociable circuits, can we observe a double dissociation in patients with lesions to the hippocampus and patients with lesions to retrosplenial cortex in the same study using the same tasks? A double dissociation – impaired function in one task relative to another task that shows the opposite pattern in the other patient group – would provide compelling evidence for their neuroanatomical differences (but see120 for a network-based explanation of double dissociations). Additionally, if navigation and memory involve at least some non-overlapping cognitive processes, then we should observe other dissociations. For example, those with vestibular lesions might be expected to show more deficits in navigation than memory while those with visual imagery deficits might be expected to show more difficulty with memory than navigation. Other important outstanding questions relate to network models of navigation and age. How can we falsify models like the PSA model and non-aggregate models that potentially allow for a large set of configurations of different brain regions to accommodate different behaviors? If age does indeed involve a subset of older adults showing difficulty with strategy switching, we should find that white matter and other functional changes better account for their difficulty navigating than loss of hippocampal gray matter. Similarly, for aging and memory, we would expect that gray matter loss might provide more explanatory power in understanding precision declines than connectivity changes. Addressing and considering these outstanding questions will help to make progress on better understanding both the similarities and differences between navigation and memory.
Acknowledgements:
The authors acknowledge funding from NIH/NINDS R01NS076856, NIH/NINDS R01NS114913, NIH/NIA AG003376 to A.D.E and the Alzheimer’s Association and Evelyn McKnight Brain Research Foundation to P.F.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Neurobiological models often assume a correspondence between memory and spatial navigation based on the idea of the cognitive map. The authors review recent literature, highlighting important distinctions between memory and navigation and discussing novel models for their independent neural underpinnings.
References
- 1.Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature neuroscience 2013;16(2):130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moscovitch M, Cabeza R, Winocur G, Nadel L. Episodic Memory and Beyond: The Hippocampus and Neocortex in Transformation. Annu Rev Psychol 2016;67:105–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farzanfar D, Spiers HJ, Moscovitch M, Rosenbaum RS. From cognitive maps to spatial schemas. Nature Reviews Neuroscience 2022:1–17. [DOI] [PubMed]
- 4.Eichenbaum H On the Integration of Space, Time, and Memory. Neuron 2017;95(5):1007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadel L The hippocampal formation and action at a distance. Proceedings of the National Academy of Sciences 2021;118(51):e2119670118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map Oxford: Clarendon Press; 1978. [Google Scholar]
- 7.Harootonian SK, Ekstrom AD, Wilson RC. Combination and competition between path integration and landmark navigation in the estimation of heading direction. PLoS computational biology 2022;18(2):e1009222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sjolund LA, Kelly JW, McNamara TP. Optimal combination of environmental cues and path integration during navigation. Mem Cognition 2018;46(1):89–99. [DOI] [PubMed] [Google Scholar]
- 9.Coutrot A, Manley E, Goodroe S, Gahnstrom C, Filomena G, Yesiltepe D, et al. Entropy of city street networks linked to future spatial navigation ability. Nature 2022;604(7904):104–10. [DOI] [PubMed] [Google Scholar]
- 10.Barhorst-Cates EM, Meneghetti C, Zhao Y, Pazzaglia F, Creem-Regehr SH. Effects of home environment structure on navigation preference and performance: A comparison in Veneto, Italy and Utah, USA. Journal of Environmental Psychology 2021;74:101580. [Google Scholar]
- 11.Hejtmanek L, Starrett M, Ferrer E, Ekstrom Arne D. How Much of What We Learn in Virtual Reality Transfers to Real-World Navigation? 2020:1. [DOI] [PubMed]
- 12.Charness N, Boot WR. A Grand Challenge for Psychology: Reducing the Age-Related Digital Divide. Current directions in psychological science 2022;31(2):187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furman AJ, Clements-Stephens AM, Marchette SA, Shelton AL. Persistent and stable biases in spatial learning mechanisms predict navigational style. Cognitive, Affective, & Behavioral Neuroscience 2014;14(4):1375–91. [DOI] [PubMed] [Google Scholar]
- 14.Weisberg SM, Ekstrom AD. Hippocampal volume and navigational ability: The map (ping) is not to scale. Neuroscience & Biobehavioral Reviews 2021;126:102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekstrom AD, Spiers HJ, Bohbot VD, Rosenbaum RS. Human Spatial Navigation: Princeton University Press; 2018. [Google Scholar]
- 16.McNeill C, Cory-Wright J, Renfrew T. Teaching Orienteering Doune, UK: Harveys; 1998. [Google Scholar]
- 17.Weisberg SM, Schinazi VR, Newcombe NS, Shipley TF, Epstein RA. Variations in cognitive maps: Understanding individual differences in navigation. Journal of Experimental Psychology: Learning, Memory, and Cognition 2014;40(3):669. [DOI] [PubMed] [Google Scholar]
- 18.Malanchini M, Rimfeld K, Shakeshaft NG, McMillan A, Schofield KL, Rodic M, et al. Evidence for a unitary structure of spatial cognition beyond general intelligence. npj Science of Learning 2020;5(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekstrom AD, Ranganath C. Space, Time and Episodic Memory: the Hippocampus is all over the Cognitive Map. Hippocampus 2018;28(9):680–7. [DOI] [PubMed] [Google Scholar]
- 20.Rolls ET, Wirth S. Spatial representations in the primate hippocampus, and their functions in memory and navigation. Progress in neurobiology 2018;171:90–113. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “Time Cells” Bridge the Gap in Memory for Discontiguous Events. Neuron 2011;71(4):737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature 1982;297:681–3. [DOI] [PubMed] [Google Scholar]
- 23.Eichenbaum H, Stewart C, Morris RG. Hippocampal representation in place learning. J Neurosci 1990;10(11):3531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolbers T, Wiener JM. Challenges in identifying the neural mechanisms that support spatial navigation: the impact of spatial scale. Frontiers in Human Neuroscience 2014;8(571):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekstrom AD, Arnold AE, Iaria G. A critical review of the allocentric spatial representation and its neural underpinnings: toward a network-based perspective. Front Hum Neurosci 2014;8:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day LB, Weisand M, Sutherland RJ, Schallert T. The hippocampus is not necessary for a place response but may be necessary for pliancy. Behav Neurosci 1999;113(5):914–24. [DOI] [PubMed] [Google Scholar]
- 27.Tulving E Episodic memory and autonoesis: Uniquely human. The missing link in cognition: Origins of self-reflective consciousness 2005:3–56.
- 28.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci 2004;27:279–306. [DOI] [PubMed] [Google Scholar]
- 29.Polyn SM, Norman KA, Kahana MJ. A context maintenance and retrieval model of organizational processes in free recall. Psychol Rev 2009;116(1):129–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekstrom AD, Huffman DJ, Starrett M. Interacting networks of brain regions underlie human spatial navigation: A review and novel synthesis of the literature. Journal of Neurophysiology 2017;118(6):3328–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefort JM, Rochefort C, Rondi-Reig L, Labex GoLimo B-P, Foundation E. Cerebellar contribution to spatial navigation: new insights into potential mechanisms. The Cerebellum 2015;14:59–62. [DOI] [PubMed] [Google Scholar]
- 32.Diamond NB, Levine B. Linking detail to temporal structure in naturalistic-event recall. Psychological Science 2020;31(12):1557–72. [DOI] [PubMed] [Google Scholar]
- 33.Starrett MJ, Huffman DJ, Ekstrom AD. Combining egoformative and alloformative cues in a novel tabletop navigation task. Psychological Research 2022:1–21. [DOI] [PMC free article] [PubMed]
- 34.Renoult L, Irish M, Moscovitch M, Rugg MD. From knowing to remembering: the semantic–episodic distinction. Trends in cognitive sciences 2019;23(12):1041–57. [DOI] [PubMed] [Google Scholar]
- 35.Teghil A, Bonavita A, Guariglia C, Boccia M. Commonalities and specificities between environmental navigation and autobiographical memory: A synthesis and a theoretical perspective. Neuroscience & Biobehavioral Reviews 2021;127:928–45. [DOI] [PubMed] [Google Scholar]
- 36.Yates FA. The art of memory: Routledge and Kegan Paul London; 1966. [Google Scholar]
- 37.Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, et al. Schema-dependent gene activation and memory encoding in neocortex. Science 2011;333(6044):891–5. [DOI] [PubMed] [Google Scholar]
- 38.Bouffard N, Stokes J, Kramer HJ, Ekstrom AD. Temporal encoding strategies result in boosts to final free recall performance comparable to spatial ones. Mem Cognition 2018;46(1):17–31. [DOI] [PubMed] [Google Scholar]
- 39.Fan CL, Abdi H, Levine B. On the relationship between trait autobiographical episodic memory and spatial navigation. Mem Cognition 2021;49(2):265–75. [DOI] [PubMed] [Google Scholar]
- 40.Fan CL, Sokolowski HM, Rosenbaum RS, Levine B. What about “space” is important for episodic memory? Wiley Interdisciplinary Reviews: Cognitive Science 2023:e1645. [DOI] [PubMed]
- 41.Waddington EE, Heisz JJ. Orienteering experts report more proficient spatial processing and memory across adulthood. PloS one 2023;18(1):e0280435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 1971;34(1):171–5. [DOI] [PubMed] [Google Scholar]
- 43.Ergorul C, Eichenbaum H. Essential role of the hippocampal formation in rapid learning of higher-order sequential associations. J Neurosci 2006;26(15):4111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milner B, Corkin S, Teuber H-L. Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of HM. Neuropsychologia 1968;6(3):215–34. [Google Scholar]
- 45.Corkin S What’s new with the amnesic patient HM? Nature Reviews Neuroscience 2002;3(2):153–60. [DOI] [PubMed] [Google Scholar]
- 46.Insausti R, Annese J, Amaral DG, Squire LR. Human amnesia and the medial temporal lobe illuminated by neuropsychological and neurohistological findings for patient E.P. Proc Natl Acad Sci U S A 2013;110(21):E1953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teng E, Squire LR. Memory for places learned long ago is intact after hippocampal damage. Nature 1999;400(6745):675–7. [DOI] [PubMed] [Google Scholar]
- 48.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 1957;20(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behavioral and Brain Sciences 1994;17(03):449–72. [Google Scholar]
- 50.Ekstrom AD, Yonelinas AP. Precision, binding, and the hippocampus: Precisely what are we talking about? Neuropsychologia 2020:107341. [DOI] [PMC free article] [PubMed]
- 51.Stark CEL, Squire LR. Recognition memory and familiarity judgments in severe amnesia: No evidence for a contribution of repetition priming. Behavioral Neuroscience 2000;114(3):459–67. [DOI] [PubMed] [Google Scholar]
- 52.Gabrieli JD, Cohen NJ, Corkin S. The impaired learning of semantic knowledge following bilateral medial temporal-lobe resection. Brain Cognition 1988;7(2):157–77. [DOI] [PubMed] [Google Scholar]
- 53.Duff MC, Covington NV, Hilverman C, Cohen NJ. Semantic memory and the hippocampus: Revisiting, reaffirming, and extending the reach of their critical relationship. Frontiers in Human Neuroscience 2020;13:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Annese J, Schenker-Ahmed NM, Bartsch H, Maechler P, Sheh C, Thomas N, et al. Postmortem examination of patient H.M.’s brain based on histological sectioning and digital 3D reconstruction. Nature communications 2014;5:3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bohbot VD, Corkin S. Posterior parahippocampal place learning in H.M. Hippocampus 2007;17(9):863–72. [DOI] [PubMed] [Google Scholar]
- 56.Bartsch T, Schonfeld R, Muller FJ, Alfke K, Leplow B, Aldenhoff J, et al. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science 2010;328(5984):1412–5. [DOI] [PubMed] [Google Scholar]
- 57.Banta-Lavenex P, Colombo F, Ribordy-Lambert F, Lavenex P. The human hippocampus beyond the cognitive map: Evidence from a densely amnesic patient. Frontiers in Human Neuroscience 2014;8(711):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kolarik BS, Baer T, Shahlaie K, Yonelinas AP, Ekstrom AD. Close but no cigar: Spatial precision deficits following medial temporal lobe lesions provide novel insight into theoretical models of navigation and memory. Hippocampus 2018;28(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kolarik BS, Shahlaie K, Hassan B, Borders AA, Kaufman K, Gurkoff G, et al. Impairments in precision, rather than spatial strategy, characterize performance on the virtual Morris Water Maze: A case study. Neuropsychologia 2016;80:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia 1998;36(11):1217–38. [DOI] [PubMed] [Google Scholar]
- 61.Herdman KA, Calarco N, Moscovitch M, Hirshhorn M, Rosenbaum RS. Impoverished descriptions of familiar routes in three cases of hippocampal/medial temporal lobe amnesia. Cortex 2015;71:248–63. [DOI] [PubMed] [Google Scholar]
- 62.Urgolites ZJ, Kim S, Hopkins RO, Squire LR. Map reading, navigating from maps, and the medial temporal lobe. P Natl Acad Sci USA 2016;113(50):14289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McAvan AS, Wank AA, Rapcsak SZ, Grilli MD, Ekstrom AD. Largely intact memory for spatial locations during navigation in an individual with dense amnesia. Neuropsychologia 2022;170:108225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Current opinion in neurobiology 2006;16(2):179–90. [DOI] [PubMed] [Google Scholar]
- 65.Bellmund JLS, Gardenfors P, Moser EI, Doeller CF. Navigating cognition: Spatial codes for human thinking. Science 2018;362(6415). [DOI] [PubMed] [Google Scholar]
- 66.Kunz L, Brandt A, Reinacher PC, Staresina BP, Reifenstein ET, Weidemann CT, et al. A neural code for egocentric spatial maps in the human medial temporal lobe. Neuron 2021;109(17):2781–96. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Constantinescu AO, O’Reilly JX, Behrens TEJ. Organizing conceptual knowledge in humans with a gridlike code. Science 2016;352(6292):1464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rinaldi L, Marelli M. Maps and space are entangled with language experience. Trends in Cognitive Sciences 2020;24(11):853–5. [DOI] [PubMed] [Google Scholar]
- 69.Ekstrom AD, Harootonian SK, Huffman DJ. Grid coding, spatial representation, and navigation: Should we assume an isomorphism? Hippocampus 2019;doi: 10.1002/hipo.23175. [DOI] [PMC free article] [PubMed]
- 70.Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, Poeppel D. Neuroscience Needs Behavior: Correcting a Reductionist Bias. Neuron 2017;93(3):480–90. [DOI] [PubMed] [Google Scholar]
- 71.Mitchell AS, Czajkowski R, Zhang N, Jeffery K, Nelson AJ. Retrosplenial cortex and its role in spatial cognition. Brain and neuroscience advances 2018;2:2398212818757098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi N, Kawamura M, Shiota J, Kasahata N, Hirayama K. Pure topographic disorientation due to right retrosplenial lesion. Neurology 1997;49(2):464–9. [DOI] [PubMed] [Google Scholar]
- 73.Hashimoto R, Tanaka Y, Nakano I. Heading disorientation: a new test and a possible underlying mechanism. Eur Neurol 2010;63(2):87–93. [DOI] [PubMed] [Google Scholar]
- 74.Mao D, Kandler S, McNaughton BL, Bonin V. Sparse orthogonal population representation of spatial context in the retrosplenial cortex. Nature communications 2017;8(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shine JP, Valdes-Herrera JP, Hegarty M, Wolbers T. The Human Retrosplenial Cortex and Thalamus Code Head Direction in a Global Reference Frame. J Neurosci 2016;36(24):6371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Westlin C, Theriault JE, Katsumi Y, Nieto-Castanon A, Kucyi A, Ruf SF, et al. Improving the study of brain-behavior relationships by revisiting basic assumptions. Trends in Cognitive Sciences 2023. [DOI] [PMC free article] [PubMed]
- 77.Hagoort P The neurobiology of language beyond single-word processing. Science 2019;366(6461):55–8. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, Olson IR. The original social network: white matter and social cognition. Trends in cognitive sciences 2018;22(6):504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alstott J, Breakspear M, Hagmann P, Cammoun L, Sporns O. Modeling the impact of lesions in the human brain. PLoS Comput Biol 2009;5(6):e1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Argyropoulos GP, Loane C, Roca-Fernandez A, Lage-Martinez C, Gurau O, Irani SR, et al. Network-wide abnormalities explain memory variability in hippocampal amnesia. eLife 2019;8(e46156):1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Warren DE, Denburg NL, Power JD, Bruss J, Waldron EJ, Sun H, et al. Brain network theory can predict whether neuropsychological outcomes will differ from clinical expectations. Arch Clin Neuropsych 2017;32(1):40–52. [DOI] [PubMed] [Google Scholar]
- 82.Iaria G, Barton JJ. Developmental Topographical Disorientation: a newly discovered cognitive disorder. Exp Brain Res 2010;206(2):189–96. [DOI] [PubMed] [Google Scholar]
- 83.Arnold AE, Protzner AB, Bray S, Levy RM, Iaria G. Neural network configuration and efficiency underlies individual differences in spatial orientation ability. J Cogn Neurosci 2014;26(2):380–94. [DOI] [PubMed] [Google Scholar]
- 84.Burles F, Iaria G. Behavioural and cognitive mechanisms of developmental topographical disorientation. Scientific reports 2020;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim JG, Aminoff EM, Kastner S, Behrmann M. A neural basis for developmental topographic disorientation. Journal of Neuroscience 2015;35(37):12954–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferguson MA, Lim C, Cooke D, Darby RR, Wu O, Rost NS, et al. A human memory circuit derived from brain lesions causing amnesia. Nature communications 2019;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Braun U, Schafer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci U S A 2015;112(37):11678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Embick D, Poeppel D. Towards a computational(ist) neurobiology of language: Correlational, integrated, and explanatory neurolinguistics. Lang Cogn Neurosci 2015;30(4):357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ritchey M, Libby LA, Ranganath C. Cortico-hippocampal systems involved in memory and cognition: the PMAT framework. Progress in brain research 2015;219:45–64. [DOI] [PubMed] [Google Scholar]
- 90.Cabeza R, Stanley ML, Moscovitch M. Process-Specific Alliances (PSAs) in Cognitive Neuroscience. Trends Cogn Sci 2018. [DOI] [PMC free article] [PubMed]
- 91.Cooper RA, Ritchey M. Cortico-hippocampal network connections support the multidimensional quality of episodic memory. Elife 2019;8. [DOI] [PMC free article] [PubMed]
- 92.Schedlbauer AM, Ekstrom AD. Flexible network community organization during the encoding and retrieval of spatiotemporal episodic memories. Network Neuroscience 2019;3(4):1070–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cabeza R, Stanley ML, Moscovitch M. Process-specific alliances (PSAs) in cognitive neuroscience. Trends in cognitive sciences 2018;22(11):996–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Misic B, Goni J, Betzel RF, Sporns O, McIntosh AR. A network convergence zone in the hippocampus. PLoS Comput Biol 2014;10(12):e1003982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mesulam M A Cortical Network for Directed Attention and Utlllateral Neglect. . Annals of Neurology 1981;10(4). [DOI] [PubMed] [Google Scholar]
- 96.Pessoa L, Medina L, Desfilis E. Refocusing neuroscience: moving away from mental categories and towards complex behaviours. Philosophical Transactions of the Royal Society B 2022;377(1844):20200534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gorbach T, Pudas S, Lundquist A, Orädd G, Josefsson M, Salami A, et al. Longitudinal association between hippocampus atrophy and episodic-memory decline. Neurobiol Aging 2017;51:167–76. [DOI] [PubMed] [Google Scholar]
- 98.Coughlan G, Laczó J, Hort J, Minihane A-M, Hornberger M. Spatial navigation deficits—overlooked cognitive marker for preclinical Alzheimer disease? Nature Reviews Neurology 2018;14(8):496–506. [DOI] [PubMed] [Google Scholar]
- 99.Lester AW, Moffat SD, Wiener JM, Barnes CA, Wolbers T. The aging navigational system. Neuron 2017;95(5):1019–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moffat SD. Aging and spatial navigation: what do we know and where do we go? Neuropsychology review 2009;19(4):478–89. [DOI] [PubMed] [Google Scholar]
- 101.Bécu M, Sheynikhovich D, Tatur G, Agathos CP, Bologna LL, Sahel J-A, et al. Age-related preference for geometric spatial cues during real-world navigation. Nature Human Behaviour 2020;4(1):88–99. [DOI] [PubMed] [Google Scholar]
- 102.Zhong JY, Magnusson KR, Swarts ME, Clendinen CA, Reynolds NC, Moffat SD. The application of a rodent-based Morris water maze (MWM) protocol to an investigation of age-related differences in human spatial learning. Behavioral Neuroscience 2017;131(6):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McAvan A, Du Y, Oyao A, Doner S, Grilli M, Ekstrom A. Older Adults Show Reduced Spatial Precision but Preserved Strategy-Use During Spatial Navigation Involving Body-Based Cues. Front Aging Neurosci 2021;13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harris MA, Wolbers T. How age-related strategy switching deficits affect wayfinding in complex environments. Neurobiol Aging 2014;35(5):1095–102. [DOI] [PubMed] [Google Scholar]
- 105.Kimura K, Reichert JF, Kelly DM, Moussavi Z. Older adults show less flexible spatial cue use when navigating in a virtual reality environment compared with younger adults. Neuroscience Insights 2019;14:2633105519896803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Merhav M, Wolbers T. Aging and spatial cues influence the updating of navigational memories. Scientific Reports 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou R, Belge T, Wolbers T. Reaching the Goal: Superior Navigators in Late Adulthood Provide a Novel Perspective into Successful Cognitive Aging. Topics in Cognitive Science 2022. [DOI] [PubMed]
- 108.Harris M, Wiener J, Wolbers T. Aging specifically impairs switching to an allocentric navigational strategy. Front Aging Neurosci 2012;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Korkki SM, Richter FR, Jeyarathnarajah P, Simons JS. Healthy ageing reduces the precision of episodic memory retrieval. Psychol Aging 2020;35(1):124. [DOI] [PubMed] [Google Scholar]
- 110.Nilakantan AS, Bridge DJ, VanHaerents S, Voss JL. Distinguishing the precision of spatial recollection from its success: Evidence from healthy aging and unilateral mesial temporal lobe resection. Neuropsychologia 2018;119:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schuck NW, Doeller CF, Polk TA, Lindenberger U, Li S-C. Human aging alters the neural computation and representation of space. Neuroimage 2015;117:141–50. [DOI] [PubMed] [Google Scholar]
- 112.Richter FR, Cooper RA, Bays PM, Simons JS. Distinct neural mechanisms underlie the success, precision, and vividness of episodic memory. Elife 2016;5:e18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koen JD, Hauck N, Rugg MD. The relationship between age, neural differentiation, and memory performance. Journal of Neuroscience 2019;39(1):149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Srokova S, Hill PF, Koen JD, King DR, Rugg MD. Neural differentiation is moderated by age in scene-selective, but not face-selective, cortical regions. ENeuro 2020;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hill PF, King DR, Rugg MD. Age differences in retrieval-related reinstatement reflect age-related dedifferentiation at encoding. Cerebral Cortex 2021;31(1):106–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Katsumi Y, Andreano JM, Barrett LF, Dickerson BC, Touroutoglou A. Greater neural differentiation in the ventral visual cortex is associated with youthful memory in superaging. Cerebral cortex 2021;31(11):5275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koen JD, Rugg MD. Neural dedifferentiation in the aging brain. Trends in Cognitive Sciences 2019;23(7):547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature 1997;388(6639):272–5. [DOI] [PubMed] [Google Scholar]
- 119.Schimanski LA, Lipa P, Barnes CA. Tracking the course of hippocampal representations during learning: when is the map required? Journal of Neuroscience 2013;33(7):3094–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Plaut DC. Double dissociation without modularity: Evidence from connectionist neuropsychology. J Clin Exp Neuropsyc 1995;17(2):291–321. [DOI] [PubMed] [Google Scholar]


