Abstract
Purpose:
The American Heart Association has identified seven modifiable cardiovascular health (CVH) metrics, including four health behaviors (body mass index, smoking, physical activity, and dietary intake) and three health factors (total cholesterol, blood pressure, and fasting glucose). We sought to examine the association between CVH metrics and depression.
Methods:
We analyzed data on 14,561 adults aged 20 years or older from the National Health and Nutrition Examination Survey 2007–2014. Depressive symptoms were assessed using the Patient Health Questionnaire; a score of 0–4, 5–9, and 10 or higher represented no or minimal, mild, moderate or severe depressive symptoms, respectively. CVH was categorized as inadequate, average, or optimum. We used multinomial logistic regression to assess the association between CVH and depression, adjusted for age, gender, race or ethnicity, education, and alcohol use.
Results:
Prevalence of inadequate, average, and optimum CVH were 6.1%, 59.7%, and 34.2%; 14.9% and 7.8% of adults had mild and moderate/severe depression, respectively. Compared with participants with optimum CVH, prevalence ratios for moderate or severe depression were 4.39 (95% confidence interval, 3.32–5.80) and 2.64 (2.15–3.24) for those with inadequate and average CVH, respectively. The corresponding prevalence ratios for mild depression were 2.11 (1.77–2.52) and 1.36 (1.19–1.55). The association appeared to be stronger for CVH behaviors.
Conclusions:
There was a graded association between CVH metrics, particularly for health behaviors, and mild and moderate/severe depression among U.S. adults.
Keywords: Cardiovascular health, Depression, NHANES, Prevalence ratio
Despite the decline in death rates over the decades, cardiovascular disease (CVD) remains the leading cause of death in the United States, accounting for 30.8% (or 800,937) of all deaths in 2013 [1]. The total direct medical costs of CVD are projected to increase to $918 billion by 2030 [1]. The substantial body of evidence demonstrated that individuals with favorable levels of major cardiovascular risk factors experienced significant reduced risk of CVD incidence and mortality [2-11]. In the 2010 “Strategic Impact Goal Through 2020 and Beyond,” the American Heart Association (AHA) published recommendations focusing on improving cardiovascular health (CVH) of all Americans by 20% in addition to reducing CVD and stroke mortality by 20% in the United States [12,13]. A set of seven metrics (body mass index [BMI], smoking, physical activity, dietary intake, total cholesterol, blood pressure, and fasting glucose) that can be modified to lower cardiovascular risk were identified and categorized into three levels (poor, intermediate, and ideal health). Studies have shown that the presence of a greater number of ideal CVH metrics was associated with a graded and significantly lower risk of CVD incidence [4] and mortality [5,11,14].
Depression is one of the most common chronic condition in general practice, involving more than 1 in 10 general patients [15]. It affects 311 million people worldwide [16]. Depression is a strong and independent risk factor for increased morbidity and mortality, lower functional status, and worse quality of life as well as increased costs [17-19]. A survey of 245,404 adults from 60 countries showed patients with comorbid depression had worse overall health than those with asthma, diabetes, arthritis, or CVD alone [20]. A few studies examined the association between CVH status and depression [21,22], but none used nationally representative samples. The present study examined the association between CVH metrics and both mild and moderate/severe depressive symptom overall and by selected subgroups (age, gender, race or ethnicity, and education), using 2007–2014 National Health and Nutrition Examination Survey (NHANES) data, the nationally representative samples.
Subjects and methods
Study participants
NHANES uses a complex, stratified, multistage probability cluster sampling, cross-sectional design to collect health and nutritional data from a representative sample of the noninstitutionalized U.S. population. The design and operation of NHANES have been described previously [23]. For the present study, we used data from the NHANES 2007–2014. From 21,030 participants aged 20 years or older with reliable first 24-hour dietary recall, we sequentially excluded 234 pregnant women, 1876 participants with missing CVH metrics scores, 1072 participants with missing depression scores, 280 participants with BMI less than 18.5 kg/m2, and 20 participants with missing value for covariates (11 for education and nine for alcohol consumption). We also excluded 1704 participants with CVD (myocardial infarction, congestive heart failure, and stroke) and 1303 participants with cancer because these diseases are associated with depression. The final analysis included 14,561 adults. Study protocols for NHANES were approved by the National Center for Health Statistics Institutional Review Board. Signed informed consent was obtained from all participants.
CVH metrics
CVH metrics included four health behaviors (smoking, physical activity, healthy dietary scores, and BMI) and three health factors (total cholesterol, blood pressure, and fasting plasma glucose) [13]. The definitions of ideal, intermediate, and poor CVH metrics for adults are presented in Table 1. We used Healthy Eating Index 2010 (HEI-2010) scores as a proxy of healthy dietary scores, which were calculated using first-day 24-hour dietary recall. HEI-2010 scores were based on a 12-component index: total fruit, whole fruit, total vegetables, grains and beans, whole grains, dairy, total protein foods, seafood and plant protein, fatty acid, refined grains, sodium, and empty calories, with total scores ranging from 0 to 100 and a higher score indicating a healthier diet [24]. Participants with an HEI-2010 score of 50 or less were assigned to poor health, those with a score of 51–80 were assigned to intermediate health, and those with a score of 81 or higher were assigned to ideal health [5].
Table 1.
Distribution of ideal, intermediate and poor CVH for each metric for adults free of CVD, NHANES 2007–2014
| Health metric | AHA definitions of CVH for each metric | Total sample (n = 14,561) |
|---|---|---|
| Smoking status | ||
| Ideal | Never or quit >12 mo ago | 11,163 |
| Intermediate | Former ≤12 mo | 290 |
| Poor | Current smoking | 3108 |
| Body mass index | ||
| Ideal | <25 kg/m2 | 4137 |
| Intermediate | 25–29 kg/m2 | 4964 |
| Poor | ≥30 kg/m2 | 5460 |
| Physical activity | ||
| Ideal | ≥150 min/wk moderate or ≥75 min/wk vigorous or ≥150 min/wk moderate + vigorous | 5084 |
| Intermediate | 1–149 min/wk moderate or 1–74 min /wk vigorous or 1–149 min/wk moderate + vigorous | 2257 |
| Poor | None | 7220 |
| Healthy diet score* | ||
| Ideal | 4–5 components | 296 |
| Intermediate | 2–3 components | 6812 |
| Poor | 0–1 components | 7453 |
| Total cholesterol | ||
| Ideal | <200 mg/dL† | 7311 |
| Intermediate | 200–239 mg/dL or treated to goal | 5299 |
| Poor | ≥240 mg/dL | 1951 |
| Blood pressure | ||
| Ideal | SBP <120 or DBP <80 mm Hg† | 6138 |
| Intermediate | SBP 120–139 or DBP 80–89 mm Hg or treated to goal | 3183 |
| Poor | SBP ≥140 or DBP ≥90 mm Hg | 5240 |
| Fasting plasma glucose | ||
| Ideal | <100 mg/dL† | 9961 |
| Intermediate | 100–125 mg/dL or treated to goal | 3256 |
| Poor | ≥126 mg/dL | 1344 |
DBP = diastolic blood pressure; SBP = systolic blood pressure; SE = standard error.
AHA’s healthy diet score includes five components: fruits and vegetables, whole grain, fish, sodium, and sugar-sweeten beverage, and a very small proportion (<0.5%) of U.S. adults meet the ideal healthy diet. HEI-2010 is a continuous score consisting of 12 components representing major food groups including fruit and vegetables, whole grains, proteins, dairy, oils, sodium, and empty calories. HEI-2010 score ranges from 0 to 100 with a higher score indicates a more healthy diet. HEI-2010 has been validated to represent the diet quality in population. We used HEI-2010 as a proxy for AHA’s healthy diet score with ideal diet: HEI-2010 >81; intermediate diet: 51–80; and poor diet: ≤50.
Untreated values.
Fasting plasma glucose was available for 63.8% participants. To maximize the sample size, we used hemoglobin A1c values less than 5.7%, 5.7%–6.4% and 6.5% or higher as a proxy for fasting plasma glucose levels less than 100 mg/dL, 100 to less than 126 mg/dL, and 126 mg/dL or more, respectively, as recommended by American Diabetes Association [25]. Participants who reported having diabetes or being treated with insulin or oral medication to lower blood glucose and had HbA1c concentration 5.7%–6.4% were categorized as intermediate health; similarly, participants who reported taking cholesterol-lowering or antihypertensive medications and were treated to goal were categorized as “intermediate,” whereas participants with these conditions who were untreated or who were not treated to goal were categorized as “poor” for that health factor. Use of antihypertensive, cholesterol-lowering, and glucose-lowering medications were self-reported. Total cholesterol and plasma glucose were measured with enzymatic method [23]. BMI was calculated as weight in kilograms divided by height in meters squared. Mean blood pressure was estimated from up to three readings, obtained under standard conditions during a single physical examination.
Each CVH component was given a point score of 0, 1, or 2 to represent poor, intermediate, or ideal health, respectively. Based on the sum of all seven components, an overall score, ranging from 0 to 14, was categorized as inadequate (0–4), average (5–9), or optimum (10–14) CVH.
Depressive symptoms
Depressive symptoms were assessed using the Patient Health Questionnaire (PHQ-9), a validated nine-item screening instrument that asks about the frequency of depressive symptoms over the past 2 weeks [26]. Response categories of “not at all,” “several days,” “more than half the day,” and “nearly every day” receive a score of 0–3, respectively. Total scores of PHQ-9 range from 0 to 27, with higher scores indicating more severe depression. PHD-9 scores of 0–4, 5–9, 10–14, 15–19, and 20–27 represent no or minimal, mild, moderate, moderately severe, and severe depressive symptom, respectively. Our analyses combined moderate, moderately severe, and severe depressive symptom for the stable estimate. The PHQ-9 scores of 10 or higher had a sensitivity of 88% and a specificity of 88% for major depression [26], a well-validated cut-point commonly used in clinical studies that measure depression.
Covariates
Study covariates included age, sex, race or ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, or others), educational attainment (<12, 12, or >12 years), alcohol consumption (0, <2, or ≥2 drinks daily for men and 0, <1, or ≥1 drinks daily for women). Heavy use of alcohol was defined as two or more drinks daily in men or one or more drinks daily in women.
Statistical analyses
Statistical analyses were performed using SUDAAN version 11 (RTI International) to take into account the complex sampling design. Data on characteristics were expressed as means and 95% confidence intervals (CI) for continuous variables or as percentages and 95% CI for categorical variables and were compared across depression categories. A t-test was used to compare among depression severity groups for continuous variables. The χ2 test was used for categorical variables. We used multinomial logistic regression to estimate the adjusted prevalence ratios (PRs) of mild and moderate/severe depressive symptom comparing average or inadequate CVH versus optimum CVH, adjusted for age, gender, race/ethnicity, education, and alcohol use. We also estimated the association between an individual component of CVH metrics and depression as well as for four health behaviors and three health factors separately. When assessing the role of individual component, we adjusted for the presence or absence of the rest of the components. As a sensitivity analysis, we examined the association between CVH metrics and depression without excluding participants with CVD and cancer, adjusted for age, gender, race/ethnicity, education, alcohol use, and history of CVD and cancer. All tests of statistical significance were two-tailed, and a probability value less than .05 was considered significant.
Results
The mean age of 14,561 participants was 44.6 years. About half of the participants were female, and 67% were non-Hispanic whites. The prevalence of inadequate, average, and optimum CVH was 6.1%, 59.7%, and 34.2%; 14.9% and 7.8% adults had mild and moderate or severe depression, respectively. Only 2.4% of participants met the ideal diet criteria. The prevalence of participants meeting the ideal level for the rest of CVH metrics were smoking, 78%; diabetes, 75%; total cholesterol, 50%; blood pressure, 45%; physical activity, 40%; BMI, 30%.
Table 2 shows the baseline characteristics of participants by the status of depressive symptoms. Women, non-Hispanic blacks, other race or ethnicity group, and participants with less than high school attainments were more likely to have self-reported depression. The prevalence of mild or moderate or severe depressive symptom was significantly lower among non-Hispanic whites and among participants with more than high school education. Ideal health behaviors, including normal weight, never smoking, healthy diet and active physical activity, and ideal diabetes status, were significantly associated with lower prevalence of mild or moderate/severe depressive symptom, whereas blood pressure and total cholesterol status were not.
Table 2.
Characteristics of participants and CVH metrics by depression status, NHANES 2007–2014*
| Characteristics | Overall (n = 14,561) |
No or minimal depression (n = 11,142) |
Mild depression (n = 2178) |
Moderate or severe depression (n = 1241) |
P value for trend |
|---|---|---|---|---|---|
| Age, y (mean, SE) | 44.6 (0.31) | 44.9 (0.33) | 43.5 (0.57) | 43.7 (0.60) | .096 |
| Female (%, SE) | 50.4 (0.54) | 48.2 (0.61) | 55.9 (1.39) | 62.1 (2.09) | <.001 |
| Race/ethnicity (%, SE) | |||||
| Non-Hispanic whites | 66.9 (1.85) | 67.7 (1.84) | 65.8 (2.23) | 61.2 (2.95) | .004 |
| Non-Hispanic blacks | 11.0 (0.87) | 10.6 (0.82) | 11.8 (1.25) | 13.2 (1.42) | .017 |
| Mexican American | 9.4 (1.00) | 9.3 (1.00) | 10.2 (1.16) | 9.1 (1.38) | .835 |
| Other | 12.7 (0.79) | 12.4 (0.76) | 12.2 (1.07) | 16.5 (1.97) | .020 |
| Heavy use of alcohol (%, SE) | 18.1 (0.61) | 17.9 (0.67) | 20.1 (1.33) | 16.4 (1.57) | .348 |
| Education (%, SE), y | |||||
| <12 | 16.4 (0.79) | 14.6 (0.78) | 19.9 (1.27) | 27.4 (2.02) | <.001 |
| 12 | 22.3 (0.79) | 21.5 (0.73) | 24.8 (1.72) | 25.6 (1.98) | .032 |
| >12 | 61.3 (1.29) | 63.9 (1.27) | 55.3 (2.21) | 47.0 (2.09) | <.001 |
| Body mass index risk (%, SE) | |||||
| Ideal | 29.9 (0.70) | 31.1 (0.83) | 25.8 (1.44) | 26.2 (1.75) | .016 |
| Intermediate | 34.4 (0.63) | 35.3 (0.76) | 32.9 (1.42) | 27.9 (1.92) | .001 |
| Poor | 35.8 (0.63) | 33.7 (0.70) | 41.3 (1.29) | 45.9 (1.71) | <.001 |
| Smoking risk (%, SE) | |||||
| Ideal | 76.9 (0.74) | 80.4 (0.67) | 69.5 (1.60) | 56.0 (2.20) | <.001 |
| Intermediate | 2.2 (0.16) | 2.1 (0.17) | 2.5 (0.47) | 2.8 (0.56) | .176 |
| Poor | 21.0 (0.70) | 17.5 (0.61) | 28.0 (1.56) | 41.2 (2.07) | <.001 |
| Physical activity risk (%, SE) | |||||
| Ideal | 39.7 (1.12) | 43.2 (1.19) | 31.3 (1.51) | 20.8 (1.79) | <.001 |
| Intermediate | 16.5 (0.47) | 17.0 (0.48) | 16.0 (1.41) | 12.8 (0.98) | <.001 |
| Poor | 43.8 (1.21) | 39.8 (1.21) | 52.7 (1.95) | 66.3 (2.13) | <.001 |
| Dietary risk (%, SE)† | |||||
| Ideal | 2.4 (0.19) | 2.6 (0.22) | 1.7 (0.44) | 1.1 (0.46) | .006 |
| Intermediate | 47.6 (1.00) | 49.5 (1.06) | 44.6 (1.68) | 34.1 (1.80) | <.001 |
| Poor | 50.1 (1.03) | 47.9 (1.08) | 53.8 (1.73) | 64.8 (1.83) | <.001 |
| Blood pressure risk (%, SE) | |||||
| Ideal | 44.8 (0.84) | 44.9 (0.92) | 43.9 (1.57) | 45.5 (1.62) | .744 |
| Intermediate | 41.7 (0.75) | 41.7 (0.83) | 41.9 (1.44) | 41.6 (1.90) | .931 |
| Poor | 13.5 (0.46) | 13.4 (0.44) | 14.2 (1.08) | 13.0 (1.24) | .725 |
| Diabetes risk (%, SE) | |||||
| Ideal | 75.0 (0.45) | 75.9 (0.53) | 72.9 (1.27) | 69.8 (1.99) | .007 |
| Intermediate | 18.7 (0.42) | 18.5 (0.53) | 18.7 (1.20) | 20.5 (1.72) | .284 |
| Poor | 6.4 (0.22) | 5.6 (0.25) | 8.5 (0.73) | 9.6 (1.00) | <.001 |
| Cholesterol risk (%, SE) | |||||
| Ideal | 49.9 (0.84) | 50.6 (0.86) | 47.4 (1.62) | 47.7 (1.97) | .143 |
| Intermediate | 36.3 (0.72) | 36.3 (0.76) | 35.7 (1.35) | 37.9 (2.05) | .443 |
| Poor† | 13.9 (0.50) | 13.2 (0.58) | 16.9 (1.20) | 14.4 (1.32) | .481 |
| HEI-2010 scores (mean, SE)† | 50.5 (0.31) | 51.3 (0.32) | 48.7 (0.53) | 45.8 (0.55) | <.001 |
| Mean scores of seven healthy metrics (mean, SE) | 3.18 (0.03) | 3.29 (0.03) | 2.92 (0.05) | 2.67 (0.05) | <.001 |
| Overall CVH metrics (%, SE) | |||||
| Inadequate (0–4) | 12.3 (0.41) | 10.5 (0.43) | 17.3 (1.10) | 20.7 (1.48) | <.001 |
| Average (5–9) | 68.3 (0.66) | 68.4 (0.74) | 66.4 (1.26) | 70.9 (1.78) | .194 |
| Optimum (10–14) | 19.4 (0.76) | 21.1 (0.84) | 16.4 (1.17) | 8.4 (0.93) | <.001 |
Mean and % are weighted.
AHA’s healthy diet score includes five components: fruits and vegetables, whole grain, fish, sodium, and sugar-sweeten beverage, and a very small proportion (<0.5%) of U.S. adults meet the ideal healthy diet. HEI-2010 is a continuous score consisting of 12 components representing major food groups including fruit and vegetables, whole grains, proteins, dairy, oils, sodium, and empty calories. HEI-2010 score ranges from 0 to 100 with a higher score indicates a more healthy diet. HEI-2010 has been validated to represent the diet quality in population. We used HEI-2010 as a proxy for AHA healthy diet score with ideal diet: HEI-2010 ≥81; intermediate diet: 51–80; and poor diet: ≤50.
Compared with those with available CVH metrics and depression scores, participants with missing CVH metrics and depression scores were younger, more likely to be women, non-Hispanic blacks or “other” race or ethnicity group, and have education less than 12 years (Supplemental Table 1).
Compared to participants with optimum CVH, the PRs for moderate or severe depression were 4.39 (95% CI, 3.32–5.80) and 2.64 (2.15–3.24), respectively, for those with inadequate and average CVH. The corresponding PRs for mild depression were 2.11 (1.77–2.52) and 1.36 (1.19–1.55; Table 3). Compared to participants meeting 3–4 ideal health behaviors, the PRs for moderate or severe depression were 4.97 (3.12–7.94) and 2.55 (1.54–4.22), respectively, for those meeting 0–1 or 2 ideal health behaviors. The corresponding PRs were 1.80 (1.47–2.21) and 1.30 (1.04–1.61) for mild depression. Ideal health factors were not associated with depressive symptom (Table 3).
Table 3.
Adjusted prevalence ratios and 95% confidence intervals for mild and moderate or severe depression associated with CVH metrics, NHANES 2007–2014 (n = 14,561)*,†
| Health metrics | Optimum CVH | Average CVH | Inadequate CVH |
|---|---|---|---|
| Moderate/severe depression | 1.00 | 2.64 (2.15–3.24) | 4.39 (3.32–5.80) |
| Mild depression | 1.00 | 1.36 (1.19–1.55) | 2.11 (1.76–2.52) |
| Number of health behaviors | |||
| Health behavior‡ | 3–4 | 2 | 0–1 |
| Moderate/severe depression | 1.00 | 2.55 (1.54–4.22) | 4.97 (3.12–7.94) |
| Mild depression | 1.00 | 1.30 (1.04–1.61) | 1.80 (1.47–2.21) |
| Number of health factors | |||
| Health factors§ | 3 | 2 | 0–1 |
| Moderate/severe depression | 1.00 | 1.27 (1.01–1.60) | 1.17 (0.94–1.46) |
| Mild depression | 1.00 | 1.01 (0.88–1.16) | 1.17 (0.99–1.39) |
Optimum CVH: CVH metrics scores 10–14; Average CVH: CVH metrics scores 5–9; Inadequate CVH: CVH metrics scores 0–4.
Adjusted by age, gender, race/ethnicity, education, and alcohol use.
Health behaviors include body mass index, smoking, physical activity, and dietary intake.
Health factors include total cholesterol, blood pressure, and fasting glucose.
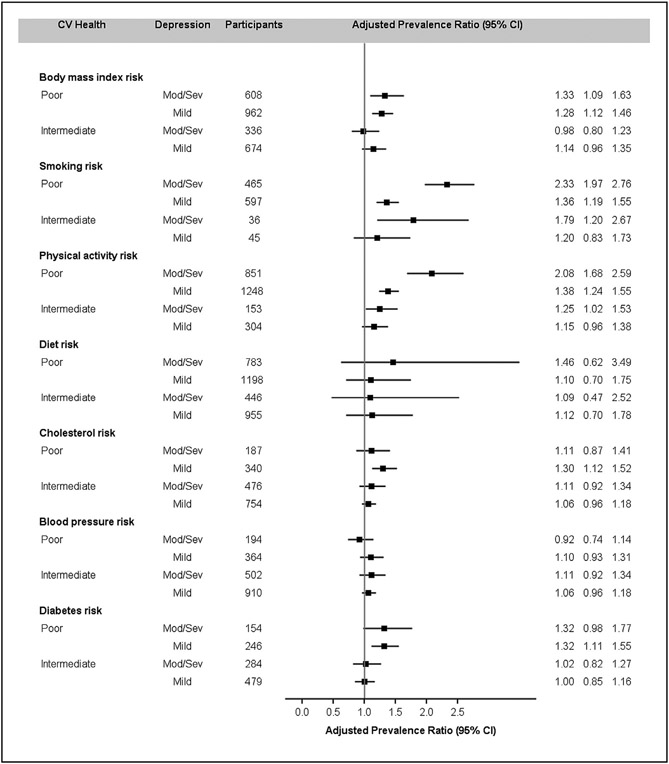
Figure 1 showed adjusted PRs (95% CI) for mild and moderate or severe depressive symptoms by individual CVH component comparing poor or intermediate to ideal health. In general, individual health behaviors were associated with moderate or severe or mild depressive symptom, whereas individual health factors were not except for diabetes status.
Fig. 1.
Adjusted prevalence ratios (95% CI) of depressive symptoms by individual component of CVH Metrics, NHANES 2007-2014. Adjusted by age, gender, race/ethnicity, education, and alcohol use. CVH = cardiovascular health; NHANES = National Health and Nutritional Examination Survey.
Table 4 presented the PRs of depression for inadequate and average health compared with optimum health by subgroups. The associations were largely consistent by age, gender, race or ethnicity, and education subgroups.
Table 4.
Adjusted prevalence ratios and 95% confidence intervals for mild and moderate/severe depression associated with CVH metrics by selected subgroups, NHANES 2007–2014*
| Characteristics | Ideal CVH | Intermediate CVH | Poor CVH |
|---|---|---|---|
| Age | |||
| 20–44 y (n = 7077) | |||
| Moderate/severe depression | 1.00 | 2.85 (2.21–3.67) | 4.58 (3.13–6.69) |
| Mild depression | 1.00 | 1.24 (1.07–1.45) | 2.00 (1.50–2.67) |
| 45–64 y (n = 5147) | |||
| Moderate/severe depression | 1.00 | 2.12 (1.30–3.46) | 3.58 (2.06–5.20) |
| Mild depression | 1.00 | 1.54 (1.11–2.14) | 2.43 (1.67–3.52) |
| ≥65 y (n = 2337) | |||
| Moderate/severe depression | 1.00 | 2.08 (0.83–5.20) | 2.57 (0.85–7.76) |
| Mild depression | 1.00 | 1.98 (1.13–3.48) | 2.45 (1.18–5.10) |
| Gender | |||
| Male (n = 7250) | |||
| Moderate/severe depression | 1.00 | 2.38 (1.58–3.59) | 2.61 (1.61–4.20) |
| Mild depression | 1.00 | 1.32 (1.06–1.64) | 2.02 (1.47–2.76) |
| Female (n = 7311) | |||
| Moderate/severe depression | 1.00 | 2.75 (2.12–3.58) | 5.53 (3.90–7.84) |
| Mild depression | 1.00 | 1.38 (1.19–1.59) | 2.17 (1.70–2.77) |
| Race/ethnicity | |||
| NHW (n = 6218) | |||
| Moderate/severe depression | 1.00 | 2.93 (2.09–4.11) | 4.15 (2.57–6.71) |
| Mild depression | 1.00 | 1.40 (1.19–1.64) | 2.52 (2.08–3.05) |
| NHB (n = 2966) | |||
| Moderate/severe depression | 1.00 | 1.46 (1.06–2.00) | 3.36 (2.18–5.16) |
| Mild depression | 1.00 | 1.26 (0.94–1.70) | 1.51 (.3–2.21) |
| MA (n = 2425) | |||
| Moderate/severe depression | 1.00 | 2.49 (1.34–4.62) | 3.89 (1.95–7.76) |
| Mild depression | 1.00 | 0.97 (0.70–1.34) | 1.04 (0.65–1.67) |
| Other (n = 2952) | |||
| Moderate/severe depression | 1.00 | 2.60 (1.82–3.71) | 5.18 (3.19–8.41) |
| Mild depression | 1.00 | 1.52 (1.09–2.12) | 1.88 (1.06–3.31) |
| Education | |||
| <12 y (n = 3548) | |||
| Moderate/severe depression | 1.00 | 2.10 (1.54–2.87) | 2.78 (1.91–4.06) |
| Mild depression | 1.00 | 1.23 (0.83–1.84) | 1.87 (1.13–3.09) |
| 12 y (n = 3294) | |||
| Moderate/severe depression | 1.00 | 1.94 (1.08–3.51) | 3.67 (1.82–7.40) |
| Mild depression | 1.00 | 1.19 (0.86–1.63) | 1.98 (1.33–2.94) |
| >12 y (n = 7719) | |||
| Moderate/severe depression | 1.00 | 3.09 (2.33–4.09) | 5.72 (3.56–9.19) |
| Mild depression | 1.00 | 1.44 (1.20–1.72) | 2.14 (1.69–2.72) |
Adjusted by age, gender, race/ethnicity, education, and alcohol use.
In a sensitivity analysis, we included participants with CVD or cancer, and results remained largely consistent with PRs excluding participants with a history of CVD or cancer (Supplemental Tables 2 and 3).
Discussion
In this nationally representative sample of adults in the United States, our analysis showed a strong and graded association between CVH metrics and both mild and moderate or severe depressive symptoms among U.S. adults. Having fewer ideal CVH metrics was associated with greater risk of having depressive symptoms. The associations were consistent across various subgroups.
Our results were consistent with several previous studies. In the Aerobics Center Longitudinal Study, Espana-Romero et al. followed 5110 participants for a mean period of 6.1 years and reported that ideal CVH, especially health behaviors, showed an inverse relationship with depressive symptoms [21]. However, most participants were male (80%) and Caucasian, relatively well-educated, and from middle to upper socioeconomic strata. A cross-sectional study of 6851 Chinese adults suggested better CVH was associated with a lower prevalence of depression, particularly among male and younger Chinese persons [22]. Participants included in this study were those with relatively high income and education. Other studies suggested that the association might be bidirectional. The Reasons for Geographic and Racial Differences in Stroke Study [27] and Mathews et al. [28] have reported that people with depressive symptoms were less likely to have optimal or adequate CVH.
The associations between depression and CVH metrics appeared to be stronger for the CVH behaviors (physical inactivity, smoking, obesity, and diet) than health factors (cholesterol, diabetes, and blood pressure). It could be that the relationship between depression and CVH behaviors is more immediate and more likely to be captured in a cross-sectional survey such as NHANES compared with the relationship between depression and health factors. For example, an episode of major depression might immediately impact a person’s likelihood of engaging in physical activity (thus leading to a stronger apparent association in a cross-sectional study), whereas a potential effect on blood glucose could take more time to develop (such as if a person experiencing depression skips a primary care appointment and thus does not have a renewed prescription for glucose-lowering medication when needed, months later). Also, it should be noted that bidirectional relationships between CVH metrics and depression are possible. Depression may lead to poorer CVH metrics, and also poor CVH metrics (such as inadequate physical activity or existing chronic conditions such as hypertension or diabetes) may lead to depression. For example, evidence suggests that having depression may increase the risk of developing diabetes, and having diabetes may increase the risk of developing depression, perhaps pertaining to increased stress and financial burden associated with having a chronic condition [29,30].
Many prior studies have examined associations between individual CVH behaviors and depression. Studies have consistently reported that physical activity has an inverse association with depressive symptom [31-33]. Physical activity likely produces endorphins (the chemicals in the brain that act as natural painkillers) and also improves the ability to sleep, which, in turn, reduces stress and depressive symptoms [34,35]. Many studies have found an association between cigarette smoking and depression, with results replicated in adolescents, adults, and the elderly [36,37]. Nicotine might damage certain pathways in the brain that regulate mood and might trigger mood swings. Nicotine’s influence on neuro-transmission pathways implicated in affective disorders provides a potential mechanism for such a relationship [38-40]. Studies have also suggested that obesity may have a direct relationship with depressive symptoms [41]. Putative mechanisms involve behavioral, physiologic, and genetic pathways, as well as iatrogenic effects of medications [42]. Nutrition factors have been found to be associated with depression symptoms; for example, Mediterranean diets might confer protection against the development of depression [43]. Consistent with the findings of previous studies, our results suggested that individual health behaviors appeared to have a stronger association with depressive symptoms in this nationally representative sample.
Few studies have examined the relationship between biological health factors and depressive symptoms. A meta-analysis showed a negative correlation between total cholesterol and depression [44]. Our study demonstrated this association for mild, but not for moderate or severe depression. Our study found the relationship between poor diabetes health and depression, which is consistent with the findings by Marano et al. [45]. However, other studies did not find associations between total cholesterol or fasting glucose and depression [21,22]. For the association between blood pressure and depression, several studies showed low or ideal blood pressure was associated with psychological or depressive symptoms [22,46,47]. A proposed mechanism for this relationship is that neurons that control blood pressure by releasing neuropeptide Y might both lower blood pressure and induce anxiety [48].
To our knowledge, this is the first study to assess the association between CVH metrics and depressive symptoms in a large nationally representative sample of U.S. adults. In addition, we were able to conduct a detailed assessment of depression and seven CVH metrics and control for several important confounding variables using NHANES data.
Our study has several limitations. First, HEI-2010 was from the first-day 24-hour dietary recall, which might not reflect individuals’ usual dietary intakes. A previous validation study using 24-hour dietary recalls suggested that energy intake may be under-estimated by as much as 11% [49]. Second, severely depressed persons may have disproportionately chosen not to participate in the survey or health examination, which included administration of the PHQ-9; therefore, the prevalence estimates in our study may underestimate the actual prevalence of depression. In addition, participants being successfully treated for depression would not be identified as having depression by the PHQ-9. Third, PHQ-9–based depressive symptoms, rather than clinical depression, can be highly variable. There could be differences in interpreting and answering the questions, especially for participants with different backgrounds and beliefs. However, PHQ-9 scores of 10 or higher have a sensitivity of 88% and a specificity of 88% for major depression [26]. Fourth, we cannot fully exclude potential effects of unmeasured confounding factors, such as anxiety disorder, general- and work-related stress, and so on, and the association between CVH and depression could be overestimated in our analysis. Fifth, smoking, physical activity, diet, and alcohol use were self-reported and subject to misclassification and recall biases. The association between CVH and depression might be over- or under-estimated due to the misclassification and recall biases of the self-reported variables. Sixth, we used HbA1c as a proxy of fasting glucose, which might misclassify the status of diabetes for some participants. Also, we used HEI-2010 as a proxy for the AHA’s healthy diet score. It is not clear how accurately the HEI-2010 healthy diet classification compared with the AHA five-component healthy diet score. However, a prior study suggested that the HEI-2010 can reliably detect the meaningful differences in diet quality in a population [50]. Furthermore, participants with missing CVH metrics and depression scores were younger, more likely to be women, non-Hispanic blacks or “other” race or ethnicity group, and have education less than 12 years. The association between CVH and depression could be biased due to the missing CVH metrics and depression scores. Finally, our study was cross-sectional; thus, the associations between CVH and depression cannot be interpreted as directly causal.
In conclusion, depression is common among U.S. adults and might be an independent risk factor for CVD. Our results suggested that there was a graded association between CVH metrics, particularly for the health behaviors, and both mild and moderate or severe depressive symptoms among U.S. adults. Our analyses extend the findings from previous studies by comprehensively assessing this relationship in a large, diverse, and nationally representative sample. Given the relationship between CVH and depression, public health efforts to achieve the AHA 2020 goals of improving CVH by 20% by 2020 might be supported by efforts to reduce the prevalence of depression.
Supplementary Material
Footnotes
The authors have no conflicts of interest to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 update: a report from the American Heart Association. Circulation 2016;133(4):e38–360. [DOI] [PubMed] [Google Scholar]
- [2].Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366(4):321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Daviglus ML, Stamler J, Pirzada A, Yan LL, Garside DB, Liu K, et al. Favorable cardiovascular risk profile in young women and long-term risk of cardiovas-cular and all-cause mortality. JAMA 2004;292(13):1588–92. [DOI] [PubMed] [Google Scholar]
- [4].Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD, for the Atherosclerosis Risk in Communities (ARIC) Study Investigators. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011;57(16):1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation 2012;125(8):987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ford ES, Zhao G, Tsai J, Li C. Low-risk lifestyle behaviors and all-cause mortality: findings from the National Health and Nutrition Examination Survey III Mortality Study. Am J Public Health 2011;101(10):1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects–Atherosclerosis Risk in Communities Study. Arch Intern Med 2007;167(6):573–9. [DOI] [PubMed] [Google Scholar]
- [8].Kulshreshtha A, Goyal A, Veledar E, McClellan W, Judd S, Eufinger SC, et al. Association between ideal cardiovascular health and carotid intima-media thickness: a twin study. J Am Heart Assoc 2014;3(1):e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D, et al. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA 1999;282(21):2012–8. [DOI] [PubMed] [Google Scholar]
- [10].Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000;343(1):16–22. [DOI] [PubMed] [Google Scholar]
- [11].Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 2012;307(12):1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Executive summary: heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation 2010;121(7):948–54. [DOI] [PubMed] [Google Scholar]
- [13].Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- [14].Artero EG, Espana-Romero V, Lee DC, Sui X, Church TS, Lavie CJ, et al. Ideal cardiovascular health and mortality: Aerobics Center Longitudinal Study. Mayo Clin Proc 2012;87(10):944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hirschfeld RM, Keller MB, Panico S, Arons BS, Barlow D, Davidoff F, et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA 1997;277(4):333–40. [PubMed] [Google Scholar]
- [16].World Health Organization. World health statistics 2017: monitoring health for the SDGs, sustainable development goals. World Health Organization; 2017. License: CC BY-NC-SA 3.0 IGO. https://www.who.int/gho/publications/world_health_statistics/2017/en/. [Accessed 1 November 2019]. [Google Scholar]
- [17].Druss BG, Rosenheck RA, Sledge WH. Health and disability costs of depressive illness in a major U.S. corporation. Am J Psychiatry 2000;157(8):1274–8. [DOI] [PubMed] [Google Scholar]
- [18].Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J 2014;35(21):1365–72. [DOI] [PubMed] [Google Scholar]
- [19].Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, et al. The functioning and well-being of depressed patients. Results from the medical outcomes study. JAMA 1989;262(7):914–9. [PubMed] [Google Scholar]
- [20].Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007;370(9590):851–8. [DOI] [PubMed] [Google Scholar]
- [21].Espana-Romero V, Artero EG, Lee DC, Sui X, Baruth M, Ruiz JR, et al. A prospective study of ideal cardiovascular health and depressive symptoms. Psychosomatics 2013;54(6):525–35. [DOI] [PubMed] [Google Scholar]
- [22].Li Z, Yang X, Wang A, Qiu J, Wang W, Song Q, et al. Association between ideal cardiovascular health metrics and depression in Chinese population: a cross-sectional study. Sci Rep 2015;5:11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].CDC website. https://www.cdc.gov/nchs/nhanes/index.htm. [Accessed 21 September 2018].
- [24].Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc 1995;95(10):1103–8. [DOI] [PubMed] [Google Scholar]
- [25].American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care 2010;33(Suppl 1):S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kronish IM, Carson AP, Davidson KW, Muntner P, Safford MM. Depressive symptoms and cardiovascular health by the American Heart Association’s definition in the reasons for geographic and racial differences in stroke (REGARDS) study. PLoS One 2012;7(12):e52771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mathews L, Ogunmoroti O, Nasir K, Blumenthal RS, Utuama OA, Rouseff M, et al. Psychological factors and their association with ideal cardiovascular health among women and men. J Womens Health (Larchmt) 2018;27(5):709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lyketsos CG. Depression and diabetes: more on what the relationship might be. Am J Psychiatry 2010;167:496–7. [DOI] [PubMed] [Google Scholar]
- [30].Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord 2012;142(suppl):S8–21. [DOI] [PubMed] [Google Scholar]
- [31].Farmer ME, Locke BZ, Moscicki EK, Dannenberg AL, Larson DB, Radloff LS. Physical activity and depressive symptoms: the NHANES I epidemiologic follow-up study. Am J Epidemiol 1988;128(6):1340–51. [DOI] [PubMed] [Google Scholar]
- [32].Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol 1991;134(4):220–31. [DOI] [PubMed] [Google Scholar]
- [33].Strohle A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm (Vienna) 2009;116(6):777–84. [DOI] [PubMed] [Google Scholar]
- [34].Moore M. Endorphins and exercise: a puzzling relationship. Phys Sportsmed 1982;10(2):111–4. [DOI] [PubMed] [Google Scholar]
- [35].Stein L, Belluzzi JD. Brain endorphins and the sense of well-being: a psycho-biological hypothesis. Adv Biochem Psychopharmacol 1978;18:299–311. [PubMed] [Google Scholar]
- [36].Bland P. Smoking cessation improves anxiety depression. Practitioner 2014;258(1769):5. [PubMed] [Google Scholar]
- [37].Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, et al. Smoking, smoking cessation, and major depression. JAMA 1990;264(12):1546–9. [PubMed] [Google Scholar]
- [38].Carmody TP. Affect regulation, nicotine addiction, and smoking cessation. J Psychoactive Drugs 1989;21(3):331–42. [DOI] [PubMed] [Google Scholar]
- [39].Pomerleau OF, Pomerleau CS. Neuroregulators and the reinforcement of smoking: towards a biobehavioral explanation. Neurosci Biobehav Rev 1984;8(4):503–13. [DOI] [PubMed] [Google Scholar]
- [40].Newhouse PA, Hughes JR. The role of nicotine and nicotinic mechanisms in neuropsychiatric disease. Br J Addict 1991;86(5):521–6. [DOI] [PubMed] [Google Scholar]
- [41].Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010;67(3):220–9. [DOI] [PubMed] [Google Scholar]
- [42].Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med 2008;70(3): 288–97. [DOI] [PubMed] [Google Scholar]
- [43].Sanchez-Villegas A, Henriquez P, Bes-Rastrollo M, Doreste J. Mediterranean diet and depression. Public Health Nutr 2006;9(8A):1104–9. [DOI] [PubMed] [Google Scholar]
- [44].Shin JY, Suls J, Martin R. Are cholesterol and depression inversely related? A meta-analysis of the association between two cardiac risk factors. Ann Behav Med 2008;36:33–43. [DOI] [PubMed] [Google Scholar]
- [45].Marano CM, Workman CI, Lyman CH, Kramer E, Hermann CR, Ma Y, et al. The relationship between fasting serum glucose and cerebral glucose metabolism in late-life depression and normal aging. Psychiatry Res 2014;222(1-2): 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Donner-Banzhoff N, Chan Y, Szalai JP, Hilditch JR. Low blood pressure associated with low mood: a red herring? J Clin Epidemiol 1997;50(10): 1175–81. [DOI] [PubMed] [Google Scholar]
- [47].Hildrum B, Mykletun A, Stordal E, Bjelland I, Dahl AA, Holmen J. Association of low blood pressure with anxiety and depression: the Nord-Trondelag Health Study. J Epidemiol Community Health 2007;61(1):53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Michalkiewicz M, Knestaut KM, Bytchkova EY, Michalkiewicz T. Hypotension and reduced catecholamines in neuropeptide Y transgenic rats. Hypertension 2003;41(5):1056–62. [DOI] [PubMed] [Google Scholar]
- [49].Espeland MA, Kumanyika S, Wilson AC, Reboussin DM, Easter L, Self M, et al. Statistical issues in analyzing 24-hour dietary recall and 24-hour urine collection data for sodium and potassium intakes. Am J Epidemiol 2001;153(10):996–1006. [DOI] [PubMed] [Google Scholar]
- [50].Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr 2014;144(3):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.