Abstract
Depression is considered a major public health concern, where existing pharmacological treatments are not equally effective across all patients. The pathogenesis of depression involves the interaction of complex biological components, such as the immune system and the microbiota‐gut‐brain axis. Adjunctive lifestyle‐oriented approaches for depression, including physical exercise and special diets are promising therapeutic options when combined with traditional antidepressants. However, the mechanisms of action of these strategies are incompletely understood. Accumulating evidence suggests that physical exercise and specific dietary regimens can modulate both the immune system and gut microbiota composition. Here, we review the current information about the strategies to alleviate depression and their crosstalk with both inflammatory mechanisms and the gut microbiome. We further discuss the role of the microbiota‐gut‐brain axis as a possible mediator for the adjunctive therapies for depression through inflammatory mechanisms. Finally, we review existing and future adjunctive strategies to manipulate the gut microbiota with potential use for depression, including physical exercise, dietary interventions, prebiotics/probiotics, and fecal microbiota transplantation.
The current increasing burden of mental illness poses significant concern for global human health, causing a negative socioeconomic impact and quality of life. 1 This has provoked increased efforts towards the development of novel drug therapies for neuropsychiatric disorders. In particular, depressive disorders affect more than 260 million people globally, 2 which in terms of disease burden is currently the leading cause of disability worldwide.
While most of the antidepressant options currently used in clinical practice involve the use of drugs that almost exclusively target serotonin and/or noradrenaline pathways, 3 clinical evidence has suggested that monoamine‐based pharmacological treatments for depression are not effective for everyone, 4 with only 50% to 65% of patients treated showing complete remission. 5 Moreover, depression has an unclear etiology, due to accumulating evidence that its pathophysiology results from the interaction of complex biological components. 6 , 7 Recently, increasing evidence has implicated both the immune system 6 and the gut microbiome 8 as key factors in depression. Moreover, the interactions between both of these and the stress system is attracting attention in both animal and human clinical research. 7
Different strategies have been adopted to increase the efficacy of antidepressant drugs, including the use of adjunctive therapies. An adjunctive therapy is used together with primary treatments to assist with efficacy, symptomatology, and side effects of the disease. 9 There is evidence to suggest that a number of adjunctive therapies, including exercise and dietary interventions, are beneficial because of their anti‐inflammatory actions. 10 Notably, the gut microbiota has been shown to be sensitive to physical exercise and diet. 11 Thus, the ability of adjunctive therapies to shape the microbiome as a mechanism for the modulation of gut‐brain‐associated inflammatory pathways may have crucial implications for the design of optimal strategies to treat depression.
Together, this review provides important information about the current strategies to alleviate depression and their crosstalk with the gut microbiome. We elaborate and discuss the role of the microbiota‐gut‐brain axis as a mediator for the adjunctive therapies for depression through inflammatory mechanisms. As part of the strategies to manipulate gut microbiota composition and diversity, we review the current preclinical and clinical evidence supporting the use of adjunctive therapies, including physical exercise, dietary interventions, prebiotics/probiotics, and fecal microbiota transplantation (FMT) (Figure 1 ).
Figure 1.
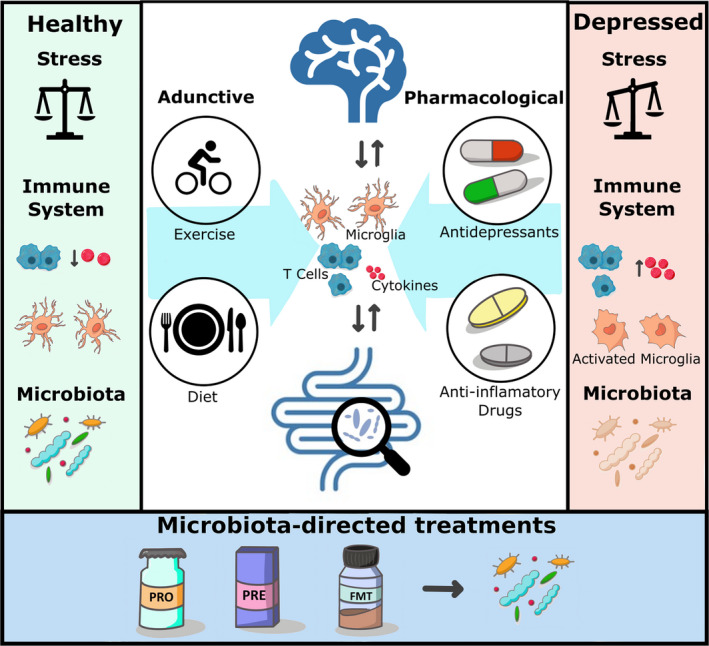
Role of the microbiota‐gut‐brain axis and the immune system in depression. In immune‐related depression, the stress response and the immune system are dysregulated; microglia are abnormally activated and there is an increased level of inflammatory cytokines in circulation and in the brain. Patients with depression display altered gut microbiota composition and diversity compared with healthy participants. Current pharmacological treatments for immune‐related depression include traditional antidepressants and anti‐inflammatory drugs. On the other hand, lifestyle factors such as exercise and diet are considered excellent adjunctive therapies for antidepressant action, which can influence the gut microbiome and the immune system. Future clinical approaches to combat depression may involve microbiota‐directed strategies such as probiotics, prebiotics and FMT. FMT, fecal microbiota transplant; PRE, prebiotics; PRO, probiotics.
THE IMMUNE SYSTEM AND DEPRESSION
The immune system is now viewed as an important component in the pathogenesis of depression 12 and a target for antidepressant strategies. 13 Increased peripheral pro‐inflammatory cytokine production, 14 dysregulation of T‐cell function, 15 and altered neuroinflammatory signaling 16 are evident in depression. The impact of inflammation on neuronal tissue and function depends on the intensity and duration of the inflammatory response. Clinical and preclinical evidence now suggest that chronic (neuro)inflammation is involved in the pathophysiology of depression. 6 , 16 Consequently, anti‐inflammatory therapies are being tested in depression. 17 , 18 While the data show some promise in certain subsets of patients, widespread use of anti‐inflammatory therapies is challenged by the fact that the immune system plays a more significant role in the pathogenesis of depression in some but not all patients. 19
Since the initial reports describing an association between depression and increased systemic levels of pro‐inflammatory cytokines, 20 numerous studies have implicated the immune system as a key element in the depressive phenotype. 16 Numerous clinical investigations have revealed low‐grade inflammation and abnormal activity of inflammatory mediators in patients with depression, which not only includes cytokines but also cytokine receptors and immune‐related cells (Table S1 ). Moreover, meta‐analysis studies assessing the effects of anti‐inflammatory drugs on major depressive disorder and depressive symptoms found that most of the nonsteroidal anti‐inflammatory drugs (NSAIDs) and cytokine inhibitors also exerted significant antidepressant effects. 21 , 22
The brain possesses its own neuroimmune machinery, including important cellular components, such as astrocytes, oligodendrocytes, and microglia. 23 Microglia are involved in the secretion of cytokines and chemokines as well as other pro‐inflammatory mediators after infection or brain damage, 24 which have been linked to neurodegenerative and psychiatric disorders, such as Alzheimer’s disease and major depressive disorder (MDD). 25 Indeed, previous studies have found abnormal activation of microglia in prefrontal cortex and anterior cingulate cortex during severe episodes of depression in human participants. 26
THE GUT MICROBIOME AND DEPRESSION
The gut microbiome is defined as a characteristic microbial community occupying the gastrointestinal tract, which has distinct physio‐chemical properties. 27 It comprises the assembly of microorganisms belonging to bacteria, archaea, protozoa, fungi, and algae, but also includes microbial‐derived structures such as metabolites and mobile genetic elements (e.g., transposons, phages, and viruses). 27 More recently, the interaction between these microbes and the brain has been given much attention, with increasing evidence supporting the importance of the microbiota‐gut‐brain axis. The microbiota‐gut‐brain axis represents the bidirectional communication between the central nervous system (CNS) and the trillions of microorganisms residing in the gastrointestinal tract. 28 The microbiota‐gut‐brain axis thus incorporates the pathways of communication between the microbes and brain, including immune regulation. 7 Alterations in gut microbiota composition and the relative abundance of specific taxa have been associated with depressive disorders. 29 , 30 Moreover, it has been proposed that the microbiota‐gut‐brain axis may utilize inflammatory mechanisms to mediate the progression of depressive behavior. 7 Recent work has shown a novel mechanism by which a quorum‐sensing molecule—quorum sensing is an important regulatory process for bacteria that enables cell‐cell communication 31 —secreted by segmented filamentous bacteria, can increase susceptibility to depressive‐like behaviors, in a helper T cell 17 (TH17)–dependent manner. 32 , 33
Clinical studies have also revealed that there are clear differences in the gut microbiota signature in patients with depression compared with healthy participants, albeit with considerable variation in the findings reported 8 , 34 (Table S2 ). A study using whole‐genome shotgun metagenomic and untargeted metabolomic analysis identified three bacteriophages, 47 bacterial species, and 50 fecal metabolites with significant differences in relative abundance between patients with depression and healthy controls. In particular, patients with depression were mainly characterized by increased abundance of the genus Bacteroides and decreased abundance of the genera Blautia and Eubacterium. 30 Changes in specific bacterial taxa have been associated with alterations in microbial‐derived metabolites and pathways with proven effects on the CNS. For example, short‐chain fatty acids such as acetate, propionate and butyrate, by‐products of the metabolism of fiber by the intestinal microbiota, have been linked to gut–brain communication. 35
In an important population‐based study evaluating the neuroactive potential of gut microbes in patients with depression, different bacteria, including Burkholderia oklahomensis, Ralstonia spp., Acinetobacter baumannii, and Klebsiella spp., correlated with the upregulation of metabolic pathways associated with gut–brain communication, including, for instance, p‐cresol degradation, serotonin synthesis, and nitric oxide synthesis. Butyrate‐producing Faecalibacterium and Coprococcus bacteria were consistently linked to higher quality‐of‐life indicators. 29 Although depression and quality‐of‐life are not synonymous, and the precise mechanism or mechanisms that allow the microbiome to mediate the development of depressive behavior is unclear, it has been demonstrated that a depressive phenotype can be successfully transferred from patients to rodents through FMT, suggesting a causal role for gut microbiota in depressive symptomology. 36 Psychiatric disorders have also been characterized by alterations in gut microbes linked to anti‐inflammatory or proinflammatory capacity, as shown in a meta‐analysis including 1,519 patients with different psychiatric disorders (MDD, bipolar disorder, psychosis and schizophrenia, anorexia nervosa, anxiety, obsessive compulsive disorder, posttraumatic stress disorder, and attention‐deficit/hyperactivity disorder), where consistent differences in β‐diversity in patients with MDD were found. Moreover, these changes were correlated with depleted levels of Faecalibacterium and Coprococcus and enriched levels of Eggerthella, suggesting that depression is characterized by a reduction of anti‐inflammatory butyrate‐producing bacteria, while pro‐inflammatory genera are enriched compared with healthy participants. 34
MICROGLIA: AT THE INTERFACE OF IMMUNE–MICROBIOME INTERACTIONS IN DEPRESSION
A potential consequence of the alteration of gut microbiota composition and diversity in patients with depression is an increase in neuroinflammation. 37 In this regard, recent findings have highlighted the relationship between the gut microbiome and microglia via microbiota‐gut‐brain axis connections. 38 , 39 It has also been suggested that a more complex intercommunication between the gut microbiome and microglia exists in MDD, denominated the microbiota‐gut‐immune‐glia axis. 40 Indeed, a complete lack of microbiota, as studied in adult germ‐free (GF) mice, induced distinct differences in microglial density and morphology (increased cell volume, segment number, branch points, and dendrite length) when compared with microglia analyzed in specific pathogen‐free (SPF) mice. 41 The same study demonstrated that colonizing these GF mice with a diverse and complex microbial community facilitated the transition to a mature microglial phenotype (as observed in adult SPF animals). 41 Moreover, results from studies in GF mice showed that the gut microbiota is crucial for microglia development in both a prenatal and postnatal period in a sex‐dependent and time‐dependent manner. 42
Immune‐associated depression has been proven to be sensitive to the manipulation of gut microbiota composition through the administration of probiotic strains and antibiotics in animal models of depression. For example, treatment with Clostridium butyricum Miyairi 588 demonstrated therapeutic potential by ameliorating depressive‐like behaviors in mice exposed to chronic social defeat stress. 43 This behavioral improvement was accompanied by reductions in interleukin 1β (IL‐1β), IL‐6, and tumor necrosis factor alpha (TNF‐α) in plasma, decreases in hippocampal microglial activation, and a significant depletion of Firmicutes abundance. 43 The antibiotic rifaximin also induced antidepressant‐like actions in adolescent rats subjected to chronic unpredictable stress, which was positively correlated with butyrate levels in the brain and anti‐inflammatory factors released by microglia. In addition, rifaximin increased the relative abundance of Ruminococcaceae and Lachnospiraceae. 44 Interestingly, minocycline, a tetracycline antibiotic, has demonstrated possible therapeutic actions through the modulation of microglia function and microbiota composition. 45 Specifically, 3 weeks of treatment with minocycline in male rats selectively bred for high anxiety‐like and depressive‐like phenotype, attenuated depressive‐like behaviors, and reduced the density of microglia. These results were accompanied by a significant decrease in plasma concentrations of interferon gamma (IFN‐γ), and increases in the abundance of Lachnospiraceae and Clostridiales Family XIII in the gut. 45 Accordingly, antidepressant augmentation with minocycline in patients with MDD with low‐grade peripheral inflammation (C‐reactive protein (CRP) ≥3 mg/L), displayed significant efficacy in reducing Hamilton Depression Rating Scale scores and decreasing plasma levels of IFN‐γ. 18
The search for the precise mechanisms underlying gut microbiota regulation of microglia function has led to interesting insights which highlight microbiome‐derived acetate as an essential modulator of microglia maturation and phagocytosis. Indeed, supplementation of acetate in GF mice reversed abnormal biological features in microglia, including increased ramification and density, high mitochondrial mass, and reduced membrane potential, like what is observed in SPF mice. 39 , 46 These findings suggest that the gut microbiome plays an important role in the regulation of microglial maturation and function. Together, deviations from microglial homeostasis associated with the pathogenesis of depression 47 and the potential involvement of the microbiota‐gut‐brain axis in the regulation of neuroinflammatory pathways have opened new discussions about the role of the microbiota‐gut‐brain axis in immune‐related depression. Thus, considering the evidence for the relationship between the microbiota‐gut‐brain axis, pro‐inflammatory markers, and microglia function with the depression phenotype, the hypothesis that alterations in the microbiota‐gut‐brain axis may contribute to the development and onset of depressive disorders via inflammatory mechanisms is gaining traction.
PHARMACOLOGICAL THERAPIES FOR DEPRESSION THROUGH GUT MICROBIOME‐MEDIATED INFLAMMATORY MECHANISMS
Most antidepressants are thought to act by primarily modulating the levels and availability of monoaminergic neurotransmitters (e.g., serotonin, dopamine, and noradrenaline) 3 or affecting N‐methyl‐d‐aspartate (NMDA) receptor activity and glutamatergic signaling 48 in the brain. Selective serotonin reuptake inhibitors (SSRIs; e.g., fluoxetine and escitalopram), serotonin/noradrenaline reuptake inhibitors (SNRIs; e.g., venlafaxine and duloxetine), tricyclic antidepressants (TCAs; e.g., desipramine and imipramine), and monoamine oxidase (MAO) inhibitors (e.g., isocarboxazid and phenelzine) 49 remain the mainstay of pharmacological treatment. Although its mechanism of action is still not fully understood, ketamine is effective in patients unresponsive to other conventional antidepressants 50 and has been recently approved by the US Food and Drug Administration (FDA) in the treatment of depression. 51 Although there are increasing data supporting the impact of antidepressants on other physiological parameters, including metabolism, 52 blood pressure, 53 and immunity, 54 little is known about the interaction of antidepressants with the microbiota‐gut‐brain axis. Nonetheless, promising data suggest the existence of a unique neural firing pattern code that is common to both chemical and bacterial vagus‐dependent antidepressant luminal stimuli, providing a novel neuronal electrical perspective as a potential explanation for the mechanisms of action of gut‐derived vagal dependent antidepressants. 55
Antidepressants impact gut microbiota parameters
The gut microbiota can be subjected to profound changes after the administration of antidepressants as previously demonstrated in preclinical and clinical investigations. 56 An in vitro approach using bacterial cultures of some typical intestinal microbes, including Bifidobacterium animalis, Bacteroides fragilis, and Faecalibacterium prausnitzii, demonstrated that several antidepressants exert significant antimicrobial activity against commensal bacteria, with desipramine and aripiprazole being the most inhibitory. 57 Antidepressants, including fluoxetine, escitalopram, venlafaxine, and duloxetine, but not desipramine, reduced richness of microbial communities. 58 Moreover, the composition of Ruminococcus flavefaciens and Adlercreutzia equolifaciens was significantly decreased after treatment with the SSRI/SNRI duloxetine when compared with the control group of mice. Simultaneous supplementation with R. flavefaciens attenuated duloxetine‐induced antidepressant‐like actions, and downregulated the expression of proteins related to neuronal plasticity, suggesting a complex mechanism involving antidepressant and microbiota‐gut‐brain axis pathways to improve depressive behavior. 58
Chronic fluoxetine treatment resulted in a significant and time‐dependent alteration of gut microbiota composition and body weight, the last one being a well‐recognized negative side effect of SSRIs. 59 The results indicated that fluoxetine treatment produced inconsistent and not significantly different changes in microbial diversity between groups, but there was a significant reduction in the abundance of Lactobacillus johnsonii and Bacteroidales S24‐7, which belong to phyla associated with body mass regulation. 59 Similarly, the effects of different psychotropic drugs on gut microbiota parameters was investigated in the rat, including the antidepressants fluoxetine and escitalopram, and other drugs used to treat depressive symptoms like valproate, lithium, and the antipsychotic aripiprazole. 60 Lithium, valproate, and aripiprazole administration significantly increased microbial species richness and diversity for several species belonging to Clostridium, Peptoclostridium, Intestinibacter, and Christenellaceae. Although fluoxetine and escitalopram demonstrated antimicrobial capacity in vitro, they were unable to significantly alter gut microbiota composition and richness in the rat. However, both fluoxetine and escitalopram induced an abnormal increase in ileum permeability. 60 Together, it is unclear whether the microbial modulatory activity of antidepressants is merely a benign collateral damage or part of a more complex systemic mechanism of antidepressant action in the gut.
Many other antipsychotics (e.g., olanzapine, aripiprazole, quetiapine, brexpiprazole, ziprasidone, risperidone, and cariprazine) are often used as adjunctive options to treat resistant depression due to their observed therapeutic effectiveness in the past century. 61 Antipsychotics can impact the growth and viability of commensal microbes as reported in an in vitro experiment using a high‐throughput drug screen on gut bacteria. 62 Intriguingly, nearly all subclasses of antipsychotic drugs exhibited antimicrobial activity despite the fact that they target serotonin and dopamine receptors in the brain, which are not expressed in bacteria. 62 The bidirectional relationship between antipsychotics and the gut microbiome has become an important point of discussion, where in fact this mutual interaction appears to be selective depending on the specific antipsychotic evaluated. For instance, the bioavailability of two commonly prescribed antipsychotics (olanzapine and risperidone) was analyzed in rats subjected to changes in gut microbial composition driven by either antibiotics or probiotics. 63 Whereas the bioavailability of olanzapine was significantly increased in rats that had undergone antibiotic‐induced gut microbiota depletion, risperidone bioavailability was unchanged. This result suggests a selective role of the gut microbiome in the biotransformation of antipsychotics with similar targets in the brain. 63
Antidepressants alter neuroinflammation
Some antidepressants have been shown to be effective in modulating the immune response. 54 For example, meta‐analyses evaluating the effect of different classes of antidepressants (such as SSRIs, SNRIs, and TCAs) on serum levels of pro‐inflammatory cytokines from patients suffering from depression showed significant reductions in IL‐1β, IL‐6, and TNF‐α. 64 Regarding neuroinflammation, the effects of different antidepressants on inflammatory mediators in the CNS have been extensively reviewed. 65 For example, the TCA desipramine produced significant reductions in the gene expression of IL‐1β and inducible nitric oxide synthase (iNOS) and decreased activity of nuclear factor kappa B (NF‐κB) in the brain cortex of rats injected with the endotoxin lipopolysaccharide (LPS). 66 The nonselective MAO inhibitor nialamide reduced neuroinflammation by attenuating the number of activated microglia and astrocytes and reduced expression of TNF‐α in the cortex of mice exposed to transient middle cerebral artery occlusion. 67
Many antidepressants have been reported to reduce microglial activation as demonstrated in preclinical investigations. 68 Fluoxetine significantly reduced microgliosis (intense reaction of microglia) and demonstrated a capacity to alleviate the expression of inflammatory markers in the hippocampus of kainic acid‐injected mice by decreasing the messenger RNA (mRNA) levels of TNF‐α, IL‐1β, and cyclooxygenase 2 (COX‐2). 69 Imipramine induced a decrease in the number of activated microglia in the hippocampal hilus in a rat model of learned helplessness, 70 and ketamine treatment effectively reduced microglial activation in the hippocampus and serum levels of TNF‐α and IL‐6 in mice subjected to the chronic restraint stress model of depression. 71 Fluoxetine alleviated the increased number of ionized calcium binding adaptor molecule 1 (Iba‐1)–positive microglia/macrophages, neutrophil infiltration, and cell death found in rats with endovascular perforation (model of subarachnoid hemorrhage). It also reduced the levels of pro‐inflammatory cytokines, downregulated the expression of toll‐like receptor 4 (TLR4) and myeloid differentiation primary response 88 (MyD88), and promoted the nuclear translocation of NF‐κB p65. 72
Other molecules with therapeutic effects for depression have been shown to regulate inflammation. For example, melatonin is a hormone released by the pineal gland and sold for medical use because of its known actions associated with control of the sleep–wake cycle, but also with antidepressant activity. 73 In a mouse model of LPS‐induced depressive‐like phenotype, treatment with melatonin abolished the effects of LPS by reducing the levels of TNFα, IL‐1β, and IL‐6, decreasing NF‐κB phosphorylation, and attenuating LPS‐induced depressive‐like behaviors. 74 Moreover, the antidepressant activity of melatonin has been associated with attenuation of neuroinflammatory mechanisms through the SIRT1/Nrf2 (sirtuin 1/nuclear factor‐erythroid factor 2‐related factor 2) pathway. 75
ANTI‐INFLAMMATORY DRUG THERAPIES FOR DEPRESSION: CONTEXT FOR THE GUT MICROBIOME
Due to the increasing evidence supporting an association between depression and inflammatory processes, 6 anti‐inflammatory drugs have been tested as both an add‐on treatment and as a monotherapy to improve depressive symptoms. In particular, cytokine inhibitors and nonsteroidal anti‐inflammatory drugs have demonstrated antidepressant actions in clinical trials. 76 Nonetheless, little is known about the interactions between anti‐inflammatory drugs and the gut microbiome in the context of depression. In this section, we provide some evidence linking the therapeutic potential of anti‐inflammatory drugs and depression; we further discuss the consequences of anti‐inflammatory treatment for gut microbiota composition and microbial‐related pathways.
Nonsteroidal anti‐inflammatory drugs
NSAIDs are a group of chemically unrelated compounds and one of the most utilized strategies to reduce inflammation in the periphery. They not only exert anti‐inflammatory actions, but also have analgesic and antipyretic actions. 77 It has been demonstrated that their main mechanism is the inhibition of COX, an enzyme crucial for the biosynthesis of prostaglandins and thromboxane and constitutively expressed in almost all tissues, including neurons and microglia. 78 The antidepressant actions of NSAIDs have been demonstrated in different clinical studies. 21 , 22 For example, a double‐blind and placebo controlled clinical trial in patients suffering from osteoarthritis demonstrated a significant decrease of depressive symptoms after treatment with ibuprofen, naproxen, and celecoxib. 79 Celecoxib is an NSAID with antidepressant efficacy, as previously demonstrated in clinical trials with patients with colorectal cancer, 80 and female patients with fibromyalgia. 81 Similarly, an animal investigation demonstrated that celecoxib, ibuprofen, and indomethacin were effective in reducing depressive‐like behaviors as observed in the forced swim test and in a model of IFNα‐induced depression in mice. 82 Although there are some promising data from a large‐scale clinical trial, NSAIDs have yet to prove effective. 83 This may be due to the fact that in this clinical trial a large proportion of patients did not display substantial inflammation, and the outcomes selected to evaluate depression might not be appropriate to assess the effectiveness of anti‐inflammatory drugs, i.e., no anhedonia‐related readouts were included. 84
It is difficult to dissociate the side effects of NSAIDs on gut function 85 from any therapeutic effects. Evidence suggests that some NSAIDs possess antibacterial activity via interference of the DNA replication and repair machinery. 86 Indeed, it has been reported that NSAIDs can impact the composition and function of the gut microbiota. However, the reported outcomes vary between studies and models. For example, indomethacin has been shown to increase Bacteroides and Clostridium in rats 87 and reduce Bacteroides and increase Firmicutes in mice. 88 Significant changes in the composition of these taxa have been positively correlated with depressive behavior in both animals and humans. 89 It is also noteworthy that commensal bacteria can induce chemical modifications to NSAIDS with critical implications for absorption and pharmacokinetics. 85 Although the antidepressant actions of NSAIDs are primarily associated with a reduction of inflammation, 90 further studies are warranted to elucidate potential gut–brain pathways linked to NSAID‐mediated antidepressant effects.
Cytokine inhibitors
The increase and activity of pro‐inflammatory cytokines have been associated with the pathophysiology of depression 91 (see Table S1 for a detailed list of cytokines altered in patients with depression). Cytokine inhibitors attenuate the inflammatory response by blocking pro‐inflammatory cytokine receptors with antagonists, or by reducing their availability with soluble receptors and antibodies. 92 Cytokine inhibitors have shown therapeutic potential by reducing depressive symptoms in some but not all studies. 17 , 21 , 22 For instance, a clinical study examining the effects of sirukumab or siltuximab (human monoclonal antibodies designed for the inhibition of IL‐6) in patients with rheumatoid arthritis or multicentric Castleman's disease showed that both inhibitors produced significant improvements in depressive symptoms. 93 These data support current insights on the role of IL‐6 in the stress response and neuroinflammation, where increased release of IL‐6 has been found to be a factor associated with depressive disorders and therapeutic response. 94 Ixekizumab, a high‐affinity antibody that selectively targets IL‐17, has also been evaluated for antidepressant capacity. An integrated analysis of different clinical studies revealed that a 12‐week treatment with ixekizumab resulted in remission of depression for ~40% of patients and improved systemic inflammation. 95
Notably, altered gut microbiota composition and diversity is linked to an aberrant immune response and abnormal production of pro‐inflammatory cytokines. 96 The impact of immunomodulatory drugs on the gut microbiota has been extensively reviewed to date. 97 For example, TNF inhibitors including neutralizing monoclonal antibodies, fusion proteins, and pegylated fragments, which have provided effective treatment against different autoimmune diseases, 98 are capable of changing the composition of the gut microbiota. Indeed, a clinical study evaluating the effects of the TNF inhibitor etanercept on the gut microbiota of patients with rheumatoid arthritis revealed that the difference between the microbial composition of healthy participants and patients was partially restored by treatment with the drug. 99 Similarly, a 6‐month drug therapy with the TNF inhibitor adalimumab significantly improved intestinal dysbiosis in Crohn’s disease patients by reducing Proteobacteria and increasing Lachnospiraceae family. 100
Taken together, although there is increasing evidence suggesting a relationship between the gut microbiome and the antidepressant actions mediated by anti‐inflammatory drugs, there remains a lack of data related to the involvement and consequences of anti‐inflammatory drugs on gut–brain communication pathways, such as vagal connection and microbial‐derived metabolites. 28 Butyrate is a short‐chain fatty acid that has been correlated with human health in different physiological contexts, 101 including modulation of mood 35 and facilitation of anti‐stress effects. 102 Therefore, it is likely that the positive effects of anti‐inflammatory drugs on depressive symptoms may be partly mediated by similar mechanisms. Future studies should include more metabolomic analyses to elevate our understanding of the role of the gut microbiome and related metabolites in anti‐inflammatory drugs for depression.
ADJUNCTIVE LIFESTYLE FACTORS: TARGETING INFLAMMATION THROUGH THE GUT MICROBIOME?
There has been increasing interest in lifestyle adjunctive strategies for managing depression symptoms. These include physical exercise and dietary changes, which have been recommended in recent years because of the increasing scientific evidence supporting their therapeutic actions in multiple diseases, including depressive disorders. 103 , 104 Although most of the benefits of adjunctive therapies are linked with multisystemic activity and influence on metabolic pathways, 105 there is increasing evidence suggesting that physical activity and appropriate diet may ameliorate the symptoms of depression through their capacity to reduce inflammation. 106 With recent mounting indications that the gut microbiome controls and influences the inflammatory response, a role for the gut microbiome in adjunctive‐mediated improvements in depression through inflammatory mechanisms has emerged.
Physical exercise and depression
The therapeutic potential of physical exercise for patients with depression has been proven due to the reported improvements in symptomatology and well‐being. 107 In this regard, a meta‐analysis examined the efficacy of physical exercise as a treatment for unipolar depression. 108 A total of 23 randomized controlled trials (with 977 participants in total) were analyzed and the main outcome was a reduction in depressive symptoms or remission. Any kind of aerobic exercise (e.g., walking, running, cycling) and nonaerobic exercise (e.g., resistance exercise, strength exercise, weightlifting) was included, even if in combination with another antidepressant treatment. Although the results of this meta‐analysis suggested that physical exercise is effective in mitigating depressive symptoms and supports its use as a viable adjunctive treatment in combination with traditional antidepressant drugs, no significant trends in therapeutic efficacy were found in terms of type, duration, and intensity of exercise. 108 This may be due to the low number of studies that included anaerobic exercises, which renders reliable conclusions difficult.
The inhibitory effects of physical exercise on inflammation and microglial activation have been extensively reviewed. 109 Therefore, a regulatory role for (neuro)inflammation in physical exercise‐induced changes in depression has gained recent attention. 110 For instance, a study conducted in healthy university students (18–30 years old) revealed that resistance exercise (stationary cycle ergometer; 18 sessions of 27.5 minutes of continuous steady‐state exercise at 40% of maximum wattage) reduced symptoms of depression and plasma levels of TNF‐α. 106 Studies in rats and mice have linked diverse neuroimmunological mechanisms to different types of physical exercise. For example, voluntary running wheel exercise for 3 weeks induced an up‐regulation of hippocampal levels of the chemokine CXCL1 (C‐X‐C motif chemokine ligand 1) in aged Tg2576 mice. 111 A reduction of the expression of toll‐like receptors and pro‐inflammatory cytokines in the hippocampus and brain cortex of rats fed with a high‐fat diet and subjected to treadmill resistance exercise for five consecutive days has also been reported. 112 Forced treadmill exercise also counteracted cognitive decline in APP/PS1 mice, possibly by shifting hippocampal microglia from M1 (classical activation, pro‐inflammatory state) to M2 (anti‐inflammatory state) phenotype. 113 Moreover, preclinical investigations revealed that endurance exercise ameliorated depression‐like behavior through inflammatory mechanisms. In ovariectomized mice, forced treadmill exercise for 1 week reduced depression‐like behaviors (as observed in the sucrose preference test and the forced swim test) by suppressing the NLP3 inflammasome and reducing IL‐1β and IL‐18 levels in the hippocampus. 114 A 10‐month treadmill exercise regime (three times a week) in TgF344‐AD rats significantly decreased depressive‐like behavior in the forced swim test and sucrose preference test, reduced hippocampal microgliosis, and suppressed the expression of NF‐κB, TNF‐α, and IL‐1β in both brain cortex and hippocampus. 115 It is worth noting that all the studies conducting exercise regimes described here assessed the concentration of cytokines in a later timepoint (not immediately after the exercise), suggesting that that the acute effects of exercise in the immune response are not evaluated.
While the current data point toward the immune system as an important component linked with the benefits of exercise in depression, what remains unclear is how the interaction between exercise and inflammation occurs to bring about an improvement in depressive symptomology. An important role for the gut microbiome in mediating the mental health benefits of exercise in patients with depression has been proposed. 116 For example, a study supporting the involvement of the gut microbiota in exercise‐mediated antidepressant actions in adolescents showed that participants with subthreshold symptoms had significantly lower β‐diversity than clinically well adolescents in both the exercise intervention and psychoeducation‐controlled arms of the study. 117 The antidepressant actions of exercise have been also associated with the gut microbiome in mice and rats. Using amyloid‐β1‐40 (Aβ1‐40) to induce an Alzheimer’s disease phenotype in mice, it was demonstrated that treadmill exercise for a 4‐week period prevented both depressive‐like behavior and Aβ1‐40‐induced gut microbiota alterations. 118 A study demonstrated that aerobic training with treadmill exercise produced a trend toward an anxiolytic effect in rats subjected to intermittent fasting. 119 These results were accompanied by significant reductions in Bifidobacterium and Lactobacillus counts compared with sedentary animals that underwent intermittent fasting. However, histological sections of hippocampus showed no differences in the number of mononuclear type inflammatory cells (macrophages and lymphocytes). IL‐1β levels in the hippocampus were also unchanged, indicating unclear implications of the immune system in the antidepressant potential of endurance exercise. 119 Future investigation is warranted to demonstrate the involvement of inflammatory mediators in a potential exercise‐microbiome pathway to alleviate depression.
Diet and depression
It is widely accepted that diet quality and dietary patterns are important factors associated with the development of depression. 120 For the same reason, dietary interventions are considered an appealing strategy to treat and support antidepressant therapies. Indeed, it has motivated the creation of a new scientific field called nutritional psychiatry. 121 A Mediterranean diet for instance—consisting of low‐fat and high‐fiber elements—has been demonstrated to possess crucial mental health benefits, such as reduction of depressive behavior and improvement of cognitive performance. 122 A randomized controlled trial demonstrated that a Mediterranean diet supplemented with fish reduced negative mood symptoms and improved mental health quality‐of‐life scores in people with depression, which were sustained during the 6 months of intervention. 123 Similarly, a systematic meta‐analysis assessing the efficacy of adjunctive administration of dietary supplements to antidepressants revealed positive effects of polyunsaturated fatty acids and zinc. 124
Besides the benefits of a Mediterranean diet on depressive behavior, this class of diet has been associated with a positive impact on the human microbiota and reduced systemic inflammation. 125 Patients with metabolic disease, which is usually comorbid with depression, 126 under an intervention with a Mediterranean diet showed significant metabolic improvements plus higher abundance of Lachnoclostridium, Oxalobacter, and genera from Christensenellaceae. 127 Adherence to a Mediterranean diet in elderly participants was associated with an increased abundance of specific bacterial taxa, such as F. prausnitzii, along with Roseburia (R. hominis), Eubacterium (E. rectale, E. eligens, and E. xylanophilum), Bacteroides thetaiotaomicron, Prevotella copri and Anaerostipes hadrus. Most of these taxa have previously been reported to possess positive health associations, including production of short‐chain fatty acids and anti‐inflammatory properties. 128 Moreover, these bacteria negatively correlated with CRP and IL‐17, supporting the role of a Mediterranean diet to promote healthier ageing through microbiome‐immune interactions. 128
Interventions rich in naturally derived biomolecules have attracted a lot of attention over many years to alleviate mood and improve mental health. 129 One of the most studied families of biomolecules for human health are polyphenols, which are phytochemicals found abundantly in plant food sources and characterized by the presence of multiple hydroxyl structural units on aromatic rings. 130 Indeed, a meta‐analysis including 18 studies (n = 1,523) found that polyphenol supplementation has an overall positive impact on improving depression, anxiety, and quality‐of‐life parameters in patients with depression. 131 However, as shown in this meta‐analysis, different types of polyphenols may have varying beneficial effects (improved fatigue and insomnia, or improvement of somatic symptoms), which suggests that different populations with depression may benefit from different polyphenols. 131 Polyphenols are also important candidates for adjunctive therapy to antidepressants as demonstrated in animal and clinical trials. For example, an adjunctive treatment with the polyphenolic compound curcumin for 12 weeks displayed significant improvements in depression rating scores in patients with MDD when combined with different antidepressants (i.e., fluoxetine, sertraline, mianserin, or trazodone). 132 Besides antidepressant drugs, adjunctive quercetin therapy with levetiracetam was demonstrated to be effective for epileptic symptoms and comorbid depression in a mouse model of epilepsy. 133 Along with the behavioral improvements, adjunctive quercetin also restored corticosterone serum levels and tryptophan turnover in both hippocampus and brain cortex. 133
Some polyphenols, like resveratrol, have been linked with the inhibition of certain inflammatory pathways in neurodegenerative diseases with risk of comorbid depression, such as Alzheimer's disease and Parkinson's disease. 134 Resveratrol has been found to inhibit the TLR4/NF‐κB and/or NLRP3 (NOD‐like receptor protein 3) and STAT (signal transducer and activator of transcription) cascade signaling pathways, 135 and to exert anti‐inflammatory effects by suppressing M1 microglial activation. 136 In a mouse model of depression induced by LPS, curcumin improved depressive‐like behaviors. Moreover, treatment with curcumin attenuated LPS‐induced microglial activation and overproduction of IL‐1β and TNF‐α, as well as the levels of iNOS and COX‐2 mRNA levels in the hippocampus and prefrontal cortex. 137 Resveratrol induced comparable results in the same animal model, as revealed by an improvement in depressive‐like behaviors and a reduction in the gene expression of IL‐1β and TNF‐α, and an amelioration of the activation of NF‐κB in the brain. 138
Diets rich in polyphenols are known to have the capacity to modulate the composition of the gut microbial community by inhibiting or stimulating the growth of certain bacteria. 139 , 140 , 141 In this regard, animal models of depression have been crucial to highlight the therapeutic potential of polyphenols through microbiota‐gut‐brain axis mechanisms. For example, rats subjected to ovariectomy (model of depression) display evident changes in gut microbiota composition and diversity, including reduced microbial richness and Bacteroides/Firmicutes ratio compared with sham animals. 142 Treatment with curcumin reversed the negative impact of ovariectomy by restoring the Bacteroides/Firmicutes ratio and increasing α‐diversity, along with increases in Pseudomonas, Shewanella, and Serratia, and reduced Helicobacter. 142 In another preclinical investigation, different polyphenols (quercetin, xanthohumol, and phlorotannins) selectively attenuated a depressive‐like phenotype induced by maternal separation. 143 Moreover, xanthohumol induced a significant increase of bacteria associated with gut–brain communication pathways as shown in a functional prediction analysis of gut–brain modules, suggesting a potential role for the microbiome in polyphenol‐mediated improvements for depressive phenotype. 143 Future clinical trials should assess changes in microbiota composition and diversity, as well as pro‐inflammatory markers in patients with depression treated with polyphenols to further determine the involvement of gut microbiota‐mediated inflammatory pathways as a mechanism underpinning the antidepressant action of polyphenols.
FUTURE PERSPECTIVES
The present review collates evidence and novel insights about the crosstalk between antidepressant therapies and the gut microbiome. Indeed, the microbiota‐gut‐brain axis appears to be an important mediator of the inflammatory mechanisms underpinning the pathophysiology of depressive disorders. We have reviewed adjunctive therapies for depression, such as physical activity and dietary interventions that have been demonstrated to modulate the immune system via regulation of gut–brain communication pathways. Thus, the gut microbiome is emerging as a critical target for the treatment of immune‐related depression and should be considered for the design of novel therapeutic strategies for this psychiatric disorder. In this section, we elaborate on microbiome‐directed treatments that shape gut bacterial composition and modulate inflammatory mechanisms, including probiotics, prebiotics, and FMT.
Probiotics
Probiotics are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. 144 Early research in the field has found important correlations between mood improvements and consumption of probiotics, and could be suggested as a potential adjuvant therapy for MDD. 145 The therapeutic capacity of probiotics in patients suffering from depression has been previously demonstrated in different animal and clinical trials, 146 where most of the studies focused in lactic acid bacteria, including Lactobacillus and Bifidobacterium. For example, a human trial conducted during pregnancy and 6 months after delivery evaluated the effects of administration of Lactobacillus rhamnosus HN001on postpartum symptoms of depression. The probiotic significantly induced lower depression and anxiety scores than those in the placebo group. 147 Nonetheless, the mechanisms by which probiotics exert their antidepressant actions have been an important point of discussion for over the last decade. 148 , 149 The microbiota‐gut‐brain axis has become crucial in connecting the changes produced in intestinal microbiota with consequences in emotion and behavior. 150
Probiotic interventions have been demonstrated to have anti‐inflammatory actions in animal and clinical investigations. Indeed, low‐grade inflammation caused by a high‐fat diet in mice was ameliorated after the consumption of a probiotic cocktail containing five strains of Lactobacillus and five strains of Enterococcus by reducing the expression of IL‐6, TNF‐α, and IL‐1β, along with evident alterations in microbiota composition and intestinal permeability. 151 A clinical trial evaluating the impact of a probiotic supplementation with Lactobacillus and Bifidobacterium spp. in women with gestational diabetes showed a significant reduction in the serum levels of CRP and TNF‐α in the probiotic group compared with placebo. 152 Interestingly, in conditions where there is a correlation between an elevated inflammatory status and depressive symptoms, probiotics have been shown to be effective in providing therapeutic improvements. For example, irritable bowel syndrome (IBS) is a disorder of gut–brain axis interactions characterized by abdominal pain, bloating, and either constipation or diarrhea, 153 and which has been linked to immune activation and low‐grade mucosal inflammation. 154 IBS has been associated with a high prevalence of psychological disorders, including anxiety and depression, 155 and neuroinflammation. The microbiota‐gut‐brain axis have been highlighted as critical components involved in its pathophysiology. 156 People suffering from IBS who are treated with probiotics not only display significant improvements in abdominal pain and severity scores, but also demonstrated positive mood changes, as shown in a meta‐analysis evaluating the effects of beneficial strains in 1,793 IBS patients. 157 Indeed, a pilot study evaluating the effects of Bifidobacterium longum in patients with IBS and mild to moderate depression showed that the probiotic treatment significantly reduced depression scores on the hospital anxiety and depression scale. 158
Although it is not clear whether the therapeutic benefits of specific probiotics in other types of depression are mediated through (anti)inflammatory mechanisms, recent evidence supports a potential immunoregulatory function of probiotics in antidepressant activity. A randomized double‐blind controlled trial evaluated the effect of probiotic treatment in individuals with depression for 4 weeks, 159 resulting in a significant improvement of psychiatric symptoms along with elevated inflammation‐regulatory pathways as observed with a Kyoto encyclopedia of genes and genomes (KEGG) metabolic analysis. Future research may need to confirm these insights experimentally, but the hypothesis that probiotics exert antidepressant actions through anti‐inflammatory mechanisms has gained attention. 160 Probiotics represent a broad group of live biotherapeutic products, and it is important to note that these benefits are strain specific. Questions about dose response, the rationale around strain selection, and the potential use of next generation probiotics outside Bifidobacteria and Lactobacillus must be addressed in order to advance the field.
Prebiotics
The term prebiotic and its definition has evolved over the last 20 years, being recognized first for its ability to manipulate the gut microbiota to confer beneficial effects to the host. 161 Today, the definition of a prebiotic has been expanded to “a substrate that is selectively utilized by host microorganisms conferring a health benefit.” 162 Most of the research that has reported significant changes in gut microbiota after prebiotic interventions used fiber‐derived oligosaccharides, particularly fructo‐oligosaccharide (FOS) and galacto‐oligosaccharide (GOS), where Bifidobacterium spp. were significantly increased. 163 Prebiotics have shown potential to ameliorate a depressive phenotype through the microbiota‐gut‐brain axis, as demonstrated in preclinical work where C57BL/6J mice treated with a FOS+GOS combination exhibited antidepressant‐like effects. 164 These behavioral effects were accompanied by an attenuated corticosterone response, changes in hippocampal expression of factors associated with depressive phenotype (i.e., brain derived neurotrophic factor (Bdnf), corticotropin releasing hormone receptor 1 (Crhr1), gamma aminobutyric acid (GABA) receptors, and NMDA receptors), increased cecal levels of acetate and propionate, and modulation of gut microbiota composition, suggesting a role for the microbiota‐gut‐brain axis. 164
In contrast, there is not much known about the antidepressant effects of prebiotics in humans. A clinical trial investigated the consequences of prebiotic consumption (FOS/GOS) on the stress response of healthy volunteers for 3 weeks and found that the salivary cortisol awakening response was significantly lower after GOS intake compared with the placebo group. Participants also showed decreased attentional vigilance to negative versus positive information, as observed in a dot‐probe task in the GOS group, while no changes were detected in the FOS group. 165 These data suggest important therapeutic implications of GOS for stress‐related neuropsychiatric disorders, including anxiety and depression. Future clinical trials should assess prebiotics as monotherapy or adjunctive therapy for depression to determine their clinical impact and potential for contribution to improve mental health.
Fecal microbiota transplantation
In clinical practice, FMT is a method for the transfer of stool from a healthy donor into the patient's gastrointestinal tract to directly change and normalize the gut microbiota composition of the recipient, for therapeutic benefit. 166 FMT procedures have been shown to be effective in treating diseases with evident disturbances in gut microbiota parameters, including ulcerative colitis, 167 IBS, 168 and in recurrent and refractory Clostridium difficile infection. 169 Given the possible role of the microbiota‐gut‐brain axis in the pathogenesis of depression, the potential application of FMT as a therapeutic option to treat this neuropsychiatric disorder has become an important focus of discussion in recent years. 170 Although there is preclinical evidence that has shown that a depressive phenotype can be transferred via FMT from patients with depression to control rats, 36 or from chronic unpredictable mild stress mice donors (model of depression) to control mice, 171 the therapeutic potential of FMT to alleviate depressive‐like behavior in animal models (i.e., FMT from healthy controls to animals with depressive‐like symptoms) has not been examined.
In clinical trials favoring FMT treatment for IBS, which is highly comorbid with common mental disorders like depression, 155 there are inconclusive results supporting the antidepressant potential of FMT in patients suffering from IBS. For example, controlled clinical trials using treatment with FMT have been effective in improving IBS and mental health subscores. 172 In contrast, other studies that performed FMT in IBS patients evaluating psychiatric outcomes did not find significant changes between FMT recipients and placebo recipients in both IBS symptom scores and psychiatric measures as determined with the hospital anxiety and depression scale. 173 Thus, the antidepressant actions of FMT need to be further investigated in large clinical trials focused on patients suffering from depression.
In previous sections we have hypothesized that the microbiota‐gut‐brain axis may be a mediator for the adjunctive therapies for depression through inflammatory mechanisms. In fact, there is evidence suggesting that FMT procedures may have the potential to reduce systemic inflammation as demonstrated in clinical and animal research. For instance, the effects of FMT on dextran sodium sulphate–induced ulcerative colitis were investigated in C57BL/6J and BALB/c mice. 174 Although no changes were found in C57BL/6J mice, FMT from healthy donor mice (BALB/c) induced improvements in colon inflammation that were paralleled by restoration of gut microbial composition and decreased mRNA levels of IL‐1, IFN‐γ, and IL‐10 in colon tissue. 174 A short‐term surveillance of pro‐inflammatory markers after FMT in patients suffering from ulcerative colitis showed a significant reduction of serum levels of CRP after 3 months of procedure. 175 A similar clinical investigation confirmed that FMT from healthy individuals induced a significant decrease of the cytokines IL‐6 and IL‐1Ra, and inflammatory chemokines IP‐10 and ENA‐78 in the serum of patients with ulcerative colitis, suggesting a potential modulation of the host immune system by FMT interventions. 176
In conclusion, this work covers important points linking the pathology of depression with the immune system and the microbiota‐gut‐brain axis, and makes an appraisal of the current strategies to combat this psychiatric disease. Lifestyle factors such as exercise and diet have demonstrated therapeutic potential as adjuncts to antidepressants likely due to their capacity to modulate inflammatory pathways and gut microbial composition. Targeting the gut microbiota may be instrumental in the design of personalized treatments for immune‐related depression. Future research should focus on the adjunctive potential of microbiota‐directed approaches such as probiotics, prebiotics, and FMT to induce specific microbial changes required to improve antidepressant efficacy.
Funding
Y.M.N. is a recipient of a Science Foundation Ireland Investigator Award (SFI/FFP/6820), and a Reta Lila Weston Trust Award under Brain Health and Microbiome program, which funds F.D. Open access funding provided by IReL.
Conflict of Interest
G.C. has received honoraria from Janssen, Probi, and Apsen as an invited speaker; is in receipt of research funding from Pharmavite and Fonterra; and is a paid consultant for Yakult, Zentiva, and Heel pharmaceuticals. J.F.C. has been an invited speaker at conferences organized by Mead Johnson, Alkermes, Janssen, Ordesa, and Yakult, and has received research funding from Mead Johnson, Cremo Nutricia, Pharmavite, Dupont, and 4D Pharma. This support neither influenced nor constrained the contents of this review. All other authors declared no competing interests for this work.
Supporting information
Table S1
Contributor Information
Yvonne M. Nolan, Email: y.nolan@ucc.ie.
Gerard Clarke, Email: g.clarke@ucc.ie.
- 1. Doran, C.M. & Kinchin, I. A review of the economic impact of mental illness. Aust. Health Rev. 43, 43–48 (2019). [DOI] [PubMed] [Google Scholar]
- 2. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hillhouse, T.M. & Porter, J.H. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp. Clin. Psychopharmacol. 23, 1–21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khoo, A.L. et al. Network meta‐analysis and cost‐effectiveness analysis of new generation antidepressants. CNS Drugs 29, 695–712 (2015). [DOI] [PubMed] [Google Scholar]
- 5. Cipriani, A. et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta‐analysis. Lancet 391, 1357–1366 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beurel, E. , Toups, M. & Nemeroff, C.B. The bidirectional relationship of depression and inflammation: double trouble. Neuron 107, 234–256 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cruz‐Pereira, J.S. , Rea, K. , Nolan, Y.M. , O'Leary, O.F. , Dinan, T.G. & Cryan, J.F. et al. Depression’s unholy trinity: dysregulated stress, immunity, and the microbiome. Annu. Rev. Psychol. 71, 49–78 (2020). [DOI] [PubMed] [Google Scholar]
- 8. Bastiaanssen, T.F.S. , Cussotto, S. , Claesson, M.J. , Clarke, G. , Dinan, T.G. & Cryan, J.F. et al. Gutted! Unraveling the role of the microbiome in major depressive disorder. Harv. Rev. Psychiatry 28, 26–39 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saeed, S.A. , Cunningham, K. & Bloch, R.M. Depression and anxiety disorders: benefits of exercise, yoga, and meditation. Am. Fam. Physician 99, 620–627 (2019). [PubMed] [Google Scholar]
- 10. Haß, U. , Herpich, C. & Norman, K. Anti‐inflammatory diets and fatigue. Nutrients 11, 2315 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gubert, C. , Kong, G. , Renoir, T. & Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: implications for neurodegenerative diseases. Neurobiol. Dis. 134, 104621 (2020). [DOI] [PubMed] [Google Scholar]
- 12. Leff‐Gelman, P. et al. The immune system and the role of inflammation in perinatal depression. Neurosci. Bull. 32, 398–420 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller, A.H. & Raison, C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim, Y.‐K. , Na, K.‐S. , Myint, A.‐M. & Leonard, B.E. The role of pro‐inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 277–284 (2016). [DOI] [PubMed] [Google Scholar]
- 15. Miller, A.H. Depression and immunity: a role for T cells? Brain Behav. Immun. 24, 1–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Troubat, R. et al. Neuroinflammation and depression: a review. Eur. J. Neurosci. 53, 151–171 (2021). [DOI] [PubMed] [Google Scholar]
- 17. Kappelmann, N. , Lewis, G. , Dantzer, R. , Jones, P.B. & Khandaker, G.M. Antidepressant activity of anti‐cytokine treatment: a systematic review and meta‐analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 23, 335–343 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nettis, M.A. et al. Augmentation therapy with minocycline in treatment‐resistant depression patients with low‐grade peripheral inflammation: results from a double‐blind randomised clinical trial. Neuropsychopharmacology 46, 939–948 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kopschina Feltes, P. et al. Anti‐inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non‐responders to standard antidepressant therapy. J. Psychopharmacol. 31, 1149–1165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith, R.S. The macrophage theory of depression. Med. Hypotheses 35, 298–306 (1991). [DOI] [PubMed] [Google Scholar]
- 21. Köhler‐Forsberg, O. , Lydholm, C.N. , Hjorthøj, C. , Nordentoft, M. , Mors, O. & Benros, M.E. Efficacy of anti‐inflammatory treatment on major depressive disorder or depressive symptoms: meta‐analysis of clinical trials. Acta Psychiatr. Scand. 139, 404–419 (2019). [DOI] [PubMed] [Google Scholar]
- 22. Bai, S. et al. Efficacy and safety of anti‐inflammatory agents for the treatment of major depressive disorder: a systematic review and meta‐analysis of randomised controlled trials. J. Neurol. Neurosurg. Psychiatry 91, 21–32 (2020). [DOI] [PubMed] [Google Scholar]
- 23. Nutma, E. , Willison, H. , Martino, G. & Amor, S. Neuroimmunology – the past, present and future. Clin. Exp. Immunol. 197, 278–293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prinz, M. , Jung, S. & Priller, J. Microglia biology: one century of evolving concepts. Cell 179, 292–311 (2019). [DOI] [PubMed] [Google Scholar]
- 25. Subhramanyam, C.S. , Wang, C. , Hu, Q. & Dheen, S.T. Microglia‐mediated neuroinflammation in neurodegenerative diseases. Semin. Cell. Dev. Biol. 94, 112–120 (2019). [DOI] [PubMed] [Google Scholar]
- 26. Deng, S.‐L. , Chen, J.‐G. & Wang, F. Microglia: a central player in depression. Curr. Med. Sci. 40, 391–400 (2020). [DOI] [PubMed] [Google Scholar]
- 27. Berg, G. et al. Microbiome definition re‐visited: old concepts and new challenges. Microbiome 8, 103 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cryan, J.F. et al. The microbiota‐gut‐brain axis. Physiol. Rev. 99, 1877–2013 (2019). [DOI] [PubMed] [Google Scholar]
- 29. Valles‐Colomer, M. et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632 (2019). [DOI] [PubMed] [Google Scholar]
- 30. Yang, J. et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 6, eaba8555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rutherford, S.T. & Bassler, B.L. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2, a012427 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cruz‐Pereira, J.S. & Cryan, J.F. In need of a quorum: from microbes to mood via the immune system. Am. J. Psychiatry 177, 895–897 (2020). [DOI] [PubMed] [Google Scholar]
- 33. Medina‐Rodriguez, E.M. et al. Identification of a signaling mechanism by which the microbiome regulates Th17 cell‐mediated depressive‐like behaviors in mice. Am. J. Psychiatry 177, 974–990 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nikolova, V.L. , Hall, M.R.B. , Hall, L.J. , Cleare, A.J. , Stone, J.M. & Young, A.H. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta‐analysis. JAMA Psychiatry 78, 1343–1354 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dalile, B. , Van Oudenhove, L. , Vervliet, B. & Verbeke, K. The role of short‐chain fatty acids in microbiota‐gut‐brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019). [DOI] [PubMed] [Google Scholar]
- 36. Kelly, J.R. et al. Transferring the blues: depression‐associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118 (2016). [DOI] [PubMed] [Google Scholar]
- 37. Rutsch, A. , Kantsjö, J.B. & Ronchi, F. The gut‐brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 11, 604179 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Agirman, G. , Yu, K.B. & Hsiao, E.Y. Signaling inflammation across the gut‐brain axis. Science 374, 1087–1092 (2021). [DOI] [PubMed] [Google Scholar]
- 39. Lynch, C.M.K. , Clarke, G. & Cryan, J.F. Powering up microbiome‐microglia interactions. Cell Metab. 33, 2097–2099 (2021). [DOI] [PubMed] [Google Scholar]
- 40. Mossad, O. & Erny, D. The microbiota‐microglia axis in central nervous system disorders. Brain Pathol. 30, 1159–1177 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thion, M.S. et al. Microbiome influences prenatal and adult microglia in a sex‐specific manner. Cell 172, 500–516.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tian, T. et al. Clostridium butyricum miyairi 588 has preventive effects on chronic social defeat stress‐induced depressive‐like behaviour and modulates microglial activation in mice. Biochem. Biophys. Res. Commun. 516, 430–436 (2019). [DOI] [PubMed] [Google Scholar]
- 44. Li, H. et al. Rifaximin‐mediated gut microbiota regulation modulates the function of microglia and protects against CUMS‐induced depression‐like behaviors in adolescent rat. J. Neuroinflammation 18, 254 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schmidtner, A.K. et al. Minocycline alters behavior, microglia and the gut microbiome in a trait‐anxiety‐dependent manner. Transl. Psychiatry 9, 223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Erny, D. et al. Microbiota‐derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 33, 2260–2276.e7 (2021). [DOI] [PubMed] [Google Scholar]
- 47. Yirmiya, R. , Rimmerman, N. & Reshef, R. Depression as a microglial disease. Trends Neurosci. 38, 637–658 (2015). [DOI] [PubMed] [Google Scholar]
- 48. Pochwat, B. , Nowak, G. & Szewczyk, B. An update on NMDA antagonists in depression. Expert Rev. Neurother. 19, 1055–1067 (2019). [DOI] [PubMed] [Google Scholar]
- 49. Beyer, J.L. The use of antidepressants in bipolar depression. Handb. Exp. Pharmacol. 250, 415–442 (2019). [DOI] [PubMed] [Google Scholar]
- 50. Matveychuk, D. et al. Ketamine as an antidepressant: overview of its mechanisms of action and potential predictive biomarkers. Ther. Adv. Psychopharmacol. 10 (2020). 10.1177/2045125320916657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Corriger, A. & Pickering, G. Ketamine and depression: a narrative review. Drug Des. Devel. Ther. 13, 3051–3067 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deuschle, M. Effects of antidepressants on glucose metabolism and diabetes mellitus type 2 in adults. Curr. Opin. Psychiatry 26, 60–65 (2013). [DOI] [PubMed] [Google Scholar]
- 53. Razavi Ratki, S.K. et al. Can antidepressant drug impact on blood pressure level in patients with psychiatric disorder and hypertension? A randomized trial. Int. J. Prev. Med. 7, 26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Szałach, Ł.P. , Lisowska, K.A. & Cubała, W.J. The influence of antidepressants on the immune system. Arch. Immunol. Ther. Exp. 67, 143–151 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. West, C.L. et al. Identification of SSRI‐evoked antidepressant sensory signals by decoding vagus nerve activity. Sci. Rep. 11, 21130 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cussotto, S. , Clarke, G. , Dinan, T.G. & Cryan, J.F. Psychotropics and the microbiome: a chamber of secrets…. Psychopharmacology 236, 1411–1432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ait Chait, Y. , Mottawea, W. , Tompkins, T.A. & Hammami, R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 10, 17878 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lukić, I. et al. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive‐like behavior. Transl. Psychiatry 9, 133 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lyte, M. , Daniels, K.M. & Schmitz‐Esser, S. Fluoxetine‐induced alteration of murine gut microbial community structure: evidence for a microbial endocrinology‐based mechanism of action responsible for fluoxetine‐induced side effects. PeerJ 7, e6199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cussotto, S. et al. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology 236, 1671–1685 (2019). [DOI] [PubMed] [Google Scholar]
- 61. Mulder, R. et al. Treating depression with adjunctive antipsychotics. Bipolar Disord. 20, 17–24 (2018). [DOI] [PubMed] [Google Scholar]
- 62. Maier, L. et al. Extensive impact of non‐antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cussotto, S. et al. The gut microbiome influences the bioavailability of olanzapine in rats. EBioMedicine 66, 103307 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hannestad, J. , DellaGioia, N. & Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta‐analysis. Neuropsychopharmacology 36, 2452–2459 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hashioka, S. Antidepressants and neuroinflammation: can antidepressants calm glial rage down? Mini Rev. Med. Chem. 11, 555–564 (2011). [DOI] [PubMed] [Google Scholar]
- 66. O’Sullivan, J.B. , Ryan, K.M. , Curtin, N.M. , Harkin, A. & Connor, T.J. Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int. J. Neuropsychopharmacol. 12, 687–699 (2009). [DOI] [PubMed] [Google Scholar]
- 67. Liu, Y. , Feng, S. , Subedi, K. & Wang, H. Attenuation of ischemic stroke‐caused brain injury by a monoamine oxidase inhibitor involves improved proteostasis and reduced neuroinflammation. Mol. Neurobiol. 57, 937–948 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jia, X. , Gao, Z. & Hu, H. Microglia in depression: current perspectives. Sci. China Life Sci. 64, 911–925 (2021). [DOI] [PubMed] [Google Scholar]
- 69. Jin, Y. et al. Fluoxetine attenuates kainic acid‐induced neuronal cell death in the mouse hippocampus. Brain Res. 1281, 108–116 (2009). [DOI] [PubMed] [Google Scholar]
- 70. Iwata, M. , Ishida, H. , Kaneko, K. & Shirayama, Y. Learned helplessness activates hippocampal microglia in rats: a potential target for the antidepressant imipramine. Pharmacol. Biochem. Behav. 150–151, 138–146 (2016). [DOI] [PubMed] [Google Scholar]
- 71. Tan, S. , Wang, Y. , Chen, K. , Long, Z. & Zou, J. Ketamine alleviates depressive‐like behaviors via down‐regulating inflammatory cytokines induced by chronic restraint stress in mice. Biol. Pharm. Bull. 40, 1260–1267 (2017). [DOI] [PubMed] [Google Scholar]
- 72. Liu, F.‐Y. et al. Fluoxetine attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage: a possible role for the regulation of TLR4/MyD88/NF‐κB signaling pathway. J. Neuroinflammation 15, 347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hoehn, R. et al. Melatonin acts as an antidepressant by inhibition of the acid sphingomyelinase/ceramide system. Neurosignals 24, 48–58 (2016). [DOI] [PubMed] [Google Scholar]
- 74. Ali, T. et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J. Pineal. Res. 69, e12667 (2020). [DOI] [PubMed] [Google Scholar]
- 75. Arioz, B.I. et al. Melatonin attenuates LPS‐induced acute depressive‐like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front. Immunol. 10, 1511 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kohler, O. , Krogh, J. , Mors, O. & Benros, M.E. Inflammation in depression and the potential for anti‐inflammatory treatment. Curr. Neuropharmacol. 14, 732–742 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bacchi, S. , Palumbo, P. , Sponta, A. & Coppolino, M.F. Clinical pharmacology of non‐steroidal anti‐inflammatory drugs: a review. Antiinflamm. Antiallergy Agents Med. Chem. 11, 52–64 (2012). [DOI] [PubMed] [Google Scholar]
- 78. Fitzpatrick, F.A. Cyclooxygenase enzymes: regulation and function. Curr. Pharm. Des. 10, 577–588 (2004). [DOI] [PubMed] [Google Scholar]
- 79. Iyengar, R.L. et al. NSAIDs are associated with lower depression scores in patients with osteoarthritis. Am. J. Med. 126, 1017 e11–e18 (2013). [DOI] [PubMed] [Google Scholar]
- 80. Alamdarsaravi, M. et al. Efficacy and safety of celecoxib monotherapy for mild to moderate depression in patients with colorectal cancer: a randomized double‐blind, placebo controlled trial. Psychiatry Res. 255, 59–65 (2017). [DOI] [PubMed] [Google Scholar]
- 81. Mahagna, H. , Amital, D. & Amital, H. A randomised, double‐blinded study comparing giving etoricoxib vs. placebo to female patients with fibromyalgia. Int. J. Clin. Pract. 70, 163–170 (2016). [DOI] [PubMed] [Google Scholar]
- 82. Mesripour, A. , Shahnooshi, S. & Hajhashemi, V. Celecoxib, ibuprofen, and indomethacin alleviate depression‐like behavior induced by interferon‐alfa in mice. J. Complement Integr. Med. 17 (2019). [DOI] [PubMed] [Google Scholar]
- 83. Husain, M.I. et al. Minocycline and celecoxib as adjunctive treatments for bipolar depression: a multicentre, factorial design randomised controlled trial. Lancet Psychiatry 7, 515–527 (2020). [DOI] [PubMed] [Google Scholar]
- 84. Miller, A.H. & Pariante, C.M. Trial failures of anti‐inflammatory drugs in depression. Lancet Psychiatry 7, 837 (2020). [DOI] [PubMed] [Google Scholar]
- 85. Maseda, D. & Ricciotti, E. NSAID–gut microbiota interactions. Front. Pharmacol. 11, 1153 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yin, Z. et al. DNA replication is the target for the antibacterial effects of nonsteroidal anti‐inflammatory drugs. Chem. Biol. 21, 481–487 (2014). [DOI] [PubMed] [Google Scholar]
- 87. Terán‐Ventura, E. , Aguilera, M. , Vergara, P. & Martínez, V. Specific changes of gut commensal microbiota and TLRs during indomethacin‐induced acute intestinal inflammation in rats. J. Crohns Colitis 8, 1043–1054 (2014). [DOI] [PubMed] [Google Scholar]
- 88. Xiao, X. , Nakatsu, G. , Jin, Y. , Wong, S. , Yu, J. & Lau, J.Y.W. Gut microbiota mediates protection against enteropathy induced by indomethacin. Sci. Rep. 7, 40317 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cheung, S.G. , Goldenthal, A.R. , Uhlemann, A.C. , Mann, J.J. , Miller, J.M. & Sublette, M.E. Systematic review of gut microbiota and major depression. Front. Psychiatry 10, 34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rapoport, S.I. & Hibbeln, J.R. Therapeutic targeting of brain arachidonic acid cascade in bipolar disorder by low dose aspirin and celecoxib. Prostaglandins Leukot Essent Fatty Acids 159, 102118 (2020). [DOI] [PubMed] [Google Scholar]
- 91. Miller, A.H. , Maletic, V. & Raison, C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nielsen, M.A. , Lomholt, S. , Mellemkjaer, A. , Andersen, M.N. , Buckley, C.D. & Kragstrup, T.W. Responses to cytokine inhibitors associated with cellular composition in models of immune‐mediated inflammatory arthritis. ACR Open Rheumatol. 2, 3–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sun, Y.U. et al. The effects of interleukin‐6 neutralizing antibodies on symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric Castleman’s disease. Brain Behav. Immun. 66, 156–164 (2017). [DOI] [PubMed] [Google Scholar]
- 94. Ting, E.Y.‐C. , Yang, A.C. & Tsai, S.‐J. Role of interleukin‐6 in depressive disorder. Int. J. Mol. Sci. 21, 2194 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Griffiths, C.E.M. et al. Impact of ixekizumab treatment on depressive symptoms and systemic inflammation in patients with moderate‐to‐severe psoriasis: an integrated analysis of three phase 3 clinical studies. Psychother. Psychosom. 86, 260–267 (2017). [DOI] [PubMed] [Google Scholar]
- 96. Schirmer, M. et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 167, 1125–1136.e8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cohen, I. , Ruff, W.E. & Longbrake, E.E. Influence of immunomodulatory drugs on the gut microbiota. Transl. Res. 233, 144–161 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lis, K. , Kuzawińska, O. & Bałkowiec‐Iskra, E. Tumor necrosis factor inhibitors — state of knowledge. Arch. Med. Sci. 10, 1175–1185 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Picchianti‐Diamanti, A. et al. Analysis of gut microbiota in rheumatoid arthritis patients: disease‐related dysbiosis and modifications induced by etanercept. Int. J. Mol. Sci. 19, 2938 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ribaldone, D.G. et al. Adalimumab therapy improves intestinal dysbiosis in Crohn’s disease. J. Clin. Med. 8, 1646 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Silva, J.P.B. et al. Protective mechanisms of butyrate on inflammatory bowel disease. Curr. Pharm. Des. 24, 4154–4166 (2018). [DOI] [PubMed] [Google Scholar]
- 102. van de Wouw, M. et al. Short‐chain fatty acids: microbial metabolites that alleviate stress‐induced brain–gut axis alterations. J. Physiol. 596, 4923–4944 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ashcraft, K.A. , Warner, A.B. , Jones, L.W. & Dewhirst, M.W. Exercise as adjunct therapy in cancer. Semin. Radiat. Oncol. 29, 16–24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Malaguarnera, L. Vitamin D3 as potential treatment adjuncts for COVID‐19. Nutrients 12, 3512 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Murphy, R.M. , Watt, M.J. & Febbraio, M.A. Metabolic communication during exercise. Nat. Metab. 2, 805–816 (2020). [DOI] [PubMed] [Google Scholar]
- 106. Paolucci, E.M. , Loukov, D. , Bowdish, D.M.E. & Heisz, J.J. Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 133, 79–84 (2018). [DOI] [PubMed] [Google Scholar]
- 107. Schuch, F.B. & Stubbs, B. The role of exercise in preventing and treating depression. Curr. Sports Med. Rep. 18, 299–304 (2019). [DOI] [PubMed] [Google Scholar]
- 108. Kvam, S. , Kleppe, C.L. , Nordhus, I.H. & Hovland, A. Exercise as a treatment for depression: a meta‐analysis. J. Affect. Disord. 202, 67–86 (2016). [DOI] [PubMed] [Google Scholar]
- 109. Seo, D.‐Y. , Heo, J.‐W. , Ko, J.R. & Kwak, H.‐B. Exercise and neuroinflammation in health and disease. Int. Neurourol. J. 23, S82–92 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ignácio, Z.M. , da Silva, R.S. , Plissari, M.E. , Quevedo, J. & Réus, G.Z. Physical exercise and neuroinflammation in major depressive disorder. Mol. Neurobiol. 56, 8323–8335 (2019). [DOI] [PubMed] [Google Scholar]
- 111. Parachikova, A. , Nichol, K.E. & Cotman, C.W. Short‐term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol. Dis. 30, 121–129 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kang, E.‐B. et al. Neuroprotective effects of endurance exercise against high‐fat diet‐induced hippocampal neuroinflammation. J. Neuroendocrinol. 28, (2016). [DOI] [PubMed] [Google Scholar]
- 113. Zhang, X. et al. Treadmill exercise decreases Aβ deposition and counteracts cognitive decline in APP/PS1 mice, possibly via hippocampal microglia modifications. Front. Aging Neurosci. 11, 78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang, Y. , Xu, Y. , Sheng, H. , Ni, X. & Lu, J. Exercise amelioration of depression‐like behavior in OVX mice is associated with suppression of NLRP3 inflammasome activation in hippocampus. Behav. Brain Res. 307, 18–24 (2016). [DOI] [PubMed] [Google Scholar]
- 115. Wu, C. et al. Effects of exercise training on anxious–depressive‐like behavior in Alzheimer rat. Med. Sci. Sports Exerc. 52, 1456–1469 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yuan, T.‐F. , Ferreira Rocha, N.B. , Paes, F. , Arias‐Carrión, O. , Machado, S. & de Sá Filho, A.S. Neural mechanisms of exercise: effects on gut miccrobiota and depression. CNS Neurol. Disord. Drug Targets 14, 1312–1314 (2015). [DOI] [PubMed] [Google Scholar]
- 117. Wang, R. et al. Effects of aerobic exercise on gut microbiota in adolescents with subthreshold mood syndromes and healthy adolescents: a 12‐week, randomized controlled trial. J. Affect. Disord. 293, 363–372 (2021). [DOI] [PubMed] [Google Scholar]
- 118. Rosa, J.M. et al. Prophylactic effect of physical exercise on Aβ1‐40‐induced depressive‐like behavior and gut dysfunction in mice. Behav. Brain Res. 393, 112791 (2020). [DOI] [PubMed] [Google Scholar]
- 119. Soares, N.L. et al. Does intermittent fasting associated with aerobic training influence parameters related to the gut‐brain axis of Wistar rats? J. Affect. Disord. 293, 176–185 (2021). [DOI] [PubMed] [Google Scholar]
- 120. Berding, K. et al. Diet and the microbiota‐gut‐brain axis: sowing the seeds of good mental health. Adv. Nutr. 12, 1239–1285 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Adan, R.A.H. et al. Nutritional psychiatry: towards improving mental health by what you eat. Eur. Neuropsychopharmacol. 29, 1321–1332 (2019). [DOI] [PubMed] [Google Scholar]
- 122. Ventriglio, A. et al. Mediterranean diet and its benefits on health and mental health: a literature review. Clin. Pract. Epidemiol. Ment. Health 16, 156–164 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Parletta, N. et al. A Mediterranean‐style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: a randomized controlled trial (HELFIMED). Nutr. Neurosci. 22, 474–487 (2019). [DOI] [PubMed] [Google Scholar]
- 124. Schefft, C. , Kilarski, L.L. , Bschor, T. & Köhler, S. Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta‐analysis. Eur. Neuropsychopharmacol. 27, 1090–1109 (2017). [DOI] [PubMed] [Google Scholar]
- 125. Merra, G. et al. Influence of Mediterranean diet on human gut microbiota. Nutrients 13, 7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dunbar, J.A. et al. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes Care 31, 2368–2373 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Galié, S. et al. Effects of Mediterranean Diet on plasma metabolites and their relationship with insulin resistance and gut microbiota composition in a crossover randomized clinical trial. Clin. Nutr. 40, 3798–3806 (2021). [DOI] [PubMed] [Google Scholar]
- 128. Ghosh, T.S. et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU‐AGE 1‐year dietary intervention across five European countries. Gut 69, 1218–1228 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Küpeli Akkol, E. , Tatlı Çankaya, I.T. , Seker Karatoprak, G. , Carpar, E. , Sobarzo‐Sánchez, E. & Capasso, R. Natural compounds as medical strategies in the prevention and treatment of psychiatric disorders seen in neurological diseases. Front. Pharmacol. 12 (2021). 10.3389/fphar.2021.669638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pontifex, M.G. , Malik, M.M.A.H. , Connell, E. , Müller, M. & Vauzour, D. Citrus Polyphenols in brain health and disease: current perspectives. Front. Neurosci. 15 (2021). 10.3389/fnins.2021.640648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lin, K. , Li, Y. , Toit, E.D. , Wendt, L. & Sun, J. Effects of polyphenol supplementations on improving depression, anxiety, and quality of life in patients with depression. Front. Psychiatry 12 (2021). 10.3389/fpsyt.2021.765485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kanchanatawan, B. et al. Add‐on treatment with curcumin has antidepressive effects in Thai patients with major depression: results of a randomized double‐blind placebo‐controlled study. Neurotox. Res. 33, 621–633 (2018). [DOI] [PubMed] [Google Scholar]
- 133. Singh, T. , Kaur, T. & Goel, R.K. Adjuvant quercetin therapy for combined treatment of epilepsy and comorbid depression. Neurochem. Int. 104, 27–33 (2017). [DOI] [PubMed] [Google Scholar]
- 134. Huang, J. et al. Signaling mechanisms underlying inhibition of neuroinflammation by resveratrol in neurodegenerative diseases. J. Nutr. Biochem. 88, 108552 (2021). [DOI] [PubMed] [Google Scholar]
- 135. Feng, L. & Zhang, L. Resveratrol suppresses Aβ‐induced microglial activation through the TXNIP/TRX/NLRP3 signaling pathway. DNA Cell Biol. 38, 874–879 (2019). [DOI] [PubMed] [Google Scholar]
- 136. Porro, C. , Cianciulli, A. , Calvello, R. & Panaro, M.A. Reviewing the role of resveratrol as a natural modulator of microglial activities. Curr. Pharm. Des. 21, 5277–5291 (2015). [DOI] [PubMed] [Google Scholar]
- 137. Wang, Z. et al. The effects of curcumin on depressive‐like behavior in mice after lipopolysaccharide administration. Behav. Brain Res. 274, 282–290 (2014). [DOI] [PubMed] [Google Scholar]
- 138. Ge, L.I. et al. Resveratrol abrogates lipopolysaccharide‐induced depressive‐like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur. J. Pharmacol. 768, 49–57 (2015). [DOI] [PubMed] [Google Scholar]
- 139. Lavefve, L. , Howard, L.R. & Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 11, 45–65 (2020). [DOI] [PubMed] [Google Scholar]
- 140. Lee, H.C. , Jenner, A.M. , Low, C.S. & Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 157, 876–884 (2006). [DOI] [PubMed] [Google Scholar]
- 141. Sorrenti, V. , Ali, S. , Manchin, L. , Davinelli, S. , Paoli, A. & Scapagnini, G. Cocoa polyphenols and gut microbiota interplay: bioavailability, prebiotic effect, and impact on human health. Nutrients 12, 1908 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang, Z. , Chen, Y. , Xiang, L. , Wang, Z. , Xiao, G.G. & Hu, J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients 9, 1146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Donoso, F. et al. Polyphenols selectively reverse early‐life stress‐induced behavioural, neurochemical and microbiota changes in the rat. Psychoneuroendocrinology 116, 104673 (2020). [DOI] [PubMed] [Google Scholar]
- 144. Hill, C. et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014). [DOI] [PubMed] [Google Scholar]
- 145. Logan, A.C. & Katzman, M. Major depressive disorder: probiotics may be an adjuvant therapy. Med. Hypotheses 64, 533–538 (2005). [DOI] [PubMed] [Google Scholar]
- 146. Liu, R.T. , Walsh, R.F.L. & Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: a systematic review and meta‐analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 102, 13–23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Slykerman, R.F. et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double‐blind placebo‐controlled trial. EBioMedicine 24, 159–165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Slyepchenko, A. , Carvalho, A.F. , Cha, D.S. , Kasper, S. & McIntyre, R.S. Gut emotions – mechanisms of action of probiotics as novel therapeutic targets for depression and anxiety disorders. CNS Neurol. Disord. Drug Targets 13, 1770–1786 (2014). [DOI] [PubMed] [Google Scholar]
- 149. Kim, Y.‐K. & Shin, C. The microbiota‐gut‐brain axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Curr. Neuropharmacol. 16, 559–573 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kim, N. , Yun, M. , Oh, Y.J. & Choi, H.‐J. Mind‐altering with the gut: Modulation of the gut‐brain axis with probiotics. J. Microbiol. 56, 172–182 (2018). [DOI] [PubMed] [Google Scholar]
- 151. Ahmadi, S. et al. A human‐origin probiotic cocktail ameliorates aging‐related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 5, e132055 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hajifaraji, M. , Jahanjou, F. , Abbasalizadeh, F. , Aghamohammadzadeh, N. , Abbasi, M.M. & Dolatkhah, N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: a randomized clinical trial. Asia Pac J. Clin. Nutr. 27, 581–591 (2018). [DOI] [PubMed] [Google Scholar]
- 153. Ford, A.C. , Lacy, B.E. & Talley, N.J. Irritable bowel syndrome. N Engl. J. Med. 376, 2566–2578 (2017). [DOI] [PubMed] [Google Scholar]
- 154. Holtmann, G.J. , Ford, A.C. & Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 1, 133–146 (2016). [DOI] [PubMed] [Google Scholar]
- 155. Fond, G. et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta‐analysis. Eur. Arch. Psychiatry Clin. Neurosci. 264, 651–660 (2014). [DOI] [PubMed] [Google Scholar]
- 156. Simpson, C.A. , Mu, A. , Haslam, N. , Schwartz, O.S. & Simmons, J.G. Feeling down? A systematic review of the gut microbiota in anxiety/depression and irritable bowel syndrome. J. Affect. Disord. 266, 429–446 (2020). [DOI] [PubMed] [Google Scholar]
- 157. Didari, T. , Mozaffari, S. , Nikfar, S. & Abdollahi, M. Effectiveness of probiotics in irritable bowel syndrome: updated systematic review with meta‐analysis. World J. Gastroenterol. 21, 3072–3084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Pinto‐Sanchez, M.I. et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153, 448–459.e8 (2017). [DOI] [PubMed] [Google Scholar]
- 159. Reininghaus, E.Z. et al. PROVIT: supplementary probiotic treatment and vitamin B7 in depression‐a randomized controlled trial. Nutrients 12, 3422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Park, C. et al. Probiotics for the treatment of depressive symptoms: an anti‐inflammatory mechanism? Brain Behav. Immun. 73, 115–124 (2018). [DOI] [PubMed] [Google Scholar]
- 161. Gibson, G. et al. Dietary prebiotics: current status and new definition. Food Sci. Technol. Bull. Funct. Foods 7, 1–19 (2010). [Google Scholar]
- 162. Gibson, G.R. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502 (2017). [DOI] [PubMed] [Google Scholar]
- 163. Liu, F. et al. Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci. Rep. 7, 11789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Burokas, A. et al. Targeting the microbiota‐gut‐brain axis: prebiotics have anxiolytic and antidepressant‐like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 82, 472–487 (2017). [DOI] [PubMed] [Google Scholar]
- 165. Schmidt, K. , Cowen, P.J. , Harmer, C.J. , Tzortzis, G. , Errington, S. & Burnet, P.W.J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 232, 1793–1801 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang, J.‐W. et al. Fecal microbiota transplantation: review and update. J. Formos. Med. Assoc. 118, S23–S31 (2019). [DOI] [PubMed] [Google Scholar]
- 167. Blanchaert, C. , Strubbe, B. & Peeters, H. Fecal microbiota transplantation in ulcerative colitis. Acta Gastroenterol. Belg. 82, 519–528 (2019). [PubMed] [Google Scholar]
- 168. Ianiro, G. et al. Fecal microbiota transplantation in gastrointestinal and extraintestinal disorders. Future Microbiol. 15, 1173–1183 (2020). [DOI] [PubMed] [Google Scholar]
- 169. Bamba, S. et al. Successful treatment by fecal microbiota transplantation for Japanese patients with refractory Clostridium difficile infection: a prospective case series. J. Microbiol. Immunol. Infect. 52, 663–666 (2019). [DOI] [PubMed] [Google Scholar]
- 170. Gheorghe, C.E. , Ritz, N.L. , Martin, J.A. , Wardill, H.R. , Cryan, J.F. & Clarke, G. Investigating causality with fecal microbiota transplantation in rodents: applications, recommendations and pitfalls. Gut. Microbes 13, 1941711 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Li, N. et al. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety‐like and depression‐like behavior in recipient mice via the gut microbiota‐inflammation‐brain axis. Stress 22, 592–602 (2019). [DOI] [PubMed] [Google Scholar]
- 172. Guo, Q. et al. Dynamic changes of intestinal flora in patients with irritable bowel syndrome combined with anxiety and depression after oral administration of enterobacteria capsules. Bioengineered 12, 11885–11897 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Aroniadis, O.C. et al. Faecal microbiota transplantation for diarrhoea‐predominant irritable bowel syndrome: a double‐blind, randomised, placebo‐controlled trial. Lancet Gastroenterol. Hepatol. 4, 675–685 (2019). [DOI] [PubMed] [Google Scholar]
- 174. Tian, Z. et al. Beneficial effects of fecal microbiota transplantation on ulcerative colitis in mice. Dig. Dis. Sci. 61, 2262–2271 (2016). [DOI] [PubMed] [Google Scholar]
- 175. Zhang, T. et al. Short‐term surveillance of cytokines and C‐reactive protein cannot predict efficacy of fecal microbiota transplantation for ulcerative colitis. PLoS One 11, e0158227 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wang, Y. , Ren, R. , Sun, G. , Peng, L. , Tian, Y. & Yang, Y. Pilot study of cytokine changes evaluation after fecal microbiota transplantation in patients with ulcerative colitis. Int. Immunopharmacol. 85, 106661 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


