Abstract
Background
Effective anti‐aging treatments are an unmet consumer need.
Aim
Ex vivoand clinical tests have evaluated the efficacy of a topical facial serum containing a proprietary blend of neuropeptides, proteins, amino acids, and marine extracts on aged skin.
Methods
In the ex vivo study the facial serum was compared to a commercially marketed face serum and to an untreated control on skin explants using microrelief, smoothness, and epidermal thickness endpoints. The 12 weeks monadic clinical study was designed for the test product to be used on the whole face. Subjects functioned as their own control; evaluating change from baseline. Skin was evaluated clinically by a Dermatologist for tolerability and for efficacy. Also part of the product assessment was skin hydration measurements, imaging, and a subject questionnaire.
Results
The facial serum improved skin condition by significant reductions in skin surface area occupied by microfolds and in skin roughness. Additionally, it increased epidermal thickness as compared to the untreated control as well as the commercially marketed face serum. The facial serum provided a statistically increased skin moisturization compared to pretreatment values. Dermatological evaluation of the skin concluded that there were statistically and clinically significant improvements in skin smoothness, wrinkles severity, fine lines visibility and lifting, and tightening effects at crow's feet area, forehead, and upper lip.
Conclusion
A facial serum, containing a proprietary blend of neuropeptides, proteins, amino acids, and marine extracts, has been shown to improve the overall quality of aged skin in a series of ex vivo and clinical tests.
Keywords: anti‐aging, moisturization, peptide, skin smoothness, wrinkles
1. INTRODUCTION
Skin shows obvious and visible signs of aging as one becomes older. 1 Aged skin is invariably dry, wrinkled, and rough. 1 , 2 Many consumers use cosmetic products that are designed to prevent or reverse the effects of aging and improve the skin to make it more moisturized, smoother, and less wrinkled. Many of the most efficacious of these products contain biologically active ingredients, such as peptides, amino acids, and marine extracts. 3 , 4 , 5 , 6 These ingredients found in many cosmeceutical products may stimulate the production of collagen and elastin, which have the potential to result in firmer, plumper, and younger looking skin. Many of these ingredients have been investigated previously, most often as individual components, but occasionally in combination. Furthermore, they may aid in smoothing the skin and making lines and wrinkles less deep. Wu et al. investigated peptide mixtures and suggested one mixture might show promise when used in anti‐aging cosmetics. 7 Baseggio Conrado et al. examined the role of hypotaurine, an amino acid with a sulfur group, in the in vitro protection against UVA‐induced damage. They concluded that this sulfur amino acid could be an effective photoprotective ingredient in cosmetic and sunscreen formulations. 8 Hameury et al. investigated a gel composed of ingredients from marine and maritime origins. They concluded that these ingredients may prevent the visible signs of skin aging and may help keep the dermis and epidermis healthy. 9 However, there has been little information on the effect of these categories of ingredients on skin aging parameters when combined in a cosmetic formulation.
Thus a new facial serum has been developed that contains a proprietary blend of neuropeptides, proteins, amino acids, and marine extracts. This research examines the impact of this facial serum on the microrelief, smoothness, and epidermal thickness of skin explants when compared to a competitor control product and to an untreated control. In addition, the facial serum was used in a 12‐week clinical study in females aged 35–55 years. Their skin was evaluated clinically by a Dermatologist for tolerability to the facial serum and for any changes to the clinical signs of skin aging. The subjects' skin was also evaluated for changes in skin hydration using a standard biophysical technique. Finally, the subjects evaluated their own perception of changes to their facial skin.
2. MATERIALS AND METHODS
2.1. Test products
The test product was a commercially available face serum (ExLinea® Pro Peptide Serum, PCA Skin®, USA) containing a proprietary blend of neuropeptides, proteins, amino acids, and marine extracts (Study Serum). A commercially marketed face serum was included in the ex vivo study for comparative purposes (Competitor Serum).
2.2. Ex vivomethod
Skin explants were taken from a 43‐year old Caucasian female with a type II phototype who was undergoing an abdominoplasty. Five circular explants, 38 mm in diameter, were prepared. The explants were held on a specifically designed support composed of a reservoir of culture medium, surmounted by a grid on which the explant was stretched. The test products were applied at a concentration of 2 mg/cm2. A TMS‐500 White‐light interferometer (Polytec GmbH, Waldbronn, Germany) used light interferometry to create a 3D image of the surface of the skin explants. Images were taken before product application (Day 0) and after three daily product applications (Day 3). The 3D images were then analyzed using Cell^D software (Olympus Soft Imaging Solutions GmbH, Münster, Germany) to measure %μF and epidermal thickness for the skin explants (Table 1). In addition, the epidermal thickness was measured three days after the final product application (D6) using standard histological techniques (Table 1). Roughness parameters (Table 1) were calculated using the TMS 3.8 software.
TABLE 1.
Evaluated parameters for roughness and histology
| Parameter | What it measures | What it means |
|---|---|---|
| % μF | % μF measures the area occupied by skin microfolds. | A decrease in %μF indicates a tensor effect. |
| % SK (μm) | % SK (μm) measures the average reduced amplitude of core roughness. It reflects the main microrelief. Defined by ISO 25178. | A decrease in %SK (μm) indicates a smoothing effect. |
| % SvK (μm) | % SvK (μm) measures the depth of folds of the microrelief. Defined by ISO 13565‐1 and 2. | A decrease in %SvK (μm) indicates a filling effect on the skin surface. |
| % Epidermal thickness | % Epidermal thickness measures the depth of the epidermal layer of the skin | An increase in % Epidermal thickness indicates a plumper, thicker skin |
2.3. In vivomethods
This clinical study was performed at I.E.C Afrique du Sud in Cape Town, South Africa, an independent research facility following ICH GCP Guidelines. The protocol including the informed consent was approved by ACEAS ‐ Independent Ethics Committee in Ahmedabad, India. Thirty three (33) subjects were enrolled in the study. Thirty‐one (31) healthy subjects who signed an informed consent, completed the study, and were included in the statistical analyses. Two (2) subjects were discontinued, one due to a non product related serious adverse event and one was lost to follow‐up.
Inclusion Criteria included
Caucasian females
All skin types
35–55 years of age
-
Presence of aged skin:
Fine line and wrinkles on the forehead, crow's feet, and upper lip
Lack of firmness/smoothness/hydration
Exclusion Criteria included
-
●
Pregnant, breastfeeding, or not willing to take the necessary precautions to avoid pregnancy during the study
-
●
Receiving an oral or a topical treatment involving tretinoin or isotretinoin within 3 months prior to the start of the study
-
●
History of intolerance or allergy to cosmetics, drugs, or other substances
-
●
Having visible traces of irritation or any other abnormality on the involved skin areas for the study
-
●
Having skin recently exposed to either natural or artificial sunlight within 2 weeks prior to the start of the study
-
●
Prior surgery on the involved skin areas for the study
-
●
Having undergone either chemical or physical treatments within 12 months and no injection within 6 months prior to the start of the study on the involved skin areas for the study
-
●
Having permanent make‐up, eyelash extensions, or false eyelashes
-
●
Modified cosmetic habits on the involved skin areas for the study within 2 weeks prior to the start of the study
-
●
Application of anti‐wrinkle, anti‐aging, or anti‐firming products within 4 weeks prior to the start of the study
-
●
Application of cosmetic or pharmaceutical products, other than their usual cleanser, to the involved skin areas for the study within 2 days prior to the start of the study
-
●
Application of cosmetic or pharmaceutical products, other than water, to the involved skin areas for the study on the starting day of the study
Subjects were supplied with the Study Serum, which was to be applied twice‐daily to their whole face along with supplied marketed support products, that is, Cetaphil Daily Facial Cleanser (Galderma Laboratories, L.P.), Neutrogena Clear Face Break‐Out Free Liquid Lotion Sunscreen Broad Spectrum SPF55 (Johnson & Johnson Consumer, Inc.), and Cetaphil Moisturizing Lotion ‐ Body & Face (Galderma Laboratories, L.P.), for a standardized skin care regimen during the 12 weeks study. Subjects' skin was evaluated before application of the Study Serum and immediately after the first application as well as after 4, 8, and 12 weeks of twice‐daily home use.
Clinical scoring was performed visually by a Dermatologist on all parameters before application, on the lifting/tightening effect, immediately after application of the Study Serum, and on wrinkles, fine lines, lifting/tightening, and smoothness after 4, 8, and 12 weeks of at‐home use. These parameters were assessed on the forehead, crow's feet and upper lip regions of the face. Wrinkles were assessed using a 0–5 scale on the forehead and a 0–6 scale on the upper lip and crow's feet areas, while the other parameters were assessed using a 0–9 scale. In addition, skin firmness was evaluated tactically by the Dermatologist on the hollow of the cheek using a 0–9 scale before application and after 4, 8, and 12 weeks of twice daily home use.
The Corneometer® (Courage + Khazaka Electronic) assessed the water content of the upper layers of the skin by electrical capacitance. 10 Three (3) measurements were captured on the forehead after acclimating for at least 15 min in a temperature and a humidity‐controlled room at all study visits. A mean was calculated for all three measurements at each time point and used for statistical analyses.
Self‐assessment questionnaires enabled the collection of effectiveness and cosmetic acceptability of the Study Serum over the course of the study. In particular, the Study Serum was evaluated by the subjects immediately after application for two parameters (lifting and tightening) and after 4, 8, and 12 weeks of home use on 26 parameters. In addition, adverse events were collected throughout the study. Most of the parameters were answered on a four point scale (agree, somewhat agree, somewhat disagree, and disagree).
Facial images were taken with the Colorface® system (Newtone Technologies, Lyon France) (Figures 1, 2, 3, 4). The images were taken using a multi‐modalities system of atrial contention, which includes a 48 colors reference chart.
FIGURE 1.
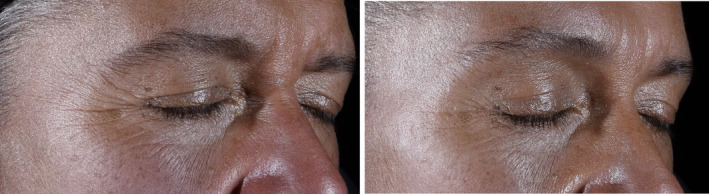
Subject 8 for Crow's feet—Pre‐application on left and Week 12 on right
FIGURE 2.

Subject 2—Forehead—Pre‐application on left and Week 12 on right
FIGURE 3.

Subject 25—Upper lip—Pre‐application on left and Week 12 on right
FIGURE 4.

Subject 29 for Immediate lifting/tightening in Crow's feet region—Pre‐application on the left—Immediately after application on the right
2.4. Statistical analysis
2.4.1. Ex vivo
Comparison of mean values to determine statistical significance was performed using the Student's t‐test. Statistical significance was reported as 95% significant (p < 0.05), and 99% significant (p < 0.01).
2.4.2. In vivo methods
The mean values were determined for each parameter at each time point in the study. The data at each post‐application time point were compared to the corresponding pre‐application data using the Wilcoxon two‐tailed test for the clinical evaluations by the Dermatologist and by the two‐tailed paired t‐test after normality was confirmed using the Shapiro–Wilk test for the skin hydration measurements by Corneometer®. Statistical significance was set at p < 0.05 for all comparisons. The binomial test was used to determine whether the percentage of favorable responses was significantly greater than 50% when evaluating the cosmetic acceptability results via the subject self‐assessment questionnaire.
3. RESULTS
3.1. Effect of study serum on skin explants
As shown in Figures 5 and 6, the Study Serum showed higher efficacy on Day 3 than the Competitor Serum and the Untreated Control in terms of a reduction in the skin microfold surface (%µF); thus demonstrating a tensor effect. The Study Serum reduced microfolds by 84.2% (p < 0.01) after 3 days of applications, while the Competitor Serum reduced microfolds by 36.5% (p < 0.01). Additionally, Figure 5 shows that Study Serum demonstrated a statistically significant smoothing effect through a reduction in average core roughness (%SK) by 25.7% (p < 0.05) and a statistically significant filling effect through a reduction in the mean depth of the folds or valleys (%SvK) by 37.1% (p < 0.05). The Competitor Serum demonstrated a statistically significant reduction of 12.2% (p < 0.05) for %SK, but there was not a statistically significant change in %SvK for the Competitor Serum. Furthermore, the Competitor Serum was not significantly different from the Untreated Control with respect to %SK. The Untreated Control showed a statistically significant reduction of 12.2% (p < 0.01) for %SK and an increase of 6.6% for (%SvK), which was not statistically significant. Finally, the Study Serum demonstrated a redensifying effect as seen through a 47.8% increase in epidermal thickness as compared to the Untreated Control (p < 0.01), while the Competitor Serum was not statistically different than the Untreated Control with regards to a change in epidermal thickness.
FIGURE 5.

Percentage change in microfold and roughness parameters on Day 3 as well as in epidermal thickness on Day 6. Statistical significance is shown for each product in comparison to pre‐treatment values for %μF, %SK, and %SvK. Statistical significance is shown for % epidermal thickness relative to the untreated control
FIGURE 6.

Tensor effect: A reduction in skin microfolds. Explants treated with Study serum showed a superior reduction of the % skin surface occupied by microfolds after 3 days of treatment as compared to explants treated with the competitor serum and as compared to the untreated control. *Wrinkle Severity at 4 weeks was statistically significant but not clinically significant
3.2. Effect of study serum in human subjects
3.2.1. Dermatologist‐reported clinical outcomes
Table 2 presents the statistical and clinical improvements as reported by the Dermatologist. In addition, pre‐application photos and photos after 12 weeks of product use of representative subjects can be seen in Figure 1 (Crow's Feet), Figure 2 (Forehead), and Figure 3 (Upper Lip). In comparison to the pre‐applications, statistically significant improvements (p < 0.05) were observed in all three regions with regards Fine Lines Visibility (up to a 66% reduction), Smoothness (up to a 203% increase), and Lifting/Tightening (up to a 218% increase) after 4, 8, and 12 weeks of twice daily application. In addition, Wrinkle Severity showed statistically significant improvement (p < 0.05) in the Crow's Feet area (32% reduction) and on the Upper Lip (up to a 34% reduction) at all three post‐application time points, and on the Forehead (up to a 25% reduction) after 8 and 12 weeks of twice‐daily application. There were also statistically significant (p < 0.05) improvements in Skin Firmness on the cheek at 8 weeks (32%) and 12 weeks (68%), but not at 4 weeks. There was a statistically significant improvement of 17% in skin tightening in the crow's feet area approximately 10 min after application as seen in the representative photo (Figure 4), but no statistically significant changes in skin tightening were observed on the forehead or upper lift immediately after product application.
TABLE 2.
Statistical and clinical improvements in the Crow's Feet area, on the forehead and on the upper lip from baseline
| Facial area | Crow's feet | Forehead | Upper lip | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical parameter | 4 weeks | 8 weeks | 12 weeks | 4 weeks | 8 weeks | 12 weeks | 4 weeks | 8 weeks | 12 weeks |
| Wrinkles severity | −7% | −17% | −32% | −3% a | −12% | −25% | −5% | −16% | −34% |
| Fine lines visibility | −13% | −33% | −66% | −8% | −28% | −62% | −7% | −27% | −53% |
| Smooth aspect | 36% | 104% | 183% | 33% | 109% | 203% | 20% | 78% | 146% |
| Lifting/tightening effect | 42% | 127% | 218% | 36% | 126% | 209% | 27% | 93% | 156% |
Wrinkle Severity at 4 weeks was statistically significant but not clinically significant.
3.2.2. Skin hydration
Twice daily use of the Study Serum caused a statistically significant increase (p < 0.001) in skin moisturization as measured by the Corneometer®. The average increase over the course of the study was 21.7% and the range was 18%–25% for the three measured time points. The values at each time point were statistically significant (p < 0.001) as compared to their pretreatment values.
3.2.3. Subjective evaluation of cosmetic acceptability and effectiveness
Subjects completed a 26‐parameter self‐assessment questionnaire. 94% of the subjects or greater either agreed or somewhat agreed with the statements that the product left their skin hydrated, firmed, and more toned at 4, 8, and 12 weeks. In addition, a statistically significant subset of the subjects believed that they experienced a tightening effect (74%) approximately 10 min after product application, which increased to more than 87% of subjects at the latter time points. Furthermore, at least 97% of subjects agreed or somewhat agreed with the statements that their skin was smoothed or that its roughness was improved at all time points. Similarly, over 77% of subjects reported that the appearance of fine lines and of wrinkles was reduced at 4, 8, and 12 weeks, and over 81% of subjects reported that they had less wrinkles at 8 and 12 weeks. (p < 0.05 for the above mentioned testing).
3.2.4. Adverse events
The Study Serum was well tolerated by the subjects. Only two subjects out of thirty‐one reported either cutaneous discomfort or irritation during the study. These reactions were not considered product‐related based on the analysis of the reactions, including the nature, location, intensity, duration, and time of appearance/emergence after Study Serum application. Furthermore, the Dermatologist did not observe any physical signs or reactions linked to the use of the Study Serum.
4. DISCUSSION
The function and appearance of skin changes with exposure to external factors, known as extrinsic aging, as well as with increasing age, also known as intrinsic aging. Aged skin can be characterized by wrinkles, age spots, and the loss of skin tone and elasticity. 11 Extrinsically aged skin, such as through sun exposure, will have a rough texture. In addition, it will have deeper wrinkles and be less elastic than intrinsically aged skin. 12 For years, consumers have sought solutions to prevent, cover‐up, or hide the signs of aging. The value of the global skin care market has been estimated to grow by 40% over the next 5 years to $88 billion US dollars. 13 Thus it is imperative that efficacious anti‐aging products are developed.
To this end, in this work, a facial serum, containing a proprietary blend of neuropeptides, proteins, amino acids, and marine extracts, has been examined individually under various experimental conditions and found to be efficacious. Neuropeptides are known to help inhibit and relax muscle contractions and to help reduce skin roughness, wrinkle depth, and the formation of facial lines. Examples of neuropeptides include Acetyl Hexapeptide‐3, Pentapeptide‐18, and Acetyl Octapeptide‐1/‐3, one of the active components in the Study Serum, whose mode of action is inhibition of muscle contraction pathways. 14
Previously conducted in vivo efficacy studies have demonstrated the varying degrees of the efficacy of the neuropeptides on wrinkle depth. A 30 days in vivo study of Acetyl Hexapeptide‐3 on 10 healthy women volunteers utilizing silicone replicas for skin topography analysis with twice daily application is shown to be efficacious on skin wrinkle depth. 15 A 10% oil‐in‐water emulsion containing 10% of Argireline, the raw material trade name of a solution of Acetyl Hexapeptide‐3, demonstrated a 30% reduction in skin wrinkle depth compared to a 10% reduction in skin wrinkle depth on the contralateral side where only an oil‐in‐water emulsion was applied.
In comparison, another in vivo study using silicone imprints of wrinkles around the eyes was conducted demonstrating and comparing the efficacy of Acetyl Hexapeptide‐3 and Pentapeptide‐18. 16 Over the course of 28 days with twice daily application, it was found a cream containing 0.05% Pentapeptide‐18 achieved a decrease of 11.64% in skin wrinkle depth and a cream containing 0.05% Acetyl Hexapeptide‐3 achieved a decrease of 16.26% in skin wrinkle depth. Additive effects were also demonstrated with a cream containing 0.05% Pentapeptide‐18 and 0.05% Acetyl Hexapeptide‐3 with a decrease of 24.62% skin wrinkle depth.
In addition, a split face in vivo efficacy study was conducted with a cream containing 3% of a 0.05% Acetyl Octapeptide‐3 solution, applied twice a day for 28 days, demonstrating wrinkle depth was lowered by up to 38%. On average, wrinkle depth decreased by 7.1%. 17 While the efficacy results of these three in vivo studies cannot be directly compared due to variables in testing conditions and rating systems, it is clear that the inclusion of neuropeptides has a positive impact on addressing the reduction of wrinkle depth.
The addition of proteins and amino acids helps the skin maintain its moisture and stay hydrated. They also help smooth and lift dry, aging skin. Finally, the marine extracts help provide a contouring effect and improve the overall appearance of sagging, loose skin. These ingredients have been formulated into a facial serum (Study Serum) whose overall benefit has been evaluated in this study for the first time.
In the experiments using skin explants, the Study Serum demonstrated a tensor effect by reducing the percentage of skin surface area occupied by microfolds in comparison to the competitor serum and to the untreated control. This finding is consistent with the observed smoothing effect of the Study Serum through a reduction in average core roughness as well as a reduction in the mean depth of the folds demonstrating a filling effect. These changes are also consistent with the histological findings, in which the Study Serum caused a statistically significant increase in epidermal thickness in comparison to the Competitor Serum and Untreated Control (Figure 7). In summary, these ex vivo experiments have demonstrated the beneficial effects that the Study Serum has on skin explants with regards to skin smoothness, less folds, and a thicker epidermis.
FIGURE 7.

Increase in epidermal thickness. Explants treated with study serum showed a significant improvement in epidermal thickness relative to the untreated control after 6 days as compared to explants treated with the Competitor Serum
The Study Serum was also evaluated during a 12 week at‐home clinical test. In this test, 31 females completed the study, aged 35–55, using the product at home under the supervision of a Dermatologist. Their skin was clinically graded by the Dermatologist before they entered the study as well as immediately after application, and every 4 weeks for 12 weeks. All‐in‐all, the Study Serum was well tolerated by the subjects and no product‐related adverse reactions were noted. All the clinical parameters evaluated by the Dermatologist directly on a subject demonstrated statistically significant improvements from baseline in all three regions of the face at all timepoints. All parameters were also considered to be clinically significant with the exception of forehead wrinkles at 4 weeks. These clinical results are supported by the photographs taken of each panelist demonstrating the benefit of the Study Serum during the 12‐week study (Figures 1, 2, 3, 4). This means that wrinkle severity was reduced, fine lines were less visible, the skin had a smooth aspect to it, and the skin demonstrated a tightening effect. These findings are supported by the ex vivo results, especially those involving smaller microfolds, less roughness, and a thicker skin. These findings are further supported when compared to the aforementioned in vivo efficacy study on Acetyl Octapeptide‐3. 17 Acetyl Octapeptide‐3 is an active component of the facial serum. Therefore, its inclusion in both the cream in the 28 days in vivo study and our facial serum in the 12 week at‐home clinical are in alignment and provide additional evidence of its efficacy in the reduction of wrinkle severity. Additionally, the Dermatologist detected a statistically significant increase in skin firmness, a tactile assessment, after 8 and 12 weeks of twice daily home use.
In addition to the clinical evaluations, the subject's skin was also evaluated for skin hydration using the Corneometer®. The Corneometer® uses skin capacitance to estimate the hydration level of the skin. It was developed more than 30 years ago and has been one of the standard instruments to measure skin hydration. 18 , 19 During the 12 weeks of this study, the increase in skin hydration averaged 21.7% and ranged from 18%−25%. It is well known that hydrated skin is one that is thicker and smoother. 20 , 21 Thus, these observations of an increased hydration level are consistent with the findings of the Dermatologist as well as the results of the ex vivo experiments.
The clinical and laboratory findings were confirmed through subject feedback over the course of the 12‐week study. As a whole, the subjects believe that their skin was more hydrated, firmed, and toned after the use of the product. Furthermore, the subjects believe that their skin was smoothed, and they reported that the appearance of fine lines and of wrinkles was reduced. Finally, subjects reported a lifting effect and a tightening effect immediately after application as well as at the later time points.
5. CONCLUSIONS
In summary, a facial serum has been developed that contains a proprietary blend of neuropeptides, proteins, amino acids, and marine extracts. A series of ex vivo and clinical tests have been used that demonstrate the improvement of the overall quality of aged skin during a 12‐week study. The beneficial effects on the skin have been observed experimentally in the lab as well as instrumentally, clinically, photographically, and subjectively in a clinical setting.
CONFLICT OF INTEREST
M. Moy, I. Diaz, E. Lesniak & G. Giancola are employees of the Colgate‐Palmolive Company.
AUTHOR CONTRIBUTIONS
Melissa Moy, MS—contributed to the study concept and design, analysis and interpretation of data, and review of the manuscript. Isabel Diaz, BA—contributed to the study concept and design, analysis and interpretation of data, and review of the manuscript. Ewelina Lesniak, BA—contributed to the study concept, analysis and interpretation of data, and review of the manuscript. Giorgiana Giancola, PhD—contributed to the study concept, analysis and interpretation of data, drafted and reviewed the manuscript.
ETHICAL APPROVAL
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The protocol including the informed consent was approved by ACEAS ‐ Independent Ethics Committee in Ahmedabad, India.
ACKNOWLEDGMENTS
The authors would like to thank Mack Morrison, Ph.D., President, Starfish Scientific Solutions, LLC for the assistance in the preparation of this manuscript. The authors also thank IEC, Institut d´Expertise Clinique (88, Boulevard des Belges – 69006 Lyon – France) for performing the clinical study and Laboratoire BIO‐EC (1, Chemin de Saulxier‐ 91160 Longjumeau ‐ France) for performing the ex‐vivo study.
Moy M, Diaz I, Lesniak E, Giancola G. Peptide‐pro complex serum: Investigating effects on aged skin. J Cosmet Dermatol. 2023;22:267–274. doi: 10.1111/jocd.14992
Funding information
The authors would like to thank the Colgate‐Palmolive Company and PCA SKIN® for the funding of this research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Haydont V, Bernard BA, Fortunel NO. Age‐related evolutions of the dermis: clinical signs, fibroblast and extracellular matrix dynamics. Mech Ageing Dev. 2019;177:150‐156. doi: 10.1016/j.mad.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 2. Augustin M, Kirsten N, Körber A, et al. Prevalence, predictors and comorbidity of dry skin in the general population. J Eur Acad Dermatol Venereol. 2019;33(1):147‐150. doi: 10.1111/jdv.15157 [DOI] [PubMed] [Google Scholar]
- 3. Alves A, Sousa E, Kijjoa A, Pinto M. Marine‐derived compounds with potential use as cosmeceuticals and nutricosmetics. Molecules. 2020;25(11):2536. doi: 10.3390/molecules25112536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorouhi F, Maibach HI. Role of topical peptides in preventing or treating aged skin. Int J Cosmet Sci. 2009;31(5):327‐345. doi: 10.1111/j.1468-2494.2009.00490.x [DOI] [PubMed] [Google Scholar]
- 5. Husein el Hadmed H, Castillo RF. Cosmeceuticals: peptides, proteins, and growth factors. J Cosmet Dermatol. 2016;15(4):514‐519. doi: 10.1111/jocd.12229 [DOI] [PubMed] [Google Scholar]
- 6. Venkatesan J, Anil S, Kim SK, Shim MS. Marine fish proteins and peptides for cosmeceuticals: a review. Marine Drugs. 2017;15(5):143. doi: 10.3390/md15050143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Y, Cao K, Zhang W, Zhang G, Zhou M. Protective and anti‐aging effects of 5 cosmeceutical peptide mixtures on hydrogen peroxide‐induced premature senescence in human skin fibroblasts. Skin Pharmacol Physiol. 2021;34(4):194‐202. doi: 10.1159/000514496 [DOI] [PubMed] [Google Scholar]
- 8. Baseggio Conrado A, Fanelli S, McGuire VA, Ibbotson SH. Role of hypotaurine in protection against UVA‐induced damage in keratinocytes. Photochem Photobiol. 2021;97(2):353‐359. doi: 10.1111/php.13334 [DOI] [PubMed] [Google Scholar]
- 9. Hameury S, Borderie L, Monneuse JM, Skorski G, Pradines D. Prediction of skin anti‐aging clinical benefits of an association of ingredients from marine and maritime origins: ex vivo evaluation using a label‐free quantitative proteomic and customized data processing approach. J Cosmet Dermatol. 2019;18(1):355‐370. doi: 10.1111/jocd.12528 [DOI] [PubMed] [Google Scholar]
- 10. Berardesca E, Mortillo S, Cameli N, Ardigo M, Mariano M. Efficacy of a shower cream and a lotion with skin‐identical lipids in healthy subjects with atopic dry skin. J Cosmet Dermatol. 2018;17(3):477‐483. doi: 10.1111/jocd.12668 [DOI] [PubMed] [Google Scholar]
- 11. Parrado C, Mercado‐Saenz S, Perez‐Davo A, Gilaberte Y, Gonzalez S, Juarranz A. Environmental stressors on skin aging. Mechanistic insights. Front Pharmacol. 2019;10:759. 10.3389/fphar.2019.00759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montagna W, Kirchner S, Carlisle K. Histology of sun‐damaged human skin. J Am Acad Dermatol. 1989;21:907‐918. 10.1016/S0190-9622(89)70276-0 [DOI] [PubMed] [Google Scholar]
- 13. Ridder M. Value of the Global Anti‐aging Market 2020‐2026. 2021. https://www.statista.com/statistics/509679/value‐of‐the‐global‐anti‐aging‐market/. Accessed May 24, 2021.
- 14. Errante F, Ledwoń P, Latajka R, Rovero P, Papini AM. Cosmeceutical peptides in the framework of sustainable wellness economy. Front Chem. 2020;8:572923. doi: 10.3389/fchem.2020.572923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blanes‐Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24:303‐310. doi: 10.1046/j.1467-2494.2002.00153.x [DOI] [PubMed] [Google Scholar]
- 16. Lipotec SA. Leuphasyl® A New Pentapeptide for Expression Wrinkles Code PD080. Date: June 2005 Revision: 1 A GMP Peptide for Cosmetic Applications. 2005. http://docplayer.net/15401907‐Leuphasyl‐a‐new‐pentapeptide‐for‐expression‐wrinkles‐code‐pd080‐date‐june‐2005‐revision‐1‐a‐gmp‐peptide‐for‐cosmetic‐applications.html. Accessed January 7, 2022.
- 17. The Lubrizol Corporation . SNAP‐8™ peptide solution C. 2022. https://www.lubrizol.com/Personal‐Care/Products/Product‐Finder/Products‐Data/SNAP‐8‐peptide‐solution‐C. Accessed January 7, 2022.
- 18. Berardesca E, Loden M, Serup J, Masson P, Rodrigues LM. The revised EEMCO guidance for the in vivo measurement of water in the skin. Skin Res Technol. 2018;24(3):351‐358. doi: 10.1111/srt.12599 [DOI] [PubMed] [Google Scholar]
- 19. Qassem M, Kyriacou P. Review of modern techniques for the assessment of skin hydration. Cosmetics. 2019;6(1):19. doi: 10.3390/cosmetics6010019 [DOI] [Google Scholar]
- 20. Crowther JM, Sieg A, Blenkiron P, et al. Measuring the effects of topical moisturizers on changes in stratum corneum thickness, water gradients and hydration in vivo. Br J Dermatol. 2008;159(3):567‐577. doi: 10.1111/j.1365-2133.2008.08703.x [DOI] [PubMed] [Google Scholar]
- 21. Dąbrowska AK, Adlhart C, Spano F, et al. In vivo confirmation of hydration‐induced changes in human‐skin thickness, roughness and interaction with the environment. Biointerphases. 2016;11(3):31015. doi: 10.1116/1.4962547 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


