Abstract
Background & Aims
Evidence for the benefit of scheduled imaging for early detection of hepatobiliary malignancies in primary sclerosing cholangitis (PSC) is limited. We aimed to compare different follow‐up strategies in PSC with the hypothesis that regular imaging improves survival.
Methods
We collected retrospective data from 2975 PSC patients from 27 centres. Patients were followed from the start of scheduled imaging or in case of clinical follow‐up from 1 January 2000, until death or last clinical follow‐up alive. The primary endpoint was all‐cause mortality.
Results
A broad variety of different follow‐up strategies were reported. All except one centre used regular imaging, ultrasound (US) and/or magnetic resonance imaging (MRI). Two centres used scheduled endoscopic retrograde cholangiopancreatography (ERCP) in addition to imaging for surveillance purposes. The overall HR (CI95%) for death, adjusted for sex, age and start year of follow‐up, was 0.61 (0.47–0.80) for scheduled imaging with and without ERCP; 0.64 (0.48–0.86) for US/MRI and 0.53 (0.37–0.75) for follow‐up strategies including scheduled ERCP. The lower risk of death remained for scheduled imaging with and without ERCP after adjustment for cholangiocarcinoma (CCA) or high‐grade dysplasia as a time‐dependent covariate, HR 0.57 (0.44–0.75). Hepatobiliary malignancy was diagnosed in 175 (5.9%) of the patients at 7.9 years of follow‐up. Asymptomatic patients (25%) with CCA had better survival if scheduled imaging had been performed.
Conclusions
Follow‐up strategies vary considerably across centres. Scheduled imaging was associated with improved survival. Multiple factors may contribute to this result including early tumour detection and increased endoscopic treatment of asymptomatic benign biliary strictures.
Keywords: cholangiocarcinoma, ERCP, follow‐up strategy, MRI, primary sclerosing cholangitis, surveillance
Abbreviations
- CA 19‐9
carbohydrate antigen 19‐9
- CT
computed tomography
- CCA
cholangiocarcinoma
- ERCP
endoscopic retrograde cholangiopancreatography
- IBD
inflammatory bowel disease
- IPSCSG
International PSC Study Group
- GBC
gallbladder cancer
- HCC
hepatocellular carcinoma
- MRI
magnetic resonance imaging
- MRCP
magnetic resonance cholangiopancreatography
- PSC
primary sclerosing cholangitis
- US
ultrasound
Lay summary.
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease with an increased risk of developing hepatobiliary malignancies. Evidence for the benefit of follow‐up with scheduled imaging in PSC is limited. Regular imaging with magnetic resonance imaging and/or ultrasound during follow‐up is associated with improved survival. Scheduled imaging during follow‐up may be beneficial for patients with PSC.
1. INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic progressive disease leading to biliary cirrhosis, liver failure and need of liver transplantation. The median time to death or liver transplantation is reported to be 21 years in a population‐based setting but shorter in cohorts reported from transplant centres. 1 Patients with PSC are also at increased risk of developing hepatobiliary malignancies including cholangiocarcinoma (CCA), gallbladder cancer (GBC) and hepatocellular carcinoma (HCC). The annual risk of CCA is 0.5%–1%, which is 400‐ to 600‐fold the risk of the general population. 1 , 2 One‐third of the CCAs are diagnosed within 1 year after the PSC diagnosis. 1 , 3 Early detection of CCA is crucial to allow for curative treatment with surgical resection or liver transplantation. CCA develops through a stepwise transformation of the biliary epithelium from biliary dysplasia to invasive CCA. 4 Regular CCA screening or surveillance may therefore be possible in PSC, for early tumour detection. A well‐founded surveillance program, however, requires appropriate means for early detection, which is sensitive, simple, safe and cost‐effective. Such tests do not exist in PSC and detection of CCA in early or premalignant stages is often very difficult and requires invasive investigation with endoscopic retrograde cholangiopancreatography (ERCP) which lacks in sensitivity and is associated with complications. 5 , 6
Follow‐up strategies for PSC have generally been focused on early detection of malignancy and are often referred to as surveillance. Regular imaging and/or ERCP also leads to the detection of high‐grade asymptomatic strictures which are often investigated and treated by ERCP to rule out malignancy and restore bile flow. Investigation of a high‐grade biliary stricture with brush cytology almost always leads to dilatation (treatment) of the stricture. The effect of dilatation of asymptomatic benign high‐grade strictures on prognosis in PSC remains unclear but recent data indicate that there might be a survival benefit of scheduled ERCP in the presence of benign high‐grade strictures. 7 Evidence for the efficacy of tumour surveillance is scarce. 8 , 9 One retrospective study showed a survival benefit in PSC patients with cancer previously included in surveillance programs. 10 Another study demonstrated that annual imaging was associated with a twofold risk reduction of hepatobiliary cancer‐related death. 11
In clinical practice, follow‐up strategies vary hugely between centres. Evaluation of the use of magnetic resonance imaging (MRI) versus ultrasound (US) for the early CCA detection has shown that the regular use of MRI may be better than US for early detection of asymptomatic CCAs. 12 Some centres have incorporated ERCP while some recommend against specific CCA surveillance. 8 , 9 , 13 A brief survey in the International PSC Study Group (IPSCSG) was performed before the initiation of this study and a variety of surveillance strategies were reported. Altogether, this urged us to study the effect of different follow‐up strategies in PSC. We hypothesize that a more vigorous follow‐up with scheduled imaging improves survival in PSC.
2. METHODS
2.1. Study design
We collected retrospective data on follow‐up strategies in PSC from 27 centres from Europe, Canada and the United States (Figure 1B). PSC patients eligible for the study were identified via the pre‐existing IPSCSG database 3 including patients diagnosed between 1 January 1980 and 31 December 2010. Additional patients diagnosed between 31 December 2010 and 03 March 2018, were identified from participating centres. PSC and inflammatory bowel disease (IBD) diagnoses were confirmed using standard criteria. 14 , 15 We restricted the collection of data to after 1 January 2000 with the aim to reduce the effect of change in different strategies over time but still have a long enough follow‐up.
FIGURE 1.
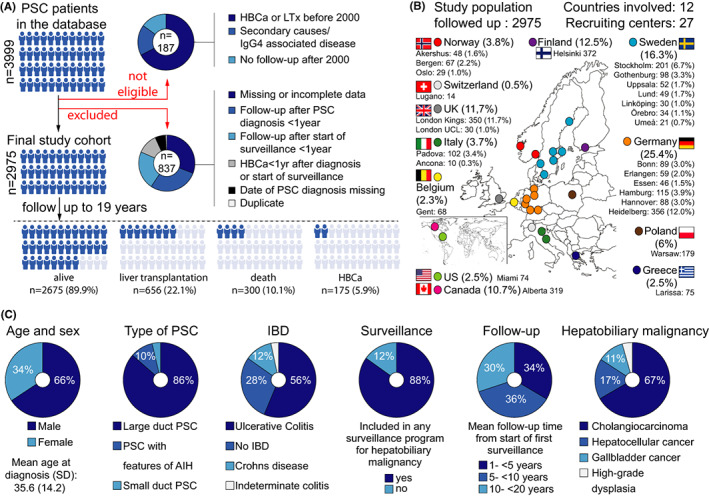
(A) Comprehensive description of the selection of the 2975 PSC patients included in the study. (B) Participating centres in the study. (C) Clinical characteristics and outcomes for all patients
All patient datasets were checked for plausibility and validity, and duplicated patient entries were removed before analysis. Patients with inadequate follow‐up duration (<1 year) or lack of data were excluded. To avoid lead‐time bias and reduce immortal‐time bias we further excluded all patients diagnosed with a hepatobiliary malignancy within 1 year after PSC diagnosis (in cases diagnosed later than 1 January 2000) or the start of follow‐up (1 January 2000). Figure 1 illustrates the final cohort for analysis and its clinical characteristics. Different follow‐up strategies were defined according to type and frequency of imaging and/or scheduled ERCP regardless of symptoms or serum liver tests.
2.2. Data collection
Data on clinical characteristics, imaging and ERCP were collected from the pre‐existing PSC database 3 and by each centre’s prospectively collected local PSC database and/or via review of medical records. Information on the participating centre's overall follow‐up strategy was noted (Table 1). Individual data on planned and performed follow‐up with imaging, CA 19‐9, the development of dominant/high‐grade strictures, biliary dysplasia, hepatobiliary malignancy, liver transplantation and death were documented. Diagnosis of hepatobiliary malignancy was made according to clinical, radiological and/or histological findings as dictated by centre‐specific protocols. The occurrence of symptoms at the time of diagnosis of hepatobiliary malignancy and the first treatment for hepatobiliary malignancy were registered. CA 19‐9 was assessed in all centres using surveillance in a variety of ways and consequently CA 19‐9 was not used as a variable to discriminate different strategies.
TABLE 1.
Follow‐up strategies for patients with primary sclerosing cholangitis (PSC) per country and centre
| Country | Centre (transplant centres underlined) | Surveillance strategy | |
|---|---|---|---|
| Modality | Scheduling time | ||
| Sweden |
Gothenburg Linköping Lund Stockholm Umeå Uppsala Örebro |
MRI with contrast CA 19‐9 |
Every 12 months Every 12 months |
| Norway | Akershus |
MRI with contrast CA 19‐9 |
Every 12 months Every 12 months |
| Bergen |
Ultrasound CA 19‐9 MRCP |
Every 12 months Every 12 months Every 36–48 months |
|
| Oslo |
Ultrasound MRI with contrast CA 19‐9 |
Every 12 months Every 12 months Every 12 months |
|
| Finland | Helsinki |
Surveillance according to a specific algorithm 19 Ultrasound ERC CA 19‐9 and CEA |
Every 6 months a Every 3, 6, 12, 48–60 months b Every 6 months |
| Germany | Bonn |
Ultrasound MRI CA19‐9 |
Every 6–12 months Every 12–24 months c Every 3–6 months |
| Erlangen |
Ultrasound MRI/MRCP |
Every 6 months Every 12 or 36 months c |
|
| Essen |
Ultrasound MRI CA 19‐9 |
Every 6–12 months c Every 12–24 months c Every 3–6 months c |
|
| Hamburg |
Ultrasound MRI (used to be with contrast, lately without contrast for asymptomatic surveillance) CA 19‐9 |
Every 6 months Every 12 months Every 6 months |
|
| Hannover |
Ultrasound MRCP CA 19‐9 |
Every 6–12 months Every 12–24 months c Every 3–6 months c |
|
| Heidelberg |
Ultrasound MRI/MRCP or ERC d CA 19‐9 |
Every 6 months Every 12 months Every 6 months |
|
| Belgium | Gent |
Ultrasound MRCP CA 19‐9 |
Every 12 months Every 4–6 months Every 6 months |
| UK | London Kings | No regular imaging | Every 6–12 months |
| London UCL | Imaging at physician's discretion | Every 6–12 months | |
| Poland | Warsaw |
MRCP CA‐19‐9 |
Every 12–24 months c Every 12 months |
| Switzerland | Lugano |
Ultrasound and MRI with contrast alternating CA 19‐9 and alphafoetoprotein |
Every 6 months Every 6 months |
| Italy | Padova |
Ultrasound MRI with contrast |
Every 12 months Every 1–2 yrs c |
| Ancona |
MRI ‐ all patients MRI in patients with dominant stenosis |
Every 12 months Every 6 months |
|
| Greece | Larissa |
Ultrasound and MRCP alternating CA 19‐9 |
Every 6 months Every 12 months |
| Canada | Alberta |
Ultrasound MRI with contrast CA 19‐9 |
Every 6 months Every 12 months Every 12 months |
| United States | Miami |
At physicians´ discretion: MRI with contrast CA 19‐9 Ultrasound a |
Every 12 months Every 12 months Every 6 months |
In patients with cirrhosis.
Depending on suspicion of dysplasia, grade of inflammation (bile fluid calprotectin), ERC‐score and need for dilatation.
The interval depends on the severity of PSC.
In patients with dominant stenosis. Balloon dilatation is repeated at 1, 3 and 6 months until the dominant stricture is resolved.
Prior to the study investigators were asked to give information on surveillance strategies of their centre (Table 1). The retrospective setting of the study preclude the differentiation between imaging performed purely as surveillance, for diagnostic purposes or as a reaction on new clinical symptoms. All patients were followed from the point of start of any follow‐up strategy (earliest 1 January 2000) or at 1 year after PSC diagnosis until death or the last clinical follow‐up alive. The primary endpoint was death.
2.3. Statistical analyses
Exposure to the type of follow‐up strategy was grouped according to the strategy offered by the different centres. We calculated mortality rate as the number of events per 1000 person‐years and used Kaplan–Meier survival curves to describe time to all‐cause mortality during follow‐up. Cox‐proportional hazard regression models adjusted for age, sex and the calendar year for the start of follow‐up were used to estimate hazard ratios (HRs) for clinical follow‐up versus follow‐up including regular imaging overall and in subgroups of patients. In additional analyses, HRs were estimated by including the first event of dominant stricture and CCA/high‐grade dysplasia as a time‐dependent covariate in the regression model. The proportional hazard assumption was tested by including an interaction term of the exposure and follow‐up time in the model. Finally, we also tested for difference in HRs between survival strategies by including an interaction term in the regression model. Data were analysed using SAS (version 9.4) and Stata (version 13). Two‐sided p‐values <.05 were considered statistically significant. Ethical approval was obtained locally by participating sites.
3. RESULTS
3.1. Participating centres and follow‐up strategies
The centres reported a broad variety of different follow‐up strategies demonstrated in Table 1. Seventeen of the participating centres (63%) are referral transplant centres. Most centres used regular measurement of CA 19‐9 and some modality of imaging—US and/or magnetic resonance cholangiopancreatography (MRCP) and/or MRI with or without contrast with the primary aim to detect early signs of hepatobiliary malignancy. Two centres used scheduled ERCP in addition to imaging for surveillance purposes. One centre did not perform scheduled imaging. ERCP was performed in all centres on clinical indications and if progressive strictures suspicious for malignancy were found at imaging in non‐symptomatic patients in line with international endoscopic guidelines. 16 In addition to centre strategies, imaging that was performed per patient was also reported. Of 2675 patients included in the follow‐up programs, 83% were subjected to regular MRI/MRCP, 49% to US and 28% to ERCP. The retrospective setting hindered a case‐based analysis on which investigations were performed with a purpose of cancer surveillance and which were done for other reasons.
3.2. Clinical characteristics of the PSC cohort
The cohort of 2975 PSC patients showed typical clinical characteristics for PSC with 65.6% (1953/2975) males, mean age (SD) at diagnosis of PSC of 35.6 (14.2) years and concomitant IBD in 71.5% (2127/2973). Large duct disease was present in 96.3% (n = 2865), and 9.8% (n = 293) were reported to have features of concomitant autoimmune hepatitis. The mean follow‐up (SD) from the study start was 7.9 (4.8) years. During follow‐up, 300 (10.1%) patients died, 656 (22.1%) were transplanted and 175 (5.9%) developed hepatobiliary malignancy. The clinical characteristics of all patients are shown in Figure 1C. Of the 300 patients that died 77 (25.7%) had a diagnosis of CCA, 7 (2%) GBC, 11 (3.7%) HCC and 3 (1%) pancreatic cancer. Twenty‐six (8.7%) who died during follow‐up had colorectal carcinoma in their medical history. Detailed data on the cause of death in all deceased patients were not possible to evaluate because of missing data.
3.3. Impact of different follow‐up strategies on mortality
Deaths were more frequent in the group that did not undergo scheduled imaging as a follow‐up strategy than in patients that underwent scheduled imaging and/or ERCP 23.4% (82/350) versus 8.3% (218/2625) (Table 2, Figure 2A). The mortality rate (CI95%) per 1000 person‐years was 23.1 (18.1–28.1) in the group that did not undergo scheduled imaging, 12.5 (10.5–14.4) in the group including MRI/MRCP/US as follow‐up strategy, and 8.4 (6.3–10.5) in follow‐up that included scheduled ERCPs. The risk of dying demonstrated as hazard ratios for death, [HR (CI95%)], was reduced in patients undergoing scheduled imaging and/or ERCP at follow‐up [HR 0.53 (0.41–0.68)] and the reduced risk remained after adjustment for sex, age and start year of follow‐up [HR 0.61 (0.47–0.80)] (Table 2). A dominant stricture can harbour an undetected malignancy; thus, some centres increase the frequency of imaging and ERCP in the presence of a dominant stricture. Therefore, adjustment for the presence of a clinically relevant or dominant stricture as a time‐dependent covariate for first such stricture was also performed. However, this did not significantly alter the overall risk of death [HR 0.64, (CI95% 0.49–0.84)] (Table 2) in comparison to adjustment for only sex, age and start year of follow‐up. We also evaluated to what extent the presence of cancer contributed to better survival by adjustment for CCA or high‐grade dysplasia as a time‐dependent covariate. The risk of death did not change considerably with a HR (CI95%) of [0.57 (0.44–0.75)] (Table 2). In addition, no clear time trend from 2000 to 2018 in 5‐year intervals, was found (Table 2).
TABLE 2.
Number of patients, deaths and mortality rate per 1000 person‐years and risk estimates for death
| Group | N (%) | N deaths (%) | Mortality rate (95% CI) per 1000 PY | Hazard ratio (95% CI) a | Hazard ratio (95% CI) b | Hazard ratio (95% CI) c | Hazard ratio (95% CI) d | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow‐up with scheduled imaging | Clinical follow‐up | Follow‐up with scheduled imaging | Clinical follow‐up | Follow‐up with scheduled imaging | Clinical follow‐up | |||||
| Overall | 2625 (100%) | 350 (100%) | 218 (8.3%) | 82 (23.4%) | 11.0 (9.5–12.4) | 23.1 (18.1–28.1) | 0.53 (0.41–0.68) | 0.61 (0.47–0.80) | 0.64 (0.49–0.84) | 0.57 (0.44–0.75) |
| Sex | ||||||||||
| Male | 1716 (65.4%) | 237 (67.7%) | 157 (9.1%) | 56 (23.6%) | 12.1 (10.2–14.0) | 23.2 (17.1–29.3) | 0.58 (0.43–0.80) | 0.69 (0.50–0.95) | 0.71 (0.52–0.99) | 0.55 (0.40–0.77) |
| Female | 907 (34.6%) | 113 (32.3%) | 61 (6.7%) | 26 (23.0%) | 8.9 (6.7–11.2) | 23.0 (14.1–31.8) | 0.42 (0.26–0.67) | 0.43 (0.26–0.70) | 0.45 (0.27–0.74) | 0.50 (0.30–0.84) |
| Age (years) at start of follow‐up | ||||||||||
| 0–<18 y | 126 (4.8%) | 20 (5.7%) | 2 (1.6%) | 1 (5.0%) | 1.6 (0.0–3.9) | 3.9 (0.0–11.4) | 0.42 (0.04–4.59) | 0.43 (0.04–4.99) | 0.40 (0.04–4.48) | 0.44 (0.04–5.01) |
| 18–<30 y | 644 (24.5%) | 69 (19.7%) | 26 (4.0%) | 8 (11.6%) | 5.3 (3.3–7.4) | 11.5 (3.5–19.4) | 0.56 (0.25–1.28) | 0.49 (0.22–1.11) | 0.51 (0.22–1.14) | 0.34 (0.14–0.82) |
| 30–<40 y | 734 (28.0%) | 65 (18.6%) | 53 (7.2%) | 13 (20.0%) | 9.1 (6.7–11.6) | 19.5 (8.9–30.1) | 0.56 (0.30–1.03) | 0.56 (0.30–1.03) | 0.55 (0.30–1.03) | 0.42 (0.22–0.79) |
| 40–<50 y | 532 (20.3%) | 81 (23.1%) | 50 (9.4%) | 25 (30.9%) | 12.1 (8.8–15.5) | 27.4 (16.7–38.2) | 0.53 (0.32–0.87) | 0.51 (0.31–0.84) | 0.50 (0.30–0.84) | 0.50 (0.30–0.83) |
| 50–<60 y | 361 (13.8%) | 65 (18.6%) | 45 (12.5%) | 18 (27.7%) | 18.1 (12.8–23.4) | 26.2 (14.1–38.3) | 0.76 (0.43–1.33) | 0.77 (0.43–1.41) | 0.82 (0.45–1.50) | 0.93 (0.50–1.74) |
| 60–<70 y | 184 (7.0%) | 41 (11.7%) | 28 (15.2%) | 12 (29.3%) | 25.1 (15.8–34.4) | 41.2 (17.9–64.6) | 0.63 (0.32–1.25) | 0.50 (0.24–1.05) | 0.59 (0.28–1.24) | 0.43 (0.20–0.92) |
| ≥70 y | 40 (1.5%) | 9 (2.6%) | 13 (32.5%) | 5 (55.6%) | 71.6 (32.7–110.6) | 156.1 (19.3–292.9) | 0.47 (0.17–1.32) | 0.21 (0.06–0.80) | 0.19 (0.04–0.88) | 0.26 (0.07–1.02) |
| Year of start of follow‐up | ||||||||||
| 2000–2004 | 688 (26.2%) | 187 (53.4%) | 94 (13.7%) | 57 (30.5%) | 11.2 (8.9–13.5) | 23.7 (17.5–29.8) | 0.50 (0.36–0.69) | 0.60 (0.42–0.84) | 0.59 (0.42–0.84) | 0.61 (0.43–0.86) |
| 2005–2009 | 604 (23.0%) | 77 (22.0%) | 66 (10.9%) | 12 (15.6%) | 12.4 (9.4–15.4) | 16.4 (7.1–25.7) | 0.78 (0.42–1.45) | 0.97 (0.52–1.81) | 1.10 (0.58–2.11) | 0.72 (0.38–1.37) |
| 2010–2014 | 898 (34.2%) | 63 (18.0%) | 50 (5.6%) | 11 (17.5%) | 10.0 (7.2–12.7) | 31.7 (13.0–50.4) | 0.32 (0.16–0.61) | 0.33 (0.17–0.64) | 0.36 (0.19–0.70) | 0.34 (0.17–0.67) |
| 2015–2018 | 435 (16.6%) | 23 (6.6%) | 8 (1.8%) | 2 (8.7%) | 7.2 (2.2–12.3) | 36.3 (0.0–86.6) | 0.16 (0.03–0.78) | 0.10 (0.02–0.52) | 0.12 (0.02–0.64) | 0.19 (0.02–1.64) |
| Main diagnosis | ||||||||||
| PSC | 2262 (86.2%) | 310 (88.6%) | 197 (8.7%) | 79 (25.5%) | 11.4 (9.8–13.0) | 25.1 (19.5–30.6) | 0.50 (0.38–0.65) | 0.58 (0.44–0.77) | 0.61 (0.46–0.80) | 0.54 (0.41–0.72) |
| Small duct PSC/possible small duct PSC | 105 (4.0%) | 5 (1.4%) | 4 (3.8%) | 0 | 4.9 (0.1–9.6) | 0 | ‐ | ‐ | ‐ | |
| PSC with features of AIH | 258 (9.8%) | 35 (10.0%) | 17 (6.6%) | 3 (8.6%) | 9.7 (5.1–14.3) | 8.2 (0.0–17.6) | 1.69 (0.48–6.01) | 2.28 (0.60–8.73) | 2.33 (0.61–8.99) | 2.19 (0.55–8.77) |
| Type of follow‐up | ||||||||||
| Scheduled MRI and/or US | 1897 (72.3%) | 350 (100%) | 158 (8.3%) | 82 (23.4%) | 12.5 (10.5–14.4) | 23.1 (18.1–28.1) | 0.61 (0.46–0.80) | 0.64 (0.48–0.86) | 0.69 (0.51–0.93) | 0.59 (0.44–0.80) |
| Follow‐up including scheduled ERCP | 728 (27.7%) | 350 (100%) | 60 (8.2%) | 82 (23.4%) | 8.4 (6.3–10.5) | 23.1 (18.1–28.1) | 0.38 (0.27–0.54) | 0.53 (0.37–0.75) | 0.51 (0.36–0.73) | 0.51 (0.36–0.72) |
Abbreviations: AIH, autoimmune hepatitis; CI, confidence intervals; ERCP, endoscopic retrograde cholangiopancreatography; MRI, Magnetic resonance imaging; PSC‐primary sclerosing cholangitis; PY, person years; US, ultrasound.
Unadjusted.
Adjusted for sex, age and start year of follow‐up.
Adjusted for sex, age, start year of follow‐up and first dominant stricture as a time‐dependent covariate.
Adjusted for sex, age, start year of follow‐up and cholangiocarcinoma or high‐grade dysplasia as a time‐dependent covariate.
FIGURE 2.
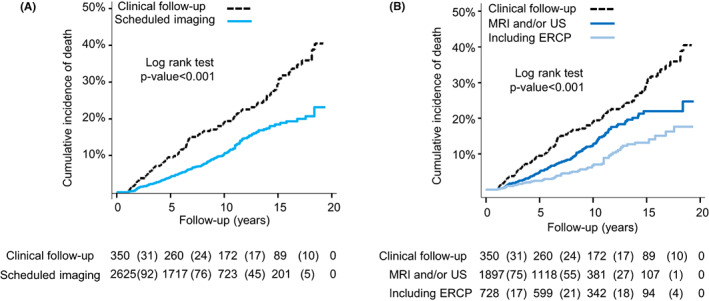
(A) Kaplan–Meier curves displaying the cumulative incidence of death for scheduled imaging versus clinical follow‐up. (B) Kaplan–Meier curves displaying the cumulative incidence of death by type of follow‐up
Furthermore, a comparison between regular imaging and strategies with scheduled ERCPs was performed. All‐cause mortality was significantly lower when ERCP was applied with HR (CI95%), [0.51 (0.36–0.73)] versus US/MRI imaging only, HR (CI95%), [0.69 (0.51–0.93)], in the model including dominant strictures as a time‐dependent covariate (p‐value for interaction = .03), but not statistically significant versus the other regression models when we adjusted for sex, age, start year of follow‐up with or without CCA or high‐grade dysplasia as a time‐dependent covariate (Table 2; Figure 2B).
3.4. Detection of hepatobiliary malignancy
Hepatobiliary malignancy was found in 175 (5.9%) patients of whom 122 (4.2%) had CCA, 21 (0.7%) GBC and 32 (1.1%) HCC. In addition, 8 (0.3%) patients were reported with high‐grade biliary dysplasia and 51 (1.7%) with low‐grade dysplasia. No information on the presence of cirrhosis was available. Only 25% (n = 31) of CCA patients were asymptomatic at CCA diagnosis (Table 3).
TABLE 3.
Treatment, surveillance and follow‐up among patients who developed hepatobiliary cancer or dysplasia
| Variable | Cholangio‐carcinoma (N = 122) | High‐grade dysplasia (N = 8) | Low‐grade dysplasia (N = 51) | Gallbladder cancer (N = 21) | Hepatocellular carcinoma (N = 32) |
|---|---|---|---|---|---|
| Frequency of cancer/dysplasia | 4.1% (122/2978) | 0.3% (8/2978) | 1.7% (51/2978) | 0.7% (21/2978) | 1.1% (32/2978) |
| Symptoms a at diagnosis of CCA/dysplasia | |||||
| Yes | 84 (68.9%) | 6 (75.0%) | 2 (3.9%) | NA | NA |
| No | 31 (25.4%) | 1 (12.5%) | 2 (3.9%) | ||
| Unknown/Missing | 7 (5.7%) | 1 (12.5%) | 47 (92.2%) | ||
| Frequency of cancer/dysplasia per type of surveillance | |||||
| None | 7.7% (27/350) | 0% (0/350) | 0% (0/350) | 0.6% (2/350) | 2.9% (10/350) |
| MRI and/or US | 3.3% (63/1900) | 0.4% (8/1900) | 0.8% (16/1900) | 0.8% (15/1900) | 0.8% (16/1900) |
| Including ERCP | 4.4% (32/728) | 0% (0/728) | 4.8% (35/728) | 0.5% (4/728) | 0.8% (6/728) |
| First treatment for CCA/dysplasia | |||||
| LTx | 31 (25.4%) | 3 (37.5%) | 2 (3.9%) | NA | NA |
| Resection | 35 (28.7%) | 2 (25.0%) | 1 (2.0%) | ||
| Chemotherapy | 23 (18.9%) | 0 | 0 | ||
| LTx and neoadjuvant brachy‐chemotherapy | 1 (0.8%) | 0 | 0 | ||
| Other | 5 (4.1%) | 1 (12.5%) | 1 (2.0%) | ||
| Best supportive care | 17 (13.9%) | 0 | 0 | ||
| Unknown/missing | 10 (8.2%) | 2 (25.0%) | 47 (92.2%) | ||
| Follow‐up b (years) | |||||
| Mean (SD) | 1.5 (2.5) | 2.9 (2.9) | 4.1 (3.4) | 2.8 (2.7) | 3.1 (3.4) |
| Median (IQR) | 0.6 (0.2–1.7) | 1.5 (1.1–6.6) | 4.3 (0.6–6.7) | 2.4 (0.9–3.6) | 1.4 (0.5–5.7) |
| Range, min‐max | 0.0–17.9 | 0.2–7.4 | 0.0–10.9 | 0.0–11.4 | 0.0–13.5 |
| Categories, n (%) | |||||
| <1y | 76 (62.3%) | 1 (12.5%) | 13 (25.5%) | 7 (33.3%) | 12 (37.5%) |
| 1–<5y | 33 (27.0%) | 4 (50.0%) | 17 (33.3%) | 9 (42.9%) | 9 (28.1%) |
| 5–<10y | 10 (8.2%) | 2 (25.0%) | 19 (37.3%) | 1 (4.8%) | 7 (21.9%) |
| 10–<20y | 2 (1.6%) | 0 | 2 (3.9%) | 1 (4.8%) | 1 (3.1%) |
| Missing cancer date | 1 (0.8%) | 1 (12.5%) | 0 | 3 (14.3%) | 3 (9.4%) |
Abbreviations: CCA, cholangiocarcinoma; ERCP, endoscopic retrograde cholangiopancreatography; IQR, interquartile range; MRI, Magnetic resonance imaging; NA, Not available; SD, standard deviation; US, ultrasound.
Weight loss, recurrent cholangitis, jaundice, pruritus, or other suspicious PSC/cancer‐related symptom.
From first cancer diagnosis to death or last of follow‐up alive.
3.5. Scheduled imaging was associated with improved survival after cancer diagnosis
More than half (66/122, 54%) of patients with CCA were treated with liver transplantation or surgical resection. One CCA patient was treated with liver transplantation and neoadjuvant brachy‐chemotherapy. Survival after diagnosis of CCA or high‐grade biliary dysplasia was better in patients with scheduled imaging and/or ERCP than in patients with clinical follow‐up, but there was no difference between follow‐up with and without ERCP (Figure 3A,B). Survival was significantly better in patients treated with liver transplantation or surgical resection with some patients having a long‐term survival (Figure 3C; Table 3).
FIGURE 3.
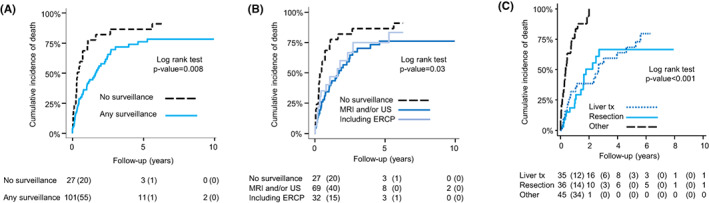
(A) Kaplan–Meier curves displaying the cumulative incidence of death for scheduled imaging versus clinical follow‐up in patients with cholangiocarcinoma or high‐grade dysplasia. (B) Kaplan–Meier curves displaying the cumulative incidence of death by type of follow‐up in patients with cholangiocarcinoma or high‐grade dysplasia. (C) Kaplan–Meier curves displaying the cumulative incidence of death from first cancer diagnosis in patients with cholangiocarcinoma or high‐grade dysplasia by type of first treatment
4. DISCUSSION
In this large multi‐centre study of 2975 PSC patients with the aim to describe and compare different follow‐up strategies and their impact on survival, we found that (1): there are a wide variety of surveillance or follow‐up strategies used globally, (2) scheduled imaging was associated with improved overall survival and (3) patients who eventually developed CCA were more often asymptomatic at time of cancer diagnosis and had better survival if scheduled imaging had been performed.
The strengths of this study are the large cohort size, international representation with varying clinical practices and long follow‐up time, enabling the comparison of different follow‐up strategies. Strategies varied considerably between centres both in frequency and choice of imaging modality. Therefore, we analysed the type of follow‐up in three major groups: (i) clinical follow‐up with imaging performed on demand, (ii) scheduled MRI/MRCP/US and (iii) scheduled imaging and ERCP on a scheduled basis. The risk for death was significantly reduced, when scheduled imaging with and without scheduled ERCP was performed and even lower for strategies including ERCP.
Multiple factors are likely to contribute to the survival benefit of scheduled imaging in this study. Selection bias with different populations from the centres may have influenced the results and therefore, these data should be interpreted with caution. However, the link between scheduled imaging and improved overall survival is an important finding as survival is the most important outcome for the patient. The risk for CCA after the first year of PSC diagnosis is much lower than death or need of liver transplantation caused by complications to benign biliary strictures and cirrhosis. 1 Follow‐up strategies should therefore not only focus on strictures suspicious for malignancy and early tumour detection, but also on the identification of significant strictures amenable to endoscopic treatment. Early endoscopic treatment of high‐grade biliary strictures without clinical symptoms or signs, may salvage liver parenchyma and prolong time to liver failure and increase survival. In this study, the majority of the CCA patients (69%) had symptomatic disease, which in clinical practice often leads to imaging and/or ERCP anyway. When mortality risk estimates were adjusted for CCA, the survival benefit from regular imaging remained. Only a minor proportion (4.1%) of the patients developed CCA during follow‐up and only 26% of the deaths were associated with CCA. There was no difference in survival after CCA diagnosis between scheduled imaging with or without ERCP. Altogether this indicates that a high proportion of the survival benefit from scheduled imaging is attributed to early detection of important and manageable benign strictures and not mainly to early tumour detection.
The invasiveness and risk for complications have in some centres led to a cautious use of ERCP. Indeed, PSC is a risk factor for ERCP‐associated complications and such procedures should only be performed by experienced endoscopists, 6 but there is increasing evidence for a more liberal use, including our data. In a retrospective study, regular ERCP with dilatation was shown to be beneficial for PSC patients with a dominant stenosis regardless of the presence of symptoms 7 and further studies with the specific aim to evaluate the role of ERCP with dilatation for long‐term prognosis in PSC are required. We found no survival benefit after diagnosis of CCA or high‐grade dysplasia in the group where regular ERCP was performed in addition to regular imaging speaking against its efficacy for early tumour detection.
There are many reviews and recommendations published supporting regular surveillance programs for early tumour detection but the evidence for their efficacy is scarce. 8 , 17 The current evidence supporting tumour surveillance mainly comes from two previous studies. One retrospective study of 79 patients with PSC and hepatobiliary cancer (54 with CCA), shows that regular surveillance before hepatobiliary cancer diagnosis is associated with a better prognosis with improved tumour recurrence‐free survival. 10 This study is affected by selection bias as the participation in a surveillance program was based on the choice of patients and their insurance status. In a register‐based study of PSC‐IBD patients, annual imaging was associated with a twofold risk reduction of hepatobiliary cancer‐related death. 11 Both studies are in keeping with our data where PSC patients who underwent regular imaging were more often asymptomatic at CCA diagnosis and had improved survival. It can not be ruled out that lead‐time bias in all these studies, including ours, is a major explanation for the survival benefit after cancer diagnosis in PSC patients undergoing regular imaging.
The broad variety of follow‐up or surveillance strategies used across centres, illustrates the need for more studies in this field. MRI has been shown to superior to US 12 and the use of liver‐specific contrast is always recommended for patients where CCA is suspected. 18 Despite the shortcomings of CA 19‐9 to identify early tumours and being non‐specific, 19 , 20 this marker is used regularly across centres. Because of heterogeneous testing and many missing variables, it was not possible to evaluate CA 19‐9 with sufficient quality in this study. However, many previous studies have shown its limited value for screening purposes and the regular use of CA 19‐9 is not recommended in recently published guidelines. 8 , 9 Early detection of hepatobiliary cancer by surveillance in PSC is also limited by the imperfect diagnostic means available to confirm cancer diagnosis in suspicious cases. To improve the diagnostic accuracy of brush samples, DNA aberrations measured by FISH (Fluorescence in situ Hybridization) is widely used 21 and next‐generation sequencing markers are suggested to add diagnostic value but has not yet reached clinical practice. 22 To improve cost‐effectiveness of surveillance, improved strategies are needed where a subset of patients at certain high‐risk of CCA (recent PSC diagnosis, high‐grade strictures, old age) are identified. In the high‐risk population, a closer follow‐up and additional diagnostic techniques such as targeted biopsies, endoscopic ultrasound, probe‐based confocal laser endomicroscopy are used, may be motivated. 23 , 24 , 25
As per all large multicentre retrospective studies, collection of data was challenging, and missing data were frequent which precluded detailed analysis of some important aspects. To decide whether imaging or ERCP was performed on clinical indication or just for the purpose of surveillance was impossible in the retrospective setting which may lead to misclassification bias. Also, some confounders have likely influenced our results. First, different centres represent different PSC populations and both tertiary referral transplant centres and population‐based cohorts are studied here. We were only able to include one centre not performing scheduled imaging which is an obvious weakness and adjustment for centre was therefore not possible. The well‐known fact that survival reported in studies from tertiary centres is shorter than from population‐based studies 1 , 26 might also have influenced our results. However, 63% of the centres are referral transplant centres including both the centres using additionally scheduled ERCP at follow‐up. Different indications for liver transplantation in the centres may also have influenced the results. We excluded the first year after PSC diagnosis or the first year after the start of surveillance to reduce the risk of coexisting undiagnosed CCA at PSC diagnosis or start of follow‐up to reduce referral bias. Like in previous published studies lead‐time bias may also be a concern.
In conclusion, we demonstrate that surveillance strategies vary considerably across centres, and that scheduled imaging is associated with improved survival in patients with PSC. Multiple factors are likely to contribute to the survival benefit of scheduled imaging including early tumour detection and more active endoscopic treatment of asymptomatic benign biliary strictures. Further data and prospective studies are required to determine optimal follow‐up and surveillance strategies, imaging modalities and scheduling of imaging to help improve outcomes for individuals with PSC.
CONFLICT OF INTEREST
Annika Bergquist received research funding from Gilead Sciences.
ACKNOWLEDGEMENTS
This study was supported by the European Reference Network for Hepatological Diseases, Swedish Hepatology Research Network (SWEHEP), Fondazione Epatocentro Ticino, Lugano, Switzerland.
Bergquist A, Weismüller TJ, Levy C, et al. Impact on follow‐up strategies in patients with primary sclerosing cholangitis. Liver Int. 2023;43:127‐138. doi: 10.1111/liv.15286
Funding informationThe Swedish Cancer Society, Stockholm County Council and the Cancer Research Funds of Radiumhemmet supported this work.
Handling Editor: Luca Valenti
REFERENCES
- 1. Boonstra K, Weersma RK, van Erpecum KJ, et al. Population‐based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58(6):2045‐2055. [DOI] [PubMed] [Google Scholar]
- 2. Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36(3):321‐327. [DOI] [PubMed] [Google Scholar]
- 3. Weismuller TJ, Trivedi PJ, Bergquist A, et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology. 2017;152(8):1975‐84 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC. Precancerous bile duct pathology in end‐stage primary sclerosing cholangitis, with and without cholangiocarcinoma. Am J Surg Pathol. 2010;34(1):27‐34. [DOI] [PubMed] [Google Scholar]
- 5. Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48(4):1106‐1117. [DOI] [PubMed] [Google Scholar]
- 6. von Seth E, Arnelo U, Enochsson L, Bergquist A. Primary sclerosing cholangitis increases the risk for pancreatitis after endoscopic retrograde cholangiopancreatography. Liver Int. 2015;35(1):254‐262. [DOI] [PubMed] [Google Scholar]
- 7. Rupp C, Hippchen T, Bruckner T, et al. Effect of scheduled endoscopic dilatation of dominant strictures on outcome in patients with primary sclerosing cholangitis. Gut. 2019;68(12):2170‐2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowlus CL, Lim JK, Lindor KD. AGA clinical practice update on surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis: expert review. Clin Gastroenterol Hepatol. 2019;17(12):2416‐2422. [DOI] [PubMed] [Google Scholar]
- 9. Chapman MH, Thorburn D, Hirschfield GM, et al. British Society of Gastroenterology and UK‐PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut. 2019;68(8):1356‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali AH, Tabibian JH, Nasser‐Ghodsi N, et al. Surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis. Hepatology. 2018;67(6):2338‐2351. [DOI] [PubMed] [Google Scholar]
- 11. Trivedi PJ, Crothers H, Mytton J, et al. Effects of primary sclerosing cholangitis on risks of cancer and death in people with inflammatory bowel disease, based on sex, race, and age. Gastroenterology. 2020;159(3):915‐928. [DOI] [PubMed] [Google Scholar]
- 12. Eaton JE, Welle CL, Bakhshi Z, et al. Early cholangiocarcinoma detection with magnetic resonance imaging versus ultrasound in primary sclerosing cholangitis. Hepatology. 2020;73:1868‐1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyd S, Mustonen H, Tenca A, Jokelainen K, Arola J, Farkkila MA. Surveillance of primary sclerosing cholangitis with ERC and brush cytology: risk factors for cholangiocarcinoma. Scand J Gastroenterol. 2017;52(2):242‐249. [DOI] [PubMed] [Google Scholar]
- 14. European Association for the Study of the Liver . EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51(2):237‐267. [DOI] [PubMed] [Google Scholar]
- 15. Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660‐678. [DOI] [PubMed] [Google Scholar]
- 16. Aabakken L, Karlsen TH, Albert J, et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the liver (EASL) clinical guideline. Endoscopy. 2017;49(6):588‐608. [DOI] [PubMed] [Google Scholar]
- 17. Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54(5):1842‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schramm C, Eaton J, Ringe KI, Venkatesh S, Yamamura J, IPSCSG MRIwgot . Recommendations on the use of magnetic resonance imaging in PSC‐A position statement from the international PSC study group. Hepatology. 2017;66(5):1675‐1688. [DOI] [PubMed] [Google Scholar]
- 19. Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19‐9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50(9):1734‐1740. [DOI] [PubMed] [Google Scholar]
- 20. Wannhoff A, Rupp C, Friedrich K, et al. Inflammation but not biliary obstruction is associated with carbohydrate antigen 19‐9 levels in patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2015;13(13):2372‐2379. [DOI] [PubMed] [Google Scholar]
- 21. von Seth E, Ouchterlony H, Dobra K, et al. Diagnostic performance of a stepwise cytological algorithm for biliary malignancy in primary sclerosing cholangitis. Liver Int. 2018;39:382‐388. [DOI] [PubMed] [Google Scholar]
- 22. Singhi AD, Nikiforova MN, Chennat J, et al. Integrating next‐generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)‐obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut. 2020;69(1):52‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han S, Kahaleh M, Sharaiah RZ, et al. Probe‐based confocal laser endomicroscopy in the evaluation of dominant strictures in patients with primary sclerosing cholangitis: results of a U.S. multicenter prospective trial. Gastrointest Endosc. 2021;94:569‐576.e1. [DOI] [PubMed] [Google Scholar]
- 24. Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta‐analysis. Gastrointest Endosc. 2015;81(1):168‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen NQ, Schoeman MN, Ruszkiewicz A. Clinical utility of EUS before cholangioscopy in the evaluation of difficult biliary strictures. Gastrointest Endosc. 2013;78(6):868‐874. [DOI] [PubMed] [Google Scholar]
- 26. Barner‐Rasmussen N, Pukkala E, Jussila A, Farkkila M. Epidemiology, risk of malignancy and patient survival in primary sclerosing cholangitis: a population‐based study in Finland. Scand J Gastroenterol. 2020;55(1):74‐81. [DOI] [PubMed] [Google Scholar]


