Abstract
Background
The rectosigmoid brake, characterised by retrograde cyclic motor patterns on high‐resolution colonic manometry, has been postulated as a contributor to the maintenance of bowel continence. Sacral neuromodulation (SNM) is an effective therapy for faecal incontinence, but its mechanism of action is unclear. This study aims to investigate the colonic motility patterns in the distal colon of patients with faecal incontinence, and how these are modulated by SNM.
Methods
A high‐resolution fibreoptic colonic manometry catheter, containing 36 sensors spaced at 1‐cm intervals, was positioned in patients with faecal incontinence undergoing stage 1 SNM. One hour of pre‐ and post meal recordings were obtained followed by pre‐ and post meal recordings with suprasensory SNM. A 700‐kcal meal was given. Data were analysed to identify propagating contractions.
Results
Fifteen patients with faecal incontinence were analysed. Patients had an abnormal meal response (fewer retrograde propagating contractions compared to controls; p = 0.027) and failed to show a post meal increase in propagating contractions (mean 17 ± 6/h premeal vs. 22 ± 9/h post meal, p = 0.438). Compared to baseline, SNM significantly increased the number of retrograde propagating contractions in the distal colon (8 ± 3/h premeal vs. 14 ± 3/h premeal with SNM, p = 0.028). Consuming a meal did not further increase the number of propagating contractions beyond the baseline upregulating effect of SNM.
Conclusion
The rectosigmoid brake was suppressed in this cohort of patients with faecal incontinence. SNM may exert a therapeutic effect by modulating this rectosigmoid brake.
Keywords: faecal incontinence, implant, rectosigmoid brake, sacral nerve stimulation, sacral neuromodulation
What does this paper add to the literature?
Patients with faecal incontinence had an attenuated rectosigmoid brake, characterised by fewer postprandial retrograde propagating contractions in the distal colon, however, the rectosigmoid brake function was improved by sacral neuromodulation.
INTRODUCTION
Faecal incontinence (FI) affects between 5%–15% of people globally [1]. It is associated with significant social embarrassment, psychological distress and economic burden [2, 3]. While causes of faecal incontinence are often multifactorial [4, 5, 6], research has predominantly focused on anorectal physiology with less emphasis on the role of colonic motility.
Sacral neuromodulation (SNM) is an effective treatment for faecal incontinence refractory to medical management [7], with sustained long‐term benefits [8]. SNM entails surgical placement of stimulating electrodes adjacent to the sacral nerve root, typically S3 [9]. Despite its clinical success, the mechanism of action has not been clearly elucidated [10, 11]. A limited understanding of the mechanism of action of SNM has meant no biomarkers exist for monitoring the treatment response to SNM which hinders improvement of the therapy and appropriate patient selection.
Sacral neuromodulation has been hypothesised to modulate afferent, central, autonomic, and somatic neural pathways [11, 12]. Chronic SNM may act through somatic afferents to reduce inhibition of sphincter function via ascending central pathways [11, 13]. Locally, external sphincter hypertrophy secondary to stimulation have also been implicated [11], however, there is little evidence that SNM affects sphincter activation, anal squeeze pressures, anal reflexes, or internal sphincter slow wave amplitudes [11, 14, 15]. Moreover, many patients benefit from SNM despite large sphincter defects [16, 17], suggesting factors other than the sphincter complex are implicated. Proximal factors such as colonic motility, which is modulated by the sacral nerves, may therefore also contribute to the pathophysiology of faecal incontinence [12, 18, 19]. By stimulating the parasympathetic innervation of the distal colon, SNM may modulate the rectosigmoid brake, a predominantly retrograde, cyclic motor pattern thought to limit rectal filling and contribute to the maintenance of continence [12, 20], a concept first postulated and demonstrated by Patton et al. [19].
In this study, we evaluated the motility of the distal colon in patients with faecal incontinence, and defined how it altered with SNM, using high‐resolution colonic manometry (HRCM).
METHODS
Ethical approval was obtained from the New Zealand Health and Disability Ethics Committee (ref: 15/NTA/175). All participants provided informed written consent. The study was registered in the Australian New Zealand Clinical Trials Registry (ACTRN12615001137583) and reported in line with the STROBE statement [21].
Eligibility criteria for sacral neuromodulation
Adult patients aged >18 years old who were referred for SNM for FI were considered. Patients were eligible for SNM if they had failed medical management for faecal incontinence and had experienced at least two episodes of faecal incontinence per week for a minimum of 12 months. This was confirmed using a daily bowel diary. All patients underwent a thorough assessment through the pelvic floor clinic at Auckland City Hospital. Baseline incontinence data assessed via Faecal Incontinence Severity Index (FISI) and modified Faecal Incontinence Quality of Life (FIQoL) scores were used to assess clinical response to SNM at Auckland City Hospital [22, 23]. The decision to perform SNM on each patient was determined by a multidisciplinary pelvic floor team. All included patients provided informed consent.
Patients that were pregnant, suffering severe metabolic, neurological, or endocrine disorders known to cause colonic dysmotility, previous colon or rectal resection, and/or major lumbosacral injury or malformations were excluded.
Healthy controls
Control data were amalgamated from a historical cohort [24], and an additional two healthy control participants were also recruited. These data were included to compare the meal response between patients with faecal incontinence and healthy controls. These participants received oral mechanical bowel preparation and underwent 2 h of pre‐ and postprandial HR colonic manometry recordings, with a 700‐kcal meal. A fibreoptic manometry catheter with 72 sensors at 1‐cm interval was used in these patients, although for consistency in comparisons with the preoperative cohort during analysis, only motor events from the most distal 36 sensors (i.e., those located in the descending colon, sigmoid colon, and rectum) were evaluated.
Interventions
The recordings were taken at the time of the first stage of SNM lead insertion. Colonic manometry recordings were taken during the in‐patient stay for the first‐stage procedure. More extensive details about the sacral nerve stimulator implant procedure can be found in the Methods section in Appendix S1. All patients with faecal incontinence were fasted from midnight on the day of the procedure. All patients received a 1 g oral paracetamol and a standardised institutional perioperative analgesia protocol. The choice of performing SNM under sedation or general anaesthesia was left to surgeon preference. Where under sedation, midazolam, remifentanil infusion and/or propofol infusion was used with medications titrated to effect; where general anaesthesia was used, fentanyl and midazolam were given as premedication. The choice of induction agent and neuromuscular blockade was left to the discretion of the anaesthetist. The neuromuscular blockade is typically a short‐acting agent, such as rocuronium (clinical duration of 33 min [25]) to allow for observation of motor responses from test nerve stimulation. Postoperative pain management was standardised and consisted of paracetamol and tramadol or Sevredol. Patients could refuse post procedural analgesia if they were not needed.
Sacral neuromodulation
Sacral neuromodulation is typically performed in two‐stages. The first stage is a temporary evaluation phase, where in this study cohort all patients had a definitive quadripolar tined lead connected to a temporary stimulator placed surgically. If clinical success was achieved (typically >50% improvement in symptoms per a bowel symptom diary at the end of a 1‐month temporary SNM period), patients would then move onto the second stage, wherein a permanent stimulator is placed.
High‐resolution colonic manometry
A fibreoptic HR manometry catheter with 36 sensors at 1‐cm intervals was used to measure distal colonic motor activity [26]. All faecal incontinence patients received one or two Fleet enemas (Fleet Laboratories) before surgery to allow for the manometry catheter. Manometry catheter placement was performed at the end of the SNM first‐stage procedure. A nylon loop was tied to the tip of the manometry catheter. A Resolution clip (Boston Scientific) was inserted into the working channel of a flexible endoscope to grasp the nylon loop and guide the placement of the manometry catheter per anus. The HR manometry catheter was advanced to a point where the last sensor was no longer visible at the anal verge. Once in position, one or two Resolution clips were used to secure the catheter via the nylon loop to the colonic mucosa. A piece of tape was also used to secure the catheter to the buttock. During the recordings, the catheter was connected to a spectral interrogator acquisition unit (FBG‐scan 804; FOS & S). A purpose‐written LabVIEW program (National Instruments) was used to record data.
Manometry study protocol
The manometry recording commenced once patients were fully awake. Abdominal x‐rays were taken approximately 4 h after the surgery to confirm the position of the manometry catheter. First, 2 h of basal recording with no stimulation were performed. After the basal recording, the implanted sacral nerve stimulator lead was connected to a temporary external stimulator, and a further 2 h of recording was undertaken using the standard therapeutic setting (suprasensory level of stimulation), as determined by the colorectal clinical nurse specialist. The setting was based on a default setting supplied by Medtronic (14 Hz and pulse width of 210 μs). The amplitude was slowly increased in 0.1 V increments until the patient perceived sensory stimulation in the perineum. The final amplitude was set at a level where the patient was aware of the stimulation but remained comfortable. After 2 h of suprasensory stimulation, patients were given a standardised 700 kcal meal, consisting of a chicken sandwich and a Nepro HP drink (Abbott Nutrition). While still receiving SNM, patients underwent a further 2 h of recording, after which the HR manometry catheter was disconnected, and the temporary external stimulator was turned off.
Hospital service requirements allowed a subgroup to undergo an extended protocol which involved providing the same 700 kcal meal after surgery, but prior to activating SNM so the baseline meal response pattern of colonic motility could be measured [27]. Only a subset of patients received this extended protocol (n = 6). Data acquisition methods for the controls have previously been published [24].
Manometric analysis
Manometric data analysis was performed using a custom‐designed software package (PlotHRM; Flinders University). One hour of data from either side of the meal and/or stimulation was extracted for analysis. This was due to unforeseen circumstances such as patients mobilising and catheter migration that meant recordings beyond 1 h were heterogeneous. Data for the healthy controls were truncated to equal length to allow for a direct comparison. Event detection and pattern recognition were based on previously described methods and definitions. Propagating contractions were defined as spatiotemporal motor patterns with pressure peaks that occur in four or more adjacent channels (i.e., ≥3 cm) and had a trough‐to‐peak amplitude of ≥5 mmHg. All propagating contractions were analysed with an additional subgroup analysis of the cyclic motor pattern (CMP). Cyclic motor pattern is defined as repetitive propagating contractions with a frequency between 2 and 8 cycles per min (cpm) for a duration of 3 min. This is the predominant motor pattern thought to underlie the rectosigmoid brake [12, 20, 24]. Event counts were averaged across multiple subjects by interpolating the data between sigmoid flexure and rectosigmoid junction of each subject to the centre line of a three‐dimensional colonic model generated using data from the Visible Human Project (US National Library of Medicine, Bethesda, Maryland, USA), then projected to the surface of the model as colour maps, as previously described [28, 29].
Sample size
Few previous studies have evaluated differences in HR colonic manometry profiles between patients with faecal incontinence and controls. We therefore powered the study based on successful precedent physiological HR colonic manometry studies in comparable patient populations [20, 27, 28, 30, 31, 32].
Statistical analysis
Data are presented as mean (standard error). Nonparametric paired Wilcoxon signed‐rank tests were used to compare pre‐ and postprandial, and pre‐ and post stimulation propagating contractions as appropriate. All statistical testing was conducted in R (R Foundation for Statistical Computing, Austria 2014) with p < 0.05 considered statistically significant.
RESULTS
Overall, 15 patients with faecal incontinence undergoing stage 1 SNM (median age 61, range 45–81; 13 female), of which six had postprandial recordings without SNM. Eleven healthy control participants were recruited (median age 52, range 30–69 years; 5 female).
The patient group consisted of five patients with urge incontinence, five patients with passive incontinence, and five patients with mixed incontinence based on pelvic floor assessments. The mean FISI score was 41.3 (range 25–61), and the mean FIQoL score was 70.9 (range 20–95). Further clinical details of patients with faecal incontinence undergoing SNM are outlined in Tables 1 and S1.
TABLE 1.
Anorectal physiology results
| Examination | Result |
|---|---|
| Endoanal ultrasound a | |
| Internal sphincter | |
| Intact | 5 |
| Defect | 6 |
| External sphincter | |
| Intact | 10 |
| Defect | 1 |
| Anorectal manometry, mmHg b | |
| Resting | 34.2 ± 19.8 |
| Squeeze | 60.6 ± 35.2 |
| Cough | 55.9 ± 36.8 |
Endoanal ultrasound results were not available for four patients.
Anal manometry results were not available for two patients.
Four patients were taking loperamide prior to the operation. No patients received antidiarrhoeal agents, including loperamide or codeine, on the day of surgery for the duration of hospitalisation. On the day of surgery, 11 patients did not receive narcotics postoperatively and four patients required more than three doses of either tramadol or Sevredol.
Ten patients underwent SNM placement under sedation while five received general anaesthesia. SNM was placed in S3 on all patients, 12 on the left side and three on the right side.
Differences in colonic motility between patients with faecal incontinence and controls
Patients with faecal incontinence had an impaired meal response with respect to the number of total (mean 17 ± 6/h premeal vs. 22 ± 9/h post‐meal, p = 0.438), antegrade (10 ± 3/h premeal vs 5 ± 2/h post meal, p = 0.916) and retrograde (8 ± 3/h premeal vs. 17 ± 9/h post‐meal, p = 0.281) propagating contractions (Figures 1, 2, and 3). As previously documented, healthy controls showed significant increases in the number of total (21 ± 14/h premeal vs. 70 ± 12/h post meal, p < 0.001), antegrade (8 ± 6/h premeal vs. 18 ± 6/h post meal, p = 0.014), and retrograde (13 ± 7/h premeal vs. 52 ± 8/h post meal, p < 0.001) propagating contractions (Figures 1, 2 and 3). Particularly, the magnitude of the delta in retrograde propagating contractions was significantly greater in controls compared to faecal incontinence patients (delta in mean retrograde contractions: 39 vs. 9, p = 0.027; Figures 2 and 3).
FIGURE 1.
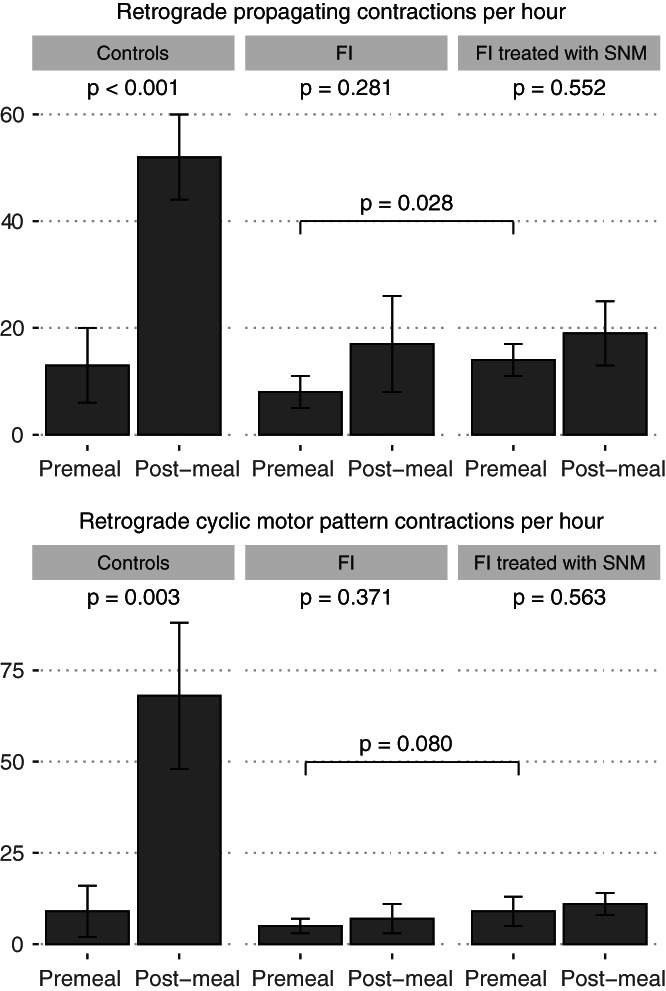
All (top) and cyclic motor pattern‐associated (bottom) retrograde propagating contractions in healthy controls, patients with faecal incontinence both before and after sacral neuromodulation. Plotting mean ± SE as per data reported in‐text.
FIGURE 2.
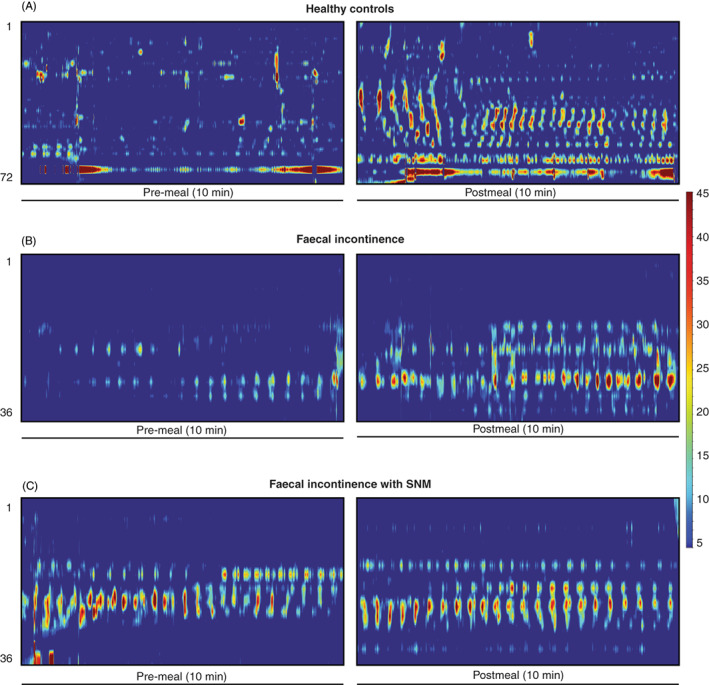
Representative examples of pre‐ and post meal high resolution colonic manometry data in 10‐min epochs. (A) Significant increase in postprandial propagating contraction frequencies in healthy controls. (B) Decreased magnitude of colonic activity meal‐response with shorter distance of propagation and decreased activity in faecal incontinence patients compared to controls. (C) Increase in propagating contractions at baseline and postprandially with SNM in patients with faecal incontinence. SNM appears to increase frequency of propagating events but not to the level seen in the healthy control meal response. Despite variation in catheter used, only data distal to the splenic flexure were analysed in all cohorts.
FIGURE 3.
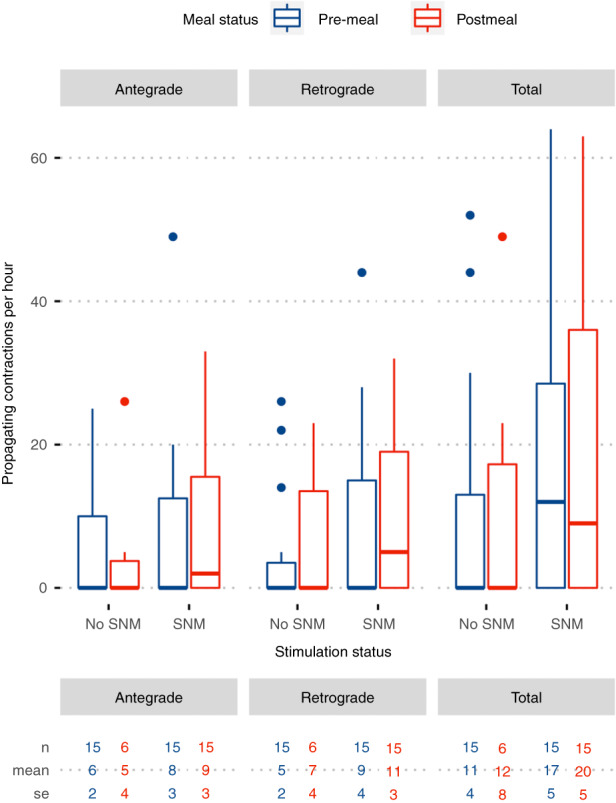
Effect of stimulation on the cyclic motor pattern in patients with faecal incontinence stratified by meal and stimulation status. Plot depicts median (IQR) to visualise the range in the raw data; paired nonparametric Wilcoxon test between pre‐SNM and full‐SNM comparisons: Total p = 0.041, antegrade p = 0.264, retrograde p = 0.011. Mean and standard error are reported in the table below to compare to data within the text.
Effect of SNM on patients with faecal incontinence
Sacral neuromodulation increased the number of propagating contractions compared to patients' baselines. Among fasted patients with faecal incontinence, introduction of SNM increased the number of total (17 ± 6/h premeal vs. 25 ± 5/h pre‐meal with SNM, p = 0.043), retrograde (8 ± 3/h premeal vs. 14 ± 3/h premeal with SNM, p = 0.028) propagating contractions (Figures 1, 2 and 3), but not antegrade propagating contractions (10 ± 3/h premeal vs. 11 ± 4/h premeal with SNM, p = 0.527). Hence, SNM was shown to partially restore the meal response that was deficient in patients with FI compared to healthy controls (Figures 2 and 3).
Sacral neuromodulation, however, did not fully restore the normal meal response. For example, in the fed state, there was no significant increase in total (22 ± 9/h post meal without SNM vs. 33 ± 8/h post meal with SNM, p = 0.156), antegrade (5 ± 2/h post meal without SNM vs. 14 ± 3/h post meal with SNM, p = 0.156) or retrograde (17 ± 9/h post meal without SNM vs. 19 ± 6/h post meal with SNM, p = 0.313) propagating contractions with SNM (Figures 2 and 3). Therefore, SNM increased the baseline frequency of propagating contractions but not significantly more so in the fed state. Hence, if patients consumed a meal while stimulation was activated there was no significant further effect in the number of propagating contractions pre‐ and post meal (p > 0.05; Table S2).
Cyclic motor pattern in faecal incontinence and health
Similar to the primary analysis of all propagating contractions, patients with faecal incontinence showed an abnormal at of the meal‐response with respect to propagating contractions associated with the cyclic motor pattern. There was no increase in the number of total (11 ± 4/h premeal vs. 12 ± 8/h post meal, p = 0.423), antegrade (6 ± 2/h premeal vs. 5 ± 4/h post meal, p = 1.00), or retrograde (5 ± 2/h premeal vs. 7 ± 4/h post meal, p = 0.371) propagating contractions associated with the cyclic motor pattern (Figure 1). Healthy controls in contrast had significant increases in the number of total (16 ± 13/h premeal vs. 87 ± 30/h post meal, p = 0.010), and retrograde (9 ± 7/h premeal vs. 68 ± 20/h post meal, p = 0.003) propagating contractions (Figure 1), but not antegrade (7 ± 6/h premeal vs. 19 ± 11/h post meal, p = 0.361) propagating contractions.
Effect of SNM on the cyclic motor pattern in patients with faecal incontinence
The effect of SNM on the number of propagating contractions associated with the cyclic motor pattern compared to baseline in patients with faecal incontinence did not reach significance. Among fasted patients with faecal incontinence, introduction of SNM resulted in a statistically insignificant increase in the number of total (11 ± 4/h premeal vs. 17 ± 5/h premeal with SNM, p = 0.107), antegrade (6 ± 2/h premeal vs. 8 ± 3/h premeal with SNM, p = 0.932), and retrograde (5 ± 2/h premeal vs. 9 ± 4/h premeal with SNM, p = 0.080) propagating contractions associated with the cyclic motor pattern (Figure 1). However, when comparing the effect of SNM irrespective of meal status (i.e., full SNM compared to pre‐SNM while amalgamating pre‐ and post meal periods), significant increases in total and retrograde propagating contractions associated with the cyclic motor pattern were seen (p < 0.05; Figure 4).
FIGURE 4.
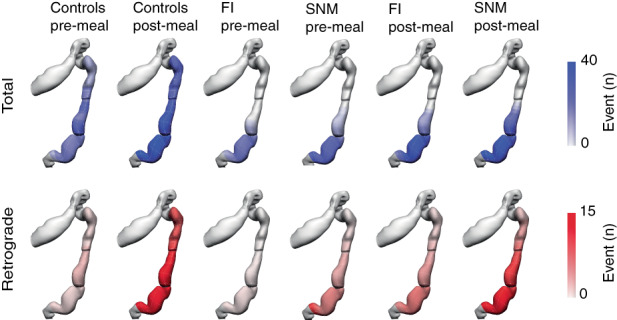
Anatomical registration of the event count distribution into a colonic geometry model, based on the estimated catheter insertion position. The colours represent the mean number of propagating events per hour. Propagating contractions were most active in the sigmoid colon. Total propagating contractions are depicted in blue and retrograde propagating contractions are depicted in red. FI, faecal incontinence; SNM, sacral neuromodulation.
Sacral neuromodulation, however, did not fully restore the normal meal response. For example, in the fed state, there was no significant increase in total (12 ± 8/h post meal without SNM vs. 20 ± 5/h post meal with SNM, p = 0.098), antegrade (5 ± 4/h post meal without SNM vs. 9 ± 3/h post meal with SNM, p = 0.098) or retrograde (7 ± 4/h post meal without SNM vs. 11 ± 3/h post meal with SNM, p = 0.098) propagating contractions with SNM. SNM therefore probably did not confer further enhancements to the rectosigmoid meal response when evaluating the cyclic motor pattern alone. However, only six patients had post meal recordings without SNM.
Clinical outcomes
Two patients did not progress to a permanent SNM implant, one due to new onset of severe constipation after stage 1 SNM, and another due to <50% improvement in incontinence symptoms. The former patient with constipation had increased frequency of propagating activity at baseline and the latter had infrequent propagating contractions at baseline which slightly increased with SNM (Figure S1). Another patient required a change in the SNM programme prior to permanent implant placement. Median follow‐up among 13 patients after second stage SNM was 47.0 (range 0.2–62.0) months. Eleven patients reported satisfaction with their bowel function whereas two patients reported deteriorating function, awaiting stimulator reprogramming.
DISCUSSION
Sacral neuromodulation is an effective treatment for faecal incontinence; however, the mechanism of action has remained uncertain, limiting therapeutic progress. This study suggests the rectosigmoid brake may have a role in the pathophysiology of faecal incontinence. Patients had fewer overall propagating contractions, and particularly retrograde propagating contractions, in response to a meal‐stimulus in comparison to healthy controls. Second, this study has shown that SNM significantly increases the total number of propagating contractions in the rectosigmoid region, particularly retrograde propagating contractions, demonstrating a likely mechanism through which SNM exerts its therapeutic benefit.
There is consistent and advancing evidence of the importance of the rectosigmoid brake in the maintenance of normal continence [12, 19, 20, 30]. A “functional sphincter” has long been recognised in this region, first attributed to O'Beirne [33]. Chen et al. [34] further characterised this “sphincter” as an intermittent pressure band lying 10–17 cm above the anal verge, which relaxes and contracts in concert with the anal sphincters in response to pressure sequences. Dinning et al. [24] applied HRCM to characterise a substantial postprandial increase in the retrograde cyclic motor pattern in the distal colon as a feature of a healthy meal response, and Lin et al. [20] subsequently localised this activity to be maximal in the same rectosigmoid region as the “functional sphincter”. Using another modality, high‐resolution impedance manometry has shown gas insufflation of the sigmoid colon initiates retrograde cyclic motor patterns to limit gas transit to the rectum [35]. These studies extended the earlier work of Rao and Welcher, who proposed periodic rectal motor activity served as an “intrinsic braking mechanism that prevents the untimely flow of contents” [36]. In addition, surgical resection of this region has recently been shown to contribute to a pathological absence of the meal response and symptoms of bowel dysfunction in patients with low anterior resection syndrome (LARS) [31]. Based on these several background studies, we hypothesised that attenuation of rectosigmoid brake activity could also be an important pathophysiological mechanism of faecal incontinence. This hypothesis was confirmed in a cohort of severe medically‐refractory incontinence patients in this study, as demonstrated by significantly reduced retrograde propagating activity in both fasting and fed states compared to controls.
Rectosigmoid motor activity has been shown to require neural innervation, as evidenced by its absence in spinal cord injury [37, 38], systemic sclerosis [39], and diabetes mellitus [40], defining a plausible pathway through which SNM may act. Our data demonstrate that SNM effectively upregulates this pathway to effect enhanced retrograde distal colonic motility in patients with faecal incontinence, thereby confirming and extending previous work by Patton et al. [19] who first applied HRCM to demonstrate this effect. Further corroborating evidence for this effect has been provided by Michelson et al. using colorectal scintigraphy, who demonstrated that SNM decreased antegrade transit and increased retrograde transit in the descending colon, thereby prolonging colonic transit time and increasing colonic storage capacity [41]. Together, these data now present a convincing body of evidence that modulation of colonic motility is a fundamental mechanism of action of SNM. Indeed, in light of the potent efficacy of SNM in many cases of anal sphincter incompetence [16, 17], it can be posited that modulation of colonic motility, probably through the parasympathetic pelvic splanchnic nerves, may be a primary mechanism of action of this therapy, working in conjunction with other coregulatory phenomena such as potentially cortical activation [11, 42, 43, 44].
The effect of a meal‐response in the context of SNM has not previously been explored with HRCM. In this study, we found that a meal did not further significantly increase the number of propagating contractions beyond its baseline upregulating effect in patients with faecal incontinence (Figure 2), when compared with controls. This is similar to the findings of Roger et al. [45] who also found no difference in the frequency of manometric waves after a meal in patients with urge faecal incontinence. Notably, the study by Roger et al. [46] employed low resolution pull‐through colonic manometry, a technique that may miss a significant proportion of propagating sequences. Our data suggest that SNM exerts its effect at the basal period as well prior to additional stimulation to the colon by a meal.
An expert consensus by Tack et al. [47]. recently proposed five criteria to qualify a putative pathophysiological mechanism in functional gastrointestinal disorders. Our findings of defective rectosigmoid brake activity in faecal incontinence can be usefully evaluated within this framework. Specifically, this study adds evidence to “Criterion 1”, which states that the pathophysiological disturbance is present in at least a subset of patients with the symptom, and the prevalence is higher than in appropriate controls. We also provide evidence for “Criterion 5”, which states that treatment, in this case through SNM, aimed at correcting the underlying disorder improves the symptom. This is evidenced by a partial restoration of the rectosigmoid brake with SNM, particularly in patients that benefited from SNM therapy. We did not investigate “Criterion 2”; whereby there should be a close temporal association between the pathophysiological disturbance and symptom occurrence, given that faecal incontinence is continuous. “Criterion 3” states that there should be a significant correlation between the presence/severity of the symptom and the presence/severity of the dysfunction, and this criterion could be the focus of future work in a larger cohort of patients with broader range of symptom severities.
Sacral neuromodulation has revolutionised the management of faecal incontinence. Over time, the threshold to offer SNM, which was once a last‐line treatment option for medically refractory patients, has been reduced [48]. However, despite its success, the lack of actionable biomarkers for the efficacy of SNM has limited progress in advancing the therapy, for example in the 10%–30% [8, 49] of nonresponders, and objective evaluation of stimulation protocols that could reduce energy consumption and prolong implant lifespan when optimised [50]. The lack of a biomarker has also inhibited the development of less‐invasive approaches, which would be applicable to a larger range of patients, such as tibial nerve stimulation [51]. While this study shows that the rectosigmoid brake may offer a key biomarker, its assessment with HRCM is notably invasive, expensive, and analytically complex, limiting its broader utility. However, novel, noninvasive approaches to measure distal colonic motility are currently emerging, notably high‐resolution electrocolonography [52].
As rectosigmoid brake is hypothesised to limit rectal filling, it is plausible that a hyperactive rectosigmoid brake may result in constipation in a subset of patients [53]. While, the role of cyclic motor patterns in constipation is incompletely understood [18], some studies have demonstrated that increased retrograde rectosigmoid motility and pressure may impair bowel motions [53, 54, 55]. Hyperactive retrograde rectosigmoid motility has also recently been shown to delay gut recovery after surgery [28], with bowel function not appearing to return until after rectosigmoid activity normalises [56]. Interestingly, the patient in our study who failed to progress to permanent SNM due to onset of marked constipation after stage one had the highest frequency of retrograde cyclic motor patterns at baseline which increased further after SNM (Figure 1). In essence, SNM may therefore have resulted in overtreatment in a patient who did not suffer from rectosigmoid brake hypoactivity. A highly active rectosigmoid brake could therefore be one approach to help predict patients unlikely to respond to SNM in order to aid patient selection. However, further data is now needed to validate this hypothesis‐generating observation from a single patient.
The present study has some limitations. Patients and controls received different bowel preparation, and patients also retained the manometric catheter in situ for longer periods, which could hypothetically lead to a confounding effect of rectal filling [57]. However, while bowel preparation alters the detection of HAPs and predefaecatory motility patterns, it is not considered to alter the overall frequency of propagating contractions and the interpretation of meal responses as was the primary focus in this study [58, 59]. There were a relatively small number of patients in this study, reflecting the invasiveness of the technique and inconvenience for participating patients. Nevertheless, the data were sufficient to demonstrate statistically‐robust effects for the primary hypothesis. The pathophysiology of incontinence is multifactorial, and this study included and analysed patients with both passive and urge incontinence together. Some patients (n = 7) had previous treatments for rectal prolapse. At the time of SNS implantation, no patients had clinically overlapping constipation symptoms and all had severe FI symptoms. Patients with both subtypes have shown improvements with SNM indicating there could be a common mechanism of action for SNM.
In conclusion, patients with faecal incontinence are shown to have an impaired rectosigmoid brake, and attenuated postprandial increase in retrograde propagating contractions. SNM upregulates the rectosigmoid brake as evidenced by increased retrograde motility, probably aiding the maintenance of bowel continence. SNM did not, however, augment the meal response. The rectosigmoid brake is probably an important contributor to faecal continence and may represent a key biomarker for the effect of SNM.
AUTHOR CONTRIBUTIONS
AL, CV, and GOG made substantial contributions to the conception and design of the work as well as acquisition, and interpretation of data; AL, CV, NP, SS, PD, IP, and GOG also contributed to drafting the work and critically revising it for important intellectual content, approve the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AL, CV, NP, PD, and SS were involved in data analysis. All authors had full access to all the data in the study.
FUNDING STATEMENT
John Mitchell Crouch Fellowship, Royal Australasian College of Surgeons; Health Research Council of New Zealand.
CONFLICT OF INTEREST
GOG is a shareholder and Director of Alimetry and The Insides Company and holds intellectual property in the field of gastrointestinal electrophysiology and therapeutics. IB is a shareholder in the Insides Company. NP and PD hold intellectual property in the field of gastric electrophysiology and is a shareholder in FlexiMap Ltd. No commercial financial support was received for this study. All remaining authors (AL, CV, NP, SS, PD) have no conflicts of interest to declare.
ETHICAL APPROVAL
Ethical approval was obtained from the New Zealand Health and Disability Ethics Committee (ref: 15/NTA/175).
Supporting information
Figure S1
Table S1‐S2
Appendix S1
ACKNOWLEDGEMENTS
The authors would like to thank the patients and volunteers who contributed to this study. Open access publishing facilitated by The University of Auckland, as part of the Wiley ‐ The University of Auckland agreement via the Council of Australian University Librarians. Open access publishing facilitated by The University of Auckland, as part of the Wiley ‐ The University of Auckland agreement via the Council of Australian University Librarians.
Lin AY, Varghese C, Paskaranandavadivel N, Seo S, Du P, Dinning P, et al. Faecal incontinence is associated with an impaired rectosigmoid brake and improved by sacral neuromodulation . Colorectal Dis. 2022;24:1556–1566. 10.1111/codi.16249
Preprint DOI: https://doi.org/10.1101/2021.11.30.21266844 (medRxiv).
Study registration: ACTRN12615001137583 (ANZCTR).
Previous communication: Presented at Royal Australasian College of Surgeons, November Annual Academic Surgery 2021.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sharma A, Yuan L, Marshall RJ, Merrie AEH, Bissett IP. Systematic review of the prevalence of faecal incontinence. Br J Surg. 2016;103:1589–97. [DOI] [PubMed] [Google Scholar]
- 2. Xu X, Menees SB, Zochowski MK, Fenner DE. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586–98. [DOI] [PubMed] [Google Scholar]
- 3. Nehra V, Bruce BK, Rath‐Harvey DM, Pemberton JH, Camilleri M. Psychological disorders in patients with evacuation disorders and constipation in a tertiary practice. Am J Gastroenterol. 2000;95:1755–8. [DOI] [PubMed] [Google Scholar]
- 4. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97. [DOI] [PubMed] [Google Scholar]
- 5. Hayden DM, Weiss EG. Fecal incontinence: etiology, evaluation, and treatment. Clin Colon Rectal Surg. 2011;24:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rao SSC. Pathophysiology of adult fecal incontinence. Gastroenterology. 2004;126:S14–22. [DOI] [PubMed] [Google Scholar]
- 7. Paquette IM, Varma MG, Kaiser AM, Steele SR, Rafferty JF. The American Society of Colon and Rectal Surgeons' clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623–36. [DOI] [PubMed] [Google Scholar]
- 8. Varghese C, Wells CI, O'Grady G, Bissett IP. Costs and outcomes of sacral nerve stimulation for faecal incontinence in New Zealand: a 10‐year observational study. ANZ J Surg. 2020;90:569–75. [DOI] [PubMed] [Google Scholar]
- 9. Matzel KE, Chartier‐Kastler E, Knowles CH, Lehur PA, Muñoz‐Duyos A, Ratto C, et al. Sacral neuromodulation: standardized electrode placement technique. Neuromodulation. 2017;20:816–24. [DOI] [PubMed] [Google Scholar]
- 10. Mirbagheri N, Sivakumaran Y, Nassar N, Gladman MA. Systematic review of the impact of sacral neuromodulation on clinical symptoms and gastrointestinal physiology. ANZ J Surg. 2016;86:232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carrington EV, Evers J, Grossi U, Dinning PG, Scott SM, O'Connell PR, et al. A systematic review of sacral nerve stimulation mechanisms in the treatment of fecal incontinence and constipation. Neurogastroenterol Motil. 2014;26:1222–37. [DOI] [PubMed] [Google Scholar]
- 12. Lin AY, Dinning PG, Milne T, Bissett IP, O'Grady G. The “rectosigmoid brake”: review of an emerging neuromodulation target for colorectal functional disorders. Clin Exp Pharmacol Physiol. 2017;44:719–28. [DOI] [PubMed] [Google Scholar]
- 13. Sheldon R, Kiff ES, Clarke A, Harris ML, Hamdy S. Sacral nerve stimulation reduces corticoanal excitability in patients with faecal incontinence. Br J Surg. 2005;92:1423–31. [DOI] [PubMed] [Google Scholar]
- 14. Devane L, O'Connell R, Jones J. Acute sacral nerve stimulation affects sensory but not motor neurones of the anal canal in an animal model: LTP83. Colorectal Dis. 2013;15:358–63. [Google Scholar]
- 15. Fowler CJ, Swinn MJ, Goodwin RJ, Oliver S, Craggs M. Studies of the latency of pelvic floor contraction during peripheral nerve evaluation show that the muscle response is reflexly mediated. J Urol. 2000;163:881–3. [PubMed] [Google Scholar]
- 16. Boyle DJ, Knowles CH, Lunniss PJ, Scott SM, Williams NS, Gill KA. Efficacy of sacral nerve stimulation for fecal incontinence in patients with anal sphincter defects. Dis Colon Rectum. 2009;52:1234–9. [DOI] [PubMed] [Google Scholar]
- 17. Ratto C, Litta F, Parello A, Donisi L, de Simone V, Zaccone G. Sacral nerve stimulation in faecal incontinence associated with an anal sphincter lesion: a systematic review. Colorectal Dis. 2012;14:e297–304. [DOI] [PubMed] [Google Scholar]
- 18. Huizinga JD, Hussain A, Chen J‐H. Interstitial cells of Cajal and human colon motility in health and disease. Am J Physiol Gastrointest Liver Physiol. 2021;321:G552–75. [DOI] [PubMed] [Google Scholar]
- 19. Patton V, Wiklendt L, Arkwright JW, Lubowski DZ, Dinning PG. The effect of sacral nerve stimulation on distal colonic motility in patients with faecal incontinence. Br J Surg. 2013;100:959–68. [DOI] [PubMed] [Google Scholar]
- 20. Lin AY, du P, Dinning PG, Arkwright JW, Kamp JP, Cheng LK, et al. High‐resolution anatomic correlation of cyclic motor patterns in the human colon: evidence of a rectosigmoid brake. Am J Physiol Gastrointest Liver Physiol. 2017;312:G508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 22. Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525–32. [DOI] [PubMed] [Google Scholar]
- 23. Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Fecal incontinence quality of life scale. Dis Colon Rectum. 2000;43:9–16. [DOI] [PubMed] [Google Scholar]
- 24. Dinning PG, Wiklendt L, Maslen L, Gibbins I, Patton V, Arkwright JW, et al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high‐resolution fiber‐optic manometry. Neurogastroenterol Motil. 2014;26:1443–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Appiah‐Ankam J, Hunter JM. Pharmacology of neuromuscular blocking drugs. Contin Educ Anaesth Crit Care Pain. 2004;4:2–7. [Google Scholar]
- 26. Arkwright JW, Blenman NG, Underhill ID, Maunder SA, Szczesniak MM, Dinning PG, et al. In‐vivo demonstration of a high resolution optical fiber manometry catheter for diagnosis of gastrointestinal motility disorders. Opt Express. 2009;17:4500–8. [DOI] [PubMed] [Google Scholar]
- 27. Vather R, O'Grady G, Arkwright JW, Rowbotham DS, Cheng LK, Dinning PG, et al. Restoration of normal colonic motor patterns and meal responses after distal colorectal resection. Br J Surg. 2016;103:451–61. [DOI] [PubMed] [Google Scholar]
- 28. Vather R, O'Grady G, Lin AY, du P, Wells CI, Rowbotham D, et al. Hyperactive cyclic motor activity in the distal colon after colonic surgery as defined by high‐resolution colonic manometry. Br J Surg. 2018;105:907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davidson JB, O'Grady G, Arkwright JW, Zarate N, Scott SM, Pullan AJ, et al. Anatomical registration and three‐dimensional visualization of low and high‐resolution pan‐colonic manometry recordings. Neurogastroenterol Motil. 2011;23:387–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hiroz P, Schlageter V, Givel J‐C, Kucera P. Colonic movements in healthy subjects as monitored by a magnet tracking system. Neurogastroenterol Motil. 2009;21:838–e57. [DOI] [PubMed] [Google Scholar]
- 31. Keane C, Paskaranandavadivel N, Vather R, Rowbotham D, Arkwright J, Dinning P, et al. Altered colonic motility is associated with low anterior resection syndrome. Colorectal Dis. 2020;23:415–23. 10.1111/codi.15465 [DOI] [PubMed] [Google Scholar]
- 32. Jaung R, Varghese C, Lin AY, Paskaranandavadivel N, Du P. High ‐ resolution colonic manometry pressure profiles are similar in asymptomatic diverticulosis and controls. Dig Dis Sci. 2020;66:832–42. 10.1007/s10620-020-06320-4 [DOI] [PubMed] [Google Scholar]
- 33. Ballantyne GH. Rectosigmoid sphincter of O'Beirne. Dis Colon Rectum. 1986;29:525–31. [DOI] [PubMed] [Google Scholar]
- 34. Chen J‐H, Nirmalathasan S, Pervez M, Milkova N, Huizinga JD. The sphincter of O'Beirne ‐ part 1: study of 18 normal subjects. Dig Dis Sci. 2021;66:3516–28. 10.1007/s10620-020-06657-w [DOI] [PubMed] [Google Scholar]
- 35. Heitmann PT, Mohd Rosli R, Maslen L, Wiklendt L, Kumar R, Omari TI, et al. High‐resolution impedance manometry characterizes the functional role of distal colonic motility in gas transit. Neurogastroenterol Motil. 2021;34(1):e14178. [DOI] [PubMed] [Google Scholar]
- 36. Rao SS, Welcher K. Periodic rectal motor activity: the intrinsic colonic gatekeeper? Am J Gastroenterol. 1996;91:890–7. [PubMed] [Google Scholar]
- 37. Aaronson MJ, Freed MM, Burakoff R. Colonic myoelectric activity in persons with spinal cord injury. Dig Dis Sci. 1985;30:295–300. [DOI] [PubMed] [Google Scholar]
- 38. Fajardo NR, Pasiliao RV, Modeste‐Duncan R, Creasey G, Bauman WA, Korsten MA. Decreased colonic motility in persons with chronic spinal cord injury. Am J Gastroenterol. 2003;98:128–34. [DOI] [PubMed] [Google Scholar]
- 39. Battle WM, Snape WJ Jr, Wright S, Sullivan MA, Cohen S, Meyers A, et al. Abnormal colonic motility in progressive systemic sclerosis. Ann Intern Med. 1981;94:749–52. [DOI] [PubMed] [Google Scholar]
- 40. Battle WM, Snape WJ Jr, Alavi A, Cohen S, Braunstein S. Colonic dysfunction in diabetes mellitus. Gastroenterology. 1980;79:1217–21. [PubMed] [Google Scholar]
- 41. Michelsen HB, Christensen P, Krogh K, Rosenkilde M, Buntzen S, Theil J, et al. Sacral nerve stimulation for faecal incontinence alters colorectal transport. Br J Surg. 2008;95:779–84. [DOI] [PubMed] [Google Scholar]
- 42. Krames E, Hunter Peckham P, Rezai AR. Neuromodulation: Comprehensive Textbook of Principles, Technologies, and Therapies. Cambridge, MA: Academic Press; 2018. [Google Scholar]
- 43. Craggs M, McFarlane J. Neuromodulation of the lower urinary tract. Exp Physiol. 1999;84:149–60. [DOI] [PubMed] [Google Scholar]
- 44. Gourcerol G, Vitton V, Leroi AM, Michot F, Abysique A, Bouvier M. How sacral nerve stimulation works in patients with faecal incontinence. Colorectal Dis. 2011;13:e203–11. [DOI] [PubMed] [Google Scholar]
- 45. Rodger CJ, Nicol L, Anderson JH, McKee RF, Finlay IG. Abnormal colonic motility: a possible association with urge fecal incontinence. Dis Colon Rectum. 2010;53:409–13. [DOI] [PubMed] [Google Scholar]
- 46. Dinning PG, Wiklendt L, Gibbins I, Patton V, Bampton P, Lubowski DZ, et al. Low‐resolution colonic manometry leads to a gross misinterpretation of the frequency and polarity of propagating sequences: initial results from fiber‐optic high‐resolution manometry studies. Neurogastroenterol Motil. 2013;25:640–9. [DOI] [PubMed] [Google Scholar]
- 47. Tack J, Corsetti M, Camilleri M, Quigley EMM, Simren M, Suzuki H, et al. Plausibility criteria for putative pathophysiological mechanisms in functional gastrointestinal disorders: a consensus of experts. Gut. 2018;67:1425–33. [DOI] [PubMed] [Google Scholar]
- 48. Matzel KE, Stadelmaier U, Hohenfellner M, Gall FP. Electrical stimulation of sacral spinal nerves for treatment of faecal incontinence. Lancet. 1995;346:1124–7. [DOI] [PubMed] [Google Scholar]
- 49. Patton V, Abraham E, Lubowski DZ. Sacral nerve stimulation for faecal incontinence: medium‐term follow‐up from a single institution. ANZ J Surg. 2017;87:462–6. [DOI] [PubMed] [Google Scholar]
- 50. Lehur PA, Sørensen M, Dudding TC, Knowles CH, de Wachter S, Engelberg S, et al. Programming algorithms for sacral neuromodulation: clinical practice and evidence‐recommendations for day‐to‐day practice. Neuromodulation. 2020;23:1121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hounsome N, Roukas C. Cost‐effectiveness of sacral nerve stimulation and percutaneous tibial nerve stimulation for faecal incontinence. Therap Adv Gastroenterol. 2018;11:1756284818802562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Erickson JC, Bruce LE, Taylor A, Richman J, Higgins C, Wells CI, et al. Electrocolonography: non‐invasive detection of colonic cyclic motor activity from multielectrode body surface recordings. IEEE Trans Biomed Eng. 2019;1:1. [DOI] [PubMed] [Google Scholar]
- 53. Chen J‐H, Collins SM, Milkova N, Pervez M, Nirmalathasan S, Tan W, et al. The sphincter of O'Beirne—part 2: report of a case of chronic constipation with autonomous dyssynergia. Dig Dis Sci. 2021;66:3529–41. 10.1007/s10620-020-06723-3 [DOI] [PubMed] [Google Scholar]
- 54. Bassotti G, Chistolini F, Battaglia E, Chiarioni G, Sietchiping Nzepa F, Dughera L, et al. Are colonic regular contractile frequency patterns in slow transit constipation a relevant pathophysiological phenomenon? Dig Liver Dis. 2003;35:552–6. [DOI] [PubMed] [Google Scholar]
- 55. Rao SS, Sadeghi P, Batterson K, Beaty J. Altered periodic rectal motor activity: a mechanism for slow transit constipation. Neurogastroenterol Motil. 2001;13:591–8. [DOI] [PubMed] [Google Scholar]
- 56. Wells CI, Penfold JA, Paskaranandavadivel N, Rowbotham D, Du P, Seo S, et al. Hyperactive distal colonic motility and recovery patterns following right colectomy: a high‐resolution manometry study. Dis Colon Rectum. 2022; 60:15–21. [DOI] [PubMed] [Google Scholar]
- 57. Narducci F, Bassotti G, Gaburri M, Morelli A. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut. 1987;28:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dinning PG, Zarate N, Szczesniak MM, Mohammed SD, Preston SL, Fairclough PD, et al. Bowel preparation affects the amplitude and spatiotemporal organization of colonic propagating sequences. Neurogastroenterol Motil. 2010;22:633–e176. [DOI] [PubMed] [Google Scholar]
- 59. Lémann M, Flourié B, Picon L, Coffin B, Jian R, Rambaud JC. Motor activity recorded in the unprepared colon of healthy humans. Gut. 1995;37:649–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wöllner J, Hampel C, Kessler TM. Surgery illustrated – surgical atlas sacral neuromodulation. BJU Int. 2012;110:146–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1‐S2
Appendix S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


