Abstract
Background
The proadaptive effects of glucagon‐like peptide‐2 (GLP‐2) include stimulation of intestinal mucosal growth as well as intestinal blood flow and angiogenesis. We have recently reported that daily subcutaneous injections of glepaglutide, a long‐acting GLP‐2 analog, improved intestinal absorptive function in patients with short bowel syndrome (SBS). As secondary and exploratory end points, the effects of glepaglutide on intestinal morphology and perfusion are reported.
Methods
The following assessments were done in 18 patients with SBS in a randomized, crossover, dose‐finding, phase 2 trial before and after three weeks of treatment with glepaglutide: plasma citrulline and mucosa biopsies to assess changes in (1) intestinal morphology by immunohistochemistry and (2) gene expressions associated with absorption, proliferation, and markers of tight‐junction integrity by quantitative polymerase chain reaction. Intestinal perfusion was assessed in stoma nipples by laser speckle contrast imaging and quantitative fluorescence angiography with indocyanine green.
Results
In the 1‐ and 10‐mg dose groups, glepaglutide significantly increased plasma citrulline by 15.3 µmol/L (P = 0.001) and 15.6 µmol/L (P = 0.001), respectively. Trends toward an increase in villus height, crypt depth, and epithelium height were seen in the same groups. No significant changes were seen in gene expressions or intestinal perfusion.
Conclusion
The increase in plasma citrulline and the morphological improvements may partly account for improvement in the intestinal absorptive function. However, the finding of a stability in perfusion after three weeks of treatment with glepaglutide may have been preceded by a more profound acute‐phase increase in intestinal perfusion at treatment initiation.
Keywords: glucagon‐like peptide‐2, intestinal failure, intestinal morphology, intestinal perfusion, plasma citrulline, research and diseases, short bowel syndrome
CLINICAL RELEVANCY STATEMENT
Our findings in the present article showed that three weeks of treatment with glepaglutide, a novel long‐acting glucagon‐like peptide‐2 analog, was associated with an increased concentration of plasma citrulline, reflecting an increased enterocyte mass and morphological improvements in the remaining intestine. Glepaglutide did not significantly affect intestinal perfusion evaluated at the end of week 3 by quantitative fluorescence angiography with indocyanine green and laser speckle contrast imaging.
Thus, glepaglutide plays a role in the restoration of intestinal structure and function, which may partly account for the improvement in the intestinal absorptive function, as reported previously.
INTRODUCTION
Patients with short bowel syndrome (SBS) have reduced intestinal absorptive surface area because of surgical resections of parts of the intestines. Important variables determining the intestinal absorption capacity include not only the remaining intestinal length but also the health, integrity, functional characteristics, and adaptive potential of the remnant intestine, as well as the underlying clinical condition and the status of the gastrointestinal motility and various upper gastrointestinal secretions. 1 , 2 , 3 , 4 , 5 Altogether, these factors determine the large spectrum of bowel dysfunction ranging from mild SBS‐associated intestinal insufficiency (SBS‐II) to severe SBS‐associated intestinal failure (SBS‐IF). 6 When SBS‐IF occurs, parenteral support (PS) of fluid, electrolytes, and/or nutrients is indicated. 7 Although life‐saving, PS is associated with reduced quality of life 8 , 9 and repeated hospitalizations, 10 not least because of life‐threatening complications, such as catheter‐related sepsis, venous occlusions, metabolic complications, and renal 11 and liver failure. 12 , 13
With the discovery of glucagon‐like peptide‐2 (GLP‐2), a new era for the treatment of patients with SBS has begun. 14 It is now established that GLP‐2 demonstrates proadaptive properties and has the potential to improve compromised intestinal function in patients with SBS. 15 , 16 , 17 Treatment with GLP‐2 seems to target different aspects of postresection bowel dysfunction that affects gastric and potentially pancreaticobiliary secretion 18 and gastrointestinal emptying, 19 intestinal blood flow and 20 barrier function, 21 as well as mucosal morphology. 16 , 17 , 22 , 23 , 24
GLP‐2 and its analogs have been shown to have potent intestinotrophic activity in numerous species including humans. 16 , 17 , 22 , 23 , 24 As an intestinal growth factor, GLP‐2 induces crypt cell proliferation in the small intestine and, to a lesser extent, in the large intestine. 15 , 23 Thus, plasma citrulline, a proposed marker of enterocyte mass, has also been shown to increase in humans following treatment with GLP‐2. 25 , 26 Moreover, several animal studies have demonstrated that treatment with GLP‐2 can improve intestinal barrier function by reducing the gastrointestinal permeability and increasing key markers of tight‐junction integrity. 21 , 27 , 28
Exogenous GLP‐2 has also been shown to stimulate proximal intestinal blood flow, 20 increase the overall splanchnic oxygenation 29 and angiogenesis, 30 , 31 as well as promote intestinal mucosal growth, 22 thereby enhancing postresectional adaptation. This has been demonstrated to enhance fluid and nutrient absorption as well as reduce malabsorption and diarrhea in both preclinical and clinical SBS settings. 15 , 32 It may also reduce or even eliminate the need for PS in patients with SBS‐IF. 33 Furthermore, GLP‐2 treatment has been proposed to have a role in gastrointestinal surgery, particularly in situations with impaired perfusion of the intestine. 34 Short‐term treatment with a GLP‐2 analog has been observed to increase small‐bowel anastomotic perfusion assessed by quantitative fluorescence angiography with indocyanine green (q‐ICG). 35 Also, a recent study in a rat model reported that the GLP‐2 analog ZP1849 improved the breaking strength of colonic anastomoses, suggesting a beneficial effect for colonic anastomotic wound healing. 36
We have recently published that three weeks of treatment with daily subcutaneous injections of 1 and 10 mg glepaglutide, a long‐acting GLP‐2 analog, reduced fecal output and resulted in improvement in intestinal wet weight absorption and macronutrient absorption in patients with SBS. 37 In the present paper on secondary and exploratory end points, we aimed to address changes in intestinal morphology as assessed by plasma citrulline and intestinal mucosa biopsies as well as intestinal perfusion evaluated by Laser Speckle Contrast Imaging (LSCI) and q‐ICG.
METHODS
Trial design
This was a randomized, double‐blind, dose‐finding, single‐center, proof‐of‐concept, phase 2 trial. The patients served as their own control. Two of three different doses (0.1, 1, and 10 mg) of glepaglutide were given to the patients as daily subcutaneous injections for a total of three weeks in a two‐period crossover design. A washout period of four to eight weeks separated the two treatment periods. The primary outcome of the trial was the absolute change from baseline in fecal output assessed by metabolic balance studies performed before and after each treatment. 37 As a secondary endpoint, this trial aimed to evaluate changes from baseline in plasma citrulline, a proposed marker of enterocyte mass following treatment with glepaglutide. Moreover, as exploratory end points, the trial aimed to assess changes in: (1) intestinal morphology assessed in mucosa biopsies by immunohistochemistry; (2) expression of genes associated with absorption, proliferation, and markers of tight‐junction integrity in mucosa biopsies; and (3) perfusion as assessed by stoma nipple examination by nontouch LSCI and q‐ICG.
All the assessments were performed during hospitalization for the metabolic balance studies prior to and following three weeks of treatment with glepaglutide.
Registrations
The trial protocol was approved by the Danish Medicines Agency and the Regional Committee on Health Research Ethics (Project ID: H‐15015411) and was registered at clinicaltrials.gov (EudraCT NCT02690025). All the procedures were conducted in accordance with the ethical standards of the Helsinki Declaration and the International Council for Harmonization Good Clinical Practice. Written consent was obtained from all patients prior to inclusion. The trial was conducted at the Department of Intestinal Failure and Liver Diseases, Rigshospitalet, Denmark between February 2016 and May 2017.
Patients
Patients with SBS were included based on malabsorption defined as fecal output ≥1500 g/day. The majority of patients (n = 17) had a stoma. One patient had jejunum in continuity with the left colon and rectum. Eligible patients were required to be stable in their SBS and PS volume if required (<25% change in volume or energy content for four weeks before randomization), without intestinal strictures or active inflammatory bowel disease or fistula, and with time from last intestinal resection of more than one year. Patients with radiation enteritis, celiac disease, or a history of colon cancer were excluded. A more detailed list of inclusion/exclusion criteria has been published previously. 37
Plasma citrulline
Blood samples were collected in EDTA tubes after an oral overnight fast. Patients with SBS‐IF could have their regular PS overnight prior to testing. After collection, the samples were immediately turned and kept on ice before centrifugation at 1590 G at 4°C. Plasma citrulline concentration was analyzed using high‐pressure liquid chromatography. 38
Biopsies
Following a separate written consent to this procedure and after an oral overnight fast, enteroscopy was performed through the stoma nipple using a thin, flexible sigmoidoscope (Olympus). Using standard 9‐mm biopsy forceps, at least two mucosa biopsies were collected approximately 10−20 cm from the stoma nipple. At least one biopsy sample was meant for histological analyses and one for gene expression analyses by real‐time quantitative polymerase chain reaction (q‐PCR).
Immunohistochemistry
The biopsies were immediately incubated in paraformaldehyde 4% in a phosphate‐buffer 0.1 M at pH 7.2. Subsequently, the samples were transferred to ethanol 70% and kept in the refrigerator until immunohistochemistry staining was performed. The samples were embedded in paraffin, cut in slide sections of 5 µM and dewaxed through xylene to tap water. To retrieve antigens, the sections were placed in a 10 mmol citric buffer of pH 6 and boiled in a microwave oven for 15 min. Next, a 10‐min preincubation in 2% bovine serum albumin was performed ahead of overnight incubation at 4°C with a primary antibody. The following antibodies were used: chromogranin A (DAKO A430, rabbit polyclonal, diluted 1:2000), as a general marker for the number of enteroendocrine cells, and GLP‐2 (rabbit polyclonal [1976‐6], in house, diluted 1:1500). The sections were incubated for 40 min with a second layer of antibodies to amplify the reaction. Biotinylated secondary immunoglobulins were used (goat antirabbit: BA‐1000; Vector Laboratories, diluted 1:200). Next, hydrogen peroxide 3% was added to block the endogenous peroxidase. The third layer consisted of a preformed Avidin and biotinylated horseradish peroxidase macromolecular complex (ABC) (code no. PK‐4000; Vector Laboratories) incubated for 30 min. Finally, the reaction was developed using 3.3‐diaminobenzidine (KEM‐EN‐TEC, cat. no. 4170) for 15 min, followed by 2‐min incubation in 0.5% copper sulphate (Merck art. no. 2790) diluted in Tris buffer 0.05% with Tween 20 (DAKO S1966), and counterstaining was performed with Mayers Hemalun. The software ImagePro plus 9.2 (Media Cybernetics) was used to determine villus and epithelium height as well as crypt depth.
Quantitative polymerase chain reaction (q‐PCR)
The biopsies were submerged in RNA‐later (Sigma Aldrich) and stored at −80°C until analysis. Details on q‐PCR analyses are published elsewhere. 39 The messenger RNA (mRNA) expressions relative to the glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) of markers of cellular proliferation (MKI67); epithelial cell dynamics; alpha smooth muscle actin (ACTA2); macrophage activation (CD68); intestinal absorption of glucose (sodium glucose cotransporter‐1 [SLC2A1], glucose transporter 2 [SLC2A2], and glucose transporter 5 [SLC2A5]); and selected tight‐junction complex proteins, claudin 7 (CLDN7), claudin 15 (CLDN15), and occludin (OCEL1) were measured.
Intestinal perfusion
For patients with a stoma, perfusion of the stoma nipple was measured by LSCI in a recumbent position after emptying the stoma bag as described previously. 40 With the patients staying in bed between the measurements, the examination was performed on two time points, t0 and t15 (given in minutes). During the treatment metabolic balance studies, the time point t0 was defined as just before administering the trial drug. The results are expressed as flux measured in arbitrary laser speckle perfusion units. 41 , 42
Perfusion was also assessed by q‐ICG after a bolus of ICG (0.25 mg/kg, 5 mg/ml) was injected intravenously and flushed with 5 ml of saline, as described earlier. 43 Results are reported as the slope of the fluorescence time curve ([Δfluorescence intensity/Δs] 43 , 44 and the normalized slope Δfluorescence intensity/Δs fluorescence/s)/maximum fluorescence intensity. 35 , 43 , 45 Considering the inter‐individual differences in stoma perfusion at baseline, results were reported as relative differences from the baseline at the end of week 3.
Statistical analysis
Because of the lack of previous pilot studies prior to our phase 2 trial, no formal sample size calculation was performed. Details on estimates of the sample size have been published previously. 37
Values at baseline are presented as either median (min; max) or mean ± SD. To assess changes from baseline, an analysis of covariance (ANCOVA) was used and results were presented with estimates, 95% confidence intervals, and P values. An exception was the results on mRNA expressions assessed by q‐PCR, where results were analyzed by the Wilcoxon signed rank test and presented as medians and interquartile range values. The analyses were performed using Statistical Analysis Software (version 9.4; SAS Institute), as detailed elsewhere. 37 Two‐sided tests were performed with a 5% significance level. Spearman rank correlations were performed for any association between variables.
The trial is registered at clinicaltrials.gov with registration number NCT02690025 and was monitored by a contract research organization (Larix A/S, Denmark).
RESULTS
During the phase 2 trial, 22 patients were screened for eligibility, 18 patients (13 patients with SBS‐IF and five with SBS‐II) were randomized and treated with glepaglutide, and 16 patients completed the trial and comprised the full analysis set group. Two patients discontinued early in treatment period 1; one patient discontinued because of adverse events (nausea, hot flushes, dizziness, and tachycardia), and another withdrew consent because of an episode of prolonged abdominal pain and subsequently sepsis. Table 1 shows the demographics and baseline characteristics of the patients randomized.
Table 1.
Individual baseline demographics
| Anatomy | Treatment | Parenteral fluid, | Body weight, | Fecal output, | Creatinine clearance, | Plasma citrulline, | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Sex/age, years | Diagnosis | SB, cm | CiC, % | Period | Glepaglutide, mg | ml/day | kg | g/day | ml/min | µmol/L | Biopsy samples collected |
| 01 | M/68 | UC | 200 | 0 | 1 | 1 | 2000 | 90 | 1667 | 59 | 78 | No |
| 2 | 0.1 | 2000 | 91 | 1483 | 58 | 79 | ||||||
| 02 | M/57 | UC | 250 | 0 | 1 | 0.1 | 0 | 70 | 2893 | 94 | 51 | No |
| 2 | 10 | 0 | 68 | 3232 | 74 | 51 | ||||||
| 03 | F/52 | CD | 300 | 0 | 1 | 0.1 | 0 | 72 | 1675 | 66 | 70 | No |
| 2 | 1 | 0 | 73 | 1850 | 68 | 52 | ||||||
| 04 | F/65 | CD | 70 | 0 | 1 | 10 | 2153 | 55 | WD | WD | WD | No |
| 2 | 0.1 | WD | WD | WD | WD | WD | ||||||
| 05 | F/61 | CD | 150 | 0 | 1 | 10 | 0 | 57 | 5207 | 85 | 42 | Yes |
| 2 | 1 | 0 | 61 | 4917 | 103 | 35 | ||||||
| 06 | M/76 | MVD | 30 | 0 | 1 | 1 | 5750 | 76 | 7577 | 44 | 39 | Yes |
| 2 | 10 | 6000 | 76 | 7457 | 49 | 38 | ||||||
| 07 | M/56 | CD | UK | 0 | 1 | 10 | 0 | 107 | 1320 | 144 | 40 | Yes |
| 2 | 0.1 | 0 | 110 | 1473 | 154 | 39 | ||||||
| 08 | F/57 | MVD | 80 | 0 | 1 | 0.1 | 3500 | 68 | 3243 | 112 | 36 | Yes |
| 2 | 1 | 3500 | 69 | 3397 | 108 | 29 | ||||||
| 09 | F/63 | MVD | 50 | 0 | 1 | 10 | 2250 | 60 | 2533 | 57 | 47 | No |
| 2 | 1 | 2250 | 60 | 2143 | 47 | 46 | ||||||
| 10 | F/73 | CD | 30 | 0 | 1 | 0.1 | 3130 | 75 | 2522 | 71 | 34 | Yes |
| 2 | 10 | 3250 | 75 | 2153 | 91 | 37 | ||||||
| 11 | F/58 | MVD | 85 | 50 | 1 | 1 | 1750 | 59 | 1927 | 114 | 39 | No |
| 2 | 0.1 | 1750 | 60 | 1675 | 108 | 35 | ||||||
| 12 | F/64 | CD | 150 | 0 | 1 | 1 | 1000 | 62 | 1497 | 130 | 53 | Yes |
| 2 | 10 | 1000 | 63 | 1520 | 120 | 54 | ||||||
| 13 | M/59 | CD | 140 | 0 | 1 | 10 | 0 | 72 | 4292 | 95 | 38 | Yes |
| 2 | 1 | 0 | 73 | 5450 | 115 | 39 | ||||||
| 14 | M/56 | CD | 70 | 0 | 1 | 1 | 2560 | 84 | 4140 | 71 | 68 | Yes |
| 2 | 10 | 2310 | 82 | 2460 | 42 | 71 | ||||||
| 15 | M/72 | CD | UK | 70 | 1 | 0.1 | 2000 | 52 | 1413 | 63 | 42 | Yes |
| 2 | 10 | 2000 | 51 | 1573 | 80 | 38 | ||||||
| 16 | M/71 | MVD | 40 | 0 | 1 | 10 | 2750 | 83 | 5493 | 106 | 13 | Yes |
| 2 | 0.1 | 3000 | 79 | 6530 | 83 | 19 | ||||||
| 17 | M/43 | SC | 70 | 0 | 1 | 0.1 | 2865 | 105 | WD | WD | WD | Yes |
| 2 | 1 | WD | WD | WD | WD | WD | ||||||
| 18 | F/55 | MVD | 50 | 0 | 1 | 1 | 3000 | 90 | 3180 | 41 | 34 | No |
| 2 | 0.1 | 3000 | 90 | 3103 | 37 | 34 | ||||||
Abbreviations: CD, Crohn's disease; CiC, colon in continuity; F, female; M, male; MVD, mesenteric vascular disease; SB, small bowel; SC, surgical complication; UC, ulcerative colitis; UK, unknown; WD, withdrawn.
Plasma citrulline
Mean plasma citrulline (± SD) was 45.3 ± 16.0 µmol/L prior to glepaglutide treatment. Following three weeks of treatment with glepaglutide, dose‐dependent increases (P = 0.013) in plasma citrulline were observed in the 1 mg (15.3 µmol/L, P = 0.001) and 10 mg (15.6 µmol/L, P = 0.001) dose groups corresponding to a relative increase of 33% and 36%, respectively. A test for carryover effect was included in the statistical analyses, showing that the changes reverted to baseline following the drug‐free washout period between the two treatment periods (Table 1); thus, no carryover effect was observed (P = 0.712). No significant change was observed in the 0.1 mg dose group (Figures 1 and 2).
Figure 1.
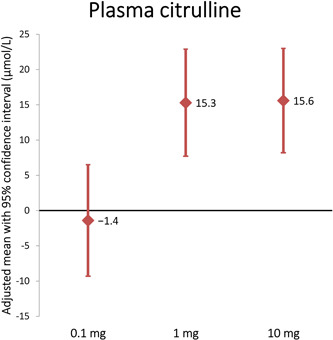
Mean changes from baseline in plasma citrulline
Figure 2.
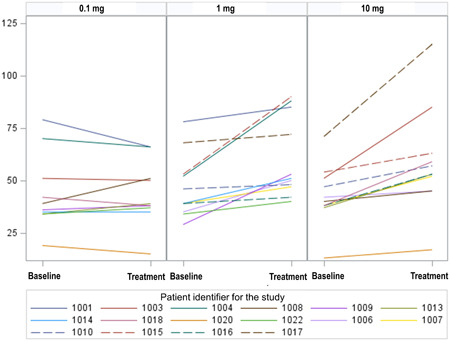
Individual changes from baseline in plasma citrulline
At baseline, plasma citrulline was correlated to small‐bowel length (r = 0.58, P = 0.0007) and macronutrient absorption 37 (carbohydrate: r = 0.38, P = 0.027; protein: r = 0.44, P = 0.010; and lipid: r = 0.44, P = 0.009). Plasma citrulline tended to correlate negatively to PS volume (r = −0.34, P = 0.050) and fecal wet weight output (−0.41, P = 0.016), and positively to wet weight absorption (r = 0.32, P = 0.067). Changes in plasma citrulline following treatment with glepaglutide were associated with reductions in fecal wet weight output (r = −0.65, P < 0.0001) and increases in intestinal wet weight absorption (r = 0.69, P < 0.0001) as well as absorption of protein (r = 0.42, P = 0.016) and lipid (0.39, P = 0.026). Plasma citrulline was not observed to correlate with villus height (P = 0.601), crypt depth (P = 0.812), epithelium height (P = 0.121), GLP‐2–positive cells (P = 0.192), or estimated glomerular filtration rate (P = 0.695).
Biopsies
Biopsy samples were collected in nine SBS patients with stoma (three SBS‐II and six SBS‐IF); of these, one patient had a sigmoid stoma and the remaining had either a jejunostomy or ileostomy. The patient who had a jejunum in continuity with the left colon and rectum did not consent to the procedure. Trends toward morphological changes were seen primarily in the 10 mg dose group (Table 2) in terms of increased crypt depth of 99 µm (P = 0.019) and epithelium height of 14 µm (P = 0.005), corresponding to a relative increase of 51% and 54%, respectively. The numerical increase in villus height of 84 µm in the 10 mg dose group, however, did not reach statistical significance (P = 0.427). For the patient with a sigmoid stoma, the crypt depth was observed to be 690 μm at baseline and 653 μm after treatment with 10 mg glepaglutide. No significant changes were observed the 1 and 0.1 mg dose groups.
Table 2.
Plasma citrulline, chromogranin A– and GLP‐2–positive cells, villus height, crypt depth, and epithelium height
| Changes from baseline, adjusted means [95% CI] | 0.1 mg/day | 1 mg/day | 10 mg/day |
|---|---|---|---|
| Plasma citrulline, µmol/L | −1.4 [−9.3 to 6.5] | 15.3 [7.7‐22.9] | 15.6 [8.2‐23.0] |
| P = 0.695 | P = 0.001 | P = 0.0009 | |
| N = 10 | N = 11 | N = 11 | |
| Chromogranin A–positive cells, per mm2 | −11 [−38 to 15] | −23 [−54 to 9] | −31 [−51 to −11] |
| P = 0.350 | P = 0.132 | P = 0.008 | |
| N = 5 | N = 4 | N = 6 | |
| GLP‐2–positive cells, per mm2 | −4 [−7 to −1] | −1 [−5 to 3] | −3 [−6 to −1] |
| P = 0.010 | P = 0.551 | P = 0.023 | |
| N = 5 | N = 4 | N = 6 | |
| Absolute villus height, µm | −59 [−332 to 214] | 167 [−177 to 511] | 84 [−179 to 347] |
| P = 0.578 | P = 0.248 | P = 0.427 | |
| N = 4 | N = 3 | N = 4 | |
| Relative villus height, % | −8 [−54 to 38] | 38 [−20 to 96] | 28 [−16 to 73] |
| P = 0.642 | P = 0.142 | P = 0.152 | |
| N = 4 | N = 3 | N = 4 | |
| Absolute crypt depth, µm | −29 [−110 to 52] | 45 [−59 to 148] | 99 [25‐174] |
| P = 0.400 | P = 0.319 | P = 0.019 | |
| N = 4 | N = 3 | N = 5 | |
| Relative crypt depth, % | −11 [−53 to 32] | 45 [−10 to 99] | 51 [12‐90] |
| P = 0.551 | P = 0.089 | P = 0.021 | |
| N = 4 | N = 3 | N = 5 | |
| Absolute epithelium height, µm | 7 [−2 to 16] | 1 [−11 to 14] | 14 [5‐22] |
| P = 0.121 | P = 0.785 | P = 0.005 | |
| N = 5 | N = 4 | N = 6 | |
| Relative epithelium height, % | 24 [−14 to 63] | 5 [−45 to 55] | 54 [19‐88] |
| P = 0.188 | P = 0.815 | P = 0.007 | |
| N = 5 | N = 4 | N = 6 |
Note: Only one biopsy per patient was included in the histology assessment.
Abbreviation: GLP‐2, glucagon‐like peptide‐2.
In the 10 mg dose group, glepaglutide treatment was associated with a reduction in the number of the chromogranin A–positive cells (by 31 cells/mm2, P = 0.008) and GLP‐2–positive cells (by 3 cells/mm2, P = 0.023). However, the number of GLP‐2–positive cells also decreased in the 0.1 mg dose group by 4 cells/mm2 (P = 0.010) (Table 2).
The mRNA expressions of MKI67, ACTA2, CD68, SLC5A1, SLC5A2, SLC5A5, and tight‐junction complex proteins CLDN7, CLDN15, and OCEL1 did not change in any dose groups (Table 3).
Table 3.
mRNA gene expressions relative to glyceraldehyde 3‐phosphate dehydrogenase of MKI67, ACTA2, CD68, SLC5A1, SLC5A2, and SLC5A5 and tight‐junction complex proteins CLDN7, CLDN15, and OCEL1
| 0.1 mg/day | 1 mg/day | 10 mg/day | |
|---|---|---|---|
| MKI67 | 1.4 [1.3‐1.5] | 0.9 [0.1‐1] | 0.7 [0.4‐1] |
| P = 0.125 | P = 0.125 | P = 0.313 | |
| N = 5 | N = 4 | N = 6 | |
| ACTA2 | 0.8 [0.7‐1.1] | 0.4 [0.1‐1.1] | 1.5 [0.3‐8.9] |
| P = 0.813 | P = 0.625 | P = 0.688 | |
| N = 5 | N = 4 | N = 6 | |
| CD68 | 1.0 [0.8‐1.1] | 1.4 [0.2‐1.7] | 1.0 [0.6‐1.6] |
| P = 0.813 | P = 0.875 | P = 1.000 | |
| N = 5 | N = 4 | N = 6 | |
| SLC5A1 | 1.2 [0.9‐1.3] | 1.3 [0.4‐1.8] | 0.6 [0.4‐1.1] |
| P = 0.875 | P = 0.875 | P = 0.438 | |
| N = 4 | N = 4 | N = 6 | |
| SLC5A2 | 0.4 [0.1‐1.6] | 0.9 [0.2‐1.4] | 1.0 [0.5‐1.1] |
| P = 0.625 | P = 0.875 | P = 1.000 | |
| N = 4 | N = 4 | N = 5 | |
| SLC5A5 | 1.2 [0.5‐1.8] | 1.0 [0.0‐1.0] | 0.7 [0.4‐0.8] |
| P = 1.000 | P = 0.500 | P = 0.438 | |
| N = 5 | N = 3 | N = 6 | |
| CLDN7 | 1.3 [0.8‐1.6] | 1.0 [0.5‐1.3] | 0.9 [0.6‐1.7] |
| P = 1.000 | P = 1.000 | P = 1.000 | |
| N = 5 | N = 4 | N = 6 | |
| CLDN15 | 0.9 [0.8‐1.8] | 0.6 [0.3‐1.2] | 1.1 [0.4‐3.8] |
| P = 1.000 | P = 0.625 | P = 0.844 | |
| N = 5 | N = 4 | N = 6 | |
| CEL1 | 0.9 [0.6‐1.5] | 0.5 [0.2‐0.7] | 1.1 [0.9‐1.5] |
| P = 0.625 | P = 0.125 | P = 0.562 | |
| N = 4 | N = 4 | N = 6 |
Note: Results are presented as fold changes from baseline in each treatment dose. Median and interquartile range values are shown. Test for difference is done by the Wilcoxon signed rank test.
Intestinal perfusion
Results on changes in intestinal perfusion evaluated by LSCI are shown in Table 4. Following treatment with glepaglutide, no significant changes from baseline (0 min baseline vs 0 min posttreatment and 15 min baseline vs 15 min posttreatment) were observed in any of the dose groups.
Table 4.
Intestinal perfusion assessed by LSCI and q‐ICG
| Changes from baseline, adjusted means [95% CI] | 0.1 mg/day | 1 mg/day | 10 mg/day | |
|---|---|---|---|---|
| LSCI, LSPU | 0 min | −45.4 [−202.0 to 111.3] | −47.1 [−193.7 to 99.5] | −30.1 [−168.4 to 108.2] |
| P = 0.555 | P = 0.513 | P = 0.657 | ||
| 15 min | 63.3 [−58.8 to 185.4] | −15.2 [−122.6 to 92.2] | −44.8 [−140.8 to 51.2] | |
| P = 0.293 | P = 0.771 | P = 0.342 | ||
| q‐ICG | Slope | 3.65 [−1.04 to 8.33] | 1.86 [−2.80 to 6.53] | 2.33 [−1.80 to 6.47] |
| P = 0.121 | P = 0.416 | P = 0.255 | ||
| Normalized slope | 0.01 [−0.02 to 0.02] | 0.01 [−0.01 to 0.03] | 0.01 [−0.01 to 0.02] | |
| P = 0.304 | P = 0.197 | P = 0.291 | ||
Abbreviations: LSCI, Laser Speckle Contrast Imaging; LSPU, laser speckle perfusion units; q‐ICG, quantitative fluorescence angiography with indocyanine green.
Likewise, no significant changes were observed in intestinal perfusion evaluated by q‐ICG in any of the dose groups (Table 4).
DISCUSSION
As recently reported, three weeks of treatment with glepaglutide in a randomized phase 2 trial resulted in a significant decrease in fecal output and an improvement in intestinal absorption in patients with SBS. 37 Based on secondary and exploratory outcomes from this phase 2 trial, we now report that glepaglutide induced morphological improvements in the remaining intestine but did not affect intestinal perfusion evaluated by q‐ICG and LSCI at the end of week 3.
Three weeks of treatment with 1 and 10 mg glepaglutide was associated with an increase in plasma citrulline and improvement in intestinal morphology in terms of increases in crypt depth, villus height, and epithelium height. The mean increase in villus height and crypt depth in the present study appear to be higher compared with previous studies in which effects of native GLP‐2 and teduglutide were assessed on the intestinal function following seven 32 and three weeks 22 of treatment, respectively. However, whereas the increase in plasma citrulline in our study was observed to be higher compared with a previous eight‐week study with native GLP‐2 treatment, 46 the increment in plasma citrulline after 24 weeks of treatment with teduglutide was higher than in our study probably because of the longer duration of treatment. 17 The increases in plasma citrulline and intestinal morphology are indicative of an increased absorptive surface area, which may be one of the responsible factors for the positive effect of glepaglutide on intestinal absorption. However, similar to other GLP‐2 analogs, the intestinotrophic effect of glepaglutide may accelerate the growth of existing neoplasms in the intestine. 47 Bearing this in mind, safety measures, including regular clinical assessments and colonoscopy prior to treatment initiation in patients with SBS and colon in continuity, should be considered in the treatment process. 48
Consistent with previous findings, low plasma citrulline was associated with the severity of SBS. Plasma citrulline was observed to correlate with small‐bowel length and intestinal macronutrient absorption, but not to renal function. In addition, correlations were observed between plasma citrulline and fecal wet weight output, PS volume, and intestinal wet weight absorption (the latter two not being significant). However, the correlations were all moderate, and no correlation was found with the intestinal morphology that might be due to the small number of biopsies.
Data in the present paper show a decrease in the chromogranin A– and GLP‐2–positive cells following treatment with glepaglutide. However, data from the present phase 2 study (to be published) indicate that no significant changes were seen in the endogenous production of GLP‐2 and GLP‐1 following three weeks of treatment with glepaglutide. Also, the expansion of the number of enterocytes and the increase in the total surface area as indicated by an increase in villus height and crypt depth should be considered when interpreting these data in a limited number of biopsies (Table 2).
No significant changes were seen in the mRNA gene expressions in intestinal mucosa biopsies assessed by q‐PCR. No inter‐individual comparison was allowed by the nature of the analysis to assess whether a transcriptional up‐ or down‐regulation of these gene expressions had taken place as expected.
Although short‐term treatment with a GLP‐2 analog has been shown to increase perfusion in intestinal anastomoses measured by q‐ICG, 26 three weeks of treatment with glepaglutide did not result in a significant change evaluated by q‐ICG and LSCI based on one measurement prior to glepaglutide injection and one at the end of week 3. Although short‐term GLP‐2 treatment and its effect on LSCI measurement have not been studied previously, the lack of change in perfusion observed by q‐ICG could be attributed to longer treatment duration and is consistent with findings in another study, in which the increase in intestinal blood flow was reduced when GLP‐2 was administered for a longer period of time. 49 However, the increased diameter and protrusion of the stoma nipple were detected within hours to a week following the injection of glepaglutide, supporting the assertion that the changes are related to the increased blood perfusion rather than the endothelial morphological improvements. This has also been described previously with native GLP‐2 treatment. The changes reversed toward baseline in the washout period when glepaglutide was discontinued. 50 This may imply that, although an increase in intestinal blood flow might still exist after three weeks of treatment, it falls under the q‐ICG detection threshold.
The data in the present study are based on findings from the secondary and exploratory end points of a phase 2 trial recently published. 37 Potential limitations, besides the absence of a placebo arm, are the relatively small number of patients and no sample size calculation, which should be considered when interpreting the results and the level of significance presented in this paper. Both type 2 and type 1 statistical errors are possible given the small sample size and the number of tests we have performed in this small sample, respectively. Another potential limitation is that two patients had received native GLP‐2 or teduglutide in previous clinical trials at least eight years prior to the study. However, previous knowledge about GLP‐2 responsiveness did not influence the objective of the study, since all patients responded to at least one dose level of glepaglutide. Furthermore, when the two patients were excluded from the data analyses, neither the magnitude nor the significance level of the primary end point were affected. 37 Some of the methods used in this study have not been used previously to assess intestinal morphology and perfusion following treatment with a GLP‐2 analog in a stable chronic SBS population. Another limitation is the lack of data on the acute changes in intestinal morphology and perfusion after glepaglutide administration. The measurements were done only at two timepoints with at least three weeks’ gap, and we have no data on the acute changes after glepaglutide administration. Moreover, the consequences of a longer/chronic treatment period should be addressed in future studies.
In conclusion, three weeks of treatment with glepaglutide in patients with SBS resulted in significant increases in plasma citrulline, reflecting an increased enterocyte mass, and induced morphological improvements in the remaining intestine. The difference between the 1 and 10 mg dose groups did not reach statistical significance. However, the changes regarding plasma citrulline and morphological improvements were primarily seen in the 10 mg dose group.
No significant changes were seen regarding mRNA gene expressions of markers of cellular proliferation MKI67, epithelial cell dynamics (ACTA2), macrophage activation (CD68), intestinal absorption of glucose (SLC5A1, SLC5A2, and SLC5A5), and selected tight‐junction complex proteins (CLDN7, CLDN15, and OCEL1). Likewise, no significant changes were seen in intestinal perfusion as measured by q‐ICG and LSCI.
Consistent with our previous findings, 37 no significant changes were observed regarding any of the abovementioned assessments for the 0.1 mg dose.
AUTHOR CONTRIBUTIONS
Authorship statement Rahim M. Naimi, Mark K. Hvistendahl, and Palle B. Jeppesen equally contributed to the conception and design of the research; Steen S. Poulsen, Hannelouise Kissow, Jens Pedersen, Nikolaj A. Nerup, Rikard Ambrus, Michael P. Achiam, and Lars B. Svendsen contributed to the acquisition and analysis of the data; Palle B. Jeppesen contributed to the interpretation of the data; and Rahim M. Naimi drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
CONFLICTS OF INTEREST
Rahim M. Naimi was an employee at Zealand Pharma as an industrial PhD fellow during the conduct of this trial. Palle B. Jeppesen serves as consultant and trial investigator for Zealand Pharma. The remaining authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This is a Zealand Pharma–sponsored study. We would like to gratefully acknowledge and thank bioanalysts Jette Christiansen, Birgitte Schou and Dorte Christensen from the Department of Intestinal Failure and Liver Diseases, Rigshospitalet, for their technical assistance. Our thanks are also extended to the trial statisticians, Henrik Wachmann from Larix A/S and Kim Mark Knudsen from Zealand Pharma A/S, for their statistical analysis and interpretation.
Naimi RM, Hvistendahl MK, Poulsen SS, et al. Effects of glepaglutide, a long‐acting glucagon‐like peptide‐2 analog, on intestinal morphology and perfusion in patients with short bowel syndrome: Findings from a randomized phase 2 trial. J Parenter Enteral Nutr. 2023;47:140‐150. 10.1002/jpen.2389
REFERENCES
- 1. Sundaram A, Koutkia P. Nutritional management of short bowel syndrome. J Clin Gastroenterol. 2002;34(3):207‐220. [DOI] [PubMed] [Google Scholar]
- 2. Wilmore DW, Byrne TA, Persinger RL. Short bowl syndrome: new therapeutic approaches. Curr Probl Surg. 1997;34(5):389‐444. [DOI] [PubMed] [Google Scholar]
- 3. Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124(4):1111‐1134. [DOI] [PubMed] [Google Scholar]
- 4. Messing B, Joly F. Guidelines for management of home parenteral support in adult chronic intestinal failure patients. Gastroenterology. 2006;130(2):43‐51. [DOI] [PubMed] [Google Scholar]
- 5. Jeppesen PB. Short bowel syndrome—characterisation of an orphan condition with many phenotypes. Expert Opin Orphan Drugs. 2013;1(7):515‐525. [Google Scholar]
- 6. Jeppesen PB. Spectrum of short bowel syndrome in adults: intestinal insufficiency to intestinal failure. JPEN J Parenter Enteral Nutr. 2014;38(1):8S‐13S. [DOI] [PubMed] [Google Scholar]
- 7. Pironi L, Arends J, Baxter J, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults Clin Nutr. 2015;34(2):171‐180. [DOI] [PubMed] [Google Scholar]
- 8. Jeppesen PB, Langholz E, Mortensen PB. Quality of life in patients receiving home parenteral nutrition. Gut. 1999;44(6):844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nordsten CB, Molsted S, Bangsgaard L, et al. High parenteral support volume is associated with reduced quality of life determined by the short‐bowel syndrome quality of life scale in nonmalignant intestinal failure patients. JPEN J Parenter Enteral Nutr. 2021;45(5):926‐932. [DOI] [PubMed] [Google Scholar]
- 10. Fuglsang KA, Brandt CF, Scheike T, Jeppesen PB. Hospitalizations in patients with nonmalignant short‐bowel syndrome receiving home parenteral support. Nutr Clin Pract. 2020;35(5):894‐902. [DOI] [PubMed] [Google Scholar]
- 11. Mathiesen SM, Fuglsang KA, Ranzato G, Scheike T, Jeppesen PB. Renal function in patients with intestinal failure receiving home parenteral support. JPEN J Parenter Enteral Nutr. 2021;46(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 12. Brandt CF, Hvistendahl M, Naimi RM, et al. Home parenteral nutrition in adult patients with chronic intestinal failure: the evolution over 4 decades in a tertiary referral center. JPEN J Parenter Enteral Nutr. 2017; 41(7):1178‐1187. [DOI] [PubMed] [Google Scholar]
- 13. Baxter JP, Fayers PM, Mckinlay AW. A review of the quality of life of adult patients treated with long‐term parenteral nutrition. Clin Nutr. 2006;25(4):543‐553. [DOI] [PubMed] [Google Scholar]
- 14. Jeppesen PB. The long road to the development of effective therapies for the short gut syndrome: a personal perspective. Dig Dis Sci. 2019;64(10):2717‐2735. [DOI] [PubMed] [Google Scholar]
- 15. Brubaker P, Izzo A, Hill M, Drucker D. Intestinal function in mice with small bowel growth induced by glucagon‐like peptide‐2. Am J Physiol. 1997;272(6):1050‐1058. [DOI] [PubMed] [Google Scholar]
- 16. Jeppesen PB, Hartmann B, Thulesen J, et al. Treatment of short bowel patients with glucagon‐like peptide 2, a newly discovered intestinotrophic, anti‐secretory, and transit modulating peptide. Gastroenterology. 2000;118(4):A178‐A179. [Google Scholar]
- 17. Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo‐controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60(7):902‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wøjdemann M, Wettergren A, Hartmann B, Hilsted L, Holst JJ. Inhibition of sham feeding‐stimulated human gastric acid secretion by glucagon‐like peptide‐2. J Clin Endocrinol Metab. 1999;84(7):2513‐2517. [DOI] [PubMed] [Google Scholar]
- 19. Wøjdemann M, Wettergren A, Hartmann B, Holst JJ. Glucagon‐like peptide‐2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol. 1998;33(8):828‐832. [DOI] [PubMed] [Google Scholar]
- 20. Bremholm L, Hornum M, Andersen UB, Hartmann B, Juul J, Bekker P. The effect of glucagon‐like peptide‐2 on mesenteric blood flow and cardiac parameters in end‐jejunostomy short bowel patients. Regul Pept. 2011;168(1‐3):32‐38. [DOI] [PubMed] [Google Scholar]
- 21. Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP‐2‐driven improvement of gut permeability. Gut. 2009;58(8):1091‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX‐0600), a dipeptidyl peptidase IV resistant qlucagon‐like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54(9):1224‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drucker DJ, Ehrlich P, Asat SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon‐like peptide 2. Proc Natl Acad Sci U S A. 1996;93(15):7911‐7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slim GM, Lansing M, Wizzard P, et al. Novel long‐acting GLP‐2 analogue, FE 203799 (apraglutide), enhances adaptation and linear intestinal growth in a neonatal piglet model of short bowel syndrome with total resection of the ileum. JPEN J Parenter Enteral Nutr. 2019;43(7):891‐898. [DOI] [PubMed] [Google Scholar]
- 25. Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143(6):1473‐1481.e3. [DOI] [PubMed] [Google Scholar]
- 26. Jeppesen PB, Gabe SM, Seidner DL, Lee HM, Olivier C. Citrulline correlations in short bowel syndrome–intestinal failure by patient stratification: analysis of 24 weeks of teduglutide treatment from a randomized controlled study. Clin Nutr. 2020;39(8):2479‐2486. [DOI] [PubMed] [Google Scholar]
- 27. Qi KK, Sun YQ, Wan J, et al. Effect of porcine glucagon‐like peptides‐2 on tight junction in GLP‐2R + IPEC‐J2 cell through the PI3k/Akt/mTOR/p70S6Ksignalling pathway. J Anim Physiol Anim Nutr. 2017;101(6):1242‐1248. [DOI] [PubMed] [Google Scholar]
- 28. Ren W, Wu J, Li L, et al. Glucagon‐like peptide‐2 improve intestinal mucosal barrier function in aged rats. J Nutr Heal Aging. 2018;22(6):731‐738. [DOI] [PubMed] [Google Scholar]
- 29. Gay AN, Lazar DA, Stoll B, et al. Near‐infrared spectroscopy measurement of abdominal tissue oxygenation is a useful indicator of intestinal blood flow and necrotizing enterocolitis in premature piglets. J Pediatr Surg. 2012;46(6):1034‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang C, Shen Y, Zhao L, et al. Glucagon‐like peptide 2 promotes angiogenesis in human umbilical vein endothelial cells via akt signaling pathway. Int J Clin Exp Med. 2017;10(3):5538‐5545. [Google Scholar]
- 31. Bulut K, Pennartz C, Felderbauer P, et al. Glucagon like peptide‐2 induces intestinal restitution through VEGF release from subepithelial myofibroblasts. Eur J Pharmacol. 2008;578(2‐3):279‐285. [DOI] [PubMed] [Google Scholar]
- 32. Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon‐like peptide 2 improves nutrient absorption and nutritional status in short‐bowel patients with no colon. Gastroenterology. 2001;120(4):806‐815. [DOI] [PubMed] [Google Scholar]
- 33. Seidner DL, Gabe SM, Lee HM, Olivier C, Jeppesen PB. Enteral autonomy and days off parenteral support with teduglutide treatment for short bowel syndrome in the STEPS Trials. JPEN J Parenter Enteral Nutr. 2020;44(4):697‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nerup N, Ambrus R, Lindhe J, Achiam MP, Jeppesen PB, Svendsen LB. The effect of glucagon‐like peptide‐1 and glucagon‐like peptide‐2 on microcirculation: a systematic review. Microcirculation. 2019;26(3):e12367. [DOI] [PubMed] [Google Scholar]
- 35. Nerup N, Ring LL, Strandby RB, et al. Quantitative perfusion assessment of intestinal anastomoses in pigs treated with glucagon‐like peptide 2. Langenbecks Arch Surg. 2018;403(7):881‐889. [DOI] [PubMed] [Google Scholar]
- 36. Kjaer M, Russell W, Schjerling P, et al. Glucagon‐like peptide‐2 analogue ZP1849 augments colonic anastomotic wound healing. Gastroenterol Res Pract. 2020;2020:8460508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Naimi RM, Hvistendahl M, Enevoldsen LH, et al. Glepaglutide, a novel long‐acting glucagon‐like peptide‐2 analogue, for patients with short bowel syndrome: a randomised phase 2 trial. Lancet Gastroenterol Hepatol. 2019;1253(19):1‐10. [DOI] [PubMed] [Google Scholar]
- 38. Fjermestad H, Hvistendahl M, Jeppesen PB. Fasting and postprandial plasma citrulline and the correlation to intestinal function evaluated by 72‐hour metabolic balance studies in short bowel jejunostomy patients with intestinal failure. JPEN J Parenter Enteral Nutr. 2017;42(2):418‐426. [DOI] [PubMed] [Google Scholar]
- 39. Hvistendahl MK, Naimi RM, Hansen SH, et al. Bile acid‐farnesoid X receptor‐fibroblast growth factor 19 axis in patients with short bowel syndrome: the randomized glepaglutide phase 2 trial. JPEN J Parenter Enteral Nutr. Published online July 21, 2021. 10.1002/jpen.2224 [DOI] [PubMed] [Google Scholar]
- 40. Rønn JH, Nerup N, Strandby RB, et al. Laser speckle contrast imaging and quantitative fluorescence angiography for perfusion assessment. Langenbecks Arch Surg. 2019;404(4):505‐515. [DOI] [PubMed] [Google Scholar]
- 41. Ambrus R, Strandby RB, Svendsen LB, Achiam MP, Steffensen JF, Svendsen MBS. Laser speckle contrast imaging for monitoring changes in microvascular blood flow. Eur Surg Res. 2016;56(3‐4):87‐96. [DOI] [PubMed] [Google Scholar]
- 42. Milstein DMJ, Ince C, Gisbertz SS, et al. Laser speckle contrast imaging identifies ischemic areas on gastric tube reconstructions following esophagectomy. Medicine. 2016;95(25):e3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nerup N, Andersen HS, Ambrus R, et al. Quantification of fluorescence angiography in a porcine model. Langenbecks Arch Surg. 2017;402(4):655‐662. [DOI] [PubMed] [Google Scholar]
- 44. Toens C, Krones CJ, Blum U, et al. Validation of IC‐VIEW fluorescence videography in a rabbit model of mesenteric ischaemia and reperfusion. Int J Colorectal Dis. 2006;21(4):332‐338. [DOI] [PubMed] [Google Scholar]
- 45. Nerup N, Knudsen KBK, Ambrus R, et al. Reproducibility and reliability of repeated quantitative fluorescence angiography. Surg Technol Int. 2017;31:35‐39. [PubMed] [Google Scholar]
- 46. Naimi RM, Madsen KB, Askov‐Hansen C, et al. A dose‐equivalent comparison of the effects of continuous subcutaneous glucagon‐like peptide 2 infusions versus meal related GLP‐2 injections in the treatment of short bowel syndrome patients. Regul Pept. 2013;184:47‐53. [DOI] [PubMed] [Google Scholar]
- 47. Thulesen J. Glucagon‐like peptide 2, an intestinotrophic mediator. Curr Protein Pept Sci. 2004;5(1):51‐65. [DOI] [PubMed] [Google Scholar]
- 48. Armstrong D, Forbes A, Jeppesen PB, Lee HM, Nagy P, Seidner DL. Colon polyps in patients with short bowel syndrome before and after teduglutide: post hoc analysis of the STEPS study series. Clin Nutr. 2020;39(6):1774‐1777. [DOI] [PubMed] [Google Scholar]
- 49. Taylor‐Edwards CC, Burrin DG, Holst JJ, McLeod KR, Harmon DL. Glucagon‐like peptide‐2 increases small intestinal blood flow and mucosal growth in ruminating calves. J Dairy Sci. 2011;94(2):888‐898. [DOI] [PubMed] [Google Scholar]
- 50. Jeppesen PB, Lund P, Gottschalck IB, et al. Short bowel patients treated for two years with glucagon‐like peptide 2 (GLP‐2): compliance, safety, and effects on quality of life. Gastroenterol Res Pract. 2009;2009:425759. [DOI] [PMC free article] [PubMed] [Google Scholar]


