Abstract
Background
Assessment of dietary intake is fundamental for evaluating the interrelationships between diet and disease. The present study aimed to develop and validate the semiquantitative Cypriot food frequency questionnaire (CyFFQ).
Methods
A 171‐item paper‐and‐pencil semiquantitative interview‐administered FFQ was developed, including local foods and culturally specific meals commonly consumed among Cypriot adults. FFQ reproducibility was assessed by comparing the energy‐adjusted daily macro‐ and micronutrients intake at baseline (FFQ1) and 1 year later (FFQ2) using a Wilcoxon matched pairs signed rank test and intraclass correlation coefficients (ICCs) in a random sample of Cypriot adults. FFQ relative validity was evaluated by comparing the intake as estimated by FFQ2 with that obtained from the average of three 24‐h recalls taken over the year between FFQ1 and FFQ2. Associations between nutrient intakes estimated using FFQ2 and the 24‐h recalls were assessed using Spearman rank correlation and Bland–Altman plots were used to assess agreement between the FFQ and the 24‐h recalls.
Results
Among eligible participants, 68 (78%) completed the study (44.1% males, aged 30.5–47.5 years). The energy‐adjusted intakes of macro‐ and micronutrients did not significantly differ between the two FFQs, excluding magnesium. The FFQ2 and the averaged 24‐h recalls were significantly correlated for most macro‐ and micronutrients. The median (interquartile) ICC for all macro‐ and micronutrients was 0.46 (0.38–0.52) (p < 0.05). Agreement was satisfactory (>30%) for most micro‐ and macronutrients. Bland–Altman plots also confirmed good agreement between the two methods.
Conclusions
The CyFFQ is a valid and reliable tool for assessing dietary consumption in Cypriot adults.
Keywords: 24‐h recall, Cyprus, dietary assessment, food frequency questionnaire, reproducibility, validity
Key points
Culturally applicable dietary intake assessment tools are necessary to evaluate the relationships between diet and health/disease aspects.
The present study aimed to develop and validate the semiquantitative Cypriot food frequency questionnaire (CyFFQ).
After developing a 171‐paper‐and‐pencil semiquantitative interview‐administered FFQ, including local and culturally specific meals, both reproducibility and relative validity were assessed in a random sample of Cypriot adults.
In statistical analysis comparing the FFQ provided to the participants at baseline and 1 year later, energy‐adjusted intakes of macro‐ and micronutrients did not significantly differ between the two FFQs, with the exception of magnesium. A comparison between the energy‐adjusted daily macro‐ and micronutrients intake 1 year later (FFQ2) and the average of three 24‐h recalls provided between baseline and FFQ2 showed significant correlation and agreement, using various methods, for most macro‐ and micronutrients.
Our findings indicate that the CyFFQ is a valid and reliable tool for assessing dietary consumption in Cypriot adults and thus can be used in future research.
A 171‐item semiquantitative food frequency questionnaire (FFQ) was developed for assessment of dietary intake of Cypriot adults. Its relative validity and reproducibility were assessed in 68 adults by repeat FFQs and 24‐h recalls. The results show that the Cypriot FFQ (CyFFQ) is a valid and reliable tool for assessing dietary consumption in Cypriot adults.

INTRODUCTION
Accurate assessment of dietary intake is fundamental for evaluating the intricate interrelationships between dietary exposures (ranging from dietary patterns to nutrient intakes) and the subsequent development and progression of nutrition‐related diseases. Food frequency questionnaires (FFQs) constitute one of several assessment tools for evaluating dietary intake, particularly within the context of epidemiological studies. They most often entail the assessment of the frequency of consumption, both within and beyond the home environment, of a wide array of food items and beverages, as well as population specific local produce, traditional food products and culture‐specific meals. Moreover, within the context of semiquantitative FFQs, additional pictorial depictions of indicative portion sizes are also employed to allow the assessment of dietary and nutrient intake and even compare intake over time. In this context and compared to alternative dietary assessment tools (e.g., food diaries), semiquantitative FFQs are often the preferred choice applied in epidemiological research. 1 This is because they may be readily and efficiently administered to large study populations, account for variations in weekly and/or seasonal variations in consumption, and, above all, provide comprehensive dietary assessments of habitual intake in a single measurement because they encompass local and culturally specific foods and meals regularly consumed by particular populations in contrast to diet records that provide a snapshot of the previous days' diet. 1 , 2 , 3
In most populations, dietary consumption is most often defined not only by the seasonal local availability of produce and food items, but also by traditional, cultural and religious practices, including traditional meals and cooking methods. Hence, culturally specific FFQs are mandated for the accurate assessment of dietary and nutrient intake. 4 Particularly in Mediterranean countries, although local populations readily adhere to the general principles of the Mediterranean diet, the latter is enriched with locally available produce and traditional food items.
In Cyprus, these include traditional cheese and meat products including ‘haloumi’ cheese, smoked pork tenderloin ‘lountza’ and fish by‐products (e.g., fish roe salad ‘taramosalata’). In addition, based on seasonal availability, traditional foods are regularly utilised for the preparation of intricate traditional dishes (e.g., taro root ‘kolokasi’) and meals (e.g., sautéed pork ‘afelia’, stuffed vine leaves ‘koupepia’, soup made with fermented goat milk and cracked wheat flour ‘trahana’ soup). Deviations between Mediterranean countries with respect to preparatory and cooking methods are also noteworthy. An example is the array of foods which compose a traditional full course meal, that is, the abundant array of appetisers, also known as ‘mzedes’, entailed in Cypriot and Greek meals, compared to their more limited adoption within other Mediterranean countries. Finally, despite recent demographic changes, religiously defined types and frequencies of meals are most notable in countries such as Cyprus and Greece, where religious customs according to the Greek Orthodox religion define transient vegan practices for up to 180–220 days of the year. 5 To account for all of the aforementioned variations in country specific dietary and culinary practices, population‐specific FFQs have been developed for several Mediterranean countries, including Greece 6 and Lebanon, 7 which have diets resembling the Cypriot cuisine and are in close proximity to Cyprus. However, to date, despite the aforementioned complex intricacies of the Cypriot cuisine, a validated and culturally specific FFQ for assessing dietary practices in Cypriots is lacking.
The present study aimed to develop and validate a comprehensive and reliable semiquantitative FFQ, for assessing the dietary intake of Cypriot adults. This new tool includes local products, traditional food items and culturally specific meals, at the same time as taking into consideration the nutritional transition towards the Western diet by including foods consumed within the Western diet.
METHODS
Study design and population
For the validation of the newly developed FFQ, a cross‐sectional study was undertaken with the participation of a random sample of Cypriot adults from the general population (N = 87 participants, 46% males) aged 18–75 years. The sample size allows estimations with a confidence of 10%, given a power of 0.80 at an α‐level of 0.05. Participants were community dwelling from all the non‐Turkish‐occupied territory of the Republic of Cyprus, with a proportional representation from rural and urban areas. Exclusion criteria included any current or chronic disease, including any disease that affects dietary intake, appetite or metabolism, as well as eating disorders, pregnancy or lactation, and use of any medication or food supplement (e.g., vitamins) at the time of recruitment or during the participation in the study. Potential participants underwent a short screening telephone interview using a questionnaire prior to recruitment to ensure eligibility. Data collection was conducted between February 2017 until May 2019. The study was approved by the Cyprus National Bioethics Committee (ΕΕΒΚ/ΕC/2016/11) before commencing and all participants provided their written informed consent before participating.
Study procedure
The study procedure is shown in Figure 1. Participants were assessed by trained investigators (nutritionists or dietitians) on five separate occasions every 3 months (0, 3, 6, 9 and 12 months) during the course of 1 year. At baseline (month 0), demographic (i.e., age, urban vs. rural area of residence, education, expressed as highest educational level attained, and marital status) and lifestyle (smoking practices and exercise) characteristics were assessed. Weight was assessed in the morning in light clothing and without shoes to the nearest 100 g using a portable scale (MBF‐6000 Digital Body Fat Analyzer; Charder) and height was assessed without shoes using a wall‐mounted stadiometer (HM‐230M; Charder). Waist circumference was measured at the height of the umbilical cord using a non‐extendible tape (Hoechstmass). Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ)‐short version validated in Greek. 8 Dietary practices were assessed by the newly developed FFQ as explained below.
Figure 1.
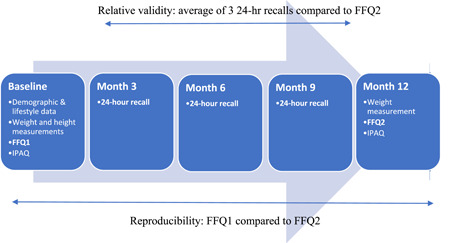
Flow chart describing the study procedure. Relative validity: average of three 24‐h recalls compared to energy‐adjusted daily macro‐ and micronutrients intake 1 year later (FFQ2) Reproducibility: energy‐adjusted daily macro‐ and micronutrients intake at baseline (FFQ1) compared to FFQ2. FFQ, food frequency questionnaire; IPAQ, International Physical Activity Questionnaire.
Follow‐up assessments were conducted at 3, 6, 9 and 12 months. During follow‐up at 3, 6 and 9 months, a detailed 24‐h recall of the food and beverage intake during the preceding 24 h was taken by the interviewer. Efforts were made to arrange the appointments on different days of the week taking into account both working and non‐working days in the 24‐h recalls. A participant's weight was measured as described above. Changes in health status were assessed and the participant was withdrawn from the study if the change fell within the exclusion criteria (e.g., a metabolic disease or pregnancy). During the final assessment at 12 months, participants completed the FFQ for a second time to assess reproducibility or reliability of the FFQ. The IPAQ‐short version was also re‐administered to assess physical activity.
FFQ
A semiquantitative paper‐and‐pencil FFQ was developed by two experienced dietitians (EP and GL) aiming to capture the dietary habits of the Cypriot population and to include most foods commonly consumed in Cyprus. To capture culturally specific foods, the methodological framework described by Sharma 4 was used, including the following steps: compilation of a complete and accurate food list, determination of culturally appropriate portion sizes, categorisation of frequencies of consumption and development of a food composition table. 1 , 2 , 4 Additionally, because of the diet transition towards a Western diet, the FFQ also included food items based on current trends, availability and cost, similar to other populations. 6 EP and GL designed the FFQ, including compilation of the food items to be included following conduction of 24‐h dietary recalls with a population‐based sample as suggested by Sharma. 4 The food list was originally drafted based on a Greek FFQ developed by Trichopoulou 9 because Cyprus and Greece share a similar cuisine. Seasonality was taken into consideration by including seasonal fruit such as watermelon and citrus fruits consumed in the summer and winter, respectively, and dishes such as ‘kleftiko’ and ‘trahana’ soup consumed in the summer and winter, respectively. The FFQ was initially piloted in the first 20 participants of the study who answered energy‐adjusted daily macro‐ and micronutrients intake at baseline (FFQ1). Each participant answered the FFQ by a face‐to‐face interview, independently and on separate occasions from all other participants. The pilot testing was considered a face and content validation and, following discussion between the dietitians who developed the FFQ, a consensus was reached to include some non‐traditional foods such as ‘peanut butter’ and some sauces such as ‘white sauce’ and ‘tomato‐based sauce’, which were not included in the first version of the questionnaire but were reported by the participants as consumed.
The final FFQ comprises of a food list and a supplement list. The food list consists of 171 items divided into the following sections: breakfast cereals, eggs, bakery goods including traditional savoury or sweet breakfast/snack items such as olive pie, ‘eliopita’, cheese pie ‘tyropita’, sweet‐tahini pie ‘tahinopita’; cereals including traditional such as bulgur wheat ‘plugouri’ and pasta including traditional ravioli filled with ‘haloumi’ cheese, ‘ravioles’; milk and milk products including traditional cheeses such as ‘anari’, ‘halloumi’ and ‘feta’ cheese, fat spreads and oils; soups including ‘trahana’ soup, meats and meat products and meat dishes including traditional preserved pork ‘lountza’, meat dishes such as stuffed vine leaves ‘koupepia’ and pasta with minced meat and bechamel sauce ‘pastitsio’; fish and fish products including breaded squid; potatoes, legumes, pulses and vegetables including beans prepared traditionally with tomato and olive oil; fresh and dried fruit of all seasons; various foods such as nuts, dips and sauces including traditional dips such as ‘tahini’ and yoghurt dip ‘tzatziki’, biscuits, sweets and savory products including traditional cakes, beverages including tea, coffee and soft drinks and alcoholic drinks. Foods usually consumed outside of the house such as kebab ‘souvlakia’ and pizza were also included in the food list. Participants were still able to report foods and beverages in open‐ended questions usually consumed but not included in the questionnaire, although items other than the ones in the main questionnaire were only rarely reported, as their consumption frequency was low.
The FFQ was administered by face‐to‐face interview by trained investigators. Any answer that seemed invalid was cross‐checked by the principal investigator. Administration of the questionnaire took approximately 1 h. For each food, participants were asked to specify the frequency of consumption in the previous year by choosing one of nine possible responses. The number of times/year or frequency factor were also specified to assist in the conceptualisation of the frequency. The frequency (times per year) were: ‘never’ (0 times per year), ‘a few times per year’ (6 times per year), ‘once per month’ (12 times per year), ‘2–3 times per month’ (30 times per year), ‘once a week’ (52 times per year), ‘twice a week’ (104 times per year), ‘3–4 times per week’ (182 times per year), ‘5–6 times per week’ (286 times per year) and ‘every day’ (365 times per year). The participants were also asked to specify the portion size for each item. This was achieved either using the Greek translation of the Block Portion Size Pictures (used with permission after the purchase of copyrights from NutritionQuest) 10 for food items such as cereals, bread, pasta, meats and so forth, tablespoons or teaspoons for dips and spreads, respectively, number of food items (e.g., for biscuits and number of glasses for beverages). The amount of food in grams or ml was calculated using the Food Patterns Equivalents Database. 11 The composition of each food was determined using Dietplan 6.0, 12 in which Cypriot traditional foods and recipes analysed by the Cyprus General Laboratory and published in the Cyprus Food Composition Tables 13 were added. Additional references were used for a limited number of For traditional bakery products, composition information as analysed using routine laboratory food analysis methods was provided by the leading bakery store, and, for some foods/beverages such as energy drinks, the nutrient profile was obtained from the US Department of Agriculture Food Data Central database. 14 In addition to the list of food items, the participants were asked to report how much of the visible fat on meat products as well as amount of chicken ‘skin’ they consumed. The options provided were: ‘all or most of i’, ‘some of it’, ‘as little as possible or none’ and ‘I don't consume meat/chicken’. The answers were taken into account in order to determine the nutritional composition of meat and meat products. As an example, if a person reported eating ‘all or most of meat fat’ in the general question, the option used for nutritional analysis of pork chops was ‘Pork chops, loin, lean and fat’, whereas, if they reported ‘some of it’, the options of ‘Pork chops, loin, lean and fat’ and ‘Pork chops, loin, lean only’ were averaged, and so on. Additionally, participants were asked whether they use sugar or honey in their beverages and the number of teaspoons used in each. These responses were multiplied by the number of hot and cold beverages (tea and coffee) they consumed per day and added to the rest of the analysis.
Average nutrient intake was computed by multiplying the specified portion of food consumed by the frequency factor and the nutrient content. Yearly energy and nutrient intake were divided to report daily intakes and thus facilitate comparison with 24‐h recall intakes. The supplement list consists of multivitamins, B‐complex vitamins, vitamin A (not beta‐carotene), beta‐carotene, vitamin C, vitamin E, folic acid, calcium, vitamin D only, vitamin D together with calcium, zinc, selenium, omega‐3 fatty acids/fish oils, probiotics, ginseng, ginkgo‐biloba, St‐John's wort, echinacea and glucosamine/chondroitin. The dose, frequency and duration of use was reported both to ensure compliance of the participants with the advice against the use of supplements during the study period, but also to collect information on supplement use in future studies.
24‐H RECALLS
To assess the relative validity of the FFQ against another dietary method, three 24‐h recalls were collected at 3, 6 and 9 months. Twenty‐four‐hour recalls are most commonly used reference method for relative validation of FFQs. 2 Each participant was interviewed by a nutritionist or dietitian and was asked to report their food and beverage intake over the 24 h preceding the interview day. The US Department of Agriculture pencil‐and‐paper multiple pass method, which has been previously validated, was used to carry out the 24‐h recalls. 15 , 16 Special attention was given to remind participants of the use of any oils, sauces, spreads and the cooking methods to obtain results which were as accurate as possible. The Greek translation of the Block Portion Size Pictures was used to estimate the portion size of each food consumed, as explained above. Data was analysed using the same database as the FFQ to estimate the nutrient intake over each 24‐h period. For each participant, intakes reported in their 24‐h recalls were averaged to obtain one daily intake measure for energy and nutrients.
Statistical analysis
Nutrient intakes were expressed in two ways: as absolute intakes and as energy‐adjusted intakes. Medians and interquartile ranges (IQRs) were calculated.
The reliability of the FFQ was assessed by comparison of nutrient intakes calculated based on the two FFQs completed by each participant. Because most nutrient intakes were not normally distributed, the Wilcoxon matched‐pairs signed rank test was used. In addition, to further evaluate agreement between the two FFQs, intraclass correlation coefficients (ICCs), using a two‐way mixed‐effects model as suggested by Koo et al. 17 were estimated to address variability as a result of differences within and between participants. The following classification was used: poor reliability/reproducibility: <0.5, moderate reliability/reproducibility: 0.75–0.90 and excellent reliability/reproducibility: >0.9 as also used in previous studies. 7 Lastly, individuals were ranked into quartiles on the basis of nutrient intakes from their two FFQs. Agreement and disagreement between the two FFQs were expressed as the proportion of participants classified, respectively, into the same and extreme (top and bottom) quartiles of the distribution, for a given nutrient intake. Agreement was examined using only energy‐adjusted nutrient intakes.
The relative validity of the FFQ was assessed by comparing mean nutrient intake over the 24‐h recalls and daily nutrient intake based on the second FFQ (energy‐adjusted daily macro‐ and micronutrients intake 1 year later [FFQ2]) completed by participants. Associations between nutrient intakes from each method, were measured using Spearman rank correlation, partialled (taking into account) for potential confounders, namely sex and age as a continuous variable. Coefficients <0.3 are considered as indicating a weak correlation, coefficients between 0.3 and 0.49 indicate a fair correlation and coefficients >0.5 indicate a good level of correlation. 18
To further assess agreement between the FFQ and the 24‐h recalls, and to detect any bias with the FFQ relative to the recalls, for each nutrient intake, differences between the two methods were plotted against the means of the two methods, as suggested by Bland and Altman. 19 This analysis was reserved for energy intake and for macro‐ and micronutrients for which the mean difference was normally distributed.
Lastly, individuals were ranked into quartiles on the basis of nutrient intakes from both dietary methods, using only energy‐adjusted nutrient intakes. As above, agreement and disagreement between the two methods was expressed as the proportion of participants classified respectively into the same and extreme (top and bottom) quartiles of the distribution for a given nutrient intake. 20
Sensitivity analyses were also carried out using energy‐adjusted intakes after exclusion of low‐energy reporters (LERs). Energy‐adjusted nutrient intake was calculated using the Atwater general factor system. 21 The basal metabolic rate (BMR) was calculated for each participant using the Henry equation 22 and this was compared to the reported daily energy intake to identify LERs (LER = [BMR × 1.2] − energy intake < −50). 23 Nominal significance was set at p <0.05. All statistical analyses were performed using STATA IC15 (StataCorp LLC).
RESULTS
Eighty‐seven participants were recruited and 68 (78%) completed the study, defined as providing two FFQs, 12 months apart. Thirteen participants dropped out because of time constraints, two participants had to withdraw because of the need to initiate medication or pregnancy and four participants were excluded from analysis because their results were not plausible (energy intake <500 kcal day–1 [n = 2] or >5000 kcal day–1 [n = 2] at the same time as maintaining a constant weight). The demographic and anthropometric characteristics of the participants completing and not completing the study are presented in Table 1. The participants not completing the study did not differ significantly from those completing it.
Table 1.
Demographic and anthropometric characteristics of participants
| Participants completing the study (n = 68) n (%) or median (IQR) | Participants not completing the study (n = 19) n (%) or median (IQR) | |
|---|---|---|
| Sex, males | 30 (44.1) | 10 (52.6) |
| Age | 37.0 (30.5–47.5) | 30.0 (24.0–36.0) |
| Weight baseline (kg) | 72.3 (56.4–81.1) | 71.2 (59.5–84.2) |
| Height baseline (m) | 1.66 (1.61–1.73) | 1.73 (1.62–1.78) |
| BMI baseline (kg m–2) | 24.3 (22.2–28.6) | 23.4 (20.1–27.7) |
| Waist circumference (cm) | 88 (80.0–99.5) | 89 (78.5–97.0) |
| Highest attained education level | ||
| Secondary school | 10 (14.7) | 1 (5.3) |
| College | 9 (13.2) | 1 (5.3) |
| University | 49 (72.1) | 17 (89.4) |
| Marital status | ||
| Not married | 27 (40.3) | 13 (68.4) |
| Married | 35 (52.2) | 6 (31.6) |
| Divorced | 5 (7.5) | 0 (0.0) |
| Smoking status | ||
| Never | 33 (49.3) | 8 (42.1) |
| Past smokers | 9 (13.4) | 7 (36.8) |
| Current | 25 (37.3) | 4 (21.1) |
| Town of residence | ||
| Nicosia (% of Cyprus population = 39%) | 30 (44.1) | 6 (31.6) |
| Limassol (% of Cyprus population = 28%) | 16 (23.5) | 6 (31.6) |
| Larnaca (% of Cyprus population = 17%) | 12 (17.6) | 2 (10.5) |
| Ammochostos (% of Cyprus population = 5.5%) | 5 (7.4) | 1 (5.3) |
| Paphos (% of Cyprus population = 10.5%) | 5 (7.4) | 4 (21.0) |
Abbreviations: BMI, body mass index; IQR, interquartile range.
Table 2 shows the median values and IQRs for reported daily intakes of energy, macro‐ and micronutrients obtained from the two FFQs. The median energy intake in FFQ2 was higher than FFQ1 in the unadjusted data, although the energy‐adjusted intakes of most macro‐ and micronutrients did not differ between the two measurements. As expected, intake distributions were narrower after energy adjustment compared to the unadjusted data. Differences in intakes were statistically significant for the majority of nutrients in the comparisons of unadjusted data, whereas, differences were mostly not significant in the comparisons of energy‐adjusted data, indicating the reliability of the FFQ. Intraclass correlation coefficients between the two FFQs ranged from 0.12 for thiamine to 0.69 for percentage saturated fat and were significant at the p < 0.05 level in all cases except thiamine (mg 1000 kcal–1), vitamin D (μg 1000 kcal–1) and percentage monounsaturated fatty acids (Table 2). Considering all macro‐ and micronutrients, the median (IQR) ICC was 0.46 (0.38–0.52).
Table 2.
Reliability (reproducibility) of the FFQ: Median daily intakes, matched pairs comparison and the intraclass correlation coefficient for the comparison between FFQ1 and FFQ2 (recorded 12 months apart) in Cypriot adults
| All results, raw intakes | All results, energy‐adjusted intakes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FFQ1 | FFQ2 | FFQ1 | FFQ2 | |||||||||||
| Median | IQR | Median | IQR | p value Wilcoxon matched pairs Rank sum test | Median | IQR | Median | IQR | p value Wilcoxon matched pairs Rank sum test | ICC coefficient | 95% CI | p value* | ||
| n | 68 | n | 68 | |||||||||||
| Energy (kcal day–1) | 2563 | 1194 | 2036 | 940 | 0.0002 | Energy (kcal day–1) | 2563 | 1194 | 2036 | 940 | 0.0002 | |||
| Protein (g day–1) | 121.7 | 62.1 | 101.7 | 61.8 | 0.0002 | % Protein | 20.4 | 5.0 | 20.7 | 4.8 | 0.3046 | 0.45 | 0.24–0.62 | <0.0001 |
| Fat (g day–1) | 108.4 | 51.0 | 94.7 | 61.0 | 0.0015 | % Fat | 39.2 | 9.4 | 40.4 | 8.0 | 0.6206 | 0.55 | 0.36–0.69 | <0.0001 |
| Sat fats (g day–1) | 33.7 | 19.4 | 29.6 | 21.4 | 0.0008 | % Sat fats | 12.7 | 3.4 | 12.6 | 3.6 | 0.8931 | 0.69 | 0.55–0.80 | <0.0001 |
| MUFA (g day–1) | 43.7 | 21.4 | 36.3 | 23.0 | 0.0025 | % MUFA | 15.5 | 5.2 | 15.4 | 5.7 | 0.9951 | 0.16 | (−0.08)−0.38 | 0.095 |
| PUFA (g day–1) | 14.6 | 8.5 | 12.1 | 9.0 | 0.0221 | % PUFA | 5.6 | 1.5 | 5.7 | 1.8 | 0.3822 | 0.64 | 0.48–0.76 | <0.0001 |
| Carbohydrate (g day–1) | 231.4 | 118.4 | 198.7 | 95.0 | 0.0003 | % Carbohydrate | 37.6 | 10.7 | 36.9 | 9.1 | 0.8690 | 0.52 | 0.33–0.68 | <0.0001 |
| Tot sugars (g day–1) | 75.1 | 44.9 | 68.1 | 39.1 | 0.1545 | % Total sugars | 12.7 | 6.1 | 12.9 | 5.9 | 0.1528 | 0.32 | 0.09–0.52 | 0.004 |
| NSP (Englyst) g day–1) | 17.0 | 10.4 | 15.0 | 11.7 | 0.0905 | NSP (Englyst) (g 1000 kcal–1) | 6.9 | 3.2 | 6.8 | 3.8 | 0.1769 | 0.39 | 0.17–0.57 | <0.0001 |
| Fibre (AOAC) (g day–1) | 20.4 | 12.2 | 18.7 | 16.4 | 0.1654 | Fibre (AOAC) (g 1000 kcal–1) | 8.6 | 3.7 | 8.7 | 5.1 | 0.1869 | 0.39 | 0.16–0.57 | 0.001 |
| Alcohol (g day–1) | 3.0 | 8.0 | 3.4 | 6.1 | 0.1550 | % Alcohol | 0.9 | 2.4 | 1.2 | 1.9 | 0.8666 | 0.52 | 0.33–0.68 | <0.0001 |
| Cholesterol (mg day–1) | 342.0 | 195.6 | 262.6 | 203.0 | <0.00001 | Cholesterol (mg 1000 kcal–1) | 137.9 | 66.8 | 135.9 | 68.0 | 0.4025 | 0.63 | 0.47–0.76 | <0.0001 |
| Sodium (mg day–1) | 2805.7 | 1948.7 | 2339.6 | 1518.4 | <0.00001 | Sodium (mg 1000 kcal–1) | 1208.0 | 331.4 | 1122.7 | 310.4 | 0.2661 | 0.46 | 0.25–0.63 | <0.0001 |
| Potassium (mg day–1) | 3872.5 | 1706.4 | 3196.9 | 1689.1 | 0.0008 | Potassium (mg 1000 kcal–1) | 1402.7 | 347.4 | 1512.5 | 377.2 | 0.2457 | 0.52 | 0.32–0.67 | <0.0001 |
| Calcium (mg day–1) | 1154.2 | 638.9 | 889.8 | 584.1 | 0.0006 | Calcium (mg 1000 kcal–1) | 462.9 | 170.5 | 428.5 | 170.6 | 0.4199 | 0.33 | 0.10–0.53 | 0.003 |
| Magnesium (mg day–1) | 408.2 | 209.5 | 338.9 | 150.5 | 0.002 | Magnesium (mg 1000 kcal–1) | 159.3 | 39.4 | 166.2 | 42.0 | 0.0477 | 0.56 | 0.37–0.70 | <0.0001 |
| Phosphorus (mg day–1) | 1807.0 | 790.0 | 1452.1 | 789.5 | 0.0002 | Phosphorus (mg 1000 kcal–1) | 699.1 | 150.8 | 718.1 | 155.0 | 0.7185 | 0.38 | 0.16–0.57 | 0.001 |
| Iron (mg day–1) | 15.6 | 5.9 | 12.7 | 6.8 | 0.0002 | Iron (mg 1000 kcal–1) | 6.4 | 2.0 | 6.0 | 1.3 | 0.1328 | 0.36 | 0.13–0.55 | 0.001 |
| Zinc (mg day–1) | 12.1 | 5.9 | 9.9 | 4.6 | 0.0007 | Zinc (mg 1000 kcal–1) | 4.8 | 1.0 | 4.9 | 0.9 | 0.4523 | 0.42 | 0.20–0.60 | <0.0001 |
| Vitamin D (μg day–1) | 2.0 | 1.4 | 1.7 | 1.4 | 0.0263 | Vitamin D (μg 1000 kcal–1) | 0.8 | 0.6 | 0.8 | 0.5 | 0.7139 | 0.18 | (−0.06)−0.40 | 0.068 |
| Vitamin E (mg day–1) | 7.3 | 5.9 | 6.7 | 7.3 | 0.3657 | Vitamin E (mg 1000 kcal–1) | 3.1 | 1.53 | 3.4 | 1.8 | 0.0816 | 0.63 | 0.46–0.75 | <0.0001 |
| Thiamin (mg day–1) | 1.9 | 0.8 | 1.6 | 0.9 | 0.0036 | Thiamin (mg 1000 kcal–1) | 0.8 | 0.2 | 0.8 | 0.2 | 0.8307 | 0.12 | (−0.12)−0.35 | 0.165 |
| Riboflavin (mg day–1) | 1.9 | 0.9 | 1.4 | 0.7 | 0.0003 | Riboflavin (mg 1000 kcal–1) | 0.7 | 0.2 | 0.7 | 0.3 | 0.3789 | 0.34 | 0.11–0.53 | 0.002 |
| Vitamin B6 (mg day–1) | 2.2 | 1.3 | 1.9 | 1.2 | 0.0006 | Vitamin B6 (mg 1000 kcal–1) | 0.9 | 0.3 | 0.9 | 0.4 | 0.6556 | 0.29 | 0.05–0.49 | 0.009 |
| Vitamin B12 (μg day–1) | 3.8 | 2.7 | 3.3 | 2.7 | 0.0111 | Vitamin B12 (μg 1000 kcal–1) | 1.6 | 0.8 | 1.7 | 1.0 | 0.8882 | 0.49 | 0.28–0.68 | <0.0001 |
| Folate (μg day–1) | 245.0 | 125.4 | 196.3 | 139.6 | 0.0008 | Folate (μg 1000 kcal–1) | 99.1 | 25.9 | 97.6 | 40.3 | 0.7461 | 0.43 | 0.21–0.60 | <0.0001 |
| Pantothenic acid (mg day–1) | 5.9 | 3.0 | 5.2 | 2.6 | 0.0003 | Pantothenic acid (mg 1000 kcal–1) | 2.5 | 0.8 | 2.4 | 0.7 | 0.6336 | 0.50 | 0.30–0.66 | <0.0001 |
| Vitamin C (mg day–1) | 77.9 | 61.3 | 67.6 | 55.2 | 0.0894 | Vitamin C (mg 1000 kcal–1) | 30.4 | 24.9 | 34.4 | 22.0 | 0.6867 | 0.24 | 0.001–0.45 | 0.025 |
| Vitamin A RAE (μg day–1) | 518.0 | 422.9 | 400.7 | 381.9 | 0.0143 | Vitamin A RAE (μg 1000 kcal–1) | 210.1 | 130.6 | 174.1 | 119.9 | 0.6511 | 0.47 | 0.26–0.64 | <0.0001 |
| Niacin equivalents (mg day–1) | 48.9 | 29.0 | 41.7 | 26.0 | 0.0002 | Niacin equivalents (mg 1000 kcal–1) | 20.2 | 5.8 | 19.3 | 7.3 | 0.1617 | 0.46 | 0.25–0.63 | <0.0001 |
p value for the ICC. p < 0.05 indicates a statistically significant agreement between FFQ1 and FFQ2.
Abbreviations: FFQ, food frequency questionnaire; FFQ1, energy‐adjusted daily macro‐ and micronutrients intake at baseline; FFQ2, energy‐adjusted daily macro‐ and micronutrients intake 1 year later; ICC, intraclass correlation coefficients; IQR, interquartile range; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; RAE, retinol activity equivalents.
A paired comparison of daily intakes of energy and nutrients from the two FFQs, excluding low energy reporters, is provided in the Supporting information (Table S1), indicating that the results of this sensitivity analysis are similar to the results of the analysis of all participants.
The level of agreement between the reported nutrient intakes in the two FFQs, as a percentage of participants whose consumption was in the same quartile for both FFQs, is provided in the Supporting information (Table S2), including and excluding LERs. Agreement was satisfactory (>30%) for most micro‐ and macronutrients. More specifically, agreement was more than 38% for protein, fats, carbohydrates, starch, total sugars, fibre, saturated fats, polyunsaturated fats, trans‐fats and alcohol.
Table 3 shows the median values and IQRs for reported daily intakes of energy, macro‐ and micronutrients obtained from the two methods (24‐h recalls and FFQ2). Even though median energy intake in FFQ2 was higher than in the 24‐h recalls, energy‐adjusted intakes of macro and micronutrients were significantly correlated between the two methods. The significant correlations, adjusted for age and sex, support the validity of the FFQ. The results were similar after exclusion of LERs, as shown in the Supporting information (Table S3).
Table 3.
Relative validity of the FFQ: Comparison of daily intakes of energy and nutrients from the average of 24‐h recalls and the FFQ2
| All results, energy‐adjusted intakes | LER excluded, energy‐adjusted intakes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24‐h recall | FFQ2 | 24‐h recall | FFQ2 | |||||||||||
| Median | IQR | Median | IQR | Spearman Partial correlation coefficient (rho) | p value Spearman partial correlation | Median | IQR | Median | IQR | Spearman partial correlation coefficient (rho) | p value Spearman partial correlation | |||
| n | 65 | 68 | 65 | 45 | 52 | 45 | ||||||||
| Energy (kcal day–1) | 1868 | 635 | 2036 | 940 | 0.24 | 0.0546 | 1881 | 731 | 2201 | 1055 | 0.42 | 0.0039 | ||
| % Protein | 19.2 | 6.0 | 20.7 | 4.8 | 0.35 | 0.0049 | 19.2 | 5.6 | 20.0 | 4.1 | 0.42 | 0.0039 | ||
| % Fat | 40.8 | 9.0 | 40.4 | 8.0 | 0.39 | 0.0013 | 41.1 | 5.8 | 40.4 | 7.9 | 0.32 | 0.0309 | ||
| % Sat fats | 12.8 | 3.5 | 12.6 | 3.6 | 0.38 | 0.002 | 12.9 | 3.8 | 12.7 | 3.9 | 0.16 | 0.2832 | ||
| % MUFA | 14.6 | 5.3 | 15.4 | 5.7 | 0.17 | 0.1909 | 14.8 | 5.5 | 15.6 | 5.5 | 0.26 | 0.0898 | ||
| % PUFA | 5.9 | 2.8 | 5.7 | 1.8 | 0.19 | 0.1338 | 5.7 | 3.1 | 5.7 | 1.8 | 0.09 | 0.5526 | ||
| % Carbohydrate | 39.4 | 8.4 | 36.9 | 9.1 | 0.45 | 0.0002 | 39.3 | 6.4 | 35.7 | 9.7 | 0.37 | 0.0115 | ||
| % Tot sugars | 13.4 | 7.1 | 12.9 | 5.9 | 0.27 | 0.0283 | 13.4 | 6.7 | 12.9 | 6.3 | 0.31 | 0.0355 | ||
| % Alcohol | 0.0 | 1.6 | 1.2 | 1.9 | 0.04 | 0.782 | 0.0 | 1.5 | 1.2 | 2.1 | 0.45 | 0.0021 | ||
| Cholesterol (mg 1000 kcal–1) | 124.3 | 91.6 | 135.9 | 68.0 | 0.40 | 0.0012 | 123.8 | 89.5 | 135.9 | 70.6 | 0.32 | 0.0296 | ||
| NSP (Englyst) (g 1000 kcal–1) | 5.8 | 4.7 | 6.8 | 3.8 | 0.46 | 0.0001 | 5.7 | 4.4 | 6.7 | 4.1 | 0.37 | 0.0118 | ||
| Dietary fibre (AOAC method) (g 1000 kcal–1) | 7.5 | 6.3 | 8.7 | 5.1 | 0.44 | 0.0003 | 6.9 | 5.9 | 8.5 | 5.3 | 0.38 | 0.011 | ||
| Sodium (mg 1000 kcal–1) | 1048.0 | 611.6 | 1122.7 | 310.4 | 0.30 | 0.0144 | 1027.8 | 606.0 | 1079.7 | 279.4 | 0.36 | 0.0149 | ||
| Potassium (mg 1000 kcal–1) | 1371.1 | 421.4 | 1512.5 | 377.2 | 0.34 | 0.0059 | 1332.9 | 390.1 | 1479.8 | 397.6 | 0.21 | 0.1624 | ||
| Calcium (mg 1000 kcal–1) | 390.6 | 195.9 | 428.5 | 170.6 | 0.40 | 0.0009 | 390.6 | 203.6 | 430.3 | 170.6 | 0.42 | 0.0038 | ||
| Magnesium (mg 1000 kcal–1) | 155.5 | 51.6 | 166.2 | 42.0 | 0.49 | <0.00001 | 155.1 | 50.7 | 161.9 | 43.5 | 0.30 | 0.0442 | ||
| Phosphorus (mg 1000 kcal–1) | 650.9 | 237.0 | 718.1 | 155.0 | 0.21 | 0.1016 | 589.7 | 229.7 | 673.1 | 146.0 | 0.23 | 0.1344 | ||
| Iron (mg 1000 kcal–1) | 6.0 | 2.5 | 6.0 | 1.3 | 0.00 | 0.9811 | 5.9 | 2.3 | 5.9 | 1.2 | 0.04 | 0.8006 | ||
| Zinc mg 1000 kcal–1) | 4.6 | 1.9 | 4.9 | 0.9 | 0.09 | 0.5014 | 4.4 | 1.6 | 4.8 | 0.9 | 0.24 | 0.1159 | ||
| Vitamin D (μg 1000 kcal–1) | 0.9 | 0.8 | 0.8 | 0.5 | 0.43 | 0.0004 | 0.7 | 0.9 | 0.8 | 0.5 | 0.35 | 0.0191 | ||
| Vitamin E (mg 1000 kcal–1) | 3.6 | 3.2 | 3.4 | 1.8 | 0.36 | 0.0034 | 3.4 | 3.7 | 3.4 | 1.8 | 0.28 | 0.059 | ||
| Thiamin (mg 1000 kcal–1) | 0.7 | 0.4 | 0.8 | 0.2 | 0.07 | 0.5677 | 0.7 | 0.3 | 0.8 | 0.2 | 0.05 | 0.7407 | ||
| Riboflavin (mg 1000 kcal–1) | 0.7 | 0.4 | 0.7 | 0.3 | 0.39 | 0.0013 | 0.6 | 0.4 | 0.7 | 0.3 | 0.55 | 0.0001 | ||
| Vitamin B6 mg 1000 kcal–1) | 0.8 | 0.3 | 0.9 | 0.4 | 0.17 | 0.1804 | 0.8 | 0.3 | 0.9 | 0.4 | 0.11 | 0.4913 | ||
| Vitamin B12 (μg 1000 kcal–1) | 1.5 | 1.4 | 1.7 | 1.0 | 0.27 | 0.0321 | 1.2 | 1.7 | 1.7 | 1.0 | 0.36 | 0.0144 | ||
| Folate (μg 1000 kcal–1) | 93.1 | 53.5 | 97.6 | 40.3 | 0.11 | 0.3986 | 83.4 | 42.2 | 97.2 | 41.4 | −0.03 | 0.8595 | ||
| Pantothenic acid (mg 1000 kcal–1) | 2.2 | 1.4 | 2.4 | 0.7 | 0.22 | 0.0797 | 2.2 | 1.4 | 2.3 | 0.7 | 0.28 | 0.0631 | ||
| Vitamin C (mg 1000 kcal–1) | 25.8 | 31.2 | 34.4 | 22.0 | 0.26 | 0.0392 | 24.9 | 30.5 | 34.4 | 22.0 | 0.27 | 0.0783 | ||
| Vitamin A RAE (μg day–1) | 142.9 | 176.6 | 580.2 | 399.5 | −0.01 | 0.9155 | 133.0 | 196.1 | 612.1 | 462.6 | 0.17 | 0.2543 | ||
| Niacin equivalents (mg 1000 kcal–1) | 18.4 | 7.2 | 19.3 | 7.3 | 0.28 | 0.0227 | 17.5 | 7.8 | 18.5 | 6.3 | 0.29 | 0.0552 | ||
Abbreviations: FFQ, food frequency questionnaire; FFQ2, energy‐adjusted daily macro‐ and micronutrients intake 1 year later; IQR, interquartile range; LER, low‐energy reporters; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; RAE, retinol activity equivalents.
The level of agreement between reported nutrient intakes in the two methods (24‐h recalls vs. FFQ2), as a percentage in the same quartile, is shown in the Supporting information (Table S4), including and excluding LERs. Agreement was satisfactory (>30%) for most micro‐ and macronutrients. More specifically, agreement was more than 30% for protein, starch, total sugars, trans‐fats and fibre. Misclassification into extreme quartile was evident, however, for saturated fat, polyunsaturated fatty acids, alcohol, potassium, iron, vitamin E and vitamin B6, folate. This is probably a result of the variability, both daily and seasonal, that naturally occurs in nutrient intake. As also shown in previous research, the reliability of 24‐h recalls for assessing some nutrients is lower than others. 24
Bland–Altman plots of energy and macronutrient intakes with normally distributed mean differences are shown in Figure 2. In each plot, the dashed horizontal line represents the mean difference in the intake measurement between the FFQ and the 24‐h recalls, and the shaded area shows the limits of agreement (mean difference ± 2 SD). Of the intake measurements shown in Figure 2, substantial over‐reporting when using the FFQ2 compared to the 24‐h recalls is evident only for energy intake (kcal day–1); however, the mean difference in energy reporting between the two methods was not normally distributed. For the remaining macronutrients, intakes display very good agreement between the two methods used. Similar results were obtained for micronutrients for which the mean difference between the two methods was normally distributed, such as iron, calcium, vitamin C and vitamin E (data not shown).
Figure 2.
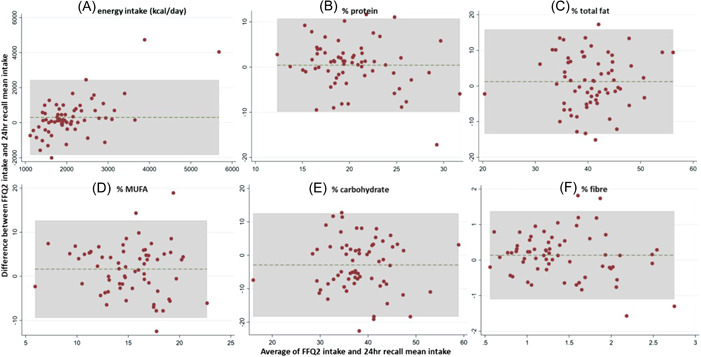
Bland–Altman plots indicating non‐significant or negligible bias when comparing the energy‐adjusted daily macro‐ and micronutrients intake 1 year later (FFQ2) with the 24‐h recall mean intakes for (a) energy (kcal day–1): mean difference (MD) = 300.98, 95% confidence interval (CI) = 30.18–571.79; (b) % protein: MD = 0.97, 95% CI = −0.87–1.74; (c) % total fat: MD = 1.23, 95% CI = −0.62, 3.08; (d) % monounsaturated fatty acids (MUFA): MD = 1.63, 95% CI = 0.24, 3.02; (e) % carbohydrate: MD = −2.89, 95% CI = −4.85 to −0.94; and (f) % fibre: MD = 0.16, 95% CI = −0.05, 0.36. The solid line represents the mean difference between the two methods used (FFQ and 24‐h recalls), whereas the dashed line represents the distance between the limits of agreement (mean difference ± 2 SD). FFQ, food frequency questionnaire.
DISCUSSION
The present study developed and validated a previously lacking FFQ for assessing dietary intake in Cypriot adults. The FFQ comprises of 171 food items commonly consumed in Cyprus including traditional foods and culturally specific dishes, as well as taking into account the transition towards a Westernised diet. Reproducibility or reliability was evaluated by comparing the same FFQ completed at baseline and 12 months (1 year) later, whereas relative validity was evaluated by comparing the nutrient intakes as estimated by the FFQ of the intake during the previous year with the intakes obtained from three 24‐h recalls completed over the year being assessed. The study has thus evaluated the extent to which the two dietary methods produce similar rankings of study participants according to the intake of individual nutrients. Good agreement was observed between the energy‐adjusted nutrient intakes calculated from the two FFQs recorded by the same participant 12 months apart, indicating the questionnaire's reliability. A high level of agreement between reported nutrient intakes in the two FFQs was also shown. Furthermore, the questionnaire's validity was confirmed by examining it against 24‐h recalls and showing significant correlations between the two dietary assessment methods. Overall, the above indicate that the FFQ is a reliable and valid dietary assessment tool to be used in both sexes in the Cypriot population.
More specifically, the results suggest that the FFQ had a high reliability because the energy‐adjusted intakes did not differ significantly between the two FFQs. The difference in energy intake is a reflection of the complexity of dietary assessment, where the mere action of (repeated) dietary reporting can lead to changes in the actual intake and recording because the person becomes more aware of their own intake by recording it.
With regard to the observed variations between the FFQ and the 24‐h recalls, our study agrees with previous reports showing that energy intake is overestimated when recorded using FFQs compared to 24‐h recalls. 25 Nevertheless, the correlation coefficients from this study are comparable, albeit on the lower end, to those for the Willett FFQ when adjusted for. 26 A previous study in an adult French population also demonstrated acceptable validity and reproducibility of a FFQ with crude Pearson correlation coefficients for relative validity ranging from 0.28 to 0.67 in men and from 0.25 to 0.55 in women. In the same study, the median Pearson correlation coefficients for reproducibility for men and women were 0.70 and 0.65, respectively. 27 The energy‐adjusted correlation coefficients between FFQ and 24‐h recalls in a study in Moroccan adults were higher, in the range 0.53–0.73. 28 For a dietary instrument to be appropriate in detecting associations between diet and disease, it has been suggested that correlations between that instrument and other dietary assessment methods need to be at least 0.3 or 0.4. 29 The correlation in the present study was >0.3 between FFQ and 24‐h recall for most macronutrients, with the exception of percentage energy from saturated and polyunsaturated fat and some micronutrients. The Bland–Altman analysis showed a high percentage of agreement between the FFQ2 and the 24‐h recall because the mean difference for key macro‐ and micronutrient intakes demonstrated non‐significant or negligible deviations from the expected value of 0. 30 Previous studies also report on similar limits of agreement. 6 , 7
A number of reasons could explain the lower correlation coefficients observed in the present study compared to other studies. One reason is the limited sample size, which, even though sufficient in reference to the Cypriot population size (0.01% of the population), in absolute numbers, it is smaller compared to other studies, such as in that carried out by Subar et al. 26 for which the sample comprised approximately 230 participants and the study by Kesse‐Guyot et al. 27 for which the sample comprised 140 participants. Moreover, the period between the administration of the two FFQs in our study was 1 year, whereas, other studies, which report higher agreements, use shorter time frames, such as 1 month 28 or 4 months. 7 As per the recommendations by Cade et al. 2 on FFQ reproducibility, the ‘interval between repeat measurements should be chosen to minimise changes over time and recall of previous answers and will depend on the reference period of the questionnaire’. Because this FFQ questionnaire was designed to assess diet over the previous year, the chosen time interval between the two assessments was 1 year. True changes in intake and variation in response over 1 year could have contributed to a reduced reproducibility. 31
A further reason could be related to the seasonal produce consumed, such as fruit and vegetables (e.g., watermelon consumed in the summer and citrus fruit consumed in the winter), as well as the seasonality of various local dishes (e.g., ‘kolokasi’, ‘kleftiko’ and others). This seasonality may be more variable compared to other populations such as the UK. Moreover, the population characteristics, as well as under‐reporting differ between studies, leading to variations in the results. Overweight and obese individuals, women and older subjects, those with a lower socio‐economic class and lower education, smoking, eating habits including dieting and psychological factors have all been found to be associated with misreporting energy and nutrient intake to various extends. 32 Biomarkers such as 24‐h urine samples or doubly‐labelled water can provide objective assessments of dietary intake because their errors in measurement are weakly correlated to those for self‐reported dietary methods and are not influenced by diet. For these reasons, further validation of this FFQ against urine biomarkers is currently being planned by the research team.
Our study has a number of strengths and limitations. With regard to strengths, to the best of our knowledge, this is the first comprehensive FFQ to be designed and used for assessing the dietary intake of Cypriot adults and includes a number of traditional and culture‐specific foods and dishes. Previous studies assessing diet of Cypriot adults either used 24‐h recalls or 3‐day food diaries. 33 or FFQs not specifically validated for this population. 34 As expected, some variation between energy intake assessed using the 24‐h recalls and the FFQ was demonstrated. By collecting three 24‐h recalls during the year, the effects of some of the inherent limitations of this diet assessment method, such as reliance on memory and high day‐to‐day variation, were reduced. Some other sources of error in this study include portion size estimation that might have also differed in different foods such as for example, seasonal foods (e.g., fruit) versus foods consumed on a daily basis (e.g., bread), types of foods and food groups (e.g, it is easier to estimate the portion size of milk compared to the portion size of breakfast cereals or rice/pasta), as well as assessment season and time (i.e., repetition of assessment). By using the Block Portion Size Pictures and assigning a standard portion size and specific amount of grams or millilitres to each food portion, we reduced the error related to conceptualising food portions, as well as limited the within‐person variation in portion size when the same food is consumed on different occasions. 35 Other possible sources of error include the inherent variations between the actual versus estimated nutrient content of the foods. Because only a limited number of Cypriot foods have been analysed and only a few nutrients are included in these analyses, 13 the main database used to analyse the FFQ and the 24‐h recalls was UK‐based to which the Cypriot food analyses were added. Nevertheless, to reduce these sources of error, we used the same food codes to analyse both FFQs and 24‐h recalls and run our analyses twice to confirm the findings. Additionally, because the day of the week may also influence the validation results, we took care to assess dietary intake both during weekdays and weekends on a proportional basis.
Another important type of response bias common in all nutritional assessment studies, and, also shown here, is LER, which was high in both assessment methods. Reporting energy intakes of <1.2 times the calculated BMR is not compatible with requirements in the long term and thus unlikely to be the true intake. 23 As mentioned above, a number of factors are associated with under‐reporting. However, we found similar results in sensitivity analyses after excluding LERs. Previous studies have also supported the use of energy‐adjusted data with the limitation that this method may obscure diet–disease relationships, if absolute intake rather than nutritional composition is driving the effect. 20 In epidemiology, however, the primary aim is often to place individuals in the correct rank order, rather than to make accurate estimates of absolute intakes, 20 something that was successfully achieved in the present study, as shown by the agreement between the two dietary assessment methods. Finally, as mentioned above, the present relative validation is limited by its subjective nature; nevertheless, further validation using urine biomarkers is planned to provide an objective assessment of dietary intake.
In conclusion, the validated FFQ provides an important tool for the assessment of dietary intake of Cypriot adults, thus allowing a comprehensive assessment of dietary intakes of important macro‐ and micronutrients in epidemiological research and public health interventions. This is of paramount importance because the FFQ will offer opportunities to investigate associations of the culture‐specific Mediterranean diet consumed in Cyprus with health outcomes and also pave the way for planning population‐tailored interventions.
AUTHOR CONTRIBUTIONS
E. Philippou is the principal investigator of the study. E. Philippou, G. Loucaides, C. A. Demetriou and K. Kyriacou conceptualised the study. E. Philippou and G. Loucaides, both experienced registered dietitians, developed the FFQ. E. Philippou, G. Loucaides, N. Solomonidou, M. Polykarpou and S. Sioulis carried out data collection and analysis. C. A. Demetriou assisted in data collection and carried out statistical analysis. E. Philippou and C. A. Demetriou wrote the paper. E. Critselis assisted in the interpretation of the findings and revised the first draft of the manuscript. A. Hadjisavvas and K. Kyriacou supervised the study. All authors read and approved the final version of the manuscript submitted for publication.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
TRANSPARENCY DECLARATION
The lead author affirms that this manuscript is an honest, accurate and transparent account of the study being reported. The reporting of this work is compliant with STROBE2 guidelines. The lead author affirms that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
ETHICAL STATEMENT
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Cyprus National Bioethics Committee (ΕΕΒΚ/ΕC/2016/11). Written informed consent was obtained from all participants prior to baseline assessment subjects.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jhn.13032
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
We are grateful to the participants for their involvement in the study, as well as to the University of Nicosia BSc and MSc students who assisted with the data collection and analysis, namely: Vasiliki Karkoni and Andriani Mixopoulou. We thank Mrs Christine Hadjiyianni for her assistance in the graphical presentation of the FFQ and Mrs Louiza Potamiti for laboratory assistance. This work was supported by Livadiotis Fruit & Nuts – G. Charalambous Ltd and by the NUTRICIA medical nutrition products distributed in Cyprus by MSJ group of companies. Internal financial support was also provided by the University of Nicosia and by the Cyprus Institute of Neurology and Genetics. The aforementioned funders had no role in the design, analysis or writing of this article.
Biographies
Dr Christiana Demetriou is an Assistant Professor of Epidemiology and Public Health at the University of Nicosia Medical School. Her research interests include neurological disease and cancer epidemiology.
Dr Elena Philippou, PhD, RD, FHEA, is an Associate Professor in Nutrition‐Dietetics at the University of Nicosia, Cyprus and a Visiting Lecturer in Nutritional Sciences at King's College London, UK. Her research interest focuses on the diet for prevention of degenerative disease specifically cognitive decline and rheumatic disease.
Dr George Loucaides, RGU Nutrition and Dietetics, is PhD from London Metropolitan University. Dietitian at the Cyprus Institute of Neurology, his interests include the effects omega 3 on MS and Parkinson's Disease, Alzheimer's Disease and intestinal bioflora.
Ms Nastazia Solomonidou is a clinical and public health dietitian.
Dr Elena Critselis is an Associate Professor in Epidemiology and Public Health and the Head of the Department of Primary Care and Population Health at the University of Nicosia Medical School.
Ms Maria Polykarpou is a clinical dietitian.
Mr Spyros Sioulis is a clinical dietitian.
Dr Andreas Hadjisavvas is a Scientist at the Department of Electron Microscopy and Molecular Pathology, The Cyprus Institute of Neurology and Genetics, and Professor at the Cyprus School of Molecular Medicine with special interest in cancer genetics.
Prof Kyriacos Kyriacou is Emeritus Professor in Biochemistry and Founder of the department of Electron Microscopy/Molecular Pathology, at the Cyprus Institute of Neurology and Genetics. He served as Dean of the Schooland he is an expert on the Molecular Pathology of Cancer.
Philippou E, Demetriou CA, Loucaides G, Solomonidou N, Critselis E, Polykarpou M, et al. Relative validity and reproducibility of the CyFFQ semiquantitative food frequency questionnaire for assessing dietary intake in Cypriot adults. J Hum Nutr Diet. 2023;36:139–153. 10.1111/jhn.13032
REFERENCES
- 1. Willett W. Nutritional epidemiology. 3rd ed. New York: Oxford University Press; 2012. [Google Scholar]
- 2. Cade J, Thompson R, Burley V, Warm D. Development, validation and utilisation of food‐frequency questionnaires ‐ a review. Public Health Nutr. 2002;5(4):567–87. [DOI] [PubMed] [Google Scholar]
- 3. Serra‐Majem L, Frost Andersen L, Henríque‐Sánchez P, Doreste‐Alonso J, Sánchez‐Villegas A, Ortiz‐Andrelluchi A, et al. Evaluating the quality of dietary intake validation studies. Br J Nutr. 2009;102:S3–9. [DOI] [PubMed] [Google Scholar]
- 4. Sharma S. Development and use of FFQ among adults in diverse settings across the globe. Proc Nutr Soc. 2011;70(2):232–51. [DOI] [PubMed] [Google Scholar]
- 5. Trepanowski JF, Bloomer RJ. The impact of religious fasting on human health. Nutr J. 2010;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Athanasiadou E, Kyrkou C, Fotiou M, Tsakoumaki F, Dimitropoulou A, Polychroniadou E, et al. Development and validation of a Mediterranean oriented culture‐specific semi‐quantitative food frequency questionnaire. Nutrients. 2016;8(9): 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aoun C, Bou Daher R, El Osta N, Papazian T, Khabbaz LR. Reproducibility and relative validity of a food frequency questionnaire to assess dietary intake of adults living in a Mediterranean country. PLoS One. 2019;14(6):e0218541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papathanasiou G, Georgoudis G, Papandreou M, Spyropoulos P, Georgakopoulos D, Kalfakakou V, et al. Reliability measures of the short International Physical Activity Questionnaire (IPAQ) in Greek young adults. Hellenic J Cardiol. 2009;50(4):283–94. [PubMed] [Google Scholar]
- 9. Gnardellis C, Trichopoulou A, Katsouyanni K, Polychronopoulos E, Rimm EB, Trichopoulos D. Reproducibility and validity of an extensive semiquantitative food frequency questionnaire among Greek school teachers. Epidemiology. 1995;6(1):74–7. [DOI] [PubMed] [Google Scholar]
- 10.NutritionQuest. Block portion size pictures. 2021. [cited 2021 Jan 10]. Available from: https://nutritionquest.com
- 11. US Department of Agriculture . Food patterns equivalents database. 2021. [cited 2021 Jan 10]. Available from: https://data.nal.usda.gov/dataset/food-patterns-equivalents-database-fped
- 12. ForeSoft . Dietplan. 2019. [cited 2019 Mar 12]. Available from: www.foresoft.co.uk
- 13. Republic of Cyprus State General Labolatory . Cyprus food composition tables. 2013. [cited 2019 Mar 13]. Available from: https://www.moh.gov.cy/MOH/SGL/sgl.nsf/All/72C8C9F6F124F979C22583C5003E694F/$file/Cyprus%20Food%20Composition%20Tables%20-%203rd%20Edition.pdf
- 14. US Department of Agriculture . FoodData central. 2021. [cited 2019 Jan 10]. Available from: https://fdc.nal.usda.gov
- 15. Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5‐step multiple‐pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77(5):1171–8. [DOI] [PubMed] [Google Scholar]
- 16. Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five‐step multiple‐pass method in men: an observational validation study. J Am Diet Assoc. 2004;104(4):595–603. [DOI] [PubMed] [Google Scholar]
- 17. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hankin JH, Wilkens LR, Kolonel LN, Yoshizawa CN. Validation of a quantitative diet history method in Hawaii. Am J Epidemiol. 1991;133(6):616–28. [DOI] [PubMed] [Google Scholar]
- 19. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 20. Brunner E, Stallone D, Juneja M, Bingham S, Marmot M. Dietary assessment in Whitehall II: comparison of 7 d diet diary and food‐frequency questionnaire and validity against biomarkers. Br J Nutr. 2001;86(3):405–14. [DOI] [PubMed] [Google Scholar]
- 21. Atwater WO, Bryant AP. The availability and fuel value of food materials. Washington, DC: US Government Printing Office; 1900. [Google Scholar]
- 22. Henry CJ. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr. 2005;8(7A):1133–52. [DOI] [PubMed] [Google Scholar]
- 23. Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, et al. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut‐off limits to identify under‐recording. Eur J Clin Nutr. 1991;45(12):569–81. [PubMed] [Google Scholar]
- 24. Pao EM, Mickle SJ, Burk MCOne‐day. and 3‐day nutrient intakes by individuals–Nationwide Food Consumption Survey findings, Spring 1977. J Am Diet Assoc. 1985;85(3):313–24. [PubMed] [Google Scholar]
- 25. Collins CE, Boggess MM, Watson JF, Guest M, Duncanson K, Pezdirc K, et al. Reproducibility and comparative validity of a food frequency questionnaire for Australian adults. Clin Nutr. 2014;33(5):906–14. [DOI] [PubMed] [Google Scholar]
- 26. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089–99. [DOI] [PubMed] [Google Scholar]
- 27. Kesse‐Guyot E, Castetbon K, Touvier M, Hercberg S, Galan P. Relative validity and reproducibility of a food frequency questionnaire designed for French adults. Ann Nutr Metab. 2010;57(3–4):153–62. [DOI] [PubMed] [Google Scholar]
- 28. El Kinany K, Garcia‐Larsen V, Khalis M, Deoula MMS, Benslimane A, Ibrahim A, et al. Adaptation and validation of a food frequency questionnaire (FFQ) to assess dietary intake in Moroccan adults. Nutr J. 2018;17(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Collins AC, Ward KD, Mirza B, Slawson DL, McClanahan BS, Vukadinovich C. Comparison of nutritional intake in US adolescent swimmers and non‐athletes. Health. 2012;4(10):873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giavarina D. Understanding Bland Altman analysis. Biochem Med (Zagreb). 2015;25(2):141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsubono Y, Nishino Y, Fukao A, Hisamichi S, Tsugane S. Temporal change in the reproducibility of a self‐administered food frequency questionnaire. Am J Epidemiol. 1995;142(11):1231–5. [DOI] [PubMed] [Google Scholar]
- 32. Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van't Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr. 2009;101(Suppl 2):S73–85. [DOI] [PubMed] [Google Scholar]
- 33. Andreou, E , Hajigeorgiou, Pg , Kyriakou. K , et al. Risk factors of obesity in a cohort of 1001 Cypriot adults: an epidemiological study. Hippokratia. 2012;16(3):256–60. [PMC free article] [PubMed] [Google Scholar]
- 34. Demetriou CA, Hadjisavvas A, Loizidou MA, Loucaides G, Neophytou I, Sieri S, et al. The Mediterranean dietary pattern and breast cancer risk in Greek‐Cypriot women: a case‐control study. BMC Cancer. 2012;12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willett WC. Invited commentary: comparison of food frequency questionnaires. Am J Epidemiol. 1998;148(12):1157–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


