Abstract
Objectives
A microneedling pen has been cleared by the US Food and Drug Administration, indicated for improving the appearance of adult facial acne scars. The objective of this study was to assess the device's effectiveness for treating wrinkles of the face area.
Materials and Methods
Healthy adults seeking to improve the appearance of face wrinkles were enrolled (N = 35), receiving four monthly microneedling procedures by a trained aesthetician who treated the face skin per manufacturer instructions. Wrinkle assessments were performed by two trained blinded raters by comparing baseline images of each subject with images obtained at 90 days post‐procedure. Subsequently, the two raters were unblinded for the Clinician's Global Aesthetic Improvement Scale (CGAIS) assessment. Subjects completed the Subject's Global Aesthetic Improvement Scale (SGAIS) and a Satisfaction Questionnaire at 30 and 90 days post‐treatment.
Results
The study was completed by 32 subjects with a mean (SD) age of 56.3 (5.0) years. Wrinkle assessments demonstrated significant improvement in the face areas (p < 0.001). The SGAIS scores showed significant improvements after 30 and 90 days post‐treatment (for each, p < 0.001). The CGAIS scores also showed significant improvements at 90 days post‐treatment (p < 0.001). Most subjects reported some level of improvement in their appearance at 30 days (73.3%) and 90 days (68.8%) post‐treatment. The satisfaction questionnaire showed high levels of improvement in wrinkles (93.8%), satisfaction with the treatment procedure (87.5%) and would recommend microneedling to friends and family members (80.6%) on the face and neck.
Conclusion
Microneedling is a viable, minimally invasive option for treating wrinkles of the face. ClinicalTrials.gov Identifier: NCT03803059.
Keywords: clinical trial, efficacy, microneedling, wrinkles
1. INTRODUCTION
During the past decade, microneedling has gained popularity as a safe, effective, and affordable aesthetic procedure. 1 Like many other rejuvenation techniques, microneedling is a method of mechanically inducing skin remodeling. 1 , 2 , 3 , 23 It is a minimally invasive procedure consisting of controlled, superficial puncturing of the skin with fine needles 4 which stimulates the normal wound healing process whereby an initial inflammatory reaction is followed by the proliferation of the extracellular matrix and remodeling of new dermal tissues. 5
Physiological changes associated with microneedling include upregulation of genes associated with tissue remodeling and wound healing, epithelial proliferation and differentiation, immune cell recruitment, and down‐regulation of pro‐inflammatory cytokines. 6 Microneedling significantly increases baseline collagen types I, III, and VII, newly synthesized collagen, and tropoelastin. 7 As a result, microneedling therapy is used to improve the appearance of facial scars, 8 , 9 , 20 stretch marks, 10 , 11 rejuvenate photoaged skin, 7 and dyschromia conditions. When used as a drug delivery system, microneedling has been used to treat alopecia, pigmentary disorders, and actinic keratoses. 12 , 22
An automated, non‐surgical microneedling pen was the first to be cleared by the US Food and Drug Administration as a microneedling device (SkinPen® Microneedling System; Crown Aesthetics, Dallas, TX) and was originally cleared with the indication as a procedure for improving the appearance of facial acne scars in adults aged 22 years or older; however, recent evidence supports the use of microneedling for treating skin rhytides. 13 , 14 , 15 Based on these promising results, the following study was performed to assess the effectiveness of the microneedling pen for treating wrinkles on the face and neck. This paper is a subjective endpoints companion to the earlier‐published objective endpoints paper, where noninvasive measurements and biopsy data of the face showed changes in skin architecture and collagen/elastin gene expression, suggesting skin rejuvenation, with new extracellular matrix production and muscle formation. 16
2. MATERIALS AND METHODS
2.1. Study subjects
Eligible subjects were healthy men and women, 35‐ to 65‐year‐old, seeking treatment to improve the appearance of wrinkles on the face and neck. Each subject expressed their willingness to comply with all study requirements and refrain from prohibited procedures including soft tissue fillers, resurfacing therapies, botulinum toxins, injectable fillers, microdermabrasion, laser and light procedures, skin tightening, or laser facial hair removal for the duration of the study. Waxing and threading were allowed. Women of childbearing potential were required to provide a negative urine pregnancy test at the baseline and 3‐month post‐treatment visits and agreed to use an acceptable method of birth control during the study.
Subjects were excluded from participation if they had known allergies to skin care products or topical lidocaine; a systemic or local disease or condition or medication affecting wound healing or any uncontrolled systemic disease; severe solar elastosis; recent trauma or scarring other than acne scars on the planned treatment area; severe or clinically significant acne on the planned treatment area, defined as >5 active inflammatory acne lesions including acne conglobate, nodules, or cysts in a planned treatment area; a history of hypertrophic or keloid scars; cancerous or pre‐cancerous lesions in the planned treatment areas or a history of skin cancer; inability to understand the instructions or provide informed consent; history of chronic drug or alcohol abuse; subjects undergoing concurrent therapy that might place the subject at risk or jeopardize study objectives; current smoker or smoked in the last 5 years; had undergone the following cosmetic treatments (time‐frame) in the planned treatment area: microdermabrasion or glycolic acid treatment (1 month), skin tightening (1 year), injectable filler including hyaluronic acid (12 months), calcium hydroxylapatite (12 months), poly‐L‐lactic (24 months) or permanent fillers (ever); neurotoxins (3 months); ablative laser resurfacing, non‐ablative rejuvenative laser or light treatment (6 months); surgical dermabrasion, deep facial peels, chemical peels or dermabrasion of the face or neck (4 weeks); isotretinoin or other systemic retinoids (6 months), topical retinoids (2 weeks) or prescription strength skin hydroquinone, AHA, BHA, and polyhydroxy acids, 4‐hydroxyanisole alone or in combination with tretinoin (4 months); nursing, pregnant, or planning to become pregnant; immune deficiency disorders or immunosuppressive medications; recently started hormone replacement therapies (<3 months) or plan on changing the dose of their therapy during the study; planned surgeries, overnight hospitalization, or invasive medical procedures during the study; participation in any other study involving the use of investigational devices or drugs (4 weeks).
2.2. Study device
The microneedling handpiece is used with sterile, individually packaged, disposable needle cartridges. The pen and needle cartridge interface with a nonsterile, disposable sheath to prevent microneedling pen contamination (SkinPen® Precision System). The 14 solid (0.25 mm) needles operate at a speed of 6300 – 7700 RPM with maximum cartridge needle extension ≤2.5mm.
2.3. Study procedures
During a 2‐week baseline period before study onset (Visit 1), subject eligibility was confirmed, overall health and wrinkle severity assessments were performed, and female subjects completed a pregnancy test. Subjects were instructed not to use topical medications or retinoids for the 2 weeks before the first treatment and to maintain their current skincare routine with regular brands of color cosmetics and makeup remover and to refrain from using any anti‐aging and acne products or devices.
Trained aestheticians treated the wrinkles of the face skin of each subject with the microneedling pen according to the manufacturer instructions 17 at depths up to 2.5 mm at Visits 2–5 on Days 1, 30, 60, and 90.
A complimentary nonmedicated hydrogel wound dressing was applied before treatment to protect against abrasion and friction during the microneedling procedure (SkinFuse® Lift HG; Crown Aesthetics, Dallas, TX) and if desired, it could be applied to prevent post‐procedure skin dryness.
A face wash (SkinFuse® PURIFY Cleansing Complex; Crown Aesthetics), moisturizer (SkinFuse® RECLAIM Hydrating Support; Crown Aesthetics), and sunscreen (SkinFuse® SHIELD Zinc Oxide 21%; Crown Aesthetics) were provided to each subject for use as needed during the study.
Makeup was removed at least 30 minutes before each clinic visit using the provided facewash. Subjects were encouraged to avoid extended periods of sun exposure and any use of tanning beds for the duration of the study. Concomitant medications and health assessments were recorded during Visits 2–5 on Days 1, 30, 60, and 90. Pregnancy testing was repeated at Visit 7 on 90 days post‐procedure. Subjects were provided with daily diaries at Visits 2 through 5 on Days 1, 30, 60, and 90. The use of the supporting products and completion of a daily diary was reviewed for safety and compliance and Subjects received a new diary and additional supporting products. Subjects were acclimated to ambient temperature and humidity conditions for 15 minutes before performing any study‐related procedures.
2.4. Imaging procedures
Before imaging procedures, study personnel ensured the face was free of makeup, and jewelry was removed from the treatment area. Subjects were provided with a black or gray matte headband to keep hair away from the face and a black or gray matte cloth was draped over subjects’ clothing.
Digital images of the face of each subject were obtained before treatment at Visit days 1, 30, 60, 90, and 30‐ and 90‐days post‐treatment (Nikon D710; Nikon Inc.).
For digital imaging, subjects were instructed to adopt neutral, non‐smiling expressions. Subjects were carefully positioned facing the camera for a center view and 45° right and left side views.
2.5. Imaging assessments
Two trained raters assessed blinded randomized images of subjects before treatment (Day 1) and 90 days post‐treatment. After completing wrinkle assessment, the two raters were unblinded to pre‐treatment images for the Clinician's Global Aesthetic Improvement Scale (CGAIS).
2.6. Subject self‐assessments
Each subject completed a sponsor‐provided self‐assessment questionnaire and the Subject's Global Aesthetic Improvement Scale (SGAIS) at the 30‐ and 90‐day post‐treatment Visits. It included questions regarding the improvement of fine lines and wrinkles, satisfaction with the treatment, and willingness to recommend the treatment to friends and family members.
2.7. Safety
At each study visit, subjects were queried about the potential adverse events using open‐ended questions and examination of the treatment area. The use of digital imaging was encouraged to document any adverse events.
2.8. Study endpoints
Clinical outcome and safety endpoints were based on the clinic assessments and evaluation of pre‐ and post‐treatment digital images, including the face. Primary efficacy endpoints included the assessment of wrinkle severity using a modified Lemperle Wrinkle Assessment Scale 18 (Table 1), and Clinician's Global Aesthetic Improvement Scale (CGAIS) scores (Table 2) at baseline and 30 and 90 days post‐treatment.
TABLE 1.
Lemperle wrinkle assessment scale
| Class 0: | No Wrinkles |
| Class 1: | Just perceptible wrinkle |
| Class 2: | Shallow wrinkles |
| Class 3: | Moderately deep wrinkle |
| Class 4: | Deep wrinkle, well‐defined edges |
| Class 5: | Very deep wrinkle, redundant fold |
TABLE 2.
Clinician's and subject's global aesthetic improvement scales
| Rating/Term | Description |
|---|---|
| 1—Very much improved | Optimal cosmetic result in this subject |
| 2—Much improved | Marked improvement in appearance from the initial condition, but not completely optimal for this subject |
| 3—Improved | Obvious improvement in appearance from initial condition, but a re‐treatment is indicated |
| 4—No change | The appearance is essentially the same as the original condition |
| 5—Worse | The appearance is worse than the original condition |
Secondary efficacy endpoints included Subject's Global Aesthetic Improvement Scale (SGAIS) scores (Table 2), a Patient Satisfaction Questionnaire at 1 and 3 months post‐treatment. Safety was assessed by reported adverse events throughout the study.
2.9. Ethics
The protocol, informed consent form, and other study‐related documents were approved by the University of Texas Southwestern Medical Center IRB (Dallas, TX) according to the 21 Code of Federal Regulations 50.25 requirements. Each enrolled subject provided a signed photography release. The study was conducted in accordance with the Declaration of Helsinki. 19 ClinicalTrials.gov Identifier: NCT03803059.
2.10. Statistical analysis population
The intent‐to‐treat population included all subjects who received a baseline and at least one treatment assessment and completed the study in accordance with the protocol. The descriptive statistical summary includes the number of observations (N), mean, median, standard deviation (SD), minimum (min), and maximum (max) of values at all applicable time points. The primary ITT analysis is based on the primary outcome of Lemperle gradings for the face at 3 months post‐treatment (study endpoint) relative to baseline (Day 1) evaluation based on the post hoc photographic ratings of two blinded evaluators. An individual study responder is defined as having attained a grading improvement of one or more grades as determined by both blinded evaluators. Overall study success (responder rate) is defined as 50% or more subjects being individual responders.
The mean change was determined for all applicable parameters. Satisfaction questionnaire results were tabulated, and a binomial (sign) test was performed to determine if the proportion of favorable responses was equal to negative responses. All statistical tests were 2‐sided with alpha = 0.05. No multiple testing corrections were considered.
3. RESULTS
Among the enrolled subjects (N = 35), 32 completed the study and formed the study population. Two subjects were lost to follow‐up and one was withdrawn for noncompliance. The demographics and baseline characteristics of the study subjects are summarized in Table 3.
TABLE 3.
Demographics and baseline characteristics
| Mean Age (SD), years | 56.3 (5.0) |
| Median Age (min, max), years | 56.5 (44, 85) |
| Gender, n (%) | |
| Female | 30 (93.8) |
| Male | 2 (6.3) |
| Race, n (%) | |
| White or Caucasian | 28 (87.5) |
| Other | 4 (12.5) |
| Ethnicity, n (%) | |
| Non‐Hispanic/Latino | 28 (87.5) |
| Hispanic/Latino | 4 (12.5) |
| Fitzpatrick skin type, n (%) | |
| II | 24 (75.0) |
| III | 4 (12.5) |
| IV | 4 (12.5) |
Analysis of the photo grading by blinded reviewers at 90 days post‐treatment revealed a decrease (improvement) in baseline scores for wrinkling on the face. The mean scores between the two blinded raters were used in the analysis. These are summarized in Table 4.
TABLE 4.
Change in baseline facial wrinkles
| Area | Improved (%) | Worsened (%) | Mean (SD) | Mean change (%) | p‐Value a |
|---|---|---|---|---|---|
| Fine lines | |||||
| Cheeks | 87.5 | 3.1 | −0.80 (0.51) | −40.5 | <0.001 |
| Forehead | 68.8 | 3.1 | −0.61 (0.58) | −33.9 | <0.001 |
| Facial wrinkling | |||||
| Forehead | 46.9 | 6.3 | −0.34 (0.55) | −17.9 | 0.001 |
| Cheek | 59.4 | 6.3 | −0.50 (0.61) | −34.8 | <0.001 |
| Chin | 65.6 | 0.0 | −0.42 (0.36) | −21.3 | <0.001 |
| Corners of mouth | 62.5 | 3.1 | −0.59 (0.71) | −18.4 | <0.001 |
| Glabellar folds | 71.9 | 6.3 | −0.56 (0.52) | −22.9 | <0.001 |
| Marionette lines | 75.0 | 6.3 | −0.53 (0.47) | −24.3 | <0.001 |
| Nasolabial fold | 59.4 | 6.3 | −0.44 (0.59) | −15.0 | <0.001 |
| Pre‐auricular areas | 56.3 | 9.4 | −0.44 (0.59) | −17.7 | <0.001 |
| Periorbital | 56.3 | 3.1 | −0.55 (0.64) | −25.5 | <0.001 |
| Upper lip | 75.0 | 3.1 | −0.64 (0.54) | −30.8 | <0.001 |
Wilcoxon signed rank test.
As for assessment using photographs by two reviewers after study completion, there was a significant improvement in the mean CGAIS at 3 months post‐treatment (p < 0.001). These results are summarized in Table 5, Figures 1, 2, 3, 4.
TABLE 5.
Descriptive statistics for global aesthetic improvement assessment
| Time post‐treatment | Mean (SD) | Median (min, max) | p‐Value a |
|---|---|---|---|
| Subject's self‐assessment for aesthetic improvement | |||
| 1 Month, N = 30 | 2.90 (0.84) | 3.0 (1.0, 4.0) | <0.001 |
| 3 Months, N = 32 | 3.16 (0.72) | 3.0 (1.0, 4.0) | <0.001 |
| Clinician's global aesthetic improvement assessment | |||
| 3 Months, N = 32 | 3.06 (0.74) | 3.0 (1.5, 4.5) | <0.001 |
Calculated using Wilcoxon signed rank test.
FIGURE 1.
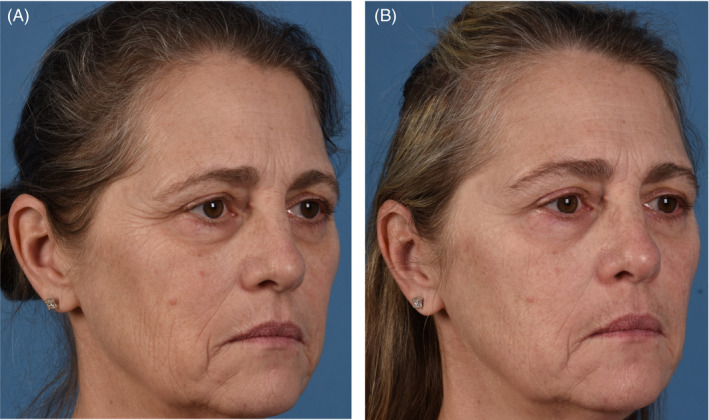
(A) 54‐year‐old female baseline photographs. (B) 54‐year‐old female 3 months post last microneedling procedure
FIGURE 2.
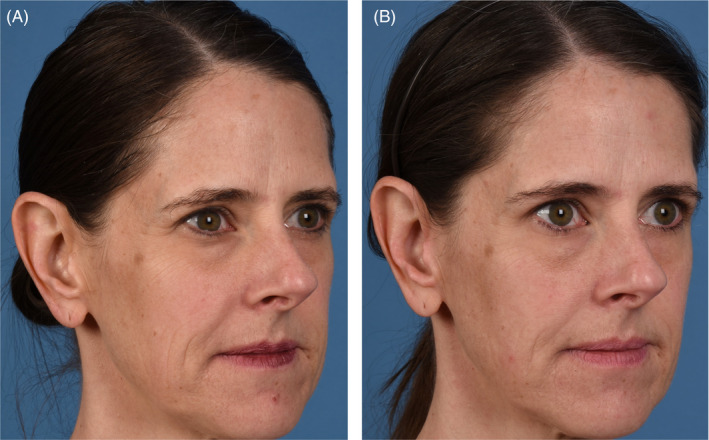
(A) 44‐year‐old female baseline photographs. (B) 44‐year‐old female post last microneedling procedure
FIGURE 3.
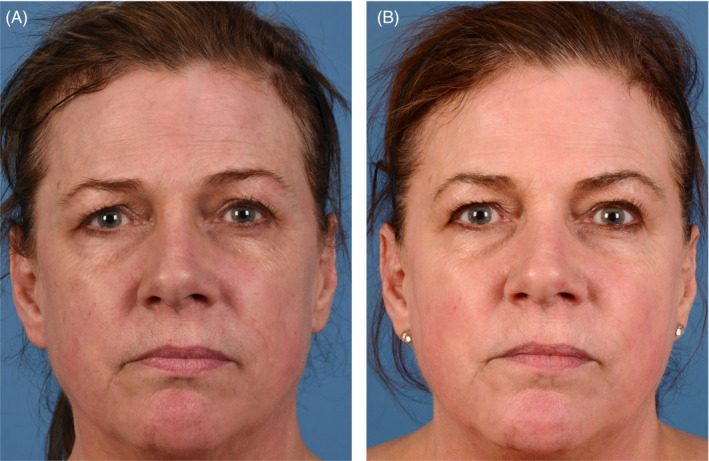
(A) 57‐year‐old female baseline photographs. (B) 57‐year‐old female post microneedling procedure
FIGURE 4.
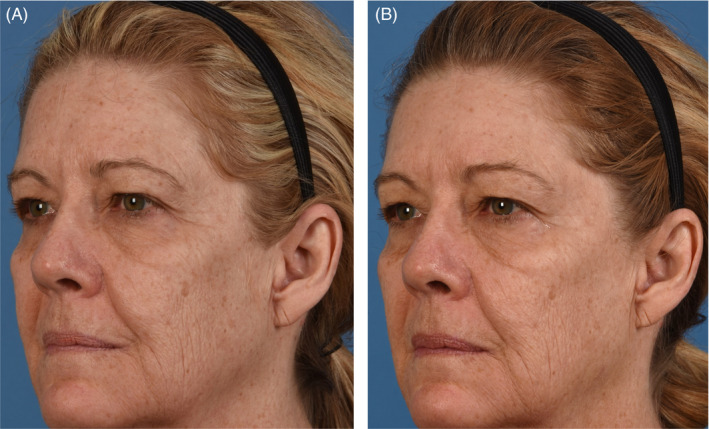
(A) 60‐year‐old female baseline photographs. (B) 60‐year‐old female post last microneedling procedure
The SGAIS scores also showed significant improvements at 30 and 90 days post‐treatment as evaluated by study subjects (for each, p < 0.001). Similarly, assessment by blinded reviewers demonstrated significant improvement in the mean CGAIS scores at 3 months post‐treatment (p < 0.001) (Table 6).
TABLE 6.
Frequency tabulation for global aesthetic improvement assessment
| Time post‐treatment | Very much improved, Much improved, Improved, n (%) | No change, n (%) | Worse, n (%) |
|---|---|---|---|
| Subject's self‐assessment for aesthetic improvement | |||
| 1 Month, N = 30 | 22 (73.3) | 8 (26.7) | 0 |
| 3 Months, N = 32 | 22 (68.8) | 10 (31.3) | 0 |
| Clinician's global aesthetic improvement assessment | |||
| 3 Months, N = 32 | 26 (81.3) | 4 (12.5) | 2 (6.3) |
A majority of subjects reported some level of improvement in their appearance at 30 days (73.3%) and 90 days (68.8%) following final treatment while most clinical assessments noted improvement after 90 days (81.3%) (Table 7).
TABLE 7.
Patient satisfaction questionnaire results
| Time post‐treatment | Favorable, n (%) | Unfavorable, n (%) | Neutral, n (%) | p‐Value a |
|---|---|---|---|---|
| “Do you notice any improvement in how your wrinkles look in the treated area?” | ||||
| 1 Month, N = 32 | 30 (93.8) | 2 (6.3) | 0 | <0.001 |
| 3 Months, N = 32 | 23 (71.9) | 9 (28.1) | 0 | 0.020 |
| “How would you characterize your satisfaction with the treatment?” | ||||
| 1 Month, N = 32 | 28 (87.5) | 3 (9.4) | 1 (3.1) | <0.001 |
| 3 Months, N = 32 | 24 (75.0) | 6 (18.8) | 2 (6.3) | <0.001 |
| “Would you recommend this treatment to your friends and family members?” | ||||
| 1 Month, N = 31 | 25 (80.6) | 6 (19.4) | 0 | <0.001 |
| 3 Months, N = 32 | 21 (65.6) | 11 (34.4) | 0 | 0.110 |
Binomial (sign) test.
The results of the subject satisfaction questionnaire showed high levels of improvement in wrinkles look in the treated area (93.8%), satisfaction with the treatment procedure (87.5%), and would recommend this microneedling procedure to their friends and family members (80.6%) (Table 8).
TABLE 8.
Response frequency for patient satisfaction questionnaire
| Time post‐treatment | Yes, n (%) | No, n (%) |
|---|---|---|
| “Do you notice any improvement in how your wrinkles look in the treated area?” | ||
| 1 Month, N = 32 | 30 (93.8) | 2 (6.3) |
| 3 Months, N = 32 | 23 (71.9) | 9 (28.1) |
| “Reduction in the number of wrinkles?” | ||
| 1 Month, N = 32 | 12 (37.5) | 20 (62.5) |
| 3 Months, N = 32 | 12 (37.5) | 20 (62.5) |
| “Reduction in the size of wrinkles?” | ||
| 1 Month, N = 32 | 25 (78.1) | 7 (21.9) |
| 3 Months, N = 32 | 17 (53.1) | 15 (46.9) |
| “Reduction in pore size?” | ||
| 1 Month, N = 32 | 11 (34.4) | 21 (65.6) |
| 3 Months, N = 32 | 11 (34.4) | 21 (65.6) |
| “Clearer skin?” | ||
| 1 Month, N = 32 | 12 (37.5) | 20 (62.5) |
| 3 Months, N = 32 | 10 (31.3) | 22 (68.8) |
| “Smoother skin texture?” | ||
| 1 Month, N = 32 | 20 (62.5) | 12 (37.5) |
| 3 Months, N = 32 | 15 (46.9) | 17 (53.1) |
| “More even skin tone/color?” | ||
| 1 Month, N = 32 | 13 (40.6) | 19 (59.4) |
| 3 Months, N = 32 | 9 (28.1) | 23 (71.9) |
| “Would you recommend this treatment to your friends and family members?” | ||
| 1 Month, N = 31 | 25 (80.6) | 6 (19.4) |
| 3 Months, N = 32 | 21 (65.6) | 11 (34.4) |
No unanticipated adverse events associated with the treatment were seen in the study.
4. DISCUSSION
Microneedling is a means to induce localized dermal tissue remodeling to improve skin texture, scars, and wrinkles. The microneedling device used in the present study is cleared as a procedure for improving the appearance of facial acne scars in adult patients; however, it also has been shown here to be a highly effective means for improving the appearance of wrinkles on the face. There were significant improvements in all measures of efficacy at 30 days following a series of four monthly microneedling procedures. The effects were durable, persisting for at least 90 days following the last treatment.
Significant improvement in the appearance of wrinkling on the face (78.1%) was observed. No subject showed a worsening of wrinkle appearance. The majority of subjects believed their overall appearance was improved after 30 days (73.3%) and 90 days (68.8%) post‐treatment and none believed it has worsened.
Most changes were noted by subjects at 30 days post‐treatment. At that time, most subjects noted improvement in wrinkles look in the treated area (93.8%), were satisfied with the treatment (87.5%), and would recommend this treatment to their friends and family members (80.6%). These results decreased but remained significant at 90 days post‐treatment.
Originally developed as a roller device for treating acne scars, 21 microneedling has advanced into more sophisticated devices using high RPMs, creating significantly more micro‐injuries per cm2 with more accurate penetration depths; all of which greatly enhances their precision and clinical outcomes. Overall, these results add to the growing body of data that support the use of microneedling for skin rejuvenation and the expanding use of this versatile procedure for numerous clinical applications.
Limitations to this study include limited population size, and a bias for white, female subjects due to the difficulty finding higher Fitzpatrick skin types meeting inclusion criteria.
5. CONCLUSION
Microneedling is a safe, viable, and minimally invasive option for treating wrinkles of the face. Significant improvements were noted as early as 30 days following four monthly treatments. Overall patient satisfaction was high. Microneedling may provide beneficial effects for other aesthetic and medical dermal conditions other than on face and neck areas requiring further studies.
CONFLICT OF INTEREST
The authors Alqam, Hitchcock, and Jones are employees of Crown Laboratories, which is the Manufacturer and Distributor of the microneedling device, and sponsor of the study. Authors, Akgul, Kenkel, and Wamsley report sponsor‐supported funding from Venus Concept (Toronto, Ontario, Canada) for research studies outside of this submitted work.
AUTHOR CONTRIBUTIONS
T.M.H., J.K., and M.L.A. developed study design. Y.A., C.E.M., and J.K. performed the clinical study. T.M.H., B.C.J., and M.L.A. were responsible for the overall direction and planning of the clinical study. T.M.H., M.L.A., and B.J. contributed to the verification of numerical study results. B.C.J., Y.A., C.E.M., J.K., M.L.A., and T.M.H. wrote the manuscript. B.C.J., Y.A., C.E.M., J.K., M.L.A., and T.M.H. contributed to and approved the final version of the manuscript.
ETHICAL APPROVAL
Approval: IRB; Code: STU 062018‐069.
ACKNOWLEDGEMENT
The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., during the preparation of this manuscript. This study was sponsored by Crown Aesthetics, Dallas, TX.
Alqam M, Wamsley CE, Hitchcock TM, Jones BC, Akgul Y, Kenkel JM. Efficacy and tolerability of a microneedling device for treating wrinkles on the face. J Cosmet Dermatol. 2023;22:206–213. doi: 10.1111/jocd.14985
Funding information
Crown Laboratories sponsored this clinical trial that was conducted at UTSW (University of Texas – Southwestern).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17(1):51‐63 doi: 10.1016/j.coms.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 2. Bhardwaj D. Collagen induction therapy with dermaroller. Community Based Med J. 2013;1:35‐37. doi: 10.3329/cbmj.v1i1.13854 [DOI] [Google Scholar]
- 3. Aust MC, Fernandes D, Kolokythas P, Kaplan HM, Vogt PM. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421‐1429. doi: 10.1097/01.prs.0000304612.72899.02 [DOI] [PubMed] [Google Scholar]
- 4. Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244‐254. doi: 10.4103/2229-5178.185468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S‐34S. doi: 10.1097/01.prs.0000225430.42531.c2 [DOI] [PubMed] [Google Scholar]
- 6. Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three‐dimensional skin model. PLoS One. 2018;13:e0204318. doi: 10.1371/journal.pone.0204318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El‐Domyati M, Barakat M, Awad S, Medhat W, El‐Fakahany H, Farag H. Multiple microneedling sessions for minimally invasive facial rejuvenation: an objective assessment. Int J Dermatol. 2015;54:1361‐1369. doi: 10.1111/ijd.12761 [DOI] [PubMed] [Google Scholar]
- 8. Bandral MR, Padgavankar PH, Japatti SR, Gir PJ, Siddegowda CY, Gir RJ. Clinical evaluation of microneedling therapy in the management of facial scar: a prospective randomized study. J Maxillofac Oral Surg. 2019;18:572‐578. doi: 10.1007/s12663-018-1155-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mujahid N, Shareef F, Maymone MBC, Vashi NA. Microneedling as a treatment for acne scarring: a systematic review. Dermatol Surg. 2020;46(1):86‐92. doi: 10.1097/DSS.0000000000002020 [DOI] [PubMed] [Google Scholar]
- 10. Alster TS, Li MK. Microneedling treatment of striae distensae in light and dark skin with long‐term follow‐up. Dermatol Surg. 2020;46(4):459‐464. doi: 10.1097/DSS.0000000000002081 [DOI] [PubMed] [Google Scholar]
- 11. Wollina U, Goldman A. Management of stretch marks (with a Focus on Striae Rubrae). J Cutan Aesthet Surg. 2017;10:124‐129. doi: 10.4103/JCAS.JCAS_118_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iriarte C, Awosika O, Rengifo‐Pardo M, Ehrlich A. Review of applications of microneedling in dermatology. Clin Cosmet Investig Dermatol. 2017;10:289‐298. doi: 10.2147/CCID.S142450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ablon G. Safety and effectiveness of an automated microneedling device in improving the signs of aging skin. J Clin Aesthet Dermatol. 2018;11:29‐34. [PMC free article] [PubMed] [Google Scholar]
- 14. Amer M, Farag F, Amer A, ElKot R, Mahmoud R. Dermapen in the treatment of wrinkles in cigarette smokers and skin aging effectively. J Cosmet Dermatol. 2018;17:1200‐1204. doi: 10.1111/jocd.12748 [DOI] [PubMed] [Google Scholar]
- 15. Haimovic A, Ibrahim O, Lee NY, Dover JS. Ensuring consistent results when microneedling perioral rhytides. Dermatol Surg. 2018;44:595‐597. doi: 10.1097/DSS.0000000000001278 [DOI] [PubMed] [Google Scholar]
- 16. Wamsley CE, Kislevitz M, Barillas J, et al. A single‐center trial to evaluate the efficacy and tolerability of four microneedling treatments on fine lines and wrinkles of facial and neck skin in subjects with Fitzpatrick skin types I‐IV: an objective assessment using non‐invasive devices and 0.33mm microbiopsies. Aesthet Surg J. 2021;41(11):NP1603‐NP1618. doi: 10.1093/asj/sjab052 [DOI] [PubMed] [Google Scholar]
- 17. SkinPen® Microneedling System Instructions for Use; Crown Aesthetics LLC. [Google Scholar]
- 18. Lemperle G, Holmes RE, Cohen SR, Lemperle SM. A classification of facial wrinkles. Plast Reconstr Surg. 2001;108:1735‐1750. doi: 10.1097/00006534-200111000-00048 [DOI] [PubMed] [Google Scholar]
- 19. World Medical Association . WMA declaration of Helsinki – Ethical principles for medical research involving human subjects. 2013. https://www.wma.net/policies‐post/wma‐declaration‐of‐helsinki‐ethical‐principles‐for‐medical‐research‐involving‐human‐subjects/ Accessed January 28, 2021. [DOI] [PubMed]
- 20. Von Dalwig‐Nolda DF, Ablon G. Safety and effectiveness of an automated microneedling device in improving acne scarring. J Clin Aesthet Dermatol. 2020;13:17‐22. [PMC free article] [PubMed] [Google Scholar]
- 21. Doddaballapur S. Microneedling with dermaroller. J Cutan Aesthet Surg. 2009;2:110‐111. doi: 10.4103/0974-2077.58529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boparai AS, Bhale G, Brar S. Evaluation of therapeutic outcome of transepidermal administration of platelet‐rich plasma with microneedling in melasma. Dermatol Ther. 2020;33:14358. doi: 10.1111/dth.14358 [DOI] [PubMed] [Google Scholar]
- 23. Merati M, Woods C, Reznik N, Parker L. An assessment of microneedling with topical growth factors for facial skin rejuvenation: a randomized controlled trial. J Clin Aesthet Dermatol. 2020;13:22‐27. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


