Abstract
To determine the prevalence of allergic sensitization in patients with vernal keratoconjunctivitis (VKC) and to provide an overview of published studies on this topic. We systematically searched 11 literature databases on 24 May 2021, for studies with cross‐sectional data on the prevalence of positive allergy tests in patients with VKC. Our main outcome of interest was the prevalence of allergic sensitization and the allergens involved. Prevalence meta‐analyses were made to provide summary estimates. We identified 33 eligible studies for qualitative review with 2122 patients with VKC. Studies were predominantly based on patients seen in ophthalmology clinics. Overall, studies reported that the most prevalent positive allergen tests were the inhaled allergens house dust mites and pollen. Twenty‐nine studies were eligible for the quantitative analysis. Here, we calculated the prevalence of allergen‐positive patients to 57.7% (95% confidence interval: 52.5%–62.8%). Subgroup analyses of pooled estimates on sensitization based on specific testing methods found prevalence estimates of 51.4% for conjunctival provocation test, 68.7% for total tear IgE, 58.9% for specific tear IgE, and 58.2% for skin prick test. The prevalence of allergic sensitization in patients with VKC is 57.7%, and mostly towards inhaled allergens. The most frequent positive allergens are house dust mites and pollen. Identifying possible clinically relevant allergens provide information that may aid in managing VKC, such as environmental allergy‐avoidance or allergy‐specific treatment.
Keywords: IgE, meta‐analysis, skin prick test, systematic review, vernal keratoconjunctivitis
1. INTRODUCTION
Vernal keratoconjunctivitis (VKC) is an ocular allergic disease, with chronic inflammation of the palpebral and/or bulbar conjunctiva, usually with bilateral manifestations. The severe and prolonged clinical course of VKC may lead to corneal changes and ultimately irreversible vision loss. It is a rare disease (3.2 per 10.000 inhabitants in Western Europe) that usually presents in childhood with a male predominance (Bremond‐Gignac et al., 2008; Leonardi et al., 2015).
Vernal keratoconjunctivitis has an important geographical variation with higher prevalence in the Mediterranean, Africa, the Middle East, Asia, and South America (Bremond‐Gignac et al., 2008; Kumar, 2009). The typical clinical course is onset in the first decade of life around the age of 5–6 years with a resolution during puberty. The disease is usually described as seasonal and lasts from spring to autumn; however, many cases do not follow this pattern and several patients have the perennial disease (Bremond‐Gignac et al., 2008; Kumar, 2009; Montan et al., 1999). The seasonal pattern during the high pollen season has led to the hypothesis that VKC may be an immunologically mediated hypersensitive reaction to environmental antigens. This antigen and allergy‐based hypothesis is further strengthened by epidemiological studies which report 46%–50% of patients have a positive history of other allergic manifestations (atopic dermatitis, asthma, and eczema). Interestingly, there is geographical variation, with very low numbers in Sub‐Saharan Africa (Bonini et al., 2004; Bremond‐Gignac et al., 2008; Leonardi et al., 2006). Evidence suggests that a positive history of allergic diseases is correlated with specific types of VKC (Leonardi et al., 2006). Despite these findings, the exact role of allergy testing in patients with VKC remains a subject of uncertainty and discussion among ophthalmologists.
The aim of this systematic review and meta‐analysis is to provide an overview of the literature on the prevalence of patients with VKC with a positive allergy test and to outline allergens identified across studies. This information is important for clinical practice.
2. METHODS
2.1. Study design
This study was designed as a systematic review with meta‐analyses following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) and the Meta‐Analysis of Observational Studies in Epidemiology (MOOSE; Moher et al., 2009; Stroup et al., 2000). Further, we followed the recommendations of the Cochrane Handbook (Higgins et al., 2021). Institutional review board approval is not required for systematic reviews according to Danish law.
2.2. Eligibility criteria
Studies were considered if they provided cross‐sectional data on the prevalence of positive allergy tests in patients with VKC aged <18 years. We included studies with any study design as long as cross‐sectional data of the outcome of interest was provided. We included relevant conference abstracts, but not studies without original data and case reports. In studies where only a part of the patient population was constituted by individuals aged <18 years, we evaluated whether it was possible to only extract data on the part of the study population aged <18 years, and if this was not possible, the study was found eligible if the mean age of the study population was <18 years. We included all kinds of allergy tests. We did not exclude studies based on patient demographics apart from the age restriction. We only considered studies disseminated in the English language.
2.3. Information sources and search strategy
We searched the literature databases PubMed/MEDLINE, EMBASE, Web of Science Core Collection, BIOSIS Previews, Current Contents Connect, Data Citation Index, Derwent Innovations Index, KCI‐Korean Journal Database, Russian Science Citation Index, SciELO Citation Index, and the Cochrane Central. The search was performed on 24 May 2021. We used search phrases specifically tailored to the individual literature databases developed by a trained investigator (author Y.S.) with details outlined in Appendix S1.
2.4. Study selection, data collection, and risk of bias assessment
One author (Y.S.) examined the titles and the abstracts from the literature search and removed duplicates as well as obviously irrelevant records. Two authors (D.T. and Y.S.) independently examined the full text of the remaining records for eligibility and reviewed reference lists for any additional relevant studies. The authors discussed study eligibility allocation and if consensus could not be reached, a third author (M.L.R.R. or L.K.) was invited for further discussions and final decision‐making. After consensus regarding study eligibility, we extracted data regarding study design and characteristics, participant characteristics, diagnostic method, and prevalence results using extraction forms. The risk of bias assessment of eligible studies was assessed using the relevant items from the Agency for Healthcare Research and Quality checklist for Cross‐Sectional/Prevalence Studies (Zeng et al., 2015). Two authors (M.D. and Y.S.) extracted data independently and compared their results afterwards to discuss any discrepancies. If consensus could not be reached, a third author (M.L.R.R. or L.K.) was to be invited for further discussions and final decision‐making.
2.5. Outcome measures, data analysis, and synthesis
The primary outcome measure was the prevalence of positive allergy tests in patients with VKC. The secondary outcome measure was the prevalence of positive test for inhaled allergens among patients with VKC. Upon review, we realized that our secondary outcome measure, could not be summarized since one individual could have a positive test for more than one allergen and the studies did not report positive allergy results on an individual level. Instead, we summarized the prevalence of individual positive allergy tests in a table to provide data that can give some information about the prevalence of positive test for inhaled allergens.
We reviewed all studies qualitatively in text and in tables. Meta‐analyses were performed using MetaXL 5.3 (EpiGear International) for Microsoft Excel 2013 (Microsoft). The random‐effects model was used to account for possible heterogeneity between the studies. In prevalence meta‐analyses, caution must be shown when numbers are close to the extremes (0% or 100%) because of variance instability, which results in studies getting erroneous weights (Barendregt et al., 2013). Therefore, prevalence numbers were transformed using the double arcsine method for analysis and then back‐transformed for interpretation. The unit of analysis was defined as per patient. Heterogeneity was assessed using Cochran's Q and quantified using I 2 (Higgins et al., 2003). Funnel plots were used to identify skewed results, for example, due to publication bias (Egger et al., 1997). We planned subgroup analyses, where we analysed results separately based on test type. Sensitivity analyses were made by removing studies in turn and evaluating the change in the results. p values below 0.05 were considered statistically significant.
3. RESULTS
3.1. Study selection
Our literature search identified 751 records. Of these, 301 were duplicates and 392 were obviously irrelevant records. The remaining 58 records were read in full text. Here, we identified two additional records from screening reference lists. Of these 60 records, 33 records were deemed eligible for the qualitative review of which 29 were eligible for quantitative review. The study selection process is outlined in Figure 1.
FIGURE 1.
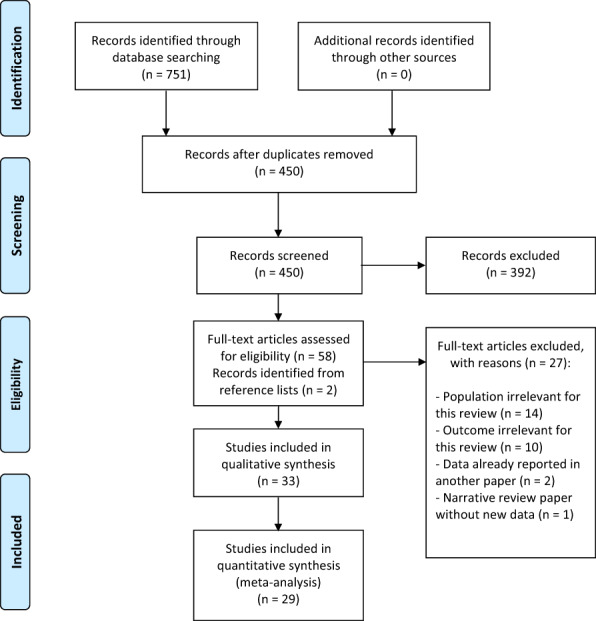
Flow diagram of the study selection process.
3.2. Study characteristics
The 33 studies included in our review summarized data on a total of 2122 patients with VKC. Studies were predominantly based on patients from ophthalmology clinics, and in seven studies patients were recruited from allergology clinics or paediatric clinics (Gómez‐Henao et al., 2018; Jongvanitpak et al., 2020; Kocaturk et al., 2012; Monzón et al., 2009; Polido et al., 2015; Tesse et al., 2010; Zicari et al., 2013). Studies were based on populations in Asia (n = 14), Europe (n = 14), South America (n = 3), and Africa (n = 2). In all studies apart from Monzón et al. (2009), the majority of the study populations were males. Further details of the study and patient characteristics are summarized in Table 1.
TABLE 1.
Study and patient characteristics in individual studies
| Reference | Country | N | Population | Age, mean ± SD | Sex, males:females |
|---|---|---|---|---|---|
| Abo‐Ali et al. (2015) | Egypt | 48 | Patients with VKC recruited from the ophthalmology clinic. Patients had not received antihistamines, steroids, or cytotoxic drugs prior to study inclusion. Patients with the following comorbidities were excluded: rhinitis, asthma, food or drug allergy, autoimmune diseases, and major system/organ failure | 15 ± 7 years | 41:7 |
| Baryishak et al. (1982) | Israel | 129 | Patients with newly diagnosed VKC or fresh attack after a quiescent period of the disease | 18 were aged 4–12 years. One was aged 20 years | 14:5 |
| Bonini et al. (2000) | Italy | 195 | Patients with newly diagnosed VKC from the ophthalmology clinic | 11 ± 6 years | 144:51 |
| Bozkurt et al., (2010) | Turkey | 27 | Patients with VKC referred to a cornea unit in a university hospital | 12 ± 4 years | 26:1 |
| Bozkurt et al. (2013) | Turkey | 67 | Patients with VKC from the ophthalmology clinic | 11 ± 4 years | 53:14 |
| Chiambaretta et al. (2012) | France | 69 | Unclear | Children, further data n/a | n/a |
| De Oliveira et al. (2006) | Brazil | 20 | Randomly chosen sample of 20 patients with VKC from the ophthalmology clinic | 11 ± 5 years | 15:5 |
| Elsurer et al. (2021) | Turkey | 39 | Patients with VKC from the ophthalmology clinic | 11 ± 3 years | 25:14 |
| Erdem et al. (2011) | Turkey | 23 | Patients with VKC from the ophthalmology clinic | 11 years (range 4–17 years) | n/a |
| Gómez‐Henao et al. (2018) | Colombia | 32 | Patients with VKC from the allergology clinic | 12 ± 3 years | 24:8 |
| Inada et al. (2007) | Japan | 13 | Patients with VKC from the ophthalmology clinic | 18 ± 8 years | 10:3 |
| Inada et al. (2009) | Japan | 15 | Patients with VKC from the ophthalmology clinic. Patients in any systemic anti‐allergic treatment were excluded | 18 ± 10 years | 13:2 |
| Jongvanitpak et al. (2020) | Thailand | 20 | Patients with VKC from the paediatric allergy clinic | 8 ± 3 years | 17:3 |
| Kocabeyoglu et al. (2008) | Turkey | 10 | Patients with VKC from the ophthalmology clinic | 14 ± 5 years | 8:2 |
| Kocaturk et al. (2012) | Turkey | 38 | Patients with VKC from the ophthalmology and the paediatric clinics | 9 ± 4 years | 24:14 |
| Kosrirukvongs et al. (2001) | Thailand | 47 | Patients with VKC from the ophthalmology clinic | 10 ± 5 years | 39:8 |
| Leonardi et al. (2006) | Italy | 406 | Patients with VKC from the ophthalmology clinic. | 83% were aged <10 years | 311:95 |
| Leonardi et al. (2015) | Italy | 10 | Patients with active VKC from the ophthalmology clinic | 12 ± 4 years | 6:4 |
| Montan et al. (1999) | Sweden | 62 | Patients with VKC from the ophthalmology clinic. | Median age 6 years | 52:10 |
| Monzón et al. (2009) | Spain | 9 | Patients with VKC from the ophthalmology and allergy clinics | 8 ± 1 years | 4:5 |
| Mumcuoglu et al. (1988) | Israel | 19 | Patients with VKC seen in the ophthalmology clinic | Mean age 6 years | 18:1 |
| Naidoo et al. (2014) | South Africa | 168 | Patients with VKC seen in the ophthalmology clinic | Range 19 months to 12.5 years | 125:43 |
| Nebbioso et al. (2015) | Italy | 26 | Patients with VKC seen in the ophthalmology clinic | All defined as children without age data | 18:8 |
| Nebbioso et al. (2018) | Italy | 47 | Patients with VKC seen in the ophthalmology clinic | 9 ± 5 years | 38:9 |
| Pokharel et al. (2009) | Nepal | 34 | Patients with VKC seen in the ophthalmology clinic | 12 patients were aged 6–10 years, overall range 2–26 years | 26:12 |
| Polido et al. (2015) | Brazil | 19 | Patients with VKC from the ophthalmology and allergy clinics | 10 ± 3 years | n/a |
| Pucci et al. (2003) | Italy | 110 | Patients with VKC seen in the ophthalmology clinic | 8 ± 3 years | 81:29 |
| Samra et al. (1984) | Israel | 27 | Patients with VKC seen in the ophthalmology clinic | 20 patients were aged 0–7 years | n/a |
| Tesse et al. (2010) | Italy | 197 | Patients with VKC seen in the paediatrics, immunology, and allergology clinic | Range 5–14 years | 126:71 |
| Tomassini et al. (1994) | Italy | 31 | Patients with VKC seen in the ophthalmology clinic. | Range 4–22 years | 24:7 |
| Tuft et al. (1989) | UK | 120 | Patients with VKC seen in the ophthalmology clinic | 87 patients were aged <16 years | 96:24 |
| Villalon Garcia et al. (2015) | Spain | 30 | Patients with VKC seen in the ophthalmology clinic | Range 4–18 years | 26:4 |
| Zicari et al. (2013) | Italy | 15 | Patients with VKC seen in the paediatrics clinic | Range 6–10 years | 10:5 |
Note: Continuous data are presented in mean ± SD unless otherwise noted. The sex distribution is presented as males:females.
Abbreviations: UK, United Kingdom; USA, United States of America.
In total, 21 studies provided data on the skin prick test from patients with VKC. Other test types identified in studies were serum‐specific IgE, tear total IgE, tear‐specific IgE, conjunctival provocation test, and nasal provocation test. These test types were reported sporadically across studies (Table 2). Eighteen studies reported total serum IgE levels, which is not an allergy test but occasionally used in relation to allergenic analyses. Similarly, peripheral blood eosinophil count is discussed in relation to allergenic analyses without being a specific allergy test. Thus, neither total serum IgE nor peripheral blood eosinophil count is included in our quantitative analyses, but briefly discussed qualitatively in relation to other allergenic analyses.
TABLE 2.
Test types and analyses reported in individual studies
| Reference | SPT | Serum specific IgE | Tear total IgE | Tear specific IgE | CPT | NPT | Serum total IgE | PBEC |
|---|---|---|---|---|---|---|---|---|
| Abo‐Ali et al. (2015) | X | X | ||||||
| Baryishak et al. (1982) | X | X | ||||||
| Bonini et al. (2000) | X | X | X | |||||
| Bozkurt et al. (2010) | X | X | X | |||||
| Bozkurt et al. (2013) | X | X | X | X | ||||
| Chiambaretta et al. (2012) | X | |||||||
| De Oliveira et al. (2006) | X | |||||||
| Elsurer et al. (2021) | X | X | ||||||
| Erdem et al. (2011) | X | X | X | X | ||||
| Gómez‐Henao et al. (2018) | X | |||||||
| Inada et al. (2007) | * | * | ||||||
| Inada et al. (2009) | X | X | ||||||
| Jongvanitpak et al. (2020) | X | |||||||
| Kocabeyoglu et al. (2008) | X | |||||||
| Kocaturk et al. (2012) | X | X | ||||||
| Kosrirukvongs et al. (2001) | X | |||||||
| Leonardi et al. (2006) | X | X | X | X | X | |||
| Leonardi et al. (2015) | X | X | X | X | X | |||
| Montan et al. (1999) | X | X | X | |||||
| Monzón et al. (2009) | X | X | X | |||||
| Mumcuoglu et al. (1988) | X | X | X | X | ||||
| Naidoo et al. (2014) | X | |||||||
| Nebbioso et al. (2015) | X | X | ||||||
| Nebbioso et al. (2018) | X | |||||||
| Pokharel et al. (2009) | X | |||||||
| Polido et al. (2015) | X | X | X | |||||
| Pucci et al. (2003) | X | X | X | X | ||||
| Samra et al. (1984) | X | X | ||||||
| Tesse et al. (2010) | X | X | X | X | ||||
| Tomassini et al. (1994) | X | |||||||
| Tuft et al. (1989) | X | X | X | X | ||||
| Villalon Garcia et al. (2015) | X | |||||||
| Zicari et al. (2013) | X |
Abbreviations: CPT, conjunctival provocation test; NPT, nasal provocation test; PBEC, peripheral blood eosinophil count; SPT, skin prick test.
Inada et al. (2007) evaluated tear total and specific IgA.
Reported allergens and their prevalence are summarized in Appendix S2. For a more clinically relevant summary, we have highlighted positive findings and stratified them according to the testing method and highlighted the population country in Table 3. The majority of studies reported data on the result of allergy testing in terms of specific serum IgE or skin prick test. Overall, studies reported that the most prevalent sensitizations were the inhaled allergens house dust mites or pollen.
TABLE 3.
Studies with allergen‐specific data reported using either a skin prick test or serum IgE
| Reference | Country | Allergens reported and their prevalence |
|---|---|---|
| Skin prick test | ||
| Abo‐Ali et al. (2015) | Egypt | Pollens (16.7%), grass (12.5%), hay dust (6.3%), mites (6.3%), moulds (6.3%), straw (4.2%), cat epithelium (2.1%), cotton dust (2.1%), candida (2.1%), cockroach (2.1%), and pigeon (2.1%). |
| Bonini et al. (2000) | Italy | Rye‐grass allergen, Parietaria officinalis, and Dermatophagoides pteronyssinus were the most common sensitizing allergens accounting for 71.1%. Data for other allergens are not specified. |
| Bozkurt et al. (2010) | Turkey | Pollen allergy (28%), food allergy (8%), and yeast allergy (4%). |
| Bozkurt et al., 2013 | Turkey | Pollen allergy was the most common (24.5%). Data for other allergens are not specified. |
| De Oliveira et al. (2006) | Brazil | House dust mites (100%), cat allergen (60%), dog allergen (40%), fungus allergen (13%), and feather allergen (7%). |
| Elsurer et al. (2021) | Turkey | House dust mites or pollens in 35.9%. Data for other allergens are not specified. |
| Gómez‐Henao et al. (2018) | Colombia | Dermatophagoides pteronyssinus (88%),Dermatophagoides farinae (84%), Blomia tropicalis (44%), ant (28%), dog dander (20%), cat dander (16%), cockroach (16%), pollen and grasses (12%), and mosquito (8%). |
| Jongvanitpak et al. (2020) | Thailand | House dust mites (68.8%), cockroaches (62.5%), dog or cat (25%), pollen (18.8%), and moulds (12.5%). |
| Kocaturk et al. (2012) | Turkey | Tree pollens (70.8%), wheat (70.8%), grass (66.7%), Festuca (54.2%), mite (54.2%), weed (45.8%), moulds (41.7%), cat (25%), and cockroach (16.7%). |
| Kosrirukvongs et al. (2001) | Thailand | House dust mite (79.5%), grass (Bermuda, Johnson, Timothy) (48.7%), house dust (42.1%), cockroach (30.8%), other grass pollen (29.5%), fungus (28.9%), food (31.6%), cat (15.4%), dog (13.1%), careless weed (10.5%), insect (10.5%), kapok (10.5%), mosquito (7.9%), and ant (2.6%). |
| Montan et al. (1999) | Sweden | Pollens (51.6%), animal dander (38.7%), food allergens (29.0%), moulds (19.4%), and mites (16.1%) |
| Naidoo et al. (2014) | South Africa | House dust mites (55.8%), grass (33.3%), cockroach (18.5%), cat (12.5%), dog (10.7%), tree pollens (5.9%), and mould (3.5%) |
| Nebbioso et al. (2015) | Italy | Dermatophagoides farinae (38%), Dermatophagoides pteronyssinus (35%), Lolium perenne (27%), grass (23%), Olea europea (19%), cat (12%), Alternaria (8%), Parietaria (8%), and dog (8%) |
| Pucci et al. (2003) | Italy | House dust mites (30.0%), grass (27.0%), olive (16.0%), cat dander (16.0%), dog dander (10.9%), Parietaria officinalis (10.9%), soybean (5.0%), peanut (2.5%), Artemisia vulgaris (2.5%), plane pollen (1.8%), cypress (1.8%), and egg (1.8%). |
| Villalon Garcia et al. (2015) | Spain | Pollen in 48% and in 22% any of the following: house dust mite, fungus, and animal epithelium. |
| Serum IgE | ||
| Elsurer et al. (2021) | Turkey | Elevated IgE levels against house dust mites or pollens in 35.9% of all patients. |
| Kocabeyoglu et al. (2008) | Turkey | House dust mites (30%), grass (30%), and animal epithelia (10%). |
| Leonardi et al. (2015) | Italy | Among 60% of VKC patients, allergen‐specific IgE in tear was found for grass, tree, mites, animal, and/or food allergens. |
| Mumcuoglu et al. (1988) | Israel | House dust mites (42%). Data for other allergens are not specified. |
| Polido et al. (2015) | Brazil | Dermatophagoides pteronyssinus (58%), Dermatophagoides farinae (58%), Blomia tropicalis (21%), Blattella germanica (21%), and mixed food/cow's milk (5%) |
| Pucci et al. (2003) | Italy | House dust mites (30.0%), grass (27.0%), olive (16.0%), cat dander (16.0%), dog dander (10.9%), Parietaria officinalis (10.9%), soybean (5.0%), peanut (2.5%), Artemisia vulgaris (2.5%), plane pollen (1.8%), cypress (1.8%), and egg (1.8%). |
Abbreviation: VKC, vernal keratoconjunctivitis.
3.3. Results of individual studies
Nine studies provided data on allergen sensitization in patients with VKC and compared it with a healthy age‐matched control group:
Baryishak et al. (1982) examined serum total IgE and tear total IgE and reported that the VKC patients had significantly higher tear total IgE than the controls (significantly elevated levels in 64% vs. 5%, respectively), whereas serum total IgE did not differ (significantly elevated levels in 8.5% vs. 0%, respectively). Leonardi et al. (2015) reported that none of the healthy controls (n = 10) had elevated specific IgE in serum or tears, whereas six out of 10 patients with VKC were positive for one or more of the 47 tested allergens. In a subset of patients, a conjunctival provocation test was performed which confirmed the specific local conjunctival reactivity (Leonardi et al., 2015). Monzón et al. (2009) reported that while none of the controls (n = 15) had a positive skin prick test for 20 inhalant allergens, a positive test was seen in three of the nine VKC patients. Mumcuoglu et al. (1988) sampled serum and tear for measuring total and specific IgE to 10 inhalant allergens and reported a positive test in 42% of patients and to house dust mites only. Pokharel et al. (2009) reported that mean total tear IgE was higher in patients with VKC than in controls, but this difference did not reach statistical significance. Pucci et al. (2003) reported that 45% of VKC patients had a positive skin prick test with house dust mites being the most prevalent allergen, and that patients had significantly higher serum total IgE and peripheral blood eosinophils compared to controls. Samra et al. (1984) compared serum total IgE and tear total IgE in VKC patients and controls and reported that both serum and tear IgE were significantly higher in patients, and that the difference was greater when measured in tears. Tomassini et al. (1994) reported that serum total IgE was significantly higher in patients than in controls. Tuft et al. (1989) reported that most patients with VKC had elevated serum and tear total IgE regardless of VKC subtype (limbal, palpebral, or mixed), and that a higher proportion of patients than controls had elevated specific IgE for grass, cat, and house dust mite.
Nine studies investigated a range of tests in patients with various diagnoses in the group of allergic keratoconjunctivitis and provided data specifically for patients with VKC:
Abo‐Ali et al. (2015) found that 67% had elevated total serum IgE, and 58% had at least one positive skin prick test, and the most prevalent positive allergen was pollens. Chiambaretta et al. (2012) performed a conjunctival provocation test and found a positive result in 57% of patients with VKC (n = 69). Inada et al. (2007) evaluated tear total IgA and tear house dust mite specific IgA and found that total IgA was significantly lower in patients with VKC than in healthy controls, but that the ratio between house dust mite specific tear IgA and total tear IgA was significantly higher in patients with VKC than in healthy controls. Inada et al. (2009) found that patients with VKC had significantly higher levels of serum and tear IgE than healthy controls and that the IgE levels were correlated with the clinical severity of the disease. Jongvanitpak et al. (2020) found that 75% of VKC patients (n = 20) had a positive skin prick test to one or more of 16 tested inhalant allergens. Kocabeyoglu et al. (2008) found that patients had a high level of specific IgE against house dust mites and mixed‐grass pollens. Kocaturk et al. (2012) reported that 71% of patients with VKC (n = 38) had at least one positive skin prick test to 30 tested allergens and that 58% had an elevated serum total IgE level. In this study, immunotherapy was administered to 17% of the patients who were all relieved of any need for further medical treatment for VKC (Kocaturk et al., 2012). Kosrirukvongs et al. (2001) found that the skin prick test was positive in 80% of all patients with VKC, and that house dust mites were the most prevalent allergens. Polido et al. (2015) found that patients had high levels of serum total IgE and peripheral blood eosinophils, and these numbers did not differ between patients with VKC and those with the other diagnoses (atopic keratoconjunctivitis, seasonal allergic conjunctivitis, and perennial allergic conjunctivitis; Polido et al., 2015).
Eleven studies provided a clinical descriptive study focused on patients with VKC:
Bonini et al. (2000) reported that 58% had any positive skin prick test, 52% had positive serum radioallergosorbent test, and 29% had IgE serum levels >200 kU/L. Rye‐grass allergen, Parietaria officinalis, and Dermatophagoides pteronyssinus were the most common sensitizing allergens (Bonini et al., 2000). Bozkurt et al. (2010) reported that 41% had elevated total serum IgE, 30% had peripheral blood eosinophilia, and 40% had a positive skin prick test. Bozkurt et al. (2013) reported a positive skin prick test in 35%, positive specific IgE in 50%, mean serum IgE of 169.8 kU/L, and mean peripheral blood eosinophil count of 334.5 cells/mm2. De Oliveira et al. (2006) found a positive skin prick test for at least one of the allergens tested in 75% of patients and a poor correlation between the clinical severity score and the sensitivity to the allergens. Elsurer et al. (2021) reported that 36% had either a positive skin prick test or elevated specific IgE levels against house dust mites or pollens. Erdem et al. (2011) reported mean total IgE levels were 496 IU/ml and a positive skin prick test for house dust mites or pollen in 30%, and a positive conjunctival or nasal provocation test in 26% and 22%, respectively. Leonardi et al. (2006) reported positive specific serum IgE in 57% of patients and a positive skin prick test to at least one allergen in 44% of patients. In 103 patients, a conjunctival provocation test was performed with the same allergens as used for the skin prick test (Leonardi et al., 2006). The conjunctival test was positive for at least one allergen in 59% of the patients (Leonardi et al., 2006). Interestingly, 42% of patients with a negative skin prick test or specific IgE had a positive conjunctival provocation test (Leonardi et al., 2006). Both the mean total serum IgE at 420 kU/L and mean peripheral blood eosinophil count at 1.2 x 109/L were high (Leonardi et al., 2006). Montan et al. (1999) reported that 60% of patients had any sensitization determined by either using a skin prick test, serum‐specific IgE, or tear‐specific IgE, and that pollen was the most prevalent allergen. Naidoo et al. (2014) used a skin prick test to identify that the most common sensitizations were house dust mites (56%) and grass (33%). Nebbioso et al. (2015) examined allergic and autoimmunological association of VKC. In this study, patients with VKC had a positive skin test in 54%, elevated IgE in 46%, and 31% had elevated serum antinuclear antibodies (Nebbioso et al., 2015). Villalon Garcia et al. (2015) reported that 70% had a positive skin prick test and that approximately half of them were commenced in allergen‐specific immunotherapy but outcomes of this therapy were not reported.
Four studies reported descriptive data on patients with VKC as part of their investigation of other aspects of the disease:
Gómez‐Henao et al. (2018) investigated the quality of life of patients with VKC and reported that skin prick tests towards inhaled allergens were positive in 78%. Nebbioso et al. (2018) investigated ocular surface before and after topical cyclosporine administration in eyes with VKC and reported that 46% had a positive skin prick test. Tesse et al. (2010) evaluated the efficacy of 1% topical cyclosporine in patients with VKC and reported that 48% of patients either had a positive skin prick test, elevated serum specific IgE, or serum total IgE. Zicari et al. (2013) investigated serum interleukin‐17 levels in patients with VKC and reported that 67% had at least one positive inhalation or food allergen on the skin prick test.
3.4. Risk of bias within studies
The evaluation of the risk of bias within studies found that overall, studies clearly defined the data source and the eligibility of participants. The time period wherein participants were identified for the studies was clearly reported in 21 studies (64%). Consecutive recruitment was clearly present in eight studies (24%). In six studies, a subset of the participants was excluded from the analyses, and only one of these studies stated an explanation for the exclusion. We realized that when evaluating allergen test outcomes, evaluation of quality assurance related to such tests could not be made in a reliable fashion based on reports of individual studies since quality aspects of such tests are often part of routine clinical quality evaluation and often made outside of an eye department, for example, a clinical biochemistry department. We therefore refrained from providing an evaluation of the quality assurance based on the full‐text manuscripts, since any such evaluation would likely not reflect the routine clinical quality evaluation made by other individuals than the study authors. The risk of bias evaluation within studies is summarized in Table 4.
TABLE 4.
Risk of bias within individual studies using the Agency for Healthcare Research and Quality checklist for cross‐sectional studies
| Reference | Defines source | Eligibility criteria | Time period | Consecutive recruitment | Quality assurance a | Explains exclusions |
|---|---|---|---|---|---|---|
| Abo‐Ali et al. (2015) | Yes | Yes | No | No | ‐ | NR |
| Baryishak et al. (1982) | No | No | No | No | ‐ | NR |
| Bonini et al. (2000) | Yes | Yes | Yes | Yes | ‐ | No |
| Bozkurt et al. (2010) | Yes | Yes | Yes | No | ‐ | No |
| Bozkurt et al. (2013) | Yes | Yes | No | No | ‐ | No |
| Chiambaretta et al. (2012) | No | No | Yes | No | ‐ | NR |
| De Oliveira et al. (2006) | Yes | Yes | No | No | ‐ | NR |
| Elsurer et al. (2021) | Yes | Yes | Yes | No | ‐ | NR |
| Erdem et al. (2011) | Yes | No | No | No | ‐ | NR |
| Gómez‐Henao et al. (2018) | Yes | Yes | Yes | Yes | ‐ | Yes |
| Inada et al. (2007) | Yes | Yes | No | No | ‐ | NR |
| Inada et al. (2009) | Yes | Yes | Yes | No | ‐ | NR |
| Jongvanitpak et al. (2020) | Yes | Yes | Yes | Yes | ‐ | NR |
| Kocabeyoglu et al. (2008) | Yes | Yes | Yes | No | ‐ | NR |
| Kocaturk et al. (2012) | Yes | No | Yes | No | ‐ | NR |
| Kosrirukvongs et al. (2001) | Yes | Yes | Yes | No | ‐ | NR |
| Leonardi et al. (2006) | Yes | Yes | Yes | Yes | ‐ | No |
| Leonardi et al. (2015) | Yes | Yes | Yes | No | ‐ | NR |
| Montan et al. (1999) | Yes | Yes | Yes | Yes | ‐ | NR |
| Monzón et al. (2009) | Yes | Yes | Yes | Yes | ‐ | NR |
| Mumcuoglu et al. (1988) | Yes | No | Yes | No | ‐ | NR |
| Naidoo et al. (2014) | Yes | Yes | No | No | ‐ | NR |
| Nebbioso et al. (2015) | Yes | Yes | No | No | ‐ | NR |
| Nebbioso et al. (2018) | Yes | Yes | No | No | ‐ | NR |
| Pokharel et al. (2009) | Yes | Yes | Yes | No | ‐ | NR |
| Polido et al. (2015) | Yes | Yes | Yes | No | ‐ | NR |
| Pucci et al. (2003) | Yes | Yes | No | No | ‐ | NR |
| Samra et al. (1984) | Yes | No | No | No | ‐ | NR |
| Tesse et al. (2010) | Yes | Yes | Yes | No | ‐ | NR |
| Tomassini et al. (1994) | Yes | Yes | Yes | Yes | ‐ | NR |
| Tuft et al. (1989) | Yes | Yes | Yes | No | ‐ | NR |
| Villalon Garcia et al. (2015) | Yes | Yes | Yes | Yes | ‐ | No |
| Zicari et al. (2013) | Yes | Yes | No | No | ‐ | NR |
Note: Studies are assessed Yes/No/Unclear/NR on items from the Agency for Healthcare Research and Quality checklist: Defines source: Defines the source of information. Eligibility criteria: lists inclusion and exclusion criteria or refers to previous publications. Time period: indicates period used for identifying participants. Consecutive recruitment: indicates whether subjects were consecutively recruited for the study. Quality assurance: describes any assessments undertaken for quality assurance purposes. Explains exclusions: explains any patient exclusions from the analysis.
Abbreviation: NR, not relevant.
We realized that when evaluating allergen test outcomes, evaluation of quality assurance related to such tests could not be made in a reliable fashion based on reports of individual studies since quality aspects of such tests are often part of routine clinical quality evaluation and often made outside of an eye department, e.g., a clinical biochemistry department.
3.5. Prevalence of VKC patients with a positive allergy test
A total of 29 studies were eligible for the quantitative analysis. These studies provided data from 1836 patients with VKC who had any test to determine any positive allergy test. The random‐effects pooled prevalence of allergy test‐positive patients with VKC was 57.7% (95% confidence interval: 52.5%–62.8%; Figure 2). Heterogeneity across studies was substantial at I2 = 76%. The Funnel plot did not suggest a strong publication bias (Appendix S3). Our sensitivity analysis underscored the robustness of the summary prevalence estimate since excluding studies by turn leads to prevalence estimates ranging only within a very narrow interval between 56.5% and 58.5% (Appendix S3).
FIGURE 2.
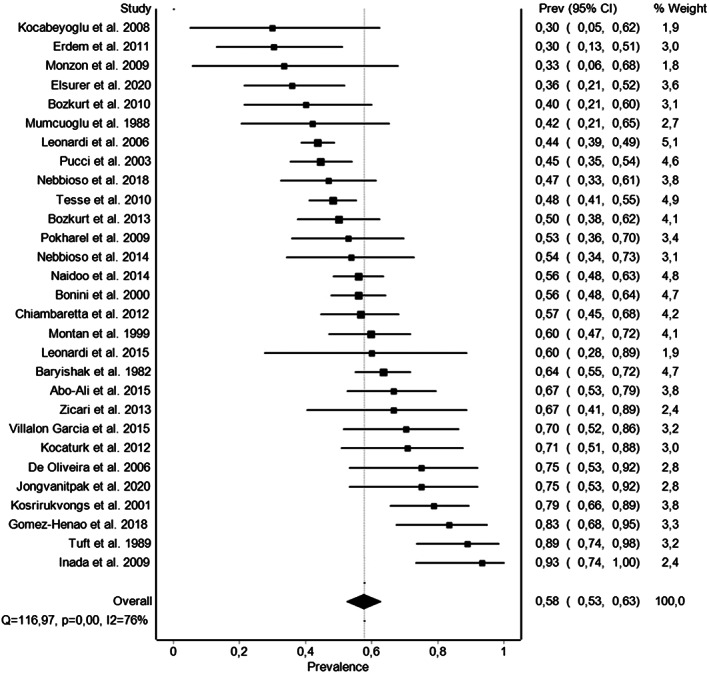
Forest plot of the meta‐analysis of the prevalence of patients with vernal keratoconjunctivitis with a positive allergy test.
We then explored our a priori defined subgroup analyses, that is, the pooled estimates focusing on sensitization based on specific testing methods, that is, conjunctival provocation test, total tear IgE, specific tear IgE, skin prick test, and specific serum IgE:
For the conjunctival provocation test, data were available from four studies on 205 patients with VKC. Here, the random‐effects pooled prevalence of positive patients was 51.4% (95% confidence interval: 38.0%–64.6%). Heterogeneity across studies was substantial at I 2 = 65%.
For the total tear IgE, data were available from five studies on 222 patients with VKC. Here, the random‐effects pooled prevalence of positive patients was 68.7% (95% confidence interval: 51.8%–83.6%). Heterogeneity across studies was substantial at I 2 = 80%.
For the specific tear IgE, data were available from three studies on 66 patients with VKC. Here, the random‐effects pooled prevalence of positive patients was 58.9% (95% confidence interval: 46.9%–70.5%). Heterogeneity across studies was low at I 2 = 0%.
For the skin prick test, data were available from 16 studies on 1173 patients with VKC. Here, the random‐effects pooled prevalence of positive patients was 58.2% (95% confidence interval: 51.0%–65.3%). Heterogeneity across studies was substantial at I 2 = 80%.
For the specific serum IgE, data were available from eight studies on 627 patients with VKC. Here, the random‐effects pooled prevalence of positive patients was 54.2% (95% confidence interval: 40.7%–67.4%). Heterogeneity across studies was considerable at I 2 = 89%.
The Funnel plots for each of these subgroup analyses did not suggest the strong presence of publication bias and the sensitivity analyses suggested robustness of the individual subgroup estimates (Appendix S4).
4. DISCUSSION
In this systematic review and meta‐analysis, we find that overall, 57.7% of patients with VKC can be expected to have a positive allergy test. The most prevalent allergens seemed to be house dust mites and pollen. A prevalence of sensitivity of 57.7% is much higher than that reported in studies with matched control groups, where ~5% of the healthy individuals had any allergens upon testing (Baryishak et al., 1982; Leonardi et al., 2015; Monzón et al., 2009). However, it should be noted that large population‐based prevalence studies of sensitivity in children have reported a prevalence of 22%–62% (Katotomichelakis et al., 2016; Majkowska‐Wojciechowska et al., 2007). Hence, the matched control groups in studies evaluated in our review may not reflect a non‐selected background population, but rather may reflect that control groups are constituted by individuals selected based on no history or symptoms of any allergy or allergy‐associated diseases. For comparison, the prevalence of a positive allergy test in patients with seasonal allergic conjunctivitis is reported in the range of ~60%–70% (Almaliotis et al., 2013; Jongvanitpak et al., 2020; Polido et al., 2015), which is slightly higher than that we find in patients with VKC.
Gold standard of allergen testing for patients with VKC is the conjunctival provocation test. We find that using the conjunctival provocation test, the pooled prevalence of positive patients was 51.4%, which is in line with the prevalence estimates identified using other testing methods. For the conjunctival provocation test, it should be noted that the estimate was calculated based on four studies and 205 patients, which is a smaller sample size in comparison to the evidence on the skin prick test with 16 studies on 1173 patients. Further, the individual studies were not without potential sources of bias, for example, in Leonardi et al. (2006) only a subset of 103 patients of 406 in total were included for the conjunctival provocation test. This was a population of both skin prick test positive and negative patients, which used an unclear testing strategy of a panel with seven allergens for the conjunctival provocation and without specifying if patients underwent conjunctival provocation testing for only known allergies identified in the skin prick test or all the seven allergens (Leonardi et al., 2006). To further add to the uncertainties surrounding this test, one should consider the risk of false‐positive tests due to irritation of the conjunctiva (Leonardi et al., 2006).
High levels of total IgE in tears are associated with allergic conjunctivitis (Monzón et al., 2009; Mumcuoglu et al., 1988; Pokharel et al., 2009). Patients with VKC are reported to have specific IgE in tears for dust mites, pollen (grass, tree), animal (cat), and food allergies (Leonardi et al., 2015; Mumcuoglu et al., 1988; Tuft et al., 1989). Mumcuoglu et al. (1988) sampled house dust from the homes of the patients and measured their specific tear IgE for dust mites, monthly for a year, and found a tear IgE increase from June to September that reflected the increase in mite levels in the summer period, which is indicative of a temporal correlation between the presence of specific IgE in tears and the flare of VKC.
Although the prevalence estimates using different methods are largely similar, it should be stressed that these tests are not interchangeable. Leonardi et al. (1993) found a positive correlation between the conjunctival provocation test and specific tear IgE levels, and a poor correlation between specific tear IgE levels and the skin prick test. These circumstances call for studies that better elucidate how best to clinically utilize different the overlap and differences between conjunctival provocation test, tear IgE testing, and other tests such as skin prick test and specific serum IgE levels.
Our review also highlights the importance of geography. We observed a clear pattern of geographic differences in sensitization, which at least to some extent may also be reflected in the variation in testing panels. Most testing panels include house dust mites and animal dander (dog and cat), but more rare allergens can be present such as tobacco, pigeon feather, cockroach, ants, and other types of insects. Geographic differences across 5 different panels are listed below for comparison:
Egypt (Abo‐Ali et al., 2015): House dust mites, animal dander (cat, dog, and horse), moulds, pollens, grass, wool, hay dust, straw, cotton dust, tobacco, pigeon feather, Candida, and American cockroach (Periplaneta americana).
Turkey (Bozkurt et al., 2010, 2013; Elsurer et al., 2021): House dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus), tree pollens, weed mix, grasses‐cereal, animal hair, moulds, and food allergens (egg and cacao). House dustmites [D. pteronyssinus (Dp) and D. farinae (Df)], a mixture of grass pollens, a mixture of grain pollens, tree pollens, moulds, Cladosporium mixture, animal hair, and cockroach.
Italy (Bonini et al., 2000): Rye grass, house dust mites, Parietaria, Olea, Artemisia, cat dander, and Alternaria allergens.
Brazil (De Oliveira et al., 2006): House dust mites D. pteronyssinus, D. farinae, and Blomia tropicalis, as well as allergens from cat, dog, fungi, and feathers.
Thailand (Kosrirukvongs et al., 2001): house‐dust mites, house dust, cockroaches, grass, insects, fungi, and food.
These geographical differences are also reflected in the variation of positive tests. Positive test for house dust mites ranges from 6% to 100%, highest in Brazil and Thailand and lowest in Europe (Abo‐Ali et al., 2015; De Oliveira et al., 2006; Kocaturk et al., 2012; Kosrirukvongs et al., 2001; Montan et al., 1999; Pucci et al., 2003). In contrast, a positive test for pollen is highest in Europe at 24%–51% and lowest in warmer geographical areas such as Egypt and Columbia at 12% (Abo‐Ali et al., 2015; Bozkurt et al., 2010, 2013; De Oliveira et al., 2006; Montan et al., 1999; Nebbioso et al., 2015; Pucci et al., 2003; Tesse et al., 2010).
Patients with VKC have a significantly higher level of serum total and specific IgE when compared to healthy controls, but similar levels when compared to other allergic or atopic conditions (Baryishak et al., 1982; Leonardi et al., 2015; Monzón et al., 2009; Polido et al., 2015; Pucci et al., 2003; Samra et al., 1984). Based on these circumstances, one could speculate that VKC, atopic keratoconjunctivitis, seasonal allergic conjunctivitis, and perennial allergenic conjunctivitis may share pathophysiology and different clinical manifestations of an allergenic disease spectrum. Our findings fall in line with other evidence reporting that approximately 50% of patients with VKC have a positive history of other allergic diseases (atopic dermatitis, asthma, and eczema) (Leonardi et al., 2006). In line with these findings is the epidemiological evidence that the presence of atopic diseases is a strong predictor of the development of VKC (Bonini et al., 2004; Bremond‐Gignac et al., 2008; Leonardi et al., 2006; Montan et al., 1999; Tesse et al., 2010). Due to the nature of the dissemination of data in the studies in the review, it was not possible to conduct reliable subgroup analyses to study the prevalence of sensibilization based on comorbidity strata.
Identifying allergens not only provides an opportunity for environmental allergy avoidance but it may also provide an opportunity for allergy‐specific treatment. House dust mite allergies and pollen allergies can be treated with allergen immunotherapy. In allergic conjunctivitis and rhinoconjunctivitis, allergen‐specific immunotherapy towards pollen and house dust mites reduces ocular symptoms (Calderon et al., 2011; Mahdy et al., 2012; Yang et al., 2018). For allergy‐related diseases, many protocols recommend re‐testing every second year. Considering that VKC is not a strictly allergic disease, and that sensitization is present in approximately half of the patients, a more practical and feasible approach is to re‐test upon new suspicion of allergies or if the severity of symptoms increases dramatically.
Limitations should be acknowledged when interpreting the results of our study. We can only rely on the authors' diagnosis of VKC. Diagnosis of VKC remains a challenge, and the pathway of diagnosis may sometimes start by finding an allergy or having a history of allergic disease. In such cases, considering VKC as a diagnosis may be more outright, whereas a mild case of VKC that responds well to mild steroids can be misdiagnosed as other types of mild chronic conjunctivitis. This source of bias potentially may overestimate the number of sensitized patients. However, one can also argue, that this reflects routine clinical practice. On the aspect of VKC diagnosis, there is another important limitation in that different centers and studies may have had different approaches and definitions of VKC. To further complicate this issue, there is also the question of whether the diagnostic criteria and approach to VKC may have changed over time. Due to the lack of such details of diagnosis, we were unable to explore such matters in this study. Another important limitation is that pooling results from studies with a variation in the testing panel introduce a certain heterogeneity, which is an important limitation in all meta‐analyses. On the point of heterogeneity, testing before and after commencement of therapy may influence the results of different testing methods, for example, since topical glucocorticosteroids can block the histamine response (Gradman & Wolthers, 2008). Although we can discuss and hypothesize on the presence of heterogeneity, our sensitivity analysis suggested overall robustness of the results and the overall trend was not dependent on the findings of single studies.
In conclusion, we find that 57.7% of the patients with VKC are sensitized. From a clinical point of view, identifying possible clinically relevant allergens provide information that may aid in managing VKC. However, it should be noted that this may only be relevant for a proportion of the patients, and when planning testing and informing patients and parents, it is important to understand that ~40% of the patients do not have any allergies.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
ACKNOWLEDGEMENT
None.
Rasmussen, M.L.R. , D’Souza, M. , Topal, D.G. , Gradman, J. , Larsen, D.A. , Lehrmann, B.B. et al. (2023) Prevalence of allergic sensitization with vernal keratoconjunctivitis: A systematic review with meta‐analyses. Acta Ophthalmologica, 101, 9–21. Available from: 10.1111/aos.15212
REFERENCES
- Abo‐Ali, F.H. , Farres, M.N. , Shahin, R.Y. , Eissa, A.M. , Ahmed, A. , Abdel‐Monsef, A. et al. (2015) Skin prick test reactivity to aeroallergens among Egyptian patients with isolated allergic conjunctival disease. The Egyptian Journal of Immunology, 22, 41–47. [PubMed] [Google Scholar]
- Almaliotis, D. , Michailopoulos, P. , Gioulekas, D. , Giouleka, P. , Papakosta, D. , Siempis, T. et al. (2013) Allergic conjunctivitis and the most common allergens in northern Greece. World Allergy Organization Journal, 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendregt, J.J. , Doi, S.A. , Lee, Y.Y. , Norman, R.E. & Vos, T. (2013) Meta‐analysis of prevalence. Journal of Epidemiology and Community Health, 67, 974‐978. [DOI] [PubMed] [Google Scholar]
- Baryishak, Y.R. , Zavaro, A. , Monselise, M. , Samra, Z. & Sompolinsky, D. (1982) Vernal keratoconjunctivitis in an Israeli group of patients and its treatment with sodium cromoglycate. The British Journal of Ophthalmology, 66, 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini, S. , Bonini, S. , Lambiase, A. , Marchi, S. , Pasqualetti, P. , Zuccaro, O. et al. (2000) Vernal keratoconjunctivitis revisited: a case series of 195 patients with long‐term followup. Ophthalmology, 107, 1157–1163. [DOI] [PubMed] [Google Scholar]
- Bonini, S. , Coassin, M. , Aronni, S. & Lambiase, A. (2004) Vernal keratoconjunctivitis. Eye, 18, 345–351. [DOI] [PubMed] [Google Scholar]
- Bozkurt, M.K. , Bozkurt, B. , Artac, H. , Arslan, N. & Reisli, I. (2010) Vernal keratoconjunctivitis—a rare but serious comorbidity of allergic rhinitis and eustachian tube dysfunction. International Journal of Pediatric Otorhinolaryngology, 74, 60–63. [DOI] [PubMed] [Google Scholar]
- Bozkurt, B. , Artac, H. , Arslan, N. , Gokturk, B. , Bozkurt, M.K. , Reisli, I. et al. (2013) Systemic atopy and immunoglobulin deficiency in Turkish patients with vernal keratoconjunctivitis. Ocular Immunology and Inflammation, 21, 28–33. [DOI] [PubMed] [Google Scholar]
- Bremond‐Gignac, D. , Donadieu, J. , Leonardi, A. , Pouliquen, P. , Doan, S. , Chiambarretta, F. et al. (2008) Prevalence of vernal keratoconjunctivitis: a rare disease? The British Journal of Ophthalmology, 92, 1097–1102. [DOI] [PubMed] [Google Scholar]
- Calderon, M.A. , Penagos, M. , Sheikh, A. , Canonica, G.W. & Durham, S.R. (2011) Sublingual immunotherapy for allergic conjunctivitis: cochrane systematic review and meta‐analysis. Clinical and Experimental Allergy, 41, 1263–1272. [DOI] [PubMed] [Google Scholar]
- Chiambaretta, F. , Taudou, C. , Fargette, F. , Viennet, A. & Fauquert, J. (2012) Adverse effects of conjunctival provocation test to inhaled allergens in children. Allergy, 67, 452–586. [Google Scholar]
- De Oliveira, L.A. , Mallozi, M.C. , Sole, D. , De Freitas, D. , De Sousa, L.B. & Mannis, M.J. (2006) Are cutaneous hypersensitivity tests to inhalant allergens a severity marker for vernal keratoconjunctivitis? Arquivos Brasileiros de Oftalmologia, 70, 991–995. [DOI] [PubMed] [Google Scholar]
- Egger, M. , Davey Smith, G. , Schneider, M. & Minder, C. (1997) Bias in meta‐analysis detected by a simple, graphical test. BMJ, 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsurer, C. , Bozkurt, B. , Aksoy, M.C. & Bozkurt, M.K. (2021) Evaluation of olfactory function in children with vernal keratoconjunctivitis. American Journal of Rhinology & Allergy, 35, 535–540. [DOI] [PubMed] [Google Scholar]
- Erdem, E. , Dogruel, D. , Yagmur, M. , Ersoz, R. , Altintas, D. , Karakoc, G. et al. (2011) Effect of bilastine on ocular symptoms in patients with allergic rhinoconjunctivitis. Allergy, 66, 1–104. [Google Scholar]
- Gómez‐Henao, C.M. , Herrera‐Morales, C.I. , Ramírez‐Giraldo, R. & Cardona‐Villa, R. (2018) Quality of life and clinical characterization of patients with vernal keratoconjunctivitis in a pediatric population in Colombia. Allergologia et Immunopathologia, 46, 370–377. [DOI] [PubMed] [Google Scholar]
- Gradman, J. & Wolthers, O.D. (2008) Suppressive effects of topical mometasone furoate and tacrolimus on skin prick testing in children. Pediatric Dermatology, 25, 269–270. [DOI] [PubMed] [Google Scholar]
- Higgins, J.P. , Thompson, S.G. , Deeks, J.J. & Altman, D.G. (2003) Measuring inconsistency in meta‐analyses. BMJ, 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. , Thomas, J. , Chandler, J. , Cumpston, M. , Li, T. , Page, M. et al. (2021) Cochrane Handbook for Systematic Reviews of Interventions version 6.0. Cochrane, p. 2021. (updated February 2021). [Accessed 30 January 2021]. Available from:. www.training.cochrane.org/handbook [Google Scholar]
- Inada, N. , Shoji, J. , Hoshino, M. & Sawa, M. (2007) Evaluation of total and allergen‐specific secretory IgA in tears of allergic conjunctival disease patients. Japanese Journal of Ophthalmology, 51, 338–342. [DOI] [PubMed] [Google Scholar]
- Inada, N. , Shoji, J. , Kato, H. , Kiely, S. , Mulyanto & Sawa, M. (2009) Clinical evaluation of total IgE in tears of patients with allergic conjunctivitis disease using a novel application of the immunochromatography method. Allergology International, 58, 585–589. [DOI] [PubMed] [Google Scholar]
- Jongvanitpak, R. , Vichyanond, P. , Jirapongsananuruk, O. , Visitsunthorn, N. & Pacharn, P. (2020) Clinical characteristics and outcomes of ocular allergy in Thai children. Asian Pacific Journal of Allergy and Immunology [ePub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Katotomichelakis, M. , Danielides, G. , Iliou, T. , Anastassopoulos, G. , Nikolaidis, C. , Kirodymos, E. et al. (2016) Allergic sensitization prevalence in a children and adolescent population of northeastern Greece region. International Journal of Pediatric Otorhinolaryngology, 89, 33–37. [DOI] [PubMed] [Google Scholar]
- Kocabeyoglu, S. , Bozkurt, B. , Bilen, O. , Irkec, M. & Orhan, M. (2008) Serum allergen specific immunoglobulin E levels in patients with allergic conjunctivitis. European Journal of Ophthalmology, 18, 675–679. [DOI] [PubMed] [Google Scholar]
- Kocaturk, T. , Kocaturk, O. , Dayanir, V. & Eliacik, K. (2012) Skin prick test and immunotherapy in children with allergic eye disease. Turkish Journal of Medical Sciences, 42, 485–490. [Google Scholar]
- Kosrirukvongs, P. , Visitsunthorn, N. , Vichyanond, P. & Bunnag, C. (2001) Allergic conjunctivitis. Asian Pacific Journal of Allergy and Immunology, 19, 237–244. [PubMed] [Google Scholar]
- Kumar, S. (2009) Vernal keratoconjunctivitis: a major review. Acta Ophthalmologica, 87, 133–147. [DOI] [PubMed] [Google Scholar]
- Leonardi, A. , Battista, M.C. , Gismondi, M. , Fregona, I.A. & Secchi, A.G. (1993) Antigen sensitivity evaluated by tear‐specific and serum‐specific IgE, skin tests, and conjunctival and nasal provocation tests in patients with ocular allergic disease. Eye (Lond), 7: 461–464. 10.1038/eye.1993.93 [DOI] [PubMed] [Google Scholar]
- Leonardi, A. , Busca, F. , Motterle, L. , Cavarzeran, F. , Fregona, I.A. , Plebani, M. et al. (2006) Case series of 406 vernal keratoconjunctivitis patients: a demographic and epidemiological study. Acta Ophthalmologica Scandinavica, 84, 406–410. [DOI] [PubMed] [Google Scholar]
- Leonardi, A. , Borghesan, F. , Faggian, D. & Plebani, M. (2015) Microarray‐based IgE detection in tears of patients with vernal keratoconjunctivitis. Pediatric Allergy and Immunology, 26, 641–645. [DOI] [PubMed] [Google Scholar]
- Mahdy, R.A.R. , Nada, W.M. & Marei, A.A. (2012) Subcutaneous allergen‐specific immunotherapy versus topical treatment in vernal keratoconjunctivitis. Cornea, 31, 525–528. [DOI] [PubMed] [Google Scholar]
- Majkowska‐Wojciechowska, B. , Pełka, J. , Korzon, L. , Kozłowska, A. , Kaczała, M. , Jarzebska, M. et al. (2007) Prevalence of allergy, patterns of allergic sensitization and allergy risk factors in rural and urban children. Allergy, 62, 1044–1050. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D.G. & PRISMA Group . (2009) Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ, 339, b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montan, P.G. , Ekström, K. , Hedlin, G. , van Hage‐Hamsten, M. , Hjern, A. & Herrmann, B. (1999) Vernal keratoconjunctivitis in a Stockholm ophthalmic Centre ‐ epidemiological, functional, and immunologic investigations. Acta Ophthalmologica Scandinavica, 77, 559–563. [DOI] [PubMed] [Google Scholar]
- Monzón, S. , Arrondo, E. , Bartra, J. , Torres, F. , Basagaña, M. , San Miguel, M.M. et al. (2009) Conjunctivitis and total IgE in lacrimal fluid: lacrytest screening. Journal of Allergy, 2009, 518903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumcuoglu, Y.K. , Zavaro, A. , Samra, Z. & Lazarowitz, Z. (1988) House dust mites and vernal keratoconjunctivitis. Ophthalmologica, 196, 175–181. [DOI] [PubMed] [Google Scholar]
- Naidoo, S. , Levin, M. , Tinley, C. & Pollock, T. (2014) Aeroallergen vensitisation and prevalence of asthma, allergic rhinitis and eczema in children with vernal keratoconjunctivitis attending red cross war memorial Children's hospital. Allergy, 69, 1–96. [Google Scholar]
- Nebbioso, M. , Zicari, A.M. , Celani, C. , Lollobrigida, V. , Grenga, R. & Duse, M. (2015) Pathogenesis of vernal keratoconjunctivitis and associated factors. Seminars in Ophthalmology, 30, 340–344. [DOI] [PubMed] [Google Scholar]
- Nebbioso, M. , Sacchetti, M. , Bianchi, G. , Zicari, A.M. , Duse, M. , del Regno, P. et al. (2018) Tear Ferning test and pathological effects on ocular surface before and after topical cyclosporine in vernal keratoconjunctivitis patients. Journal of Ophthalmology, 2018, 1061276–1061211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokharel, S. , Shah, D.N. , Joshi, S.N. & Choudhary, M. (2009) Tearfilm immunoglobulin E (IgE) level in vernal keratoconjunctivitis by ELISA. Kathmandu University Medical Journal, 7, 104–108. [DOI] [PubMed] [Google Scholar]
- Polido, J.G. , Cabral, T. , Perini Pde, R. , Fernandes Mde, F. , de Freitas, D. , dos Santos Araújo, M.E. et al. (2015) Correlations between allergen‐specific IgE serum levels in patients with ocular allergy. Cornea, 34, 1092–1097. [DOI] [PubMed] [Google Scholar]
- Pucci, N. , Novembre, E. , Lombardi, E. , Cianferoni, A. , Bernardini, R. , Massai, C. et al. (2003) Atopy and serum eosinophil cationic protein in 110 white children with vernal keratoconjunctivitis: differences between tarsal and limbal forms. Clinical and Experimental Allergy, 33, 325–330. [DOI] [PubMed] [Google Scholar]
- Samra, Z. , Zavaro, A. , Barishak, Y. & Sompolinsky, D. (1984) Vernal keratoconjunctivitis: the significance of immunoglobulin E levels in tears and serum. International Archives of Allergy and Applied Immunology, 74, 158–164. [DOI] [PubMed] [Google Scholar]
- Stroup, D.F. , Berlin, J.A. , Morton, S.C. , Olkin, I. , Williamson, G.D. , Rennie, D. et al. (2000) Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA, 283, 2008–2012. [DOI] [PubMed] [Google Scholar]
- Tesse, R. , Spadavecchia, L. , Fanelli, P. , Rizzo, G. , Procoli, U. , Brunetti, L. et al. (2010) Treatment of severe vernal keratoconjunctivitis with 1% topical cyclosporine in an Italian cohort of 197 children. Pediatric Allergy and Immunology, 21, 330–335. [DOI] [PubMed] [Google Scholar]
- Tomassini, M. , Magrini, L. , Bonini, S. , Lambiase, A. & Bonini, S. (1994) Increased serum levels of eosinophil cationic protein and eosinophil‐derived neurotoxin (protein X) in vernal keratoconjunctivitis. Ophthalmology, 101, 1808–1811. 10.1016/s0161-6420(94)31097-9 [DOI] [PubMed] [Google Scholar]
- Tuft, S.J. , Dart, J.K. & Kemeny, M. (1989) Limbal vernal keratoconjunctivitis: clinical characteristics and immunoglobulin E expression compared with palpebral vernal. Eye (Lond), 3, 420–427. 10.1038/eye.1989.63 [DOI] [PubMed] [Google Scholar]
- Villalon Garcia, A.L. , Alarcon Tomas, M. , Vasquez Bautista, A.A. , Rodriguez Cabreros, M. , Martin Carribero, R. & Rodriquez Mosquera, M. (2015) Epidemiology of vernal keratoconjunctivitis in a Spanish pediatric population. Allergy, 70, 527–613. [Google Scholar]
- Yang, J. , Zhang, L. , Zhao, Z. & Liao, S. (2018) Sublingual immunotherapy for pediatric allergic conjunctivitis: a meta‐analysis of randomized controlled trials. International Forum of Allergy & Rhinology, 8, 1253–1259. [DOI] [PubMed] [Google Scholar]
- Zeng, X. , Zhang, Y. , Kwong, J.S. , Zhang, C. , Li, S. , Sun, F. et al. (2015) The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta‐analysis, and clinical practice guideline: a systematic review. Journal of Evidence‐Based Medicine, 8, 2–10. [DOI] [PubMed] [Google Scholar]
- Zicari, A.M. , Nebbioso, M. , Zicari, A. , Mari, E. , Celani, C. , Occasi, F. et al. (2013) Serum levels of IL‐17 in patients with vernal keratoconjunctivitis: a preliminary report. European Review for Medical and Pharmacological Sciences, 17, 1242–1244. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4


