Abstract
Objectives
Mucosal melanoma (MM) is a rare malignancy that can present in the head and neck (H&N). The Oral cavity is the second most common primary site in the H&N after sinonasal mucosa. This study investigates the impact of demographic and clinical factors on survival in oral cavity MM. Further, it investigates the outcomes and utility of elective neck dissections (END) in the management of oral MM.
Methods
The National Cancer Database was used to evaluate 432 patients with oral cavity MM from 2004 to 2016. Kaplan‐Meir and Cox regression analyses were used to determine variables associated with survival.
Results
The mean age was 64.0 ± 16.0 years. Most patients were white (85.1%) and male (60.0%). Gingiva (37.6%) and hard palate (36.1%) were the most common primary subsites in the oral cavity. Five‐year overall survival was 31.0%. Age (Hazards Ratio [95% Confidence Interval], 1.03 [1.01–1.06]), N‐stage (1.94 [1.10–3.42]), M‐stage (10.13 [3.33–30.86]), male sex (1.79 [1.06–3.03]), and African‐American race (2.63 [1.14–6.11]) were significantly associated with worse survival. 199 patients (46.9%) underwent neck dissection including 118 with lymph node yield (LNY) ≥ 18. The rate of occult nodal positivity was 45.4% for LNY ≥ 18 and 28.3% for LNY ≥ 1. ENDs were not associated with improved outcomes. However, occult lymph node involvement was associated with worse overall survival (p = 0.004).
Conclusions
Oral cavity MM has a poor prognosis. Lymph node involvement, distant metastasis, age, race, and male sex are associated with worse outcomes. Performing an END did not improve survival. However, END may have a prognostic role and help select patients for treatment intensification.
Level of Evidence
4 Laryngoscope, 133:317–326, 2023
Keywords: mucosal melanoma, oral cavity, national cancer database, neck dissection
This study uses the National Cancer Database to analyze factors associated with survival in oral cavity mucosal melanoma, as well as the role of elective neck dissections. Lymph node involvement, distant metastasis, age, race, and male sex were associated with worse outcomes. While elective neck dissections did not improve overall survival, they may have a prognostic role and help select patients for treatment intensification.

INTRODUCTION
Mucosal melanoma (MM) is a rare subtype of melanoma, accounting for less than 1.3% of all melanomas, with the majority occurring in the head and neck. 1 Primary mucosal melanomas are believed to arise from noncutaneous melanocytes. 1 While most cutaneous melanomas are diagnosed in the radial growth phase, mucosal melanomas are more likely to be found in the invasive, vertical growth phase, and are thus associated with worse outcomes. 2 , 3 Among head and neck melanomas, the nasal cavity and paranasal sinuses are the most common primary site (70%) followed by the oral cavity (25%). 4
Mucosal melanomas make up less than 1% of all oral cavity malignancies. 5 Early diagnosis and treatment are essential due to the associated poor survival. Five‐year overall survival (OS) for head and neck mucosal melanomas is approximately 25%. Oral and nasal cavity primary sites are associated with higher 5‐year OS at approximately 35%, compared to the nasopharynx, oropharynx, and paranasal sinuses. 6 Despite the low prevalence of MM, a trend of increasing incidence during the last several decades is remarkable, highlighting the increasing clinical relevance of this disease. 5
Oral MM often presents as a painless, rapidly growing mass; though, ulceration, bleeding, swelling, loose teeth, and ill‐fitting dentures may also occur in the early stages. 4 , 7 Compared to sinonasal MM, oral cavity MMs are more likely to have evidence of nodal metastasis on presentation. 4 , 8 Because of the higher likelihood of nodal metastasis, the National Comprehensive Cancer Network (NCCN) recommends elective neck dissections for mucosal melanomas of the oral cavity, though not of other primary sites. 9 However, a study of 74 patients in South Korea found there to be no survival benefit in patients undergoing neck dissection. 10 To our knowledge, there are no published data on the rate of positive nodal metastasis in elective neck dissections for this malignancy.
Prior studies have used national multicenter databases to investigate factors associated with survival among head and neck MMs. The primary site, age, tumor size, and nodal and distant metastases were associated with OS, while treatment modality was not. 6 When considering sinonasal mucosal melanoma specifically, age, T‐stage, M‐stage, and margin status were associated with survival outcomes. 11 However, there have been no prior population‐based studies of mucosal melanoma‐specific to the oral cavity. This study utilizes the national cancer database (NCDB) to describe demographics, clinicopathologic factors, and treatment modalities in oral cavity mucosal melanoma, and their associations with overall survival. Further, it investigates the outcomes and utility of elective neck dissections in this cohort.
METHODS
This study is a retrospective, population‐based analysis of the NCDB—a national clinical oncology registry sponsored by the Commission on Cancer of the American Academy of Surgeons, and the American Cancer Society. 12 The NCDB contains data from over 1500 Commission on Cancer accredited programs. Because the data is de‐identified, the study was exempt from our institution's Institutional Review Board.
The NCDB was investigated to collect patients with mucosal melanoma of the oral cavity between 2004 and 2016. Malignant MM was selected using the International Classification of Diseases for Oncology, 3rd Edition (ICD‐O‐3) histology codes 8720–8780. Patients with primary malignant oral cavity MM were selected based on ICD‐O‐3 topography codes. The oral cavity subsite was categorized into the following: lip mucosa, tongue, gingiva, floor of mouth, hard palate, buccal mucosa, vestibule of mouth, retromolar trigone, and other/unspecified (Table S1).
Demographic data included in the analysis were patient age, sex, insurance status, household median income, facility type, and degree of urbanization. The race was classified as White, African American, Asian, and other. Insurance status was classified as uninsured, Medicaid, Medicare, private insurance, and other government insurance. Household median income was categorized into quartiles based on the median income in the patient's zip code. Facility type was grouped as community cancer programs, academic/research programs, and integrated network cancer programs. The degree of urbanization was categorized as metropolitan, urban, and rural‐based on population.
Clinicopathological variables were defined based on the American Joint Committee on Cancer (AJCC) T stage (T3 or T4), N Stage (N0 or N1), M stage (M0 or M1), and clinical stage (III or IV). Charlson‐Deyo comorbidity index was used to categorize the severity of patients' comorbidities and was reported as 0, 1, 2, or 3+. Treatment variables analyzed included surgery and surgical margin status, radiation, chemotherapy, immunotherapy, and neck dissection.
Patients were considered to have undergone a neck dissection if at least 18 lymph nodes were examined—a validated indicator of an adequate lymph node dissection in head and neck cancers. 13 Because lymph node yield (LNY) of 18 nodes was validated on cases of squamous cell carcinoma of the head and neck and not MM, we also performed analyses when any number of lymph nodes were sampled, excluding lymph node aspirations (LNY ≥ 1). Elective neck dissections (END) were defined as neck dissections performed in patients who had no clinical evidence of nodal (N0) or distant (M0) metastases. Occult lymph node positivity was defined as the percentage of these patients who had lymph nodes examined and were found to have positive pathologic lymph node involvement.
Overall survival was calculated for the cohort and stratified by the above variables. Patients lost to follow up or alive at the end of the study period in 2016 were coded as right‐censored data. Statistical analyses were performed using SPSS statistical software version 25 (IBM Corporation, Armonk, NY). Kaplan–Meier (KM) univariate analysis was used to determine variables associated with OS. Multivariable analysis was completed using Cox regression to determine factors independently associated with OS. To avoid overfitting, only age, sex, and variables with p < 0.10 on univariate analysis were included in the model. Hazards ratios (HR) with 95% confidence intervals (CI) were reported for Cox regression. We then repeated the above analyses in the cohort of patients with N0 M0 disease to determine factors associated with survival in patients without clinical nodal or distant metastases. Statistical significance was established as p < 0.05 for univariate and multivariable analyses.
RESULTS
Study Population and Demographics
There were 432 identified cases of primary oral cavity MM. Demographics, clinicopathologic characteristics, and treatment in the cohort are shown in Table I. The most common primary subsite within the oral cavity was the gingiva (37.6%) followed by the hard palate (36.1%). On presentation, most oral cavity MMs had a T stage of 3 (57.3%). Clinical nodal metastasis was present in 36.9% of cases and distant metastasis was present in 13.4% of cases. The majority of patients received surgery (83.8%) with negative surgical margins (83.0%). Radiation (44.7%) was the second most common treatment provided, followed by immunotherapy (15.1%) and chemotherapy (13.5%). Neck dissection with a lymph node yield (LNY) of at least 18 nodes was performed in 28.2% of patients. Neck dissection with an LNY of at least one node was performed in 46.9% of patients. Mean ± SD LNY was 11.9 ± 18.7 nodes and the mean number of pathologically positive nodes was 2.2 ± 3.6.
TABLE I.
Demographic and Clinicopathologic Factors of Oral Cavity Mucosal Melanoma.
| Variable | No. | % |
|---|---|---|
| Total | 432 | 100 |
| Age, mean (±SD), years | 64 (SD ± 16) | |
| Sex | ||
| Male | 259 | 60.0 |
| Female | 173 | 40.0 |
| Race | ||
| White | 365 | 85.1 |
| Black | 34 | 7.9 |
| Asian/Pacific Islander | 21 | 4.9 |
| Other | 9 | 2.1 |
| Unknown | 3 | |
| Insurance status | ||
| Not insured | 19 | 4.6 |
| Private Insurance | 160 | 39.0 |
| Medicaid | 25 | 6.1 |
| Medicare | 202 | 49.3 |
| Other Government Insurance | 4 | 1.0 |
| Unknown | 22 | — |
| Median Household Income | ||
| <$40,227 | 72 | 16.8 |
| $40,227–50,353 | 89 | 20.8 |
| $50,354–63,332 | 103 | 24.1 |
| > = $63,333 | 164 | 38.3 |
| Unknown | 4 | — |
| Degree of Urbanization | ||
| Metropolitan | 335 | 80.5 |
| Urban | 71 | 17.1 |
| Rural | 10 | 2.4 |
| Unknown | 16 | — |
| Facility Type | ||
| Community | 108 | 27.1 |
| Academic/Research | 253 | 63.6 |
| Integrated Network | 37 | 9.3 |
| Unknown | 34 | |
| Charlson‐Deyo Score | ||
| 0 | 351 | 81.3 |
| 1 | 65 | 15 |
| 2 | 11 | 2.5 |
| 3+ | 5 | 1.2 |
| Primary Site | ||
| Lip Mucosa | 31 | 7.9 |
| Tongue | 13 | 3.3 |
| Gingiva | 147 | 37.6 |
| Floor of Mouth | 13 | 3.3 |
| Hard Palate | 141 | 36.1 |
| Buccal Mucosa | 30 | 7.7 |
| Vestibule | 7 | 1.8 |
| Retromolar Trigone | 9 | 2.3 |
| Other/Unknown | 41 | — |
| T Stage | ||
| T3 | 133 | 57.3 |
| T4 | 99 | 42.7 |
| Unknown | 200 | — |
| N Stage | ||
| N0 | 154 | 63.1 |
| N1 | 90 | 36.9 |
| Unknown | 188 | — |
| M Stage | ||
| M0 | 226 | 86.6 |
| M1 | 35 | 13.4 |
| Unknown | 171 | — |
| AJCC Stage | ||
| 3 | 88 | 37.4 |
| 4 | 147 | 62.6 |
| Unknown | 197 | — |
| Surgery | ||
| Yes | 361 | 83.8 |
| No | 70 | 16.2 |
| Unknown | 1 | — |
| Surgical Margins | ||
| Negative | 278 | 83.0 |
| Positive | 57 | 17.0 |
| Unknown | 26 | — |
| Radiation | ||
| Yes | 193 | 44.7 |
| No | 239 | 55.3 |
| Chemotherapy | ||
| Yes | 56 | 13.5 |
| No | 359 | 86.5 |
| Unknown | 17 | — |
| Immunotherapy | ||
| Yes | 65 | 15.1 |
| No | 366 | 84.9 |
| Unknown | 1 | — |
| Lymph Node Dissection (LNY ≥ 18) | ||
| Yes | 118 | 28.2 |
| No | 300 | 71.8 |
| Unknown | 14 | — |
| Lymph Node Dissection (LNY ≥ 1) | ||
| Yes | 199 | 46.9 |
| No | 225 | 53.1 |
| Unknown | 8 | — |
LNY = lymph node yield; SD = standard deviation.
Univariate Survival Analysis
Median survival time was 32.9 months and five‐year overall survival was 31.0% (Fig. 1). On Kaplan–Meier univariate analysis, age (p < 0.001), race (p = 0.002), clinical T stage (p = 0.009), clinical N stage (p < 0.001), clinical M stage (p < 0.001), and AJCC clinical stage (p < 0.001) were associated with OS (Table II). Figure 2 demonstrates overall survival by T, N, and M stages. No surgery (p < 0.001), positive margin status (p = 0.01), undergoing chemotherapy (p = 0.002), and undergoing neck dissections (p = 0.02) were associated with worse OS on unmatched KM analysis (Table II). Radiotherapy (p = 0.07) and immunotherapy (p = 0.46) were not associated with survival.
Fig. 1.
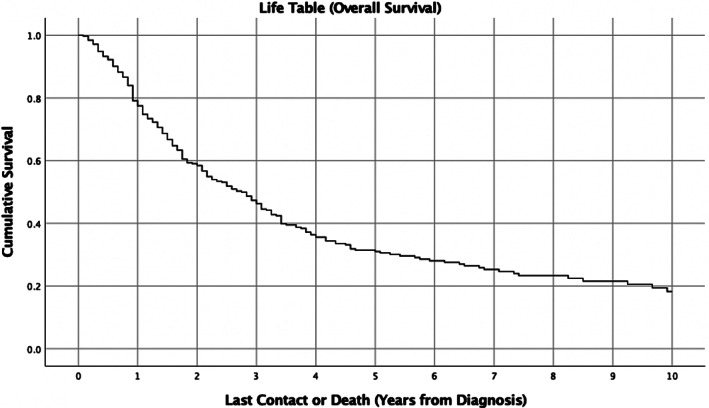
Kaplan–Meier survival curve of overall survival in oral cavity mucosal melanoma. Five‐year overall survival is 31.0% and median survival time is 32.9 months.
TABLE II.
Patient Characteristics and Univariate Kaplan–Meier Analysis of Overall Survival.
| Total Cohort | Local Disease Only (Clinical N0 M0) | ||
|---|---|---|---|
| Total | 389 | Total | 118 |
| Overall Survival (OS) | % | Overall Survival (OS) | % |
| 1‐Yr | 77.5 | 1‐Yr | 90.3 |
| 3‐Yr | 46.3 | 3‐Yr | 63.5 |
| 5‐Yr | 31.0 | 5‐Yr | 34.2 |
| 10‐Yr | 18.2 | 10‐Yr | — |
| Median | 32.9 Months | Median | 43.2 Months |
| Variable | N | 5‐Yr OS (%) | p value | Variable | N | 5‐YR OS (%) | p value |
|---|---|---|---|---|---|---|---|
| Total | 389 | 31.0 | Total | 118 | 34.2 | ||
| Age | <0.001 | Age | 0.002 | ||||
| 18–59 | 140 | 34.7 | 18–59 | 36 | 38.8 | ||
| 60–69 | 90 | 32.9 | 60–69 | 32 | 47.5 | ||
| 70–79 | 89 | 30.3 | 70–79 | 32 | 25.9 | ||
| 80+ | 67 | 17.1 | 80+ | 17 | 23.1 | ||
| Sex | 0.13 | Sex | 0.03 | ||||
| Male | 226 | 27.5 | Male | 69 | 28.8 | ||
| Female | 163 | 35.8 | Female | 49 | 35.4 | ||
| Race | 0.002 | Race | 0.15 | ||||
| White | 327 | 32.5 | White | 104 | 36.3 | ||
| African American | 31 | 14.5 | African American | 8 | 31.3 | ||
| Asian‐Pacific Islander | 21 | 43.9 | Asian‐Pacific Islander | 3 | 33.3 | ||
| Other | 7 | 14.3 | Other | 1 | 0 | ||
| Primary Payer | 0.11 | Primary Payer | 0.31 | ||||
| Not insured | 18 | 0 | Not insured | 2 | 0 | ||
| Private Insurance | 143 | 37.2 | Private Insurance | 46 | 46.5 | ||
| Medicaid | 23 | 42.9 | Medicaid | 8 | 62.5 | ||
| Medicare | 181 | 26.4 | Medicare | 56 | 24.5 | ||
| Other Government Insurance | 3 | 0 | Other Government Insurance | 1 | 0 | ||
| Median Income Quartile | 0.14 | Median Income Quartile | 0.92 | ||||
| <$40,227 | 61 | 23.48 | <$40,227 | 18 | 33.3 | ||
| $40,227–50,353 | 81 | 39.24 | $40,227–50,353 | 27 | 37.9 | ||
| $50,354–63,332 | 97 | 30.33 | $50,354–63,332 | 29 | 46.2 | ||
| >= $63,333 | 146 | 30.4 | >= $63,333 | 43 | 29.2 | ||
| Facility Type | 0.80 | Facility Type | 0.12 | ||||
| Community | 96 | 28.3 | Community | 29 | 28.2 | ||
| Academic/Research | 228 | 31.2 | Academic/Research | 67 | 38.6 | ||
| Integrated Network | 35 | 22.9 | Integrated Network | 12 | 13.9 | ||
| Degree of Urbanization | 0.22 | Degree of Urbanization | 0.54 | ||||
| Metropolitan | 303 | 31.7 | Metropolitan | 87 | 33.1 | ||
| Urban | 62 | 26.6 | Urban | 25 | 27.5 | ||
| Rural | 8 | 66.7 | Rural | 4 | 66.7 | ||
| Primary Site | 0.57 | Primary Site | 0.22 | ||||
| Lip Mucosa | 25 | 54 | Lip Mucosa | 12 | 65.6 | ||
| Tongue | 12 | 32.1 | Tongue | 2 | 0 | ||
| Gingiva | 129 | 28.1 | Gingiva | 32 | 27 | ||
| Floor of Mouth | 12 | 25 | Floor of Mouth | 4 | 33.3 | ||
| Hard Palate | 133 | 31.2 | Hard Palate | 44 | 32 | ||
| Buccal Mucosa | 29 | 45.1 | Buccal Mucosa | 12 | 53.6 | ||
| Vestibule | 6 | 25 | Vestibule | 1 | — | ||
| Retromolar Trigone | 8 | 37.5 | Retromolar Trigone | 3 | 66.7 | ||
| Charlson‐Deyo Score | 0.50 | Charlson‐Deyo Score | 0.84 | ||||
| 0 | 315 | 30.9 | 0 | 94 | 32.6 | ||
| 1 | 60 | 32.6 | 1 | 21 | 43.1 | ||
| 2 | 10 | 13.3 | 2 | 2 | 0 | ||
| 3+ | 4 | 50 | 3+ | 1 | — | ||
| T Stage | 0.009 | T Stage | 0.004 | ||||
| T3 | 108 | 32.9 | T3 | 68 | 44.8 | ||
| T4 | 86 | 13.4 | T4 | 36 | 10.2 | ||
| N Stage | <0.001 | ||||||
| N0 | 131 | 33 | |||||
| N1 | 74 | 15.3 | |||||
| M Stage | <0.001 | ||||||
| M0 | 194 | 29.9 | |||||
| M1 | 27 | 0 | |||||
| AJCC Stage | <0.001 | AJCC Stage | 0.003 | ||||
| III | 73 | 44.7 | III | 68 | 44.8 | ||
| IV | 124 | 11 | IV | 35 | 9.9 | ||
| Surgery | <0.001 | Surgery | 0.42 | ||||
| No | 62 | 15.2 | No | 7 | 42.9 | ||
| Yes | 326 | 34.2 | Yes | 111 | 33.7 | ||
| Radiation | 0.07 | Radiation | 0.11 | ||||
| No | 211 | 36.1 | No | 68 | 43.5 | ||
| Yes | 178 | 25.2 | Yes | 50 | 23.7 | ||
| Chemotherapy | 0.002 | Chemotherapy | 0.80 | ||||
| No | 325 | 32.4 | No | 108 | 31.9 | ||
| Yes | 51 | 23.1 | Yes | 5 | 40.0 | ||
| Immunotherapy | 0.46 | Immunotherapy | 0.73 | ||||
| No | 339 | 31.2 | No | 107 | 32.8 | ||
| Yes | 49 | 30.7 | Yes | 11 | 51.9 | ||
| Surgical Margins | 0.01 | Surgical Margins | 0.10 | ||||
| Negative | 253 | 36.6 | Negative | 94 | 33.6 | ||
| Positive | 49 | 23 | Positive | 15 | 30.5 | ||
| Lymph Node Dissection (LNY ≥ 18) | 0.02 | Lymph Node Dissection (LNY ≥ 18) (Elective) | 0.24 | ||||
| No | 272 | 33.9 | No | 85 | 37.5 | ||
| Yes | 103 | 24.2 | Yes | 30 | 21.1 | ||
| Lymph Node Dissection (LNY ≥ 1) | 0.79 | Lymph Node Dissection (LNY ≥ 1) (Elective) | 0.80 | ||||
| No | 206 | 29.7 | No | 65 | 32.7 | ||
| Yes | 175 | 32.7 | Yes | 53 | 36.6 | ||
| Occult LN involvement | 0.004 | ||||||
| No | 38 | 45.1 | |||||
| Yes | 15 | 0 | |||||
| Treatment Regimen | 0.03 | ||||||
| Surgery Alone | 54 | 42.8 | |||||
| Surgery + RT | 37 | 11.5 |
Note: Bold indicates P‐value < 0.05.
LNY = lymph node yield.
Fig. 2.
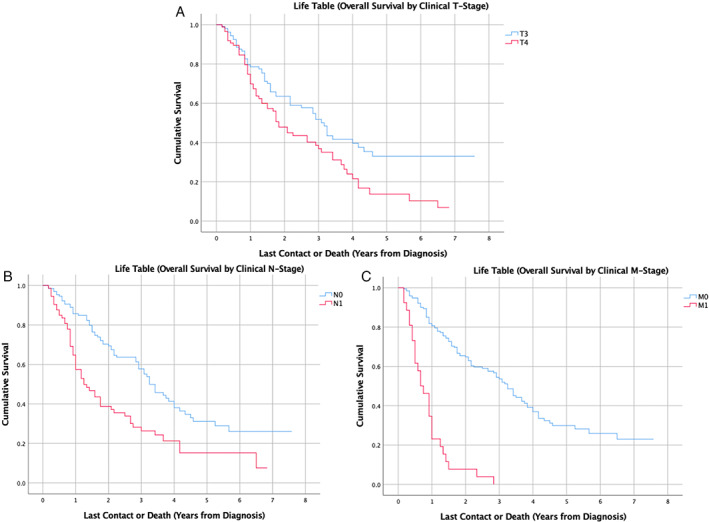
Kaplan–Meier survival curve of overall survival in oral cavity mucosal melanoma stratified by (A) Clinical T‐Stage (B) Clinical N‐Stage and (C) Clinical M‐stage. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Multivariable Survival Analysis
Cox regression analysis was performed with age, sex, race, T‐stage, N‐stage, M‐stage, treatment modality, margin status, and neck dissection (LNY ≥ 18) included as covariates. Advanced age (HR: 1.03, 95% CI: [1.01–1.06]), African American race (2.63 [1.14–6.11]), nodal metastasis (1.94 [1.10–3.42]), distant metastasis (10.13 [3.33–30.86]), and neck dissections with LNY ≥ 18 (2.23 [1.17–4.24]) were independently associated with worse survival (Table III). Female sex (0.56 [0.33–0.94]) was associated with improved survival outcomes. T‐stage, treatment modality and margin status were not associated with OS.
TABLE III.
Cox Proportional Hazards Multivariable Regression of Overall Survival in Oral Cavity Mucosal Melanoma.
| Covariate | Total (N = 120) | Hazard Ratio (95% CI) | p value |
|---|---|---|---|
| Age | 1.03 (1.01–1.06) | 0.003 | |
| Sex | |||
| Male | 70 | 1.00 (reference) | |
| Female | 50 | 0.56 (0.33–0.94) | 0.03 |
| Race | |||
| White | 103 | 1.00 (reference) | |
| Black | 11 | 2.63 (1.14–6.11) | 0.02 |
| Asian/Pacific Islander | 6 | 1.55 (0.45–5.22) | 0.48 |
| T Stage | |||
| T3 | 70 | 1.00 (reference) | |
| T4 | 50 | 0.95 (0.57–1.82) | 0.95 |
| N Stage | |||
| N0 | 82 | 1.00 (reference) | |
| N1 | 38 | 1.94 (1.10–3.42) | 0.02 |
| M Stage | |||
| M0 | 115 | 1.00 (reference) | |
| M1 | 5 | 10.13 (3.33–30.86) | <0.001 |
| Treatment | — | ||
| Surgery | 56 | 1.00 (reference) | |
| Surgery + RT | 48 | 1.57 (0.88–2.78) | 0.12 |
| Surgery + Chemotherapy | 4 | 1.94 (0.54–6.94) | 0.31 |
| Surgery + Immunotherapy | 5 | 2.24 (0.48–10.41) | 0.31 |
| Surgery + CRT | 7 | 1.09 (0.38–3.19) | 0.87 |
| Margins | |||
| Negative | 98 | 1.00 (reference) | |
| Positive | 22 | 1.07 (0.56–2.03) | 0.85 |
| Neck Dissection (LNY ≥ 18) | |||
| No | 76 | 1.00 (reference) | |
| Yes | 44 | 2.23 (1.17–4.24) | 0.02 |
| Neck Dissection (LNY ≥ 1)* | |||
| No | 50 | 1.00 (reference) | |
| Yes | 73 | 1.54 (0.81–2.93) | 0.19 |
Note: Bold indicates P‐value <0.05.
CI = confidence interval; CRT = chemoradiotherapy; LNY = lymph node yield; RT = radiation therapy.
For regression with Neck Dissection (LNY ≥ 1), N = 123;
The above analysis was repeated with neck dissections defined as LNY ≥ 1, as neck dissections with LNY ≥ 18 were validated on cases of squamous cell carcinoma of the head and neck and not MM. There was no association between neck dissection with LNY ≥ 1 and OS (1.54 [0.81–2.93]).
Clinical N0 and M0 Disease
There were 138 cases identified without clinical nodal or distant metastatic disease. Median survival was 43.2 months and five‐year overall survival was 34.2%. On Kaplan–Meier univariate analysis, advanced age (p = 0.002), male sex (p = 0.03), T‐stage (p = 0.004), AJCC stage (p = 0.003), and postoperative radiation (p = 0.03) were associated with worse OS (Table II). On multivariable Cox regression, older age (HR 1.07 [1.01–1.11]), T4 tumors (3.38 [1.37–8.32]), and undergoing neck dissection with LNY ≥ 18 (2.92 [1.04–8.19]) were associated with worse OS (Table IV). There was no difference in survival between patients undergoing surgery with radiation versus surgery alone. Margin status, race, and sex did not impact OS. When repeating the analysis of neck dissection with LNY ≥ 1, neck dissections were marginally associated with worse outcomes (1.95 [0.99–4.30]).
TABLE IV.
Cox Proportional Hazards Multivariable Regression of Overall Survival in Clinical N0 M0 Oral Cavity Mucosal Melanoma.
| Covariate | Total (N = 71) | Hazard Ratio (95% CI) | p value |
|---|---|---|---|
| Age | 1.07 (1.03–1.11) | <0.001 | |
| Sex | |||
| Male | 46 | 1.00 (reference) | |
| Female | 25 | 0.52 (0.23–1.16) | 0.11 |
| Race | |||
| White | 66 | 1.00 (reference) | |
| Black | 5 | 1.51 (0.40–5.78) | 0.55 |
| T Stage | |||
| T3 | 45 | 1.00 (reference) | |
| T4 | 26 | 3.38 (1.37–8.32) | 0.008 |
| Treatment | — | ||
| Surgery | 40 | 1.00 (reference) | |
| Surgery + RT | 31 | 0.93 (0.38–2.26) | 0.88 |
| Margins | |||
| Negative | 59 | 1.00 (reference) | |
| Positive | 12 | 0.93 (0.35–2.52) | 0.89 |
| Elective Neck Dissection (LNY ≥ 18) | |||
| No | 54 | 1.00 (reference) | |
| Yes | 17 | 2.92 (1.04–8.19) | 0.04 |
| Elective Neck Dissection (LNY ≥ 1)* | |||
| No | 42 | 1.00 (reference) | |
| Yes | 31 | 1.95 (0.88–4.30) | 0.10 |
Note: Bold indicates P‐value <0.05.
CI = confidence interval; LNY = lymph node yield; RT = radiation therapy.
For the regression including LNY ≥ 1, N = 73.
Elective Neck Dissections (END)
Of patients with clinical N0 M0 disease, 24.4% underwent END with an LNY ≥ 18, and 44.9% underwent a neck dissection of at least 1 node (Table V). On Kaplan–Meier analysis, there was no difference in survival between patients who did and did not undergo END, regardless of LNY (Table II). Of the 62 patients identified as having ENDs of at least 1 node, 17 (28.3%) yielded positive pathologic lymph node involvement. Among these patients, 7 had T3 tumors, 7 had T4 tumors, and the tumor stage was unknown for 3 patients. The rate of occult node positivity in patients undergoing neck dissection with LNY ≥ 18 was 45.4%. Positive lymph node involvement was associated with worse survival outcomes (p = 0.004; Table II).
TABLE V.
Elective Neck Dissections and Occult Nodal Involvement in Oral Cavity Mucosal Melanoma.
| LNY ≥ 18 | LNY ≥ 1 | |||||
|---|---|---|---|---|---|---|
| Number | % | 5‐Yr OS (%) | Number | % | 5‐Yr OS (%) | |
| Lymph Node Dissection | ||||||
| Yes | 118 | 28.2 | 24.1 | 199 | 46.9 | 32.7 |
| No | 300 | 71.8 | 33.9 | 225 | 53.1 | 29.7 |
| Unknown | 14 | — | 8 | — | ||
| Elective Neck Dissection | ||||||
| Yes | 33 | 24.4 | 21.1 | 62 | 44.9 | 36.6 |
| No | 102 | 75.6 | 37.5 | 76 | 55.1 | 32.7 |
| Unknown | 3 | — | — | 0 | — | |
| Elective Neck Dissection Result | ||||||
| No Positive LN | 18 | 54.5 | 44.4 | 43 | 71.7 | 45.1 |
| Positive LN | 15 | 45.4 | 0.0 | 17 | 28.3 | 0.0 |
| Missing | 0 | — | 2 | — | ||
LNY = lymph node yield; OS = overall survival.
DISCUSSION
To our knowledge, this is the largest study to systematically examine the associations of patient demographics, tumor characteristics, and treatment modality with overall survival in patients with primary MM of the oral cavity. Mucosal melanoma is a rare malignancy with a poor prognosis, and the oral cavity is the second most common subsite in the head and neck. 4 Our findings suggest that the 5‐year OS rate is 31.0%, which is consistent with some of the largest published retrospective and epidemiological studies that have proposed 5‐year survival rates ranging from 15 to 35%. 4 , 14 , 15
Patient Demographics
The average age of patients in this cohort was 64 years, consistent with previous studies of patients with oral cavity MM. 4 , 5 , 16 , 17 Most patients were male and White. As demonstrated in previous studies, the most common anatomical subsites were the gingiva and hard palate. 4 , 5 , 15 Advanced age, male sex, and African American race were each associated with poorer survival on multivariable analysis (Table III). In the United States, the incidence of MM has been found to be lower in African American than White populations. 18 However, prior studies of head and neck MMs overall and specific to the oral cavity have not found discrepancies in survival by race. 6 , 11 , 19 Further research is warranted to determine the reason for worse survival outcomes among African American patients with oral cavity MM. Possible contributing factors may include different tumor biology, later presentation, and healthcare disparities.
Treatment Modalities
Wide surgical resection with negative margins is the primary treatment for mucosal melanoma stage III through IVA, as it has been shown to optimize local control and survival. 20 , 21 , 22 Most patients (83.8%) in our study received surgery as part of their treatment. Surgery and negative margins were associated with improved survival on univariate analysis (Table II). However, on multivariable analysis, there was no difference in overall survival between treatment regimens or margin status (Tables III and IV). Jethanamest et al. demonstrated similar results, showing no difference in survival by treatment modality. 6
Surgical resection is considered the mainstay treatment for oral MM, with adjuvant radiotherapy recommended in selected cases to improve locoregional control. 9 , 23 Postoperative radiation for head and neck MM is recommended in cases of extracapsular nodal disease, involvement of at least 2 cervical or intraparotid lymph nodes, any node 3 cm or greater, nodal excision without neck dissection, or locoregional recurrence. 9 The T staging for mucosal melanoma omits the early T stage, so by definition, every MM is either T3 or T4. Therefore, all MM is considered an advanced T stage, which can be another justification for adjuvant RT. For oral cavity melanomas, adjuvant radiation may be used more often due to the higher likelihood of nodal metastasis. 9
Overall, 44.7% of patients underwent radiotherapy as part of their treatment. Among N0 and M0 patients, 51.2% of patients underwent surgery alone and 33.1% underwent surgery with radiotherapy. Our results demonstrated that in N0 M0 disease, adjuvant radiation therapy was not associated with improved survival outcomes (Tables III and IV). This is similar to prior studies on head and neck MM. Although radiotherapy was associated with longer local disease‐free survival and improved locoregional control, there was no significant impact of postoperative radiotherapy on the likelihood of developing distant metastases or overall survival. 14 , 24 , 25
In our analysis, 15.1% of patients received immunotherapy. Immunotherapy did not demonstrate any significant difference in overall survival on univariate analysis. Immunotherapy has a clearly established role in cutaneous melanoma, however, the benefit of immunotherapy in mucosal melanoma is less clear. 11 , 26 , 27 Current immunotherapy options for melanoma include cytokine treatment (INF‐a and IL‐2), as well as treatments targeting immune checkpoints including CTLA‐4, PD‐1, and PDL‐1. 10 In a pooled analysis that included 86 mucosal melanoma patients with unresectable disease receiving immunotherapy, the median progression‐free survival, was only 3 months and the objective response rate was only 23.3%. 28
Oral cavity mucosal melanomas have been found to have a higher proportion of c‐Kit aberrations compared to cutaneous melanomas. 29 Therapies targeting KIT such as Imatinib have been found to have a response rate of 30% in oral mucosal melanoma. 29 Immunotherapy with the combination of monoclonal antibodies nivolumab and ipilimumab have been shown to improve progression‐free survival over either agent alone in late‐stage MM. Though these regimens are more effective in cutaneous melanoma, results in clinical trials for MM are promising and further studies are warranted. 28 Given the poor prognosis for these patients, treatment intensification with novel agents should continue to be investigated.
Lymph Node Metastasis and Management of the Neck
The risk of cervical lymph node involvement is 50%–60% higher in oral cavity MM than in other MM of the head and neck. 5 , 9 , 26 , 27 Nodal metastasis has been found to be associated with a worse survival outcomes in oral cavity MM, and this was confirmed in our study. 30 Elective neck dissection (END) in N0 M0 oral cavity mucosal melanoma and radical, modified, or selective neck dissection in N1 M0 cases are typically recommended to maximize locoregional control. 9 , 31 A lymph node yield of at least 18 has been defined as a quality metric of adequate neck dissection in head and neck malignancies. 13 We found that 28.2% of patients underwent a neck dissection with LNY ≥ 18. This demonstrates that the majority of surgeons treating oral cavity MM are not following NCCN guidelines as they are omitting neck dissection. However, undergoing neck dissection with LNY ≥ 18 was associated with worse prognosis on univariate and multivariable analyses. Given that a minority of patients underwent neck dissection with LNY ≥ 18, it is probable that these neck dissections were performed in cases of more advanced local disease. This was likely a significant confounding factor that influenced these results. We found that the rate of occult node positivity in oral cavity mucosal melanoma in patients with LNY ≥ 18 was 45.4%, which is well above the threshold of 20% typically discussed for assessing the utility of neck dissection.
The NCCN advocates for considering elective neck dissection (END) for any case of oral cavity mucosal melanoma. 9 END with LNY ≥ 18 was performed in 24.4% of patients with clinical N0 M0 disease, and END with LNY ≥ 1 was performed in 44.9% of patients (Table V). Pathological nodes were found in 28.3% of ENDs overall and in 45.4% with LNY ≥ 18. This demonstrates that a comprehensive neck dissection allows for adequate and accurate staging, which offers important prognostic information to the patient. While there was no survival benefit of END on univariate or multivariable analyses, cases where pathological nodes were found were associated with worse survival (Table II). This suggests that while the therapeutic benefit of END is uncertain, it may serve a prognostic role. Prior studies showed no direct mortality benefit of END in oral cavity MM. 10 , 32 Wu et al. found that while there was no overall survival benefit of END in oral cavity MM, patients with nodular‐subtype tumors experienced a survival benefit. 30 Despite the uncertain survival benefit of END in oral cavity MM, it may be useful for staging and identifying candidates for treatment intensification in the form of adjuvant therapies.
Limitations
There are several limitations to this study. The accuracy of data in the NCDB is dependent on the integrity of data entry from the many contributing centers, and there are some missing or incomplete data fields. The NCDB includes overall survival data but does not have information on disease‐specific survival or locoregional control. Although to our knowledge, this is the largest analysis of oral mucosal melanoma, it is nevertheless a rare disease and the sample size is limited. The NCDB does not report specific data regarding chemotherapy and immunotherapy agents used during treatment. As new agents are being approved for the treatment of melanoma, their effect on MM will need to be elucidated in the setting of clinical trials. While neck dissection was a reported variable, the timing of neck dissection and relation to additional therapy was not reported.
CONCLUSIONS
This study analyzed the largest cohort of patients with mucosal melanoma of the oral cavity. Advanced age, male sex, and African American race were independently associated with worse survival outcomes. Treatment modality did not impact survival. For oral cavity mucosal melanoma, the rate of occult nodal positivity is 45.4%. While elective neck dissection did not improve survival, the finding of pathologically positive lymph nodes was associated with worse survival outcomes. Elective lymph node dissections may serve a prognostic role and help select patients for additional adjuvant treatments, despite the lack of mortality benefit at this time.
Supporting information
Table S1. ICD‐O‐3 Topography Codes and Primary Subsite Classification for Oral Cavity Mucosal Melanoma.
Editor's Note: This Manuscript was accepted for publication on April 05, 2022.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Presented as a poster presentation at the 2022 Triological Society, Combined Selection Meeting, January 20–22. Coronado, CA. (Abstract #2493).
REFERENCES
- 1. Chang AE, Karnell LH, Menck HR. The national cancer data base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664‐1678. [DOI] [PubMed] [Google Scholar]
- 2. Batsakis JG, Suarez P, El‐Naggar AK. Mucosal melanomas of the head and neck. Ann Otol Rhinol Laryngol. 1998;107:626‐630. [DOI] [PubMed] [Google Scholar]
- 3. Barker BF, Carpenter WM, Daniels TE, et al. Oral mucosal melanomas: the WESTOP Banff workshop proceedings. Western Society of Teachers of Oral pathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83(6):972‐979. [DOI] [PubMed] [Google Scholar]
- 4. Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24(3):247‐257. [DOI] [PubMed] [Google Scholar]
- 5. López F, Rodrigo JP, Cardesa A, et al. Update on primary head and neck mucosal melanoma. Head Neck. 2016;38:147‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jethanamest D, Vila PM, Sikora AG, Morris LG. Predictors of survival in mucosal melanoma of the head and neck. Ann Surg Oncol. 2011;18(10):2748‐2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Femiano F, Lanza A, Buonaiuto C, et al. Oral malignant melanoma. J Oral Pathol Med. 2008;37(7):383‐388. [DOI] [PubMed] [Google Scholar]
- 8. McLean N, Tighiouart M, Muller S. Primary mucosal melanoma of the head and neck. Comparison of clinical presentation and histopathologic features of oral and sinonasal melanoma. Oral Oncol. 2008;44(11):1039‐1046. [DOI] [PubMed] [Google Scholar]
- 9. Pfister DG, Ang K, Brizel DM, et al. Mucosal melanoma of the head and neck. J Natl Compr Cancer Netw. 2012;10(3):320‐338. [DOI] [PubMed] [Google Scholar]
- 10. Chae YS, Lee JY, Lee JW, Park JY, Kim SM, Lee JH. Survival of oral mucosal melanoma according to treatment, tumour resection margin, and metastases. Br J Oral Maxillofac Surg. 2020;58(9):1097‐1102. [DOI] [PubMed] [Google Scholar]
- 11. Ganti A, Raman A, Shay A, et al. Treatment modalities in sinonasal mucosal melanoma: a national cancer database analysis. Laryngoscope. 2020;130:275‐282. [DOI] [PubMed] [Google Scholar]
- 12. Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Divi V, Chen M, Nussenbaum B, et al. Lymph node count from neck dissection predicts mortality in head and neck cancer. J Clin Oncol. 2016;34(32):2892‐2897. [DOI] [PubMed] [Google Scholar]
- 14. Temam S, Mamelle G, Marandas P, et al. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer. 2005;103:313‐319. [DOI] [PubMed] [Google Scholar]
- 15. Bachar G, Loh KS, O'Sullivan B, et al. Mucosal melanomas of the head and neck: the Princess Margaret hospital experience. Head Neck J Sci Spec. 2008;30:1325‐1331. [DOI] [PubMed] [Google Scholar]
- 16. Hicks MJ, Flaitz CM. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncol. 2000;36(2):152‐169. [DOI] [PubMed] [Google Scholar]
- 17. Manolidis S, Donald PJ. Malignant mucosal melanoma of the head and neck: review of the literature and report of 14 patients. Cancer. 1997;80:1373‐1386. [DOI] [PubMed] [Google Scholar]
- 18. McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005;103(5):1000‐1007. [DOI] [PubMed] [Google Scholar]
- 19. Altieri L, Eguchi M, Peng DH, Cockburn M. Predictors of mucosal melanoma survival in population‐based setting. J Am Acad Dermatol. 2019;81(1):136‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andersen LJ, Berthelsen A, Hansen HS. Malignant melanoma of the upper respiratory tract and the oral cavity. J Otolaryngol. 1992;21:180‐185. [PubMed] [Google Scholar]
- 21. Lee SP, Shimizu KT, Tran LM, Juillard G, Calcaterra TC. Mucosal melanoma of the head and neck: the impact of local control on survival. Laryngoscope. 1994;104:121‐126. [DOI] [PubMed] [Google Scholar]
- 22. Stern SJ, Guillamondegui OM. Mucosal melanoma of the head and neck. Head Neck. 1991;13:22‐27. [DOI] [PubMed] [Google Scholar]
- 23. Yii NW, Eisen T, A'Hern R, Rhys‐Evans P, Archer D, et al. Mucosal malignant melanoma of the head and neck: the Marsden experience over half a century. Clin Oncol. 2003;15(4):199‐204. [DOI] [PubMed] [Google Scholar]
- 24. Benlyazid A, Thariat J, Temam S, et al. Postoperative radiotherapy in head and neck mucosal melanoma. Arch Otolaryngol Head Neck Surg. 2010;136(12):1219‐1225. [DOI] [PubMed] [Google Scholar]
- 25. Li W, Yu U, Wang H, Yan A, Jiang Z. Evaluation of the prognostic impact of postoperative adjuvant radiotherapy on head and neck mucosal melanoma: a meta‐analysis. BMC Cancer. 2015;15:758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chatzistefanou I, Kolokythas A, Vahtsevanos K, Antoniades K. Primary mucosal melanoma of the oral cavity: current therapy and future directions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(1):17‐27. [DOI] [PubMed] [Google Scholar]
- 27. Ascierto PA, Accorona R, Botti G, et al. Mucosal melanoma of the head and neck. Crit Rev Oncol Hematol. 2017;112:136‐152. [DOI] [PubMed] [Google Scholar]
- 28. D'Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of Nivolumab alone or in combination with Ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35(2):226‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsiambas E, Mastronikolis NS, Ragos V, Fotiades PP. c kit in oral mucosal melanoma. J BUON. 2018;23(6):1927‐1928. [PubMed] [Google Scholar]
- 30. Wu Y, Zhong Y, Li C, Song H, Guo W, Ren G. Neck dissection for oral mucosal melanoma: caution for nodular lesion. Oral Oncol. 2014;50(4):319‐324. [DOI] [PubMed] [Google Scholar]
- 31. Mendenhall WM, Amdur RJ, Hinerman RW, Werning JW, Villaret D, Mendenhall NP. Head and neck mucosal melanoma. Am J Clin Oncol. 2005;28(6):626‐630. [DOI] [PubMed] [Google Scholar]
- 32. Wang X, Wu HM, Ren GX, Tang J, Guo W. Primary oral mucosal melanoma: advocate a wait‐and‐see policy in the clinically N0 patient. J Oral Maxillofac Surg. 2012;70:1192‐1198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ICD‐O‐3 Topography Codes and Primary Subsite Classification for Oral Cavity Mucosal Melanoma.


