Abstract
Objective
The UK incidence of oropharyngeal cancer has risen sharply over the last 30 years with an increase in human papillomavirus (HPV) associated diagnoses, most prevalent in younger, working age populations. This meta‐ethnography explores the psychosocial needs of HPV+ve oropharyngeal cancer patients during early recovery following (chemo)radiotherapy.
Methods
Meta‐ethnography methods were used, based on the approach of Noblit and Hare. Systematic searches for relevant qualitative studies were conducted in five electronic databases (MEDLINE, PubMed, CINAHL, PsycINFO and Cochrane database) between 2010 and 2021, followed by citation searching.
Results
Twenty‐three papers exploring the psychosocial needs of HPV+ve oropharyngeal cancer patients after treatment were included. Findings were synthesised to develop five constructs: ‘gaps in continuity of support from healthcare professionals’ reflecting unmet needs; ‘changes to self‐identity’ revealing the comprehensive disruption of this disease and treatment; ‘unrealistic expectations of recovery’ highlighting the difficulty of preparing for the impact of treatment; ‘finding ways to cope’ describing the distinct complexity of this experience; and ‘adjusting to life after the end of treatment’ exploring how coping strategies helped patients to regain control of their lives.
Conclusions
Completing (chemo)radiotherapy signalled a transition from hospital‐based care to home‐based support, challenging patients to address the constructs identified. An unexpectedly difficult and complex recovery meant that despite a favourable prognosis, poor psychosocial well‐being may threaten a successful outcome. The provision of tailored support is essential to facilitate positive adjustment.
Keywords: cancer, head and neck cancer, human papillomavirus, meta‐ethnography, oncology, oropharyngeal cancer, Psycho‐Oncology, psychosocial, qualitative, radiotherapy
1. BACKGROUND
Head and neck cancers (HNC) are the 6th most common cancer type globally. The UK incidence of oropharyngeal cancer quadrupled between 1990 and 2010 1 and continues to rise 2 with up to 80% now linked to human papillomavirus (HPV). 3 , 4 Disease onset in this sub‐group is typically during middle age (40–55 years) 5 and younger than that previously seen in HNC (65 years plus) traditionally associated with tobacco and alcohol consumption. Although HPV+ve oropharyngeal disease is often locally advanced when diagnosed, it is responsive to radiotherapy, often given concurrently with chemotherapy, that is, chemoradiotherapy, (75%–80% surviving 5 years). 6 However, poor quality of life due to severe treatment side effects is common for example, excessively dry mouth and difficult or painful swallowing, 7 resulting in significant post‐treatment support needs.
Over the last decade, qualitative research has explored HNC patients' experiences, resulting in four pertinent reviews. The first 8 revealed disruption in all aspects of life, diminishing a sense of self, managed by finding support, re‐evaluating what was important and adapting to the future. Mixed HNC populations included those who had surgery, with different experiences to those primarily receiving (chemo)radiotherapy (e.g., facial disfigurement, oral reconstruction, and prosthetics). A review of the psychosocial impact of HPV+ve HNC diagnosis 9 found quality of life lowest after 2‐3 months, commonly when radiotherapy is scheduled, 1 but included only one qualitative study, 10 revealing a sense of stigma and negative impact upon relationships, 1–5 years later.
Two recent reviews selected (chemo)radiotherapy studies but again included mixed HNC populations and different time points along the illness trajectory, from diagnosis to long‐term survival. A meta‐ethnography of 8 studies exploring HNC radiotherapy experiences, 11 described unmet needs related to isolation, making sense of the experience, disrupted life, waiting and uncertainty. Although some needs were met during treatment, the importance of therapeutic radiographers in building relationships with patients was emphasised to aid coping and understanding. A review of 13 studies sought to understand the impact of the lived experience of treatment upon patients. 12 Although incorporating evaluation of both early and late recovery phases, combining differing temporal perspectives and ‘response shifts’, 13 areas for psychosocial research were identified, including approaches to address feelings of post‐treatment abandonment.
Reviews of mixed HNC populations leave gaps in understanding about the distinct psychosocial experiences and support needs of the growing population of HPV+ve oropharyngeal cancer patients following (chemo)radiotherapy. Management guidelines for this population in the UK following the PETNeck trial 14 mean patients wait for a 12‐week post‐treatment response assessment scan to determine if a neck dissection is required. 15 This time of waiting encompasses a transition from hospital‐based support for management of side effects as they peak, to home‐based self‐care with hospital follow up. Early research of this ‘hidden experience’, 16 when patients need a ‘hand to hold’ 17 alongside increased understanding of treatment sequelae, 18 has contributed to developments in MDT support including pre‐ and post‐habilitation for example, physical exercise, nutrition and swallowing. 19 , 20 Contemporaneous adoption of Intensity Modulated Radiotherapy (IMRT), a targeted form of treatment, has also changed patients' experiences. 21 It is therefore timely for this review of patients' experiences during early recovery, addressing the question: ‘What are the psychosocial experiences and needs of HPV+ve oropharyngeal cancer patients following (chemo)radiotherapy?’
2. METHOD
Meta‐ethnography methods, as set out by Noblit and Hare, 22 were used to synthesise existing qualitative research. New insight was developed through translation of one study into that of others in order to develop a ‘line of argument’ through reciprocal, or potentially refutational interpretation. 23
2.1. Search strategy
The search strategy was developed using Population, Exposure, Outcome (PEO) 24 and keywords identified in preliminary searches. The resultant free‐text, Medical Subject Headings (MeSH) and thesaurus terms were used in searches adapted for MEDLINE, PubMed, CINAHL, PsycINFO and Cochrane databases. Multiple psychosocial terms related to the emotional, psychological, and social consequences of HPV+ve oropharyngeal cancer were included to ensure a sensitive search (Table S1). Citation searching included pertinent papers alongside the ‘Web of Science’ database.
2.2. Eligibility criteria
Criteria were developed through discussion between the authors (Table 1).
TABLE 1.
Eligibility criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Qualitative HNC studies with analysis of psychosocial experience or support needs of patients during early recovery following (chemo)radiotherapy | Quantitative or mixed methodologies focused on physical or functional QoL without analysis of psychosocial needs |
| Explorations of patient experience only at diagnosis, during (chemo)radiotherapy or long‐term survivorship | |
| (Chemo)radiotherapy HNC studies with at least 1/3rd oropharyngeal patients, or if site not given, at least 1/3rd study's patients aged 40–65 (typical of oropharyngeal patients) a | Studies including mixed cancers or only surgical or palliative patients |
| Primary studies, peer reviewed | Healthcare professionals' experiences only |
| Published: Jan 2010–Sep 2021, reflecting ongoing rising incidence of HPV+ve oropharyngeal cancer and widespread adoption of IMRT | Evaluations of different (chemo)radiotherapy treatments or of rehabilitation or self‐management interventions |
| English language | Expert opinion papers or conference abstracts |
| Patients 18 years and above |
For studies including surgical patients but not specifying HNC site, the sample was assumed to reflect the UK HNC population that is, 25% oropharyngeal cancers 4 .
2.3. Selection of studies
Titles and abstracts were screened for eligibility by SM, with a random 5% sample of excluded papers confirmed by the co‐authors.
2.4. Quality appraisal
Eligible studies were appraised using the Critical Appraisal Skills Programme (CASP) qualitative checklist 25 to assess reliability for inclusion. This commonly used checklist enabled structured assessment of 10 items of study quality and insight into content.
2.5. Data extraction, synthesis, and translation
A ‘Synthesis table’ listed the selected papers chronologically and by research foci, demonstrating knowledge and practice development with the authors' 2nd order constructs entered alongside representative quotations (1st order constructs) from which they were derived (Table S2). Included quotations were those attributable to patients under 65 years (more likely to be HPV+ve), whilst those related to experiences during treatment or surgery were disregarded, as were caregivers' quotations. SM re‐read the papers enabling immersion in their meaning and considered quotations in terms of psychosocial experiences and consequent needs. The resultant interpretations or ‘metaphors’ were entered into the table, ensuring transparency within this inherently subjective process. 22 Following debate between authors, terminology was agreed, underpinned by theoretical knowledge. Metaphors developed were organised into the table's columns and the relationships and commonality between them considered iteratively, resulting in the evolution of ‘3rd order constructs’ which appeared later as the headings of 5 columns. Sufficient similarity was found between the studies for reciprocal translation and the development of a line of argument describing the relationships between the constructs. 22 Any refutations were described.
3. RESULTS
3.1. Search results
Following the retrieval of 4398 papers, duplicates were removed leaving 3643, with 10 added from citation searching (Figure 1). SM used the eligibility criteria to screen titles and abstracts, selecting 30 papers for full‐text review. A further 7 were ineligible following discussion with the co‐authors. 23 papers were therefore chosen, including two papers based on the same study population, but with different foci. 26 , 27 Quality appraisal found acceptable methodological rigor: authors set out research aims, design and findings well, ethical issues were considered but reflexivity was often not explained. Maximal purposive sampling ensured diversity within some studies, 26 , 27 , 28 whilst others chose convenience sampling 29 , 30 , 31 to address research questions.
FIGURE 1.
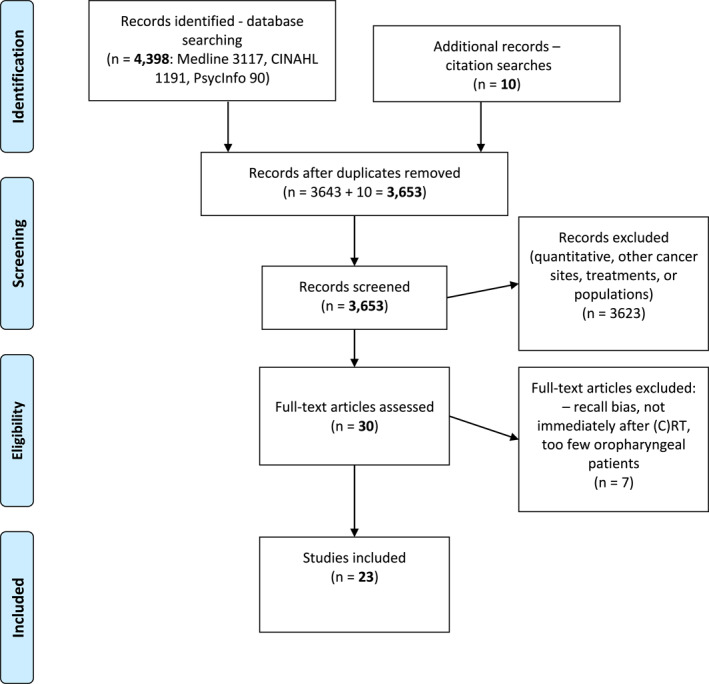
Prisma flow diagram
3.2. Study characteristics
The research foci of the 23 papers fell into two groups: (Table S3).
Experience and psychosocial support needs (n.11), three of which addressed typical features of HPV+ve oropharyngeal cancer that is, HNC in middle adulthood, the impact of HPV and HNC as parents of young children. 32 , 33 , 34
Experience of physical effects following radiotherapy and psychosocial impact (n.12), with 9 related to nutritional consequences (e.g., dysphagia, enteral feeding, xerostomia).
Most studies reported qualitative data at one time point (n.19) and were descriptive (n.20) using various methods for example, ‘Interpretative description’ (n.4), 35 enabling deeper understanding built upon existing knowledge. Other research designs were Interpretative Phenomenological Analysis of the lived experience of xerostomia, 36 ethnographic observation of eating behaviour 37 and Grounded Theory development of a model of adjustment. 38 Differing epistemological positions necessitated careful interpretation. 39 Commonly, semi‐structured interviews were analysed thematically.
3.3. Results of synthesis
Five interrelated 3rd order constructs were derived: ‘gaps in continuity of support from healthcare professionals’, ‘changes to self‐identity’, ‘unrealistic expectations of recovery’, ‘finding ways to cope’, and ‘adjusting to life after treatment’ and are depicted in Figure 2.
FIGURE 2.
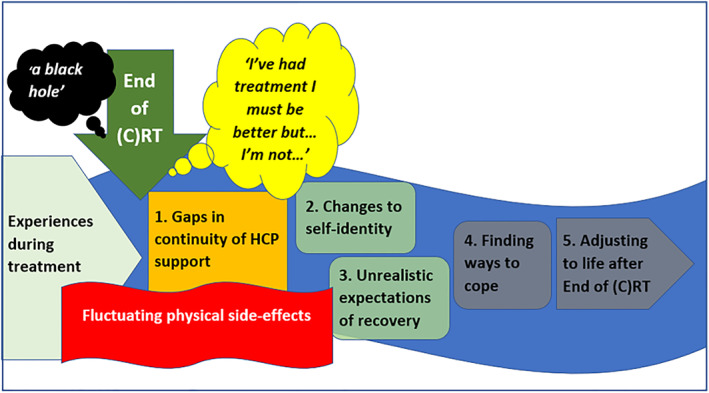
A conceptual model
3.4. Gaps in continuity of support from healthcare professionals (HCPs)
This construct revealed gaps in emotional support and information needs. Completing treatment presented patients with an illness milestone, focussing thoughts upon recovery whilst processing their treatment experiences. However, the loss of daily contact with HCPs meant fewer opportunities for support or to ask time‐sensitive questions about fluctuating side effects. Although it marked a release from intense, hospital‐imposed schedules it also brought feelings of abandonment 27 , 40 , 41 and a ‘void’ of emotional support 31 as the umbrella of care seemed removed:
When the radiotherapy started… I received support from many staff [members]…after the final radiation… then everyone disappeared. 40
Despite patients knowing they could contact HCPs some chose to be stoic which, alongside uncertainty about when to make contact, created a barrier:
As the nurse practitioner says: “If something is wrong, just give a call.” But you don’t want to do that too soon. Still a kind of threshold, I think. You first wait for better times, just one more week. 41
On‐going enteral feeding enabled continuity in support from trusted HCPs in recent studies. 41 , 42 , 43 Unmet needs following a lack of empathy, poor communication and insufficient symptom management advice were reported in older studies where patients had ‘to learn through the school of hard knocks’. 31 In the absence of HCPs to ask pertinent questions to assuage concerns about the origins and disease course of HPV, 33 patients sought written information, but found it was inapplicable:
… either aimed at preventing young people from contracting HPV or preventing older people from getting cancer through HPV. 33
Younger patients who were parents wanted to know how to support children 34 and HNC information seemed aimed at older patients. Where access to support for specific and timely information was perceived as inadequate, patients proposed strategies including more frequent follow ups. 40 , 41
3.5. Changes to self‐identity
This concept characterised how experiences challenged and diminished self‐identity, as lives were profoundly disrupted. Roles as parents and partners, and as providers for others were affected:
…there were some dark days where, …I didn’t think about anybody ‐ I didn’t think about my kids, I didn’t think about my wife. I thought about myself and how I had to get through this . . . And that was hard for her to hear. 32
Disruption to daily living included pain, 36 , 40 lack of sleep 29 , 31 , 32 , 36 , 42 , 44 , 45 and considerable time required for self‐care to manage symptoms, 42 , 43 , 46 likening it to a full‐time job.
Social identity was affected by functional changes in speaking, eating, and drinking, causing social embarrassment. Enteral feeding was isolating, patients felt unhygienic 42 and restricted in their physical activity. 43 Xerostomia‐related dysphasia, 36 the presence of a nasogastric tube 42 or dysphagia 37 resulted in social avoidance and consequent loss of opportunities for support 31 , 46 :
I declined invitations… I did not want to ruin the appetite for everyone at the dinner table. 46
Perceived stigma of an HPV+ve diagnosis also affected social identity. 33 Likened to the ‘elephant in the room’ 32 patients withheld information: ‘…you don't go around broadcasting that something's sexually transmitted’, 33 decreasing self‐esteem and affecting relationships:
When they told me it was because of HPV I don’t think I told him for ages … it was easier to tell other people why as opposed to him.’ ‘My partner (sic) …. “said something that made me feel really dirty. 33
Its significance was reduced for some when its contagious nature and prevalence was understood. 33 However, self‐identity within strained relationships was also challenged by changing roles 26 , 43 :
…it sort of becomes a bit like a child mother relationship… I found it hard to move back to being an equal adult. 26
Attempts to return to the role of family provider to alleviate financial concerns were thwarted by treatment consequences, 32 , 42 , 43 , 44 such as communication difficulties attributed with being perceived as less intelligent. 32 These wide‐reaching changes affected a sense of self, exacerbated by an inability to undertake fundamental functions of living, 27 , 29 previously taken for granted or valued. 26 , 32 , 36 The pleasure of eating was replaced with fear and dread, as it hurt to eat. 26 , 36 , 37 , 47 Being unable to enjoy the taste of culturally normative foods could also threaten identity. 46 , 48 Sleep deprivation and lack of energy had a significant influence on a person's sense of self 32 , 36 :
It really does take your breath away and it takes your spirit away, which is even worse…. 32
The after‐treatment period was likened to ‘a black hole’ 41 and the accumulation of such disabling and distressing effects resulted in loss of interest and self‐worth:
I gave it up. I didn’t feel and I was not willing to do anything, I didn’t recognize myself anymore, this thing had left a shell of my previous self, a self that I despised. 36
There was belief that the cancer was undeserved and unfair in a previously healthy person. 40 Some did assume an illness identity, 42 , 48 which could be permanent, 43 affecting future plans and life expectations. 28 , 31 , 32 This view was reinforced by shifts in body image due to dental extraction, 42 weight loss 37 , 48 and facial lymphoedema, also affecting sleep and ability to undertake swallowing exercises. 45 , 49 Self‐esteem was reduced and self‐consciousness raised, affecting social identity, particularly for female patients:
I try to cover it up when I go out… so that people don’t look at me. 45
These clustered treatment consequences heightened their impact upon self‐identity.
3.6. Unrealistic expectations of recovery
This construct captured the emotional consequences of unmet expectations of recovery. Patients described the first weeks after treatment ‘….like a nightmare’, 29 including a long struggle, 31 , 46 feelings of failure 43 and depression 31 , 36 , 42 :
You’re still mentally in a very bad place, and physically in a bad place… it took me a long time to come out of that bad place. I went through depression and all sorts of terrible things in that six months after treatment. 31
The unexpected difficulty of recovering physically was amplified when considerable side effects during treatment initially worsened, rather than improved. 26 , 27 , 36 Patients' hopes for a quick recovery were unfulfilled, creating uncertainty about the future 28 , 29 , 37 , 41 , 42 , 43 , 45 , 46 , 47 , 50 :
…I didn’t expect it to be going on as long as it did… I thought that once the treatment had finished a couple of weeks and I’d be fine. 50
Those expecting a long recovery were frustrated by a lack of progress, reporting difficulty sustaining motivation for rehabilitation. 45 Discord was magnified by optimistic information provided regarding side effect likelihood and duration, such as mucous and saliva production and normal eating patterns 46 , 47 , 50 :
Most people tried to sort of sugar coat it a little bit and say ‘‘these things can happen but they do not always happen and they might not happen to you.” 50
Although patients conceded they might not have absorbed all of the information given at clinic appointments 50 their lived reality did not correspond with what they heard or read. 47 This was echoed by those who sought to prepare, finding information had limited meaning in advance of the actual experience:
I could not imagine how it should be so I asked the doctor if there was something special to think about concerning the food, and the doctor said to me: that you eat. I could not understand how right that was, how difficult it should be. 46
The fluctuating nature of side effects intensified uncertainty, raising doubt about improvement or permanent change, the meaning behind new symptoms and recovery time 27 , 29 , 37 , 45 , 49 :
What they say to you is you’re going to be very poorly and for a couple of weeks after, then things will start picking up. Well two weeks after, then a month after and you think well I’m still not eating, it’s on your mind, am I lagging behind people? 37
Patients tried to assess the normality of their recovery but hearing ‘everyone's different’ 31 did not restore confidence. Concerns about the future included fears for the family, and existential thoughts of potentially not being there as a parent. 34 , 42 Anxieties about survival 40 were intensified for those with unmet information needs regarding HPV:
… is it [cancer] more likely to come back because of this [HPV]? Is it something that stays in your body? 33
Patients wanted to be better informed, 27 proposing fellow patients could help. 41 Some would want to know the worst that would happen, 50 but others felt otherwise:
It was for the best that I did not know because then it would have felt impossible that I should be sick until June…. I thought all the time – next Friday it will be better. 46
3.7. Finding ways to cope
The emotional rollercoaster patients described meant having to seek ways to cope, the fourth construct, often with assistance from others. 29 , 41 , 46 Patients monitored for small signs of improvement, to bolster confidence. 46 Self‐compassion was evident, 38 although emotions were checked:
You have to be careful not to become a victim or have a victim mentality. You have to really work hard to say “Ok, I’ve got these things, I’ve to live with them now, let’s get on with it.” But that’s something you have to, almost on a daily basis; I find I’ve got to remind myself. 31
Patients cultivated an optimistic, hopeful attitude 27 , 28 , 29 , 30 , 32 , 41 , 42 , 43 , 46 , 47 despite the uncertainty of recovery. 28 Some were able to positively reframe difficulties:
I’m still the same bloke. The only little problem I’ve got, and I treat it as little, is my eating problem. 27
Other coping strategies included goal‐setting 32 (e.g., tube removal and eating orally 47 ) and adopting a fighting spirit. 30 , 38 , 48 Downward social comparison including positive self‐appraisal helped patients to situate themselves and compare themselves favourably. 28 , 30 , 43 , 44 Some found returning to work enabled resumption of some normality and aided coping, whilst others had to return to work before adequate recovery, due to financial obligations. 32 Maladaptive coping strategies included disengagement through avoidance, fantasy thinking, self‐blame, or denial. 30 Past coping strategies were not always available, such as eating, 48 whilst social avoidance compromised support 31 , 36 , 46 :
I was afraid to talk to anyone, not from a close distance at least, I was afraid of their reaction to my bad breath…I didn’t know if I could handle this kind of rejection. It seemed better to be alone and silent and keep my dignity…perhaps the only thing that the treatment hadn’t take away from me, yet. 36
However, social contact conveyed others cared for example, in the parental role 34 , 42 or through maintaining social traditions 32 , 37 :
Went to the pub as before, despite having profound swallowing difficulties 37
The support of fellow patients for sharing experiences was valued. Insightful peers enabled emotional expression, information provision, resolution of uncertainty and making sense of experiences 27 , 31 , 32 , 41 , 43 , 50 :
When I met [another support group member] he said to me “it gets better”. And that was probably one of the best things that ever happened to me because at that stage I didn’t think it was ever going to get better. 31
Informal caregivers, often partners, supported patients who notably described coping using the pronoun ‘we’. 26 , 33 , 41 Accepting help was vital following treatment, 31 as managing independently as before was impossible. 26 , 27 , 30 , 41 , 42 Recognition of this was common 26 , 27 :
My wife forced me to eat. She said: “Stop tube feeding. We are going to eat now.” .… My wife and children have pulled me through. 41
However, some described a lack of understanding by family and friends reducing available support, 26 , 27 , 41 , 48 whilst caregiving was threatened by patients' fluctuating needs as side effect severity varied. 26 , 27 , 32 Desire to reassert control during recovery could strain relationships, as patients' acceptance of practical help lessened:
Finally I said “Hey, back off . . . There are some things now that I want to be able to do.” 32
Others reported shared experiences strengthened relationships, 32 but awareness of the impact of the cancer diagnosis on the whole family and the need to comfort and support them, increased coping burden. 40 Enteral feeding emerged as a significant feature within intimate relationships but could provide an opportunity to involve family in practical tasks. 43
3.8. Adjusting to life after treatment
This construct identified how patients sought to adjust and regain control of their changed lives, for example, solving practical problems and adapting to physical changes 27 , 31 , 42 , 43 , 45 , 48 , 49 :
I can’t open my jaw wide enough, … when I eat a spoon full of something, I can’t. I have to put the spoon in sideways. 48
Although acceptance of enteral feeding was perceived as losing control in an early study, 48 more recently, increased empowerment through HCP‐led enteral feeding self‐management programs was expressed, enabling relief from worries about nutrition and weight loss. 42 , 43 Patients described opting for a Percutaneous Endoscopic Gastrostomy (‘G‐tube’) to live a normal life again, fostering a sense of control, and reducing social isolation as they re‐engaged with support networks. 43 Understanding their own experience resulted in acceptance evolving 26 , 27 , 32 , 42 , 45 , 49 :
I don’t really worry about it anymore . . . no point. If I can’t eat it I can’t eat it. 27
However, for some, adjustment to what had been lost was harder 28 , 36 , 43 :
…he told me that it [G‐tube] was going to be permanent…I just can't imagine never eating again… 43
The process of adjustment observed in some studies, particularly those with varied data collection time points included looking for signs of treatment success, or otherwise, 29 , 42 for example, changes in texture, swallowable portion sizes 37 and less tangible signs, such as ‘getting better’. 41 Although individual time scales varied, this could help trigger a ‘Turning point’, 38 which could be supported by others 32 , 48 :
I said to [a friend] … “this is my new normal. . . do I necessarily like it? No, but it’s a whole lot better than the alternative”. He looked at me and said, “You know, that’s not a bad way to look at things.” And that’s the way I am. 32
This ability to modify one's perspective, or psychological flexibility, 51 was also demonstrated by those cautiously learning to live with uncertainty. 28 A yearning to return to the activities of daily living was evident, 40 whilst the significance of patients' families in the reassessment of priorities within the ‘new normal’ was clear 32 , 34 , 38 , 40 :
Your values change. You value drinking and going out with you mates and working…. Then you step back from that and say, ‘Hold on’. Everybody thinks kids are important, but it makes them doubly important. 34
And for some, ‘moving on’ could lead to benefits from ‘giving back’. 32 , 43
4. DISCUSSION
This review illustrated that after treatment HPV+ve oropharyngeal cancer patients experienced ‘gaps in continuity of support from HCPs’, ‘changes to self‐identity’ and ‘unrealistic expectations of recovery’, challenging them to ‘find ways to cope’, which if successful could enable them to begin ‘adjusting to life after treatment’. Figure 2 depicts these findings building upon past research, 8 , 11 , 12 conveying ‘what it is like’ during early recovery for this population following (chemo)radiotherapy.
Awareness of gaps in continuity of support demonstrated unmet psychosocial needs. Opportunities to obtain timely information were limited by patients' reluctance to contact HCPs, likely due to stoicism. HCP‐led support for enteral feeding, reflecting UK practice development, 52 did meet needs, 43 indicating the benefits of a formalised approach. Although of secondary importance to cancer, HPV was a worry, which if unaddressed, persisted into survivorship. 33 Recognition of this by the European HNC Society's ‘Make Sense Campaign’ has resulted in HCP patient support guidance, 53 acknowledged as required in the UK. 9 , 54
Middle adulthood 55 is a life stage when expectations and goals (e.g., raising a family and work plans) are often formulated and realised. This working age population experienced considerable interlinked shifts in their self‐identity and life assumptions, termed ‘biographical disruption’, 56 previously recognised within HNC. 8 There was a contrast between the challenges of having to depend on others and severe side effects, after limited pre‐treatment symptoms and news of an optimistic prognosis. Disappointment when hopes for a swift recovery were not met, exacerbated uncertainty about the future, following an unexpected cancer diagnosis. HPV+ve status, visible signs of illness, such as a nasogastric tube, loss of control and the sudden switch in role from full time employment to full time self‐management, also threatened identity. Subsequent social isolation could be amplified by withholding an HPV+ve diagnosis, 33 threatening supportive relationships, and potentially causing further disruption.
Although patients may have received information, they had unrealistic expectations of recovery within their own personal, family, work, and social context, and a discordance between being told what may happen and lived reality persisted. Assessment by patients of uncertainties as ‘dangerous’, 57 such as HPV‐associated recurrence risk, fluctuating side effects or unknown permanence of changes, challenged their management, impacting coping. Uncertainty has been linked to lower quality of life in HNC 58 and prostate cancer. 59 Additionally, focus upon physical effects after (chemo)radiotherapy, during ‘Survive mode’ 38 may mask emotional support needs, previously found to be greater in younger HNC patients than older. 60 Depression was disclosed by patients within this review 31 , 36 , 42 and previously in up to one third of HNC patients following radiotherapy. 61 High levels of distress have been associated with avoidant coping strategies such as distancing and disengagement 62 and reduced quality of life, 63 pertinent for this population requiring motivation to undertake self‐management, such as swallowing exercises. 64 Support includes re‐habilitation programmes, stress management, relaxation therapy 53 and person‐centred interventions. 65 Counselling and Cognitive Behavioural Therapy (CBT) have also been widely used to treat depression in cancer patients, 66 but require further research within HNC. 67
Self‐appraisal, as patients sought ways to cope, was evident throughout these studies for example, questioning if experiences were normal, reluctance to contact HCPs ‘too soon’ and goal monitoring. Past experiences and personality disposition types affected individual coping styles and patients' information needs. Without personalised assessment (e.g., ‘Patient Concerns Inventory’ (PCI) 68 and Macmillan Cancer Support Holistic Needs Assessment (HNA) 69 ) these may be unmet, causing greater anxiety than that related to side effect severity. 70
Finding ways to cope enabled responses to the biographical disruption experienced, determined by different cognitive models of self, world, and others, including role flexibility, priority reassessment and social comparison. This adaptation of their ‘Assumptive world’ 71 made a positive transition possible, if needs such as HPV knowledge and returning to work challenges were met. Such potential barriers to ‘finding a path’ 8 following treatment were portrayed here, and previously, 12 as a ‘sense of abandonment’. Subsequent searching for meaning, whilst facing loss of control and ongoing uncertainty, have previously been described 8 following a past exploration of ‘liminality in illness’. 72 Interviewed colorectal cancer patients were able to avoid the sense of alienation, or ‘separateness’ conceptualised if supported by someone who had undergone similar experiences. That ‘traumatic experiences are indescribable until they have been experienced’ has been demonstrated within HNC, 70 where expectations during recovery were revised. Peer support has enhanced coping during HNC radiotherapy, 73 providing peers were well‐matched, and was echoed here, after treatment. Patients were able to attribute personal meaning to credible information from peers, reducing uncertainty. Sharing experiences in support groups, where social confidence could be regained, or during chance conversations in clinic established a sense of commonality, normalising experiences and creating precedence: a ‘frame of reference’ 74 . Within the development of their ‘Information seeking behaviour theory’, these researchers found that information from HCPs ‘took a backseat’ to the importance placed on patients' lived experience. It seems that through shared social reality, peers are able to convey information in a unique way, bridging gaps in support.
The ability to modify one's relationship with experiences, or psychological flexibility, may also help meet the psychosocial needs of this population, some of whom were able to reframe experiences positively (e.g., ‘a new normal’ and strengthened relationships). Additionally, parents of young children and those returning to work provided examples of a drive to adjust to a changed self, a ‘Turning Point’, 38 reflecting Leventhal's ‘Self‐Regulatory Model’. 75 Acceptance and Commitment Therapy (ACT), 51 a supportive intervention to enhance psychological flexibility, may help facilitate adjustment to the complex psychosocial experiences identified here.
4.1. Study limitations
This meta‐ethnography interpreted the findings of studies posing varied research questions to reveal psychosocial experiences and support needs of HPV+ve oropharyngeal cancer patients following (chemo)radiotherapy. Any preconceptions in interpretations by the first author, a therapeutic radiographer, were addressed by confirming findings with the co‐authors. As qualitative research includes potential biases, for example, study participant self‐selection and recall of experiences, findings were not generalisable to all HNC patients. Trustworthiness, including credibility and dependability, were demonstrated through rich description and confirmation of constructs, for example, psychosocial impact conveyed when each paper or topic occurred within every construct, such as experience related to food and eating. However, synthesis of papers with varied research approaches or with specific foci (evident in more recent studies e.g., HPV diagnosis, enteral feeding, lymphoedema) meant not all papers demonstrated every construct, thus limiting their influence (e.g., some included few quotations, 29 , 38 , 44 whilst deductive approaches were less likely to reflect every construct 31 , 41 ).
Study characteristics (Table S3) were used to monitor and limit influence, for example, older studies including surgery only patients 34 , 44 or untypically large female populations. 36 , 47 Populations were mainly Caucasian and male with an average age below 65 years, reflecting past HPV+ve oropharyngeal cancer incidence, however, experiences may not represent those of the growing number of female patients. 2 Practice development differences such as pre‐habilitation and HCP role in fostering self‐management over time and between countries, created inconsistencies, affecting the strength of some constructs.
4.2. Clinical implications
The UK HNC MDT guidelines 76 were introduced during the timespan of these studies, enhancing support. Personalised care 77 has been facilitated by tools (e.g., PCI, 68 HNA 69 ) to ascertain unmet needs and engage patients in self‐management. Additionally, this review found that HPV+ve oropharyngeal cancer patients require:
Specific support to cope with severe side effects, changes in self‐identity and treatment impact (e.g., disruption, sense of uncertainty) to promote and sustain self‐management and adjustment.
Clear information guiding expectations around recovery timelines, side effect profile and return to work implications to mitigate gaps in support and work towards alignment of expectations and experiences.
Access to tailored HPV‐related information, involving HCP education. 54
Support for patients as parents, for relationships and families.
Facilitation of well‐matched peer support for example, support groups, buddy schemes.
Enhanced psychological support for example, CBT, ACT.
5. CONCLUSIONS
This meta‐ethnography has highlighted the considerable psychosocial needs of HPV+ve oropharyngeal cancer patients during early recovery following (chemo)radiotherapy. It builds upon past HNC reviews by revealing the experience of this population within five interlinked constructs, conveying the complexity of experience, and describing ‘what it is like’. Despite often receiving support from others and a favourable prognosis, an emotional rollercoaster meant patients struggled to reconcile their experiences with expectations of recovery. Gaps in timely, tailored information and available support challenged their ability to cope after treatment. Poor psychosocial well‐being, in what may be long cancer survivorship could threaten an otherwise successful outcome, which may require enhanced provision. Evolving understanding of HPV+ve oropharyngeal cancer patients' experiences following (chemo)radiotherapy should enable facilitated adjustment through personalised support and tailored interventions.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ETHICS APPROVAL
Ethical approval was not required as this was a review.
Supporting information
Table S1
Table S2
Table S3
ACKNOWLEDGEMENTS
This meta‐ethnography was undertaken by SM, with support from the co‐authors during a MPhil/PhD studentship at the Oxford Institute of Nursing, Midwifery and Allied Health Research (OxINMAHR), Faculty of Health and Life Sciences, Oxford Brookes University.
Matthews S, Brett J, Ramluggun P, Watson E. The psychosocial experiences of human papillomavirus (HPV) positive oropharyngeal cancer patients following (chemo)radiotherapy: a systematic review and meta‐ethnography. Psychooncology. 2022;31(12):2009‐2019. 10.1002/pon.5984
DATA AVAILABILITY STATEMENT
The data that support the findings of this review are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mehanna H, Evans M, Beasley M, et al. Oropharyngeal cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016;130(S2):S90‐S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Office National Statistics. 2018. [17.11.21]. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/adhocs/008721incidenceoforalcavityandoropharyngealcancerbysexinengland2000to2016 [Google Scholar]
- 3. Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer‐‐systematic review and meta‐analysis of trends by time and region. Head Neck. 2013;35(5):747‐755. [DOI] [PubMed] [Google Scholar]
- 4. Cancer Research UK. Head and Neck Cancer Statistics. [17.09.21]. https://www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/head‐and‐neck‐cancers [Google Scholar]
- 5. Marur S, D'Souza G, Westra WH, Forastiere AA. HPV‐associated head and neck cancer: a virus‐related cancer epidemic. Lancet Oncol. 2010;11(8):781‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Semple CJ, Dunwoody L, George Kernohan W, McCaughan E, Sullivan K. Changes and challenges to patients’ lifestyle patterns following treatment for head and neck cancer. J Adv Nurs. 2008;63(1):85‐93. [DOI] [PubMed] [Google Scholar]
- 8. Lang H, France E, Williams B, Humphris G, Wells M. The psychological experience of living with head and neck cancer: a systematic review and meta‐synthesis. Psycho Oncol. 2013;22(12):2648‐2663. [DOI] [PubMed] [Google Scholar]
- 9. Dodd RH, Waller J, Marlow LA. Human papillomavirus and head and neck cancer: psychosocial impact in patients and knowledge of the link ‐ a systematic review. Clin Oncol. 2016;28(7):421‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baxi SS, Shuman AG, Corner GW, et al. Sharing a diagnosis of HPV‐related head and neck cancer: the emotions, the confusion, and what patients want to know. Head Neck. 2013;35(11):1534‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fitchett RC, Aldus EJ, Fitchett LR, Cross J. The lived experience of head and neck cancer patients receiving curative radiotherapy: a systematic review and meta‐ethnography. Psycho Oncol. 2018;27(9):2077‐2086. [DOI] [PubMed] [Google Scholar]
- 12. Qualizza M, Bressan V, Rizzuto A, et al. Listening to the voice of patients with head and neck cancer: a systematic review and meta‐synthesis. Eur J Cancer Care. 2019;28(3):1‐13. [DOI] [PubMed] [Google Scholar]
- 13. Sprangers MAG, Schwartz CE. Integrating response shift into health‐related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507‐1515. [DOI] [PubMed] [Google Scholar]
- 14. Mehanna H, Wong WL, McConkey CC, et al. PET‐CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med. 2016;374(15):1444‐1454. [DOI] [PubMed] [Google Scholar]
- 15. NICE. Cancer of the Upper Aerodigestive Tract Cancer: Assessment and Management in People Aged 16 and over. 2016. [17.10.2021]. https://www.nice.org.uk/guidance/ng36 [PubMed] [Google Scholar]
- 16. Wells M. The hidden experience of radiotherapy to the head and neck: a qualitative study of patients after completion of treatment. J Adv Nurs. 1998;28(4):840‐848. [DOI] [PubMed] [Google Scholar]
- 17. Larsson M, Hedelin B, Athlin E. Needing a hand to hold: lived experiences during the trajectory of care for patients with head and neck cancer treated with radiotherapy. Cancer Nurs. 2007;30(4):324‐334. [DOI] [PubMed] [Google Scholar]
- 18. Wilson JA, Carding PN, Patterson JM. Dysphagia after nonsurgical head and neck cancer treatment: patients' perspectives. Otolaryngol Head Neck Surg. 2011;145(5):767‐771. [DOI] [PubMed] [Google Scholar]
- 19. Clarke P, Radford K, Coffey M, Stewart M. Speech and swallow rehabilitation in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S176‐S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sandmael JA, Bye A, Solheim TS, et al. Feasibility and preliminary effects of resistance training and nutritional supplements during versus after radiotherapy in patients with head and neck cancer: a pilot randomized trial. Cancer. 2017;123(22):4440‐4448. [DOI] [PubMed] [Google Scholar]
- 21. Nutting CM, Morden JP, Harrington KJ, et al. Parotid‐sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noblit GW, Hare RD. Meta‐ethnography: Synthesizing Qualitative Studies. SAGE Publications; 1988. [Google Scholar]
- 23. France EF, Uny I, Ring N, et al. A methodological systematic review of meta‐ethnography conduct to articulate the complex analytical phases. BMC Med Res Methodol. 2019;19(1). 10.1186/s12874-019-0670-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. University of Suffolk . PEO and PICO. [27.05.21]. https://libguides.uos.ac.uk/ld.php?content_id=30159737 [Google Scholar]
- 25. CASP.Critical Appraisal Skills Programme . 2018. Qualitative checklist [17.11.2021]. https://casp‐uk.net/casp‐tools‐checklists/
- 26. Nund RL, Ward EC, Scarinci NA, Cartmill B, Kuipers P, Porceddu SV. The lived experience of dysphagia following non‐surgical treatment for head and neck cancer. Int J Speech Lang Pathol. 2014;16(3):282‐289. [DOI] [PubMed] [Google Scholar]
- 27. Nund RL, Ward EC, Scarinci NA, Cartmill B, Kuipers P, Porceddu SV. Survivors' experiences of dysphagia‐related services following head and neck cancer: implications for clinical practice. Int J Lang Commun Disord. 2014;49(3):354‐363. [DOI] [PubMed] [Google Scholar]
- 28. McQuestion M, Fitch M. Patients' experience of receiving radiation treatment for head and neck cancer: before, during and after treatment. Can Oncol Nurs J. 2016;26(4):325‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haisfield‐Wolfe ME, McGuire DB, Krumm S. Perspectives on coping among patients with head and neck cancer receiving radiation. Oncol Nurs Forum. 2012;39(3):E249‐E57. [DOI] [PubMed] [Google Scholar]
- 30. Andersen KS, Jarden M. Coping with radiotherapy for head and neck squamous cell carcinoma: a qualitative exploration. Nordic J Nurs Res Clin Stud / Vård i Norden. 2012;32(2):25‐29. [Google Scholar]
- 31. Moore KA, Ford PJ, Farah CS. “I have quality of life…but…”: exploring support needs important to quality of life in head and neck cancer. Eur J Oncol Nurs. 2014;18(2):192‐200. [DOI] [PubMed] [Google Scholar]
- 32. Grattan K, Kubrak C, Caine V, O'Connell DA, Olson K. Experiences of head and neck cancer patients in middle adulthood: consequences and coping. Global Qualitative Nurs Res. 2018;5:1‐13. 10.1177/2333393618760337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dodd RH, Forster AS, Marlow LAV, Waller J. Psychosocial impact of human papillomavirus‐related head and neck cancer on patients and their partners: a qualitative interview study. Eur J Cancer Care. 2019;28(2):e12999. 10.1111/ecc.12999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Semple CJ, McCance T. Experience of parents with head and neck cancer who are caring for young children Experience of parents with head and neck cancer. J Adv Nurs. 2010;66(6):1280‐1290. [DOI] [PubMed] [Google Scholar]
- 35. Thorne S, Kirkham SR, MacDonald‐Emes J. Interpretive description: a noncategorical qualitative alternative for developing nursing knowledge. Res Nurs Health. 1997;20(2):169‐177. [DOI] [PubMed] [Google Scholar]
- 36. Charalambous A. Hermeneutic phenomenological interpretations of patients with head and neck neoplasm experiences living with radiation‐induced xerostomia: the price to pay? Eur J Oncol Nurs. 2014;18(5):512‐520. [DOI] [PubMed] [Google Scholar]
- 37. Patterson JM, McColl E, Wilson J, Carding P, Rapley T. Head and neck cancer patients' perceptions of swallowing following chemoradiotherapy. Support Care Cancer. 2015;23(12):3531‐3538. [DOI] [PubMed] [Google Scholar]
- 38. Calver L, Tickle A, Biswas S, Moghaddam N. How patients adjust psychologically to the experience of head and neck cancer: a grounded theory. Eur J Cancer Care. 2019;28(4). 10.1111/ecc.13068 [DOI] [PubMed] [Google Scholar]
- 39. Atkins S, Lewin S, Smith H, Engel M, Fretheim A, Volmink J. Conducting a meta‐ethnography of qualitative literature: lessons learnt. BMC Med Res Methodol [Internet]. 2008;8(1):21. 10.1186/1471-2288-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schaller A, Larsson B, Lindblad M, Liedberg GM. Experiences of pain: a longitudinal, qualitative study of patients with head and neck cancer recently treated with radiotherapy. Pain Manag Nurs. 2015;16(3):336‐345. [DOI] [PubMed] [Google Scholar]
- 41. Peeters MAC, Braat C, Been‐Dahmen JMJ, Verduijn GM, Oldenmenger WH, van Staa A. Support needs of people with head and neck cancer regarding the disease and its treatment. Oncol Nurs Forum. 2018;45(5):587‐596. [DOI] [PubMed] [Google Scholar]
- 42. Ehrsson YT, Sundberg K, Laurell G, Langius‐Eklöf A. Head and neck cancer patients' perceptions of quality of life and how it is affected by the disease and enteral tube feeding during treatment. Ups J Med Sci. 2015;120(4):280‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Williams GF, White H, Sen M, Prestwich RJD. Patients' experience of enteral feeding following (chemo)radiotherapy for head and neck cancer: a qualitative study. Clin Nutr. 2019;38(3):1382‐1389. [DOI] [PubMed] [Google Scholar]
- 44. Molassiotis A, Rogers M. Symptom experience and regaining normality in the first year following a diagnosis of head and neck cancer: a qualitative longitudinal study. Palliat Support Care. 2012;10(3):197‐204. [DOI] [PubMed] [Google Scholar]
- 45. Jeans C, Ward EC, Cartmill B, et al. Patient perceptions of living with head and neck lymphoedema and the impacts to swallowing, voice and speech function. Eur J Cancer Care. 2019;28(1):e12894. 10.1111/ecc.12894 [DOI] [PubMed] [Google Scholar]
- 46. Ottosson S, Laurell G, Olsson C. The experience of food, eating and meals following radiotherapy for head and neck cancer: a qualitative study. J Clin Nurs. 2013;22(7‐8):1034‐1043. [DOI] [PubMed] [Google Scholar]
- 47. Sandmael JA, Sand K, Bye A, Solheim TS, Oldervoll L, Helvik A‐S. Nutritional experiences in head and neck cancer patients. Eur J Cancer Care. 2019;28(6):e13168. [DOI] [PubMed] [Google Scholar]
- 48. McQuestion M, Fitch M, Howell D. The changed meaning of food: physical, social and emotional loss for patients having received radiation treatment for head and neck cancer. Eur J Oncol Nurs. 2011;15(2):145‐151. [DOI] [PubMed] [Google Scholar]
- 49. McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Lymphoedema following treatment for head and neck cancer: impact on patients, and beliefs of health professionals. Eur J Cancer Care. 2014;23(3):317‐327. [DOI] [PubMed] [Google Scholar]
- 50. Brockbank S, Miller N, Owen S, Patterson JM. Pretreatment information on dysphagia: exploring the views of head and neck cancer patients. J Pain Symptom Manage. 2015;49(1):89‐97. [DOI] [PubMed] [Google Scholar]
- 51. Hulbert‐Williams NJ, Storey L, Wilson KG. Psychological interventions for patients with cancer: psychological flexibility and the potential utility of Acceptance and Commitment Therapy. Eur J Cancer Care. 2015;24(1):15‐27. [DOI] [PubMed] [Google Scholar]
- 52. Talwar B, Donnelly R, Skelly R, Donaldson M. Nutritional management in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J laryngology otology. 2016;130(S2):S32‐S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reich M. Best practice guidelines in the psychosocial management of HPV‐related head and neck cancer: recommendations from the European Head and Neck Cancer Society's Make Sense Campaign. Ann Oncol. 2016;27(10):1848‐1854. [DOI] [PubMed] [Google Scholar]
- 54. Jopson R, Callender J. Information provided to Patients Diagnosed with Human Papilloma Virus Positive Head and Neck Cancers: An Exploratory Study of Current Practice; 2019. Poster session presented at UKIO. [Google Scholar]
- 55. Erikson EH, Erikson JM. The Life Cycle Completed. Extended Version/Ed. W.W. Norton; 1997. [Google Scholar]
- 56. Bury M. Chronic illness as biographical disruption. Sociol Health Illn. 1982;4(2):167‐182. [DOI] [PubMed] [Google Scholar]
- 57. Mishel MH. Uncertainty in illness. J Nurs Scholarsh. 1988;20(4):225‐232. [DOI] [PubMed] [Google Scholar]
- 58. Suzuki M. Quality of life, uncertainty, and perceived involvement in decision making in patients with head and neck cancer. Oncol Nurs Forum. 2012;39(6):541‐548. [DOI] [PubMed] [Google Scholar]
- 59. Guan T, Santacroce SJ, Chen D‐G, Song L. Illness uncertainty, coping, and quality of life among patients with prostate cancer. Psycho Oncol. 2020;29(6):1019‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Papadakos J, McQuestion M, Gokhale A, et al. Informational needs of head and neck cancer patients. J Cancer Educ. 2018;33(4):847‐856. [DOI] [PubMed] [Google Scholar]
- 61. Neilson KA, Pollard AC, Boonzaier AM, et al. Psychological distress (depression and anxiety) in people with head and neck cancers. Med J Aust. 2010;193(S5):S48‐S51. [DOI] [PubMed] [Google Scholar]
- 62. Morris N, Moghaddam N, Tickle A, Biswas S. The relationship between coping style and psychological distress in people with head and neck cancer: a systematic review. Psycho Oncol. 2018;27(3):734‐747. [DOI] [PubMed] [Google Scholar]
- 63. Dunne S, Mooney O, Coffey L, et al. Psychological variables associated with quality of life following primary treatment for head and neck cancer: a systematic review of the literature from 2004 to 2015. Psycho Oncol. 2017;26(2):149‐160. [DOI] [PubMed] [Google Scholar]
- 64. Bond SM, Hawkins DK, Murphy BA. Caregiver‐reported neuropsychiatric symptoms in patients undergoing treatment for head and neck cancer: a pilot study. Cancer Nurs. 2014;37(3):227‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Koinberg I, Olofsson EH, Carlström E, Olsson L‐E. Impact of a person‐centered intervention for patients with head and neck cancer: a qualitative exploration. BMC Nurs [Internet]. 2018;17(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Howren MB, Christensen AJ, Karnell LH, Funk GF. Psychological factors associated with head and neck cancer treatment and survivorship: evidence and opportunities for behavioral medicine. J Consult Clin Psychol. 2013;81(2):299‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Richardson AE, Broadbent E, Morton RP. A systematic review of psychological interventions for patients with head and neck cancer. Support Care Cancer. 2019;27(6):2007‐2021. [DOI] [PubMed] [Google Scholar]
- 68. Rogers SN, El‐Sheikha J, Lowe D. The development of a Patients Concerns Inventory (PCI) to help reveal patients concerns in the head and neck clinic. Oral Oncol. 2009;45(7):555‐561. [DOI] [PubMed] [Google Scholar]
- 69. Macmillan UK. Personalised Care for People Living with Cancer 2021. [06.06.21]. https://www.macmillan.org.uk/healthcare‐professionals/innovation‐in‐cancer‐care/personalised‐care [Google Scholar]
- 70. Llewellyn CD, McGurk M, Weinman J. Striking the right balance: a qualitative pilot study examining the role of information on the development of expectations in patients treated for head and neck cancer. Psychol Health Med. 2005;10(2):180‐193. [Google Scholar]
- 71. Brennan J. Adjustment to cancer ‐ coping or personal transition? Psycho Oncol. 2001;10(1):1‐18. [DOI] [PubMed] [Google Scholar]
- 72. Little M, Jordens CF, Paul K, Montgomery K, Philipson B. Liminality: a major category of the experience of cancer illness. Soc Sci Med. 1998;47(10):1485‐1494. [DOI] [PubMed] [Google Scholar]
- 73. Egestad H. The significance of fellow patients for head and neck cancer patients in the radiation treatment period. Eur J Oncol Nurs. 2013;17(5):618‐624. [DOI] [PubMed] [Google Scholar]
- 74. McCaughan E, McKenna H. Never‐ending making sense: towards a substantive theory of the information‐seeking behaviour of newly diagnosed cancer patients. J Clin Nurs. 2007;16(11):2096‐2104. [DOI] [PubMed] [Google Scholar]
- 75. Ogden J. Health Psychology. 6th ed. McGraw‐Hill Education; 2019. [Google Scholar]
- 76. Paleri V, Rowland N. Head and neck cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. NHS . Long Term Plan 2015. [17.11.21]. https://www.longtermplan.nhs.uk/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Data Availability Statement
The data that support the findings of this review are available from the corresponding author upon reasonable request.


