Abstract
Aim
To establish the efficacy of oscillating‐rotating power toothbrush (OR‐PTB) compared to high‐frequency sonic power toothbrush (HFS‐PTB) on improving parameters of plaque and gingival inflammation. Safety and participants' preference were secondary interests.
Materials and methods
MEDLINE‐PubMed and Cochrane‐CENTRAL databases were searched, up to April 2021. Inclusion criteria were (randomized)controlled clinical trials that evaluated healthy humans brushing with an OR‐PTB compared to a HFS‐PTB. Evaluation for a minimum of 4 weeks, of one or more of the following parameters: plaque index scores (PI), bleeding scores (BS), number of bleeding sites (NoB) and gingival index scores (GI).
Results
Thirty two publications involving 38 comparisons were included after the independent screening. The descriptive analysis showed that in 54% of the comparisons, a significant difference in favour of the OR‐PTB was found for PI, BS and GI scores. The Quigley and Hein index showed a significant difference of means (DiffM) between the end scores (DiffM 0.13, 95% CI [0.05;0.21] p < 0.001), as well as for the Rustogi‐modified Navy index (DiffM 0.01, 95% CI [0.01;0.03] p = 0.002). This is in line with the meta‐analysis for BS (DiffM 0.09, 95% CI [0.03;0.14] p = 0.003), for which the results were in favour of the OR‐PTB and considered potentially clinically relevant. NoB showed a significant difference in favour of the OR‐PTB for the end scores (DiffM 3.61, 95% CI [2.63;4.58] p < 0.00001). No difference in safety was indicated, 78% of participants preferred the OR‐PTB.
Conclusion
For patients to maintain good plaque control and improve gingival health, there is a small but significant difference based on longer‐term studies between OR‐PTB and HFS‐PTB. This difference is potentially clinically relevant.
Keywords: dental plaque, gingival health, oscillating‐rotating toothbrush, power toothbrush, sonic toothbrush, systematic review
1. INTRODUCTION
There is general agreement that the greatest contributor to oral health is regular and thorough dental plaque removal, typically by means of a toothbrush. 1 Toothbrushes are available in many different designs, varying in the shape of the handle, brush head, arrangement of bristles and filament shapes. The brush head in particular is continuously under revision in order to improve effectiveness. Powered toothbrushes (PTBs) have been in use since the 1940s, starting with devices with a circular brush head or a rectangular\brush head. Today PTBs are widely used and demonstrate benefits with regard to reducing dental plaque and gingivitis in comparison to manual toothbrushes (MTBs) in both short‐ and long‐term observations. 2 Because of this cleaning performance and ease of use, PTBs are becoming increasingly accepted. 3 Several types of PTBs with different brush head configurations and modes of actions are available on the market. The high‐frequency sonic power toothbrush (HFS‐PTB) with a side‐to‐side bristle movement and the oscillating‐rotating power toothbrush (OR‐PTB) are the most retailed and researched modes of action worldwide.
Almost a decade ago, a solid Cochrane systematic review 3 conducted a direct comparison between PTBs with different modes of actions in regards to plaque and gingivitis reduction. At that time, no definitive conclusions could be drawn regarding the superiority of one type of PTB over another. A network meta‐analysis was recently published on the effects of OR‐PTBs, HFS‐PTBs and MTBs. The meta‐analysis was limited to randomized control trial (RCT) studies with a duration of up to 3 months in the period 2007–2017 and available in the database of a PTB manufacturer. 4 The analysis ultimately recommended that patients with various degrees of gingival bleeding use OR‐PTBs over HFS‐PTBs to improve plaque control and parameters of gingival inflammation. 4 Another systematic review compared PTBs to MTBs for oral health with no limitation to study duration. This review concluded that a PTB is more effective in reducing dental plaque, gingivitis and bleeding compared with a MTB. 5 Based on studies solely from the last decade, a recent systematic review further concluded that there is some evidence to suggest that OR‐PTBs might remove more plaque and reduce the number of bleeding sites better than other PTBs, including HFS‐PTBs. 6 Based on single brushing exercises, recent evidence also indicates that the use of OR‐PTBs results in a larger reduction of plaque scores than the use of HFS‐PTBs. 7 The authors of the study, with moderate certainty, recommended a OR‐PTB over a HFS‐PTB. However, the authors noted that the clinical relevance of the observed difference deserved further evaluation in longer‐term studies.
All other recent systematic reviews 6 , 7 on this topic were characterized by various restrictions, such as a specific research design, 7 year of publication, 6 or manufacturer database. 4 Therefore, based on that and the suggestion of a SR for long‐term studies there is a need for a SR that included and updates the findings of the dated Cochrane systematic review Deacon et al. (2010) 3 with respect to the comparison of OR and HFS‐PTB. Thus, the aim of this systematic review is to evaluate direct comparisons of the oscillating‐rotating power toothbrush versus high‐frequency sonic power toothbrush mode of action with respect to parameters of plaque and gingival inflammation using data from studies with a minimum duration of 4 weeks. The recommendations that emerge from this review may guide dental care professionals in providing evidence‐based advice concerning power toothbrush use to their patients.
2. MATERIALS AND METHODS
The systematic review and meta‐analyses in this study were prepared and reported in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. 8 In addition, this research adhered to the guidelines of Transparent Reporting of Systematic Reviews and Meta‐analyses 9 , 10 and Assessing the Methodology Quality of Systematic Reviews. 11 , 12
The protocol for this systematic review was developed a priori and registered with the International Prospective Register of Systematic Reviews 13 under registration number CRD42020161883.
2.1. Focused questions
The questions that guided the systematic review, which is focused on healthy participants, were the following.
Primary question:
What is the efficacy of oscillating‐rotating power toothbrush compared to high‐frequency sonic power toothbrush in improving parameters of dental plaque and gingival inflammation?
Secondary questions:
What is the safety of oscillating‐rotating power toothbrush compared to high‐frequency sonic power toothbrush for oral soft tissues and hard tissues?
What are participants' preferences between oscillating‐rotating power toothbrush and high‐frequency sonic power toothbrush?
2.2. Search strategy
For the comprehensive search strategy that was in place through April 2021, three electronic databases were utilized to search for appropriate papers that satisfied the study purpose. The National Library of Medicine, Washington, D.C. (MEDLINE‐PubMed) and the Cochrane Central Register of Controlled Trials (CENTRAL) were used to identify eligible publications. Table 1 presents the search strategy, including the search terms. All references cited in the papers that were selected for this review were scrutinized to identify additional relevant studies. Further hand searching was not performed other than as part of the Cochrane Worldwide Hand Searching Program and uploaded to CENTRAL.
TABLE 1.
Search terms used for the search strategy
| The following strategy was used in the search: {(intervention) AND (specification)} |
|---|
| {(<Intervention:> |
| Toothbrush* OR ‘Toothbrushing’[Mesh]) |
| AND |
| (<specification:> |
| power* OR electric*)} |
Note: The asterisk (*) was used as a truncation symbol.
2.3. Screening and selection
Two reviewers (NLHH and EvdS) screened the titles and abstracts of publications obtained from the search process using the Rayyan web application. 14 , 15 Possible duplicates were flagged and checked by the two reviewers to eliminate identical studies. Disagreements in the screening and selection process were resolved by consensus or, if disagreement persisted, by arbitration through a third reviewer (DES). The reviewers read titles and abstracts in detail to screen for suitability and categorized them as included, excluded or undecided. Once the list of included titles and abstracts was obtained, full‐text versions of the papers were retrieved and screened for suitability. After the independent screening process, the search was unblinded and the ‘conflicts’ that were identified by Rayyan were resolved by the reviewers. The reviewers worked independently and were blinded from each other's results during the screening process.
The eligibility criteria for published studies were the following:
Publications written in the English language.
(Randomized) controlled clinical trials (CCT or RCTs).
- Studies conducted on humans who met the following conditions:
- ≥18 years old
- In good general health, which means having no systemic disorders
- Participant brushing
- Without an orthodontic fixed appliance
Study duration of a minimum of 4 weeks.
Intervention: A high‐frequency sonic power toothbrush (HFS‐PTB) with a side‐to‐side bristle movement.
Comparison: An oscillating‐rotating power toothbrush (OR‐PTB). Only power toothbrushes with a rechargeable battery and a handle containing a motor that provides mechanical movement to a brush head with filaments. 16
Primary parameters of interest: Plaque index scores (PI), bleeding scores (BS), number of bleeding sites (NoB), gingival index scores (GI).
Secondary parameters of interest: Oral soft tissue assessments (OST), gingival abrasion scores (GA), adverse events (AE) and/or panellist questionnaires.
2.4. Assessment of heterogeneity
The heterogeneity of the primary outcome parameters across publications was detailed according to the following factors:
Study design and participant characteristics.
Study procedures and products.
Plaque and gingivitis indices and their modifications.
To evaluate methodological heterogeneity, the study designs and toothbrushing regimens were evaluated. When clinical or methodological heterogeneity was considered to be high across publications, the sources of heterogeneity were explored by subgroup analyses and sensitivity analysis.
2.5. Risk of bias assessment and grading
Two reviewers (NLHH and DES) individually scored the methodological qualities and ethical aspects 7 of the included publications according to the method described in detail by Van der Weijden (2009) 17 and Keukenmeester et al. (2012). 18 When random allocation, defined eligibility criteria, masking of examiners, masking of participants, balanced experimental groups, identical treatment between groups (except for the intervention) and reporting of follow‐up were present, the study was classified as having an estimated low risk of bias. When one of these criteria was missing, the study was considered to have an estimated moderate risk of bias. When two or more of these criteria were missing, the study was estimated to have a high risk of bias. Disagreements in the quality assessments were resolved by consensus or, if disagreement persisted, by arbitration through a third reviewer (FvdW).
2.6. Statistical analyses
2.6.1. Data extraction
The data from the publications that met the selection criteria were extracted and processed for further analysis. Two reviewers (EvdS and NLHH) evaluated the selected publications for mean baseline, end and incremental difference scores and standard deviations (SDs). To ensure an accurate estimate, any data approximation in figures was avoided. Some of the studies provided a standard error (SE) of the mean. These values were converted to SDs based on the sample size (SE = SD/√N). Numbers with three digits were rounded to two digits. In cases of missing data or undetermined information, attempts were made to contact the first or corresponding author of the included publications for clarification or to retrieve additional data. For studies that had multiple treatment arms and for which data from the control group were compared with more than one other group, the number of participants (N) in the control group was divided by the number of comparisons. Disagreements in the data extraction were resolved by consensus or, if disagreement persisted, by arbitration through a third reviewer (DES).
2.6.2. Data analysis
Descriptive analysis
A descriptive data analysis was conducted for all included studies to summarize the results. The primary variables of interest were plaque index scores (PI), bleeding scores (BS) and/or gingival index scores (GI). The data were summarized and analysed using vote counting. 19 For the secondary parameters of interest, an overview was presented.
Meta‐analysis
When appropriate, a meta‐analysis was performed, and the difference of means (DiffM) was calculated using an inverse variance method (review manager 2014) in Review Manager [RevMan Version 5.3.Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014] 20 with either the fixed or random‐effects model, as appropriate. For the analysis, the assumption was made that summary data were missing at random, and therefore, all available data were included. The data were separated by the following primary indices: Modified Quigley and Hein Plaque index, 21 , 22 , 23 Rustogi Modified Navy Plaque index, 24 papillary bleeding index, 25 angular bleeding Index/bleeding on marginal probing, 26 , 27 , 28 NoB, gingival bleeding index, 29 or the (modified) gingival index. 30
Heterogeneity was tested using the chi‐square test and the I2 statistic. 8 A chi‐square test resulting in a p‐value <0.1 was considered to be an indication of significant statistical heterogeneity. As an approximate guide for assessing the degree of inconsistency across studies, an I2 statistic of 0%–40% was interpreted as might not be important, a statistic of 40%–60% indicated moderate heterogeneity, 60%–80% indicated substantial heterogeneity, and 80%–100% indicated considerable heterogeneity. 31
Publication bias
If the meta‐analysis comprised sufficient trials to make visual inspection of the plot meaningful (10 trials minimum), funnel plots were used for assessment of publication bias. The presence of asymmetry in the inverted funnel may suggest publication bias. 8 , 32
Additional analysis
The Lan‐DeMets version 33 of the O'Brien‐Fleming function 34 was used for calculating the trial sequential monitoring boundaries (TSMBs). TSA software version 0.9.5.10 Beta (Copenhagen Trial Unit) was used. 35 , 36 , 37 , 38
Furthermore, distribution‐based methods were applied in order to determine the clinical relevance of the study results. 39 , 40 , 41 , 42 , 43 , 44 Using the distribution‐based method, the clinical relevance was scored as not clinically relevant, potentially clinically relevant or clinically relevant, based on the relationship among the mean difference of the variable, minimal important difference (MID) and effect size 44 was interpreted as small, 0.50 (0.40–0.79) as medium and ≥0.80 as large. The MID was determined by multiplying the effect size of the difference between groups by the pooled baseline SD of the two groups. 39 The MID was calculated by multiplying either 0.2 or 0.5 by the pooled baseline standard deviation. 41
Evaluation of the clinical relevance of the results on the variable plaque indices, BOP, MGI and GBI was performed based on the distribution‐based method using the ES, 39 MID 39 , 45 and clinical judgement. 42
2.7. Grading the body of evidence
The Grading of Recommendations Assessment, Development and Evaluation system, as proposed by the GRADE working group, was used to rank and grade the evidence emerging from this review. 45 , 46 Two reviewers (FvdW and DES) rated the certainty of the evidence as well as the strength and direction of recommendations according to the following aspects: risk of bias of the individual studies, consistency and precision among the study outcomes, directness of the study results and detection of publication bias. Any disagreement between the two reviewers was resolved after additional discussion with the third reviewer (EvdS).
3. RESULTS
3.1. Search and selection results
Figure 1 presents the search and selection results and the included publications. Among the unique titles and abstracts, there were 14 conflicts (<2%) scored by the reviewers. The comprehensive search of the databases resulted in 32 publications including 38 comparisons that were eligible for inclusion. 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78
FIGURE 1.
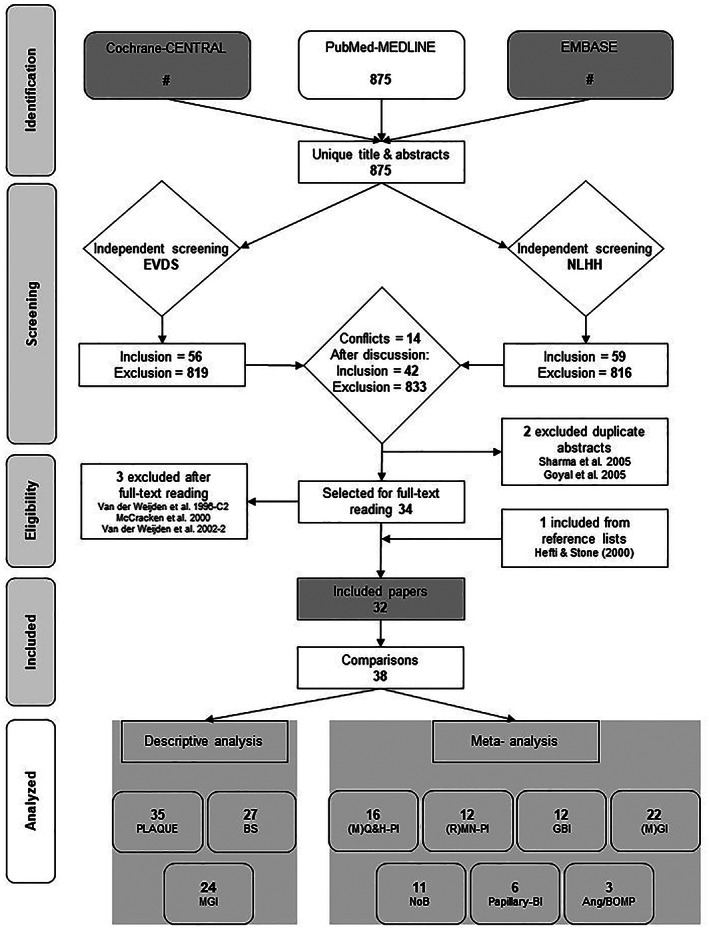
Search and selection results. (M)GI, Modified gingival index; (M)Q&H‐PI, modified Quigley & Hein plaque index; (R)MN‐PI, Rustogi Modification of the Navy plaque index; Ang/BOMP, Angular Bleeding index/ Bleeding on marginal probing; BI, Bleeding index; BS, Bleeding scores; GBI, Gingival bleeding index; MGI, Modified gingival Index; NoB, Number of bleeding sites
3.2. Study characteristics and heterogeneity assessment
Considerable heterogeneity was observed in the 32 papers with respect to study design; evaluation period; study population and number, gender and age of participants. With the exception of two comparisons 51 , 58 that used a cross‐over design, all other included studies were RCTs and had a parallel design. Four comparisons 48 , 53 , 54 , 57 utilized a split‐mouth method in which one comparison 48 selected two contralateral quadrants and the other three comparisons 53 , 54 , 57 divided the dentitions into the left and the right side. The total study duration varied from 30 days to 6 months. The total number of participants ranged from 30–284. The approximate mean age of the 2805 participants was 40 and varied from 18–83 years old. Information regarding the study characteristics is provided in an overview in online Appendix S1A and in detail in online Appendices S1B,C.
3.3. Risk of bias assessment
The quality assessments including internal validity, external validity, statistical validity and clinical and ethical considerations are presented in the online Appendices S3A,B. The estimated potential risk of bias is moderate in one publication 64 and low in the other 31 publications. 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 Compared to the older studies, the five ethical aspects were more frequently reported in the publications from the last decade.
3.3.1. Ethical aspects
IRB approval was reported for all studies since 2002, with the exception of one study. 64 From the same year onward, all participants provided written informed consent. The presence of a conflict of interest (COI) was reported in 16 out of 32 publications. 60 , 62 , 63 , 64 , 65 , 67 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 Of the 16 publications, 10 publications disclosed a combination of non‐industry authors and industry‐related authors. 60 , 62 , 65 , 67 , 69 , 72 , 74 , 76 , 77 , 78 Only the non‐industry authors declared not having a COI. The source of funding was reported in 22 publications 49 , 53 , 54 , 56 , 57 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 and of those, two publications 70 , 73 stated that products were supplied by one or more manufacturers.
3.4. Results of study outcomes
The online Appendices S4, S5 and S6 detail the results from the data extraction for the primary parameters of interest: PI, BS, PBI, GBI and the Modified Gingival Index scores (MGI).
3.4.1. Descriptive analysis
Tables 2 and 3 present an overview of the descriptive analysis of the 32 publications and 38 comparisons. Table 2 indicates statistical significance results of OR‐PTBs compared to HFS‐PTBs. The overall difference was significant in favour of the OR‐PTB in 54% of the comparisons that evaluated PI, 58% with respect to BS and 48% with respect to MGI scores. In total, 22 out of the 98 primary outcome parameters of interest indicated no difference between both toothbrushes (Details are shown in online Appendix S2). Table 3 presents an overview of the secondary parameters of interest. In the included studies, there was no difference between the toothbrushes with respect to safety (Online Appendices S7 and S8), and participants in seven out of the nine publications preferred the OR‐PTB (Online Appendix S9).
TABLE 2.
Overview of the descriptive summary of the 32 studies and 38 comparisons with the number and percentages of statistical significance of the OR‐PTB compared to the HFS‐PTB in numbers and percentages
| Outcome | Number of comparisons | Significant difference in favour of the comparison (OR‐PTB) | Significant difference in favour of the intervention (HFS‐PTB) | No difference | Unknown (?) | Online appendix number |
|---|---|---|---|---|---|---|
| Plaque index scores | 35 | 19 (54%) | 3 (9%) | 11 (31%) | 2 (6%) | S2 |
| Bleeding scores | 38 a | 22 (58%) | 8 (21%) | 4 (10.5%) | 4 (10.5%) | S2 |
| (modified) Gingival index scores | 25 | 12 (48%) | 5 (20%) | 7 (28%) | 1 (4%) | S2 |
| Overall | 98 | 53 (54%) | 16 (16%) | 22 (22%) | 7 (7%) |
Abbreviations: HF‐PTB, High‐frequency sonic power toothbrush; OR‐PTB, Oscillating‐rotating power toothbrush.
Twelve comparisons evaluated two measures of bleeding scores.
TABLE 3.
Overview of the descriptive summary of safety assessments and panel list questionnaire comparing OR‐PTB with HFS‐PTB
| Outcome | Number of publications | Number of publications and the result | Online appendix number |
|---|---|---|---|
| Intra‐oral safety assessments | 23 |
15 No difference 8 Unknown |
S7 |
| Gingiva abrasion score | 1 | No difference between the products | NA |
| Adverse events | 22 |
18 Reported no AE 10 Reported AE assessed as possibly related to the study product 1 Reported AE unrelated to the products |
S8 |
| Panellist questionnaire | 9 |
5 Significant in favour of OR‐PTB 3 Numerically in favour for OR‐PTB 1 Unknown |
S9 |
Abbreviations: AE, Adverse Event; HFS‐PTB, High‐frequency sonic power toothbrush; NA, not applicable; OR‐PTB, Oscillating‐rotating power toothbrush.
3.4.2. Meta‐analysis
Primary outcomes
Tables 4, 5, 6, 7, 8, 9, 10 present an overview of the outcomes of the meta‐analysis, and the forest plots are displayed in online Appendices S10 to S16. The meta‐analysis was based on 16 comparisons using the modified Quigley and Hein Plaque index (MQHPI). The results revealed that the Difference of Means (DiffM) was significant (p < 0.001) in favour of the OR‐PTB for end scores (DiffM = 0.13, 95% CI [0.05; 0.21]) (Table 4). In addition, the (Rustogi) modified Navy Plaque index (RMNPI) demonstrated a statistically significant DiffM (p = 0.002) for the end scores (DiffM = 0.01 (95% CI [0.00; 0.03]) in favour of the OR‐PTB (Table 5).
TABLE 4.
Meta‐analysis for the modified Quigley and Hein Plaque index using a random model
| Modified Quigley and Hein Plaque index | Number of comparisons | Comparison | Effect size | Heterogeneity | Online Appendix number | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DiffM | 95% CI | p‐Value | I 2 | p‐Value | Forrest plot | Funnel plot | TSA | ||||
| Number | TSA does research the RIS or not (yes/no) | ||||||||||
|
Grossman et al. (1995) 47 Robinson et al. (1997) 49 Yankell & Emling (1997) 50 Putt et al. (2001) 53 Van der Weijden et al. (2002–1) 54 Goyal et al. (2005) 55 2x Patters et al. (2005) 56 Rosema et al. (2005) 57 Williams et al. (2009) 60 Büchel et al. (2014) 65 Schmickler et al. (2016) 70 2x Schmalz et al. (2017‐AJD) 71 Schmalz et al. (2018‐COI) 73 2x |
16 | Baseline | −0.03 | (−0.08; 0.02) | 0.19 | 0% | 0.95 | S10A | S10B | NA | NA |
| End | 0.13 | (0.05; 0.21) | <0.001 | 54% | 0.008 | S10C | S10D | S10E | Yes | ||
| Difference | ? | ? | ? | ? | ? | NA | NA | NA | NA | ||
Note: p‐Values are presented in bold if p ≤ 0.05.
Abbreviations: ?, Unclear; NA, Not applicable; RIS, Required Information Size; TSA, Trial sequential analysis.
TABLE 5.
Meta‐analysis for the Rustogi modified Navy Plaque index using a random model
| Rustogi modified Navy Plaque index | Number of comparisons | Comparison | Effect size | Heterogeneity | Online Appendix number | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DiffM | 95% CI | p‐Value | I 2 | p‐Value | Forrest plot | Funnel plot | TSA | ||||
| Number | TSA does research the RIS or not (yes/no) | ||||||||||
|
Goyal et al. (2009) 55 Ayad et al. (2012) 61 2x Klukowska et al. (2012) 62 Klukowska et al. (2013) 63 Klukowska et al. (2014‐12w) 66 Klukowska et al. (2014‐6wCOL) 67 Klukowska et al. (2014‐6wPH) 68 Ccahuana‐Vasquez et al. (2015) 69 Ccahuana‐Vasquez et al. (2018) 74 Adam et al. (2020) 77 Goyal et al. (2021) 78 |
12 | Baseline | −0.00 | (−0.01; 0.00) | 0.08 | 0% | 0.81 | S11A | S11B | NA | NA |
| End | 0.01 | (0.00; 0.03) | 0.02 | 85% | <0.00001 | S11C | S11D | S11E | Yes | ||
|
Goyal et al. (2009) 55 Ayad et al. (2012) 61 2x Klukowska et al. (2012) 62 Klukowska et al. (2013) 63 Klukowska et al. (2014‐12w) 66 Klukowska et al. (2014‐6wCOL) 67 Klukowska et al. (2014‐6wPH) 68 Ccahuana‐Vasquez et al. (2015) 69 Ccahuana‐Vasquez et al. (2018) 74 Lv et al. (2018) 75 2x Adam et al. (2020) 77 Goyal et al. (2021) 78 |
14 | Difference | 0.01 | (0.00; 0.02) | 0.01 | 81% | <0.00001 | S11F | S11G | NA | NA |
Note: p‐Values are presented in bold if p ≤ 0.05.
Abbreviations: ?, Unclear; NA, Not applicable; RIS, Required Information Size; TSA, Trial sequential analysis.
TABLE 6.
Meta‐analysis for the Papillary Bleeding index using a random model
| Papillary Bleeding index | Number of comparisons | Comparison | Effect size | Heterogeneity | Online appendix number | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DiffM | 95% CI | p‐Value | I 2 | p‐Value | Forrest plot | Funnel plot | TSA | ||||
| Number | TSA does research the RIS or not (yes/no) | ||||||||||
|
Robinson et al. (1997) 49 Schmickler et al. (2016) 70 2x Schmalz et al. (2017‐AJD) 71 Schmalz et al. (2018‐COI) 73 2x |
6 | Baseline | 0.05 | (−0.04; 0.15) | 0.28 | 29% | 0.22 | S12A | NA | NA | NA |
| End | −0.08 | (−0.18; 0.02) | 0.13 | 55% | 0.05 | S12B | NA | S12C | No | ||
| Difference | ? | ? | ? | ? | ? | NA | NA | NA | NA | ||
Note: p‐Values are presented in bold if p ≤ 0.05.
Abbreviations: ?, Unclear; NA, Not applicable; RIS, Required Information Size; TSA, Trial sequential analysis.
TABLE 7.
Meta‐analysis for the Angular Bleeding index/ Bleeding On Marginal Probing using a fixed model
| Angular Bleeding index/Bleeding On Marginal Probing | Number of comparisons | Comparison | Effect size | Heterogeneity | Online Appendix number | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DiffM | 95% CI | p‐Value | I 2 | p‐Value | Forrest plot | Funnel plot | TSA | ||||
| Number | TSA does research the RIS or not (yes/no) | ||||||||||
|
Putt et al. (2001) 53 Van der Weijden et al. (2002–1) 54 Rosema et al. (2005) 57 |
3 | Baseline | 0.00 | (−0.06; 0.07) | 0.93 | 0% | 0.77 | S13A | NA | NA | NA |
| End | 0.09 | (0.03; 0.14) | 0.003 | 40% | 0.19 | S13B | NA | S13C | no | ||
| Difference | ? | ? | ? | ? | ? | NA | NA | NA | NA | ||
Note: p‐Values are presented in bold if p ≤ 0.05.
Abbreviations: ?, Unclear; NA, Not applicable; RIS, Required Information Size; TSA, Trial sequential analysis.
TABLE 8.
Meta‐analysis for the number of bleeding sites using a random model
| Number of bleeding sites | Number of comparisons | Comparison | Effect size | Heterogeneity | Online Appendix number | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DiffM | 95% CI | p‐Value | I 2 | p‐Value | Forrest plot | Funnel plot | TSA | ||||
| Number | TSA does research the RIS or not (yes/no) | ||||||||||
|
Goyal et al. (2009) 55 Williams et al. (2009) 60 Klukowska et al. (2012) 62 Klukowska et al. (2013) 63 Klukowska et al. (2014‐12w) 66 Klukowska et al. (2014‐6wCOL) 67 Klukowska et al. (2014‐6wPH) 68 Ccahuana‐Vasquez et al. (2015) 69 Ccahuana‐Vasquez et al. (2018) 74 Adam et al. (2020) 77 Goyal et al. (2021) 78 |
11 | Baseline | 0.94 | (−0.30; 2.18) | 0.14 | 0% | 0.72 | S14A | S14B | NA | NA |
| End | 3.61 | (2.63; 4.58) | <0.00001 | 1% | <0.00001 | S14C | S14D | S14E | Yes | ||
| Difference | 3.61 | (2.64; 4.59) | <0.00001 | 81% | <0.00001 | S14F | S14G | NA | NA | ||
Note: p‐Values are presented in bold if p ≤ 0.05.
Abbreviations: ?, Unclear; NA, Not applicable; RIS, Required Information Size; TSA, Trial sequential analysis.
TABLE 9.
Meta‐analysis for the Gingival Bleeding index using a random model
| Gingival Bleeding Index | Number of comparisons | Comparison | Effect size | Heterogeneity | Online Appendix number | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DiffM | 95% CI | p‐Value | I 2 | p‐Value | Forrest plot | Funnel plot | TSA | ||||
| Number | TSA does research the RIS or not (yes/no) | ||||||||||
|
Goyal et al. (2009) 55 Klukowska et al. (2012) 62 Klukowska et al. (2013) 63 Klukowska et al. (2014‐12w) 66 Klukowska et al. (2014‐6wCOL) 67 Klukowska et al. (2014‐6wPH) 68 Ccahuana‐Vasquez et al. (2015) 69 Starke et al. (2017) 72 Ccahuana‐Vasquez et al. (2018) 74 Mirza et al. (2019) 76 Adam et al. (2020) 77 Goyal et al. (2021) 78 |
12 | Baseline | −0.01 | (−0.03; 0.01) | 0.48 | 82% | <0.00001 | S15A | S15B | NA | NA |
| End | 0.01 | (−0.01; 0.02) | 0.29 | 93% | <0.00001 | S15C | S15D | S15E | No | ||
| Difference | 0.01 | (−0.00; 0.02) | 0.08 | 90% | <0.00001 | S15F | S15G | NA | NA | ||
Note: p‐values are presented in bold if p ≤ 0.05.
Abbreviations: ?, Unclear; NA, Not applicable; RIS, Required Information Size; TSA, Trial sequential analysis.
TABLE 10.
Meta‐analysis for the (modified) Gingival index using a random model
| (Modified) Gingival index | Number of comparisons | Comparison | Effect size | Heterogeneity | Online Appendix number | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DiffM | 95% CI | p‐Value | I 2 | p‐Value | Forrest plot | Funnel plot | TSA | ||||
| Number | TSA does research the RIS or not (yes/no) | ||||||||||
|
Grossman et al. (1995) 47 Yankell & Emling (1997) 50 Putt et al. (2001) 53 Goyal et al. (2009) 59 Williams et al. (2009) 60 Ayad et al. (2012) 61 2x Klukowska et al. (2012) 62 Klukowska et al. (2013) 63 Klukowska et al. (2014‐12w) 66 Klukowska et al. (2014‐6wCOL) 67 Klukowska et al. (2014‐6wPH) 68 Ccahuana‐Vasquez et al. (2015) 69 Schmickler et al. (2016‐non‐rep) 70 2x Starke et al. (2017) 72 Schmalz et al. (2018‐COI instr) 73 2x Ccahuana‐Vasquez et al. (2018) 74 Mirza et al. (2019) 76 Adam et al. (2020) 77 Goyal et al. (2021) 78 |
22 | Baseline | 0.00 | (−0.00; 0.01) | 0.27 | 0% | 0.71 | S16A | S16B | NA | NA |
| End | 0.02 | (−0.01; 0.05) | 0.24 | 94% | <0.00001 | S16C | S16D | S16E | Yes | ||
|
Goyal et al. (2009) 59 Williams et al. (2009) 60 Ayad et al. (2012) 61 2x Klukowska et al. (2012) 62 Klukowska et al. (2013) 63 Klukowska et al. (2014‐12w) 66 Klukowska et al. (2014‐6wCOL) 67 Klukowska et al. (2014‐6wPH) 68 Ccahuana‐Vasquez et al. (2015) 69 Starke et al. (2017) 72 Ccahuana‐Vasquez et al. (2018) 74 Lv et al. (2018) 75 2x Mirza et al. (2019) 76 Adam et al. (2020) 77 Goyal et al. (2021) 78 |
17 | Difference | 0.00 | (−0.04; 0.04) | 0.89 | 97% | <0.00001 | S16F | S16G | NA | NA |
Note: p‐Values are presented in bold if p ≤ 0.05. Interpretation of the heterogeneity: Potentially not important: 0%–40%; Moderate heterogeneity: 40%–60%; Substantial heterogeneity: 60%–80%; Considerable heterogeneity: 80%–100%. According to Sälzer et al. 201531.
Abbreviations: ?, Unclear; NA, Not applicable; RIS, Required Information Size; TSA, Trial sequential analysis.
The Angular Bleeding index Bleeding On Marginal Probing indices showed a significant difference in the end scores (DiffM = 0.09; 95% CI [0.03; 0.14] p = 0.003) in favour of the OR‐PTB (Table 7). Regarding gingival bleeding tendency, meta‐analysis for Number of Bleeding (NoB) was significant in favour of the OR‐PTB for the end scores (DiffM = 3.61 (95% CI [2.63; 4.58] p < 0.0001)) and the incremental difference scores (DiffM = 3.61 (95% CI [2.64; 4.59] p < 0.0001; Table 8). For the PBI, GBI and MGI, the baseline, end and mean differences were not significantly different (Tables 6, 9, 10).
Publication bias
It was possible to draw funnel plots for 14 trials, which can be found in the online Appendices S10B,D, S11B,D,G, S14B,D,G, S15B,D,G and S16B,D,G. Most outcomes are located at the top of the funnel plots, with the exception of one funnel plot of the end scores of the MQ&HPI (S16G). The presence of this asymmetry in the inverted tunnels is suggestive of a form of publication bias. 8 , 32
3.5. Additional analysis
The TSA graphs for each index are presented in the online Appendices S10E, S11E, S12C, S13C, S14E, S15C,E and S16E. The TSA suggests that for the indices of plaque scores, NoB scores and MGI, the required information size (RIS) was reached. The RIS was not fulfilled for the PBI, angular bleeding index/ bleeding on marginal probing indices or GBI.
Evaluation of the clinical relevance of the results on parameters of gingival inflammation was performed according to the distribution‐based method using the ES, 39 MID 41 , 45 and clinical judgement. 42 The results of the clinical relevance assessments indicate that the difference between the OR‐PTB and the HFS‐PTB was potentially clinically relevant (see online Appendices S17A,B). Of the clinical relevance assessments for the PI scores, 34% and 31% of the included experiments were potentially clinically relevant and clinically relevant, respectively. For the bleeding/gingivitis assessment, the percentages were 28% and 30%.
3.6. Evidence profile
Table 11 presents a summary of the various factors used to rate the quality of evidence and appraise the strength and direction of recommendations according to GRADE, 45 , 46 including the level of certainty. 79 All 32 studies in this review were RCTs with a crossover or parallel design. The directness of the studies is generalizable, as all studies had a minimum duration of 4 weeks. The risk of bias varied from low to moderate, and as many studies were industry financed, reporting bias cannot be ruled out. According to the descriptive analyses and meta‐analyses, the outcomes are relatively consistent and precise. There is a small difference in the clinical parameters of gingival inflammation in favour of the OR‐PTB. The strength of the recommendation was estimated to be moderate. Therefore, the direction of the recommendation is that there is a moderate certainty that there is a small but clinically relevant advantage of OR‐PTB over HFS‐PTB.
TABLE 11.
Estimated evidence profile appraisal of the strength of the recommendation, and the direction regarding the efficacy of the OR‐PTB compared to the HFS‐PTB on dental plaque removal and parameters of gingival inflammation
| Determinants of quality | Plaque Index Score | Bleeding Index Score | Gingival Index Score |
|---|---|---|---|
| Study design (Online Appendix S1A,B) | RCT crossover or parallel design | RCT crossover or parallel design | RCT crossover or parallel design |
| # Studies N = 32 | N = 38 | N = 51 | N = 38 |
| # Comparisons N = 38 (Figure 1) | |||
| # Studies in MA & TSA & CR | |||
| Risk of bias (Online Appendix S3A,B) | Low‐Moderate | Low‐Moderate | Low |
| Consistency (Table 2, Online Appendix S3A,B) | Rather consistent | Rather consistent | Rather consistent |
| Directness (Longer term use) | Rather generalizable | Rather generalizable | Rather generalizable |
| Precision (Tables 4, 5, 6, 7, 8, 9, 10) | Precise | Precise | Precise |
| Reporting bias (Online appendix S10B,D, S11B,D,G, S14B,D,G, S15B,D,G and S16B,D,G) | Likely | Likely | Likely |
| Magnitude of the effect (Tables 4, 5, 6, 7, 8, 9, 10) | Very small | Very small | None or very small |
| Strength of the recommendation based on the quality and body of evidence | Moderate | ||
| Direction of recommendation | There is a moderate certainty of a very small clinical relevant beneficial effect for an OR‐PTB over a HFS‐PTB. | ||
Abbreviations: CR, Clinical relevance; HFS‐PTB, High‐frequency sonic power toothbrush; MA, Meta‐analysis; OR‐PTB, Oscillating‐rotating power toothbrush; TSA, Trial sequential analysis.
4. DISCUSSION
The purpose of this systematic review was to comprehensively summarize the available scientific literature with respect to the efficacy of oscillating rotating (OR) and high‐frequency sonic (HFS) PTBs in reducing dental plaque and gingival inflammation. More than a decade ago this was also evaluated and at that time, the authors concluded that there is some evidence that OR‐PTBs reduce plaque and gingivitis more than side‐to‐side brushes in the short term, although the difference was small and the clinical importance was unclear. 3 In order to come to a better current understanding of the different potential benefits between PTB modes of action, there was a need for an up to date comprehensive review that systematically appraises and synthesizes the published evidence from longer‐term evaluations without restrictions. The current review has demonstrated that both types of PTB are effective in dental plaque removal and in improving the parameters of gingival inflammation. With a moderate degree of certainty, this review, therefore, concludes that there is a small but clinically relevant significant difference in favour of OR‐PTB over HFS‐PTB.
4.1. Toothbrushes and mode of action
Powered toothbrushes have electrically driven brush heads developed to improve plaque removal efficacy. The role of the user is mainly to guide the brush filaments across the dentition. Several PTBs are currently in the market, with variations in brush head design, filament pattern and speed of motion. 4 The studies included in this review frequently do not provide specific details on the toothbrush (head) design and variables related to the mode of action. The studies often note little beyond the brand and type. Thus, while marketing claims suggest that brush head design is important with respect to efficacy, this has been less researched than effects of the modes of action of specific brush technologies. The factors that distinguish different brush heads are filament arrangement, orientation, size, shape and flexibility, brush head size and shape. The development of different brush heads is driven by consumer preference and thus expected to help lead to more compliant behaviour. Also, a pressure sensor or a timer can contribute to the improvement of brushing behaviours, which could lead to improved efficacy.
However, with limited reporting on most of these details, it was impossible to isolate and analyse outcomes related to these factors. 80
4.2. Longer‐term effect designs
It has been suggested that studies up to 4 weeks in duration can be used for evaluating the effect on plaque scores and that longer‐term studies are necessary for measurements regarding parameters of gingival inflammation, such as BS and GI. 81 , 82 The inclusion criterion for the present study was, therefore, a study duration of a minimum of 4 weeks. This choice is in line with the American Dental Association guidelines for Seals concerning toothbrushes, 83 which require studies to be of at least a 30‐day duration to assess efficacy. With respect to short term and long term, there are variations in interpretations. Previous systematic reviews comparing PTBs to MTBs have used a minimum duration of 1 month, 84 , 85 and the criterion for long term was a follow‐up beyond 3 months. 3 , 83 In the current review, 12 studies had an evaluation period of 3 months or longer.
4.3. Bias and ethical considerations
The results of a systematic review are strongly dependent on the quality of the methodology, validity and estimated risk of bias of the included studies. In a recent systematic review, 7 five ethical aspects were introduced as part of the risk of bias assessment, including reported funding or a declared (or absent) COI. Details on the ethical aspects of the present review are presented in online Appendices S3A,B. In general, approval by a medical committee, informed consent, declaration of a COI and disclosed funding are well reported in studies from 2000 onwards. Trial registration is less reported and has only reported in studies since 2016.
Although reporting ethical aspects may not be fundamental to a trial, the Consolidated Standards of Reporting Trials (CONSORT) Statement 86 first published in 1996 recommends that factors such as the study registration, protocol and funding and approval by an institutional ethical review board are reported. In addition, funding bodies and medical journals strictly enforce ethical review. 86 The International Committee on Medical Journal Editors, therefore, has prepared a form to disclose financial and non‐financial relationships and activities and COIs. 87 This disclosure is currently standard when a manuscript is submitted to a scientific journal. As such, taking ethical aspects as part of the quality assessment in a systematic review is a judicious choice.
4.4. Conflicts of interest and sources of funding
A potential for a COI exists when professional judgement concerning a primary interest, such as patients' welfare or the validity of research, may be influenced by a secondary interest, such as financial gain. 87 Financial relationships are often judged to undermine the credibility of a journal, the authors, or the science itself. 87 Other interests, such as personal relationships or rivalries, academic competition and intellectual beliefs, 87 may also present conflicts. Authors, reviewers, editors and editorial board members of journals in the peer‐review and publication process must, therefore, consider and disclose their relationships and activities when fulfilling their different roles. Transparent and complete disclosures are required to help maintain public trust in the scientific process. In the current systematic review, 14 (45%) publications reported a variety of COIs. Nine papers 66 , 67 , 69 , 72 , 74 , 76 , 77 , 78 were written in conjunction with authors related to industry. The rate of COIs in the present study is much lower than the 76% that was found in a systematic review of RCTs from six dental journals published between January 2011 and March 2012. 88 This difference may be explained by the fact that the included studies in the present review were published over a decade ago when COI reporting was not common.
As presented in the online Appendices S3A,B, 22 of 32 of the included publications reported a source of funding. COIs and funding sources are often reported under the same heading, although these are separate items. Funding does not always indicate a problematic influence, but authors should carefully consider agreements with study sponsors, both for‐profit and non‐profit, and discuss terms such as authors' access to all of the study's data, their ability to analyse and interpret the data and to prepare and publish manuscripts independently, taking into account the principle of academic freedom. From toothpaste trials, it is evident that COIs are not associated with positive conclusions. 89 As toothpaste and toothbrushes are the most recommended products in oral care, the results on COIs and positive conclusions regarding toothpaste can be considered to apply to toothbrush studies as well. 89
4.5. Clinical relevance and clinical judgement
The evaluation of clinical relevance in research is key to simplify the transfer of knowledge from research into daily practice. 90 It is possible to have statistical significance without having clinical relevance. 43 Concluding that a result is statistically significant alone is inadequate because it does not speak to the magnitude of the effect. There is variation in the estimation of clinical relevance, potential clinical relevance and clinical irrelevance. When clinical relevance is translated to clinical judgement, it is unlikely that dental care professionals in routine examination would be able to detect small differences, 43 such as the difference between the two PTB products in the present review. Clinical judgement based on clinical knowledge takes precedence over the calculated methods for determining clinical relevance. 43 Thus, given that there is only a small statistically significant difference between the PTBs, this study does not firmly support the recommendation of one particular PTB over another. As such, the personal preferences of dental care professionals and patients will be decisive in choosing the best‐suited PTB in a given case.
4.6. Limitations and recommendations
Clinical observations are valid for the study conditions and applicable for the study population. The results may not predict how well an intervention will work in daily practice for a large variety of patients. 90 However, the recommendations that emerge from this review can help guide dental care professionals in providing evidence‐based advice to their patients concerning PTB use.
There are several limitations to this study:
Intra‐oral safety assessments were evaluated and a summary of the outcomes can be found in online Appendix S7. However, none of the studies included provided specific information on variables such as the impact of pressure indicators.
Although the present review includes 2805 total participants, the results of this systematic review should be extrapolated to specific patient populations with caution. Patients who have dental implants or orthodontic appliances, elderly patients and young children, for example, were not participants in any of the included studies.
Evidence suggests that oral prophylaxis and oral hygiene instructions may improve oral health. 91 Oral prophylaxis was provided in 8 out of the 32 included studies, which may have affected the results.
As mentioned in the Cochrane systematic review, 3 the cost and reliability of toothbrushes are important issues to consider beyond efficacy. However, those items were not reported in their included trials and could, therefore, not be part of this review. Patient preference is one of the three components of making an evidence based decision and should, therefore, be included in clinical studies and systematic reviews. 3
Technical improvements to PTBs are made over time. One direction for further research could be to evaluate the effects on plaque removal and parameters of gingival inflammation of PTBs over time to estimate the impact of these advances.
5. CONCLUSION
For patients to maintain good plaque control and improve gingival health, there is a small but significant difference between oscillating‐rotating power toothbrush and high‐frequency sonic toothbrush as based on longer‐term use studies. The direction of the recommendation is, therefore, that there is a moderate certainty of a small but clinically relevant advantage of OR‐PTB over HFS‐PTB. Both products were found to be safe, and 78% of participants prefer the OR‐PTB.
6. CLINICAL RELEVANCE
6.1. Scientific rationale for the study
There is a need for a systematic evaluation of direct comparisons on the most commonly used power toothbrush designs for their effects on plaque control and parameters of gingival inflammation.
6.2. Principal findings
With moderate certainty, there is a small but potentially clinically significant difference between the two types of power toothbrushes on PI, BS, GI scores.
6.3. Practical Implications
Both types of power toothbrushes appear to be effective in improving plaque control and parameters of gingival inflammation. This study recommends, with moderate certainty, the OR‐PTB over the HFS‐PTB.
AUTHOR CONTRIBUTIONS
EvdS contributed to design, search and selection, analysis and interpretation and drafted the manuscript. DES contributed to conception and design, search and selection, analysis and interpretation and critically revised the manuscript. NLHH contributed to design, search and selection, analysis and interpretation and drafted the manuscript. CV contributed to analysis, interpretation and critically revised the manuscript. FvdW contributed to conception and design, analysis and interpretation and critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work ensuring integrity and accuracy.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
Ethical approval was not required; the protocol was registered at PROSPERO (number CRD42020161883).
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
This study was sponsored in part by an unrestricted educational grant by Procter and Gamble Company Worldwide Clinical Investigations—Oral Care. The company had no say in the design of this review nor did they influence the reporting and publishing of the findings. The authors also thank the following authors for their responses, time and effort to collect additional data: dr. Grender for the Goyal (2021) and Adam (2020) studies, ms. Sagel for the Grossmann (1995) and Putt (2001, 2008) studies, dr. Ward, dr. Jenkins, dr. Nelson for the Mirza (2019) and Starke (2017) studies, dr. Ricci, dr Robinson, dr. Rosema, dr. Ziebolz for the Schmickler (2016), Schmalz (2018) and Schmalz (2017) studies. The authors have previously received either external advisor fees, lecturer fees or research grants from toothbrush manufacturers. Those manufacturers included Colgate, Dentaid, GABA, Lactona, Oral‐B, Procter & Gamble, Sunstar, Sara Lee, Philips, Waterpik and Unilever. Open access funding enabled and organized by ProjektDEAL.
van der Sluijs E, Slot DE, Hennequin‐Hoenderdos NL, Valkenburg C, van der Weijden F(A. The efficacy of an oscillating‐rotating power toothbrush compared to a high‐frequency sonic power toothbrush on parameters of dental plaque and gingival inflammation: A systematic review and meta‐analysis. Int J Dent Hygiene. 2023;21:77‐94. doi: 10.1111/idh.12597
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the Supplementary Material of this article.
REFERENCES
- 1. Kinane DF, Attstrom R. Advances in the pathogenesis of periodontitis. Group B consensus report of the fifth European workshop in periodontology. J Clin Periodontol. 2005;32(Suppl 6):130‐131. [DOI] [PubMed] [Google Scholar]
- 2. Murakami S, Mealey BL, Mariotti A, Chapple ILC. Dental plaque–induced gingival conditions. J Periodontol. 2018;89:S17‐S27. [DOI] [PubMed] [Google Scholar]
- 3. Deacon SA, Glenny AM, Deery C, et al. Different powered toothbrushes for plaque control and gingival health. Cochrane Database Syst Rev. 2010;2010:CD004971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grender J, Adam R, Zou Y. The effects of oscillating‐rotating electric toothbrushes on plaque and gingival health: a meta‐analysis. Am J Dent. 2020;33:3‐11. [PubMed] [Google Scholar]
- 5. Wang P, Xu Y, Zhang J, et al. Comparison of the effectiveness between power toothbrushes and manual toothbrushes for oral health: a systematic review and meta‐analysis. Acta Odontol Scand. 2020;78:265‐274. [DOI] [PubMed] [Google Scholar]
- 6. Clark‐Perry D, Levin L. Systematic review and meta‐analysis of randomized controlled studies comparing oscillating‐rotating and other powered toothbrushes. J Am Dent Assoc. 2020;151:265‐275. [DOI] [PubMed] [Google Scholar]
- 7. Van der Sluijs E, Slot DE, Hennequin‐Hoenderdos NL, Valkenburg C, van der Weijden F. Dental plaque score reduction with an oscillating‐rotating power toothbrush and a high‐frequency sonic power toothbrush: a systematic review and meta‐analysis of single‐brushing exercises. Int J Dent Hyg. 2021;19:78‐92. doi: 10.1111/idh.12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins JPT, Thomas J, Chandler J, et al. (eds). Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane, 2019. Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training. Accessed July 27, 2021.
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006‐1012. [DOI] [PubMed] [Google Scholar]
- 10. PRISMA Statement . Preferred reporting items for systematic reviews and meta‐analysis. PRISMA. Accessed July 27, 2021. prisma‐statement.org
- 11. AMSTAR . A measurement tool to assess systematic reviews. AMSTAR – Assessing the Methodological Quality of Systematic Reviews. Accessed July 27, 2021. https://amstar.ca/
- 12. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. PROSPERO . International prospective register of systematic reviews. 2014. Accessed July 27, 2021. https://www.crd.york.ac.uk/PROSPERO/
- 14. Ryan R, Cochrane Consumers and Communication Review Group . Cochrane consumers and communication review group: data synthesis and analysis. 2013. Accessed July 27, 2021. http://cccrg.cochr ane.org
- 15. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosema NAM, Slot DE, van Palenstein Helderman WH, Wiggelinkhuizen L, Van der Weijden GA. The efficacy of powered toothbrushes following a brushing exercise: a systematic review. Int J Dent Hyg. 2016;14:29‐41. [DOI] [PubMed] [Google Scholar]
- 17. Van der Weijden F, Dell'Acqua F, Slot DE. Alveolar bone dimensional changes of post‐extraction sockets in humans: a systematic review. J Clin Periodontol. 2009;36:1048‐1058. [DOI] [PubMed] [Google Scholar]
- 18. Keukenmeester RS, Slot DE, Rosema NA, Van der Weijden GA. Determination of a comfortable volume of mouthwash for rinsing. Int J Dent Hyg. 2012;10:169‐174. [DOI] [PubMed] [Google Scholar]
- 19. Light RJ, Smith PV. Accumulating evidence: procedures for resolving contradictions among research studies. Harv Educ Rev. 1971;41:429‐471. [Google Scholar]
- 20. Review Manager (RevMan) . Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 21. Quigley GA, Hein JW. Comparative cleansing efficiency of manual and power brushing. J Am Dent Assoc. 1962;65:26‐29. [DOI] [PubMed] [Google Scholar]
- 22. Turesky S, Gilmore ND, Glickman L. Reduced formation by chloromethyl analogue of vitamin C. J Periodontol. 1970;41:41‐43. [DOI] [PubMed] [Google Scholar]
- 23. Lobene RR, Soparker PM, Newman BS. Use of dental floss. Effect of plaque and gingivitis. Clin Prev Dent. 1982;4:5‐8. [PubMed] [Google Scholar]
- 24. Rustogi KN, Curtis JP, Volpe AR, Kemp JH, McCool JJ, Korn LR. Refinement of the modified navy plaque index to increase plaque scoring efficiency in gumline and interproximal tooth areas. J Clin Dent. 1992;3:C9‐C12. [PubMed] [Google Scholar]
- 25. Lange DE, Plagmann HC, Eenboom A, Promesberger A. Clinical methods for the objective evaluation of oral hygiene. Dtsch Zahnarztl Z. 1977;32:44‐47. [PubMed] [Google Scholar]
- 26. Van der Weijden GA, Timmerman MF, Saxton CA, Russell JI, Huntington E, Van der Velden U. Intra−/ inter‐examiner reproducibility study of gingival bleeding. J Periodontal Res. 1994;29:236‐241. [DOI] [PubMed] [Google Scholar]
- 27. Van der Weijden GA, Timmerman MF, Reijerse E, Nijboer A, Van der Velden U. Comparison of different approaches to assess bleeding on probing as indicators of gingivitis. J Clin Periodontol. 1994;21:589‐594. [DOI] [PubMed] [Google Scholar]
- 28. Lie MA, Timmerman MF, van der Velden U, van der Weijden GA. Evaluation of 2 methods to assess gingival bleeding in smokers and non‐smokers in natural and experimental gingivitis. J Clin Periodontol. 1998;25:695‐700. [DOI] [PubMed] [Google Scholar]
- 29. Saxton CA, van der Ouderaa FJ. The effect of a dentifrice containing zinc citrate and Triclosan on developing gingivitis. J Periodontal Res. 1989;24:75‐80. [DOI] [PubMed] [Google Scholar]
- 30. Lobene RR, Weatherford T, Ross NM, Lamm RA, Menaker L. A modified gingival index for use in clinical trials. Clin Prev Dent. 1986;8:3‐6. [PubMed] [Google Scholar]
- 31. Sälzer S, Slot DE, Van der Weijden FA, Dörfer CE. Efficacy of inter‐ dental mechanical plaque control in managing gingivitis–a meta‐ review. J Clin Periodontol. 2015;42:S92‐S105. [DOI] [PubMed] [Google Scholar]
- 32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994;13:1341‐1352. discussion 53–56. [DOI] [PubMed] [Google Scholar]
- 34. O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549‐556. [PubMed] [Google Scholar]
- 35. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. J Clin Epidemiol. 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
- 36. Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive – trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. Int J Epidemiol. 2009;38:287‐298. [DOI] [PubMed] [Google Scholar]
- 37. Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clin Epidemiol. 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thorlund K, Engstrøm J, Wetterslev J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). Copenhagen Trial Unit; 2011. Centre for Clinical Intervention Research. 2017: 1‐115. [Google Scholar]
- 39. Cohen J. A power primer. Psychol Bull. 1992;112:155‐159. [DOI] [PubMed] [Google Scholar]
- 40. Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Clinical significance consensus meeting G. methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77:371‐383. [DOI] [PubMed] [Google Scholar]
- 41. Lemieux J, Beaton DE, Hogg‐Johnson S, Bordeleau LJ, Goodwin PJ. Three methods for minimally important difference: no relationship was found with the net proportion of patients improving. J Clin Epidemiol. 2007;60:448‐455. [DOI] [PubMed] [Google Scholar]
- 42. Musselman KE. Clinical significance testing in rehabilitation research: what, why, and how? Phys Ther Rev. 2007;12:287‐296. [Google Scholar]
- 43. Armijo‐Olivo S, Warren S, Fuentes J, Magee DJ. Clinical relevance vs. statistical significance: using neck outcomes in patients with temporomandibular disorders as an example. Man Ther. 2011;16:563‐572. [DOI] [PubMed] [Google Scholar]
- 44. Ferracini GN, Chaves TC, Dach F, Bevilaqua‐Grossi D, Fernández‐de‐Las‐Peñas C, Speciali JG. Analysis of the cranio‐cervical curvatures in subjects with migraine with and without neck pain. Physiotherapy. 2017;103:392‐399. [DOI] [PubMed] [Google Scholar]
- 45. Guyatt GH, Oxman AD, Kunz R, et al. Incorporating considerations of resources use into grading recommendations. BMJ. 2008;336:1170‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. GRADE . Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group. 2016. Accessed July 27, 2021. http://www.gradeworkinggroup.org/
- 47. Grossman E, Dembling W, Proskin HM. A comparative clinical investigation of the safety and efficacy of an oscillating/rotating electric toothbrush and a sonic toothbrush. J Clin Dent. 1995;6:108‐112. [PubMed] [Google Scholar]
- 48. Van der Weijden GA, Timmerman MF, Reijerse E, Snoek CM, Van der Velden U. Comparison of an oscillating/rotating electric toothbrush and a 'sonic' toothbrush in plaque‐removing ability. A professional toothbrushing and supervised brushing study. J Clin Periodontol. 1996;23:407‐411. [DOI] [PubMed] [Google Scholar]
- 49. Robinson PJ, Maddalozzo D, Breslin S. A six‐month clinical comparison of the efficacy of the Sonicare and the Braun Oral‐B electric toothbrushes on improving periodontal health in adult periodontitis patients. J Clin Dent. 1997;8:4‐9. [PubMed] [Google Scholar]
- 50. Yankell SL, Emling RC. A thirty‐day safety and efficacy evaluation of the Rowenta, Braun and Sonicare powered toothbrushes and a manual toothbrush. J Clin Dent. 1997;8:120‐123. [PubMed] [Google Scholar]
- 51. Isaacs RL, Beiswanger BB, Rosenfield ST, et al. A crossover clinical investigation of the safety and efficacy of a new oscillating/rotating electric toothbrush and a high‐frequency electric toothbrush. Am J Dent. 1998;11:7‐12. [PubMed] [Google Scholar]
- 52. Hefti AF, Stone C. Power toothbrushes, gender, and dentin hypersensitivity. Clin Oral Investig. 2000;4:91‐97. [DOI] [PubMed] [Google Scholar]
- 53. Putt MS, Milleman JL, Davidson KR, Kleber CJ, Cugini M. A split‐mouth comparison of a three‐dimensional‐action electric toothbrush and a high‐frequency electric toothbrush for reducing plaque and gingivitis. J Int Acad Periodontol. 2001;3(4):95‐103. [PubMed] [Google Scholar]
- 54. van der Weijden GA, Timmerman MF, Piscaer M, Ijzerman Y, van der Velden U. A clinical comparison of three powered toothbrushes. J Clin Periodontol. 2002;29:1042‐1047. [DOI] [PubMed] [Google Scholar]
- 55. Goyal CR, Sharma NC, Qaqish JG, Cugini MA, Thompson MC, Warren PR. Efficacy of a novel brush head in the comparison of two power toothbrushes on removal of plaque and naturally occurring extrinsic stain. J Dent. 2005;33:37‐43. [PubMed] [Google Scholar]
- 56. Patters MR, Bland PS, Shiloah J, Blankenship JA, Scarbecz M. Comparison of the Hydrabrush powered toothbrush with two commercially‐available powered toothbrushes. J Int Acad Periodontol. 2005;7:80‐89. [PubMed] [Google Scholar]
- 57. Rosema NA, Timmerman MF, Piscaer M, et al. An oscillating/pulsating electric toothbrush versus a high‐frequency electric toothbrush in the treatment of gingivitis. J Dent. 2005;33:29‐36. [DOI] [PubMed] [Google Scholar]
- 58. Sharma NC, Goyal CR, Qaqish JG, Cugini MA, Thompson MC, Warren PR. Single‐use plaque removal efficacy of three power toothbrushes. J Dent. 2005;33:11‐15. [PubMed] [Google Scholar]
- 59. Goyal CR, Qaqish J, He T, Grender J, Walters P, Biesbrock AR. A randomized 12‐week study to compare the gingivitis and plaque reduction benefits of a rotation‐oscillation power toothbrush and a sonic power toothbrush. J Clin Dent. 2009;20:93‐98. [PubMed] [Google Scholar]
- 60. Williams K, Rapley K, Haun J, et al. Comparison of rotation/oscillation and sonic power toothbrushes on plaque and gingivitis for 10 weeks. Am J Dent. 2009;22:345‐349. [PubMed] [Google Scholar]
- 61. Ayad F, Petrone DM, Wachs GN, Mateo LR, Chaknis P, Panagakos F. Comparative efficacy of a specially engineered sonic powered toothbrush with unique sensing and control technologies to two commercially available power toothbrushes on established plaque and gingivitis. J Clin Dent. 2012;23 Spec No A:A5‐A10. [PubMed] [Google Scholar]
- 62. Klukowska M, Grender JM, Goyal CR, Mandl C, Biesbrock AR. 12‐week clinical evaluation of a rotation/oscillation power toothbrush versus a new sonic power toothbrush in reducing gingivitis and plaque. Am J Dent. 2012;25:287‐292. [PubMed] [Google Scholar]
- 63. Klukowska M, Grender JM, Conde E, Goyal CR. A 12‐week clinical comparison of an oscillating‐rotating power brush versus a marketed sonic brush with self‐adjusting technology in reducing plaque and gingivitis. J Clin Dent. 2013;24:55‐61. [PubMed] [Google Scholar]
- 64. Ricci M, Marchisio O, Genovesi AM, et al. Comparison between oscillating‐twisting rotating of brush head vs a characteristic sweeping bristles motion on reducing oral inflammation. Minerva Stomatol. 2014;62: 27‐31. [PubMed] [Google Scholar]
- 65. Buchel B, Reise M, Klukowska M, et al. A 4‐week clinical comparison of an oscillating‐rotating power brush versus a marketed sonic brush in reducing dental plaque. Am J Dent. 2014;27:56‐60. [PubMed] [Google Scholar]
- 66. Klukowska M, Grender JM, Conde E, Ccahuana‐Vasquez RA, Goyal CR. A randomized 12‐week clinical comparison of an oscillating‐rotating toothbrush to a new sonic brush in the reduction of gingivitis and plaque. J Clin Dent. 2014;25:26‐31. [PubMed] [Google Scholar]
- 67. Klukowska M, Grender JM, Conde E, Ccahuana‐Vasquez RA, Ram GC. A randomized clinical trial evaluating gingivitis and plaque reduction of an oscillating‐rotating power brush with a new brush head with angled bristles versus a marketed sonic brush with self‐adjusting technology. Am J Dent. 2014;27:179‐184. [PubMed] [Google Scholar]
- 68. Klukowska M, Grender JM, Conde E, Goyal CR, Qaqish J. A six‐week clinical evaluation of the plaque and gingivitis efficacy of an oscillating‐rotating power toothbrush with a novel brush head utilizing angled CrissCross bristles versus a sonic toothbrush. J Clin Dent. 2014;25:6‐12. [PubMed] [Google Scholar]
- 69. Ccahuana‐Vasquez RA, Conde E, Grender JM, Cunningham P, Qaqish J, Goyal CR. An eight‐week clinical evaluation of an oscillating‐rotating power toothbrush with a brush head utilizing angled bristles compared with a sonic toothbrush in the reduction of gingivitis and plaque. J Clin Dent. 2015;26:80‐85. [PubMed] [Google Scholar]
- 70. Schmickler J, Wurbs S, Wurbs S, et al. The influence of the utilization time of brush heads from different types of power toothbrushes on oral hygiene assessed over a 6‐month observation period: a randomized clinical trial. Am J Dent. 2016;29:307‐314. [PubMed] [Google Scholar]
- 71. Schmalz G, Mller M, Schmickler J, et al. Influence of manual and power toothbrushes on clinical and microbiological findings in initial treatment of periodontitis ‐ a randomized clinical study. Am J Dent. 2017;30:40‐46. [PubMed] [Google Scholar]
- 72. Starke M, Delaurenti M, Ward M, Souza S, Milleman KR, Milleman JL. A comparison of the effect of two power toothbrushes on the gingival health and plaque status of subjects with moderate gingivitis. J Clin Dent. 2017;28:A29‐A35. [PubMed] [Google Scholar]
- 73. Schmalz G, Kiehl K, Schmickler J, et al. No difference between manual and different power toothbrushes with and without specific instructions in young, oral healthy adults‐results of a randomized clinical trial. Clin Oral Investig. 2018;22:1147‐1155. [DOI] [PubMed] [Google Scholar]
- 74. Ccahuana‐Vasquez RA, Conde EL, Cunningham P, Grender JM, Goyal CR, Qaqish J. An 8‐week clinical comparison of an oscillating‐rotating electric rechargeable toothbrush and a sonic toothbrush in the reduction of gingivitis and plaque. J Clin Dent. 2018;29:27‐32. [PubMed] [Google Scholar]
- 75. Lv J, Guo B, Ling J. A 6‐month clinical evaluation of a high frequency sonic toothbrush in comparison with an oscillating‐rotating power toothbrush and a traditional sonic toothbrush in reducing gingivitis and plaque. Am J Dent. 2018;31:171‐176. [PubMed] [Google Scholar]
- 76. Mirza F, Argosino K, Ward M, Ou SS, Milleman KR, Milleman JL. A comparison of the effect of two power toothbrushes on the reduction of gingival inflammation and Supragingival plaque. J Clin Dent. 2019;30:A9‐A15. [PubMed] [Google Scholar]
- 77. Adam R, Ram Goyal C, Qaqish J, Grender J. Evaluation of an oscillating‐rotating toothbrush with micro‐vibrations versus a sonic toothbrush for the reduction of plaque and gingivitis: results from a randomized controlled trial. Int Dent J. 2020;70:S16‐S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Goyal CR, Adam R, Timm H, Grender J, Qaqish J. A 6‐month randomized controlled trial evaluating a novel smart‐connected oscillating‐rotating toothbrush versus a smart‐connected sonic toothbrush for the reduction of plaque and gingivitis. Am J Dent. 2021;34:54‐60. [PubMed] [Google Scholar]
- 79. Smiley CJ, Tracy SL, Abt E, et al. Evidence‐based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 2015;146:525‐535. [DOI] [PubMed] [Google Scholar]
- 80. Warren PR, Cugini MA, Chater BV, Strate J. A review of the clinical efficacy of the Oral‐B oscillating/rotating power toothbrush and the Philips Sonicare toothbrush in normal subject populations. Int Dent J. 2004;54:429‐437. [DOI] [PubMed] [Google Scholar]
- 81. Hossainian N, Slot DE, Afennich F, Van der Weijden GA. The effects of hydrogen peroxide mouthwashes on the prevention of plaque and gingival inflammation: a systematic review. Int J Dent Hyg. 2011;9:171‐181. [DOI] [PubMed] [Google Scholar]
- 82. Van Leeuwen MP, Slot DE, Van der Weijden GA. Essential oils compared to chlorhexidine with respect to plaque and parameters of gingival inflammation: a systematic review. J Periodontol. 2011;82:174‐194. [DOI] [PubMed] [Google Scholar]
- 83. ADA . Acceptance Program Product Requirements: Toothbrushes: American Dental Association; 2016. Accessed on July 27, 2021 https://www.ada.org/en [Google Scholar]
- 84. Yaacob M, Worthington HV, Deacon SA, et al. Powered versus manual toothbrushing for oral health. Cochrane Database Syst Rev. 2014;2014:CD002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. de Jager M, Rmaile A, Darch O, Bikker JW. The effectiveness of manual versus high‐frequency, high‐amplitude sonic powered toothbrushes for Oral health: a meta‐analysis. J Clin Dent. 2017;28:A13‐A28. [PubMed] [Google Scholar]
- 86. CONSORT . Accessed on July 27, 2021. http://www.consort‐statement.org/about‐consort/history
- 87. The International Committee on Medical Journal Editor . Accessed July 27, 2021. http://www.icmje.org/recommendations/browse/roles‐and‐responsibilities/author‐responsibilities‐‐conflicts‐of‐interest.html
- 88. Beyari MM, Hak A, Li CS, Lamfon HA. Conflict of interest reporting in dentistry randomized controlled trials: a systematic review. J Evid Based Dent Pract. 2014;14:158‐164. [DOI] [PubMed] [Google Scholar]
- 89. Martins CC, Riva JJ, Firmino RT, et al. Conflict of interest is not associated with positive conclusions in toothpaste trials: a systematic survey. J Clin Epidemiol. 2019;108:141‐143. [DOI] [PubMed] [Google Scholar]
- 90. Markowitz K, Roberts E, Strickland M. Dental products and evidence‐based dentistry. Quintessence Int. 2019;50:402‐411. [DOI] [PubMed] [Google Scholar]
- 91. Van der Weijden GA, Hioe KP. A systematic review of the effectiveness of self‐performed mechanical plaque removal in adults with gingivitis using a manual toothbrush. J Clin Periodontol. 2005;32:214‐228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that supports the findings of this study are available in the Supplementary Material of this article.


