Abstract
Termites have physiological and behavioral immunities that make them highly resistant to pathogen infections, which complicates biocontrol efforts. However, the stimuli that trigger the pathogen‐avoidance behaviors of termites are still unclear. Our study shows that workers of Coptotermes formosanus exposed to the conidia of Metarhizium anisopliae exhibited a significantly higher frequency and longer duration of allogrooming behaviors compared with untreated termites. Volatile compounds in the cuticle of control termites and termites previously exposed to a suspension of M. anisopliae conidia were analyzed and compared using a gas chromatography‐mass spectrometer (GC‐MS). Our results showed that the amount of ergosterol differed between the fungus‐exposed and control termites. Choice tests showed that termites significantly preferred to stay on filter paper treated with ergosterol (0.05, 0.1, or 1.0 mg/mL) compared with control filter paper. In addition, termites exposed to ergosterol followed by M. anisopliae conidia were allogroomed at a significantly higher frequency and for a longer duration than termites exposed to alcohol (the solvent used with the ergosterol in the ergosterol trials) alone followed by M. anisopliae conidia. These results showed that ergosterol may enhance the allogrooming behavior of termites in the presence of entomopathogenic fungi.
Keywords: allogrooming behavior, entomopathogenic fungus, ergosterol, recognition, social immunity, termite
Ergosterol is a significantly different volatile matter in the entomopathogenic fungus‐exposed termites. Ergosterol attracts termites and enhances the allogrooming behavior of termites in the presence of entomopathogenic fungi.
Introduction
The Formosan subterranean termite, Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae) is one of the most destructive pests of wooden structures and living plants worldwide (Lax & Osbrink, 2003; Ahmad et al., 2021; Blumenfeld et al., 2021). These termites are often controlled using entomopathogenic fungi as alternative agents to chemical controls; however, these agents have not always been as successful as the chemical methods (Chouvenc et al., 2011). This is because of a series of social immunity mechanisms evolved by the termites that promote pathogen resistance via a number of behaviors, including chemical communication, hygienic behavior, and physiological immune responses (Liu et al., 2020, 2019a; Zhao et al., 2020). With hygienic behaviors, the pathogens can be directly eliminated from individuals’ cuticles by allogrooming, thereby improving the disease resistance of the entire termite colony (Shimizu & Yamaji, 2003; Yanagawa & Shimizu, 2007; Liu et al., 2019b). Additionally, the allogrooming contact causes the pathogens to quickly spread throughout the nest; as a result, termite individuals obtain only a few fungal conidia, triggering mild infections and an increase in immune gene expression and enhancement of antifungal activity (Konrad et al., 2012; Liu et al., 2015). Therefore, identifying the cues that stimulate the termites to allogroom fungi‐infected termites may assist in developing a reliable method of biocontrol.
Worker termites are blind, sensing nest‐mates and the environment through chemical communication (Watson & Gay, 1991; Funaro et al., 2018). Recent studies suggest that termites can distinguish fungi and respond sensitively to the musty odors of many species of entomopathogenic fungi (Yanagawa et al., 2009, 2010). These studies have identified certain components of the fungal odor, such as 1‐octen‐3‐ol, 3‐octanone, 3‐methyl‐1‐butanol, and 3‐octanol (Yanagawa et al., 2012, 2011). A study by Yanagawa et al. (2012) confirmed the termites demonstrate a significant aversion to the odor of 3‐octanone and indifference to the odors of 1‐octen‐3‐ol and 3‐methyl‐1‐butanol. Although the termites showed inconsistent aversion to the odor of the fungi, they groomed the termites infected with fungal conidia (Yanagawa et al., 2012, 2015). However, recent studies have all focused exclusively on the fungi rather than the infected termites; therefore, identification of the odor components that trigger the termites to allogroom entomopathogenic fungi‐infected termites may be incomplete.
The entomopathogenic fungus Metarhizium anisopliae is a widely used agent for controlling biological pests and an ideal experimental fungus for studying immunity in social insects (Liu et al., 2015; Konrad et al., 2018; Zhou et al., 2021). Here, the workers of C. formosanus and the fungal conidia of M. anisopliae were used as a host‐pathogen system to identify the chemical components that can trigger the termites’ allogrooming by focusing directly on the infected termites. First, the cuticle components of termites that had either been exposed or unexposed to M. anisopliae conidia were measured by gas chromatograph‐mass spectrometry (GC‐MS), and second, the effect of the chosen components was assessed based on the result of the GC‐MS.
Materials and methods
Termite collection and rearing
In this study, we used colonies of C. formosanus collected using a lure collection device (a plastic basket with pine blocks) in Longdong Forest Park, Guangzhou, China. These colonies were fed with wet pinewood and maintained in a greenhouse (26 ± 1 °C, 70%–80% relative humidity) under total darkness.
Preparation of the fungal conidial suspensions
The entomopathogenic fungus, M. anisopliae (strain no.: IBCCM321.93), was purchased from the Guangdong Microbial Culture Collection Center, Guangzhou, China. The fungus was cultivated on potato dextrose agar (PDA, HuanKai Microbial, Guangzhou, China) at 25 ± 1 °C for approximately 10 d. The conidia were harvested by washing each plate with sterile 0.1% Tween 80 solution and stored at 4 °C for a maximum of 10 d before using. The number of conidia was counted using a Thoma hemocytometer (Sail Brand, Yancheng, China) and diluted to the required concentration (0, 1 × 105, 5 × 105, 1 × 106, 5 × 106, and 1 × 108 conidia/mL) using 0.1% Tween 80.
Termite susceptibility to different concentrations of M. anisopliae
To select 2 significantly different concentrations of M. anisopliae conidial suspensions, susceptibility of the termites to different concentrations of the fungus was tested. Twenty‐eight workers were each placed separately into a 2.0‐mL microcentrifuge tube containing a conidial suspension (500 μL) and submerged in the suspension for 10 s; these termites were then transferred onto filter paper and dried for 60 s to absorb excess fluid (Bulmer et al., 2019). The bioassay arena was a 24‐well plate (Corning‐Costar, Beijing, China; diameter = 15.6 mm) with a piece of small circular filter paper (diameter = 15.6 mm) moistened with 20 μL of water on the bottom of each well. A worker was put in each well, and a total of 28 workers were used for each concentration of conidial suspension. A total of 504 wells (28 workers × 6 concentrations × 3 colonies) from 21 24‐well plates were used. Termite deaths were recorded daily for 10 d. The sensitivity of the termites to the concentration of M. anisopliae was assessed in median lethal time (LT50). Three colonies (28 termites for each colony) were used.
Termites allogrooming behavior toward M. anisopliae‐exposed termites
The allogrooming behavior of the termites responding to 2 concentrations (1 × 106 or 1 × 108 conidia/mL, selected from the above experiment) of M. anisopliae were evaluated. The 0.1% Tween 80 solution was used as the control. Eight workers from each colony were treated with 150 μL of M. anisopliae conidial suspension or a control suspension in a 2.0‐mL microcentrifuge tube. The method of treating termites was as outlined above. Sixteen workers from 2 colonies were used for each concentration. Each worker was then placed in a single well of a 24‐well plate containing filter paper for 1 h of exposure. After exposure, 1 worker termite previously exposed to M. anisopliae conidia was brushed using green pigment on the dorsal surface of the abdomen and placed with 1 control worker (previously exposed to a control suspension) that had been brushed with red pigment, 1 blank worker (without any treatment), and 1 blank soldier (without any treatment). These termites were placed on a 5.5‐cm diameter Petri dish with a piece of wet filter paper (diameter = 5.5 cm) and allowed to acclimatize for 15 min (Yanagawa et al., 2010). The behaviors of the termites were recorded for 30 min using a video camera. When the body surface of the worker was touched by the mouthpart of another worker, this was recorded as 1 allogrooming event (Liu et al., 2019b). Allogrooming events were counted by recording allogrooming frequency and total duration (the sum of each allogrooming event) of each worker. The experiment was repeated 16 times (8 times × 2 colonies).
Identification of volatiles in the M. anisopliae‐exposed termites
To identify the chemical volatiles on the surface of M. anisopliae conidia‐treated termites, N‐hexane was used to extract the volatiles directly. Our preliminary experiment showed that it was difficult to detect the difference between samples with a small number of termites (n < 100). Therefore, the number of treated termites was increased to 300. Sixty workers from each colony were treated with 1 mL of 1 × 108 conidia/mL M. anisopliae conidial suspension or 0.1% Tween 80 in a 2.0‐mL microcentrifuge tube. The method of treating termites was as outlined above, and a total of 300 workers from each colony was used for each treatment. Then, each worker was placed in a single well of a 24‐well plate containing filter paper for 1 h of exposure. A total of 1 800 wells (300 workers × 3 treatments × 2 colonies) from 75 24‐well plates was used. After exposure, a total of 300 workers that had been exposed to M. anisopliae conidia was considered as the treatment group, 300 termites treated with 0.1% Tween 80 as the control group, and 300 untreated termites as the blank group. Then, 300 termites from each group were put into a 4‐mL sample injection bottle and 1.2 mL of N‐hexane were added to extract the volatiles. After 10 min, 200 μL of supernatant were drawn up into a 2‐mL sample injection bottle and blow‐dried using nitrogen gas, followed by the addition of 100 μL of N‐hexane to resolubilize the sample. Fifty microliters of resolubilized sample were drawn up into a glass‐lined pipe in a 2‐mL sample injection bottle, and the chemical volatiles on the surface of the treated termites were identified using GC‐MS (Agilent 7890B‐5977B, Wilmington, USA). The column oven was programmed to hold at 45 °C for 3 min, followed by an increase of 4 °C/min to 200 °C and hold for 3 min, then 6 °C/min to 300 °C, finally holding for 2 min at 300 °C. Each group was repeated for 2 colonies.
Termite preference for ergosterol
The ergosterol reference substance (Macklin, Shanghai, China) was dissolved in pure alcohol, and 5 concentrations (0, 0.01, 0.05, 0.1, and 1.0 mg/mL) of ergosterol were obtained. Two pieces of filter paper (diameter = 3.5 cm) were symmetrically pasted on the 2 sides of a Petri dish (diameter = 9.0 cm) with double‐sided tape. One filter paper was soaked with 40 μL of alcohol, and the other one was soaked with 40 μL of ergosterol (0, 0.01, 0.05, 0.1, or 1.0 mg/mL). After 5 min, 30 workers were placed into the center of each Petri dish. Photographs were taken every 30 s for a 15‐min period, and the numbers of termites that stayed on each filter paper were counted. The experiment for each concentration of ergosterol was repeated 16 times (8 times × 2 colonies).
Termite allogrooming behavior in response to ergosterol
This experiment was used to verify whether ergosterol enhanced termite allogrooming in response to M. anisopliae. Because alcohol can affect the activity of termites and M. anisopliae, we used a 2‐step spray method to treat termites. Workers were randomly divided into 4 groups (Table 1), and (1) exposed to ergosterol followed by M. anisopliae conidial suspensions; 16 termites from each colony were placed into a 5.5‐cm diameter Petri dish padded with a piece of wet filter paper and sprayed with 300 μL of ergosterol at a concentration of 0.1 mg/mL using a plastic sprinkling can. Then, each termite was transferred to 1 well in the 24‐well plate for 30 min of exposure. Subsequently, all termites were transferred to the same Petri dish sprayed with 300 μL of 1×108 conidia/ml M. anisopliae conidial suspension, and then each termite was transferred to 1 well in a 24‐well plate for 30 min of exposure. Finally, we selected the most active 8 termites of each group of 16 termites, and brushed them with green pigment for subsequent experiments. (2) The remaining termites were exposed to alcohol followed by M. anisopliae conidial suspensions: the method was similar to that outlined above, but the termites were placed on the Petri dish sprayed with alcohol first. All termites were brushed with black pigment, and (3) exposed to alcohol followed by 0.1% Tween 80: a similar method to that outlined above was used, but the termites were placed on the Petri dish sprayed with alcohol first, and then placed on the Petri dish sprayed with 0.1% Tween 80. All termites were brushed with red pigment. (4) Four untreated termites from the 4 treatment groups were placed on a 5.5‐cm diameter Petri dish with a piece of wet filter paper (diameter = 5.5 cm) and allowed to acclimatize for 15 min. The allogrooming behavior (total frequency and total duration of each allogrooming event) of each termite was recorded for 30 min using a video camera. This experiment was repeated 16 times (8 times × 2 colonies).
Table 1.
Experimental treatments of allogrooming behavior of termites Coptotermes formosanus in response to ergosterol
| Method | ||||||
|---|---|---|---|---|---|---|
| Treatment | Abbreviation | First spraying | Exposing time | Second spraying | Exposing time | Marked color |
| Ergosterol + Metarhizium anisopliae conidia | E + M | 300 μL 0.1 mg/mL ergosterol | Each termite 1 well in the 24‐well plate for 30 min | 300 μL 1 × 108 conidia/mL M. anisopliae | Each termite 1 well in the 24‐well plate for 30 min | Green |
| Alcohol + M. anisopliae conidia | A + M | 300 μL alcohol | Same as above | 300 μL 1 × 108 conidia/mL M. anisopliae | Same as above | Black |
| Alcohol + 0.1% Tween 80 | A + T | 300 μL alcohol | Same as above | 300 μL 0.1% Tween 80 | Same as above | Red |
| The untreated termites | CK | Untreated | Same as above | Untreated | Same as above | Unmarked |
Data analysis
The total frequency of allogrooming behavior in the behavioral experiments was analyzed using a generalized linear model (Poisson error structure, log link function). The termite mortality data were binary; therefore, a generalized linear model (binomial error structure, log link function) was used to examine termite mortality in the susceptibility experiment. Additionally, the time of 50% termite death (LT50) was used for predicting the doses for the binomial assay model and analyzed using the R package MASS (Venables & Ripley, 2002). A one‐way analysis of variance (ANOVA) was used to examine the total duration of allogrooming. To determine the preference of the termites for the piece of paper containing ergosterol or the control in each Petri dish, the data were divided into 2 treatment groups. One group comprised the ergosterol concentrations (0, 0.01, 0.05, 0.1, and 1.0 mg/mL), while the other group comprised ergosterol and alcohol (alcohol and alcohol + ergosterol). The data on preferences of the termites (Poisson error structure, log link function) were analyzed using a generalized linear mixed‐effects model (GLMM), and the random intercepts constituted the recording of the time‐by‐Petri dish repeat interaction. The R package lme4 was used to construct the GLMM (Bates et al., 2015). A visual inspection of the residual plots did not reveal any obvious deviations from homoscedasticity or normality. When a significant effect was detected, the pairwise contrasts between the treatments were made using the Tukey post hoc comparison procedure with the emmeans R package (Lenth, 2018). All the data analyses were conducted using R 4.0.2 statistical software (R Development Core Team, 2020), and the figures were created using the R package ggplot2 (Wickham, 2016).
Results
Termite mortality at different M. anisopliae concentrations
At all 6 concentrations of M. anisopliae conidial suspensions, termite mortality increased as the postexposure time increased (χ 2 = 48.85, P < 0.001; χ 2 = 85.07, P < 0.001; χ 2 = 266.85, P < 0.001; χ 2 = 413.32, P < 0.001; χ 2 = 587.95, P < 0.001; χ 2 = 247.09, P < 0.001 for 0, 1 × 105, 5 × 105, 1 × 106, 5 × 106, and 1 × 108 conidia/mL, respectively). As the concentration increased, the time of termite death gradually advanced (LT50: 0 > 1 × 105 > 5 × 105 > 1 × 106 > 5 × 106 > 1 × 108 conidia/mL). Considering the difference in the termites’ LT50, the concentrations of 1 × 108 conidia/mL (LT50 = 1.01 ± 1.00 d, mean ± standard error) and 1 × 106 conidia/mL (LT50 = 4.28 ± 0.14 d) were selected as 2 different susceptibility concentrations for subsequent experiments; while the concentration of 0 conidia/mL (LT50 = 13.34 ± 1.05 d) was used as the control concentration (Fig. 1).
Fig. 1.
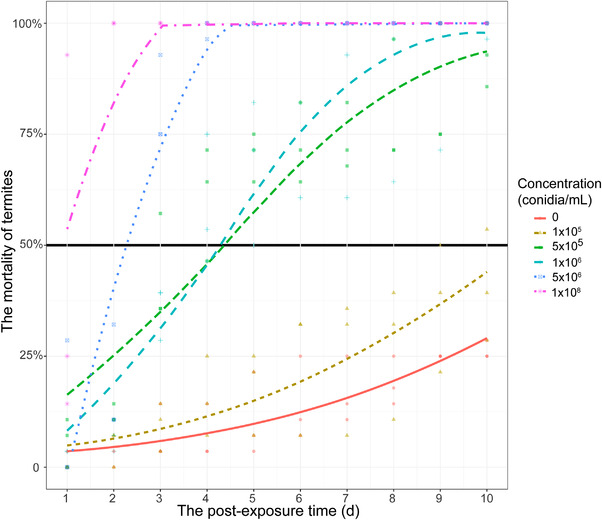
Termite (Coptotermes formosanus) mortality at different concentrations of Metarhizium anisopliae. The day of intersection of each colored line and the black line represents the LT50 of termites in each treatment.
Termite allogrooming behavior toward M. anisopliae‐exposed termites
Frequencies of worker allogrooming behavior were significantly affected by fungal concentration (χ 2 = 28.07, P < 0.001), worker treatment (χ 2 = 428.28, P < 0.001), and their interaction (χ 2 = 21.10, P < 0.001) (Fig. 2). Workers treated with 1 × 106 and 1 × 108 conidia/mL were allogroomed at a significantly higher frequency than workers treated with 0.1% Tween 80 (z = 3.24, P = 0.003; z = 8.43, P < 0.001, respectively) or untreated workers (z = 10.08, P < 0.001; z = 16.16, P < 0.001, respectively).
Fig. 2.
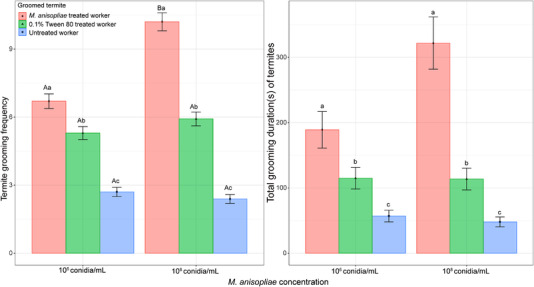
Frequency and duration (mean ± SE) of termite (Coptotermes formosanus) allogrooming behavior at 2 concentrations of Metarhizium anisopliae. Different capital letters indicate significant differences between the 2 concentrations in the same group of groomed termites. Different lowercase letters indicate significant differences among 3 groups of groomed termites at the same concentration (Tukey's honestly significant difference [HSD] test, P < 0.05).
The total grooming duration of workers was significantly affected by the workers’ treatment (F 2,299 = 51.88, P < 0.001), but not by the fungal concentration (F 1,299 = 1.12, P = 0.29) or their interaction (F 2,299 = 3.01, P < 0.05) (Fig. 2). The workers exposed to M. anisopliae conidia allogroomed for longer than workers treated with 0.1% Tween 80 (t = 5.27, P < 0.001), or untreated workers (t = 10.16, P < 0.001).
Identification of the volatiles of M. anisopliae‐exposed termites
There was a significant difference in the composition of the volatiles in M. anisopliae‐treated termites at 51.04 min in both the colonies: this difference was attributed to ergosterol, which was identified using a mass spectrometry system (Fig. 3).
Fig. 3.
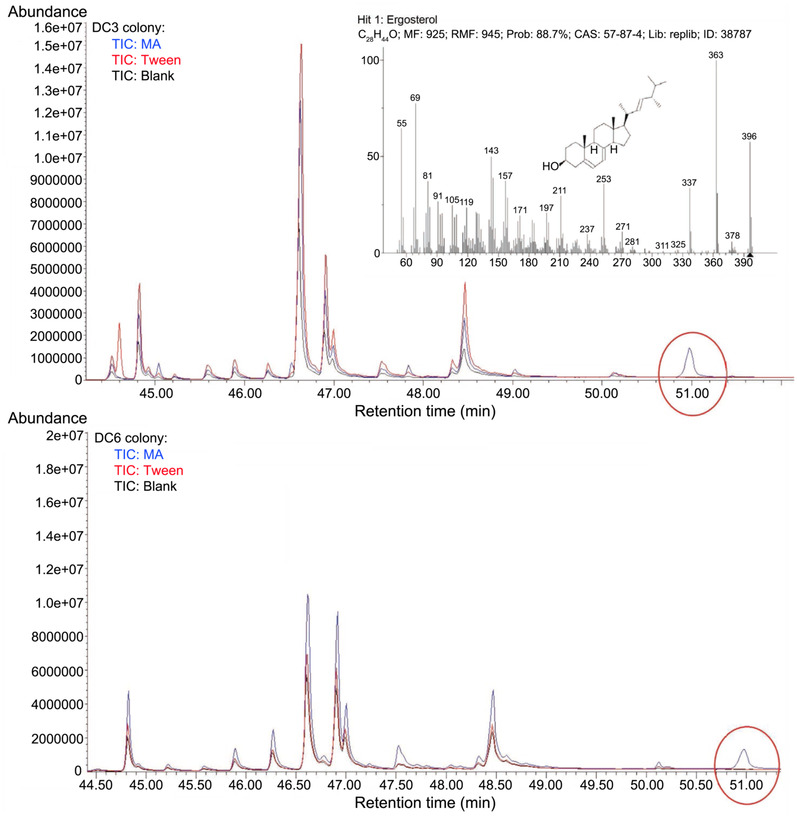
Gas chromatogram of volatiles from the Metarhizium anisopliae‐exposed termites (Coptotermes formosanus). MA: 1 × 108 conidia/mL M. anisopliae‐treated workers; Tween: 0.1% Tween 80‐treated workers; blank: untreated workers; the red circle shows the significantly different volatile (ergosterol).
Aggregation preference of the termites under different ergosterol treatments
Aggregation preference of the termites was significantly affected by the treatment (χ 2 = 268.40, P < 0.001), concentration (χ 2 = 744.97, P < 0.001), and their interaction (χ 2 = 314.94, P < 0.001) (Fig. 4). At a concentration of 0 and 0.1 mg/mL, there was no significant difference between the number of termites that stayed on the filter paper treated with alcohol or ergosterol (z = 0.32, P = 0.75; z = 1.88, P = 0.06, respectively). However, a significantly higher number of termites preferred filter paper treated with ergosterol at the concentration of 0.05 (z = 12.78, P < 0.001), 0.10 (z = 15.68, P < 0.001), or 1.0 mg/mL (z = 13.05, P < 0.001) than filter paper treated with alcohol.
Fig. 4.
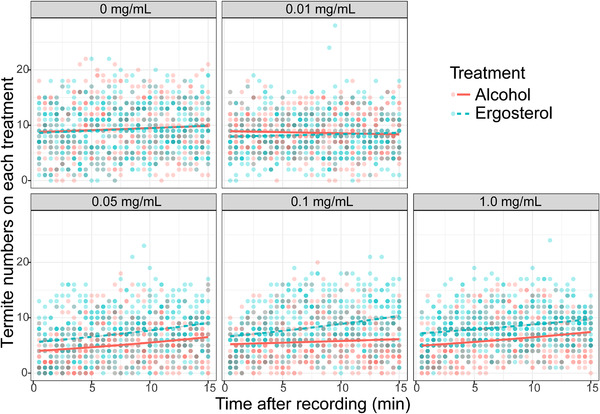
Aggregation preference of termites (Coptotermes formosanus) under different ergosterol treatments. Dots represent raw data; deeper colors reflect overlapping data.
Termite allogrooming behavior in response to ergosterol
The frequencies of termite allogrooming behavior were found to be significantly affected by the treatment (χ 2 = 51.67, P < 0.001) (Fig. 5). Termites treated with ergosterol followed by M. anisopliae conidia (E + M) were allogroomed at a significantly higher frequency than termites treated with alcohol followed by M. anisopliae conidia (A + M) (z = 3.05, P = 0.013), termites treated with alcohol followed by 0.1% Tween 80 (A + T) (z = 5.21, P < 0.001), or untreated termites (CK) (z = 5.51, P < 0.001). Termites treated with alcohol followed by M. anisopliae conidia (A + M) were allogroomed at a significantly higher frequency than termites treated with alcohol followed by 0.1% Tween 80 (A + T) (z = 2.41, P = 0.020), or untreated termites (CK) (z = 3.42, P = 0.004).
Fig. 5.
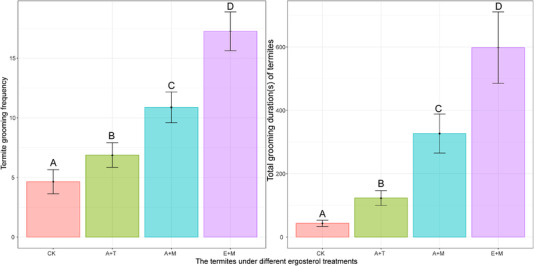
Frequency and duration (mean ± SE) of termite (Coptotermes formosanus) allogrooming behavior under different ergosterol treatments. CK: untreated worker; A + T: alcohol + 0.1% Tween 80 treated worker; A + M: alcohol + Metarhizium anisopliae conidia treated worker; E + M: ergosterol + M. anisopliae conidia treated worker. Different capital letters above the bars indicate significant differences between the treatment groups (Tukey's honestly significant difference [HSD] test, P < 0.05).
The total grooming duration of termites was significantly affected by the treatment (F 3, 55 = 30.87, P < 0.001) (Fig. 5). Termites treated with ergosterol followed by M. anisopliae conidia (E + M) were allogroomed for a significantly longer duration than termites treated with alcohol followed by M. anisopliae conidia (A + M) (t = 2.27, P = 0.027), termites treated with alcohol followed by 0.1% Tween 80 (A + T) (t = 5.93, P < 0.001), or untreated termites (CK) (z = 8.90, P < 0.001). Termites treated with alcohol followed by M. anisopliae conidia (A + M) were allogroomed for a significantly longer duration than termites treated with alcohol followed by 0.1% Tween 80 (A + T) (t = 3.66, P = 0.003), or untreated termites (CK) (t = 6.85, P < 0.001).
Discussion
Termites are attracted to ergosterol (Mauldin & Rich, 1975), and the present study indicates this attraction enhances social immunity of termites carrying M. anisopliae conidia. Ergosterol could therefore be a special component that induces the termites to allogroom entomopathogenic fungi‐exposed termites.
Sterols can perform various functions in several insect–fungus symbioses. The insects in these relationships are dependent on the sterols provided by the associated fungus (Maurer et al., 1992; Mondy & Corio‐Costet, 2000; Jing & Behmer, 2020). For instance, the fungus associated with the diet of the ambrosia beetle, Xyleborus ferrugineus, is required for oviposition, larval development, and pupation (Norris & Baker, 1967; French & Roeper, 1975). Some researchers have found that ergosterol can act as a pheromone; for example, female rock lizards (Iberolacerta cyreni) prefer the scent of males with more ergosterol, and male rock lizards may attract more females and mate more often by increasing the proportion of ergosterol in their scent when scent‐marking their territories (Lopez et al., 2006; Martin & Lopez, 2012). A study by Zhu et al. (2017) explored the metabolites secreted by the fungus beetle Xylographus bostrichoides, and stellasterol and ergosterol peroxide were found to act as pheromones or defensins in this species.
In termites, ergosterol has been identified as a feeding stimulant and has been patented as such for C. formosanus (Henderson et al., 1999), where it is incorporated into a bait matrix for controlling the subterranean termites (Rojas & Morales‐Ramos, 2001). In our study, the termites’ aggregation preference was evaluated in response to a series of ergosterol concentrations. The results showed concentrations of 0.05, 0.10, and 1.0 mg/mL of ergosterol to be attractive to the termites, which is similar to the results of a study by Cornelius (2003), where ergosterol was found to stimulate feeding at a concentration of 1 mg of ergosterol per gram of paper. Conversely, Cornelius et al. (2004) found that termites showed no preference for sawdust upon treatment with ergosterol, and the tunneling activity of the termites in the sawdust remained unaffected by ergosterol treatment. However, the response time (<1 h) may be too short for the termite to synthesize ergosterol (C28H44O). Of course, the presence of ergosterol precursors in termites cannot be ruled out. Nevertheless, in our research, we hypothesize that the most likely explanation is that the treated termites acquire this chemical by contacting the fungus. Hence, the termites would encounter ergosterol in virtually any soil type, and it is not surprising that the tunneling activity of termites was unaffected by adding ergosterol to the sawdust in Cornelius et al.’s (2004) study. Therefore, although termites can be significantly attracted by ergosterol, there must be additional reasons why ergosterol stimulates the termites.
In our research, the allogrooming frequency and duration for ergosterol + M. anisopliae conidia‐treated termites were higher, compared to alcohol + M. anisopliae conidia‐treated termites (Fig. 5). This indicates that the termites increased their allogrooming behavior by recognizing the ergosterol in the presence of M. anisopliae. Social insects such as termites, ants, and bees are reported to exhibit increased susceptibility to the pathogens surrounding them (Traniello et al., 2002; Ugelvig & Cremer, 2007; Hamilton et al., 2011; Pusceddu et al., 2019). In termites, some studies have indicated that entomopathogenic fungi have a notably lethal effect (Zoberi, 1995; Jones et al., 1996; Rath, 2000). The present study also indicated the termites were susceptible to a high concentration of M. anisopliae (Fig. 1). However, efficient control in termite colonies via the use of M. anisopliae has not been realized (Shimizu & Yamaji, 2003; Yanagawa et al., 2009; Chouvenc & Su, 2010; Liu et al., 2019a). Therefore, the effect of individual innate immunity in termites may be limited. Colony‐level disease defenses are important for resisting infection by pathogens and can quickly react to potential threats and benefit the fitness of the whole colony (Cremer et al., 2018, 2007; Rosengaus et al., 2011). Allogrooming behavior is an important component of colony‐level hygienic behavior and can remove external pathogens from the termites’ cuticles, thereby disinfecting them and inhibiting pathogen growth during the early stage of infection (Yanagawa & Shimizu, 2007; Hamilton & Bulmer, 2012; Konrad et al., 2018; Liu et al., 2019a). Recent studies have revealed that termites respond sensitively to the musty odors of many species of entomopathogenic fungi (Yanagawa et al., 2009, 2010). While one study found that termites avoid the fungal odors (Yanagawa et al., 2012), another study showed that the termites had an aversion toward the fungal odor, but could groom termites that had been exposed to the fungal conidia (Yanagawa et al., 2012, 2015). Contrary to the odor components of previous studies, we found that ergosterol, which is a component of the cell wall in most types of fungi, appeared to be a novel odor via which the termites could recognize various entomopathogenic fungi and allogroom fungi‐exposed termites. This serves to benefit the fitness of the whole colony by enabling the termites to recognize the ergosterol and increase their allogrooming behavior when individuals are infected by entomopathogenic fungi. Therefore, the results of this study indicate that ergosterol may serve as an important stimulant for the termites.
In conclusion, our study showed that termites can recognize M. anisopliae conidia‐exposed termites, and ergosterol can enhance the allogrooming behavior of termites in the presence of the entomopathogenic fungus. Ergosterol may therefore be the cue that stimulates the termites to allogroom infected termites.
Disclosure
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by the GDAS Special Project of Science and Technology Development (2020GDASYL‐20200103091), Guangdong Basic and Applied Basic Research Foundation (2020A1515110416), Science and Technology Planning Project of Guangzhou (202102021018), and Science and Technology Planning Key Project of Guangzhou (201904020002). The authors would like to thank all organizations that funded our research, and Hui Peng (Institute of Zoology, Guangdong Academy of Sciences), Rui Tang (Institute of Zoology, Guangdong Academy of Sciences), and Jiajia Zhao (Nanjing Agriculture University) for assisting in GC‐MS operation. The authors really appreciate the suggestions of editors and reviewers for this research.
References
- Ahmad, F. , Fouad, H. , Liang, S.Y. , Hu, Y. and Mo, J.C. (2021) Termites and Chinese agricultural system: applications and advances in integrated termite management and chemical control. Insect Science, 28, 2‒20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, D. , Maechler, M. , Bolker, B. and Walker, S. (2015) Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1‒48. [Google Scholar]
- Blumenfeld, A.J. , Eyer, P.A. , Husseneder, C. , Mo, J. , Johnson, L.N.L. , Wang, C. et al. (2021) Bridgehead effect and multiple introductions shape the global invasion history of a termite. Communications Biology, 4, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer, M.S. , Franco, B.A. and Fields, E.G. (2019) Subterranean termite social alarm and hygienic responses to fungal pathogens. Insects, 10, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouvenc, T. and Su, N.Y. (2010) Apparent synergy among defense mechanisms in subterranean termites (Rhinotermitidae) against epizootic events: limits and potential for biological control. Journal of Economic Entomology, 103, 1327‒1337. [DOI] [PubMed] [Google Scholar]
- Chouvenc, T. , Su, N.Y. and Grace, J.K. (2011) Fifty years of attempted biological control of termites – analysis of a failure. Biological Control, 59, 69‒82. [Google Scholar]
- Cornelius, M.L. , Bland, J.M. , Daigle, D.J. , Williams, K.S. , Lovisa, M.P. , Connick, W.J. et al. (2004) Effect of a lignin‐degrading fungus on feeding preferences of formosan subterranean termite (Isoptera: Rhinotermitidae) for different commercial lumber. Journal of Economic Entomology, 97, 1025‒1035. [DOI] [PubMed] [Google Scholar]
- Cornelius, M.L. (2003) Evaluation of semiochemicals as feeding stimulants for the formosan subterranean termite (Isoptera: Rhinotermidae). Sociobiology, 41, 583‒591. [Google Scholar]
- Cremer, S. , Pull, C.D. and Fürst, M.A. (2018) Social immunity: emergence and evolution of colony‐level disease protection. Annual Review of Entomology, 63, 105‒123. [DOI] [PubMed] [Google Scholar]
- Cremer, S. , Armitage, S.A. and Schmid‐Hempel, P. (2007) Social immunity. Current Biology, 17, R693–R702. [DOI] [PubMed] [Google Scholar]
- French, J. and Roeper, R.A. (1975) Studies on the biology of the ambrosia beetle Xyleborus dispar (F.) (Coleoptera: Scolytidae). Zeitschrift Für Angewandte Entomologie, 78, 241–247. [Google Scholar]
- Funaro, C.F. , Boroczky, K. , Vargo, E.L. and Schal, C. (2018) Identification of a queen and king recognition pheromone in the subterranean termite Reticulitermes flavipes . Proceedings of the National Academy of Sciences USA, 115, 3888‒3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, C. and Bulmer, M.S. (2012) Molecular antifungal defenses in subterranean termites: RNA interference reveals in vivo roles of termicins and GNBPs against a naturally encountered pathogen. Developmental and Comparative Immunology, 36, 372–377. [DOI] [PubMed] [Google Scholar]
- Hamilton, C. , Lejeune, B.T. and Rosengaus, R.B. (2011) Trophallaxis and prophylaxis: social immunity in the carpenter ant Camponotus pennsylvanicus . Biology Letters, 7, 89‒92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, G. , Chen, J. and Laine, R.A. (1999) Compositions and methods for detecting and killing termites. United States Patent US5874097.
- Jing, X. and Behmer, S.T. (2020) Insect sterol nutrition: physiological mechanisms, ecology, and applications. Annual Review of Entomology, 65, 251‒271. [DOI] [PubMed] [Google Scholar]
- Jones, W.E. , Grace, J.K. and Tamashiro, M. (1996) Virulence of seven isolates of Beauveria bassiana and Metarhizium anisopliae to Coptotermes formosanus (Isoptera: Rhinotermitidae). Environmental Entomology, 25, 481‒487. [Google Scholar]
- Konrad, M. , Vyleta, M.L. , Theis, F.J. , Stock, M. , Tragust, S. , Klatt, M. et al. (2012) Social transfer of pathogenic fungus promotes active immunisation in ant colonies. PLoS Biology, 10, e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad, M. , Pull, C.D. , Metzler, S. , Seif, K. , Naderlinger, E. , Grasse, A.V. et al. (2018) Ants avoid superinfections by performing risk‐adjusted sanitary care. Proceedings of the National Academy of Sciences USA, 115, 2782‒2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth, R . Emmeans, Estimated Marginal Means, Aka Least‐Squares Means. R Package Version 1.2.3. (2018) https://CRAN.R‐project.org/package=emmeans.
- Liu, L. , Li, G. , Sun, P. , Lei, C. and Huang, Q. (2015) Experimental verification and molecular basis of active immunization against fungal pathogens in termites. Scientific Reports, 5, 15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Wang, W. , Liu, Y.L. , Sun, P.D. , Lei, C.L. and Huang, Q.Y. (2019b) The influence of allogrooming behavior on individual innate immunity in the subterranean termite Reticulitermes chinensis (Isoptera: Rhinotermitidae). Journal of Insect Science, 19, 1‒6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Wang, C. , Zhao, X. , Guan, J. , Lei, C. and Huang, Q. (2020) Isocitrate dehydrogenase‐mediated metabolic disorders disrupt active immunization against fungal pathogens in eusocial termites. Journal of Pest Science, 93, 291‒301. [Google Scholar]
- Liu, L. , Zhao, X.Y. , Tang, Q.B. , Lei, C.L. and Huang, Q.Y. (2019a) The mechanisms of social immunity against fungal infections in eusocial insects. Toxins, 11, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, P. , Amo, L. and Martin, J. (2006) Reliable signaling by chemical cues of male traits and health state in male lizards, lacerta monticola. Journal of Chemical Ecology, 32, 473‒488. [DOI] [PubMed] [Google Scholar]
- Lax, A.R. and Osbrink, W.L. (2003) United states department of agriculture—agriculture research service research on targeted management of the formosan subterranean termite Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). Pest Management Science, 59, 788‒800. [DOI] [PubMed] [Google Scholar]
- Martin, J. and Lopez, P. (2012) Supplementation of male pheromone on rock substrates attracts female rock lizards to the territories of males: a field experiment. PLoS ONE, 7, e30108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauldin, J.K. and Rich, N.M. (1975) Rearing two subterranean termites, Reticulitermes flavipes and Coptotermes formosanus, on artificial diets. Annals of the Entomological Society of America, 68, 454‒456. [Google Scholar]
- Maurer, P. , Debieu, D., Leroux, P. , Malosse, C. and Riba, G. (1992) Sterols and symbiosis in the leaf‐cutting ant Acromyrmex octospinosus (Reich) (Hymenoptera, Formicidae: Attini). Archives of Insect Biochemistry and Physiology, 20, 13‒21. [Google Scholar]
- Mondy, N. and Corio‐Costet, M.F. (2000) The response of the grape berry moth (Lobesia botrana) to a dietary phytopathogenic fungus (Botrytis cinerea): the significance of fungus sterols. Journal of Insect Physiology, 46, 1557‒1564. [DOI] [PubMed] [Google Scholar]
- Norris, D.M. and Baker, J.K. (1967) Symbiosis: effects of a mutualistic fungus upon the growth and reproduction of Xyleborus ferrugineus . Science, 156, 1120‒1122. [DOI] [PubMed] [Google Scholar]
- Pusceddu, M. , Piluzza, G. , Theodorou, P. , Buffa, F. , Ruiu, L. , Bullitta, S. et al. (2019)Resin foraging dynamics in Varroa destructor‐infested hives: a case of medication of kin? Insect Science, 26, 297‒310. [DOI] [PubMed] [Google Scholar]
- R Core Team (2020) R, A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. http://www.r‐project.org/. [Google Scholar]
- Rath, A.C. (2000) The use of entomopathogenic fungi for control of termites. Biocontrol Science and Technology, 10, 563‒581. [Google Scholar]
- Rojas, M.G. and Morales‐Ramos, J.A. (2001) Bait matrix for delivery of chitin synthesis inhibitors to the Formosan subterranean termite (Isoptera: Rhinotermitidae). Journal Economic Entomology, 94, 506‒510. [DOI] [PubMed] [Google Scholar]
- Rosengaus, R.B. , Traniello, J.F.A. and Bulmer, M.S. (2011) Ecology, behavior and evolution of disease resistance in termites. In: Biology of Termites: A Modern Synthesis (eds. Bignell D.E., Roisin Y. & Lo N.), pp. 165‒191. Springer, Dordrecht, the Netherlands. [Google Scholar]
- Shimizu, S. and Yamaji, M. (2003) Effect of density of the termite, Reticulitermes speratus Kolbe (Isoptera: Rhinotermitidae), on the susceptibilities to Metarhizium anisopliae . Applied Entomology and Zoology, 38, 125‒130. [Google Scholar]
- Traniello, J.F.A. , Rosengaus, R.B. and Savoie, K. (2002) The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proceedings of the National Academy of Sciences USA, 99, 6838‒6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugelvig, L.V. and Cremer, S. (2007) Social prophylaxis: group interaction promotes collective immunity in ant colonies. Current Biology, 17, 1967‒1971. [DOI] [PubMed] [Google Scholar]
- Venables, W.N. and Ripley, B.D. (2002) Modern Applied Statistics with S. Springer New York. [Google Scholar]
- Watson, J.A.L. and Gay, F.J. (1991) Isoptera (Termites). In: The Insects of Australia, 2nd edn, Vol. 20. pp. 330‒347. Melbourne University Press, Carlton, Vic. [Google Scholar]
- Wickham, H. (2016) ggplot2, Elegant Graphics for Data Analysis. Springer‐Verlag, New York. https://ggplot2.tidyverse.org. [Google Scholar]
- Yanagawa, A. and Shimizu, S. (2007) Resistance of the termite, Coptotermes formosanus Shiraki to Metarhizium anisopliae due to grooming. BioControl, 52, 75‒85. [Google Scholar]
- Yanagawa, A. , Yokohari, F. and Shimizu, S. (2009) The role of antennae in removing entomopathogenic fungi from cuticle of the termite, Coptotermes formosanus . Journal of Insect Science, 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa, A. , Yokohari, F. and Shimizu, S. (2010) Influence of fungal odor on grooming behavior of the termite, Coptotermes formosanus . Journal of Insect Science, 10, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa, A. , Fujiwara‐Tsujii, N. , Akino, T. , Yoshimura, T. , Yanagawa, T. and Shimizu, S. (2012) Odor aversion and pathogen‐removal efficiency in grooming behavior of the termite Coptotermes formosanus . PLoS ONE, e47412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa, A. , Fujiwara‐Tsujii, N. , Akino, T. , Yoshimura, T. , Yanagawa, T. and Shimizu, S. (2011) Musty odor of entomopathogens enhances disease‐prevention behaviors in the termite Coptotermes formosanus . Journal of Invertebrate Pathology, 108, 1‒6. [DOI] [PubMed] [Google Scholar]
- Yanagawa, A. , Imai, T. , Akino, T. , Toh, Y. and Yoshimura, T. (2015) Olfactory cues from pathogenic fungus affect the direction of motion of termites, Coptotermes formosanus . Journal of Chemical Ecology, 41, 1118‒1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Liu, L. , Zhou, W. , Cai, Q. and Huang, Q. (2020) Roles of selenoprotein T and transglutaminase in active immunization against entomopathogenic fungi in the termite Reticulitermes chinensis . Journal of Insect Physiology, 125, 104085. [DOI] [PubMed] [Google Scholar]
- Zhou, W. , Huang, Q.Y. , Zhao, X.Y. , Liu, L. and Mehmood, N. (2021) Silencing of selenium‐binding protein disrupted the active immunization of the termite Reticulitermes chinensisand improved the lethal effect of the entomopathogenic fungus Metarhizium anisopliae . Biological Control, 157, 104588. [Google Scholar]
- Zhu, F. , Li, J. , Xie, W. , Wang, C. and Liu, Y. (2017) Identification and antibacterial activity of two steroids secreted by the fungus beetle Xylographus bostrichoides (dufour, 1843). Bangladesh Journal of Botany, 46, 1171‒1176. [Google Scholar]
- Zoberi, M.H. (1995) Metarhizium anisopliae, a fungal pathogen of Reticulitermes flavipes (Isoptera: Rhinotermitidae). Mycologia, 87, 354‒359. [Google Scholar]


