Abstract
Objectives
To assess the clinical significance of repeat transurethral resection (reTUR) and surgical margin status after en bloc resection of bladder tumour (ERBT) for pathological T1 (pT1) bladder cancer.
Patients and Methods
We retrospectively analysed the record of 106 patients with pT1 high‐grade bladder cancer who underwent ERBT between April 2013 and February 2021 at multiple institutions. All specimens were reviewed by a genitourinary pathologist. The primary outcome measures were recurrence‐free survival (RFS) and progression‐free survival (PFS) between patients with and those without reTUR. We also analysed the predictive value of surgical margin on the likelihood of residual tumour on reTUR.
Results
A reTUR was performed in 50 of the 106 patients. The 2‐year RFS and 3‐year PFS were comparable between patients who underwent reTUR and those who did not (55.1% vs 59.9%, P = 0.6, 80.6% vs 82.6%, P = 0.6, respectively). No patient was upstaged to pT2 on reTUR. Regarding the surgical margin status, there were no recurrences at the original site in 51 patients with negative horizontal margins. Cox proportional hazard analysis revealed that a positive vertical margin was an independent prognostic factor of worse PFS. On reTUR, six pTa/is residues were detected in patients with a positive horizontal margin, and three pT1 residues were detected in one patient with a positive vertical margin or other adverse pathological features.
Conclusions
A reTUR after ERBT for pT1 bladder cancer appears not to improve either recurrence or progression. Surgical margin status affects prognosis and reTUR outcomes. A reTUR can be omitted after ERBT in patients with pT1 bladder cancer and negative margins; for those with positive horizontal or vertical margins, reTUR should remain the standard until proven otherwise.
Keywords: Bladder cancer, pT1, en bloc TUR, repeat TURs, recurrence, progression
Abbreviations
- BCa
Bladder cancer
- CIS
Carcinoma in situ
- TURBT
Transurethral resection of bladder tumour
- ERBT
En bloc resection of bladder tumour
- HG
High‐grade
- HR
Hazard ratio
- IQR
Interquartile range
- LP
Lamina propria
- LVI
Lymphovascular invasion
- MM
Muscularis mucosae
- MP
Muscularis propria
- NMIBC
non‐muscle‐invasive bladder cancer
- OS
Overall survival
- PFS
Progression‐free survival rate
- RCT
Randomised controlled trial
- ReTUR
Repeat transurethral resection
- RFS
Recurrence‐free survival rate
- TURBT
transurethral resection of bladder tumour
Introduction
Stage T1 bladder cancer (BCa) has relatively high progression and fatality rates [1, 2]. Very high rates of patients who underwent a transurethral resection of bladder tumour (TURBT) for T1 BCa have disease persistence and are upstaged to T2 BCa [2]. Therefore, a repeat transurethral resection (reTUR) after initial TURBT is recommended in clinical guidelines [3]. Indeed, several studies have shown that a reTUR significantly improves recurrence‐free survival (RFS) [4, 5], progression‐free survival (PFS) and overall survival (OS) in patients with pathological stage T1 (pT1) BCa [4, 5, 6, 7]. However, not all the published studies agree on the long‐term oncological value of reTUR [6, 8]. Furthermore, every resection is invasive, costly, and associated with an increased risk of complications given the fact that the site has been already resected.
Recently, there has been increasing evidence to support the clinical benefit of en bloc resection of bladder tumour (ERBT) in BCa [9], even for pT1 BCa regarding the clinical significance of reTUR after ERBT [10, 11]. A recent multicentric study comprising 300 pT1 BCa demonstrated that ERBT was independently associated with the likelihood of no residual tumour on reTUR [11]. We, therefore, hypothesised that ERBT may allow the omission of an unnecessary, and potentially harmful reTUR in at least some patients with pT1 BCa. Therefore, we set out to perform a multicentre retrospective study of reTUR or not in patients diagnosed with pT1 BCa on initial ERBT. We investigated the impact of predictive factors such as surgical margins of initial ERBT.
Patients and Methods
Patient Selection
The study was approved by the Ethics Committee (32–189[10270]). We retrospectively analysed the records of 130 consecutive patients with pT1 high‐grade (HG) BCa who underwent ERBT at the Jikei University Kashiwa Hospital (n = 63), the Ureshino Medical Center (n = 40), and the Saitama Medical Center (n = 27) between April 2013 and February 2021. All specimens were corrected and reviewed by the same genitourinary pathologist (S.S.). After pathological review, diagnostic change was noted in 18 cases (change to pTa: 17 cases, change to pT2: one), thus these were excluded. Then, four patients who underwent early radical cystectomy and two who had a cancer at the vesico‐ureteric junction were excluded. This left a total of 106 eligible patients for analysis. Our endpoints of interest were: (i) comparison of RFS and PFS between the patients treated with reTUR and those who did not, (ii) comparison of RFS and PFS between the patients who had positive surgical margin and those who did not, and (iii) evaluation the predictive value of histopathological features on the initial ERBT with the likelihood of residual cancer on reTUR.
Indication and time of reTUR (4–6 weeks after the primary resection) were followed according to the standards of each institution. In the Jikei University Kashiwa Hospital, surgeons recommended reTUR to all patients in principle, following the guidelines [3], but reTUR was not necessarily performed in the other institutions. Application of immediate single bladder instillation therapy and/or BCG instillation therapy were dependent on physician’s preference. Patients were followed up with subsequent cystoscopy and cytology every 3 months for 2 years, every 6 months thereafter until 5 years, and then yearly. Recurrence was defined as the reappearance of a pathologically confirmed urothelial carcinoma in the bladder after the reTUR in patients who had a reTUR and after initial ERBT in those who did not have a reTUR. Progression was defined as an advance in pathological stage (e.g., upstaging to ≥T2), identification of metastasis, or death caused by BCa.
Pathological Evaluation
The diagnosis of all BCa was performed according to the TNM classification and the European Association of Urology (EAU) guidelines [3]. Histological grade was adjudicated according to the 2016 WHO classification system. pT1 sub‐staging and surgical margin status and location was allocated by an experienced genitourinary pathologist (S.S.). The sub‐staging system was based on the invasion into the muscularis mucosae (MM) (pT1a/b) [12, 13]. Horizontal margin status was defined as the presence or absence of cancer at the edge of the continuous mucosa. Vertical margin status was defined as the presence or absence of cancer at the edge of the continuous lamina propria (LP) or muscularis propria (MP) [14].
En Bloc Resection Of Bladder Tumour Procedure
The ERBT was performed using a bipolar TURis system (Olympus Medical Systems, Tokyo, Japan) with needle or loop‐type electrode (Video S1). After marking at least a 5‐mm circular margin, we then cut through the LP to the MP and dissected around the entire circumference of the tumour. Finally, we identified and incised the shallow MP layer and removed the tumour en bloc, including the MP. Biopsy forceps were used to extract the specimen [15]. When the tumour was too large to extract through the outer cylinder, we cut the surface of the tumour with a conventional loop‐type electrode (n = 23).
Statistical Analysis
Continuous parametric variables were reported as median and interquartile range (IQR) or mean (± SD). The chi‐square test, Fisher’s exact test, Student’s t‐test, and Mann–Whitney U‐test were used to compare features of each form of treatment. A two‐sided P < 0.05 was considered to be statistically significant. The Kaplan–Meier method was used to estimate RFS and PFS and differences were tested using the log‐rank test. A Cox proportional‐hazard model was used to analyse prognostic factors for RFS and PFS. All statistical analyses were performed with R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Comparison between the Patients Who Underwent reTUR or Not
Patient Demographics and Pathological Findings
Among the 106 patients, 50 underwent a reTUR and 56 did not. Patient demographics were comparable between the two groups (Table 1 ). The median age was 74 years in the reTUR group and 76 years in the no reTUR group (P = 0.4). The mean tumour size was not different between the reTUR and the no reTUR groups (mean [SD] 20.6 [9.7] vs 20.8 [10.9] mm, P = 0.9). The mean number of tumours was also not different between the two groups (mean [SD] 2.22 [2.26] vs 2.16 [1.91], P = 0.9).
Table 1.
Patient demographics, pathological and oncological outcomes of the reTUR and no reTUR groups.
| Variable | ReTUR | No reTUR | P |
|---|---|---|---|
| Number of patients | 50 | 56 | |
| Patient demographics | |||
| Age, years, median (IQR) | 74 (70.25–78) | 76 (69–82.25) | 0.43 |
| Sex, n (%) | |||
| Male | 33 (66) | 43 (77) | 0.28 |
| Female | 17 (34) | 13 (23) | |
| History of UTUC, n (%) | 6 (12) | 6 (11) | 1 |
| Recurrent tumours, n (%) | 6 (12) | 10 (18) | 0.43 |
| Positive cytology, n (%) | 14 (28) | 9 (20) | 0.34 |
| Multiple tumours, n (%) | 25 (50) | 27 (48) | 1 |
| Non‐papillary tumours, n (%) | 8 (16) | 7 (12) | 0.78 |
| Sessile tumours, n (%) | 21 (42) | 17 (30) | 0.23 |
| Tumour diameter, mm, mean (SD) | 20.6 (9.7) | 20.8 (10.9) | 0.93 |
| Number of tumours, mean (SD) | 2.22 (2.26) | 2.16 (1.91) | 0.88 |
| Pathological diagnosis at initial ERBT | |||
| Concomitant CIS, n (%) | 22 (44) | 15 (27) | 0.07 |
| Adequate sampling of MP, n (%) | 47 (94) | 52 (93) | 1 |
| pT1a/b sub‐staging, n (%) | |||
| pT1a | 36 (72) | 38 (68) | 0.68 |
| pT1b | 14 (28) | 18 (32) | |
| Positive horizontal margin, n (%) | 21 (42) | 15 (37) | 0.52 |
| Positive vertical margin, n (%) | 4 (8) | 3 (5.4) | 1 |
| Pathological diagnosis at repeat resection | |||
| Residual pTa/is | 6 (12) | ‐ | |
| Residual pT1 | 3 (6) | ‐ | |
| Upstaging to pT2 | 0 | ‐ | |
| Treatment and oncological outcomes | |||
| Immediate bladder instillation therapy, n (%) | 33 (66) | 42 (75) | 0.39 |
| Maintenance bladder instillation therapy, n (%) | 36 (72) | 30 (54) | 0.07 |
| Follow‐up, months, median (IQR) | 23 (13–35) | 22.5 (11.75–42.25) | 0.79 |
| Recurrence, n (%) | 18 (36) | 18 (32) | 0.68 |
| Progression, n (%) | 7 (14) | 6 (11) | 0.77 |
| 2‐year RFS, % (95% CI) | 55.1 (37.2–69.8) | 59.9 (43.1–73.2) | 0.55 |
| 3‐year PFS, % (95% CI) | 80.6 (59.5–91.4) | 82.6 (63.7–92.2) | 0.64 |
UTUC, upper urinary tract urothelial carcinoma.
As for pathological findings, the rate of adequate sampling of MP was 94% in the reTUR group compared to 93% in the no reTUR group on initial ERBT (P = 1). There were further no statistical differences in the distribution of additional pathological features such as pT1a/b sub‐staging and surgical margins between the two groups.
Oncological Outcomes
During the median follow‐up of 23 months in the reTUR group and 22.5 months in the no reTUR group, recurrence was noted in 18 patients each who underwent reTUR and those who did not. Progression was detected in six patients each who underwent reTUR and those who did not (Table 1). The 2‐year RFS estimates were 55.1% (95% CI 37.2–69.8) in the reTUR group and 59.9% (95% CI 43.1–73.2) in the no reTUR group (P = 0.6). The 3‐year PFS estimates were 80.6% (95% CI 59.5–91.4) in the reTUR group and 82.6% (95% CI 63.7–92.2) in the no reTUR group (P = 0.6) (Fig. 1).
Fig. 1.
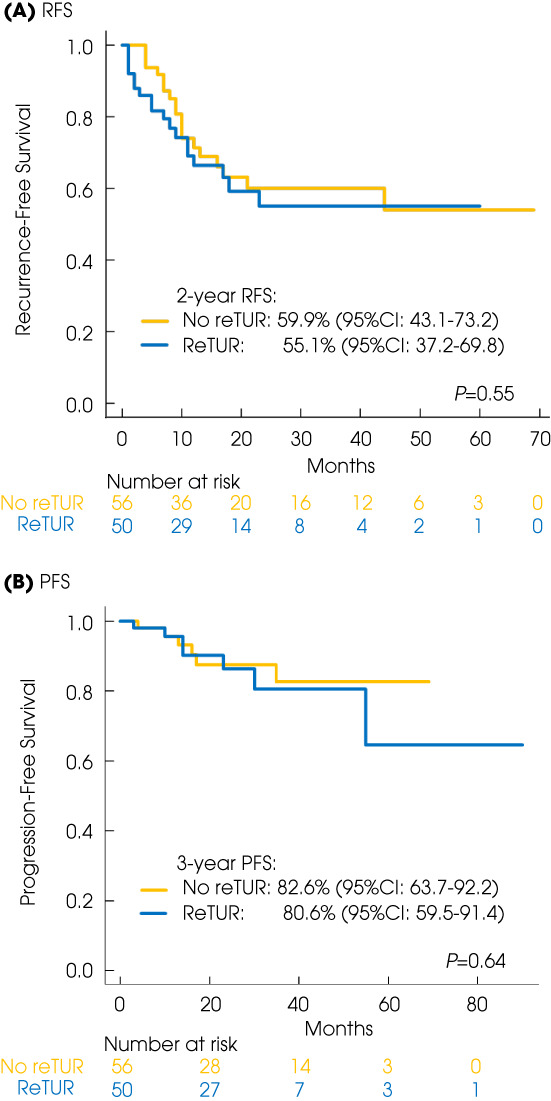
Kaplan–Meyer projection of the RFS (A) and the PFS (B) in the reTUR group vs no reTUR group.
Analysis of Surgical Margins
Positive vs Negative Horizontal Margins
Among the 106 patients, horizontal margin status was detected in 89 (84%). The reasons for undetectable horizontal margins were as follows: exfoliation and damage at the end of the mucosa (65%) and fragmentation (35%). Among 89 patients in whom horizontal margins were able to be diagnosed accurately, 51 patients had negative horizontal margins while 38 had positive horizontal margins. Table 2 shows patients’ demographics and oncological outcomes according to the two groups. The rate of concomitant carcinoma in situ (CIS) in the peripheral area of original tumour was significantly higher in the positive horizontal margin group (61% vs 24%, P < 0.001).
Table 2.
Patients demographics and oncologic outcomes of negative versus positive horizontal margins.
| Variable | Negative | Positive | P |
|---|---|---|---|
| Number of patients | 51 | 38 | |
| Age, years, median (IQR) | 73 (68–79.5) | 76 (71–78) | 0.66 |
| Sex, n (%) | |||
| Male | 37 (73) | 29 (76) | 0.79 |
| Female | 14 (27) | 9 (24) | |
| Recurrent tumours, n (%) | 9 (18) | 4 (11) | 0.38 |
| Positive cytology, n (%) | 11 (23) | 9 (28) | 0.79 |
| Non‐papillary tumours, n (%) | 8 (16) | 4 (11) | 0.55 |
| Sessile tumours, n (%) | 18 (35) | 13 (34) | 1 |
| Multiple tumours, n (%) | 20 (39) | 23 (61) | 0.06 |
| Tumour diameter, mm, mean (SD) | 19.5 (9.3) | 21.4 (11.5) | 0.38 |
| Concomitant CIS, n (%) | 12 (24) | 23 (61) | <0.001 |
| Follow‐up, months, median (IQR) | 23 (11–37.5) | 20 (13.25–33.75) | 0.95 |
| Recurrence, n (%) | 14 (28) | 18 (47) | 0.07 |
| Location of recurrence, n (%) | |||
| Original site | 0 | 7 (39) | 0.01 |
| Distant | 14 (100) | 11 (61) | |
| 2‐year RFS, % (95% CI) | 67.3 (49.7–79.9) | 41.6 (22.6–59.6) | 0.12 |
Recurrences were noted in 14 patients with negative horizontal margins and 18 with positive horizontal margins. The 2‐year RFS estimates were 67.3% (95% CI 49.7–79.9) in the negative horizontal margin group and 41.6% (95% CI 22.6–59.6) in the positive horizontal margin group (P = 0.1; Fig. 2). In the negative horizontal margin group, all recurrences occurred at distant areas (not previous resection site). In contrast, there were seven recurrences (39%) at the original ERBT site in the positive horizontal margin group (P = 0.01; Table 2).
Fig. 2.
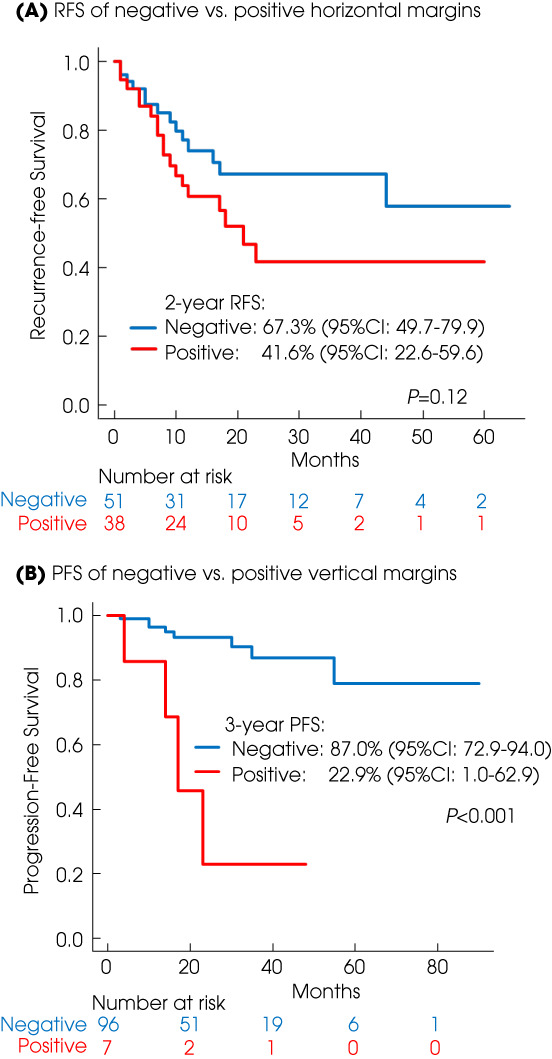
Kaplan–Meyer projection of the RFS of negative vs positive horizontal margins (A) and the PFS of negative vs positive vertical margins (B).
Multivariable analysis did not show a positive horizontal margin as an independent prognostic factor for worse RFS (Table 3).
Table 3.
Impact of possible prognostic factors on recurrence‐free survival and progression‐free survival in 106 patients.
| Recurrence | Progression | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | P | Multivariable | P | Univariable | P | Multivariable | P | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| Sex (male vs female) | 1.04 (0.50–2.16) | 0.92 | 0.49 (0.17–1.48) | 0.21 | ||||
| Positive cytology | 1.24 (0.57–2.67) | 0.59 | 0.46 (0.10–2.08) | 0.31 | ||||
| Multiple tumours | 1.01 (0.53–1.95) | 0.97 | 1.38 (0.45–4.25) | 0.57 | ||||
| Tumour diameter >30 mm | 0.85 (0.37–1.96) | 0.71 | 1.56 (0.42–5.83) | 0.51 | ||||
| Non‐papillary tumour | 0.68 (0.24–1.93) | 0.47 | 1.90 (0.52–6.92) | 0.33 | ||||
| Sessile tumour | 0.96 (0.48–1.89) | 0.9 | 1.27 (0.43–3.81) | 0.67 | ||||
| Recurrent tumour | 2.34 (1.06–5.18) | 0.035 | 1.60 (0.59–4.37) | 0.36 | 2.02 (0.55–7.38) | 0.29 | ||
| Concomitant CIS | 1.06 (0.57–1.96) | 0.86 | 0.97 (0.46–2.08) | 0.94 | 0.78 (0.26–2.35) | 0.65 | ||
| History of UTUC | 2.74 (1.25–6.02) | 0.01 | 2.47 (0.97–6.30) | 0.06 | 1.81 (0.50–6.61) | 0.37 | ||
| Maintenance therapy | 0.52 (0.27–1.01) | 0.052 | 0.51 (0.24–1.06) | 0.07 | 0.36 (0.12–1.11) | 0.08 | 0.45 (0.14–1.43) | 0.18 |
| pT1a/b | 0.88 (0.42–1.82) | 0.73 | 4.93 (1.60–15.2) | 0.006 | 3.18 (0.93–10.9) | 0.07 | ||
| Positive horizontal margin | 1.74 (0.86–3.5) | 0.12 | 2.17 (0.94–5.03) | 0.07 | ‐ | ‐ | ‐ | ‐ |
| Positive vertical margin | 1.55 (0.54–4.39) | 0.41 | 9.78 (2.82–33.9) | <0.001 | 6.93 (1.84–26.1) | 0.004 | ||
| ReTUR | 1.29 (0.63–2.35) | 0.55 | 1.10 (0.35–3.42) | 0.87 | ||||
UTUC, upper urinary tract urothelial carcinoma.
Positive vs Negative Vertical Margins
Among the106 patients, vertical margin status was able to be attributed in 103 (97%). Among the 103 patients, 97 patients had a negative horizontal margin and six had a positive vertical margin. The 3‐year PFS estimates were 87.0% (95% CI 72.9–94.0) in the negative vertical margin group and 22.9% (95% CI 1.0–62.9) in the positive vertical margin group (P < 0.001; Fig. 2). In multivariable analysis, a positive vertical margin was an independent prognostic factor for worse PFS (Table 3 ).
Results of reTUR Stratified by Surgical Margin Status
Among 50 patients who underwent reTUR, six (12%) had residual pTa/is and three (6%) had residual pT1. No patient was upstaged to pT2 (Table 1).
Regarding the association between horizontal margin and results of reTUR, 23 patients were diagnosed with positive horizontal margins and 25 were diagnosed with negative horizontal margins. Among them, all pTa/is residues were noted in the positive horizontal margin group. In contrast, none of the patients with negative horizontal margins had residual tumour at the original site (Fig. 3A).
Fig. 3.
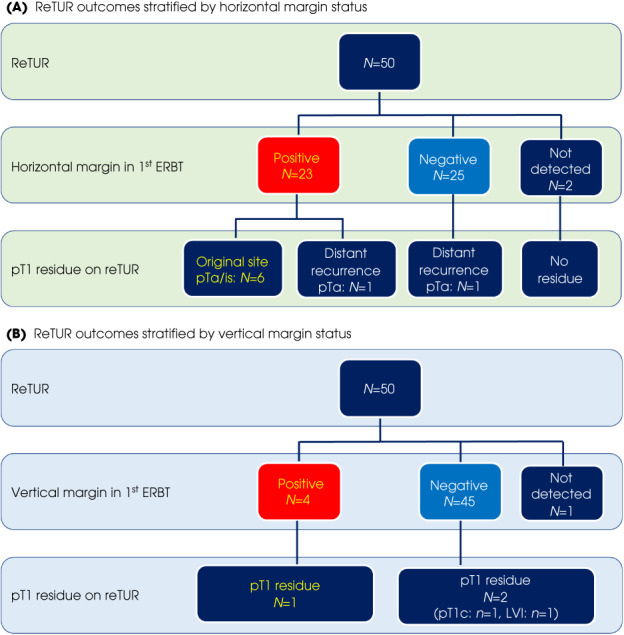
Flow charts of reTUR outcomes stratified by horizontal margin status (A) and vertical margin status (B).
Four patients were diagnosed with positive vertical margins. Among these patients, one had pT1 disease. On the other hand, among the 46 patients with negative vertical margins, only two had residual pT1 disease. In addition, these two patients had adverse pathological features such as progression beyond the MM and/or lymphovascular invasion (LVI) (Fig. 3B).
Discussion
In this retrospective study, we found no significant benefits in terms of RFS and PFS to performing a reTUR in all patients with pT1 on initial ERBT. No patient was upstaged to pT2 on the reTUR, supporting that initial ERBT attributed the correct, accurate pathological stage. These findings suggest that standard reTUR is not routinely required in patients with after ERBT for pT1 BCa on initial ERBT.
In 2010, a randomised controlled trial (RCT) with 210 patients with newly diagnosed T1 BCa conducted by Divrik et al. [16] demonstrated that reTUR significantly decreases the recurrence and progression rates, as well as the number of cancer deaths compared to no reTUR after initial conventional TURBT. Indeed, reTUR is recommended for all pT1 BCa in clinical guidelines [3]. More recently, Wettstein et al. [7] analysed 2162 patients who underwent reTUR among a total of 7666 patients and found that reported that reTUR was significantly associated with lower overall mortality after adjusting for the effects of all assumed confounders (hazard ratio [HR] 0.88, 95% CI 0.81–0.95; P < 0.001).
However, despite reTUR for pT1 BCa being a guideline‐endorsed intervention, clinical application of reTUR varies widely, in part because of its potential harm. Indeed, the compliance rates of reTUR for HG non‐muscle‐invasive BCa (NMIBC) were reported as high as 43% in a systematic review, not mentioning the cost associated with it [17]. Further, theoretically, the clinical impact of reTUR depends on the quality of the initial TURBT based on surgeons’ experience and dedication. A recent systematic review of reTUR found a negligible risk of upstaging in many more recent studies (1–4%) [2]. These results emphasise a possible impact of surgeons’ experience and the importance of the quality of initial resection for pT1 BCa [2]. Others and we suggest that ERBT enables a more complete and higher quality specimen in the initial resection, decreasing recurrence and progression as well as up‐staging to pT2 on reTUR.
The presence of MP in the TURBT specimen has been previously established as a quality indicator of the resection, impacting RFS and upstaging [18, 19]. Gontero et al. [6], in a large series of 2451 patients with T1 HG BCa treated with BCG, found that reTUR resulted in a survival advantage only in patients without the presence of MP in the initial TURBT specimen. Furthermore, Soria et al. [11] demonstrated that the presence of MP was an independent predictor of negative histology at reTUR. These previous studies reported the rates of MP presence of approximately 70%–80% with conventional TURBT [6, 11, 18, 19]. On the other hand, the rate of MP presence was 93% in our study, which is in accordance with the more than 90% reported in a recent meta‐analysis [20]. Thus, in the present study, a higher rate of presence of MP seems to be one of the primary reasons for the lack of reTUR impacting RFS and PFS.
Theoretically, ERBT allows the assessment of horizontal and vertical margins. However, only a few studies reported the diagnostic rate of surgical margins with a success range from 63% to 95% [14, 21, 22]. The present study showed that the diagnostic rate of horizontal and vertical margins was 84% and 97%, respectively. These rates are higher than those in previous reports probably owing to the diagnostic effort by our dedicated genitourinary pathologist.
We found no residual disease and no recurrence overtime at the initial ERBT site in patients with negative horizontal margins. The association between surgical margins and oncological outcomes in NMIBC remain poorly investigated/understood [14, 21, 22]. Gakis et al. [21] showed that patients with NMIBC who underwent ERBT with negative surgical margins were less likely to experience intravesical recurrence, resulting in a favourable 12‐month RFS compared to those with positive surgical margins. Consistent with this, we found that the 2‐year RFS estimates were higher but not statistically significant in the patients with negative horizontal margins compared to those with positive horizontal margins. In addition, there were no recurrences at the original site in patients with negative horizontal margins. Moreover, at reTUR, none of the patients with negative horizontal margins exhibited residual tumour of pTa/is at the original site. This suggests, after validation in larger well‐designed studies, the negative horizontal margin is a potential indicator of complete resection.
Furthermore, the present study is the first to demonstrate that a positive vertical margin is an independent prognosticator of worse PFS. In addition, among the patients who underwent reTUR, one of four patients with positive vertical margins harboured residual pT1 disease. On the other hand, among 45 patients with negative vertical margins, only two patients (4.4%) who had adverse pathological features, such as invasion beyond the MM and/or LVI, harboured residual pT1 disease. Thus, positive vertical margins appear to reflect the invasion degree and aggressiveness of pT1 BCa leading to worse PFS, helping clinical decision‐making regarding the need of immediate radical cystectomy or at least reTUR.
This study has several limitations that should be taken into account. First, it was a retrospective cohort study with a limited number of patients as well as events of recurrence and progression, increasing the risk of overfitting the results on multivariable analysis. Second, although there were no statistical differences in patient demographics between the reTUR and no reTUR group, confounders that may affect oncological outcomes were not matched in our study. Third, ERBT has several technical limitations, including the fact that large tumours must be resected in pieces. Regarding this aspect, ERBT cannot be applied for all pT1 BCa. Therefore, large tumours, likely to have more aggressive pathological features, were not included in this study. This might lead to a selection bias. Finally, surgical margin diagnosis is limited by specimen processing, e.g., whether the circumferential mucosal edge should be pinned or inked for better orientation and better histological assessment, is still unclear.
Conclusions
Our study suggests that reTUR after ERBT does not improve either recurrence or progression rates in the patients with pT1 HG BCa on initial ERBT. Negative horizontal margin seems to be a quality indicator for complete resection, resulting in absence of recurrence at the original site and no residual disease on reTUR. Positive vertical margins were associated with a poor progression rate. Taken together, the presented data comply with our hypothesis partially, reTUR can be omitted for patients with pT1 BCa treated with initial ERBT who harbour negative horizontal and vertical margins. The possible insufficient power of our analyses is likely, which makes drawing conclusions difficult. Therefore, confirmation and validation by dedicated well‐designed studies are needed.
Conflict of Interest
Takahiro Kimura is a paid consultant/advisor of Astellas, Bayer, Janssen and Sanofi. Shahrokh F. Shariat received as follows: Honoraria: Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Roche, Takeda. Consulting or Advisory Role: Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Pierre Fabre, Roche, Takeda. Speakers Bureau: Astellas, Astra Zeneca, Bayer, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Richard Wolf, Roche, Takeda. Shin Egawa is a paid consultant/advisor of Takeda, Astellas, AstraZeneca, Sanofi, Janssen, and Pfizer. The other authors declare no conflicts of interest associated with this manuscript.
Author Contributions
Takafumi Yanagisawa contributed to the protocol and project development, data collection and management, data analysis, and manuscript writing and editing. Shun Sato contributed to pathological diagnosis. Yasushi Hayashida, Yohei Okada, Kosuke Iwatani and Akihiro Matsukawa contributed to data collection. Takahiro Kimura, Hiroyuki Takahashi, Shin Egawa and Shahrokh F. Shariat contributed to manuscript editing. Jun Miki contributed to the protocol and project development and supervision.
Supporting information
Video S1
Acknowledgement
None.
References
- 1. Nieder AM, Brausi M, Lamm D et al. Management of stage T1 tumors of the bladder: International consensus panel. Urology 2005; 66: 108–25 [DOI] [PubMed] [Google Scholar]
- 2. Cumberbatch MGK, Foerster B, Catto JWF et al. Repeat transurethral resection in non‐muscle‐invasive bladder cancer: A systematic review. Eur Urol 2018; 73: 925–33 [DOI] [PubMed] [Google Scholar]
- 3. Babjuk M, Burger M, Capoun O et al. European Association of Urology guidelines on non‐muscle‐invasive bladder cancer (ta, T1, and carcinoma in situ). Eur Urol 2022; 81: 75–94 [DOI] [PubMed] [Google Scholar]
- 4. Divrik RT, Yildirim U, Zorlu F, Ozen H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: A prospective, randomized clinical trial. J Urol 2006; 175: 1641–4 [DOI] [PubMed] [Google Scholar]
- 5. Grimm MO, Steinhoff C, Simon X et al. Effect of routine repeat transurethral resection for superficial bladder cancer: A long‐term observational study. J Urol 2003; 170: 433–7 [DOI] [PubMed] [Google Scholar]
- 6. Gontero P, Sylvester R, Pisano F et al. The impact of re‐transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high‐grade/grade 3 bladder cancer treated with bacille Calmette‐Guerin. BJU Int 2016; 118: 44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wettstein MS, Baxter NN, Sutradhar R et al. Oncological benefit of re‐resection for T1 bladder cancer: A comparative effectiveness study. BJU Int 2022; 129: 258–68 [DOI] [PubMed] [Google Scholar]
- 8. Angulo JC, Palou J, Garcia‐Tello A et al. Second transurethral resection and prognosis of high‐grade non‐muscle invasive bladder cancer in patients not receiving bacillus Calmette‐Guerin. Actas Urol Esp 2014; 38: 164–71 [DOI] [PubMed] [Google Scholar]
- 9. Yanagisawa T, Mori K, Motlagh RS et al. En bloc resection for bladder tumors: An updated systematic review and meta‐analysis of its differential effect on its safety, recurrence, and histopathology. J Urol 2022; 207: 754–68 [DOI] [PubMed] [Google Scholar]
- 10. Zhou W, Wang W, Wu W et al. Can a second resection be avoided after initial thulium laser endoscopic en bloc resection for non‐muscle invasive bladder cancer? A retrospective single‐center study of 251 patients. BMC Urol 2020; 20: 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soria F, D'Andrea D, Moschini M et al. Predictive factors of the absence of residual disease at repeated transurethral resection of the bladder. Is there a possibility to avoid it in well‐selected patients? Urol Oncol 2020; 38: e71–77.e77 [DOI] [PubMed] [Google Scholar]
- 12. van Rhijn BW, van der Kwast TH, Alkhateeb SS et al. A new and highly prognostic system to discern T1 bladder cancer substage. Eur Urol 2012; 61: 378–84 [DOI] [PubMed] [Google Scholar]
- 13. Younes M, Sussman J, True LD. The usefulness of the level of the muscularis mucosae in the staging of invasive transitional cell carcinoma of the urinary bladder. Cancer 1990; 66: 543–8 [DOI] [PubMed] [Google Scholar]
- 14. Yanagisawa T, Miki J, Sakanaka K et al. Clinical significance of horizontal and vertical margin of En bloc resection for nonmuscle invasive bladder cancer. J Urol 2021; 206: 252–9 [DOI] [PubMed] [Google Scholar]
- 15. Yanagisawa T, Miki J, Yorozu T et al. Vertical lamina propria invasion diagnosed by En bloc transurethral resection is a significant predictor of progression for pT1 bladder cancer. J Urol 2021; 205: 1622–8 [DOI] [PubMed] [Google Scholar]
- 16. Divrik RT, Sahin AF, Yildirim U et al. Impact of routine second transurethral resection on the long‐term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease‐specific survival: A prospective randomised clinical trial. Eur Urol 2010; 58: 185–90 [DOI] [PubMed] [Google Scholar]
- 17. Mori K, Miura N, Babjuk M et al. Low compliance to guidelines in nonmuscle‐invasive bladder carcinoma: A systematic review. Urol Oncol 2020; 38: 774–82 [DOI] [PubMed] [Google Scholar]
- 18. Mariappan P, Zachou A, Grigor KM, Edinburgh Uro‐Oncology Group . Detrusor muscle in the first, apparently complete transurethral resection of bladder tumour specimen is a surrogate marker of resection quality, predicts risk of early recurrence, and is dependent on operator experience. Eur Urol 2010; 57: 843–9 [DOI] [PubMed] [Google Scholar]
- 19. Shoshany O, Mano R, Margel D, Baniel J, Yossepowitch O. Presence of detrusor muscle in bladder tumor specimens‐‐predictors and effect on outcome as a measure of resection quality. Urol Oncol 2014; 32: 40e17–22 [DOI] [PubMed] [Google Scholar]
- 20. Sari Motlagh R, Rajwa P, Mori K et al. Comparison of clinicopathologic and oncological outcomes between trans‐urethral En bloc (TUEB) resection and conventional trans‐urethral resection of bladder tumor (cTURBT): A systematic review, meta‐analysis and network meta‐analysis with focus on different energy sources. J Endourol 2022; 36: 535–47 [DOI] [PubMed] [Google Scholar]
- 21. Gakis G, Karl A, Bertz S et al. Transurethral en bloc submucosal hydrodissection vs conventional resection for resection of non‐muscle‐invasive bladder cancer (HYBRIDBLUE): a randomised, multicentre trial. BJU Int 2020; 126: 509–19 [DOI] [PubMed] [Google Scholar]
- 22. Krasny SA, Masanskiy IL. Comparison of the safety and efficacy of the new method of en‐bloc and conventional monopolar transurethral resection in the management of primary non‐muscle‐invasive bladder cancer. Onkourologiya 2019; 15: 102–12 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1


