Abstract
Objectives
Cleft lip and/or palate (CLP) is a common craniofacial birth defect caused by genetic as well as environmental factors. The phenotypic spectrum of CLP also includes submucous clefts with a defect in the palatal bone. To elucidate the contribution of vitamin A, we evaluated the effects of the vitamin A metabolite all‐trans retinoic acid (ATRA) on the osteogenic differentiation and mineralization of mouse embryonic palatal mesenchymal cells (MEPM).
Setting and Sample Population
MEPM cells were isolated from the prefusion palates of E13 mouse embryos from three different litters.
Materials and Methods
MEPM cells were cultured with and without 0.5 μM ATRA in osteogenic medium. Differentiation was analysed by the expression of osteogenic marker genes and alkaline phosphatase (ALP) activity after 1, 2, and 7 days. The expression of Wnt marker genes was also analysed. Mineralization was assessed by alizarin red staining after 7, 14, 21, and 28 days.
Results
The bone marker genes Sp7, Runx2, Alpl, and Col1a1 were inhibited 10% ± 2%, 59% ± 7%, 79% ± 12% and 57% ± 20% (P < .05) at day 7. ALP activity was inhibited at days 1 and 7 by 35 ± 0% (P < .05) and 23 ± 6% (P < .001). ATRA also inhibited mineralization at 3 and 4 weeks. Finally, expression of the universal Wnt marker gene Axin2 was strongly reduced, by 31 ± 18% (P < .001), at day 7.
Conclusion
Our data indicate that ATRA (vitamin A) inhibits bone formation by reducing Wnt signalling. This might contribute to the molecular aetiology of submucous clefting.
Keywords: mineralization, mouse embryonic palatal mesenchymal cells, osteoblast differentiation, retinoic acid, WNT signalling
Suggested mechanism of excess vitamin A: Excess vitamin A inhibits WNT signalling and thus reduces bone formation in the developing palate. This may lead to a submucous cleft palate. In this study, mesenchymal cells from the pre‐fusion embryonic mouse palate are cultured with retinoci acid (the active metabolite of vitamin A) in conditions that induce osteoblast differentiation and mineralization. The levels of WNT signalling and osteoblast differentiation are determined by expression analyses of marker genes. In addition, alkaline phosphatase activity and mineral deposition are quantified.
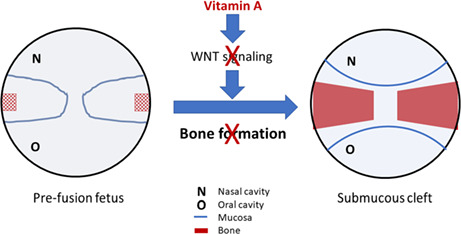
1. INTRODUCTION
A common craniofacial birth defect in humans is cleft lip and/or palate (CLP), with an occurrence rate of 1/700 live births. 1 CLP aetiology includes both genetic and environmental factors, but the mechanisms of clefting of the primary (hard) palate that involve the lip and/or palate seem to be different from those of the secondary (soft) palate. 2 Use of medication, tobacco, or alcohol, or a vitamin imbalance during pregnancy, increases the risk of CLP. 1 CLP can involve the palate, alveolus, and/or lip, and can be divided into unilateral or bilateral CLP, which can vary in severity. 1 CLP can further be nonsyndromic or syndromic. 1 A submucosal cleft only involves defects in the bone and muscles of the palate, while the etiological risk factors appear to overlap partially with CLP and CP. 3
The formation of the secondary palate involves downward growth of the palatal shelves, elevation above the tongue, and fusion in the midline. 4 Subsequently, intramembranous ossification occurs. In humans, the palate is formed between embryonic weeks 6 and 12 and is similar in all mammals. 4 Hence, mice are often used in research on palatogenesis and CLP. 4 In mice, the palate develops from embryonic day 11.5 to day 15.5. 5 During ossification, mesenchymal cells differentiate into osteoblasts initiated by, among others, the transcription factors runt‐related transcription factor 2 (RUNX2) and SRY‐box transcription factor 9 (SOX9). 6 Further differentiation and mineralization are regulated by factors such as osterix (SP7), osteocalcin (BGLAP), and wingless‐type MMTV integration site family (Wnt) signalling. 6
Wnt proteins bind to the frizzled receptor and co‐receptor LRP5/6, causing β‐catenin to translocate to the nucleus and activate transcription factors of the T cell‐specific transcription factor/lymphoid enhancer binding factor (TCF/LEF) family, which control the expression of target genes. 7 Wnt signalling generally increases bone formation in rats and mice by stimulating osteoblast differentiation. 8
All‐trans retinoic acid (ATRA), the active derivate of vitamin A, is a crucial regulator of embryonic development, including palatogenesis. 9 Intracellular ATRA can be degraded by Cyp26b1; alternatively, it binds to cellular retinoic acid‐binding proteins (CRABPs) and is then translocated to the nucleus. 10 There, ATRA binds to retinoic acid receptors (RARs) such as Rarb, which form a heterodimer with retinoid X receptors (RXRs) to induce target gene expression. 10 Epidemiological studies show an increased risk of CLP when excessive or insufficient amounts of vitamin A are used by the pregnant mother. 11 , 12 In vivo studies in rats and mice also show a causative role for ATRA in cleft palate. 13 , 14 In vitro studies on chondroprogenitor cells, mouse adipose‐derived mesenchymal cells, and mouse embryonic palatal mesenchymal cells (MEPM cells) show an inhibition of cell proliferation, differentiation, and mineralization by ATRA. 15 , 16 , 17 , 18 Interestingly, studies in certain non‐primary cell lines indicate this might be caused by inhibition of Wnt signalling. 19 , 20 An in vivo study in mice also shows that Wnt signalling in the developing palate is inhibited by ATRA and causes CLP. 21 Since ATRA interacts with Wnt signalling in several in vivo and in vitro models, we set out to investigate this in primary MEPM cells isolated from mouse embryos just before the fusion stage. We hypothesized that ATRA inhibits the osteogenic differentiation and mineralization of MEPM cells by reducing Wnt signalling.
2. MATERIAL AND METHODS
2.1. Cell culture
MEPM cells were isolated from E13.5 foetuses from three pregnant mice. E13.5 is the embryonic stage just before the fusion of the palatal shelves. The experiments were approved by the board for animal experiments of Radboud University, Nijmegen (RUDEC 2015‐0080). Cells were isolated from the palates by enzymatic digestion and plastic adherence. All cells were mixed and frozen in liquid nitrogen after two passages. For the dose–response study, MEPM cells were cultured in proliferation medium composed of minimum essential medium α (MEM α; Gibco) with 10% fetal bovine serum (FBS; Gibco), 10 000 U/mL penicillin, 10 000 μg/mL streptomycin (2% Pen Strep, Gibco) and cultured at 37°C in a humidified atmosphere of 5% CO2 in air. Next, the cells were distributed in 24‐well plates at a density of 25 000 cells/well and grown until confluent. The medium was then switched to osteogenic medium composed of MEM α (Gibco), 10% FBS, 2% Pen Strep, 10 mM β‐glycerophosphate (Sigma‐Aldrich), 50 μg/mL vitamin C (Sigma‐Aldrich) with 0, 0.1, 0.5, or 1 μM ATRA (Sigma‐Aldrich), and a final concentration of 0.05% dimethyl sulfoxide (DMSO). As no significant differences between control groups with and without DMSO were observed, only data from the control group with DMSO are shown. The medium was changed every other day for up to 7 days. Cultures for the analysis of gene expression were grown in triplicate.
In the main study, MEPM cells were distributed in 24‐well plates as described above. Upon confluency, the medium was switched to osteogenic medium with 0.0 or 0.5 μM ATRA. Only the control group with DMSO is shown, because no significant differences between groups with and without DMSO were observed. Cells were cultured for up to 28 days. Cultures for the alkaline phosphatase (ALP) activity assay and gene expression analyses were grown in triplicate and cells were harvested after 1, 2, and 7 days. Cultures for the mineralization assay were performed in duplicate and cells were harvested on days 7, 14, 21, and 28.
2.2. Alkaline phosphatase activity assay
ALP activity was measured with a colorimetric assay to quantify the hydrolysis of p‐nitrophenol (p‐NP). Cells were washed once with Dulbecco's phosphate‐buffered saline (DPBS) (Gibco), then lysed with two freeze–thaw cycles in 1 mL of water and stored at −20°C. Next, 100 μL of standard (0–25 nM serial dilutions of 4‐NP (Sigma‐Aldrich)) or 80 μL of sample mixed with 20 μL of buffer solution (0.5 M 2‐amino‐2‐methyl‐1‐propanol, pH 10.3; Sigma‐Aldrich) was mixed with 100 μL of substrate solution (5 mM p‐nitrophenyl phosphate (Sigma‐Aldrich) in a 96‐well plate and incubated at 37°C for 60 min. The reaction was stopped by adding 100 μL of 0.3 M NaOH, and absorbance was measured in a Synergy HTX Multi‐Mode Microplate Reader at 405 nm. Total DNA was determined using the Quantifluor dsDNA kit (Promega). ALP activity values were expressed as nmol 4‐NP/h/ng DNA.
2.3. Quantitative real‐time PCR
Total RNA was isolated using the miRNeasy Micro Kit (Qiagen). Equal amounts of RNA from each sample were reverse transcribed using the iScript cDNA Synthesis Kit (Bio‐Rad). RT‐qPCR reactions were carried out in 25 μL containing 5 μL diluted cDNA, 4.5 μL RNAse‐free water, 3 μL of 2.5 μM forward and reverse primers, and 12.5 μL SYBR Green Supermix (Bio‐Rad). The amplifications were performed in a CFX96 Real‐Time System (Bio‐Rad) using the following conditions: initial denaturalization at 95°C for 3 min, followed by 39 cycles performed at 95°C for 15 s and 60°C for 30 s. All data were normalized to the expression of three reference genes (Gapdh, Actb, and 18S rRNA). Relative expression was calculated according to the method. Primer (Sigma‐Aldrich; Biolegio) sequences are shown in Table 1.
TABLE 1.
Primer sequences
| Gene category | Symbol | Forward primer (5'‐3') | Reverse primer (5'‐3') |
|---|---|---|---|
| Housekeeping genes | Gapdh | GGCAAATTCAACGGCACA | GTTAGTGGGGTCTCGCTCCTG |
| Actb | CGGTTCCGATGCCCTGAGGCTCTT | CGTCACACTTCATGATGGAATTGA | |
| 18S rRNA | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | |
| Osteogenic markers | Runx2 | CGGACGAGGCAAGAGTTTCA | GGATGAGGAATGCGCCCTAA |
| Sp7 | CCTACCCAGGAAGAAGCTCAC | TGGTTTTGGGGGCTGAAAGG | |
| Alpl | GCACCTGCCTTACCAACTCT | GTGGAGACGCCCATACCATC | |
| Col1a1 | AAGGTGCCAATGGTGCTC | ACCAGTGTCTCCTTTGTTGC | |
| Bglap | CCGGGAGCAGTGTGAGCTTA | CCATACTGGTCTGATAGCTC | |
| ATRA‐responsive genes | Rarb | GAAAACGACGACCCAGCAAG | TTACACGTTCGGCACCTTTC |
| Cyp26b1 | GATCCTACTGGGCGAACACC | GGAGAAGACCTTGCGCTTGT | |
| Crabp2 | GCTAAAAGCTCTGGGGGTGAA | TGATCTCGACTGCTGGCTTG | |
| Wnt components | Axin2 | GGTTCCGGCTATGTCTTTGC | CAGTGCGTCGCTGGATAACTC |
| Myc | GGTTTGCCTCTTCTCCACAG | TCCTGTACCTCGTCCGATTC | |
| Lef1 | TATGAACAGCGACCCGTACA | TCGTCGCTGTAGGTGATGAG | |
| Lrp5 | ACGTCCCGTAAGGTTCTCTTC | GCCAGTAAATGTCGGAGTCTAC | |
| Lrp6 | TGGGGCAAGCACACTGATAAAAA | TGGGGAGAAGTGCCAAAGATAGAAC |
2.4. Mineralization assay
Staining of the cells was performed with the Osteogenesis Quantitation Kit (Merck KGaA). Pictures were taken to monitor mineralization. To quantify mineral deposition, incorporated alizarin red was solubilized and absorbance was compared with a standard curve of alizarin red concentrations (0–2 mM). Alizarin red data are expressed as mM.
2.5. Statistical analysis
Each culture experiment was performed in triplicate (except for the mineralization assay) and the results are presented as mean ± SD. Differences between the groups in the dose–response study were evaluated at day 7 by one‐way analysis of variance (ANOVA), with the control group set at 100%. Differences between the groups in the main study were evaluated by two‐way ANOVA (except for the mineralization assay, where N was too small). Post‐hoc comparisons were made using the Bonferroni test. Differences were considered significant for values of P < .05. All statistical tests were performed with Graphpad Prism 5 software (Graphpad Software).
3. RESULTS
3.1. Dose–response effects of ATRA
To decide which concentration to use for the main study, we analysed the gene expression of a retinoic acid–responsive gene, an osteogenic differentiation marker, and a Wnt signalling marker gene (retinoic acid receptor beta [Rarb], Sp7, and axis inhibition protein 2 [Axin2], respectively) using 0.1, 0.5, and 1 μM ATRA. The expression of Rarb was upregulated about 40‐fold in all three ATRA‐treated groups as compared with the controls (P < .01, Figure 1A). In contrast, the expression of Sp7 was downregulated about 6‐fold in the three ATRA‐treated groups (P < .01, Figure 1B). The expression of Axin2 was downregulated more than 2‐fold only in the groups treated with 0.1 and 0.5 μM ATRA (P < .01, Figure 1C). There were no significant differences between the control groups with and without DMSO for any of the genes and there were no significant differences between the three ATRA concentrations. We therefore chose the average concentration of 0.5 μM ATRA for the main experiments.
FIGURE 1.
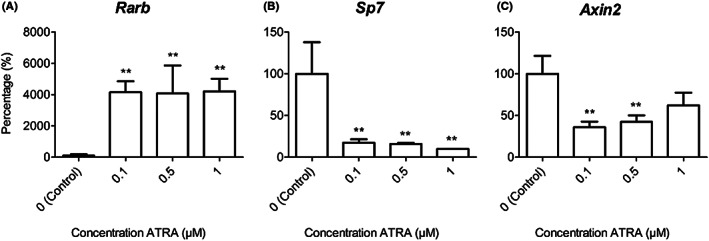
Effects of different ATRA concentrations on gene expression. MEPM cells were treated with 0, 0.1, 0.5, or 1 μM ATRA for 1 week and compared with the control group; expression of Rarb (A), Sp7 (B), and Axin2 (C) was analysed. Data are presented as mean ± SD in percentages of the control groups (N = 3). **P < .01, compared with the control group at day 7
3.2. ATRA inhibits MEPM cell mineralization
MEPM cells were cultured with and without 0.5 μM of ATRA in osteogenic medium for up to 28 days and stained with alizarin red to examine mineralization. The controls show a clear increase in alizarin red staining after 28 days, whereas the treated cells remained unstained (Figure 2A). After 4 weeks, extraction of the dye also showed an increase of alizarin red staining in the controls, whereas the cells treated with ATRA showed no staining (Figure 2B).
FIGURE 2.
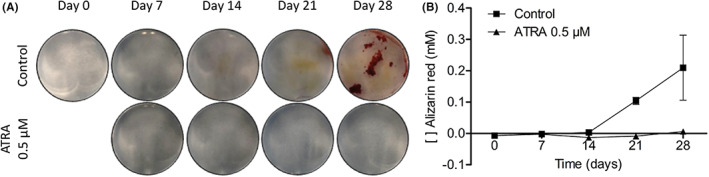
Mineralization of MEPM cells. MEPM cells were cultured with and without 0.5 μM ATRA for up to 28 d. Representative photographs of the alizarin red staining (A) and quantitative analysis of the alizarin red staining (B) on days 7, 14, 21, and 28 are shown. Data of the quantitative analysis are presented as mean ± SD (N = 2)
3.3. ATRA upregulates ATRA‐responsive genes
We evaluated the effects of ATRA on the expression levels of the genes Rarb, cytochrome P450 family 26 subfamily B member 1 (Cyp26b1), and cellular retinoic acid binding protein 2 (Crabp2), which are involved in retinol‐dependent signalling. The expression of Rarb in the controls was constantly low, whereas it increased up to 50‐fold in the ATRA group after 7 days (P < .001, Figure 3A). Similar effects were observed for Cyp26b1 expression in the controls, whereas it increased about 13‐fold in the ATRA group at day 2 (P < .01, Figure 3B). After 7 days, Cyp26b1 expression was 70 times higher than in the controls (P < .001, Figure 3B). The expression of Crabp2 in the controls increased linearly during the 7 days of culture. In the ATRA‐treated group, Crabp2 expression showed significant upregulation at days 1 and 2 (P < .001), but was normalized at day 7 (Figure 3C). There were no significant differences between the control groups with and without DMSO for any of the genes (data not shown).
FIGURE 3.
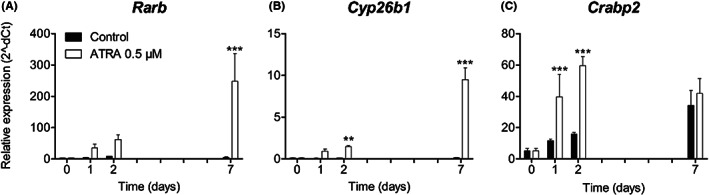
Expression of ATRA‐responsive genes. MEPM cells were cultured with and without 0.5 μM ATRA for up to 7 days. Expression of the genes Rarb (A), Cyp26b1 (B) and Crabp2 (C) is shown. Data are presented as mean ± SD (N = 3). **P < .01, ***P < .001, compared with the control group at each time point
3.4. ATRA downregulates osteogenic marker genes and ALP activity
We investigated the effects of ATRA on the expression of early (alkaline phosphatase, liver/bone/kidney [Alpl], Sp7, and Runx2) and late (collagen type I, alpha 1 [Col1a1] and Bglap) osteoblast marker genes. Alpl expression increased gradually over 7 days in the controls and in the ATRA‐treated group, but ATRA downregulated Alpl significantly at day 2 compared with its expression in the controls (P < .001, Figure 4A). Sp7 did not show a consistent expression pattern (Figure 4B). At days 2 and 7, ATRA reduced Sp7 expression compared with the control levels (P < .001, Figure 4B). Runx2 expression was more or less constant in the controls, but was decreased in the ATRA‐treated groups at days 2 and 7 (P < .05, Figure 4C). Col1a1 expression showed a gradual increase over 7 days in the control group, while ATRA significantly downregulated Col1a1 at day 7 (P < .05, Figure 4D). Bglap expression was more or less stable over 7 days and was not affected by ATRA (Figure 4E). There were no significant differences between the control groups with and without DMSO for any of the genes (data not shown). We also evaluated the effects of ATRA on ALP activity in the cultured MEPM cells after 7 days to assess early osteoblast differentiation (Figure 4F). ALP activity in the control group increased gradually up to day 7 (0.10 ± 0.00 nmol 4‐NP/h/ng DNA). Similar results were observed in the MEPM cells treated with DMSO only (data not shown). MEPM cells treated with ATRA showed slightly increased ALP activity, but it was significantly reduced at days 1 (P < .05) and 7 (0.02 ± 0.00 nmol 4‐NP/h/ng DNA, P < .001) compared with the controls.
FIGURE 4.
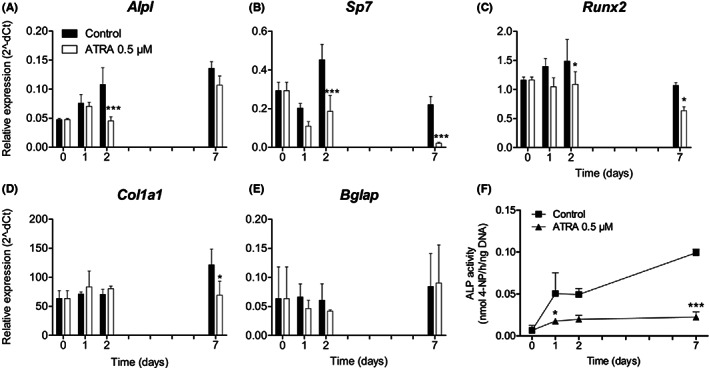
Expression of osteogenic differentiation genes and ALP activity. MEPM cells were cultured with and without 0.5 μM ATRA for up to 7 d. Expression of the early osteogenic marker genes Alpl (A), Sp7 (B) and Runx2 (C) and late osteogenic marker genes Col1a1 (D) and Bglap (E) is shown. Data are represented as mean ± SD (N = 3). *P < .05, ***P < .001, compared with the control group at each time point. ALP activity in MEPM cells with and without 0.5 μM ATRA for up to 7 d (F). Data are presented as mean ± SD (N = 3). *P < .05, ***P < .001, compared with the control group at each time point
3.5. ATRA inhibits Wnt signalling
To assess the effects of ATRA on Wnt signalling during osteogenic differentiation, the expression of several Wnt‐related genes was analysed. Axin2 was upregulated more than 2‐fold over 7 days in the control group, while ATRA significantly inhibited Axin2 expression at day 7 (P < .001, Figure 5A). There was no difference in the expression of the Wnt co‐receptors Lrp5 and Lrp6 between the ATRA‐treated groups and the control groups (Figure 5B and C). The expression of Lrp5 was more or less constant over time in the controls and ATRA‐treated groups, while Lrp6 expression showed a slight increase over time with a dip at day 1. In the first 2 days, expression of the Wnt target gene Myc was upregulated, and by day 7 it had declined (Figure 5D). ATRA had no influence on Myc expression. The expression of Lef1 in the controls was upregulated in the first 2 days, but declined by day 7 (Figure 5E). ATRA significantly upregulated the expression of Lef1 at 1, 2, and 7 days compared with its expression in the controls (P < .001). There were no significant differences between the control groups with and without DMSO for any of the genes (data not shown).
FIGURE 5.
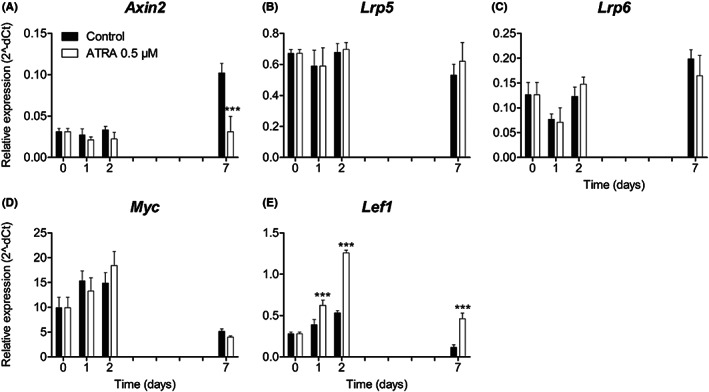
Expression of Wnt‐related genes. MEPM cells were cultured with and without 0.5 μM ATRA for up to 7 d. The expression of the Wnt‐related gene Axin2 (A), the Wnt co‐receptors Lrp5 (B) and Lrp6 (C), the Wnt target gene Myc (D), and the Wnt signalling transcription factor Lef1 (E) are shown. Data are presented as mean ± SD (N = 3). ***P < .001, compared with the control group at each time point
4. DISCUSSION
ATRA is a key regulator of embryonic development. Epidemiological and animal studies indicate that either an excess or a shortage of ATRA can induce CLP and cleft palate (CP). 22 , 23 We investigated the effects of ATRA on the osteogenic differentiation and mineralization of primary MEPM cells to clarify the molecular aetiology of submucous clefting. We hypothesized that ATRA inhibits osteoblast differentiation and mineralization by disrupting Wnt signalling.
In our initial dose–response experiments, ATRA (0.1, 0.5, and 1 μM) strongly upregulated the expression of the well‐known ATRA‐responsive gene Rarb. Additionally, we found that ATRA strongly downregulated the expression of the osteoblast transcription factor Sp7 and the Wnt marker gene Axin2. Sp7 is an important transcription factor for osteoblast differentiation, 24 while Axin2 is a universal marker gene for Wnt signalling in vertebrates. 25 The effects were maximal even at the lowest concentration of ATRA, confirming that the MEPM cells are highly sensitive to ATRA.
Studies on other pre‐osteoblasts have generally used ATRA concentrations in the range of 0.004 to 10 μM. 26 , 27 , 28 , 29 At low ATRA doses (1–10 nM) the osteogenic gene expression response seems to reach a plateau. In contrast, more supra‐physiological doses of ATRA (≥10 μM) can produce opposite effects. 10 , 30 Higher concentrations (up to 100 μM) of ATRA generally show clear dose‐dependent effects. 16 , 29 , 31 A study with intermediate concentrations of ATRA (10–50 nM) also reported a dose‐dependent effect on osteogenic gene expression in chondroprogenitor cells. 15 Our data might not show a clear dose–response effect because at the lowest concentration of 0.1 μM the ATRA effect had already reached a plateau.
MEPM cultures started to mineralize after about 3 weeks, and this was completely inhibited by ATRA. In addition, ALP activity at day 7 was also reduced. This indicates that the differentiation of MEPM cells was strongly inhibited by ATRA. Similar results for mineralization and ALP activity are reported for osteoblasts and chondroprogenitor cells from humans, rats, and mice treated with ATRA. 17 , 19 , 26 , 29 , 32 Together these data show that ATRA strongly reduces the differentiation and mineralization of MEPM cells and several other types of pre‐osteoblasts.
The enhanced expression of three ATRA‐responsive genes (Rarb, Cyp26b1, and Crabp2) confirms the activation of the ATRA signalling pathway. Intracellular ATRA levels are regulated by the synthesis and degradation of ATRA. If ATRA is not degraded by Cyp26b1, it can bind to cellular retinoic acid–binding proteins (CRABPs) and be translocated to the nucleus, where it activates nuclear receptors such as Rarb to induce transcription of target genes. 10
As expected, the early osteoblast differentiation genes Alpl, Sp7, and Runx2 were inhibited by ATRA, as been reported in other osteoblast precursor cells. 17 , 19 , 26 , 29 We also found that expression of the late differentiation gene Col1a1 was downregulated in the first week, but Bglap expression was unchanged. Other studies report downregulation of Bglap in the second week in osteoblast precursor cells, 19 , 20 , 26 , 29 but we analysed gene expression only in the first week. These data indicate that osteoblast differentiation is strongly inhibited by ATRA in MEPM cells, which is in line with the observed inhibition of ALP activity and mineralization.
A further finding was that ATRA strongly reduced the expression of Axin2, which is considered a universal Wnt marker gene in vertebrates. 25 In contrast, the expression of Lef1 was stimulated. Lef1 is a transcription factor from the TCF/LEF family that binds to β‐catenin in the nucleus after Wnt activation and then induces the transcription of Wnt target genes. 25 Lef1 is also known to activate the expression of epithelial‐mesenchymal transformation–related target genes independently from β‐catenin in cancer cells, increasing their proliferation and invasiveness. 33 Moreover, in pre‐osteoblast‐like MC‐3 T3 cells, overexpression of Lef1 inhibits terminal differentiation. 34 The overexpression of Lef1 induced by ATRA in MEPM cells may thus enhance the β‐catenin‐dependent inhibition of terminal differentiation. The expression of more indirectly Wnt‐related genes (Lrp5, Lrp6, Myc) was not affected by ATRA.
5. CONCLUSIONS
In summary, our data indicate that ATRA inhibits the differentiation of MEPM cells into osteoblasts by inhibiting Wnt signalling. In vivo, this might contribute to vitamin A‐dependent CLP and CP in both animal models and humans. More specifically, the bone‐related effects of vitamin A might contribute to the aetiology of submucous clefts. However, the exact molecular mechanisms of vitamin A excess and deficiency in the aetiology of CLP and CP remain to be established.
AUTHOR CONTRIBUTIONS
CK: performed experiments, data analysis, figures, discussion of data and manuscript writing. LR: Did experimental design and isolated the cells. MB: performed part of the experiments. JH: Did experimental design, discussion of data and manuscript writing. All authors contributed to the article and approved the submitted version.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This research was supported in part by the Departamento Administrativo de Ciencia Tecnología e Innovación (Colciencias), Colombia.
Krutzen CLJ, Roa LA, Bloemen M, Von den Hoff JW. Excess vitamin a might contribute to submucous clefting by inhibiting WNT‐mediated bone formation. Orthod Craniofac Res. 2023;26:132‐139. doi: 10.1111/ocr.12594
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dixon MJ, Marazita ML, Beaty TH, Murray JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2011;12(3):167‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray JC. Gene/environment causes of cleft lip and/or palate. Clin Genet. 2002;61(4):248‐256. [DOI] [PubMed] [Google Scholar]
- 3. Reiter R, Bosch S, Lüdeke M, et al. Genetic and environmental risk factors for submucous cleft palate. Eur J Oral Sci. 2012;120(2):97‐103. [DOI] [PubMed] [Google Scholar]
- 4. Li C, Lan Y, Jiang R. Molecular and cellular mechanisms of palate development. J Dent Res. 2017;96(11):1184‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meng L, Bian Z, Torensma R, Von den Hoff JW. Biological mechanisms in palatogenesis and cleft palate. J Dent Res. 2009;88(1):22‐33. [DOI] [PubMed] [Google Scholar]
- 6. Rutkovskiy A, Stenslokken KO, Vaage IJ. Osteoblast differentiation at a glance. Med Sci Monit Basic Res. 2016;22:95‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maeda K, Kobayashi Y, Koide M, et al. The regulation of bone metabolism and disorders by Wnt signaling. Int J Mol Sci. 2019;20(22):5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116(5):1202‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mammadova A, Zhou H, Carels CE, Von den Hoff JW. Retinoic acid signalling in the development of the epidermis, the limbs and the secondary palate. Differentiation. 2016;92(5):326‐335. [DOI] [PubMed] [Google Scholar]
- 10. Henning P, Conaway HH, Lerner UH. Retinoid receptors in bone and their role in bone remodeling. Front Endocrinol (Lausanne). 2015;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johansen AM, Lie RT, Wilcox AJ, Andersen LF, Drevon CA. Maternal dietary intake of vitamin a and risk of orofacial clefts: a population‐based case‐control study in Norway. Am J Epidemiol. 2008;167(10):1164‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krapels IP, van Rooij IA, Ocke MC, West CE, van der Horst CM, Steegres‐Theunissen RP. Maternal nutritional status and the risk for orofacial cleft offspring in humans. J Nutr. 2004;134(11):3106‐3113. [DOI] [PubMed] [Google Scholar]
- 13. Choi JW, Park HW, Kwon YJ, Park BY. Role of apoptosis in retinoic acid‐induced cleft palate. J Craniofac Surg. 2011;22(5):1567‐1571. [DOI] [PubMed] [Google Scholar]
- 14. Nugent P, Sucov HM, Pisano MM, Greene RM. The role of RXR‐alpha in retinoic acid‐induced cleft palate as assessed with the RXR‐alpha knockout mouse. Int J Dev Biol. 1999;43(6):567‐570. [PubMed] [Google Scholar]
- 15. Kirimoto A, Takagi Y, Ohya K, Shimokawa H. Effects of retinoic acid on the differentiation of chondrogenic progenitor cells, ATDC5. J Med Dent Sci. 2005;52(3):153‐162. [PubMed] [Google Scholar]
- 16. Malladi P, Xu Y, Yang GP, Longaker MT. Functions of vitamin D, retinoic acid, and dexamethasone in mouse adipose‐derived mesenchymal cells. Tissue Eng. 2006;12(7):2031‐2040. [DOI] [PubMed] [Google Scholar]
- 17. Chen M, Huang HZ, Wang M, Wang AX. Retinoic acid inhibits osteogenic differentiation of mouse embryonic palate mesenchymal cells. Birth Defects Res A Clin Mol Teratol. 2010;88(11):965‐970. [DOI] [PubMed] [Google Scholar]
- 18. Yu Z, Xing Y. All‐trans retinoic acid inhibited chondrogenesis of mouse embryonic palate mesenchymal cells by down‐regulation of TGF‐beta/Smad signaling. Biochem Biophys Res Commun. 2006;340(3):929‐934. [DOI] [PubMed] [Google Scholar]
- 19. Green AC, Kocovski P. Retinoic acid receptor signalling directly regulates osteoblast and adipocyte differentiation from mesenchymal progenitor cells. Exp Cell Res. 2017;350(1):284‐297. [DOI] [PubMed] [Google Scholar]
- 20. Roa LA, Bloemen M, Carels CEL, Wagener FADTG, Von den Hoff JW. Retinoic acid disrupts osteogenesis in pre‐osteoblasts by down‐regulating WNT signaling. Int J Biochem Cell Biol. 2019;116:105597. [DOI] [PubMed] [Google Scholar]
- 21. Hu X, Gao J, Liao Y, Tang S, Lu F. Retinoic acid alters the proliferation and survival of the epithelium and mesenchyme and suppresses Wnt/beta‐catenin signaling in developing cleft palate. Cell Death Dis. 2013;4:e898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang YD, Dong SY, Huang HZ. Inhibition of periderm removal in all‐trans retinoic acid‐induced cleft palate in mice. Exp Ther Med. 2017;14(4):3393‐3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abbott BD, Harris MW, Birnbaum LS. Etiology of retinoic acid‐induced cleft palate varies with the embryonic stage. Teratology. 1989;40(6):533‐553. [DOI] [PubMed] [Google Scholar]
- 24. Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183‐196. [DOI] [PubMed] [Google Scholar]
- 25. Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. 2012;4(11):a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lind T, Sundqvist A. Vitamin a is a negative regulator of osteoblast mineralization. PLoS One. 2013;8(12):e82388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li N, Xu Y, Zhang H, et al. Excessive retinoic acid impaired proliferation and differentiation of human fetal palatal chondrocytes (hFPCs). Birth Defects Res B Dev Reprod Toxicol. 2014;101(3):276‐282. [DOI] [PubMed] [Google Scholar]
- 28. James AW, Levi B, Xu Y, Carre AL, Longaker MT. Retinoic acid enhances osteogenesis in cranial suture‐derived mesenchymal cells: potential mechanisms of retinoid‐induced craniosynostosis. Plast Reconstr Surg. 2010;125(5):1352‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mattinzoli D, Messa P, Corbelli A, et al. A novel model of in vitro osteocytogenesis induced by retinoic acid treatment. Eur Cell Mater. 2012;24:403‐425. [DOI] [PubMed] [Google Scholar]
- 30. Green AC, Martin TJ, Purton LE. The role of vitamin a and retinoic acid receptor signaling in post‐natal maintenance of bone. J Steroid Biochem Mol Biol. 2016;155(Pt A):135‐146. [DOI] [PubMed] [Google Scholar]
- 31. Song HM, Nacamuli RP, Xia W, et al. High‐dose retinoic acid modulates rat calvarial osteoblast biology. J Cell Physiol. 2005;202(1):255‐262. [DOI] [PubMed] [Google Scholar]
- 32. Wang A, Ding X, Sheng S, Yao Z. Retinoic acid inhibits osteogenic differentiation of rat bone marrow stromal cells. Biochem Biophys Res Commun. 2008;375(3):435‐449. [DOI] [PubMed] [Google Scholar]
- 33. Santiago L, Daniels G, Wang D, Deng FM, Lee P. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am J Cancer Res. 2017;7(6):1389‐1406. [PMC free article] [PubMed] [Google Scholar]
- 34. Kahler RA, Galindo M, Lian J, Stein GS, van Wijnen AJ, Westendorf JJ. Lymphocyte enhancer‐binding factor 1 (Lef1) inhibits terminal differentiation of osteoblasts. J Cell Biochem. 2006;97(5):969‐983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


