Abstract
Nerve growth factor (NGF) is a neurotrophin that has been implicated in pain signaling, apoptosis, inflammation and proliferation. The resultant effects depend on interaction with two different receptors; tyrosine kinase A (TrkA) and p75NTR. NGF increases in synovial fluid from osteoarthritic joints, and monoclonal antibody therapy is trialed to treat osteoarthritis (OA)‐related pain. Investigation of the complex and somewhat contradictory signaling pathways of NGF is conducted in neural research, but has not followed through to orthopaedic studies. The objectives of this study were to compare the expression of NGF receptors and the downstream regulator BAX in synovial membranes from joints in various stages of OA. The horse was used as a model. Synovial membranes were harvested from five healthy horses postmortem and from clinical cases with spontaneous OA undergoing arthroscopic surgery for lameness. Four horses with synovitis without gross cartilage changes, four horses with synovitis and cartilage damage, and four horses with synovitis and intracarpal fractures were included. Samples were investigated by immunohistochemistry and results showed that nuclear staining of TrkA, p75NTR and BAX increases in OA‐associated synovitis. TrkA expression increased in early disease stages whereas increases in p75NTR were most prominent in later disease stages with cartilage damage and fibrosis. Clinical significance: Suppression of NGF may result in varied effects depending on different stages of the osteoarthritic disease process.
Keywords: immunohistochemistry, nerve growth factor, osteoarthritis, p75, TrkA
1. INTRODUCTION
Nerve growth factor (NGF) is a neurotrophin that since its discovery in the 50s 1 has been implicated in a wide range of physiological and pathological processes such as nerve development, growth and survival, pain signaling and inflammation. 2 , 3 , 4 The resultant effects of NGF depend on receptor interaction with two different receptors; tyrosine kinase A (TrkA) and pan‐neurotrophin receptor (p75NTR). NGF is secreted in both a mature form (mNGF) and in a proform (proNGF), although many studies do not specify what forms are studied. The current understanding is that mNGF binds preferentially to TrkA, while proNGF has been shown to have greater affinity for the p75NTR receptor. 5 , 6 TrkA binding promotes nerve growth and survival. p75NTR binding to proNGF in the absence of TrkA can reduce nerve growth or induce apoptosis via activation of Bcl‐2‐Associated X protein (BAX). 7 , 8 , 9 Both receptors impact on transmission of pain signals 10 and NGF has been shown to cause direct neuronal sensitization 11 and nerve sprouting, 12 which could cause increased nociception.
Human patients with osteoarthritis (OA) of the knee have been shown to have increasing synovial fluid concentrations of NGF with increased disease severity, 13 and serum NGF has been shown to be higher in OA patients compared to healthy controls. 14 Since OA is a whole joint disease and not only defined by articular cartilage pathology, the synovial membrane likely has an integral role in the OA disease process. 15 , 16 Synoviocytes have been shown to express NGF and NGF receptors and the expression was increased after stimulation with interleukin‐1 and tumor necrosis factor‐α, indicating an influence of inflammation. 14 , 17
The horse has been suggested as an animal model for studying disease mechanisms in human OA. 18 , 19 Horses have a genome structure with substantial synteny with humans, 20 develop OA spontaneously without iatrogenic intervention and provide an approximation for human OA in terms of cartilage thickness and morphology. 18 , 21 The possibility of harvesting cartilage and synovial membrane specimens and chondrocytes from articular cartilage from euthanized horses immediately post mortem provides good access to material, both from healthy and diseased joints. Moreover, the horse has been shown to have increased concentrations of NGF in articular chondrocytes, synovial membranes and synovial fluid of OA joints compared to healthy joints, 22 , 23 which is in agreement with findings in humans. 13 , 24
Most published studies so far use commercial antibodies that are directed at epitopes on the mature form of NGF, and these do not distinguish between mature and proNGF. In addition to this, it has been shown that proNGF function can change according to the relative expression of TrkA and p75NTR 7 and further investigation of receptor expression is therefore important to increase understanding of the effects of NGF. Several immunohistochemistry studies have examined NGF receptor expression in rheumatoid arthritis, 17 , 25 , 26 but information on receptor expression in spontaneous OA including comparisons with a healthy control group is, to the best of the authors' knowledge, lacking.
This study aimed to compare the expression of NGF receptors and the downstream regulator BAX in synovial membranes from healthy equine joints and from equine joints in various stages of symptomatic OA determined by the morphological phenotype that can be visualized by arthroscopic surgery.
The hypothesis was that receptor expression would increase in synovial membranes with morphological changes consistent with synovitis, compared to synovial membranes harvested from healthy joints. The hypothesis was also that receptor expression would vary in different disease stages of OA and that BAX expression would increase with increased p75NTR 27 in more advanced OA.
2. METHODS
Sample collection was approved by the Ethical Committee on Animal Experiments, Uppsala, Sweden (Dnr: 5.8.18‐02896/2018).
2.1. Horses
Synovial membranes from healthy and OA carpal joints were sampled for immunohistochemistry and owner consent was obtained before sampling. Healthy joints were sampled from horses euthanized for reasons unrelated to the musculoskeletal system. Joints had macroscopically normal cartilage on post mortem arthrotomy. Synovial membranes and full‐thickness cartilage samples from the radial facet of the third carpal bone (C3) were obtained within 1 h of euthanasia and placed in 10% neutral buffered formalin for 2–4 days before paraffin embedding. Before inclusion of healthy joints, cartilage samples were stained with hematoxylin and eosin (H&E) and examined by light microscopy to rule out changes consistent with early OA. Osteoarthritic carpal joints were sampled from clinical cases during arthroscopic surgery. Three groups of horses were included: horses with synovitis but no gross cartilage changes, horses with synovitis and macroscopically visible cartilage damage and horses with synovitis and intra‐carpal fractures. Horses were grouped according to the clinical diagnosis made by the attending surgeon.
2.2. Synovial membrane grading
All synovial membranes were sectioned and stained with H&E. They were evaluated for degree of cellular infiltration, vascularity, intimal hyperplasia, subintimal edema and subintimal fibrosis. Grading of these parameters was performed according to a previously published method 28 by a board certified veterinary pathologist (SE) blinded to sample identity. The total score was used to divide specimens into groups based on severity of synovitis; normal (score 0−4), mild to moderate synovitis (score 5–9) and severe synovitis (score ≥10). Fibrosis was selected as an additional way of evaluating chronicity and three groups were identified; no or very slight fibrosis (score 0–1), mild fibrosis (score 2) and moderate to marked fibrosis (score 3–4).
2.3. Immunohistochemistry
Slides (4 µm) were deparaffinized and rehydrated. Phosphate buffered saline was used for all washes. After antigen retrieval in a 60°C water bath for 2 h, endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 5 min. Nonspecific binding was blocked with Normal Goat Serum (X0907; Dako) for 30 min. Primary antibodies were diluted and added to the sections; TrkA: 1:1500 (polyclonal, LS‐C389392; Nordic BioSite), p75NTR: 1:2000 (polyclonal, ABIN1917233; Antibodies Online) and BAX: 1:800 (AF820; R&D systems). Rabbit IgG (X0903; Dako) was run in parallel on all sections as a negative isotype control, using identical protein concentrations as for the primary antibodies. The sections were incubated at 4°C overnight, thereafter incubated with secondary antibody (EnVision K4003; Dako) for 30 min and stained with 3,3′‐diaminobenzidine tetrahydrochloride (DAB) in organic solvent for 2 or 6 min, respectively, for TrkA and p75NTR/BAX. Nuclei were counter‐stained with Mayer's hematoxylin. Finally, sections were dehydrated and mounted. Equine spinal cord was used as a positive control for TrkA and p75NTR, a lymph node with malignant lymphoma was used as a positive control for BAX. 29
All slides were stained simultaneously for each antibody, to decrease batch‐to‐batch variation in staining outcomes.
For estimation of the amount of positive synoviocytes in each sample, 100 cells in each of 10 randomly selected high power fields (HPF, ×60 magnification) were evaluated for positive intranuclear immunohistochemistry staining. 30 , 31 When there were not enough cells in one HPF to fill the quota, counting was continued in an immediately adjacent field. Slides were coded to decrease the risk of evaluator bias. In addition, to evaluate total staining (cytoplasmic and nuclear), slides were analyzed with ImageJ software (Fiji Downloads, ImageJ, National Institute of health, Bethesda, MD). Ten HPF (×40 magnification) were photographed from each slide. 31 Images were colour deconvoluted to separate the DAB positive areas. The software set the thresholds and the same settings were used for all images within the same antibody staining. Relevant areas containing synoviocytes were manually annotated, avoiding areas of vessels or subsynovial fibrous tissue. The mean percentage (%) stained area was then calculated.
2.4. Statistical analysis
Statistical analyses were performed using a commercial statistical software program, JMP Pro 16.0 (JMP Nordics). Residuals were confirmed to be normally distributed. The total synovitis score and the percentage of stained nuclei or total area staining in the four clinical groups were compared using analysis of variance and least square means estimates. Total synovitis score or percentage of positive staining was selected as the response variable with clinical diagnosis selected as model effect. Tukey's test was used for testing pairwise differences. In addition, fibrosis scores were compared between clinical groups and total and nuclear staining in groups based on total synovitis score or fibrosis score were compared as described above. The same analysis was used to investigate the impact of sex. Correlation between age and positive staining was tested by bivariate analysis.
The results are presented as mean ± SD and significance level was set at p < 0.05.
3. RESULTS
3.1. Horses
Sampled horses were 2–12 years old and four different breeds were represented. Stallions, geldings and mares were sampled. A total of 17 carpal joints were included in the study; five healthy, four with synovitis and macroscopically normal articular cartilage, four with synovitis and articular cartilage damage and four with synovitis and intra‐articular fractures with associated articular cartilage and subchondral bone damage. Three samples were obtained from radiocarpal joints, and the rest were sampled from intercarpal joints. All horses with synovitis were clinically lame at the time of sampling. For demographic data, see Table S1.
3.2. Synovial membrane grading
The healthy joints had lower total synovitis scores than the OA joints (p < 0.01), but there were no statistical differences for mean total synovitis score between the OA groups (p = 0.60). Fibrosis scores were not significantly different between the synovitis groups (p = 0.16). Total scores and fibrosis scores for the different groups are presented in Figure 1. The complete synovitis scoring of the synovial membranes is presented in Table S2.
Figure 1.
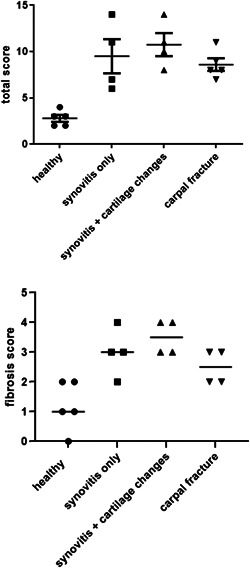
Total synovitis score and fibrosis score for the healthy and OA groups classified according to McIlwraith et al. 28 Bars show means ± SD. Mean total score for healthy joints was lower than means for OA joints (p < 0.01) but the OA group means did not differ from each other (p = 0.50). Mean fibrosis score was lower for healthy joints than for OA joints (p < 0.001) but the OA group means did not differ from each other (p = 0.16). OA, osteoarthritis
3.3. Immunohistochemistry
The % positive nuclear staining for TrkA was higher in horses with synovitis only and in horses with synovitis and articular cartilage changes, compared to healthy synovial membranes. The staining in horses with intracarpal fractures was inconsistent with scarce staining in some individuals and abundant staining in others (Figures 2, 3, 4 and Figure S3).
Figure 2.
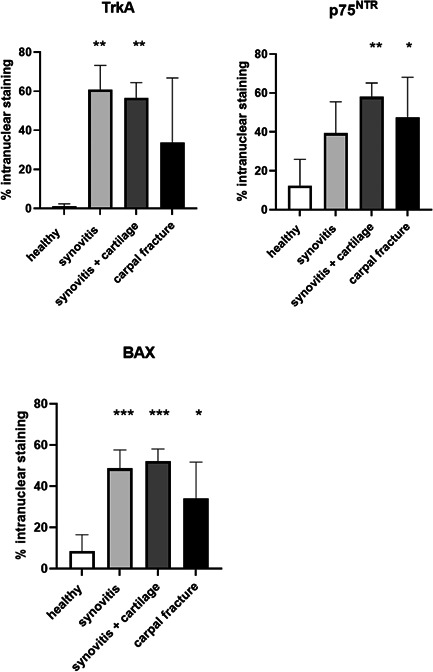
TrkA: Quantification of nuclear staining of TrkA. Staining is increased in the groups with synovitis (61% ± 12) and synovitis + articular cartilage changes (57% ± 8) compared to healthy (1% ± 1). p75NTR: Quantification of nuclear staining of p75NTR. Staining is increased in the synovitis + articular cartilage changes group (58% ± 7) and in the intracarpal fracture group (48% ± 21) compared to healthy (12% ± 14). BAX: Quantification of nuclear staining of BAX. Staining is increased in all synovitis groups (49% ± 9 for synovitis only, 52% ± 6 for synovitis and articular cartilage changes and 34% ± 18 for intracarpal fractures) compared to healthy (9% ± 8). There were no statistical differences in mean nuclear staining between different synovitis groups for either receptor. Bars indicate SD from mean. Asterisks indicate statistically significant difference from healthy; *p < 0.05, **p < 0.01, ***p < 0.001. BAX, Bcl‐2‐Associated X protein; p75NTR, pan‐neurotrophin receptor; TrkA, tyrosine kinase A
Figure 3.
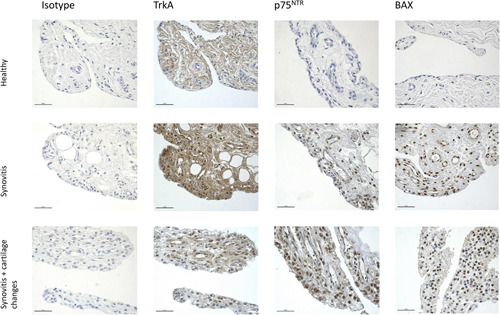
Expression of TrkA, p75NTR and BAX in synovial membranes from a healthy horse, a horse with synovitis without visible articular cartilage changes, and a horse with synovitis + visible articular cartilage changes. Isotype controls are selected from the TrkA staining but are representative of isotypes from all experiments (×40, scale bar: 50 µm).BAX, Bcl‐2‐Associated X protein; p75NTR, pan‐neurotrophin receptor;TrkA, tyrosine kinase A
Figure 4.
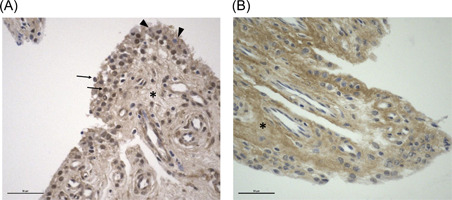
Expression of TrkA in synovial membranes from two horses with intracarpal fractures representing different nuclear staining patterns. (A) Synoviocytes with abundant intranuclear staining (arrows) and cytoplasmic staining only (arrowhead). (B) Synoviocytes with cytoplasmic staining only. In both (A) and (B) there is a marked extracellular matrix staining (asterisks). Total synovitis score for the two horses were 9 (left) and 8 (right) (×40, scale bar: 50 µm). BAX, Bcl‐2‐Associated X protein; p75NTR, pan‐neurotrophin receptor;TrkA, tyrosine kinase A
The % positive nuclear staining for p75NTR was higher in horses wit more advanced disease of the cartilage; the group with synovitis and cartilage changes as well as the carpal fracture group had significantly more abundant nuclear staining compared to the healthy group (Figures 2 and 3 and Figure S3).
The % positive nuclear staining for BAX was higher in all synovitis groups compared to healthy synovial membranes (Figures 2 and 3 and Figure S3).
There were no statistically significant differences in total TrkA, p75NTR or BAX staining between OA groups (nuclear + cytoplasmic area stain evaluated by ImageJ analysis).
When horses were grouped according to the histologic synovitis scores instead of according to the clinical diagnosis, TrkA, p75NTR and BAX nuclear staining was significantly increased in synovial membranes with total synovitis scores >5 compared to those with scores <5. When groups were based on fibrosis score only, nuclear TrkA staining was increased in groups with scores ≥2, compared to the group with scores 0–1. For p75NTR and BAX, nuclear staining was only significantly increased in the group with the highest fibrosis scores of 3–4 (Figure 5). There were no statistical differences in total staining (nuclear + cytoplasmic area stain) between groups (data not shown). There was no correlation between age and % staining and there was no difference due to sex for any of the receptors (data not shown).
Figure 5.
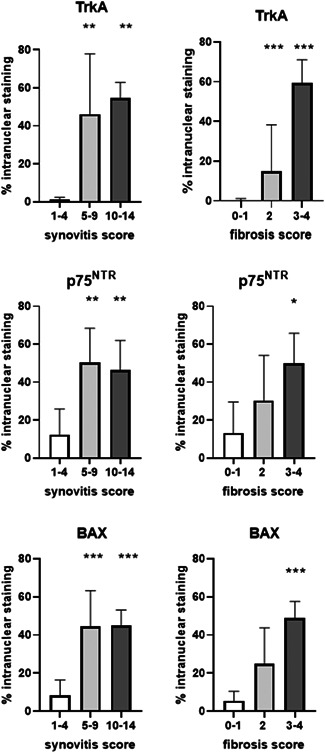
Nuclear staining for TrkA, p75NTR and BAX in groups with increasing synovitis severity (synovitis score) and with increasing fibrosis (fibrosis score). Staining was significantly increased for all three in horses with synovitis compared to healthy horses. TrkA was increased in all horses with increased fibrosis, but p75NTR and BAX was only significantly increased in the horses with moderate to severe fibrotic changes. Bars indicate SD from mean. Asterisks indicate statistically significant difference from synovitis score 1–4 or from fibrosis score 0–1; *p < 0.05, **p < 0.01, ***p < 0.001. BAX, Bcl‐2‐Associated X protein; p75NTR, pan‐neurotrophin receptor; TrkA, tyrosine kinase A
4. DISCUSSION
This is the first study to show NGF receptor expression in equine synovial membranes, and to show differences in receptor expression in different stages of OA.
TrkA staining was increased in early OA disease stages represented by synovitis without gross articular cartilage changes, whereas p75NTR increases were most prominent in later stages with articular cartilage damage and synovial fibrosis. These results are in line with previous findings that expression of inflammatory markers in synovial membranes differ between early and late OA. 32
Quantification of nuclear staining was utilized as a way of indicating receptor activation 33 , 34 and this was increased for TrkA in early OA disease stages without macroscopic cartilage lesions. The p75NTR receptor staining was only significantly increased in synovial membranes from joints with macroscopic articular cartilage changes, a later stage of OA.
Our results showed an increased expression of BAX in all synovitis groups, compared to healthy synovial membranes. NGF binding to p75NTR without involvement of TrkA can activate the c‐Jun N‐teminal kinase (JNK) pathway. 9 JNK causes a conformational change and polymerization of cytoplasmic BAX. These polymers form pores in the mitochondrial membranes, leading to the release of cytochrome C and subsequent activation of caspases, initiating apoptosis. 8 However, it was not the synoviocyte cytoplasm but the nucleus that showed a marked increase in BAX staining in diseased synovial membranes. The apoptotic effects of BAX have been shown to vary in different cell types 35 and regulatory functions of cell growth and differentiation have been suggested for intranuclear BAX. 36
Immunohistochemistry provides a snapshot image of the receptor expression in the synovial membranes. Although epitopes are identified, it is not possible from the present study to discern the activation of downstream signaling. Not only membrane bound receptors, but also cleaved internal fragments can be biologically active in the complex signaling systems. p75NTR can bind to several neurotrophins (e.g., BDNF, NT‐3, and NT‐4) and interacts with a multitude of coreceptors, creating varied downstream signaling events (reviewed in 37 , 38 ). TrkA is more specific for NGF and is activated by phosphorylation of the cytoplasmic domain after binding. The NGF‐bound TrkA is also internalized via endocytosis and can be transported retrograde toward the soma in nerve cells (reviewed in Marlin & Li 39 ). This can promote several downstream signaling pathways and TrkA receptor stimulation can increase cell proliferation in synoviocytes. 14 TrkA binding to NGF is enhanced by the presence of p75NTR. 40 It is possible that intranuclear staining of p75NTR represents pathways with TrkA interaction, as well as with other co‐receptors. The multiple possible pathways could be the reason why differences were not detected between groups when evaluated for total staining, as this will represent receptors in various stages; nuclear (active signaling), cytoplasmic (active signaling and/or endocytosed receptor fragments) and membrane bound receptors (active signaling as well as unbound, inactive receptors).
In immunohistochemistry, batch‐to‐batch variation in staining intensity can vary considerably. 41 As the TrkA and p75NTR receptor expression has not previously been quantified in different stages of OA, an effort was made to only compare within‐batch stainings for a specific antibody. Due to this, the sample sizes are small and it is possible that some differences in receptor expression between healthy and inflamed synovial membranes were missed.
Receptor expression in different stages of OA warrants further research and failure to acknowledge the complexity of NGF receptor signaling may create problems in a future clinical setting. Anti‐NGF antibody has shown promise as a novel treatment for OA‐associated pain in patients refractory to traditional analgesic treatments. 42 In 2010, trials were temporarily halted due to adverse events related to unexplained, rapidly progressing OA in some test subjects. The risk of occurrence had a dose‐response relationship and also seemed to be increased with concurrent NSAID treatment. 43 Phase III trials have since been resumed with measures such as restricted concurrent NSAID use, close monitoring of OA progression and use of lower treatment doses. Despite this, 1–3% of the subjects in the treatment groups experienced rapidly progressing OA in one phase III trial with long‐term follow‐up. 42 The reason for this is still unclear and no drugs have so far (2022) been approved for clinical use. If the relative TrkA/p75NTR receptor expression in different stages of OA varies, or if individual differences in receptor expression occur, the effect of inhibiting NGF could also be varied. Anti‐NGF therapy in a state of TrkA receptor dominance could inhibit signaling of growth and repair, while it could inhibit apoptosis in the presence of abundant p75NTR receptor expression, as has been shown in other cell types. 2
Performing the study on clinical, client‐owned cases had the disadvantage that it was not possible to fully control for factors such as prior treatment or level of training, and microscopic evaluation of articular cartilage was not possible. Using client‐owned horses instead of experimental animals also limited the information on disease duration. It is very common for owners and trainers to be unaware of mild lameness in horses 44 and as this could create information bias, collection of data on disease duration was not attempted. For six horses a full treatment history could not be obtained hence it is possible that some of these individuals had received intra‐articular treatments some weeks before surgery. However, all OA horses were lame at the time of sampling, and synovial membrane histology confirmed the presence of synovitis. The carpal fracture group had a high degree of intranuclear p75NTR, but total p75NTR stain was inconsistent (Figure S3). This could be due to individual variation or to the fact that the p75NTR receptor has many different ligands that it may respond to, making expression less relatable to NGF dynamics only. However, intranuclear TrkA staining also varied markedly in this group as demonstrated in Figure 4. It is possible that, although the carpal fracture group shared a common phenotype and all had evidence of synovitis, the pathogenesis and disease duration for the fractures could be different. Horses were of different breeds and two of the horses in the carpal fracture group were used for racing whereas the two Warmbloods would have been intended for lighter work such as pleasure riding. Some horses could have had progressive OA with secondary fractures, whereas some could have been traumatic fractures caused by excessive forces during exercise. 45 The groups were considered too small to provide conclusive data on the impact of breed on receptor expression.
There are substantial advantages of using clinical cases as supposed to creating adjuvant inflammation for the study of receptor expression when aiming to make inferences to human OA. Creating adjuvant inflammation will produce an acute inflammatory process in a previously healthy joint. This may cause different pathological processes than those represented in naturally occurring OA disease. The same applies to models of traumatic OA, where acute destabilization and/or joint surface incongruity in a previously healthy joint is not equal to spontaneous and often slowly progressing disease involving all joint structures. Innate immunity is involved in disease progression in OA and the systemic inflammatory processes of chronic OA 46 is not present in OA models using healthy research animals. The clinical cases in the present study represent naturally occurring OA disease in varied stages and include individual variation. Also, no animals were subjected to any procedures for the sole purpose of research, in line with the 3Rs.
In summary, the results of this study show that the NGF receptor and BAX expression in equine synovial membranes is increased in synovitis, and that there may be disease stage‐related differences in the relative receptor expression. This study provides information that can be used for planning of study protocols and sample size calculations for further research on TrkA and p75NTR expression and signaling pathways in human OA.
AUTHOR CONTRIBUTIONS
All authors: contributed to sample collection, read and approved the final version. Anna Kendall: performed the laboratory work and all authors analysed the data and contributed to drafting the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
This study was supported by a grant from Formas (2019‐02069). The authors would like to thank Albin Normal and Vidar Andersson for excellent laboratory support.
Kendall A, Ekman S, Skiöldebrand E. Nerve growth factor receptors in equine synovial membranes vary with osteoarthritic disease severity. J Orthop Res. 2023;41:316‐324. 10.1002/jor.25382
REFERENCES
- 1. Levi‐Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116:321‐361. [DOI] [PubMed] [Google Scholar]
- 2. Masoudi R, Ioannou MS, Coughlin MD, et al. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. J Biol Chem. 2009;284:18424‐18433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nilsson G, Forsberg‐Nilsson K, Xiang Z, Hallböök F, Nilsson K, Metcalfe DD. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997;27:2295‐2301. [DOI] [PubMed] [Google Scholar]
- 4. Indo Y, Tsuruta M, Hayashida Y, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet. 1996;13:485‐488. [DOI] [PubMed] [Google Scholar]
- 5. Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945‐1948. [DOI] [PubMed] [Google Scholar]
- 6. Clewes O, Fahey MS, Tyler SJ, et al. Human ProNGF: biological effects and binding profiles at TrkA, P75(NTR) and sortilin. J Neurochem. 2008;107:1124‐1135. [DOI] [PubMed] [Google Scholar]
- 7. Ioannou MS, Fahnestock M. ProNGF, but Not NGF, switches from neurotrophic to apoptotic activity in response to reductions in TrkA receptor levels. Int J Mol Sci. 2017;18(3):599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papadakis ES, Finegan KG, Wang X, et al. The regulation of Bax by c‐Jun N‐terminal protein kinase (JNK) is a prerequisite to the mitochondrial‐induced apoptotic pathway. FEBS Lett. 2006;580:1320‐1326. [DOI] [PubMed] [Google Scholar]
- 9. Casaccia‐Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716‐719. [DOI] [PubMed] [Google Scholar]
- 10. Sung K, Ferrari LF, Yang W, et al. Swedish nerve growth factor mutation (NGF(R100W)) defines a role for TrkA and p75(NTR) in nociception. J Neurosci. 2018;38:3394‐3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirth M, Rukwied R, Gromann A, et al. Nerve growth factor induces sensitization of nociceptors without evidence for increased intraepidermal nerve fiber density. Pain. 2013;154:2500‐2511. [DOI] [PubMed] [Google Scholar]
- 12. Jones MG, Munson JB, Thompson SWN. A role for nerve growth factor in sympathetic sprouting in rat dorsal root ganglia. Pain. 1999;79:21‐29. [DOI] [PubMed] [Google Scholar]
- 13. Montagnoli C, Tiribuzi R, Crispoltoni L, et al. Beta‐NGF and beta‐NGF receptor upregulation in blood and synovial fluid in osteoarthritis. Biol Chem. 2017;398:1045‐1054. [DOI] [PubMed] [Google Scholar]
- 14. Raychaudhuri SP, Raychaudhuri SK. The regulatory role of nerve growth factor and its receptor system in fibroblast‐like synovial cells. Scand J Rheumatol. 2009;38:207‐215. [DOI] [PubMed] [Google Scholar]
- 15. de Lange‐Brokaar BJ, Ioan‐Facsinay A, van Osch GJ, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20:1484‐1499. [DOI] [PubMed] [Google Scholar]
- 16. Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361‐367. [DOI] [PubMed] [Google Scholar]
- 17. Rihl M. Involvement of neurotrophins and their receptors in spondyloarthritis synovitis: relation to inflammation and response to treatment. Ann Rheum Dis. 2005;64:1542‐1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malda J, Benders KE, Klein TJ, et al. Comparative study of depth‐dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthr Cartil. 2012;20:1147‐1151. [DOI] [PubMed] [Google Scholar]
- 19. McIlwraith CW, Fortier LA, Frisbie DD, Nixon AJ. Equine models of articular cartilage repair. Cartilage. 2011;2:317‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wade CM, Giulotto E, Sigurdsson S, et al. Genome sequence, comparative analysis, and population genetics of the domestic horse. Science. 2009;326:865‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frisbie DD, Cross MW, McIlwraith CW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre‐clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006;19:142‐146. [PubMed] [Google Scholar]
- 22. Kendall A, Nyström S, Ekman S, et al. Nerve growth factor in the equine joint. Vet J. 2021;267:105579. [DOI] [PubMed] [Google Scholar]
- 23. Henriksson S 2016. Localization of nerve growth factor (NGF) and interleukin1β (IL‐1β) in cartilage and synovial membranes from normal and osteoarthritic equine joints. Masters Thesis, Swedish University of Agricultural Science: Available from https://stud.epsilon.slu.se/8869/11/henriksson_s_160225.pdf
- 24. Iannone F, De Bari C, Dell'Accio F, et al. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology. 2002;41:1413‐1418. [DOI] [PubMed] [Google Scholar]
- 25. Deleuran BW, Chu CQ, Field M, et al. Localization of tumor necrosis factor receptors in the synovial tissue and cartilage‐pannus junction in patients with rheumatoid arthritis. Implications for local actions of tumor necrosis factor alpha. Arthritis Rheum. 1992;35:1170‐1178. [DOI] [PubMed] [Google Scholar]
- 26. Wu Z, Nagata K, Iijima T. Immunohistochemical study of NGF and its receptors in the synovial membrane of the ankle joint of adjuvant‐induced arthritic rats. Histochem Cell Biol. 2000;114:453‐459. [DOI] [PubMed] [Google Scholar]
- 27. Coassin M, Lambiase A, Sposato V, Micera A, Bonini S, Aloe L. Retinal p75 and bax overexpression is associated with retinal ganglion cells apoptosis in a rat model of glaucoma. Graefes Arch Clin Exp Ophthalmol. 2008;246:1743‐1749. [DOI] [PubMed] [Google Scholar]
- 28. McIlwraith CW, Frisbie DD, Kawcak CE, Fuller CJ, Hurtig M, Cruz A. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the horse. Osteoarthr Cartil. 2010;18(Suppl 3):S93‐S105. [DOI] [PubMed] [Google Scholar]
- 29. Carpenter R, Brady MF 2021. BAX Gene. StatPearls. Treasure Island (FL). [PubMed]
- 30. Karamitopoulou E, Zlobec I, Kölzer V, et al. Proposal for a 10‐high‐power‐fields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol. 2013;26:295‐301. [DOI] [PubMed] [Google Scholar]
- 31. Mofidi R, Walsh R, Ridgway PF, et al. Objective measurement of breast cancer oestrogen receptor status through digital image analysis. Eur J Surg Oncol. 2003;29:20‐24. [DOI] [PubMed] [Google Scholar]
- 32. Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonacchi A, Taddei ML, Petrai I, et al. Nuclear localization of TRK‐A in liver cells. Histol Histopathol. 2008;23:327‐340. [DOI] [PubMed] [Google Scholar]
- 34. Parkhurst CN, Zampieri N, Chao MV. Nuclear localization of the p75 neurotrophin receptor intracellular domain. J Biol Chem. 2010;285:5361‐5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Middleton G, Davies AM. Populations of NGF‐dependent neurones differ in their requirement for BAX to undergo apoptosis in the absence of NGF/TrkA signalling in vivo. Development. 2001;128:4715‐4728. [DOI] [PubMed] [Google Scholar]
- 36. Brayer S, Joannes A, Jaillet M, et al. The pro‐apoptotic BAX protein influences cell growth and differentiation from the nucleus in healthy interphasic cells. Cell Cycle. 2017;16:2108‐2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vilar M. Structural characterization of the p75 neurotrophin receptor: a stranger in the TNFR superfamily. Vitam Horm. 2017;104:57‐87. [DOI] [PubMed] [Google Scholar]
- 38. Barrett GL. The p75 neurotrophin receptor and neuronal apoptosis. Prog Neurobiol. 2000;61:205‐229. [DOI] [PubMed] [Google Scholar]
- 39. Marlin MC, Li G. Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol. 2015;314:239‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mahadeo D, Kaplan L, Chao MV, Hempstead BL. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi‐subunit polypeptide receptors. J Biol Chem. 1994;269:6884‐6891. [PubMed] [Google Scholar]
- 41. Grube D. Constants and variables in immunohistochemistry. Arch Histol Cytol. 2004;67:115‐134. [DOI] [PubMed] [Google Scholar]
- 42. Berenbaum F, Blanco FJ, Guermazi A, et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24‐week randomised phase III study with a 24‐week follow‐up period. Ann Rheum Dis. 2020;79:800‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hochberg MC. Serious joint‐related adverse events in randomized controlled trials of anti‐nerve growth factor monoclonal antibodies. Osteoarthr Cartilage. 2015;23:S18‐S21. [DOI] [PubMed] [Google Scholar]
- 44. Greve L, Dyson SJ. The interrelationship of lameness, saddle slip and back shape in the general sports horse population. Equine Vet J. 2014;46:687‐694. [DOI] [PubMed] [Google Scholar]
- 45. Palmer JL, Bertone AL, Litsky AS. Contact area and pressure distribution changes of the equine third carpal bone during loading. Equine Vet J. 1994;26:197‐202. [DOI] [PubMed] [Google Scholar]
- 46. Robinson WH, Lepus CM, Wang Q, et al. Low‐grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.


