Abstract
Neuromelanins are compounds accumulating in neurons of human and animal brain during aging, with neurons of substantia nigra and locus coeruleus having the highest levels of neuromelanins. These compounds have melanic, lipid, peptide, and inorganic components and are contained inside special autolysosomes. Neuromelanins can participate in neuroprotective or toxic processes occurring in Parkinson's disease according to cellular environment. Their synthesis depends on the concentration of cytosolic catechols and is a protective process since it prevents the toxic accumulation of catechols‐derived reactive compounds. Neuromelanins can be neuroprotective also by binding reactive/toxic metals to produce stable and non‐toxic complexes. Extraneuronal neuromelanin released by dying dopamine neurons in Parkinson's disease activates microglia which generate reactive oxygen species, reactive nitrogen species, and proinflammatory molecules, thus producing still neuroinflammation and neuronal death. Synthetic neuromelanins have been prepared with melanic, protein structure, and metal content closely mimicking the natural brain pigment, and these models are also able to activate microglia. Neuromelanins have different structure, synthesis, cellular/subcellular distribution, and role than melanins of hair, skin, and other tissues. The main common aspect between brain neuromelanin and peripheral melanin is the presence of eumelanin and/or pheomelanin moieties in their structure.
Keywords: iron, melanins, microglia, neuroinflammation/neurodegeneration, neuromelanins, Parkinson's disease, synthetic neuromelanins
Abbreviations
- AD
Alzheimers disease
- DA
dopamine
- EPR
electron paramagnetic resonance
- LC
locus coeruleus
- L‐DOPA
L‐3,4‐dihydroxyphenylalanine
- NM
neuromelanin
- PD
Parkinsons disease
- SN
substantia nigra
- VMAT2
vesicular monoamine transporter 2
- VTA
ventral tegmental area
1. INTRODUCTION
Neuromelanins (NMs) are a family of compounds present in neurons of many regions of the human and animal brain as shown by morphological and neurochemical studies. These compounds are mostly abundant in human substantia nigra (SN) and locus coeruleus (LC) catecholaminergic neurons. 1 , 2 Both these regions are early targeted in Parkinson's disease (PD), while in Alzheimer's disease (AD) only LC is early damaged. 3 , 4 In AD and PD, the NM‐containing neurons are preferentially lost compared to those without NM. Considering the presence of NM in vulnerable neurons of these diseases, it has been discussed for long time whether NMs are protective or toxic. 5 , 6 During normal aging there is a continuous accumulation of NMs in neurons, especially in SN and LC, so that an increasing pigmentation is observed, 7 while in PD and AD there is a severe decrease of pigmentation, visible to the eye and at histopathological examination. 3
The synthesis of NMs requires high neuronal content of catecholamines. 8 Several enzymes have been hypothesized to be involved in the oxidative phase of NM synthesis, however none of them was shown to play a key role. 2 , 9 NMs are accumulated within autolysosomal organelles together with several proteins and lipid bodies mainly containing dolichols. 10 Isolated NM pigments are dark‐brown and are insoluble in water, organic solvents and are slightly soluble in basic solutions. Purified NMs contain melanic, lipid and protein components and inorganics. 11 , 12 In the inorganic component, an important role is played by metals like iron, copper, and zinc. 1 NMs shares several aspects with amyloid‐β protein like insolubility, accumulation in aging, affinity for metals, ultrastructural features, synthesis that removes toxic precursors and neurotoxic effect through activation of microglia. 13
Investigation of NM structure and role has been always a challenge since it can be isolated and purified in low amount from human brain. Moreover, the low solubility of NM prevents its use in important structural methods. Then, adequate models that mimic the structure and behavior of natural NMs have been synthesized also providing a reasonable solubility. 14 , 15 Considering structure, synthesis, interactions, and behavior, the brain NMs are different from melanins present in peripheral tissues like skin, hair, eyes, and others. Unlike brain NM, in these tissues the melanin synthesis is depending on the presence of tyrosinase, and two types of melanins can be identified: brown‐black eumelanin and yellow‐red pheomelanin. 2 , 16
2. SYNTHESIS AND ACCUMULATION OF NMs IN NEURONS DURING AGING
NMs are synthesized and accumulated during lifespan in neurons of several brain regions of human and animal brain. The main accumulation of NM, reaching levels as high as 3–4 mg/g brain tissue, is observed in catecholamine neurons of SN and LC of elderly humans. 7 The NMs have been described in SN of primates, cats, dogs, sheep, goats, and horses in variable amount depending on age and type of animal, with the lowest or undetectable amount in rodents and the highest in primates. These differences are related to the different dopamine (DA) metabolism and the slow age‐related process of accumulation. 17 , 18 , 19 , 20
In cultured DA neurons of rat SN, it was shown that NM biosynthesis is depending on cytosolic concentration of DA not accumulated in synaptic vesicles, so that neurons overexpressing vesicular monoamine transporter 2 (VMAT2) have low cytosolic DA and therefore low NM synthesis. 8 In murine neurons of LC, the disruption of VMAT2 induces an increase of norepinephrine cytosolic content and synthesis of NM, suggesting an inverse relationship between VMAT2 expression and NM content in LC. 21 , 22 Then, the synthesis of NMs in SN and LC is clearly a protective process that removes excess of cytosolic catechols that otherwise will be oxidized to reactive/toxic quinones, which in turn could react with proteins altering their structure and function. In human SN and LC, it was demonstrated that precursors of NM synthesis are DA, norepinephrine, and their metabolites. 23
Interestingly, an inverse relationship has been observed between NM content and neuronal vulnerability on one side, and VMAT2 expression on the other side in human midbrain neurons. 24 , 25 Indeed, regions like ventral tegmental area (VTA), SN pars reticulata and SN pars compacta, in the mentioned order, have increasing content of NM and increasing neuronal vulnerability, while VMAT2 expression is decreasing from VTA to SN pars reticulata and pars compacta. 24 In principle, the accumulation and toxic role of NM (as explained in a next section), as well as related neuronal vulnerability, could be counteracted by treatments overexpressing VMAT2, which would reduce the high level of cytosolic DA, then DA‐derived toxicity, and also synthesis of NM with its neurodegenerative effects would be decreased. 8 , 25
In other brain regions like putamen, premotor cortex and cerebellum the presence of NMs was also reported. 1 There are neurons in these regions expressing tyrosine hydroxylase which converts L‐tyrosine into L‐3,4‐dihydroxyphenylalanine (L‐DOPA). 26 , 27 Indeed, it was demonstrated that NM isolated from these regions mainly derive from oxidation of DOPA and not from catecholamines, since these neurons expressing tyrosine hydroxylase do not express aromatic L‐amino acid decarboxylase necessary for DA biosynthesis, so that only DOPA is synthesized. 1 , 27
The synthesis of NM starts with iron/copper‐mediated oxidation of catechols to quinones which reacts with cysteine, histidine and lysine of fibrillated proteins that form seeds of aggregated oligomers of peptides/proteins, then further oxidation to melanin takes place. Among the fibrillated proteins that can interact with quinones, alpha‐synuclein and glycoprotein nonmetastatic melanoma protein B were identified in NM‐containing organelles of SN, although other proteins able to form insoluble fibrils with cross β‐structure are likely involved. The complexes iron/copper‐melanin‐cross β sheet proteins aggregates are not degradable by proteasome system, and therefore are incorporated by double membranes to form an autophagosome, which fuses with lysosome producing an autolysosome. This autolysosome again fuses with other vesicles that contain further proteins and lipids. At the end, inside autolysosomes the adduct iron/copper‐melanin‐β sheet proteins will react with lipids to generate the final NM pigment within the organelle. 10 , 12 The mechanism through which lipids are bound to the melanin‐protein moieties of NM is still completely unknown.
In addition to iron and copper, 7 large amounts of zinc have been found into human NMs of SN and other brain regions. 1 , 28 , 29 Smaller quantities of other metals like aluminum, manganese, chromium, molybdenum, lead, and mercury are present in NMs isolated from different regions of the human brain and part of these metals arises from environmental exposure. 1 Then, human NM pigment can bind reactive iron and copper, as well as other metals, forming stable complexes and blocking their neurotoxic effects that could take place through redox reactions and other processes. These observations indicate a neuroprotective role of NM against metal‐derived toxicity particularly in brain areas highly enriched of NM pigment, like SN and LC regions involved in neurodegenerative processes of PD and AD. 7 These metals could be incorporated into NM during the first steps of NM biosynthesis participating to the oxidation of catechols to quinones or they can be carried by proteins in the formation of complexes with NM pigment. It is interesting that in NM‐containing neurons reactive iron is not found, while neurons without NM have several deposits of reactive iron 2 , 7 : this suggests that when reactive iron is produced, the NM immediately chelate it forming a stable complex buffering iron‐related toxicity. In NMs, iron(III) is present in sites of different nuclearity. The simplest sites are constituted by mononuclear iron sites, where the metal is six‐coordinated by oxygen atoms of catechol moieties, and possibly by water or hydroxo groups, in a distorted octahedral arrangement and high‐spin configuration, so that these centers are detectable by electron paramagnetic resonance (EPR) spectroscopy. These sites are of low or moderate affinity and the metal can be removed at least partially by treatment with chelating agents. Most of iron is bound to NM in multinuclear clusters of likely variable nuclearity, where iron(III) centers are linked by oxo and/or hydroxo bridges and through catechol groups. These sites are of high affinity and contain strongly coupled and EPR silent iron(III) centers. 2 , 30 The NM‐iron complex was shown to be the major player of the contrast mechanisms in magnetic resonance imaging of NM in human SN, thus allowing to image the loss of DA neurons containing NM and to diagnose PD. 31 , 32
In NM‐containing organelle of the human SN many lysosomal proteins of soluble and membrane type have been found, with large number of peptidases, sulfatases, and esterases but low amount of glycosylases and lipases, suggesting that this special organelle has less degradation capacity than typical lysosome. 10 , 33 Important aspects concerning the last steps of NM synthesis and its storing as final form of NM should be considered. The NM‐containing organelle has a macroautophagic genesis, as shown by its double membrane and the presence of proteins like microtubule‐associated proteins 1A/1B light chain 3B, autophagic adaptor sequestosome‐1, alpha‐crystallin B chain, heat shock protein HSP 90‐alpha, tubulin polymerization‐promoting protein, glycoprotein nonmetastatic melanoma protein B and ubiquitins, all involved in macroautophagy and bulk degradation processes. 10
The NM biosynthesis involves also the lipids stored in lipid bodies of NM‐containing organelles, that are mainly dolichols and dolichoic acids with smaller amounts of glycerophospholipids, sphingolipids, and free fatty acids. 10 Dolichols and dolichoic acids, the prevalent molecules of lipid bodies which are also strictly associated with NM pigment, 34 , 35 are likely transported inside NM‐containing organelles as dolichyl esters by lysosome membrane protein 2 and apolipoprotein D present in the organelle, and later hydrolyzed to dolichols by abundant esterases, and can also be transported inside NM‐containing organelles by vesicle transport and membrane fusion with other oganelles. 10
During life NM‐containing organelles accumulate undegradable NM pigment, proteins, lipids, and metals. The NM itself and the particular feature of its organelle inhibits peptidase enzyme activity, then several of above‐mentioned proteins can be accumulated. Electron microscopy images show that autolysosomal organelles that contain NMs are 0.5–3.0 μm in size and may have slightly different shapes depending on the neuron and region where they are observed. 1 , 10 , 30 Studies with scanning electron microscopy on the NMs pigments isolated from different brain regions reveal aggregates of spherical units with about 200 to 400 nm diameter. 36 , 37 Images obtained by atomic force microscopy show that these units are composed of substructures whose size is about 30 nm. 1 , 36 , 37 The SN and LC have the highest number of pigmented neurons of the brain, and each neuron has more NM‐containing organelles than the neurons of putamen, premotor cortex, and cerebellum, caudate nucleus, globus pallidus, occipital, parietal, and temporal cortexes. 1 , 12
Outside central nervous system it was reported in few cases the presence of pigmented nodules in the adrenal gland, with cells showing positive reaction for Fontana‐melanin staining. Electron microscopy showed the presence of few NM‐like organelles but with morphology very different from that of NM‐containing organelles observed in neurons of human SN. 38 In any case, further investigation is required to identify the nature of this pigment and its related organelle.
3. STRUCTURAL CHARACTERISTICS OF NMs OF THE HUMAN BRAIN
For a number of years, the structural features of brain NMs have been described in terms of that of the more broadly diffused peripheral melanins. 16 , 39 This is generally referred to as the “casing model” of mixed melanogenesis, 40 which basically represents NM as composed of spheroidal particles containing a core of pheomelanin surrounded by an external coat of eumelanin. 36 The latter is more chemically resistant and photoprotective, which is an extremely important property for skin melanin. 16 These properties depend on the peculiar organization of the eumelanic component, which is synthesized enzymatically by tyrosinase producing L‐DOPA or catecholamine oligomers assembled in layers with aromatic stacking interactions. 41 The layers are kept close to each other at a distance of 3.5 Å, typical of aromatic stacks, but this feature is completely absent in the X‐ray powder spectra of the NMs isolated from all brain regions, indicating that human brain NM must be organized in a different way. An important clue to unveiling the different structural organization of human brain NMs came from the X‐ray powder data, that systematically showed a 4.7 Å motif (corresponding to the lateral distance between adjacent backbone peptide chains) typical of cross‐β structure of fibrillated proteins. 1 This shows that the melanic component of brain NMs is not structurally organized and a different biosynthetic model is then necessary to describe its formation, as a random chemical rather than enzymatic process, and assuming that the core of NM particles must be constituted by protein fibrils rather than only pheomelanin.
The first indication of a possible way to build up an unstructured melanic coat around the protein core came from a study of dopamination of myoglobin, a protein with a rigid α‐helical structure. 42 Analysis of the modified fragments after proteolysis, indeed, showed that after initial attack of nucleophilic residues from cysteine (in human myoglobin) or histidine (in horse heart myoglobin) to DA‐quinone, the resulting electron‐rich catechol drives further melanization upon oxidation to quinone and addition of DA molecules in a sequence of oxidation/condensation reactions. 30 As it is known that cysteine residues are by far more reactive than other amino acids toward quinones, 30 , 43 the pathway involving cysteine thiol is represented in Figure 1. This process was systematically confirmed in our subsequent attempts to synthesize models for the melanin‐protein core of brain NM, using serum albumin, 14 or β‐lactoglobulin. 15 Although, we were interested to obtain soluble melanin‐protein conjugates and, therefore, we limited the protein dopamination to oligomers of limited size, the melanization process inevitably leads to insoluble products when allowed to proceed for extended time. In all these melanin‐protein conjugates the melanic portion is unstructured and it was important to find that when fibrillated β‐lactoglobulin was used in the synthesis, the isolated conjugate showed that DA melanization did not disrupt the fibrillar core of the protein, yielding the characteristic pattern of cross‐β fibrils in the X‐ray powder spectra. 15
FIGURE 1.

Initial steps of the biosynthetic pathway to the melanin‐protein core of NM. The DA quinone formed by metal catalyzed DA oxidation undergoes nucleophilic attack by the side chains of polar amino acids, indicated as Protein^XH (i.e., cysteine –SH; histidine –NH; or also lysine –NH2), exposed on the surface of fibril seeds. As cysteine thiols are by far the most reactive groups against the quinone, the reaction of this particular protein residue is shown in the figure. Melanization then proceeds by a sequence of oxidation/condensation of the electron rich catechol residues attached to the protein fibrils.
We strongly believe that the pathway to melanization indicated by our model studies reflects the biosynthetic pathway of the initial core of human brain NM as it occurs in the cytosol starting from seeds of protein fibrils, which better expose the polar side chains of amino acids on the surface, making the nucleophilic attack to catecholamine quinones easier. The excess of DA (and other catecholamines) present in the environment continues the reaction chain to generate the unstructured melanin core. The process of further maturation and lipidation of the initial melanin‐protein core has been described elsewhere. 2 , 10
4. ROLE OF HUMAN NMs IN NEUROINFLAMMATION AND NEURODEGENERATION: APPLICATIONS OF MODEL NMs IN IN VITRO AND IN VIVO NEURODEGENERATIVE SYSTEMS
The massive presence of NM pigment inside dopaminergic neurons of the SN, the brain area mostly damaged in PD, has indicated NM as key factor for selective vulnerability of these neurons. 44 The loss of NM‐containing neurons of the SN during PD, as demonstrated by histopathological studies, 6 , 45 was also confirmed by the huge decrease of NM concentration measured in the SN of PD patients. 46 Many studies described a common phenomenon in the SN of PD subjects: the presence of several extracellular NM deposits, as result of severe degeneration of pigmented SN dopaminergic neurons over the disease. 47 Notably, these extracellular NM debris have been observed in close proximity to activated microglia in the SN of patients with PD and parkinsonism with dementia, 47 , 48 or toxin‐induced parkinsonism. 49 Phagocytic microglia are responsible for the disappearance of NM pigment in the SN following to neuronal death, since NM debris have been observed inside reactive microglia. 47 Interestingly, extracellular NM in PD behaves like amyloid‐β protein in AD, since both compounds can activate microglia leading to exacerbation of neurodegenerative processes. 13 Then, SN dopaminergic neurons seem to be particularly vulnerable to inflammatory insult that is mainly generated by glia, mostly microglia and astroglia, since reactive astrocytes and microglia have been reported in PD SN indicating a robust inflammatory state. 47 , 50
All these data converge on the key role of NM in glia activation and disease progression. To deeply understand these mechanisms, NM purified from human SN has been used to study the role of NM in driving this inflammatory state in in vitro and in vivo models. An early report demonstrated that human NM added to microglia cultures was able to induce positive chemotactic effects, microglial activation with subsequent release of proinflammatory and neurotoxic mediators like tumor necrosis factor α, interleukin 6, and nitric oxide. 51 This model was later improved showing that NM, rapidly phagocytized and degraded by microglia, induced microglial activation ensuing production of reactive oxygen and nitrogen species, together with several proinflammatory factors. Moreover, this updated in vitro model demonstrated that NM induced severe neurons degeneration in neurons/microglia co‐cultures, showing that this microglia‐mediated process likely involves macrophage antigen complex‐1 and phagocytic oxidase of microglia. 52 Indeed, microglia derived from mice deficient in phagocytic oxidase or macrophage antigen complex‐1, when activated by human NM induced less neuronal degeneration. Then, drugs blocking these microglial proteins could have neuroprotective effects reducing microglia‐mediated toxic effect of NM. 52
These neuroinflammatory/neurodegenerative effects have been also investigated by using in vivo models: injection of human NM into rat SN caused strong microglia activation with significant degeneration of dopaminergic neurons. 52 , 53 In the intranigral injection area, NM was also able to induce a moderate astrocytosis. 52 Then, the role of NM on human astroglial cells was investigated in vitro showing that human NM exposure caused inhibition of the tumor necrosis factor α induced expression of the chemokine interferon γ inducible protein‐10, 54 demonstrating that NM can affect inflammatory signaling also in human astroglial cells, even though this interplay deserves further studies.
In addition to the activation of tissue‐resident cells like microglia and astroglia, NM could have effects on other immune cells: an in vitro study reported that after exposure to human NM, dendritic cells effectively phagocytized NM, developed mature phenotype while secreting proinflammatory cytokines and triggering T cell proliferation and response. 55 Moreover, a novel inflammatory T cell‐mediated degenerative mechanism has been suggested to involve the major histocompatibility complex class I, which is highly concentrated in NM and expressed in dopaminergic neurons containing NM of the SN mostly targeted in PD. 56 In this regard, high expression of the major histocompatibility complex class I was also observed in NM‐containing neurons of the LC, the norepinephrine brain area targeted in PD and early damaged in AD. The major histocompatibility complex class I can bind antigenic peptides derived by cleavage of foreign or native proteins and present them on plasma membrane, then these NM‐containing neurons can be particularly susceptible to T cell‐mediated cytotoxic attack inducing selective neurodegeneration. 56
These findings explain the role of NM in neuroinflammatory/neurodegenerative processes of PD: after an initial neuronal damage, NM released from dying neurons induces microglial activation with production of neurotoxic molecules which damage other neurons with further release of NM, establishing a vicious condition of neuroinflammation and neurodegeneration. In addition, due to its poor solubility, NM could remain for long time in the extracellular milieu of SN sustaining this chronic inflammatory process that can be exacerbated also by release of toxic compounds and redox active metals immobilized into NM structure. 57 , 58
Synthetic melanins have been used to induce and study neurodegenerative and neuroinflammatory processes in vitro and in vivo, replacing classic exogenous toxins which cause only acute damage that is far from what really happens in a PD brain. These synthetic models, obtained by DA autoxidation, have been used to study effects on dendritic cells, with very little effect on their phenotype and function, since these compounds were just synthetic melanins with structure and composition very different from human NM. 55 A later in vitro and in vivo study showed that similar synthetic model of melanin with the addition of cysteine induced microglia activation with up‐regulation of a variety proinflammatory mediators, in a caspase‐dependent manner. 59 Both studies provided an approach in the study of molecular mechanisms underlying neuroinflammation of PD, although used very simple analogues. This limitation was later overcome with the synthesis of new NM models, comprising a more realistic melanic portion with amyloid cross‐β protein core and iron components, which were used in vitro (Figure 2), showing that these models were able to activate microglia cells stimulating the proinflammatory response as human NM does. 15
FIGURE 2.
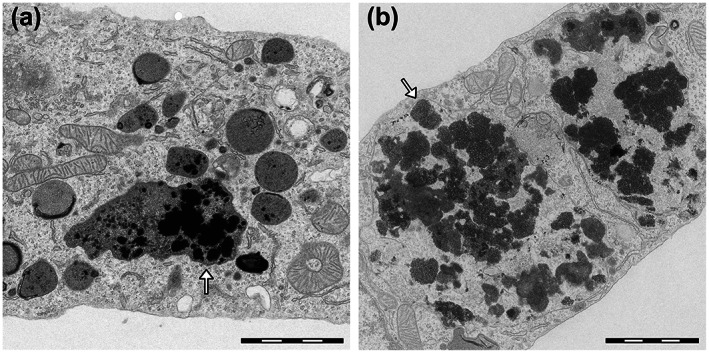
Transmission electron microscopy images of rat microglia cultures exposed to synthetic NM models (a) and human NM pigment isolated from SN (b). Both synthetic and natural NM appear in microscopy images as black and electron‐dense pigments (arrows). The synthetic NM used in this in vitro model has a composition similar to that of the natural pigment, with a melanin‐protein core obtained by melanization of fibrillated β‐lactoglobulin with the presence of iron. Both compounds were actively phagocytosed by microglia cells and engulfed inside specialized phagosomes (arrows) for future breakdown and degradation of the pigments. During this process, this synthetic NM, as well as similar analogues with minor differences in their constituents, were able to promote microglia activation that was assessed by analysis of activation of typical proinflammatory markers in a manner that was similar to that of human NM. Scale bar = 2 μm. Reprinted (adapted) with permission from ref. 15 Copyright 2017 American Chemical Society
5. COMPARISON BETWEEN NM AND PERIPHERAL MELANINS
Melanin behaves as a redox system, containing multiple reducing and oxidizing groups. 60 Reverse engineering methodology, based on mediated electrochemical probing employing appropriate redox probes, showed that eumelanin redox potential was about 0.3 V versus normal hydrogen electrode, while that of pheomelanin was about 0.6 V versus normal hydrogen electrode. 61 The researchers concluded that melanin could act as an electron transfer catalyst and pheomelanin, in particular, could exert pro‐oxidizing activity through redox‐buffering mechanism. 61 Based on free electron laser‐photoelectron emission microscopy measurements of threshold photoionization potential of hair eumelanosomes and pheomelanosomes, 62 their oxidation potentials were estimated to be about −0.2 V for the former and +0.5 V versus normal hydrogen electrode, for the latter, suggesting that pheomelanosomes were stronger reductants than eumelanosomes, and even in the dark pheomelanosomes could reduce molecular oxygen to superoxide anion. On the other hand, the interaction of synthetic eumelanin and pheomelanin models with different radicals, generated by pulse radiolysis, suggested that the one‐electron reduction potential of the majority of redox groups of cysteinyl‐DOPA‐melanin was less negative than −0.31 V, while that of DOPA‐melanin was more negative than −0.45 V. 63 Distinct pro‐oxidizing activity of synthetic pheomelanins was demonstrated in a study, in which the efficiency of melanin containing different amount of benzothiazole and benzothiazine, to photo‐oxidize reduced glutathione was compared. 64 The researchers found that benzothiazole‐rich pheomelanin was more efficient in depleting glutathione upon irradiation with ultraviolet A than benzothiazine‐rich melanin.
As described in a previous paragraph, the structure and biogenesis of NM strongly differs from that of peripheral melanins. However, the external melanic coat of NM is essentially constituted of unstructured eumelanic oligomers and we expect that the redox properties of NM should be similar to that of eumelanin. Indeed, photoelectron emission microscopy measurements revealed that the oxidation potential of NM pigment isolated from human SN was about −0.1 V ± 0.2 V versus normal hydrogen electrode, 36 which compares favorably with the oxidation potential of human eumelanosomes (−0.2 V ± 0.2 V) determined in an independent study. 62 Although oxidation potential of NM shell is not sufficiently reductive to induce a high level of oxidative stress, it is sufficient to induce redox cycling with paraquat, a redox active environmental toxin, as demonstrated by electrochemistry‐based reverse engineering methodology. 65 Redox cycling of paraquat, with the formation of its one‐electron reduction product, which via interaction with molecular oxygen yields superoxide anion, could account for possible neurotoxicity mediated by NM. 65
One of the most distinct properties of melanin is its ability to bind metal ions. 66 The binding sites of synthetic catechol‐ and DOPA‐melanin, analyzed by EPR spectroscopy, using 63‐copper(II) as a paramagnetic molecular probe, revealed that at weakly acidic pH, only monodentate carboxyl complexes were formed, while at near neutral pH bidentate phenolic hydroxy complexes were observed. At higher pH, also bidentate nitrogen‐carboxyl complexes were formed. Copper complexes with natural melanin from bovine choroid, at weakly acidic pH, were the same as in synthetic DOPA‐melanin; however, at pH above 7 the EPR spectra of copper(II) suggested possible formation of bidentate nitrogen complexes. There is little doubt that the catechol hydroxy, amine and carboxylic groups are involved in binding of monovalent and divalent alkali metal ions, divalent and trivalent transition metal ions and lanthanide ions by natural and synthetic eumelanins. 66
Assuming that typical melanic units of NM are reasonably well approximated by dihydroxyindole, benzothiazine and benzothiazole units, 67 the binding of these units with iron(II) and iron(III) was analyzed by potentiometric and spectrophotometric titration. 68 The study indicated that under neutral and mildly acidic conditions, iron(III) preferable forms with dihydroxyindole species bidentate complexes.
Iron and copper ions are the biologically most relevant redox‐active transition metal ions; however, when in complexes with certain low‐molecular weight ligands, these metal ions can be toxic due to their ability to catalyze free radical decomposition of hydrogen peroxide. 66 Binding of ferric ions by NM from SN and by a synthetic NM model was studied by EPR spectroscopy. 69 Later, it was shown that intraneuronal NM buffered reactive iron and blocked iron‐mediated oxidation of ascorbate and DA. 70 The study also revealed that NM, by sequestering iron ions, inhibited in a dose‐dependent manner the formation of hydroxyl radicals by Fenton's reaction. Distinct antioxidant action of a synthetic and natural NM was found in a model system by monitoring the rate and extent of lipid peroxidation induced by ferrous ions and free radical initiators. 71 Although both free radical scavenging activity of NM and its metal‐ion sequestering ability were considered, it was concluded that the predominant mechanism of melanin protection against oxidative damage was sequestration of iron. Similar protective role of retinal pigment epithelium melanin, based on its ability to sequester iron ions, was reported elsewhere. 72 Interestingly, retinal pigment epithelium melanin granules, after experimental photoaging exhibited substantially reduced antioxidant properties and even accelerated iron‐induced peroxidation of unsaturated lipids. 72 In view of the in situ photoaging of retinal pigment epithelium melanin demonstrated in samples from older human donors, 73 the reduced ability of photoaged melanin to sequester redox‐active metal ions and to scavenge reactive oxygen species is of considerable interest. Although it is unknown if NM undergoes with age similar physicochemical changes that could compromise its antioxidant and protective functions, chronic oxidative modifications of NM are expected considering its long‐time exposure to high flux of hydrogen peroxide formed in DA metabolism. 2 , 9
6. SYNTHESIS AND STRUCTURE OF MODELS NMs
Our knowledge on the biosynthesis, composition, structure, activities (as metal chelating agent, remover of toxic species, activator/quencher of redox reactions and as activator of microglia and astrocytes) is continuously increasing based on the studies of the natural NM pigment. Nevertheless, a detailed characterization is hampered by the fact that NM is substantially insoluble and can only be extracted in small amounts from the aged human brain (4–5 brains of elderly subjects are required to obtain ~1 mg of NM from SN). Due to scarce availability of human NM, the use of synthetic analogues is really promising. Synthetic models of this pigment are needed in order to have access to techniques where large amount of substance is required, and when the insolubility does not allow the acquisition of data. The best synthetic models would require a similar structure and composition of NM: in particular considering the eumelanin/pheomelanin ratio, the metal content, the accessibility and association of the metal ions, the protein content with cross‐β structure core and, finally, covalently bound lipid residues. Presently, it is hard to obtain all these features in synthetic models.
The optimization then required a stepwise process. Synthetic NMs were prepared by oxidative polymerization of DA and/or cysteine/DA: by changing the reagents ratio, the benzothiazine to dihydroxyindole (the pheomelanin and the eumelanin oligomer units, respectively) ratio can be controlled. 1 , 67 , 74 When the synthesis of the pigment is performed in the presence of iron(II) as catalyst, a faster reaction is observed and the formed NM contain the metal ion, in its iron(III) form, strongly bound in clusters to catecholate residues, with spectroscopic (i.e., EPR) features that closely resemble those of the natural NM pigment. 1 , 69
These latter synthetic NMs are almost completely insoluble and difficult to study. A big improvement in the models was obtained by the addition of protein moieties in the compounds. At first albumin was used as the protein component. 14 Through the change of the relative ratios between the reagents (DA, cysteine, albumin, iron) during the synthesis, it has been possible to obtain derivatives with different eumelanin/pheomelanin, metal and protein contents. The characterization of these synthetic NMs showed the presence of two types of iron binding sites. 14 A small fraction of the metal ion is weakly bound to catecholato groups of the melanic component in mononuclear sites, which give rise to EPR detectable signals. The larger amount of iron is also bound to the melanic moiety but forming multinuclear clusters with additional oxo‐hydroxo bridges which keep the iron centers strongly bound to each other and electronically coupled, so that these centers are non‐detectable by EPR spectroscopy. 14 The organization of iron centers in these synthetic NM analogues is therefore similar to that occurring in natural NM of human brain. 1 , 2 , 30 A radical, coupled to the iron, is also observed in the melanic moiety, 14 similarly to what observed in the natural NM pigment. 1 Nevertheless, the most important aspect of the models containing albumin is the possibility to obtain soluble conjugates, facilitating the spectroscopic and activity studies.
The proteolytic digestion of the conjugates followed by mass spectrometry analysis allowed to clarify the principal mechanism of protein covalent link to the melanic portion, as a nucleophilic addition to DA quinone with a reactivity rank as follow: –SH (cysteine) >> –imidazole (histidine) > –NH2 (lysine). The nuclear magnetic resonance analysis on the conjugate showed that the melanin covalent link to the protein has different effect on the conformational mobility of the residues according to whether the nucleophilic addition occurs on small melanic oligomers (in this case the protein gains in conformational freedom) or on extended polymers (thus leading to slow rotational freedom). 14
A good NM model should consider that NM biosynthesis does not involve the enzymatic DA oxidation catalyzed by tyrosinase or other enzymes, but involves the interaction of fibrillar protein nuclei with DA (and metabolites) present in the cytosol of neurons. DA is oxidized in the presence of traces of metal ions (particularly iron and copper), and in this way melanin‐protein conjugates with an unstructured melanic component are thus generated. In support of this thesis, there is the fact that unlike the peripheral melanins, the only structural element recognized in human NM is the fibrillar protein nucleus, as above described, from which the melanic portion grows. 1 , 60
In order to obtain more sophisticated models that best mimic the structure of human NM, we have replaced albumin with β‐lactoglobulin, a protein capable of forming fibrils. 75 Soluble melanin‐protein conjugates were also obtained by melanization of fibrillated β‐lactoglobulin. 15 These new soluble models allow analysis using various techniques including mass spectrometry, nuclear magnetic resonance, EPR and ultraviolet‐visible spectroscopies, and circular dichroism. The melanic portion of the conjugates contains either eumelanic or mixed eumelanic/pheomelanic composition, with the latter better simulating natural NMs. In addition, the conjugates can be loaded with controlled amounts of iron. These conjugates maintain the amyloid cross‐β protein core as the only structurally organized element, as in human brain NM, together with similar iron environment as shown by the EPR spectra, thus acting as good models. 15
Moreover, it is important to underline that, in addition to DA, other catecholamines participate in the biosynthesis of NM. 23 Indeed, our group recently developed the synthesis of models that mimic the NM of human LC. These NM models, unlike the previous ones that were synthetized from DA, were prepared starting from norepinephrine and with iron and copper ions in a ratio similar to that found in natural NM from LC. 7 These new synthetic analogues mimicking the NM of LC could be used to develop in vitro and in vivo models to study neurodegenerative and neuroinflammatory mechanism of diseases that affect LC neurons containing NM, primarily AD and PD.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ACKNOWLEDGMENTS
The authors thank Dr. Maria Carla Panzeri (Advanced Light and Electron Microscopy BioImaging Center, an advanced microscopy laboratory established by IRCCS San Raffaele Hospital and Vita‐Salute San Raffaele University, Milan, Italy) for expert assistance in electron microscopy imaging. Authors also thank the Section of Legal Medicine and Insurances, Department of Biomedical Sciences for Health at University of Milan, for providing brain tissue samples. The financial support by the Grigioni Foundation for Parkinson's Disease (Milan, Italy) is also acknowledged. Open Access Funding provided by Consiglio Nazionale delle Ricerche within the CRUI‐CARE Agreement.
Zucca FA, Capucciati A, Bellei C, Sarna M, Sarna T, Monzani E, et al. Neuromelanins in brain aging and Parkinson's disease: synthesis, structure, neuroinflammatory, and neurodegenerative role. IUBMB Life. 2023;75(1):55–65. 10.1002/iub.2654
Fabio A. Zucca and Andrea Capucciati contributed equally to this work and wish to be considered as co‐first authors.
Funding information Grigioni Foundation for Parkinson's Disease
REFERENCES
- 1. Zecca L, Bellei C, Costi P, et al. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc Natl Acad Sci USA. 2008;105:17567–17572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zucca FA, Segura‐Aguilar J, Ferrari E, et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson's disease. Prog Neurobiol. 2017;155:96–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. [DOI] [PubMed] [Google Scholar]
- 4. Theofilas P, Ehrenberg AJ, Dunlop S, et al. Locus coeruleus volume and cell population changes during Alzheimer's disease progression: A stereological study in human postmortem brains with potential implication for early‐stage biomarker discovery. Alzheimers Dement. 2017;13:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibb WR. Melanin, tyrosine hydroxylase, calbindin and substance P in the human midbrain and substantia nigra in relation to nigrostriatal projections and differential neuronal susceptibility in Parkinson's disease. Brain Res. 1992;581:283–291. [DOI] [PubMed] [Google Scholar]
- 6. Kastner A, Hirsch EC, Lejeune O, Javoy‐Agid F, Rascol O, Agid Y. Is the vulnerability of neurons in the substantia nigra of patients with Parkinson's disease related to their neuromelanin content? J Neurochem. 1992;59(1):1080–1089. [DOI] [PubMed] [Google Scholar]
- 7. Zecca L, Stroppolo A, Gatti A, et al. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc Natl Acad Sci USA. 2004;101:9843–9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sulzer D, Bogulavsky J, Larsen KE, et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci USA. 2000;97:11869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Segura‐Aguilar J, Paris I, Muñoz P, Ferrari E, Zecca L, Zucca FA. Protective and toxic roles of dopamine in Parkinson's disease. J Neurochem. 2014;129:898–915. [DOI] [PubMed] [Google Scholar]
- 10. Zucca FA, Vanna R, Cupaioli FA, et al. Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson's disease. NPJ Parkinsons Dis. 2018;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zecca L, Costi P, Mecacci C, Ito S, Terreni M, Sonnino S. Interaction of human substantia nigra neuromelanin with lipids and peptides. J Neurochem. 2000;74:1758–1765. [DOI] [PubMed] [Google Scholar]
- 12. Engelen M, Vanna R, Bellei C, et al. Neuromelanins of human brain have soluble and insoluble components with dolichols attached to the melanic structure. PLoS One. 2012;7:e48490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rao KS, Hegde ML, Anitha S, et al. Amyloid beta and neuromelanin‐‐toxic or protective molecules? The cellular context makes the difference. Prog Neurobiol. 2006;78:364–373. [DOI] [PubMed] [Google Scholar]
- 14. Ferrari E, Engelen M, Monzani E, et al. Synthesis and structural characterization of soluble neuromelanin analogs provides important clues to its biosynthesis. J Biol Inorg Chem. 2013;18:81–93. [DOI] [PubMed] [Google Scholar]
- 15. Ferrari E, Capucciati A, Prada I, et al. Synthesis, structure characterization, and evaluation in microglia cultures of neuromelanin analogues suitable for modeling Parkinson's disease. ACS Chem Neurosci. 2017;8:501–512. [DOI] [PubMed] [Google Scholar]
- 16. d'Ischia M, Wakamatsu K, Cicoira F, et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015;28:520–544. [DOI] [PubMed] [Google Scholar]
- 17. Marsden CD. Pigmentation in the nucleus substantiae nigrae of mammals. J Anat. 1961;95:256–261. [PMC free article] [PubMed] [Google Scholar]
- 18. Merighi A, Peirone SM. Histochemical and ultrastructural features of neuronal pigment in some encephalic nuclei of ruminants. Exp Biol. 1985;44:109–121. [PubMed] [Google Scholar]
- 19. DeMattei M, Levi AC, Fariello RG. Neuromelanic pigment in substantia nigra neurons of rats and dogs. Neurosci Lett. 1986;72:37–42. [DOI] [PubMed] [Google Scholar]
- 20. Cozzi B, Pellegrini M, Droghi A. Neuromelanin in the substantia nigra of adult horses. Anat Anz. 1988;166:53–61. [PubMed] [Google Scholar]
- 21. Pasbakhsh P, Omidi N, Mehrannia K, et al. The protective effect of vitamin E on locus coeruleus in early model of Parkinson's disease in rat: Immunoreactivity evidence. Iran Biomed J. 2008;12:217–222. [PubMed] [Google Scholar]
- 22. Taylor TN, Alter SP, Wang M, Goldstein DS, Miller GW. Reduced vesicular storage of catecholamines causes progressive degeneration in the locus ceruleus. Neuropharmacology. 2014;76:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wakamatsu K, Tabuchi K, Ojika M, Zucca FA, Zecca L, Ito S. Norepinephrine and its metabolites are involved in the synthesis of neuromelanin derived from the locus coeruleus. J Neurochem. 2015;135:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang CL, Nelson O, Yazdani U, Pasbakhsh P, German DC. Inverse relationship between the contents of neuromelanin pigment and the vesicular monoamine transporter‐2: Human midbrain dopamine neurons. J Comp Neurol. 2004;473:97–106. [DOI] [PubMed] [Google Scholar]
- 25. Segura‐Aguilar J, Sulzer D, Zucca FA, Zecca L. Overexpression of vesicular monoamine transporter‐2 may block neurotoxic metabolites from cytosolic dopamine: A potential neuroprotective therapy for Parkinson's disease. Clin Pharmacol Transl Med. 2019;3:143–148. [PMC free article] [PubMed] [Google Scholar]
- 26. Betarbet R, Turner R, Chockkan V, et al. Dopaminergic neurons intrinsic to the primate striatum. J Neurosci. 1997;17:6761–6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ikemoto K, Kitahama K, Nishimura A, et al. Tyrosine hydroxylase and aromatic L‐amino acid decarboxylase do not coexist in neurons in the human anterior cingulate cortex. Neurosci Lett. 1999;269:37–40. [DOI] [PubMed] [Google Scholar]
- 28. Biesemeier A, Eibl O, Eswara S, et al. Elemental mapping of Neuromelanin organelles of human Substantia Nigra: Correlative ultrastructural and chemical analysis by analytical transmission electron microscopy and nano‐secondary ion mass spectrometry. J Neurochem. 2016;138:339–353. [DOI] [PubMed] [Google Scholar]
- 29. Brooks J, Everett J, Lermyte F, et al. Label‐free nanoimaging of neuromelanin in the brain by soft X‐ray spectromicroscopy. Angew Chem Int Ed Engl. 2020;59:11984–11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Monzani E, Nicolis S, Dell'Acqua S, et al. Dopamine oxidative stress and protein‐quinone modifications in Parkinson's and other neurodegenerative diseases. Angew Chem Int Ed Engl. 2019;58:6512–6527. [DOI] [PubMed] [Google Scholar]
- 31. Trujillo P, Summers PE, Ferrari E, et al. Contrast mechanisms associated with neuromelanin‐MRI. Magn Reson Med. 2017;78:1790–1800. [DOI] [PubMed] [Google Scholar]
- 32. Möller HE, Bossoni L, Connor JR, et al. Iron, myelin, and the brain: Neuroimaging meets neurobiology. Trends Neurosci. 2019;42:384–401. [DOI] [PubMed] [Google Scholar]
- 33. Tribl F, Gerlach M, Marcus K, et al. “Subcellular proteomics” of neuromelanin granules isolated from the human brain. Mol Cell Proteomics. 2005;4:945–957. [DOI] [PubMed] [Google Scholar]
- 34. Ward WC, Guan Z, Zucca FA, et al. Identification and quantification of dolichol and dolichoic acid in neuromelanin from substantia nigra of the human brain. J Lipid Res. 2007;48:1457–1462. [DOI] [PubMed] [Google Scholar]
- 35. Ward WC, Zucca FA, Bellei C, Zecca L, Simon JD. Neuromelanins in various regions of human brain are associated with native and oxidized isoprenoid lipids. Arch Biochem Biophys. 2009;484:94–99. [DOI] [PubMed] [Google Scholar]
- 36. Bush WD, Garguilo J, Zucca FA, et al. The surface oxidation potential of human neuromelanin reveals a spherical architecture with a pheomelanin core and a eumelanin surface. Proc Natl Acad Sci USA. 2006;103:14785–14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bush WD, Garguilo J, Zucca FA, et al. Neuromelanins isolated from different regions of the human brain exhibit a common surface photoionization threshold. Photochem Photobiol. 2009;85:387–390. [DOI] [PubMed] [Google Scholar]
- 38. Damron TA, Schelper RL, Sorensen L. Cytochemical demonstration of neuromelanin in black pigmented adrenal nodules. Am J Clin Pathol. 1987;87:334–341. [DOI] [PubMed] [Google Scholar]
- 39. Cao W, Zhou X, McCallum NC, et al. Unraveling the structure and function of melanin through synthesis. J Am Chem Soc. 2021;143:2622–2637. [DOI] [PubMed] [Google Scholar]
- 40. Ito S. Encapsulation of a reactive core in neuromelanin. Proc Natl Acad Sci USA. 2006;103:14647–14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. d'Ischia M, Napolitano A, Ball V, Chen CT, Buehler MJ. Polydopamine and eumelanin: From structure–property relationships to a unified tailoring strategy. Acc Chem Res. 2014;47:3541–3550. [DOI] [PubMed] [Google Scholar]
- 42. Nicolis S, Zucchelli M, Monzani E, Casella L. Myoglobin modification by enzyme‐generated dopamine reactive species. Chemistry. 2008;14:8661–8673. [DOI] [PubMed] [Google Scholar]
- 43. Ito S, Sugumaran M, Wakamatsu K. Chemical reactivities of ortho‐quinones produced in living organisms: fate of quinonoid products formed by tyrosinase and phenoloxidase action on phenols and catechols. Int J Mol Sci. 2020;21:6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haining RL, Achat‐Mendes C. Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator. Neural Regen Res. 2017;12:372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pakkenberg B, Møller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54:30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zecca L, Fariello R, Riederer P, Sulzer D, Gatti A, Tampellini D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson's disease. FEBS Lett. 2002;510:216–220. [DOI] [PubMed] [Google Scholar]
- 47. McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA‐DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. [DOI] [PubMed] [Google Scholar]
- 48. Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II‐positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. 2003;106:518–526. [DOI] [PubMed] [Google Scholar]
- 49. Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. [DOI] [PubMed] [Google Scholar]
- 50. McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008;23:474–483. [DOI] [PubMed] [Google Scholar]
- 51. Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF‐kappaB dependent and involves p38 mitogen‐activated protein kinase: Implications for Parkinson's disease. FASEB J. 2003;17:500–502. [DOI] [PubMed] [Google Scholar]
- 52. Zhang W, Phillips K, Wielgus AR, et al. Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: Implications for progression of Parkinson's disease. Neurotox Res. 2011;19:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zecca L, Wilms H, Geick S, et al. Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: Implications for Parkinson's disease. Acta Neuropathol. 2008;116:47–55. [DOI] [PubMed] [Google Scholar]
- 54. Tousi NS, Buck DJ, Zecca L, Davis RL. Neuromelanin inhibits CXCL10 expression in human astroglial cells. Neurosci Lett. 2010;486:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oberländer U, Pletinckx K, Döhler A, et al. Neuromelanin is an immune stimulator for dendritic cells in vitro. BMC Neurosci. 2011;12:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cebrián C, Zucca FA, Mauri P, et al. MHC‐I expression renders catecholaminergic neurons susceptible to T‐cell‐mediated degeneration. Nat Commun. 2014;5:3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fink J, Pathak H, Smith J, Achat‐Mendes C, Haining RL. Development of a competition‐binding assay to determine binding affinity of molecules to neuromelanin via fluorescence spectroscopy. Biomolecules. 2019;9:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Capucciati A, Zucca FA, Monzani E, Zecca L, Casella L, Hofer T. Interaction of neuromelanin with xenobiotics and consequences for neurodegeneration; promising experimental models. Antioxidants (Basel). 2021;10:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Viceconte N, Burguillos MA, Herrera AJ, et al. Neuromelanin activates proinflammatory microglia through a caspase‐8‐dependent mechanism. J Neuroinflammation. 2015;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Meredith P, Sarna T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006;19:572–594. [DOI] [PubMed] [Google Scholar]
- 61. Kang M, Kim E, Temoçin Z, et al. Reverse engineering to characterize redox properties: Revealing Melanin's redox activity through mediated electrochemical probing. Chem Mater. 2018;30:5814–5826. [Google Scholar]
- 62. Samokhvalov A, Hong L, Liu Y, et al. Oxidation potentials of human eumelanosomes and pheomelanosomes. Photochem Photobiol. 2005;81:145–148. [DOI] [PubMed] [Google Scholar]
- 63. Rózanowska M, Sarna T, Land EJ, Truscott TG. Free radical scavenging properties of melanin interaction of eu‐ and pheo‐melanin models with reducing and oxidising radicals. Free Radic Biol Med. 1999;26:518–525. [DOI] [PubMed] [Google Scholar]
- 64. Tanaka H, Yamashita Y, Umezawa K, Hirobe T, Ito S, Wakamatsu K. The pro‐oxidant activity of pheomelanin is significantly enhanced by UVA irradiation: Benzothiazole moieties are more reactive than benzothiazine moieties. Int J Mol Sci. 2018;19:2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim E, Leverage WT, Liu Y, et al. Paraquat‐melanin redox‐cycling: Evidence from electrochemical reverse engineering. ACS Chem Neurosci. 2016;7:1057–1067. [DOI] [PubMed] [Google Scholar]
- 66. Sarna T, Swartz HM, Zadlo A. Interaction of melanin with metal ions modulates their cytotoxic potential. Appl Magn Reson. 2022;53:105–121. [Google Scholar]
- 67. Wakamatsu K, Murase T, Zucca FA, Zecca L, Ito S. Biosynthetic pathway to neuromelanin and its aging process. Pigment Cell Melanoma Res. 2012;25:792–803. [DOI] [PubMed] [Google Scholar]
- 68. Charkoudian LK, Franz KJ. Fe(III)‐coordination properties of neuromelanin components: 5,6‐dihydroxyindole and 5,6‐dihydroxyindole‐2‐carboxylic acid. Inorg Chem. 2006;45:3657–3664. [DOI] [PubMed] [Google Scholar]
- 69. Shima T, Sarna T, Swartz HM, Stroppolo A, Gerbasi R, Zecca L. Binding of iron to neuromelanin of human substantia nigra and synthetic melanin: An electron paramagnetic resonance spectroscopy study. Free Radic Biol Med. 1997;23:110–119. [DOI] [PubMed] [Google Scholar]
- 70. Zecca L, Casella L, Albertini A, et al. Neuromelanin can protect against iron‐mediated oxidative damage in system modeling iron overload of brain aging and Parkinson's disease. J Neurochem. 2008;106:1866–1875. [DOI] [PubMed] [Google Scholar]
- 71. Korytowski W, Sarna T, Zareba M. Antioxidant action of neuromelanin: The mechanism of inhibitory effect on lipid peroxidation. Arch Biochem Biophys. 1995;319:142–148. [DOI] [PubMed] [Google Scholar]
- 72. Zadlo A, Rozanowska MB, Burke JM, Sarna TJ. Photobleaching of retinal pigment epithelium melanosomes reduces their ability to inhibit iron‐induced peroxidation of lipids. Pigment Cell Res. 2007;20:52–60. [DOI] [PubMed] [Google Scholar]
- 73. Ito S, Pilat A, Gerwat W, et al. Photoaging of human retinal pigment epithelium is accompanied by oxidative modifications of its eumelanin. Pigment Cell Melanoma Res. 2013;26:357–366. [DOI] [PubMed] [Google Scholar]
- 74. Wakamatsu K, Fujikawa K, Zucca FA, Zecca L, Ito S. The structure of neuromelanin as studied by chemical degradative methods. J Neurochem. 2003;86:1015–1023. [DOI] [PubMed] [Google Scholar]
- 75. Gosal WS, Clark AH, Ross‐Murphy SB. Fibrillar beta‐lactoglobulin gels: Part 1. Fibril formation and structure. Biomacromolecules. 2004;5:2408–2419. [DOI] [PubMed] [Google Scholar]


