Abstract
Background
Autologous conditioned serum (ACS) is used to treat osteoarthritis in horses, although its effects are not fully investigated.
Objectives
To investigate the effects of equine serum and conditioned serum on chondrocytes stimulated with interleukin (IL)‐1β and cartilage explants with mild osteoarthritis.
Study design
In vitro experimental study.
Methods
The effect of three different serum preparations (unincubated control [PS], serum incubated 24 h [PS24h] and serum incubated 24 h in ACS containers [PCS]) pooled from lame horses were tested in two in vitro models. IL‐1β and IL‐1 receptor antagonist (IL‐1Ra) concentrations were measured in all sera. In model 1, chondrocyte pellet cultures were stimulated with IL‐1β prior to treatment with the serum preparations for 2 and 48 h. Microarray, polymerase chain reaction, and matrix metallopeptidase‐13 analyses were performed. In model 2, cartilage explants from horses with structural osteoarthritis were treated with PS or PCS on days 0, 6 and 12, or left untreated, and evaluated at day 24 using the OARSI grading scale for histological evaluation of articular cartilage.
Results
The IL‐1Ra concentration in PS24h and PCS was significantly higher than in PS. In model 1, inflammation‐ and cartilage matrix degradation‐related genes were upregulated after 48 h in all treatment groups versus untreated controls. Cartilage matrix molecules, aggrecan and collagens, were downregulated in PS24h‐ and PCS‐treated pellets versus untreated controls. Growth factor signalling genes were upregulated—FGF7 in all treatment groups, BMP2 in PS24h‐, and INHBA in PCS‐treated—compared with untreated controls. In model 2, the OARSI score at day 24 was not significantly different between treatment groups.
Main limitations
Results from in vitro models cannot be directly translated to in vivo situations.
Conclusions
In vitro treatment with conditioned serum did not alleviate IL‐1β‐induced responses in chondrocyte pellets or lead to morphological improvement in osteoarthritic cartilage explants.
Keywords: autologous conditioned serum, horse, IL‐1 receptor antagonist, inflammation, interleukin 1β, osteoarthritis
Resumen
Historial
Suero autólogo acondicionado (ACS) es usado para tartar osteoartritis en caballos, aunque sus efectos no han sido completamente investigados.
Objetivos
Investigar los efectos de suero equino y suero acondicionado en condrocitos estimulados con interleukina (IL)‐1β y explantes de cartílago con osteoartritis leve.
Diseño del Estudio
Estudio experimental in vitro.
Métodos
El efecto de tres preparaciones séricas diferentes (control no incubado (PS), suero incubado 24 h (PS24h), y suero incubado 24 h en frascos ACS (PCS)) combinados y obtenidos de caballos cojos fueron probados en dos modelos in vitro. Las concentraciones de IL‐1β y de receptor antagonista de IL‐1 (IL‐1Ra) fueron medidas en todos los sueros. En el modelo 1, los cultivos de pellets de condrocitos fueron estimulados con IL‐1β antes de ser tratados con las preparaciones séricas durante 2 y 48 h. Se realizaron análisis de micromatrices, reacciones de polimerasa en cadena y de matriz de metalopeptidasa‐13. En el modelo 2, explantaciones de cartílago proveniente de caballos con osteoartritis estructural fueron tratados con PS o PCS en los días 0, 6 y 12, o dejados sin tartar, y evaluados al día 24 usando la escala de graduación OARSI para evaluación histológica de cartílago articular.
Resultados
La concentración de IL‐1Ra en PS24h y PCS fue significativamente mayor que en PS. En el modelo 1, los genes relacionados a la inflamación y a la degradación de la matriz cartilaginosa estaban aumentados después de 48 h en todos los grupos tratados en comparación a los controles no tratados. Las moléculas de matriz cartilaginosa, agrecanos y colágenos estaban disminuidos en los pellets PS24h y PCS versus los controles no tratados. Los genes de señales de factores de crecimiento FGF7 estaban aumentados en todos los grupos tratados, BMP2 en PS24h y INHBA in PCS en comparación con los controles no tratados. En el modelo 2, la escala OARSI al día 24 no fue significativamente distinta entre los grupos de tratamientos.
Limitaciones Principales
Los resultados de modelos in vitro no pueden ser directamente aplicados a situaciones in vivo.
Conclusiones
El tratamiento in vitro con suero acondicionado no alivió las respuestas inducidas por IL‐1β en pellets de condrocitos o llevo a mejoramiento morfológico en explantes de cartílago con osteoartritis.
1. INTRODUCTION
An effective and truly disease‐modifying treatment for osteoarthritis (OA), the most common cause of lameness in horses, 1 is still lacking. One focal point in equine OA research is to block the interleukin (IL)‐1 receptor with an IL‐1 receptor antagonist (IL‐1Ra), aiming to prevent the driving effects of IL‐1β on the OA process. 2 Indeed, clinical and histological signs of OA improve after intra‐articular treatment with IL‐1Ra gene transfer in an experimentally induced OA model. 2 Biological treatments such as autologous conditioned serum (ACS) have been proposed to have an anti‐inflammatory effect through increased expression of IL‐1Ra. 3 ACS preparation involves incubation of whole blood in commercial tubes containing glass beads followed by centrifugation and collection of serum, which is then returned to the subject, 3 , 4 and this method has been used to treat OA in both humans and horses. 5 , 6 Compared with regular serum, ACS has been shown to contain higher levels of both anti‐ and pro‐inflammatory cytokines as well as growth factors; however, there are large individual variations in the concentration of the measured constituents, and the complete composition of ACS is yet to be determined. 3 , 4 , 7 , 8 Clinical effects of ACS treatment in naturally occurring OA were recently linked to the ACS concentration of IL‐1Ra and insulin growth factor‐1 (IGF‐1), 9 pointing out the previously reported large inter‐individual variations in ACS cytokine and growth factor content as one of the major drawbacks of its clinical use. 4 , 7
Although the scientific knowledge regarding ACS is growing, its effects on articular cartilage remain poorly understood. 3 , 4 , 7 , 8 Moreover, previous studies have demonstrated increased levels of cytokines and growth factors in equine serum incubated in regular red‐top tubes, even exceeding IL‐1Ra levels found in ACS prepared in specialised ACS containers. 4 , 7 , 9
In this study, we aimed to investigate the effects of ACS and equine serum on IL‐1β stimulated equine chondrocyte pellets (model 1) and cartilage explants (model 2) from joints with structural OA. We hypothesised that treatment with incubated and conditioned serum in model 1 would upregulate growth factor genes and downregulate inflammatory genes. In model 2, we hypothesised that treatment with conditioned serum would improve histological scores of OA.
2. MATERIALS AND METHODS
2.1. Serum collection and processing
The effect of three different serum preparations was tested in two separate in vitro models. For the chondrocyte pellet culture model (model 1), serum was obtained from a mixed sex (three mares and two geldings) cohort including five client‐owned horses (4–22 years of age; mean age 12 years) which were deemed healthy but lame based on a thorough clinical examination. Lameness was located to one or more joints with the use of intra‐articular diagnostic anaesthesia (defined as a ≥80% reduction in lameness post anaesthesia). The horses had not received any systemic medication in the last 6 months prior to sampling. Samples were collected after obtaining informed written consent from horse owners. Blood was aseptically drawn into serum tubes (Greiner Bio‐One GmbH), left to clot at room temperature (20–22°C) for 1 h before centrifugation (unincubated control), aseptically drawn into serum tubes (Greiner Bio‐One GmbH) and incubated for 24 h at 37°C (incubated serum) or aseptically drawn into commercially available ACS containers containing borosilicate glass beads (Arthrex VET Systems) and incubated for 24 h at 37°C (conditioned serum). All samples were centrifuged at 2500×g for 10 min before serum harvesting and filtering through a 0.22 μm filter; samples were stored at −80°C until analysis. The different serum preparations were pooled from all five horses and referred to as pooled serum (PS, unincubated control), pooled incubated serum (PS24h) and pooled conditioned serum (PCS).
For the cartilage explant model (model 2), serum was obtained from 20 client‐owned harness racehorses of mixed sex (age 2–7 years, mean age 5 years) that were deemed healthy but lame (based on a thorough clinical examination including a comprehensive subjective and objective work‐up, and haematology and serum amyloid A analysis). Included horses had detectable lameness that could be abolished or significantly improved with intra‐articular anaesthesia. The horses were enrolled in another study, and additional clinical details including signalment and results from the lameness work‐up are available elsewhere. 9 The different serum preparations were pooled from all 20 horses and PS and PCS were used.
The concentration of IL‐1Ra and IL‐1β was measured in all individual and PS samples using Equine IL‐Ra DuoSet sandwich ELISA (R&D Systems) and Equine IL‐1β VetSet sandwich ELISA (Kingfisher Biotech Inc.), respectively; all assays were performed according to the manufacturer's instructions. Samples were assayed in duplicate on one plate with an intra‐assay coefficient of variation (CV) of 2.5% and 1.5%, respectively. For both cytokines, the lower limit of quantification was 0.31 ng/ml.
2.2. Chondrocyte pellet culture (model 1)
Macroscopically normal, full‐thickness cartilage was aseptically harvested from the radial facet of the third carpal bone from five non‐lame Icelandic horses (mixed sex [three mares and two stallions], all approximately 30 months old) immediately after euthanasia as a part of a slaughter cohort in another study. 10 Isolation, expansion and 3D pellet chondrocyte culturing were performed as previously described. 11 In brief, cartilage was mechanically minced and digested by type II collagenase 0.8 mg/ml (Worthington Biochemicals) for 20–24 h at 37°C in 7% CO2. Chondrocytes were thereafter grown in monolayer until 80% confluence and thereafter stored in liquid nitrogen until pellet culture. After thawing, chondrocytes were expanded once before pellet culture, where 200 000 cells were seeded in the wells of flat bottom 96‐well culture plates (Corning Life Sciences) to form 3D pellet cultures. After 14 days of culturing at 37°C in 7% CO2 with pellet medium changed every second day, the cultures were stimulated for 48 h at 37°C with pellet medium (unstimulated controls) or pellet medium containing 5 ng/ml recombinant equine IL‐1β (R&D Systems; stimulated cultures; Figure 1), which previously has been shown to induce a catabolic response in pellet cultures. 11
FIGURE 1.
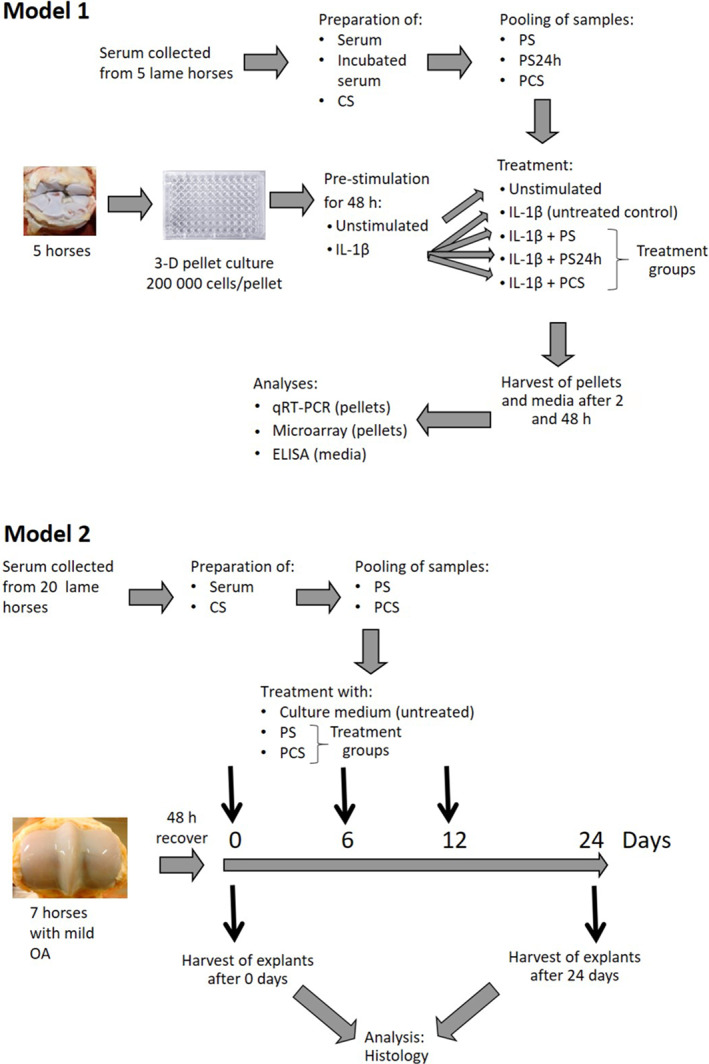
In vitro models. Chondrocyte pellet cultures (model 1): Serum was collected from five lame horses and then processed to prepare serum, incubated serum and conditioned serum (CS); serum from these horses were pooled into pooled serum (PS), pooled serum incubated 24 h (PS24h), or pooled conditioned serum (PCS). Cartilage samples from the third carpal bone were harvested from five non‐lame horses at an abattoir; horses were free of macroscopic evidence of joint pathology. Chondrocytes were isolated and expanded to 3D pellet cultures. The pellets were pre‐stimulated with interleukin (IL)‐1β for 48 h and thereafter treated PS, PS24h or PCS. Pellets and culture media were harvested after 2 and 48 h. Gene expression in pellets was analysed using qRT‐PCR and microarray analysis, and matrixmetallopeptidare‐13 levels in culture media were measured. Cartilage explant culture (model 2): Serum was collected from 20 lame horses and thereafter processed to prepare serum and conditioned serum (CS); serum from these horses were pooled into PS or PCS for use in model 2. Cartilage explants were collected from the third metacarpal condyle from seven horses with mild osteoarthritis (OA) sourced from an abattoir. The explants were allowed to recover for 48 h in culture medium and then treated with culture media, PS, or PCS on days 0, 6 and 12. Explants were harvested on day 0 and 24 and subjected to histological analysis.
After 48 h of stimulation, the medium was replaced with fresh pellet medium for unstimulated controls, whereas stimulated cultures were divided into four treatment groups as illustrated in Figure 1 and treated with 5 ng/ml IL‐1β (concentration in total treatment volume) either alone (untreated control) or combined with the treatments PS (40% V/V); PS24h (40% V/V), or PCS (40% V/V); cultures were incubated at 37°C until harvesting at 2 and 48 h post treatment. At harvesting, the pellets were washed in phosphate buffered saline, snap frozen in liquid nitrogen and stored at −80°C for gene expression analysis. The supernatant pellet media were centrifuged at 200×g for 5 min to remove cell debris and stored at −80°C for further analysis.
To confirm induction of inflammation, matrix metallopeptidase‐13 (MMP‐13) was measured in pellet medium using Fluorokine E Human Active MMP‐13 Fluorescent Assay (R&D Systems); analyses were performed according to the manufacturer's instructions. p‐Aminophenylmercuric acetate was used to activate any potentially active forms of MMP‐13. The samples were assayed in duplicate, and samples from each horse were run on separate plates with an intra‐assay and inter‐assay CV of 4.7% and 6.7%, respectively. The lower limit of quantification was 0.25 ng/ml.
RNA was isolated from chondrocyte pellets as previously described. 11 The total RNA (100 ng) was used for cDNA synthesis using a High‐Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer's instructions. Quantitative reverse transcription polymerase chain reaction (qRT‐PCR) was performed on Rotor‐Gene 3000 (Corbett Research) using TaqMan Gene Expression Assays (Applied Biosystems) for aggrecan (ACAN; Ec03469667_m1) and collagen type II alpha 1 (COL2A1; Ec03467378_m1) and TaqMan Gene Expression Master Mix (Applied Biosystems) according to the manufacturer's instructions. Relative gene expression was determined using the 2−(ΔΔct) method. 12 Two reference genes, glucuronidase beta (forward primer, 5′‐GTGACCAACTCCAACTATGAAGCA‐3′; reverse primer, 5′‐AGGAGTAGTAACTATTCACACAGATGACA‐3′; probe, 6‐carboxyfluorescein‐CATATGGCGCCCCTAGGTC‐dihydrocyclopyrroloindole tripeptide minor groove binder, Custom TaqMan Gene Expression Assays, Applied Biosystems) and hypoxanthine phosphoribosyltransferase 1 (Ec03470217_m1, TaqMan Gene Expression Assays, Applied Biosystems), were selected for the normalisation of target gene expression after evaluating reference genes using GenEx Enterprise 5.2.3.13 data analysis software (MultiD analyses).
The total RNA (100 ng) isolated from chondrocyte pellets was also subjected to microarray analysis, with a single replicate from each horse. The RNA was used to generate and amplify biotinylated sense‐strand cDNA from the entire expressed genome using the GeneChip WT PLUS Reagent Kit (Affymetrix Inc.). GeneChip Equine Gene 1.0 ST Arrays (Affymetrix Inc.) containing 25 923 probe sets were hybridised for 16 h at 45°C and rotated at 60 rpm. The arrays were washed and stained using the Fluidics Station 450 (Affymetrix Inc.) per the instructions in GeneChip Expression Wash, Stain and Scan Manual P/N 702731 Rev 3 (Affymetrix Inc.) and finally scanned using the GeneChip Scanner 3000 7G (Affymetrix Inc.). Of special interest were genes involved in inflammation, genes involved in cartilage matrix organisation and growth factor genes.
2.3. Cartilage explant (model 2)
Articular cartilage from the lateral and medial third metacarpal condyle was aseptically harvested from seven horses (age 7–16 years) at a local abattoir within 5 h of death; the included horses had macroscopic evidence of mild metacarpophalangeal joint OA characterised by the presence of wear lines, erosions, and palmar arthrosis (total OARSI score ≤2 for any variable). 13 Explants of non‐calcified articular cartilage were harvested from each joint using a 5 mm biopsy punch; tissues from each horse were run as separate explant replicates throughout the experiment. Three explants from each joint were cut into six segments each and allowed to recover for 48 h in culture medium containing DMEM (5 mM glucose), bovine serum albumin (0.1 mg/ml) and ascorbic acid (0.1 mg/ml). Six of the 18 segments from each horse were evaluated by light microscopy at day 0 as described below. Twelve segments were used for culturing experiments: four were left untreated (culture media), four were treated with PS (40% V/V), and four with PCS (40% V/V); culture media were changed every third day with the addition of PS or PCS on days 0, 6 and 12 until harvesting on day 24, when the explants were processed for histological analysis. The explants were fixed in 10% neutral buffered formalin for 48 h, dehydrated, and embedded in paraffin. Tissue sections (4 μm thickness) were stained with haematoxylin and eosin (H&E) and 0.1% aqueous safranin‐O, and counterstained with 0.1% aqueous fast green (SOFG). The sections were evaluated by a board‐certified pathologist (S.E.) blinded to the treatment groups, and scored according to the OARSI grading scale for histological evaluation of articular cartilage. 13 In brief, this grading scale is based on evaluating each of the following parameters on a 0–4 scale (0 = normal; 4 = worst possible change): chondrocyte necrosis, chondrone formation, fibrillation/fissuring, focal cell loss and SOFG stain uptake.
2.4. Data analyses
2.4.1. Analysis of cytokine data, MMP‐13 in culture media and qRT PCR of gene expression in pellets (model 1)
Cytokine data (concentration of IL‐1Ra and IL‐1β) were analysed as a one‐factor randomised block experiment 14 with “horse” as a block using the Mixed procedure in the SAS package according to the SAS/Stat User's Guide Version 9.4. (SAS Institute Inc.). Multiple comparisons were adjusted for multiplicity using Tukey's method. The concentration of MMP‐13 and the qRT‐PCR data were analysed using a repeated‐measures mixed model 15 and the mixed procedure in SAS software (SAS Institute Inc.). The fixed part of the model included treatment, time and the interaction between them. Relationships between observations within units were modelled using a compound symmetric covariance structure. The assumptions underlying these analyses were checked using diagnostic plots. No apparent deviations from normality or homoscedasticity were detected. Differences were considered significant at p < 0.05.
2.4.2. Microarray data analysis (model 1)
Controls were evaluated and raw data from the microarray analyses were normalised in an Expression Console (Affymetrix Inc.) using the robust multi‐array average method described previously. 16 , 17 Subsequent analyses of gene expression data were performed using the statistical computing language R (www.r-project.org) and packages available from the Bioconductor project (www.bioconductor.org). The differentially expressed genes (DEGs) between untreated controls (IL‐1β‐stimulated pellets) and the other groups (unstimulated, IL‐1β + PS, IL‐1β + PS24h and IL‐1β + PCS) were identified by a paired empirical Bayes moderated t test using the limma package. 18 , 19 To address the problem of multiple testing, p values were adjusted using the Benjamini and Hochberg method. 20 Differences between the groups were considered significant if the logarithmic fold change (log 2 FC) was ≥1 or ≤−1 with adjusted p < 0.05.
2.4.3. Histological analysis (model 2)
Histological OARSI scores on day 24 were checked for normality using the D'Agostino–Pearson omnibus test. Differences between treatment groups on day 24 were evaluated using a repeated‐measures one‐way ANOVA with Greenhouse–Geisser correction. Analyses were performed in GraphPad Prism 9.3.1 (GraphPad Software). Data are presented as mean (±SD) and significance was set at p < 0.05.
3. RESULTS
3.1. IL‐1Ra and IL‐1β concentrations in serum preparations
In the individual serum samples, IL‐1Ra concentration was significantly higher in incubated serum (31.1 ± 11.2 ng/ml) versus conditioned serum (15.8 ± 9.94 ng/ml; p = 0.02), and there was significantly higher IL‐1Ra concentration in incubated serum and conditioned serum versus unincubated serum (0.412 ± 0.493 ng/ml; p < 0.001 and p = 0.02, respectively). The IL‐1Ra concentration in the pooled PS, PS24h and PCS samples used for model 1 was 0.4, 36.9 and 12.2 ng/ml, respectively. IL‐1β was not detected in any unincubated or conditioned serum sample; it was, however, detected in one individual incubated serum sample (7.7 ng/ml) and, consequently, also in the pooled PS24h sample (1.2 ng/ml).
3.2. Chondrocyte pellet culture model (model 1)
Stimulation with IL‐1β resulted in a significantly higher cell media MMP‐13 concentration at 48 h of stimulation (p = 0.02) versus unstimulated controls; however, there were no differences between treatment groups (Table S1). Stimulation with IL‐1β also resulted in a significant reduction in ACAN expression at 2 and 48 h and in COL2A1 expression at 48 h versus unstimulated controls; however, there were no differences in either gene expression between treatment groups (Tables 1 and 2).
TABLE 1.
Gene expression of aggrecan in unstimulated pellets, untreated controls (interleukin [IL]‐1β‐stimulated pellets) and pellets treated with addition of pooled serum (PS), pooled serum incubated for 24 h (PS24h) or pooled conditioned serum (PCS) sampled after 2 and 48 h
| 2 h | 48 h | |||
|---|---|---|---|---|
| 2−(ΔΔct) ± SD | p | 2−(ΔΔct) ± SD | p | |
| Unstimulated control | 1.36 ± 1.05 | 0.01 | 1.69 ± 1.07 | 0.008 |
| Untreated control (IL‐1β) | 0.300 ± 0.170 | 0.575 ± 0.542 | ||
| IL‐1β + PS | 0.424 ± 0.315 | 0.8 | 0.266 ± 0.330 | 0.5 |
| IL‐1β + PS24h | 0.374 ± 0.263 | 0.9 | 0.188 ± 0.172 | 0.3 |
| IL‐1β + PCS | 0.284 ± 0.136 | 0.97 | 0.151 ± 0.120 | 0.3 |
Note: Values are presented as relative gene expression 2−(ΔΔct) ± SD and p values for all groups were compared against untreated controls at each time point. Results were considered statistically significant at p < 0.05, n = 5.
TABLE 2.
Gene expression of collagen type 2 alpha 1 in unstimulated pellets, untreated controls (interleukin [IL]‐1β‐stimulated pellets), and pellets treated with addition of pooled serum (PS), pooled serum incubated 24 h (PS24h), or pooled conditioned serum (PCS) sampled after 2 and 48 h
| 2 h | 48 h | |||
|---|---|---|---|---|
| 2−(ΔΔct) ± SD | p | 2−(ΔΔct) ± SD | p | |
| Unstimulated control | 0.884 ± 1.02 | 0.5 | 1.94 ± 2.93 | 0.006 |
| Untreated control (IL‐1β) | 0.416 ± 0.471 | 0.090 ± 0.098 | ||
| IL‐1β + PS | 0.659 ± 0.538 | 0.7 | 0.207 ± 0.358 | 0.9 |
| IL‐1β + PS24h | 0.652 ± 0.516 | 0.7 | 0.144 ± 0.180 | 0.9 |
| IL‐1β + PCS | 0.265 ± 0.184 | 0.8 | 0.125 ± 0.164 | >0.9 |
Note: Values are presented as relative gene expression 2−(ΔΔct) ± SD and p values for all treatments were compared against untreated controls at each time point. Results were considered statistically significant at p < 0.05, n = 5.
Evaluation of controls in the Affymetrix Expression Console (Affymetrix Inc.) indicated successful expression profiling for all samples included in the microarray analysis. Differential expression of 672 genes at 2 h (Table S2) and 279 genes at 48 h (Table S3) was found between unstimulated and untreated controls (IL‐1β‐stimulated pellets). The number of DEGs in PS‐, PS24h‐ and PCS‐treated pellets compared with that in untreated controls is shown as Venn diagrams (Figure 2). The DEGs in the treatment groups are listed in Table S4 (upregulated and downregulated after 2 h), Table S5 (upregulated after 48 h) and Table S6 (downregulated after 48 h).
FIGURE 2.
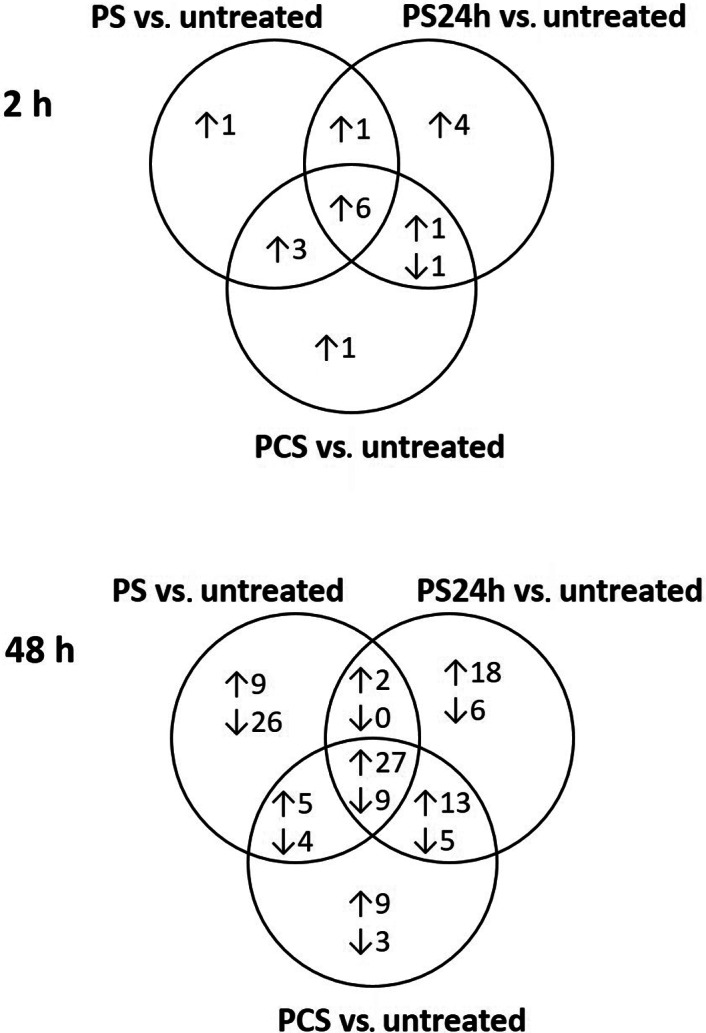
Differentially expressed genes. Venn diagrams of genes with significantly different expression in the treated (pooled serum [PS], pooled serum incubated for 24 h [PS24h] and pooled conditioned serum [PCS]) pellets compared with those in untreated controls (interleukin [IL]‐1β‐stimulated pellets) after 2 and 48 h. Numbers after arrows pointing upwards indicate the number of upregulated genes and numbers after arrows pointing downwards indicate the number of downregulated genes compared with those in untreated controls.
Compared with untreated controls, IL‐6 gene expression was upregulated after 2 h (Table S4) and 48 h (Table S5), whereas IL‐11 gene expression was upregulated after 48 h (Table S5) in all treatment groups. The chemokine (C‐X‐C motif) ligand 13 (CXCL13) gene was upregulated in the PS and PCS versus the untreated controls at 48 h (Table S5). IL‐1 receptor type II (IL‐1RII) gene expression was upregulated in all treatment groups compared with untreated controls at 48 h (Table S5). Collagen type XI alpha 1 and 2 (COL11A1 and COL11A2) genes were downregulated at 48 h in pellets treated with PS24h and PCS versus untreated controls (Table S6). The metalloproteinase‐disintegrin genes ADAMTS1 and ADAMTS5 were upregulated in all treatment groups at 48 h. Tissue inhibitor metalloproteinase 3 (TIMP3) expression was upregulated in pellets treated with PS24h and PCS at 48 h versus untreated controls (Table S5).
The expression of fibroblast growth factor 7 (FGF7) was upregulated in all treatment groups versus untreated controls, and expression of platelet‐derived growth factor D (PDGFD) was upregulated at 48 h in the PS‐treated pellets versus untreated controls (Table S5). Expression of bone morphogenetic protein 2 (BMP2), growth/differentiation factor‐10 (GDF10) and inhibin beta A (INHBA) were upregulated compared with those in the untreated controls after 48 h in PS24h, PS and PCS, respectively (Table S5). Expression of inhibin beta E (INHBE) was downregulated in the PS24h and PCS groups versus untreated controls at 48 h (Table S6).
3.3. Explant cultures (model 2)
On day 0, the cartilage explants were assigned an overall histological OARSI score of 9.1 (±2.4). At harvesting at day 24, there were no differences in histological score between the treatment groups (overall OARSI score of 8.0 ± 2.4, 8.8 ± 1.8 and 8.5 ± 2.2 for the untreated controls, the PS‐treated explants and the PCS‐treated explants, respectively; Table 3).
TABLE 3.
Histological grading of cartilage explants (model 2) at days 0 and 24
| Macroscopy: wear lines, erosion, palmar arthrosis | Microscopy H&E: Chondrocyte necrosis, chondrone formation, fissuring, focal cell loss SOFG: Stain uptake | |||||||
|---|---|---|---|---|---|---|---|---|
| ID | Age (years) | Sex | Day 0 | Day 24 control | Day 24 PS | Day 24 PCS | ||
| 1 | 12 | Not known | 1, 0, 2 | H&E | 4, 1, 2, 3 = 10 | 4, 1, 1, 3 = 9 | 4, 1, 1, 3 = 9 | 4, 1, 1, 3 = 9 |
| SOFG | 1 | 2 | 2 | 2 | ||||
| 2 | 7 | Mare | 0, 0, 1 | H&E | 2, 0, 0, 2 = 4 | 3, 0, 0, 3 = 6 | 3, 1, 0, 3 = 7 | 4, 1, 0, 3 = 8 |
| SOFG | 1 | 2 | 3 | 4 | ||||
| 3 | 14 | Mare | 1, 0, 0 | H&E | 4, 1, 1, 3 = 9 | 4, 1, 0, 3 = 8 | 4, 2, 1, 3 = 10 | 4, 1, 1, 3 = 9 |
| SOFG | 1 | 2 | 2 | 3 | ||||
| 4A | 14 | Mare | 0, 0, 1 | H&E | 4, 1, 1, 3 = 9 | 3, 1, 0, 2 = 6 | 3, 1, 1, 3 = 8 | 3, 1, 0, 3 = 7 |
| SOFG | 2 | 3 | 2 | 3 | ||||
| 4B | 14 | Mare | 1, 2, 2 | H&E | 4, 2, 2, 3 = 11 | 4, 3, 2, 2 = 11 | 4, 3, 1, 3 = 11 | 4, 3, 1, 3 = 11 |
| SOFG | 1 | 2 | 3 | 2 | ||||
| 5 | 8 | Gelding | 2, 0, 1 | H&E | 4, 3, 2, 3 = 12 | 4, 3, 2, 3 = 12 | 4, 2, 2, 3 = 11 | 4, 3, 2, 3 = 12 |
| SOFG | 3 | 3 | 2 | 2 | ||||
| 6 | 16 | Mare | 0, 0, 1 | H&E | 3, 1, 1, 3 = 8 | 3, 1, 0, 2 = 6 | 3, 1, 0, 2 = 6 | 3, 1, 0, 2 = 6 |
| SOFG | 1 | 3 | 3 | 2 | ||||
| 7 | 17 | Gelding | 1, 0, 1 | H&E | 4, 2, 1, 3 = 10 | 3, 1, 0, 2 = 6 | 4, 1, 0, 3 = 8 | 3, 1, 0, 2 = 6 |
| SOFG | 2 | 3 | 3 | 3 | ||||
| Mean H&E | 9.1 | 8 | 8.8 | 8.5 | ||||
| Standard deviation H&E | 2.4 | 2.4 | 1.8 | 2.2 | ||||
Note: Explants were treated with control medium, pooled serum (PS) and pooled autologous serum (PCS) on days 0, 6 and 12 and harvested at day 24. Macroscopic staging of wear lines, erosions and palmar arthrosis; microscopic assessment of chondrocyte necrosis, cluster formation, fissuring and focal cell loss; and extra cellular matrix staining were performed as previously described 11 by haematoxylin & eosin (H&E) and safranin‐O fast green (SOFG) staining.
4. DISCUSSION
The proposed mechanism of intra‐articular treatment with conditioned serum is to reduce the detrimental effects of IL‐1β, which is believed to be the main driver of the OA process. Therefore, we aimed to investigate whether the incubated serum could decrease or inhibit IL‐1β‐induced effects and enhance cartilage regeneration in 3D chondrocyte pellets. To the best of our knowledge, this study is the first to use microarrays to measure global gene expression in chondrocyte pellets to obtain a broader understanding of genes involved. Moreover, to assess the potential healing of diseased cartilage, we evaluated the treatment effects in OA explants.
Cartilage metabolism in healthy cartilage involves a fine balance between catabolic and anabolic processes. In chondrocyte pellets, IL‐1β stimulation mediates a majority of catabolic events. We identified genes involved in inflammation among those showing significant differential expression. Specifically, two cytokines in the IL‐6 family (IL‐6 and IL‐11) were upregulated in all treatment groups compared with those in the untreated controls (IL‐1β‐stimulated pellets). IL‐6 is a regulatory cytokine with dual roles in OA; its effects can be either pro‐ or anti‐inflammatory. 21 IL‐6, along with IL‐1α, induces collagen release from cartilage explants, 22 and both IL‐6 and IL‐11 inhibit proteoglycan synthesis in porcine cartilage explants. 23 Increased levels of IL‐6 have been observed in human synovial fluid and serum and are suggested to have a catabolic and inflammatory role in joint tissues via trans‐signalling. 24 Interestingly, IL‐6 prevents proteoglycan destruction in mice, 25 whereas IL‐11 has a protective effect in human cartilage. 26 Protective effects of IL‐6 are suggested to be mediated via classical IL‐6 signalling. 24 Previous studies of IL‐6 stimulated equine 3D chondrocyte pellets show signs of anabolic activities such as upregulation of GDF5 and chondrocyte differentiation. 11 , 27 Although we mainly observed catabolic events such as increased expression of degrading enzymes in our study, we cannot make conclusions about the effects of IL‐6 signalling. To the best of our knowledge, the effects of IL‐11 have not been studied in equine cartilage. The expression of IL‐11 in IL‐1β stimulated equine cartilage explants is not different from unstimulated controls. 28 Gene expression of IL‐11 has also been studied in equine lamellae and is increased in a model of laminitis. 29
The chemokine CXCL13, upregulated in the PS‐ and PCS‐treated groups after 48 h in the present study, might contribute to a catabolic state as it reportedly increases in the synovial fluid of OA patients with severe cartilage damage, whereas reduced levels are found after surgery. 30 , 31 IL‐1RII serves as an inhibitor of IL‐1 signalling, 32 and its increased expression after 48 h of stimulation suggests the potential of all treatments to reduce IL‐1β signalling.
Here, IL‐1β stimulation resulted in increased MMP‐13 concentration in the pellet culture media in untreated IL‐1β stimulated controls compared with unstimulated pellets. Treatment with the incubated sera did not reduce the MMP‐13 concentration, suggesting that the treatments did not inhibit matrix‐degrading enzymes at the protein level in the stimulated chondrocyte pellets. The degradation of proteoglycans, such as aggrecan, followed by degradation of collagens occurs gradually during OA. 33 The reduced expression of ACAN in all treatment groups and that of COL11A1 and COL11A2 in PS24h‐ and PCS‐treated pellets indicate a catabolic state, which is corroborated by increased expression of ADAMTS1 and ADAMTS5 after treatment. However, the matrix metallopeptidase inhibitor TIMP3, suggested to protect cartilage, 34 was upregulated in PS24h‐ and PCS‐treated pellets after 48 h.
Among the DEGs that may affect OA, several growth factors were identified. For example, FGF7, upregulated in all treatment groups after 48 h compared with IL‐1β‐stimulated pellets, is thought to stimulate the proliferation of chondrocytes and aid healing of damaged cartilage. 35 Growth factors BMP2, GDF10 and INHBA, involved in TGF‐β signalling, 36 , 37 were also significantly upregulated compared with IL‐1β‐stimulated pellets. TGF‐β is a potential therapeutic target in OA treatment because it is involved in the development, growth, maintenance and repair of articular cartilage. 38 TGF‐β inhibition is associated with the degeneration of cartilage and terminal differentiation of chondrocytes. 39 The regenerative effects of TGF‐β include chondrocyte differentiation and cartilage formation. 38 However, adverse effects, such as fibrosis and osteophyte formation, have been associated with TGF‐β treatment. 39 BMP2 is thought to be involved in cartilage repair because increased proteoglycan synthesis has been observed after BMP2 stimulation. 40 A combination of TGF‐β and BMP2 showed potential to induce a chondrogenic phenotype in mesenchymal stem cells. 41 Interestingly, TGF‐β/BMP signalling reportedly has both protective and deleterious effects 42 and needs to be further studied in the context of ACS treatment. PDGFD, significantly upregulated in the PS‐treated group, is involved in wound healing and angiogenesis. 43 It reportedly plays a role in rheumatoid arthritis as it stimulates synovial fibroblast proliferation and MMP‐1 expression. 44 Thus, the induction of several growth factors may be favourable for cartilage regeneration.
IL‐1Ra is suggested to be a potential anti‐inflammatory protein 33 and is thought to be the major anti‐inflammatory cytokine induced by the preparation of ACS. 3 We observed significantly higher IL‐1Ra concentration in serum samples incubated for 24 h at 37°C, irrespective of the presence (ACS) or absence (incubated serum) of borosilicate glass beads, than in the non‐incubated serum sample. These findings are consistent with those of previous studies, where increased IL‐1Ra level was detected in equine, 4 , 7 , 45 , 46 canine 47 and human 3 , 8 serum samples after incubation with or without glass beads for 24 h. Interestingly, the IL‐1Ra concentration was significantly higher in the serum incubated for 24 h (without beads) than in ACS (with beads). A previous study reported no significant difference in the IL‐1Ra concentrations between ACS and serum samples incubated for 24 and 36 h from lame or non‐lame horses, using a sandwich ELISA specific for equine IL‐1Ra. 45 The authors of the study concluded that whole blood incubation in glass tubes and that using specialised equine commercial ACS kits result in similar concentrations of IL‐1Ra. Another study reported similar results, wherein a comparison of serum samples collected from non‐lame horses before and after castration showed no significant difference in the IL‐1Ra concentration between serum and ACS samples incubated for 24 h. 7 In contrast, significantly higher IL‐1Ra concentrations were found in sera from healthy horses prepared using one commercially available preparation (IRAP II/Arthrex) than those prepared using another commercial preparation (IRAP/Orthokine) as well as control serum incubated for 24 h without glass beads. 4 Similarly, the IL‐1Ra concentration was significantly higher in human sera (obtained from healthy volunteer donors) incubated with 3.5 mm polished beads than in sera incubated with beads of other sizes and non‐incubated sera. 48 The different results in these studies may be attributed to the different ACS preparation kits and ELISA methods used for analysing the IL‐1Ra concentration and/or the clinical status of the horses. Our findings are in accordance with the previously reported results, suggesting that an increased IL‐Ra concentration can be achieved by the incubation of whole blood in glass tubes, even without the use of commercial systems containing glass beads. Interestingly, the levels of IL‐1Ra in ACS vary between the studies. The levels in our study (15.8 ± 9.94 ng/ml) are comparable to those of other studies using the same system (ABPS/Arthrex), 9 , 45 , 49 whereas Hraha et al. reported values approximately 10 times lower 4 and Linardi et al. reported a value of 89.9 ± 88.8 ng/ml. 46 The high values in the study by Fjordbakk et al. can be explained by the use of other commercial systems for ACS preparation. 7 Individual differences in cytokine/growth factor content of the conditioned sera are another important aspect. Differences in cytokine content have previously been associated with surgical stress 7 indicating the influence of health status. Notably, a previous study shows no difference in concentration of IL‐1Ra, IL‐1β and IGF‐1 between ACS preparations from OA and control non‐lame horses. 45 Further studies of individual differences in IL‐1Ra concentration as well as other components would be of interest to identify individuals with the potential to produce ACS with beneficial effects. The wide age range of the horses used for preparation of serum in model 1 (4–22 years) might have influenced the levels in our study, but there was no clear relation between age and IL‐Ra concentration. However, age might influence other components of the serum and the number of studied horses were low.
In a previous study, IL‐1β‐stimulated healthy equine cartilage co‐cultured with synovial explants was treated with ACS for 96 h, which resulted in significant downregulation of IL‐1β in cartilage compared with that after triamcinolone treatment. 50 In addition, a trend toward upregulated IL‐10 expression in the synovium and that of type II collagen and aggrecan expression in the cartilage was observed. 50 However, treating the diseased cartilage explants, cultured for 24 days with PS and PCS at days 0, 6 and 12 in the present study, did not result in increased staining intensity for proteoglycans or changes in histological grading at day 24 compared with those of the untreated controls. These results are in accordance with the findings of an in vivo study, 5 reporting a lack of effective treatments for articular cartilage in equine experimental OA. Differences in treatment results can be explained by different time points for treatment and analysis. Our protocol with addition of treatments at days 0, 6 and 12 followed by evaluation of effects at day 24 was an attempt to mimic in vivo treatment protocols, where three treatments with 1–2‐week intervals is widely used. 9 , 49 However, Lasarzik et al. suggested that a two‐day interval might be preferable. 49 The time points selected for treatment and analysis in our study is a known weakness of the study, and response at other time points cannot be ruled out.
Carlson et al. studied the effects of unincubated serum and ACS on equine chondrocyte pellets, and reported no clear beneficial effects. 51 An important difference from our study was that Carlson et al. studied blood samples from healthy horses, whereas we evaluated samples from horses with naturally occurring lameness, mimicking the real‐life situation in which ACS would be used. As joint pathology might possibly influence ACS content, we chose to use client‐owned lame horses as blood donors for the serum preparations studied. Another difference was that we pre‐treated the chondrocyte pellets with IL‐1β to induce catabolic responses, whereas Carlson et al. simultaneously treated the pellets with IL‐1β and sera. In addition, we used 40% of the total stimulation volume for PS, PS24h and PCS treatments, a volume that was calculated to be relevant for in vivo joint treatment, compared with 10% or 20% of the total stimulation volume used by Carlson et al. Moreover, we used a lower concentration of recombinant equine IL‐1β (5 ng/ml) compared with 10 ng/ml recombinant human IL‐1β used by Carlson et al. Our rationale for this was that a higher percentage of serum and ACS combined with a lower concentration of IL‐1β would mimic in vivo conditions and improve the treatment efficacy. However, no clear anti‐inflammatory effects were observed in our study although genes with regenerative potential were identified, the expression analysis of genes involved in matrix degradation indicated an increased catabolic state in the chondrocytes treated with PS, PS24h and PCS.
This study had some limitations. The chondrocyte pellets were cultured from healthy horses, and stimulation with IL‐1β induced acute cellular responses, which is not comparable to clinical OA where the disease process is of low‐grade chronic nature. However, our cartilage explants were harvested from OA joints, representing chronic inflammation. One may also argue that a concentration curve for IL‐1β would have been of value to select an appropriate concentration for the stimulation. Hence, the IL‐1β concentration used here may not represent the concentration in vivo. Measured IL‐1β concentrations in OA synovial fluid in vivo is variable, and <2 ng/ml has been measured in human, porcine and canine samples. 52 Mean concentration in equine synovial fluid was 35.3 pg/ml in mild OA and 329.91 pg/ml in advanced OA. 53 Concentrations used for stimulation in vitro is up to 100 ng/ml. 52 The concentration used in our study (5 ng/ml) has previously been observed to induce a catabolic response in the same 3D pellet culture system. 11 It cannot be ruled out that other effects of ACS treatment may be observed at other concentrations of IL‐1β and %V/V serum.
In conclusion, we did not observe any inhibition of IL‐1β‐induced responses in 3D chondrocyte pellets or morphological improvement in OA cartilage explants after PCS treatment. To the best of our knowledge, this is the first study to determine the global expression of genes involved in inflammation and cartilage matrix degradation in IL‐1β stimulated chondrocytes and histological staging of OA cartilage explants to study the treatment effects of autologous sera. We found that serum incubated for 24 h contained significantly higher IL‐1Ra concentration than ACS, which questions the reported use of commercial systems to achieve high levels of IL‐1Ra. However, neither PS24h nor PCS showed any evidence of overall anti‐inflammatory effects on the inflamed chondrocyte pellets or regeneration in the OA cartilage explants. Hence, the presented results do not support the disease‐modifying properties of ACS on articular cartilage in vitro. Future studies to determine the efficacy of ACS for the treatment of joint pain and its potential ability to reduce clinical lameness may be of value.
AUTHOR CONTRIBUTIONS
Maria Löfgren, Stina Ekman, Eva Skiöldebrand designed the project; acquired, analysed, and interpreted the results; and wrote the manuscript with contributions from the other authors. Josefine Ekholm, Mona Engström and Emilia Svala contributed to experimental design and performed the experiments. Karin Holm Forsström contributed to the design and implementation of the study and collected the samples. Cathrine T. Fjordbakk contributed to implementation of the research and collected the samples. Anders Lindahl contributed to the design and implementation of the research, and the analysis of the results. All authors approved the final version of the manuscript.
FUNDING INFORMATION
Swedish‐Norwegian Foundation for Equine Research, Svelands Stiftelse and Valborg Jacobssons Fond.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/evj.13582.
ETHICS STATEMENT
The study was approved by the Ethical Committee on Animal Experiments, Uppsala, Sweden (No. C62/13 and 5.8.18‐02896/2018). Cartilage for pellet culture was collected as a part of another study, with ethical approval from the Iceland National Animal Research Committee 2007.
Supporting information
TABLE S1 Matrixmetallopeptidase‐13 (MMP‐13) concentration in the media from unstimulated pellets, untreated controls (interleukin [IL]‐1β‐stimulated pellets) and pellets treated with pooled serum (PS), pooled serum incubated for 24 h (PS24h), or pooled conditioned serum (PCS) sampled after 2 and 48 h. Values are presented as mean ± SD and p values for all treatments were compared against untreated controls at each time point. Results were considered statistically significant at p < 0.05, n = 5.
TABLE S2 Significantly differentially expressed genes between unstimulated (culture media) and untreated controls (interleukin [IL]‐1β‐stimulated pellets) after 2 h.
TABLE S3 Significantly differentially expressed genes between unstimulated (culture media) and untreated controls (interleukin [IL]‐1β‐stimulated pellets) after 48 h.
TABLE S4 Gene expression in pellets treated with pooled serum (PS), pooled serum incubated for 24 h (PS24h) and pooled conditioned serum (PCS) compared with that in untreated controls (interleukin [IL]‐1β‐stimulated pellets) after 2 h. Genes are grouped based on significant expression in the treatment groups.
TABLE S5 Gene expression in pellets treated with pooled serum (PS), pooled serum incubated for 24 h (PS24h) and pooled conditioned serum (PCS) compared with that in untreated controls (interleukin [IL]‐1β‐stimulated pellets) after 48 h. Genes were grouped based on significantly upregulated expression in the treatment groups.
TABLE S6 Gene expression in pellets treated with pooled serum (PS), pooled serum incubated for 24 h (PS24h) and pooled conditioned serum (PCS) compared with that in untreated controls (interleukin [IL]‐1β‐stimulated pellets) after 48 h. Genes were grouped based on significantly downregulated expression in the treatment groups.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Christina Nilsson and Beate Hillmann for performing the histological preparations and Ulf Olsson (Centre for Statistics, Swedish University of Agricultural Sciences, Uppsala) for statistical consultation. The microarray analysis was performed by the Array and Analysis Facility, Science for Life Laboratory at Uppsala Biomedical Centre, Uppsala, Sweden.
Löfgren M, Ekman S, Ekholm J, Engström M, Fjordbakk CT, Svala E, et al. Conditioned serum in vitro treatment of chondrocyte pellets and osteoarthritic explants. Equine Vet J. 2023;55(2):325–335. 10.1111/evj.13582
[Correction added on 29 Jun 2022, after initial online publication. A duplicate of this article was published under the 10.1111/evj.13852. This duplicate has now been deleted and its DOI redirected to this version of the article.]
Funding information Valborg Jacobssons Fond; Svelands Stiftelse; Swedish‐Norwegian Foundation for Equine Research, Grant/Award Number: H‐16‐47‐182
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Gene Expression Omnibus at www.ncbi.nlm.nih.gov/geo/ reference number GSE152253.
REFERENCES
- 1. Kidd JA, Fuller C, Barr RS. Osteoarthritis in the horse. Equine Vet Educ. 2001;13:160–8. [Google Scholar]
- 2. Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith C. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin‐1 receptor antagonist gene. Gene Ther. 2002;9(1):12–20. [DOI] [PubMed] [Google Scholar]
- 3. Meijer H, Reinecke J, Becker C, Tholen G, Wehling P. The production of anti‐inflammatory cytokines in whole blood by physico‐chemical induction. Inflamm Res. 2003;52:404–7. [DOI] [PubMed] [Google Scholar]
- 4. Hraha TH, Doremus KM, McIlwraith CW, Frisbie DD. Autologous conditioned serum: the comparative cytokine profiles of two commercial methods (IRAP and IRAP II) using equine blood. Equine Vet J. 2011;43:516–21. [DOI] [PubMed] [Google Scholar]
- 5. Frisbie DD, Kawcak CE, Werpy NM, Park RD, McIlwraith CW. Clinical, biochemical, and histologic effects of intra‐articular administration of autologous conditioned serum in horses with experimental induced osteoarthritis. Am J Vet Res. 2007;68:290–6. [DOI] [PubMed] [Google Scholar]
- 6. Wehling P, Moser C, Frisbie D, McIlwraith CW, Kawcak CE, Krauspe R, et al. Autologous conditioned serum in the treatment of orthopedic diseases. BioDrugs. 2007;21(5):323–32. [DOI] [PubMed] [Google Scholar]
- 7. Fjordbakk CT, Johansen GM, Løvås AC, Oppegård KL, Storset AK. Surgical stress influences cytokine content in autologous conditioned serum. Equine Vet J. 2014;47:212–7. [DOI] [PubMed] [Google Scholar]
- 8. Rutgers M, Saris DB, Wouter WJ, Creemers LB. Cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra‐articular levels after injection. Arthritis Res Ther. 2010;12:R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marques‐Smith P, Kallerud AS, Johansen GM, Boysen P, Jacobsen AM, Reitan KM, et al. Is clinical effect of autologous conditioned serum in spontaneously occurring equine articular lameness related to ACS cytokine profile? BMC Vet Res. 2020;16:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ley CJ, Ekman S, Dahlberg LE, Björnsdóttir S, Hansson K. Evaluation of osteochondral sample collection guided by computed tomography and magnetic resonance imaging for early detection of osteoarthritis in centrodistal joints of young Icelandic horses. Am J Vet Res. 2013;74:874–87. [DOI] [PubMed] [Google Scholar]
- 11. Ley C, Svala E, Nilton A, Lindahl A, Eloranta ML, Ekman S, et al. Effects of high mobility group box protein‐1, interleukin‐1β, and interleukin‐6 on cartilage matrix metabolism in three‐dimensional equine chondrocyte cultures. Connect Tissue Res. 2011;52:290–300. [DOI] [PubMed] [Google Scholar]
- 12. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta C[T]) method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 13. McIlwraith CW, Frisbie DD, Kawcak CE, Fuller CJ, Hurtig M, Cruz A. The OARSI histopathology initiative–recommendations for histological assessments of osteoarthritis in the horse. Osteoarthr Cartil. 2010;18:S93–S105. [DOI] [PubMed] [Google Scholar]
- 14. Olsson U. Statistics for Life Science 1. Lund, Studentlitteratur; 2011.
- 15. Littell R, Milliken G, Stroup W, Wolfinger R, Schabenberger O. SAS for mixed models. 2nd ed. Cary, NC: SAS Institute Inc.; 2006. [Google Scholar]
- 16. Li C, Wong WH. Model‐based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001;98:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Irizarry RA, Hobbs B, Collin F, Beazer‐Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. [DOI] [PubMed] [Google Scholar]
- 18. Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:3. 10.2202/1544-6115.1027 [DOI] [PubMed] [Google Scholar]
- 19. Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, editors. Bioinformatics and computational biology solutions using R and Bioconductor. New York: Springer; 2005. p. 397–420. [Google Scholar]
- 20. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995;57:289–300. [Google Scholar]
- 21. Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti‐inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat Inflamm. 2014;2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rowan AD, Koshy PJT, Shingleton WD, Degnan BA, Heath JK, Vernallis AB, et al. Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin‐1 on cartilage collagen breakdown. Arthritis Rheum. 2001;44:1620–32. [DOI] [PubMed] [Google Scholar]
- 23. Hui W, Bell M, Carroll G. Oncostatin M (OSM) stimulates resorption and inhibits synthesis of proteoglycan in porcine articular cartilage explants. Cytokine. 1996;8:495–500. [DOI] [PubMed] [Google Scholar]
- 24. Wiegertjes R, van de Loo FAJ, Blaney Davidson EN. A roadmap to target interleukin‐6 in osteoarthritis. Rheumatology (Oxford). 2020;59(10):2681–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van de Loo FA, Kuiper S, van Enckevort FH, Arntz OJ, van den Berg W. Interleukin‐6 reduces cartilage destruction during experimental arthritis. A study in interleukin‐6‐deficient mice. Am J Pathol. 1997;151:177–91. [PMC free article] [PubMed] [Google Scholar]
- 26. Maier R, Ganu V, Lotz M. Interleukin‐11, an inducible cytokine in human articular chondrocytes and synoviocytes, stimulates the production of the tissue inhibitor of metalloproteinases. J Biol Chem. 1993;268:21527–32. [PubMed] [Google Scholar]
- 27. Svala E, Thorfve AI, Ley C, Henriksson HKB, Synnergren JM, Lindahl AH, et al. Effects of interleukin‐6 and interleukin‐1β on expression of growth differentiation factor‐5 and Wnt signaling pathway genes in equine chondrocytes. Am J Vet Res. 2014;75(2):132–40. [DOI] [PubMed] [Google Scholar]
- 28. Löfgren M, Svala E, Lindahl A, Skiöldebrand E, Ekman S. Time‐dependent changes in gene expression induced in vitro by interleukin‐1β in equine articular cartilage. Res Vet Sci. 2018;118:466–76. [DOI] [PubMed] [Google Scholar]
- 29. Watts MR, Hegedus OC, Eades SC, Belknap JK, Burns TA. Association of sustained supraphysiologic hyperinsulinemia and inflammatory signaling within the digital lamellae in light‐breed horses. J Vet Intern Med. 2019;33(3):1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li ZM, Li M. Improvement in orthopedic outcome score and reduction in IL‐1β, CXCL13, and TNF‐α in synovial fluid of osteoarthritis patients following arthroscopic knee surgery. Genet Mol Res. 2017;16(3). 10.4238/gmr16039487 [DOI] [PubMed] [Google Scholar]
- 31. Ye G, Peng C, Gao Z, Xiao J, Mei L. Effects of arthroscopic knee surgery on IL‐1β, CXCL13 and TNF‐α in the knee joint fluid of knee osteoarthritis patients and their correlation with clinical outcomes. Int J Clin Exp Pathol. 2017;10:1690–6. [Google Scholar]
- 32. Peters VA, Joesting JJ, Freund GG. IL‐1 receptor 2 (IL‐1R2) and its role in immune regulation. Brain Behav Immun. 2013;32:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. [DOI] [PubMed] [Google Scholar]
- 34. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abo T, Nagayasu T, Hishikawa Y, Tagawa T, Nanashima A, Yamayoshi T, et al. Expression of keratinocyte growth factor and its receptor in rat tracheal cartilage: possible involvement in wound healing of the damaged cartilage. Acta Histochem Cytochem. 2010;43:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cunningham NS, Jenkins NA, Gilbert DJ, Copeland NG, Reddi AH, Lee SJ. Growth/differentiation factor‐10: a new member of the transforming growth factor‐beta superfamily related to bone morphogenetic protein‐3. Growth Factors. 1995;12:99–109. [DOI] [PubMed] [Google Scholar]
- 37. Hopwood B, Tsykin A, Findlay DM, Fazzalari NL. Microarray gene expression profiling of osteoarthritic bone suggests altered bone remodelling, WNT and transforming growth factor‐beta/bone morphogenic protein signalling. Arthritis Res Ther. 2007;9:R100. 10.1186/ar2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Finnson KW, Chi Y, Bou‐Gharios G, Leask A, Philip A. TGF‐b signaling in cartilage homeostasis and osteoarthritis. Front Biosci (Schol Ed). 2012;4:251–68. [DOI] [PubMed] [Google Scholar]
- 39. Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF‐beta and osteoarthritis. Osteoarthr Cartil. 2007;15:597–604. [DOI] [PubMed] [Google Scholar]
- 40. Blaney Davidson EN, Vitters EL, van Lent PL, van de Loo FAJ, van den Berg WB, van der Kraan PM. Elevated extracellular matrix production and degradation upon bone morphogenetic protein‐2 (BMP‐2) stimulation point toward a role for BMP‐2 in cartilage repair and remodeling. Arthritis Res Ther. 2007;9:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Legendre F, Ollitrault D, Gomez‐Leduc T, Bouyoucef M, Hervieu M, Gruchy N, et al. Enhanced chondrogenesis of bone marrow‐derived stem cells by using a combinatory cell therapy strategy with BMP‐2/TGF‐β1, hypoxia, and COL1A1/HtrA1 siRNAs. Sci Rep. 2017;7:3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhai G, Doré J, Rahman P. TGF‐β signal transduction pathways and osteoarthritis. Rheumatol Int. 2015;35:1283–92. [DOI] [PubMed] [Google Scholar]
- 43. Uutela M, Wirzenius M, Paavonen K, Rajantie I, He Y, Karpanen T, et al. PDGF‐D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood. 2004;104:3198–204. [DOI] [PubMed] [Google Scholar]
- 44. Pohlers D, Huber R, Ukena B, Kinne RW. Expression of platelet‐derived growth factors C and D in the synovial membrane of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 2006;54:788–94. [DOI] [PubMed] [Google Scholar]
- 45. Lasarzik de Ascurra J, Ehrle A, Einspanier R, Lischer C. Influence of incubation time and incubation tube on the cytokine and growth factor concentrations of autologous conditioned serum in horses. J Equine Vet Sci. 2019;75:30–4. [DOI] [PubMed] [Google Scholar]
- 46. Linardi RL, Dodson ME, Moss KL, King WJ, Ortved KF. The effect of autologous protein solution on the inflammatory cascade in stimulated equine chondrocytes. Front Vet Sci. 2019;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sawyere DM, Lanz OI, Dahlgren LA, Barry SL, Nichols AC, Werre SR. Cytokine and growth factor concentrations in canine autologous conditioned serum. Vet Surg. 2016;45:582–6. [DOI] [PubMed] [Google Scholar]
- 48. Magalon J, Bausset O, Veran J, Giraudo L, Serratrice N, Magalon G, et al. Physico‐chemical factors influencing autologous conditioned serum purification. Biores Open Access. 2014;3:35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lasarzik J, Bondzio A, Rettig M, Estrada R, Klaus C, Ehrle A, et al. Evaluation of two protocols using autologous conditioned serum for intra‐articular therapy of equine osteoarthritis: a pilot study monitoring cytokines and cartilage‐specific biomarkers. J Equine Vet Sci. 2018;60:35–42. [Google Scholar]
- 50. Alvarez AV, Boone LH, Pondugula SR, Caldwell F, Wooldridge AA. Effects of autologous conditioned serum, autologous protein solution, and triamcinolone on inflammatory and catabolic gene expression in equine cartilage and synovial explants treated with IL‐1β in co‐culture. Front Vet Sci. 2020;7:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carlson ER, Stewart AA, Carlson KL, Durgam SS, Pondenis HC. Effects of serum and autologous conditioned serum on equine articular chondrocytes treated with interleukin‐1 β. Am J Vet Res. 2013;74:700–5. [DOI] [PubMed] [Google Scholar]
- 52. Johnson CI, Argyle DJ, Clements DN. In vitro models for the study of osteoarthritis. Vet J. 2016;209:40–9. [DOI] [PubMed] [Google Scholar]
- 53. Ehrle A, Lischer CJ, Lasarzik J, Einspanier R, Bondzio A. Synovial fluid and serum concentrations of interleukin‐1 receptor antagonist and interleukin‐1ß in naturally occurring equine osteoarthritis and septic arthritis. J Equine Vet Sci. 2015;35(10):815–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Matrixmetallopeptidase‐13 (MMP‐13) concentration in the media from unstimulated pellets, untreated controls (interleukin [IL]‐1β‐stimulated pellets) and pellets treated with pooled serum (PS), pooled serum incubated for 24 h (PS24h), or pooled conditioned serum (PCS) sampled after 2 and 48 h. Values are presented as mean ± SD and p values for all treatments were compared against untreated controls at each time point. Results were considered statistically significant at p < 0.05, n = 5.
TABLE S2 Significantly differentially expressed genes between unstimulated (culture media) and untreated controls (interleukin [IL]‐1β‐stimulated pellets) after 2 h.
TABLE S3 Significantly differentially expressed genes between unstimulated (culture media) and untreated controls (interleukin [IL]‐1β‐stimulated pellets) after 48 h.
TABLE S4 Gene expression in pellets treated with pooled serum (PS), pooled serum incubated for 24 h (PS24h) and pooled conditioned serum (PCS) compared with that in untreated controls (interleukin [IL]‐1β‐stimulated pellets) after 2 h. Genes are grouped based on significant expression in the treatment groups.
TABLE S5 Gene expression in pellets treated with pooled serum (PS), pooled serum incubated for 24 h (PS24h) and pooled conditioned serum (PCS) compared with that in untreated controls (interleukin [IL]‐1β‐stimulated pellets) after 48 h. Genes were grouped based on significantly upregulated expression in the treatment groups.
TABLE S6 Gene expression in pellets treated with pooled serum (PS), pooled serum incubated for 24 h (PS24h) and pooled conditioned serum (PCS) compared with that in untreated controls (interleukin [IL]‐1β‐stimulated pellets) after 48 h. Genes were grouped based on significantly downregulated expression in the treatment groups.
Data Availability Statement
The data that support the findings of this study are openly available in Gene Expression Omnibus at www.ncbi.nlm.nih.gov/geo/ reference number GSE152253.


