Abstract
Background
Early childhood caries (ECC) remains one of the most prevalent childhood diseases in Australia, disproportionately affecting disadvantaged populations.
Aim
To investigate the ECC experience including risk factors, incidence of caries, pain and infection as well as relapse rates of caries and secondary dental general anaesthesia (GA).
Design
A retrospective cohort study included dental records of children with ECC, aged <72 months at an Australian public dental hospital paediatric dentistry department from 2013 to 2015 (n = 102). Dental caries, pain, infection, referral patterns, demographic and caries risk factor data were recorded for 24 months. Descriptive statistics were used for patient characteristics and clinical data, and Kaplan–Meier curves and parametric exponential survival models for time‐to‐event series.
Results
The study population demonstrated higher‐than‐national average dmft and disease progression at baseline. Major risk factors for the development of caries, pain and infection were daily consumption of sweetened beverages, poor oral hygiene, residing in lower socio‐economic areas, older age and being male. Rates of caries relapse and new referral for secondary treatment under general anaesthesia were relatively high.
Conclusion
A high degree of ECC progression and recurrence in this population indicates a need for a more comprehensive approach to ECC addressing multilevel root causes and systemic risk factors.
Keywords: early childhood caries, oral health, oral health promotion, paediatric population, paediatric dentistry
Why this paper is important to paediatric dentists.
Children who attended an Australian public dental hospital had disproportionately high rates of caries and associated dental sequelae with major risk factors being daily consumption of sweetened beverages, poor oral hygiene, residing in lower socio‐economic areas, older age at presentation and being male.
High caries relapse rates and repeat referrals for treatment under general anaesthesia indicate that more comprehensive, multidisciplinary strategies that assess systemic and individual risk factors are required.
A chronic disease management approach to ECC, which addresses multilevel aetiologic factors and is based on individualised caries risk, could reduce disease burden and even the need for dental GA in children.
1. INTRODUCTION
Early childhood caries (ECC), though largely preventable, is one of the most prevalent diseases in childhood and is one of the strongest predictors of poor oral health in adulthood. 1 In Australia, 34% of children have caries by age 5, with higher caries prevalence in children of Indigenous background, low‐income households and in regional and remote areas. 2 Data on global burden of diseases indicate that oral diseases are highly prevalent in children worldwide. 1
ECC can lead to pain, infection and reduced quality of life for children and their families. 3 Complications may result in school absenteeism, poor nutritional intake, effects on growth and development, and frequent emergency visits and hospitalisations. In Australia, the rate of dental hospitalisations related to potentially avoidable dental conditions is rising—4.7 per 1000 in the 0–4 years age group in 2013–2014 increased to 5.7 per 1000 in 2016. For the 5–9 years age group, this number rose from 9.3 to 10.7 per 1000 in the same timeframe. 2 , 4
Dental treatment of young children is complicated due to age‐related cognitive and communication limitations, 1 and therefore, general anaesthesia (GA) is widely used to facilitate complex or extensive dental treatment for children. In Australia, the demand for paediatric dental GA is increasing. Some jurisdictions have waiting lists of up to two years. 5 Many children who undergo dental GA continue to develop new carious lesions and symptoms, with a reported relapse rate of up to 79%, some of whom require further treatment under GA. 6 The public health implications cannot be ignored, as dental treatment under GA for young children is costly and not without risks.
In the state of New South Wales in Australia, public dental clinics and hospitals serve a significant portion of the paediatric population where routine dental treatment is at no cost to the patient's family under Medicare, a type of universal healthcare programme. For a paediatric patient to receive dental treatment under GA, however, a government‐issued, means‐tested concession card is required. 7 As private dental options also exist, public clinics tend to see populations from lower socio‐economic backgrounds. In order to improve equity in health outcomes, it is vital to study the most at‐risk patients.
As dental caries has multifactorial aetiology, a clear understanding of determinants is necessary in order to implement effective preventive measures. It has been shown that targeted approaches, based on caries risk that take into account community and family levels of influence, are promising in reducing the burden of ECC. 1 , 8 Further defining the risk factors, scope of the problem of ECC and its burden on public health system will aid in shaping policy priorities and clinical practice.
Currently, there is little information available on the major risk factors specific to the population that is referred to public dental services in Australia. In order to target this group with specific tailored strategies to reduce the burden of dental disease, this study aimed to investigate the caries experience—including risk factors, incidence of caries, pain and infection—during treatment and while on waiting lists, as well as post‐treatment relapse rates in children with ECC who attended a department of paediatric dentistry in an Australian public dental hospital.
2. MATERIALS AND METHODS
2.1. Study design
This study was a single‐centre retrospective cohort study approved by the Human Research Ethics Committee, RPAH Zone (Protocol Numbers: X18‐0365 & LNR/18/RPAH/515).
2.2. Study setting and participants
Data were collected from dental records at a department of paediatric dentistry at a public dental hospital in Sydney, New South Wales, Australia. The specialist department services children aged 0–18 years from the Sydney metropolitan area and the wider state population. As a tertiary referral‐only clinic, patients need to be first seen at a local public community or private clinic, and must then fulfil referral criteria based on the type of management required and urgency. Once an initial consultation is completed in the department, and a treatment plan is created, dental treatment may commence then, or the patient will be rebooked for the appropriate number of restorative dental appointments. If the treatment under GA is the chosen option, and the family holds the government‐issued, means‐tested concession card, the patient is placed on a waiting list in one of three treatment urgency categories. After treatment completion, either during the in‐chair treatment course or while on the waiting list for treatment under GA, patients are seen for recall appointments and, when appropriate, are discharged back to the referring clinician or clinic. Types of patients referred consist mostly of patients with ECC and previous behaviour management difficulties, special needs, dental anomalies, complex medical backgrounds or those who have had dental trauma. All patients have access to a dietician, oral health therapist and preventive modalities such as fluoride varnish, fissure sealants, interim therapeutic restorations and oral hygiene instructions. Higher strength fluoride adjuncts are provided to high caries risk patients.
Participant inclusion criteria included the following: children who were under age 72 months at their initial consultation in the department between 1 January 2013 and 31 December 2015, who presented with at least one cavitation at the initial visit and had at least one recall visit within a minimum 12‐month follow‐up period from treatment completion (defined as completion of restorative and preventive treatment planned at initial visit). Participants' records were gathered starting with the 2013 records, which continued into the subsequent years until numbers required for an adequately powered study were reached. Records were excluded if no caries were charted at initial visit, if there was less than 12‐month follow‐up or if there were no recall visits.
The sample size was calculated for the comparison of the difference in the proportion of children with incidence of dental caries between baseline and 18 months with and without implementing a prospective caries disease management programme. The primary outcome was the percentage of children with new cavitations. Assuming an alpha of 0.05, a power of 0.8 and an expected small effect size (Cohen's convention measure f = 0.10), a study with ANOVA repeated measures within–between interaction required a sample size of 100 participants in each group. Correlations among repeated measures are assumed to be 0.5, and non‐sphericity correction is assumed to be 1. Accounting for a 50% dropout rate for the programme, a minimum of 200 participants are required for the future prospective intervention group and 100 participants in the retrospective control group (the current study).
2.3. Collected variables
For each participant, number of new cavitations, pain, infection (abscess, parulis, intra‐ or extraoral swelling), dmft (decayed, missing or filled primary teeth) and referrals for treatment under GA or sedation throughout the entire study period, and the dates at which these occurred, were recorded from notes in the patient's hard‐copy records. A cut‐off period of 24 months from initial visit was made. Patient demographic characteristics (including gender, presence of concession card and postcode), caries risk factors (including oral hygiene, water fluoridation, diet and special healthcare needs), time on GA waitlist and the number of treatment and disease management appointments (the latter were defined as oral hygiene and dietary consultations, fluoride varnish, fissure sealants with no restorative treatment) were recorded. Socio‐economic status was derived from the patient's postcode using the Socio‐economic Indexes for Areas (SEIFA), which is a product developed by the Australian Bureau of Statistics that ranks areas in Australia according to relative socio‐economic advantage and disadvantage. 9
2.4. Data collection
A data collection tool was designed to record relevant data from patient files. Three researchers (SB, CD and RD) performed the data extraction. Initial inter‐rater reliability for data entry was (69%) using 10 test charts. Initial discrepancies were harmonised through discussion with the project leaders (CT and HK), and a consensus on a set of rules for data collection and entry was created after which a more acceptable inter‐rater reliability was reached (91%). Eight weeks later, intra‐rater reliability was found to be 91%. Following the patient privacy protocol, password‐protected data were transferred to a spreadsheet (Microsoft Excel, version 16.34) for statistical analysis.
2.5. Data analysis
Descriptive analyses of patient characteristics were summarised using frequencies for categorical variables and means for continuous variables. The risk factors associated with the number of new cavitations were examined in multivariate linear regressions (coefficients reported). Poisson regressions were used for sensitivity analyses. The risk factors associated with new pain, new infection and referral to GA were estimated in logistic regressions (odds ratios reported). The risk factors considered included age, gender, area socio‐economics, concession card status, special healthcare need status, number of disease management appointments, number of restorative appointments, number of fluoride applications, whether the patient had dietitian or OHT session, whether the patient had more than three sweet snacks or sweetened beverages daily, fluoride toothpaste use status and oral hygiene status. Kaplan–Meier curves and a parametric exponential survival model, allowing for multiple records, were used to examine time‐to‐event series for new cavitation, new pain and new infection (hazard ratios reported). Survival models with other distributions were conducted as sensitivity checks. Heteroscedastic robust standard errors were applied in cross‐sectional data for models of caries outcomes at initial and recall visits. Cluster robust standard errors were used in longitudinal data for models of caries outcomes at subsequent visits to correct for within‐individual clustering due to multiple follow‐ups. 10 The data were analysed in Stata version 14.0.
3. RESULTS
3.1. Baseline demographic characteristics and clinical findings
A total of 102 children, 48 female and 54 male, were included in the study (Table 1). The mean age was 52.2 ± 11.6 months (range: 23–71 months). Nearly half of participants (48%) resided in the bottom two Socioeconomic Indexes for Areas (SEIFA) quartiles, whereas most (90.9%) held a government‐issued, means‐tested concession card.
TABLE 1.
Patient characteristics at initial visit
| Demographic characteristics | Mean (SD) |
| Age (months) | 52.2 (11.6) |
| Sex | n (%) |
| Female | 48 (47.1) |
| Male | 54 (52.9) |
| Area socio‐economic status | |
| 1st quartile (most disadvantaged) | 25 (24.5) |
| 2nd quartile | 24 (23.5) |
| 3rd quartile | 14 (13.7) |
| 4th quartile (most advantaged) | 39 (38.2) |
| Concession Card | |
| Yes | 90 (90.9) |
| No | 9 (9.1) |
| Clinical characteristics | |
| Pain | n (%) |
| Yes | 48 (47.5) |
| No | 53 (52.5) |
| Infection | |
| Yes | 23 (23.7) |
| No | 74 (76.3) |
| Referred to GA | |
| Yes | 73 (73.0) |
| No | 27 (27.0) |
| Mean | |
| Number cavitations | 7 (9.9) |
| dmft | 4.1 (8.0) |
| Caries risk factors | |
| Living in fluoridated area | n (%) |
| Yes | 98 (98.0) |
| No | 2 (2.0) |
| Toothpaste use | |
| Non‐fluoridated | 24 (28.9) |
| <1000 ppm | 49 (59.0) |
| 1000+ ppm | 10 (12.0) |
| Sweetened beverages daily | |
| Yes | 65 (72.2) |
| No | 25 (27.8) |
| Nurses on demand | |
| Yes | 25 (26.6) |
| No | 69 (73.4) |
| 3+ sweet snacks/day | |
| Yes | 60 (66.7) |
| No | 30 (33.3) |
| Reported oral hygiene | |
| Good | 8 (8.1) |
| Fair | 49 (49.5) |
| Poor | 42 (42.4) |
| Special healthcare needs | |
| Yes | 16 (16.0) |
| No | 84 (84.0) |
| Referral reason | |
| Behaviour management | 53 (64.6) |
| Expected treatment | 29 (35.4) |
| GA | 21 (67.7) |
| IV sedation | 3 (9.7) |
| Oral sedation | 2 (6.5) |
| Nitrous oxide | 1 (3.2) |
| Other | 4 (12.9) |
| Previous dental GA | |
| Yes | 1 (1.2) |
| No | 85 (98.8) |
Nearly all participants resided in areas with community water fluoridation (98%), whereas 71% reported using a fluoridated toothpaste. Sweetened beverages such as soft drink, cordial and fruit juice were consumed by 72.2% of children on a daily basis, whereas 26.6% were currently nursing on demand. Two‐thirds of participants consumed more than three snacks per day (66.7%). Only 8% of clinician‐reported oral hygiene practices were considered ‘Good’, whereas 49.5% reported ‘Fair’ and 42.4% ‘Poor’. Sixteen per cent of participants were identified as having special healthcare needs such as physical disabilities or medical conditions affecting dental management.
The reason for referral was ‘treatment required under general anaesthesia’ in 73% of the cases.
At initial visit, the mean number of cavitations was 9.9 (SD = 7; maximum 28) and the mean dmft was 8 (SD = 4.1; maximum 18 teeth) (Table 1). Nearly half (47.5%) reported pain at initial visit, and nearly a quarter (23.7%) had at least one dental infection.
At initial visit, older age (months) was significantly associated with experiencing pain and higher number of cavitations (Table 2). The association between age and pain incidence is sizable, which reflects disproportionate pain development among older children. Residing in the 2nd and 3rd SEIFA quartile was negatively associated with pain reported at initial visit. Other factors such as gender, concession card status, sweetened beverage consumption and oral hygiene were not significant correlates at initial visit.
TABLE 2.
Factors associated with pain, infection, cavitation and dmft at initial visit
| Had pain | Had infection | No. of cavitations | No. of dmft | |
|---|---|---|---|---|
| OR (CI) | OR (CI) | Coef (CI) | Coef. (CI) | |
| Age (months) | 1.14** (1.06,1.23) | 1.04 (0.96,1.14) | 0.18*(0.01,0.35) | 0.09 (−0.01,0.20) |
| Male (vs. female) | 0.83 (0.21,3.21) | 1.28 (0.32,5.10) | 0.53 (−3.31,4.38) | 1.20 (−1.10,3.51) |
| Had concession card | 1.45 (0.21,9.89) | – | 0.74 (−9.26,10.75) | −0.42 (−6.76,5.91) |
| SEIFA (base: most disadvantaged) | ||||
| 2nd quartile | 0.11*(0.01,0.99) | 0.81 (0.14,4.77) | 4.94 (−0.35,10.23) | 1.31 (−1.70,4.32) |
| 3rd quartile | 0.07 (0.01,1.12) | 2.36 (−3.57,8.29) | 0.56 (−3.16,4.28) | |
| highest quartile | 0.51 (0.08,3.29) | 1.25 (0.10,15.47) | 1.51 (−3.53,6.54) | 0.10 (−2.99,3.20) |
| Had 3+ sweet snacks/day | 5.89* (1.22,28.5) | 6.40 (0.70,58.52) | −3.76 (−9.45,1.93) | −0.39 (−3.62,2.84) |
| Had sweetened beverages daily | 0.69 (0.13,3.76) | 0.38 (0.04,3.76) | 1.60 (−3.42,6.62) | 0.38 (−2.63,3.38) |
| Poor oral hygiene (vs. otherwise) | 0.48 (0.13,1.82) | 0.92 (0.14,5.93) | 1.40 (−2.21,5.01) | 1.27 (−1.03,3.57) |
| Nurses on demand | 2.17 (0.43,11.10) | 1.53 (0.23,10.27) | 1.72 (−2.94,6.39) | −0.59 (−3.28,2.10) |
Note: Models for pain and infection were estimated using logit logistic regressions and models of numbers of cavitations and dmft were estimated using multivariate linear regressions, controlling for all risk factors available at initial visit. Odds rations (OR) or coefficients and 95% confidence intervals (CI) using robust standard errors are reported. Sensitivity analyses using Poisson regressions were also conducted to account for potential positively skewed distribution of dmft and caries data (Appendix S1). CI: confidence interval. *p < .05 ** p < .01.
3.2. Appointment summaries
Patients had varying numbers of appointments during the study period. The mean number of disease management and restorative appointments was 4.7 (SD = 2.1) and 2.9 (SD = 2.2), respectively, for a total mean number of appointments of 7.6 (SD = 2.6). The mean waiting time for treatment under GA was 394 days (SD = 205). When outliers with confirmed extenuating circumstances reasons were removed, the mean waiting time was just over 1 year (367 days [SD = 167]).
3.3. Dental sequelae during subsequent visits
Nearly one quarter (24.8%) of patients reported new pain either during the in‐chair treatment course or while on the waiting list for treatment under GA. New infections arose in 17.8% of participants during this time. More patients experienced new pain during the GA waiting time than those receiving treatment in‐chair (29.3% and 10.7%); the incidence of new infection, however, was similar (17.3% and 17.9%).
At subsequent appointments, new cavitations were positively associated with residing in the 2nd lowest SEIFA quartile (Coef = 1.27; 95% CI 0.22, 2.31, p = .019) and negatively associated with more disease management appointments (Coef = −0.23; 95% CI −0.46, −0.003, p = .047). Higher risk for new infections was associated with poor oral hygiene (OR = 6.06; 95% CI 0.97, 37.63, p = .053), residing in the 3rd SEIFA quartile (OR = 0.16; 95% CI 0.03, 0.80, p = .025), being male (OR = 17.16; 95% CI 3.44, 85.62, p = .001) and having sweetened beverages daily (OR = 15.46; 95% CI 1.36, 176.13, p = .027) (Table 3).
TABLE 3.
Factors associated with new cavitations, pain and infection during treatment wait time
| No. new cavitations | Had new pain | Had new infection | |
|---|---|---|---|
| Coef (CI) | OR (CI) | OR (CI) | |
| GA (vs. non‐GA) | −0.20 (−1.26,0.85) | 1.20 (0.16,9.02) | 1.10 (0.17,6.98) |
| Age (months) | −0.04 (−0.08,0) | 0.96 (0.91,1.02) | 0.98 (0.91,1.04) |
| Male (vs. female) | −0.02 (−0.98,0.95) | 0.35 (0.06,1.87) | 17.16** (3.44,85.62) |
| Had concession card | −1.15 (−2.91,0.60) | – | – |
| SEIFA (base: most disadvantaged) | |||
| 2nd quartile | 1.27* (0.22,2.31) | 2.57 (0.47,13.96) | 0.22 (0.04,1.17) |
| 3rd quartile | 0.57 (−.41,1.55) | 3.26 (0.49,21.63) | 0.16* (0.03,0.80) |
| 4th (highest) quartile | 0.97 (−0.19,2.14) | 0.64 (0.09,4.72) | 0.31 (0.02,5.35) |
| No. of disease management appointments | −0.23* (−0.46,0) | 1.17 (0.88,1.55) | 0.94 (0.70,1.27) |
| No. of restorative appointments | 1.49 (−0.11,3.1) | 0.97 (0.70,1.37) | 1.20 (0.76,1.90) |
| Had appointment with dietician | 0.75 (−0.11,1.61) | 0.37 (0.10,1.42) | 0.58 (0.16,2.09) |
| Had appointment with OHT | 0.18 (−0.77,1.13) | 0.29 (0.04,2.39) | 0.82 (0.22,3.01) |
| No. of fluoride applications | −0.04 (−0.21,0.13) | 1.42 (0.97,2.06) | ‐ |
| Had 3+ sweet snacks/day | 1.08 (−0.15,2.30) | 0.33 (0.02,7.26) | 0.08 (0.01,1.04) |
| Had sweetened beverages daily | −1.08* (−2.11,‐0.05) | 3.00 (0.24,37.17) | 15.46* (1.36176.13) |
| Poor oral hygiene (vs otherwise) | −0.33 (−1.17,0.51) | 1.56 (0.54,4.54) | 6.06 (0.98,37.63) |
| Non‐fluoride toothpaste (vs otherwise) | −0.33 (−1.35,0.70) | 0.51 (0.10,2.69) | 0.19 (0.03,1.09) |
| Baseline pain/infection/cavitation | 0.01 (−0.04,0.06) | 1.87 (0.35,10.11) | 0.30 (0.06,1.44) |
Note: Some variables are omitted due to perfect prediction or a relatively large number of missing values. Coefficients or odds ratios (OR) and 95% confidence intervals (CI) using robust standard errors are reported. *p < .05 **p < .01.
The Kaplan–Meier survival functions (Figure 1) showed that 50% of all patients experienced new cavitation after approximately 380 days (mean time: 268 days); 20% of all patients reported new pain at 300 days (mean time: 273 days); and 20% of all patients presented with new infection at 350 days (mean time: 192 days). The mean number of new cavitations was 1.4 during the treatment or waiting period before treatment was complete.
FIGURE 1.
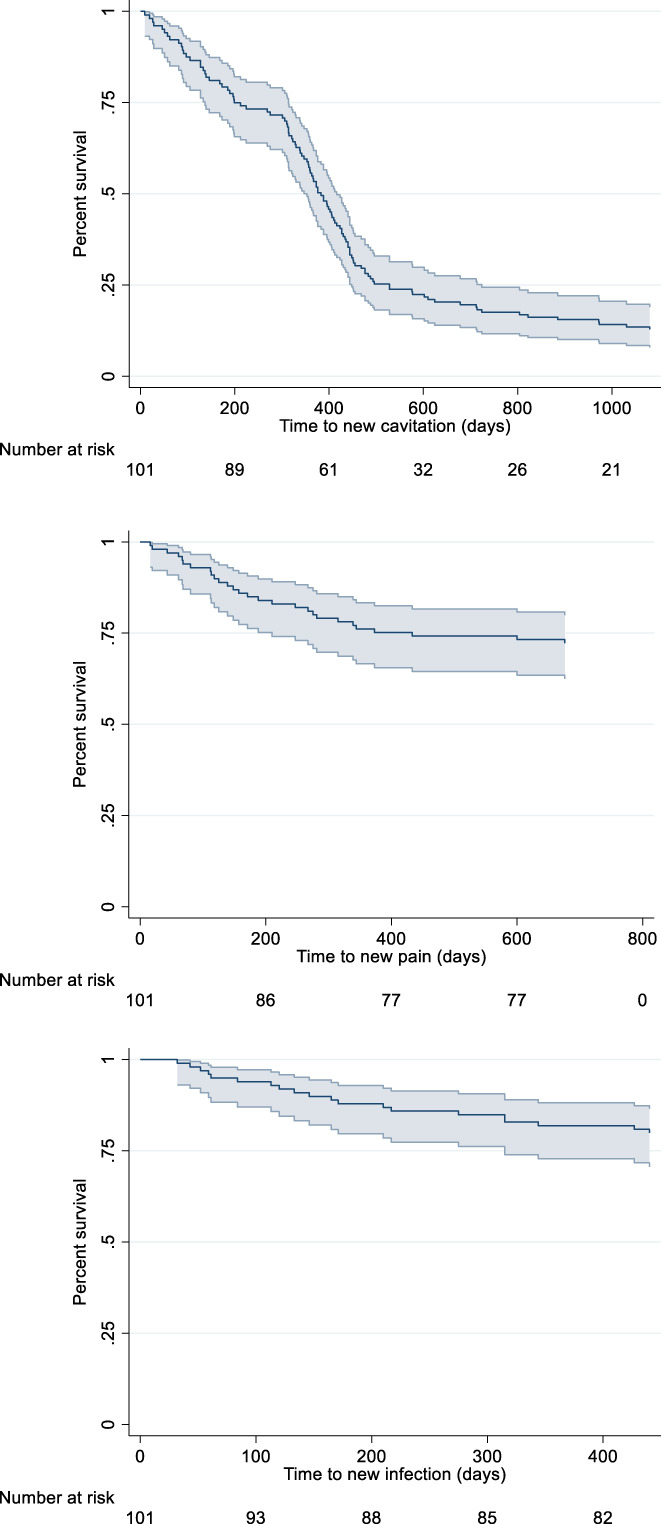
Kaplan–Meier survival functions for new cavitation, pain and infection
According to the survival analysis, the time from initial visit to reporting of pain was significantly shorter for patients with special healthcare needs (HR 3.465; 95% CI 1.567, 7.663, p = .002). Time to new infection was associated with older age (HR 1.058; 95% CI 1.016, 1.102, p = .006), poor oral hygiene (HR 2.128; 95% CI 1.072, 4.223, p = .031) and having a dietetic appointment (HR 3.175; 95% CI 1.010, 9.978, p = .048). Male patients (HR 1.262; 95% CI 0.980, 1.624, p = .071) and those who had restorative appointments (HR 1.074; 95% CI 1.000, 1.155, p = .051) were slightly more likely to experience new cavitations sooner. Importantly, disease management appointments (HR 0.686; 95%CI 0.522, 0.901, p = .007) were associated with a significantly longer time before the experience of infection (Table 4).
TABLE 4.
Survival model results for days to new pain, infection and cavitation
| Time to new pain | Time to new infection | Time to new cavitation | |
|---|---|---|---|
| Haz. Ratio (CI) | |||
| GA (vs. non‐GA) | 1.14 (0.28,4.61) | 0.45 (0.19,1.09) | 1.27 (0.78,2.07) |
| Age (months) | 1.04 (0.98,1.10) | 1.06** (1.02,1.10) | 1.00 (0.98,1.01) |
| Male (vs. female) | 1.06 (0.52,2.15) | 0.36 (0.09,1.38) | 1.26 (0.98,1.62) |
| Had concession card | – | – | 1.06 (0.83,1.37) |
| Special healthcare needs | 3.46** (1.57,7.66) | 0.15* (0.03,0.72) | 1.23 (0.78,1.92) |
| Poor oral hygiene | 1.54 (0.73,3.24) | 2.13* (1.07,4.22) | 0.99 (0.73,1.34) |
| Appointment with dietician | 0.92 (0.31,2.77) | 3.18* (1.01,9.98) | 0.84 (0.63,1.12) |
| Appointment with OHT | 0.73 (0.19,2.76) | 1.19 (0.38,3.71) | 1.06 (0.77,1.46) |
| No. of disease management appointments in 24 months | 1.14 (0.86,1.53) | 0.69** (0.52,0.90) | 1.02 (0.95,1.10) |
| No. of restorative appointments in 24 months | 1.04 (0.83,1.29) | 0.98 (0.81,1.18) | 1.07 (1.00,1.16) |
Note: Due to limited sample size, only variables with at most 10 missing values were included. Concession card was dropped in some models due to collinearity. Hazard ratios and 95% confidence intervals (CI) are reported. *p < .05 **p < .01.
3.4. Post‐treatment dental sequelae and repeat GA referrals
After treatment was complete and for up to 24 months of available follow‐up, 7.9% of patients experienced new pain, whereas 5% experienced a new infection. Patients who underwent treatment under GA had fewer instances of new pain (6.7%) and infection (2.7%) than those receiving treatment in clinic (10.7% and 10.7%) in the follow‐up period. A high number of patients (41% of GA patients and 59.2% of in‐chair treatment patients) experienced new caries (mean 1.4 cavitations in GA patients and 1.9 non‐GA) in this period with a mean caries incidence time of 436 days for GA patients and 267 days for non‐GA patients.
In the recall period, relapse rates were relatively high with 13.3% of all participants referred for treatment under GA for new dental needs. This comprised 13.7% of patients who underwent a prior treatment under GA and 11.1% of non‐GA patients. These patients had a mean age of 54.3 months and 43.5 months at initial consultation, respectively. Being a GA patient was associated with a slightly higher risk of referral for a secondary dental GA in the recall period at a marginal significance level of 10% (OR 5.51; 95% CI 0.83, 36.46, p = .076).
Residing in the 2nd lowest SEIFA quartile (Coef = 2.47; 95% CI 0.44, 4.50, p = .018) was a risk factor for new cavitations at recall; older age (OR = 0.908; 95% CI 0.83, 0.99, p = .049) was associated with lower risk of recurrence of pain; and being a GA patient (OR = 0.023; 95%CI 0.001, 0.896, p = .044) was associated with a lowered risk of relapsed infection. Additionally, the risk of experiencing pain at recall was higher for those who experienced pain at baseline although with marginal significance (OR = 10.9; 95%CI 0.86, 139.42, p = .066) (Table 5).
TABLE 5.
Factors associated with new pain, infection and cavitation at recall (post‐treatment completion)
| No. of new cavitations | Had new pain | Had new infection | |
|---|---|---|---|
| Coef (CI) | OR (CI) | OR (CI) | |
| Had dental general anaesthesia | −0.10 (−2.53,2.33) | 0.68 (0.03,14.27) | 0.023 (0,1.51) |
| Age (months) | −0.01 (−0.08,0.06) | 0.908* (0.80,1.03) | 1.01 (0.94,1.08) |
| Male (vs. female) | 0.87 (−0.61,2.35) | 0.74 (0.12,4.73) | – |
| Had health/pension care card | −0.11 (−2.53,2.31) | – | – |
| SEIFA (Reference: most disadvantaged) | |||
| 2nd quartile | 2.47** (0.64,4.30) | 4.99 (0.37,67.56) | – |
| 3rd quartile | 0.43 (−1.57,2.43) | 5.07 (0.10251.79) | 3.47 (0.03394.71) |
| Highest quartile | 1.56 (−0.16,3.28) | 3.33 (0.28,39.72) | 3.40 (0.02530.31) |
| No. of disease management appointment | −0.33 (−0.84,0.18)) | 0.79 (0.50,1.25) | 0.80 (0.36,1.78) |
| No. of restorative appointments | 1.67 (−1.55,4.89) | 0.95 (0.58,1.55) | 0.20* (0.05,0.82) |
| Had appointment with dietician | 0.24 (−1.34,1.82) | 0.17 (0.02,1.89) | – |
| Had appointment with OHT | 0 (−1.73,1.72) | – | – |
| No. of fluoride applications | −0.08 (−0.37,0.22) | – | – |
| Had 3+ sweet snacks/day | 1.66 (−0.75,4.06) | – | – |
| Had sweetened beverages daily | −1.94 (−3.92,0.04) | – | – |
| Poor oral hygiene (vs. otherwise) | −0.24 (−1.86,1.37) | 0.90 (0.06,14.59) | 10.98* (1.30,92.92) |
| Non‐fluoride toothpaste (vs. otherwise) | −0.84 (−2.94,1.27) | – | – |
| Baseline pain/infection/cavitation | 0.06 (−0.03,0.15) | 10.92* (1.09109.73) | 9.79 (0.49197.73) |
Note: Some variables are omitted due to perfect prediction or a relatively large number of missing values. Odds rations (OR) or coefficients and 95% confidence intervals (CI) using robust standard errors are reported. *p < .05 **p < .01.
4. DISCUSSION
This study involved a retrospective cohort of young children with ECC who were referred to the paediatric dentistry specialist department in an Australian public dental hospital, mostly for dental care that was beyond the scope of dental care available in the community or for financial reasons in the case of referrals for GA. Although not a comprehensive epidemiological study, these children demonstrated a higher dmft than the national average (8 vs. 1.3 teeth). 2 Upon initial visit, half were already experiencing dental pain and a quarter exhibiting localised dental infection. This group had a relatively high caries relapse rate and high referral for repeat dental GA. We determined that the major risk factors for the development of caries, pain and infection in this cohort were daily consumption of sweetened beverages, poor oral hygiene, residing in lower socio‐economic areas, older age at presentation and being male.
The findings of sweetened beverage consumption and poor oral hygiene as risk factors for caries and its sequelae in our cohort are aligned with a wide breadth of previous research. Dietary factors, especially high‐frequency consumption of foods and drinks high in sugar (usually more than once per day), as well as poor oral hygiene, have routinely been cited as being associated with higher risk of ECC. 5 , 11 , 12 , 13 , 14 , 15 , 16 , 17 As these risk factors seem to persist despite education from dental professionals and patient knowledge of their harms, it is apparent that different approaches need to be taken to translate oral health education into long‐lasting health behaviour change, especially for populations from lower socio‐economic backgrounds, like the cohort in this study.
Studies have found the distribution of caries across countries of varying developmental statuses to be increasingly skewed, with a small fraction of disadvantaged individuals experiencing the majority of the lesions or restorations. 18 Our findings that residing in the second lowest SEIFA quartile was a risk factor for new cavitations during treatment, and subsequent visits, and also during recall, are consistent with a systematic review on risk factors for ECC, which found that of sociodemographic factors studied, low household income and gender (male) were found to be most frequently implicated in ECC. 12 Some potential explanations or mediating pathways for this relationship have been suggested, including parental education determining income and access to resources and treatment; health literacy and behaviour; health service utilisation frequency and patterns; and social position and social support. These potential pathways are often intertwined in a ‘complex “eco‐social” framework of macroenvironmental, community level, family and individual level factors’. 18 Effective strategies to address this issue should be similarly broad and comprehensive in scope.
Boys have often been cited being at higher risk for ECC. 12 , 19 Although some explanatory models exist, including the decreased desire to improve oral health care than girls, to the pushing back on the perceived ‘femininity’ of good hygiene, 19 further research is required to understand this apparent gender bias in children's oral health as it has implications for future caries development into older childhood, adolescence and beyond.
This study found that older age was a risk factor for more severe dental disease in our study, which points to the importance of prevention, early detection and timely referrals. Despite recommendations for first dental visits to be by the age of 1 year, or within 6 months of eruption of the first tooth, 20 the mean age of presentation in this study was 4 years. Although these patients were all referred and hence had a dental visit prior, given the advanced stages of ECC noted, it is likely that even earlier visits to a dental practitioner would have led to earlier detection of dental disease and therefore the ability to reduce the rate of disease process.
The relapse rate for new caries (41%) following the treatment under GA in this study was relatively lower than the rates reported in other studies, which ranged from 53% to 68% at 2‐year follow‐up. 21 , 22 Meanwhile, the rate of referral for a repeat dental GA in this study, which was a proxy for ‘repeat GA’, within a 24‐month recall period (nearly 14%) was in the middle of the range in the literature (from 4.2% to 23%). 6 , 21 , 22
These types of relapses can be partially explained by the very high caries risk nature of our cohort—all participants were referred for specialist treatment of caries, and within this cohort, nearly three‐fourths were referred specifically for treatment under general anaesthesia. Although increasingly it is being acknowledged that surgical interventions alone do not address the underlying aetiology or prevent new caries development. 23
A shift from the focus on biological, dietary and oral hygiene influences on their own to models that explore oral health outcomes using a broader framework, incorporating psychosocial and environmental predictors, has occurred in recent years. 24 Conceptual models of children's oral health that outline the multilevel nature of determinants can help researchers and programme designers to understand the complex social milieu that children's oral health exists in, which will in turn help to guide more effective caries prevention programmes. For example, programmes that incorporate measures to improve family self‐efficacy for health behaviour practices, as well as community level changes such as improving supportive social and food environments as well as characteristics of healthcare system, can facilitate the adoption of optimal health behavior. 25 The risk of dental disease in a child cannot be separated from the risk of disease in the family and community, and therefore, programmes must be designed in such a way that they incorporate multilevel perspectives. 24
There is a need for a paradigm shift in how we manage caries in children. One particular novel approach to ECC has moved beyond the traditional view of dental caries as an acute surgical problem requiring restoration and rehabilitation to one that characterises ECC as a chronic disease requiring individually tailored management of multilevel aetiologic factors according to disease severity and patient needs. 26 Chronic disease management (CDM) of caries uses a structured multidisciplinary approach and is guided by individualised caries risk with a significant focus on patient self‐management. 27 It emphasises family engagement and empowerment to adopt effective day‐to‐day behaviour modifications (e.g. dietary control, tooth brushing and topical fluorides) that address disease aetiology with clinicians and families working collaboratively to develop and commit to a set of self‐management goals at each visit. 8
Emerging evidence supports the efficacy of a CDM approach for ECC in a hospital‐based dental clinic. One group employing this model, the ‘ECC Collaborative’, showed a 65.3% decrease in the number of new cavitations, 32.8% decrease in new pain and a 47.8% decrease in referrals for GA in children treated with CDM protocols compared with historical controls after 30 months of intervention. 28 A second phase involving an additional five new sites across the United States replicated these results after 18 months. Moreover, the study demonstrated the cost‐effectiveness of the programme on the healthcare system, Medicaid (the public insurance) and societal perspectives. 29
In this retrospective study, it is encouraging to see the protective value of disease management appointments in preventing new cavitations and prolonging the incidence of new infection. These visits included oral hygiene and dietetic consultations and fluoride varnish and fissure sealant application. One interesting finding was the association between dietetic appointments and quicker time to new infection. This can be potentially explained by the referral of children with worse dental disease to this service. Moving forward, the Sydney Dental Hospital has begun trialling a CDM protocol similar to that of the ECC Collaborative for its high‐risk patient population and is currently assessing the efficacy of the programme. Further research is needed to show any reductions in caries and associated sequelae as well as repeat dental GA from the implementation of this strategy over time.
Lengthy waiting times for dental GA, mean 367 days in this cohort, is an unfortunate reality as the system tries to keep up with the high rates of ECC. An extensive policy critique and discussion regarding waiting times for dental GAs is warranted. Nevertheless, although the waiting lists remain long, it is envisioned that the adoption of a CDM by the Sydney Dental Hospital can reduce disease burden for its young patients on waiting lists by reducing the development of new caries and pain for those on waiting lists, or even prevent the need for treatment under GA as shown in other paediatric populations.
The limited sample size of this study in one location restricts validity to the larger population. A further study conducted at a larger scale and including a larger sample size (especially for less frequent outcomes) would enhance the precision and external validity of the analysis. In addition, an inherent limitation of this retrospective study was the dependence on the non‐standardised, hand‐written clinical notes from a set of clinicians. Despite this, the strengths of this study were the sufficient sample size and ability to assess a relatively long follow‐up time to assess relapse, other dental sequelae and any repeat referrals for GA in a high‐risk population at a major dental referral centre. This comprehensive study in a cohort of a highly vulnerable paediatric population in Australia is one of the first steps to developing strategies to improve oral health outcomes for this section of this community.
The high‐risk population of this study experienced high rates of progression of caries during treatment and waiting time, as well as relatively high relapse rates in the post‐treatment period. Several major risk factors for the development of caries, pain and infection in this cohort, before, during and after treatment included daily consumption of sweetened beverages, poor oral hygiene, residing in lower socio‐economic areas, older age at presentation and being male. Although the multilevel aetiologies of ECC are increasingly being recognised, the prevailing focus on surgical treatment alone has failed to relieve the significant burden of disease for some of the most vulnerable members of our population and their families. These findings support the need for a comprehensive, chronic disease management approach to addressing ECC in high‐risk patients, in a collaborative effort that will support children and their families to feel empowered and ready to make the necessary lifestyle changes to prevent ECC progression in order to improve both oral and general health outcomes, as well as quality of life.
AUTHOR CONTRIBUTIONS
H.K. and C.T. conceived the ideas; S.B., C.D. and R.E.L. collected the data; A.L. analysed the data; C.T. led the writing; A.L, A.K., M.I. and H.K. provided a significant contribution to the manuscript editing.
CONFLICT OF INTEREST
The authors listed certify that they have no financial or non‐financial conflicts of interest in the subject matter or materials discussed in this manuscript.
Supporting information
Appendix S1
ACKNOWLEDGEMENT
Open Access Funding provided by The University of Sydney within the CAUL Agreement. [Correction added on July 04, 2022 after first online publication : Funding information under CAUL Agreement has been updated.]
Tsai C, Li A, Brown S, et al. Early childhood caries sequelae and relapse rates in an Australian public dental hospital. Int J Paediatr Dent. 2023;33:1‐11. doi: 10.1111/ipd.12969
Funding information
There was no funding for this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not available as they are personal patient health information.
REFERENCES
- 1. Soares RC, da Rosa SV, Moysés ST, et al. Methods for prevention of early childhood caries: overview of systematic reviews. Int J Paediatr Dent. 2021;31(3):394‐421. doi: 10.1111/ipd.12766 [DOI] [PubMed] [Google Scholar]
- 2. Australian Institute of Health and Welfare . Oral Health and Dental Care in Australia 2019 . 2019. Accessed 31 August 2021. https://www.aihw.gov.au/reports/dental‐oral‐health/oral‐health‐and‐dental‐care‐in‐australia/notes
- 3. Alantali K, Al‐Halabi M, Hussein I, El‐Tatari A, Hassan A, Kowash M. Changes in preschool children's oral health‐related quality of life following restorative dental general anaesthesia. Br Dent J. 2020;229(10):670‐676. doi: 10.1038/s41415-020-2335-7 [DOI] [PubMed] [Google Scholar]
- 4. Australian Institute of Health and Welfare . Oral Health and Dental Care in Australia 2015 . 016. Accessed 31 August 2021. https://www.aihw.gov.au/reports/dental‐oral‐health/oral‐health‐dental‐care‐in‐australia‐2015
- 5. Alcaino E, Kilpatrick NM, Smith ED. Utilization of day stay general anaesthesia for the provision of dental treatment to children in New South Wales, Australia. Int J Paediatr Dent Sep. 2000;10(3):206‐212. doi: 10.1046/j.1365-263x.2000.00193.x [DOI] [PubMed] [Google Scholar]
- 6. Almeida AG, Roseman MM, Sheff M, Huntington N, Hughes CV. Future caries susceptibility in children with early childhood caries following treatment under general anesthesia. Pediatr Dent. 2000;22(4):302‐306. [PubMed] [Google Scholar]
- 7. Centre for Oral Health Strategy . Oral health 2020: A Strategic Framework for Dental Health in NSW. Centre for Oral Health Strategy; 2013. [Google Scholar]
- 8. Ng MW, Ramos‐Gomez F, Lieberman M, et al. Disease management of early childhood caries: ECC collaborative project. Int J Dent. 2014;2014:327801. doi: 10.1155/2014/327801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. 2039.0 ‐ Information Paper: An Introduction to Socio‐Economic Indexes for Areas (SEIFA), 2006 (Australian Government) (2008).
- 10. Cameron AM. DL a practitioner's guide to cluster‐robust inference. J Hum Resour. 2015;50(2):317‐372. [Google Scholar]
- 11. Ghazal T, Levy SM, Childers NK, et al. Factors associated with early childhood caries incidence among high caries‐risk children. Community Dent Oral Epidemiol. 2015;43(4):366‐374. doi: 10.1111/cdoe.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirthiga M, Murugan M, Saikia A, Kirubakaran R. Risk factors for early childhood caries: a systematic review and meta‐analysis of case control and cohort studies. Pediatr Dent. 2019;41(2):95‐112. [PMC free article] [PubMed] [Google Scholar]
- 13. Seow WK. Early childhood caries. Pediatr Clin North Am. 2018;65(5):941‐954. doi: 10.1016/j.pcl.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 14. Chanpum P, Duangthip D, Trairatvorakul C, Songsiripradubboon S. Early childhood caries and its associated factors among 9‐ to 18‐month old exclusively breastfed children in Thailand: a cross‐sectional study. Int J Environ Res Public Health. 2020;17(9):3194. doi: 10.3390/ijerph17093194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peltzer K, Mongkolchati A, Satchaiyan G, Rajchagool S, Pimpak T. Sociobehavioral factors associated with caries increment: a longitudinal study from 24 to 36 months old children in Thailand. Int J Environ Res Public Health. 2014;11(10):10838‐10850. doi: 10.3390/ijerph111010838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin M, Li J, Zhang S, Ma W. Risk factors for severe early childhood caries in children younger than 4 years old in Beijing, China. Pediatr Dent. 2008;30(2):122‐128. [PubMed] [Google Scholar]
- 17. Winter J, Glaser M, Heinzel‐Gutenbrunner M, Pieper K. Association of caries increment in preschool children with nutritional and preventive variables. Clin Oral Investig. 2015;19(8):1913‐1919. doi: 10.1007/s00784-015-1419-2 [DOI] [PubMed] [Google Scholar]
- 18. Schwendicke F, Dörfer CE, Schlattmann P, Foster Page L, Thomson WM, Paris S. Socioeconomic inequality and caries: a systematic review and meta‐analysis. J Dent Res. 2015;94(1):10‐18. doi: 10.1177/0022034514557546 [DOI] [PubMed] [Google Scholar]
- 19. Drummond M, Drummond C. Boys and their teeth: a qualitative investigation of boys' oral health in early childhood. J Child Health Care. 2012;16(3):284‐292. doi: 10.1177/1367493511435427 [DOI] [PubMed] [Google Scholar]
- 20. American Academy of Pediatric Dentistry . The reference manual of pediatric dentistry. Policy on early childhood caries (ECC): classifications, consequences, and preventive strategies. American Academy of Pediatric Dentistry; 2020. [Google Scholar]
- 21. Amin MS, Bedard D, Gamble J. Early childhood caries: recurrence after comprehensive dental treatment under general anaesthesia. Eur Arch Paediatr Dent. 2010;11(6):269‐273. doi: 10.1007/bf03262761 [DOI] [PubMed] [Google Scholar]
- 22. El Batawi HY. Factors affecting clinical outcome following treatment of early childhood caries under general anaesthesia: a two‐year follow‐up. Eur Arch Paediatr Dent. 2014;15(3):183‐189. doi: 10.1007/s40368-013-0081-0 [DOI] [PubMed] [Google Scholar]
- 23. Fontana M, Wolff M. Translating the caries management paradigm into practice: challenges and opportunities. J Calif Dent Assoc. 2011;39(10):702‐708. [PubMed] [Google Scholar]
- 24. Fisher‐Owens SA, Gansky SA, Platt LJ, et al. Influences on children's oral health: a conceptual model. Pediatrics. 2007;120(3):e510‐e520. doi: 10.1542/peds.2006-3084 [DOI] [PubMed] [Google Scholar]
- 25. Tsai C, Blinkhorn A, Irving M. Oral health Programmes in indigenous communities worldwide‐lessons learned from the field: a qualitative systematic review. Community Dent Oral Epidemiol. 2017;45(5):389‐397. doi: 10.1111/cdoe.12302 [DOI] [PubMed] [Google Scholar]
- 26. Edelstein BL, Ng MW. Chronic disease management strategies of early childhood caries: support from the medical and dental literature. Pediatr Dent. 2015;37(3):281‐287. [PubMed] [Google Scholar]
- 27. Ng MW, Torresyap G, White A, et al. Disease management of early childhood caries: results of a pilot quality improvement project. J Health Care Poor Underserved. 2012;23(3 Suppl):193‐209. doi: 10.1353/hpu.2012.0122 [DOI] [PubMed] [Google Scholar]
- 28. Ng MW, Fida Z. Dental hygienist‐led chronic disease management system to control early childhood caries. J Evid Based Dent Pract. 2016;16(Suppl):20‐33. doi: 10.1016/j.jebdp.2016.01.015 [DOI] [PubMed] [Google Scholar]
- 29. Samnaliev M, Wijeratne R, Kwon EG, Ohiomoba H, Ng MW. Cost‐effectiveness of a disease management program for early childhood caries. J Public Health Dent Winter. 2015;75(1):24‐33. doi: 10.1111/jphd.12067 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are not available as they are personal patient health information.


