Abstract
Objective
Patients with congenital adrenal hyperplasia (CAH) in developing countries have limited access to appropriate laboratory facilities for diagnosis and follow‐up. The aim of this study is to evaluate steroid measurement in hair as a diagnostic tool to identify and monitor CAH in these patients.
Design
A method was developed to measure steroids in hair, the stability of steroids in hair was assessed, and the concentration range in healthy volunteers was determined. Hair samples of patients, before and after starting therapy, were transported at ambient temperature to The Netherlands for analysis.
Patients
Twenty‐two Indonesian CAH patients and 84 healthy volunteers participated.
Measurements
Cortisol, 17‐hydroxyprogesterone (17OHP), androstenedione, and testosterone in hair were measured by liquid chromatography with tandem mass spectrometry.
Results
Steroids in hair could be measured and remained stable (<4.9% deviation) for at least 3 weeks at 4°C and 30°C. In each of the untreated patients, hair concentrations of 17OHP (9.43–1135 pmol/g), androstenedione (36.1–432 pmol/g), and testosterone (2.85–69.2 pmol/g) were all above the upper limit of the corresponding range in healthy volunteers; 5.5 pmol/g, 13 pmol/g, and 1.8 pmol/g, respectively. After starting glucocorticoid treatment, the steroid concentrations in the hair of CAH patients decreased significantly for androstenedione (73%) and testosterone (59%) after 6 months.
Conclusions
CAH could be confirmed in Indonesian patients based on the concentration of 17OHP, androstenedione, and testosterone in hair, and a treatment effect was observed. These findings open up opportunities to diagnose and/or monitor CAH in developing countries with a simple noninvasive technique.
Keywords: 17OHP, androstenedione, congenital adrenal hyperplasia, hair, mass spectrometry, steroids, testosterone
1. BACKGROUND
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders of the adrenal gland with impaired cortisol synthesis, most frequently caused by mutations in CYP21A2 or CYP11B1. 1 CAH has a worldwide estimated incidence between 1:10,000–1:20,000. 2 Due to the decreased or lack of negative feedback of cortisol to the pituitary gland, adrenocorticotrophic hormone (ACTH) concentration is elevated and chronically stimulates the adrenal cortex. The elevated ACTH levels lead to increased concentrations of steroid precursors upstream of the affected enzyme, notably 17‐hydroxyprogesterone (17OHP). 3 Patients present with a clinical spectrum from a severe (classic) to a mild (non‐classic) form of CAH depending on the residual enzymatic activity. The accumulated precursor steroids are partially shunted into adrenal androgen synthesis pathways leading to excess androgens. Treatment of patients with CAH consists of glucocorticoid therapy aiming to substitute for cortisol deficiency and consequently lower adrenal androgen.
CAH is generally diagnosed by measurement of 17OHP and androstenedione concentrations in serum. Measurement of 17OHP and androstenedione in serum is limited in developing countries due to logistical and technical challenges. Serum samples must be frozen for long‐distance transport from remote outpatient clinics to clinical laboratory facilities, 4 while access to long‐distance transport of frozen samples is limited in developing countries and, if available, substantially increases costs. Others have shown that measuring the cortisol concentration in human hair is technically feasible by liquid chromatography with tandem mass spectrometry (LC‐MS/MS) and may be used to diagnose and monitor Cushing's disease in patients, who suffer from increased cortisol exposure. 5 Analogous, in CAH patients, the measurement of steroid hormones in hair, specifically 17OHP and androstenedione, but also testosterone, may be of interest for diagnosis and treatment monitoring of CAH and could solve the aforementioned logistical problems and reduce costs. For this purpose, steroids in hair samples have to remain stable during transport by regular post at ambient temperature to a clinical laboratory with expertize in steroid measurement by LC‐MS/MS.
In this study, we explored the technical feasibility and clinical utility of measurement of steroid concentrations in hair from an Indonesian cohort of untreated CAH patients after a long‐distance transport at ambient temperature to The Netherlands. We also investigated the effect of glucocorticoid treatment on 17OHP, androstenedione, and testosterone concentrations in the hair of these patients. For these purposes, we developed and validated an LC‐MS/MS‐based method to measure steroids in hair, including assessing the long‐term stability of the steroids at ambient temperature. We analyzed hair samples of healthy volunteers and determined the ranges in which these hormones are present. Subsequently, we investigated hair samples of the patients of the Indonesian cohort over a period of a year, both before and after starting therapy.
2. SUBJECTS AND METHODS
2.1. Patients and hair sampling
Hair samples were obtained from 22 untreated CAH patients (CYP21A2 mutation n = 17; CYP11B1 mutation n = 5, age 3–46 years, all 46XX) from the Center for Biomedical Research, Faculty of Medicine Diponegoro University, Semarang, Indonesia. These samples were transported to The Netherlands as part of a collaborative project. This cohort was clinically evaluated by the local pediatric endocrinologist, mutation analysis and karyotyping were performed and serum steroids were measured before and after a standard ACTH stimulation test as earlier described by us. 6 The study was in compliance with institutional guidelines and the declaration of Helsinki and was approved by the local ethical committee of the Diponegoro University (No 713/EC/FK‐RSDK/2016). Oral and written informed consent was obtained after a full explanation of the purpose and nature of all procedures.
Hair samples from the posterior vertex were cut as close as possible to the scalp with scissors. No special hair treatment before the collection was required. The hair sample was attached to a paper form with patient identification and time of collection in a fixed proximal‐distal orientation. Samples were transported at ambient temperature from Indonesia to The Netherlands in regular paper envelopes without any additions and upon arrival stored at 4°C until analysis. Hair segments of 10 mg consisting of multiple hairs of 3 cm from the proximal end were analyzed. For the first time of hair sample collection, a standard synacthen test (0.25 mg) was performed on all patients to determine serum cortisol, 17OHP, androstenedione, and testosterone concentrations. Furthermore, hair samples were collected at two time intervals (3 months and 6 months) after the start of glucocorticoid treatment in 12 patients. Treatment consisted of prednisone, hydrocortisone, or dexamethasone after the diagnosis of CAH was confirmed. 6 Hair samples were collected from 84 healthy volunteers in The Netherlands (age 0–56 years, both female and male). We aimed for a separate prepubertal cohort based upon age since some steroids are present in a (much) lower concentration during this period of life. The healthy volunteers were divided into four groups based on their gender and estimated pubertal status based on Dutch population data (prepuberty vs. puberty + postpuberty); female <8 years, female ≥8 years, male <9 years, and male ≥9 years. 7
2.2. Measurement of cortisol, 17OHP, androstenedione, and testosterone in serum by LC‐MS/MS
The steroid measurements in serum were measured with LC‐MS/MS as described 8 with the following additional information. Internal standard for cortisol, 17OHP, and androstenedione were [13C3]‐cortisol, [13C3]‐17OHP, and [13C3]‐androstenedione, respectively (Isoscience). Retention time was 1.47 min for cortisol, 4.81 min for 17OHP, and 3.70 min for androstenedione. Transitions (Q1 > Q3) were m/z 363.4 > 121.1 (25 V) and m/z 363.4 > 97.1 (34 V) for cortisol; m/z 366.4 > 124.1 (25 V) and m/z 366.4 > 100.1 (35 V) for [13C3]‐cortisol; m/z 331.3 > 97.1 (31 V) and m/z 331.3 > 109.1 (31 V) for 17OHP; m/z 334.3 > 100.1 (30 V) and m/z 334.3 > 112.1 (33 V) for [13C3]‐17OHP; m/z 287.2 > 97.1 (23 V) and m/z 287.2 > 109.1 (26 V) for androstenedione; and m/z 290.2 > 100.1 (21 V) and m/z 290.2 > 112.1 (26 V) for [13C3]‐androstenedione. Dwell time was 100 ms, 60 ms, and 100 ms for cortisol, 17OHP, and androstenedione, respectively. For cortisol, total coefficient of variation (CV) is 4.5% at 270 nmol/L and 4.5% at 870 nmol/L. For 17OHP, total CV is 5.3% at 3.25 nmol/L and 6.1% at 80.0 nmol/L. For androstenedione, total CV is 5.2% at 2.60 nmol/L and 4.9% at 19.8 nmol/L.
Reference ranges in serum are 60–550 nmol/L for cortisol, the upper limit for 17OHP is 7.4 nmol/L for children up to 8 years old and 12.7 nmol/L for females, and the upper limit for androstenedione is 0.98 nmol/L for females between 6 months and 7 years old and 7.4 nmol/L for females of 8 years and older, the upper limit for testosterone is 0.3 nmol/L for prepubertal children and 2.0 nmol/L for adult females.
2.3. Sample preparation and LC‐MS/MS method steroids in hair samples
Cortisol, 17OHP, androstenedione, and testosterone were analyzed by LC‐MS/MS after grinding hair samples (in an MM400 Mixer Mill for 2 h at 20 Hz) followed by extraction with supported liquid extraction (SLE) and solid‐phase extraction (SPE) columns. A 9‐point calibration series was prepared (Sigma‐Aldrich), aliquoted, and stored at −40°C until analysis. The range of the calibration was 0–115 nmol/L for cortisol, 0–49.5 nmol/L for androstenedione, 0–23 nmol/L for testosterone, and 0–59.9 nmol/L for 17OHP. In every run, one aliquot per calibration point was used. Calibrators, in‐house hair quality controls, as well as patient samples, were prepared in duplicate. Sample preparation consisted of internal standard addition [13C3]‐cortisol, [13C3]‐androstenedione, [13C3]‐testosterone, and [13C3]‐17OHP (Isosciences) to 1200 µl grinded hair extract and subsequent SLE using 1 ml Biotage Isolute® columns and SPE using Oasis® HLB 1cc cartridges (Waters Corp). Columns were preequilibrated with 1 ml methanol:isopropanol (95:5) and subsequently washed with 1 ml H2O. After application of the sample, columns were washed two times with 1 ml H2O, 1 ml water + 2% formic acid, two times with 1 ml methanol:H2O (10:90), 1 ml methanol:water (30:70), and 0.5 ml methanol:water (40:60) + 5% NH4OH. The 300 µl eluate methanol:isopropanol (95:5) was dried under a stream of N2 gas, reconstituted in methanol:H2O (30:70), and injected (20 µl) into an Agilent Technologies 1290 Infinity VL UHPLC‐System (Agilent Technologies) equipped with a BEH C18 (1.7 μm 2.1 × 50 mm) analytical column (Waters Corp.) at 60°C. Mobile phase A (methanol:water 20:80 + 2 mM NH4CH3COO + 0.1% formic acid) and B (methanol:water 98:2 + 2 mM NH4CH3COO + 0.1% formic acid) were run in a gradient (0.4 ml/min). The gradient programme was as follows: Start gradient 70:30 A:B for 2.5 min; then to 60:40 A:B in 2 min; followed by a gradient in 2.5 min to 35:65 and a subsequent gradient in 0.5 min to 2:98 to remain such for 0.5 min and thereafter to 70:30 A:B in 0.5 min. The retention time was 1.5 (cortisol) min, 3.6 (androstenedione) min, 4.3 (testosterone) min, and 5.0 (17OHP) min, respectively, with a total run time of 9 min.
An Agilent 6490 tandem mass spectrometer (Agilent Technologies) was operated in the electrospray positive ion mode, with a capillary voltage 3.5 kV, fragmentor voltage 380 V, sheath gas temperature 350°C, and gas temperature 150°C with N2 collision gas. The collision energy was optimized for all analytes. Two mass transitions were monitored for each analyte and the internal standards. The first transition was used for quantification, the second for confirmation. The transitions (Q1 > Q3), collision energy (between brackets), and dwell time were m/z 363 > 121 (25 eV) and m/z 363 > 97 (34 eV) for cortisol (50 ms); m/z 366 > 124 (25 eV) and m/z 366 > 100 (35 eV) for [13C3]‐cortisol; m/z 287 > 97 (23 eV) and m/z 287 > 109 (26 eV) for androstenedione (50 ms); m/z 290 > 100 (21 eV) and m/z 290 > 112 (26 eV) for [13C3]‐AD; m/z 289 > 97 (30 eV) and m/z 289 > 109 (30 eV) for testosterone (50 ms); m/z 292 > 100 (30 eV) and m/z 292 > 112 (30 eV) for [13C3]‐testosterone; m/z 331 > 97 (31 eV) and m/z 331 > 109 (31 eV) for 17OHP (50 ms); and m/z 334 > 100 (30 eV) and m/z 334 > 112 (33 eV) for [13C3]‐17OHP.
The concentration of the analytes is calculated based on an added internal standard.
2.4. Method validation
Ion suppression was assessed by continuous infusion of the labelled steroid. The abundance was compared between a hair matrix and mobile phase at the retention time of the steroid. Intra‐ and interassay imprecision was assessed by adapted CLSI‐EP5 protocol with a homogeneous hair sample and based on duplicate measurements (n = 11); the limit of quantification (LoQ) was assessed by measuring a sample with a low concentration 12–25 times in separate experiments. Carry over was tested by measuring blank samples after calibration curves were carried out. No measurable amount of the steroids were detected in these samples. To assess the stability of steroids in hair samples, five patient samples were split into portions. Of each patient three portions were measured directly, three portions were kept at 4°C for 3 weeks before measuring, and three portions were kept at 30°C for 3 weeks before measuring.
2.5. Statistical analyses
GraphPad Prism software version 5.0 for Windows and EP Evaluator 11.0 were used for statistical analyses. To analyze whether there was a difference in cortisol and 17OHP levels in the different gender/age groups of healthy volunteers, a Kruskal–Wallis test was performed followed by a Dunns posttest. The same tests were used to determine whether there was a difference between steroid concentrations in the hair of patients with CAH before and after starting treatment.
3. RESULTS
3.1. The cohort of untreated CAH patients
All patients (CYP21A2 mutation n = 17; CYP11B1 mutation n = 5) were diagnosed with classic CAH based on phenotypic, biochemical, and genetic analysis as previously published. 6 Eight patients had male sex of rearing and one patient had yet undefined sex of rearing, while all patients had a 46XX karyotype. None of the patients received glucocorticoid treatment before testing. Five patients reported hospital admissions because of salt‐wasting crisis or episodes of vomiting and/or seizures. 6 The synacthen test revealed that all the patients in our cohort had an inadequate cortisol response of <240 nmol/L in serum (interquartile range: 64–214 nmol/L; reference value >500 nmol/L). 6 , 9
3.2. Development of the steroid measurements in hair by LC‐MS/MS
First, the characteristics of our method were determined. The variation was assessed by running an adapted EP5 protocol with 11–13 measurements per steroid at a mean concentration of 14.5 pmol/g for cortisol, 18.3 pmol/g for androstenedione, 7.3 pmol/g for testosterone, and 4.6 pmol/g for 17OHP. This resulted in an intra‐assay CV of 4.8% for cortisol, 3.0% for androstenedione, 5.0% for testosterone, and 3.7% for 17OHP and an inter‐assay CV of 8.7%, 3.4%, 6.3%, and 7.9%, respectively. The trueness of the method was assessed by calculating the recovery of steroids from an additional experiment performed in triplo. A mean addition of 7.1 pmol cortisol per gram of hair resulted in a recovery of 106% and a mean addition of 190 pmol/g resulted in a recovery of 98%. For androstenedione, the recovery amounted to 105% at a concentration of 3.1 pmol/g and 101% at 82 pmol/g. The addition of testosterone had a recovery of 114% at 1.4 pmol/g and 99% at 38 pmol/g. For 17OHP, the recovery was 108% at 3.7 pmol/g and 103% at the addition of 99 pmol/g. On the basis of the CV of the control, samples measured 12–25 times, we concluded that the LoQ is below 6.1 pmol/g (CV: 10.9%) for cortisol, below 0.9 pmol/g (CV: 15.4%) for 17OHP, below 5.0 pmol/g (CV: 8.7%) for androstenedione, and below 0.74 pmol/g (CV: 16%) for testosterone.
The stability of the steroids in hair samples was examined by calculating the CV of the control samples stored at room temperature over a period of 8 months, resulting in a CV of 8.7%, 7.9%, 3.4%, and 6.3% for cortisol, 17OHP, androstenedione, and testosterone, respectively. No apparent drift was observed during this period. To simulate transport conditions of hair collection in Indonesia to the laboratory in The Netherlands, one control and five patient samples were split and stored at 4°C and 30°C for a period of 3 weeks. The difference between the mean concentration at 4°C and 30°C was 1.0% (−8.2% to 9.4%) for cortisol, 1.1% (−2.8% to 6.9%) for 17OHP, 1.5% (−10.5% to 20.8%) for androstenedione, and 4.9% (−10.4% to 22.8%) for testosterone which demonstrates its stability at 4°C and 30°C.
Medians and the upper limit of the 95% range of steroid concentrations in the hair of healthy volunteers were established (Table 1). For androstenedione and testosterone, the medians and upper limits of the 95% range were calculated for four groups (female 0–7 years, female 8–56 years, male 1–8 years, and male 9–51 years). For cortisol and 17OHP, it was determined whether the results of the same four gender/age groups were significantly different from each other. For cortisol, the results of the four gender/age groups were pooled since no significant difference was found. For 17OHP, there was no significant gender difference, but there was a significant age difference. Therefore, the results of young females and young males were pooled and the results of the older females and males were pooled using the age groupings stated above.
Table 1.
Ranges of steroid concentrations in hair of healthy volunteers.
| Steroid | N | Female/Male | Age (year) | Median (pmol/g) | Upper limit 95% range (pmol/g) |
|---|---|---|---|---|---|
| Cortisol | 84 | Both | 0–56 | 12 | 61 |
| 17OHP | 19 |
Female Male |
0–7 1–8 |
0.47 | 2.1a |
| 17OHP | 57 |
Female Male |
8–56 9–51 |
2.0 | 5.5 |
| Androstenedione | 10 | Female | 0–7 | 0.89 | 1.7a |
| Androstenedione | 42 | Female | 8–56 | 3.0 | 11 |
| Androstenedione | 12 | Male | 1–8 | 2.8 | 4.6a |
| Androstenedione | 19 | Male | 9–51 | 5.8 | 13a |
| Testosterone | 8 | Female | 0–7 | 0.27 | 0.75a |
| Testosterone | 38 | Female | 8–56 | 0.52 | 1.8 |
| Testosterone | 15 | Male | 1–8 | 0.88 | 2.7a |
| Testosterone | 18 | Male | 9–51 | 1.7 | 6.3a |
Note: The volunteers are divided into four groups based on gender and age.
Abbreviation: 17OHP, 17‐hydroxyprogesterone.
Data transformation did not result in a normal distribution.
3.3. Elevated 17OHP, androstenedione, and testosterone concentrations in hair from untreated CAH patients
The described LC‐MS/MS technique was used to measure steroid levels in the hair of our cohort of untreated CAH patients (Figure 1). The mean cortisol level was 19.8 pmol/g. All cortisol results were within the 95% range of healthy volunteers (2.7–61 pmol/g). All 17OHP, androstenedione, and testosterone results in the total cohort of CAH patients were above the upper limit of the 95% range in healthy female volunteers of the matching age groups, distinguishing the CAH cohort (either CYP21A2 or CYP11B1 deficient) from healthy volunteers. The overall mean 17OHP concentration was 501 pmol/g. A significant difference (p = .0011) was observed between patients with CYP21A2 deficiency (ranging from 247 to 1135 pmol/g 17OHP) and CYP11B1 deficiency (ranging from 9.43 to 20.5 pmol/g 17OHP), allowing to distinguish between CYP21A2 and CYP11B1 affected CAH patients. While in CYP11B1 deficiency 17OHP can be converted into 11‐deoxycortisol, 17OHP is the direct upstream precursor in CYP21A2 deficiency. The mean androstenedione concentration in the CAH patients was 208.6 pmol/g and the mean testosterone level in the CAH patients was 16.8 pmol/g.
Figure 1.
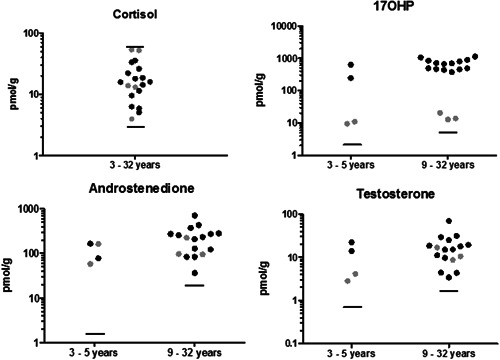
Steroid levels in hair of untreated CAH patients. Results of patients with mutated CYP21A2 are depicted in black and the results of patients with a CYP11B1 mutation are in grey. The upper limit of the 95% range in female healthy volunteers is indicated by the horizontal lines. For cortisol, the lower limit of the 95% range in female healthy volunteers is indicated as well. 17OHP, 17‐hydroxyprogesterone; CAH, congenital adrenal hyperplasia.
As CAH patients can be diagnosed and their treatment monitored by measurement of 17OHP and androstenedione concentrations in serum, we investigated how serum values and hair concentrations of CAH patients correlated. The Spearman r of the correlation between hair and serum levels was 0.48 for cortisol, 0.64 for 17OHP, 0.78 for androstenedione, and 0.78 for testosterone (Figure 2).
Figure 2.
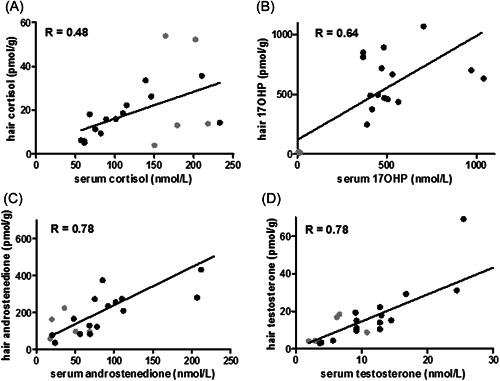
Correlation of steroid levels from hair and serum measurements. Results of patients with mutated CYP21A2 are depicted in black and the results of patients with a CYP11B1 mutation are in grey. 17OHP, 17‐hydroxyprogesterone.
3.4. Decrease of androstenedione, testosterone, and 17OHP level in hair after glucocorticoid treatment of patients with CAH
Of a subset of 12 patients, who started glucocorticoid treatment with either prednisone, hydrocortisone, or dexamethasone after the diagnosis CAH was confirmed, hair samples were collected just before the start of treatment and 3 and 6 months after starting treatment, and androstenedione, testosterone, and 17OHP concentrations were measured (Figure 3). A mean decrease in the concentration of androstenedione was observed at 44% after 3 months and 73% after 6 months of treatment (ranging from 10.4 to 398.2 pmol/g with a median of 39.6 pmol/g after 6 months of treatment; compare to the highest upper limit in healthy female volunteers of 11 pmol/g). For testosterone, the average decrease was 40% after 3 months and 59% after 6 months (ranging from 1.1 to 29.7 pmol/g with a median of 4.15 pmol/g after 6 months of treatment; compared to the highest upper limit in healthy female volunteers of 1.8 pmol/g). The average decrease of 17OHP concentration was 32% and 53%, respectively (ranging from 4.7 to 855 pmol/g with a median of 179 pmol/g after 6 months of treatment; compared to the highest upper limit in healthy female volunteers of 5.5 pmol/g). The decrease in androstenedione and testosterone over 6 months was significant (p < .05).
Figure 3.
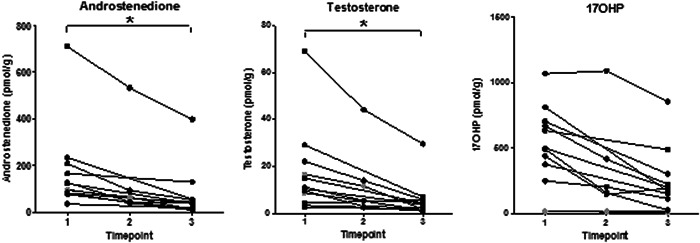
Effect glucocorticoid treatment in CAH patients on hair steroid levels. Timepoint 1 is before starting treatment, Timepoint 2 is 3 months after starting treatment, and Timepoint 3 is 6 months after starting treatment (*p < .05). Results of patients with mutated CYP21A2 are depicted in black and results of patients with a CYP11B1 mutation are in grey. 17OHP, 17‐hydroxyprogesterone; CAH, congenital adrenal hyperplasia.
4. DISCUSSION
Here, we examined the suitability of sampling and transporting scalp hair to distant laboratories for steroid analysis to enable diagnosis and/or monitoring of CAH in patients from areas with limited access to current conventional serum‐based diagnostics, like developing countries and remote areas. The assessed stability of steroids in hair and the opportunity to study a unique untreated CAH patient cohort were key in this study. We developed a robust method to measure steroids in hair, confirmed the stability of steroids in hair for at least 3 weeks at 4°C and 30°C, and determined concentration ranges in healthy volunteers. With this method, we were able to measure elevated 17OHP, androstenedione, and testosterone concentrations in a well‐defined cohort of untreated Indonesian patients with CAH. The concentration of cortisol in hair samples was similar to the concentrations in healthy volunteers. Possibly the constant high ACTH stimulus on the adrenal glands in this cohort of classical CAH patients with severe virilization results in low levels of cortisol despite the enzyme block. The measured steroid concentration in hair correlates with the corresponding serum levels. We note that a serum measurement reflects a limited time span of steroid status in a patient, while steroid concentrations in hair samples may reflect the average exposure to these steroids over a longer time frame during hair growth. This difference might explain the relatively low correlation. After starting glucocorticoid treatment, the levels of steroids in hair decreased.
In the past decade, the measurement of steroids in human hair with LC‐MS/MS has been performed in several laboratories. In 2012, it was shown that cortisol concentration in hair can be used as a tool in diagnosing and following up on Cushing's disease. 5 Since then research on hair cortisol concentrations has developed rapidly with a wide variety of clinical and research applications. 10 Noppe et al. 11 published an explorative study in 2016, investigating the potential of measuring steroids in hair to monitor therapy in CAH patients. They and others concluded that hair 17OHP and androstenedione concentrations seem to be a promising parameter for treatment monitoring in CAH patients. 11 , 12 Further studies would be needed to gain insight into optimal monitoring with hair steroid analysis and to set appropriate target levels for steroid concentrations in hair.
An additional advantage of measuring steroids in hair could be the possibility to monitor long‐term steroid exposure, while measurement in serum or saliva only shows the steroid levels at the moment of blood draw. The incorporation of steroids from the blood into hair is thought to occur in the hair follicles during its growth. 13 It is unclear whether other sources, such as sweat, might also contribute to the hair steroid concentration. 13 With a growth of about 1 cm a month on average, 14 the analysis of steroid concentrations in human hair samples of 3 cm display a 3‐month steroid exposure period. This may provide a better reflection of the average steroid status of a patient compared to serum measurements (for diagnosis) and be used to monitor treatment compliance. Samples of shorter length may also be collected. In that case, a shorter period of steroid exposure is reflected. Sending hair samples by regular mail simplifies logistics considerably compared to serum samples which would need to be transported on dry ice, decreasing transport costs comparable to sending a regular letter by post.
There are some limitations of our study design. First, the Dutch cohort of volunteers and the Indonesian cohort of CAH patients have different ethnic backgrounds. Unfortunately, we were not able to include an Indonesian cohort of volunteers. However, there are no findings suggesting a difference in steroid levels or steroid incorporation in hair between people from Asian and Caucasian backgrounds. 15 Second, patients in which treatment was started, received a variety of different treatment/corticoids and dosages. Therefore, a more systematic analysis of monitoring therapy was not possible. Further research is needed to address this. Third, although 11‐deoxycortisol may be used as a marker of CAH due to CYP11B1 deficiency, we have not included the measurement of this steroid in human hair. However, with the measurement of both 17OHP and androstenedione, it is possible to distinguish CYP21A2‐ and CYP11B1‐deficient CAH patients from healthy volunteers. 17OHP concentrations alone allow distinguishing between CYP21A2 and CYP11B1 deficiency. Lastly, without scalp hair the measurements cannot be performed, therefore it is unlikely that it can be a standard method in newborn screening programmes for CAH.
For the first time, hair samples were collected from untreated CAH patients and subsequently transported over a long distance at ambient temperatures after which the diagnosis could be confirmed by measuring the steroid concentrations. Our study shows great potential to open up access to health care in developing countries and remote areas for people with CAH, both for identification and monitoring of the disease in a noninvasive manner. Possibly a similar approach can be developed for other analytes and diseases.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Waaijers S, Utari A, van der Doelen RHA, et al. Measuring steroids in hair opens up possibilities to identify congenital adrenal hyperplasia in developing countries. Clin Endocrinol (Oxf). 2023;98:41‐48. 10.1111/cen.14754
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Claahsen‐van der Grinten HL, Stikkelbroeck N, Falhammar H, Reisch N. Management of endocrine disease: gonadal dysfunction in congenital adrenal hyperplasia. Eur J Endocrinol. 2021;184(3):R85‐R97. 10.1530/EJE-20-1093 [DOI] [PubMed] [Google Scholar]
- 2. Han TS, Walker BR, Arlt W, Ross RJ. Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nat Rev Endocrinol. 2014;10(2):115‐124. 10.1038/nrendo.2013.239 [DOI] [PubMed] [Google Scholar]
- 3. El‐Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390(10108):2194‐2210. 10.1016/S0140-6736(17)31431-9 [DOI] [PubMed] [Google Scholar]
- 4. Kley HK, Rick W. The effect of storage and temperature on the analysis of steroids in plasma and blood. J Clin Chem Clin Biochem. 1984;22(5):371‐378. [PubMed] [Google Scholar]
- 5. Manenschijn L, Koper JW, van den Akker EL, et al. A novel tool in the diagnosis and follow‐up of (cyclic) Cushing's syndrome: measurement of long‐term cortisol in scalp hair. J Clin Endocrinol Metab. 2012;97(10):E1836‐E1843. 10.1210/jc.2012-1852 [DOI] [PubMed] [Google Scholar]
- 6. Engels M, Pijnenburg‐Kleizen KJ, Utari A, et al. Glucocorticoid activity of adrenal steroid precursors in untreated patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2019;104(11):5065‐5072. 10.1210/jc.2019-00547 [DOI] [PubMed] [Google Scholar]
- 7. Mul D, Fredriks AM, van Buuren S, Oostdijk W, Verloove‐Vanhorick SP, Wit JM. Pubertal development in The Netherlands 1965‐1997. Pediatr Res. 2001;50(4):479‐486. 10.1203/00006450-200110000-00010 [DOI] [PubMed] [Google Scholar]
- 8. Ter Horst R, Jaeger M, Smeekens SP, et al. Host and environmental factors influencing individual human cytokine responses. Cell. 2016;167(4):1111‐1124. 10.1016/j.cell.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stoupa A, González‐Briceño L, Pinto G, et al. Inadequate cortisol response to the tetracosactide (Synacthen®) test in non‐classic congenital adrenal hyperplasia: an exception to the rule? Horm Res Paediatr. 2015;83(4):262‐267. 10.1159/000369901 [DOI] [PubMed] [Google Scholar]
- 10. Greff MJE, Levine JM, Abuzgaia AM, Elzagallaai AA, Rieder MJ, van Uum SHM. Hair cortisol analysis: an update on methodological considerations and clinical applications. Clin Biochem. 2019;63:1‐9. 10.1016/j.clinbiochem.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 11. Noppe G, de Rijke YB, Koper JW, van Rossum EF, van den Akker EL. Scalp hair 17‐hydroxyprogesterone and androstenedione as a long‐term therapy monitoring tool in congenital adrenal hyperplasia. Clin Endocrinol. 2016;85(4):522‐527. 10.1111/cen.13078 [DOI] [PubMed] [Google Scholar]
- 12. Auer M, Krumbholz A, Bidlingmaier M, Thieme D, Reisch N. Steroid 17‐hydroxyprogesterone in hair is a potential long‐term biomarker of androgen control in congenital adrenal hyperplasia due to 21‐hydroxylase deficiency. Neuroendocrinology. 2019;110:938‐949. [DOI] [PubMed] [Google Scholar]
- 13. Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37(5):589‐601. 10.1016/j.psyneuen.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 14. Griffiths WA, Reshad H. Hair and nail growth: an investigation of the role of left‐ and right‐handedness. Clin Exp Dermatol. 1983;8(2):129‐133. 10.1111/j.1365-2230.1983.tb01756.x [DOI] [PubMed] [Google Scholar]
- 15. Santner SJ, Albertson B, Zhang GY, et al. Comparative rates of androgen production and metabolism in Caucasian and Chinese subjects. J Clin Endocrinol Metab. 1998;83(6):2104‐2109. 10.1210/jcem.83.6.4898 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


