Abstract
Background
Microsurgical free tissue transfer (FTT) is a widely employed surgical modality utilized for reconstruction of a broad range of defects, including head and neck, extremity, and breast. Flap survival is reported to be 90%–95%. When FTT fails, salvage procedures aim at establishing reperfusion while limiting ischemia time—with salvage rates between 22% and 67%. There are limited data‐driven predictors of successful salvage present in the literature. This systematic review aims to identify predictors of flap salvage.
Methods
A systematic literature review was conducted per PRISMA guidelines. Articles included in the final analysis were limited to those investigating FTT salvage procedures and included factors impacting outcomes. Cohort and case series (>5 flaps) studies up until March 2021 were included. Chi‐square tests and linear regression modeling was completed for analysis.
Results
The patient‐specific factors significantly associated with salvage included the absence of hypercoagulability (p < .00001) and no previous salvage attempts (p < .00001). Case‐specific factors significantly associated with salvage included trunk/breast flaps (p < .00001), fasciocutaneous/osteocutaneous flaps (p = .006), venous compromise (p < .00001), and shorter time from index procedure to salvage attempt (R = .746). Radiation in the head and neck population was significantly associated with flap salvage failure.
Conclusions
Given the complexity and challenges surrounding free flap salvage procedures, the goal of this manuscript was to present data helping guide surgical decision‐making. Based on our findings, patients without documented hypercoagulability, no previous salvage attempts, fasciocutaneous/osteocutaneous flaps, trunk/breast flaps, and a shorter time interval post‐index operation are the best candidates for a salvage attempt.
1. INTRODUCTION
Microsurgical free tissue transfer (FTT) is a widely employed operation utilized for reconstruction of various defects. FTT can be used for wound coverage in various anatomic locations, including the head/neck, lower and upper extremity, and breast (Cho et al., 2018; Eckart & Fokas, 2003; Gill et al., 2004). Survival of FTT is reported to be between 90% and 97% (Cho et al., 2018; Eckart & Fokas, 2003; Gill et al., 2004; Yang et al., 2014; Zhao et al., 2020), making it a reliable tool for the reconstructive surgeon. However, complications do arise, which can present as venous or arterial thrombosis, hematoma, or infection. Most commonly, thrombosis or hematoma can result in acute flap compromise, requiring salvage operation—a procedure involving exploration of the anastomosis, thrombectomy, thrombolysis, re‐do of the anastomosis, or combinations (Ho et al., 2012; Pu et al., 2004). Based on the literature, FTT salvage procedure success is reported to range from 22% to 67% (Mirzabeigi et al., 2012; Saint‐Cyr et al., 2007; Tall et al., 2015; Wang et al., 2019), which necessitates the exploration of nuances which predict FTT salvage.
Efforts to maximize success of salvage procedures manifest as clinical monitoring following primary FTT. Most commonly, ancillary staff perform clinical assessments of the flap—noting characteristics such as color, turgor, and capillary refill—as well as Doppler ultrasonography to assess adequate perfusion and drainage of the flap (Abdel‐Galil & Mitchell, 2009; Disa et al., 1999). This system of flap monitoring is rooted in the knowledge that salvage success rates drop precipitously as time between FTT and return to the operating room (RTOR) increases (Smit et al., 2007). While clinical monitoring is standard of care for most institutions, it remains a costly endeavor and does not eliminate salvage failure (Jablonka et al., 2019). Shen et al. reviewed 11 studies and showed that salvage rates decrease by postoperative day and anatomic location, with breast flaps having the highest salvage rates (Shen et al., 2021).
To our knowledge, no systematic reviews or meta‐analyses have examined patient‐ and operation‐specific factors which impact FTT salvage outcomes other than time and anatomic location. Therefore, the goals of this study were to conduct a systematic review of the literature to (1) identify any factors that impact FTT salvage outcomes and (2) aid the reconstructive surgeon in counseling patients on chances of a successful salvage.
2. MATERIALS AND METHODS
2.1. Search strategy
A systematic review of publications evaluating FTT salvage outcomes and factors associated with outcomes was conducted using the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidelines (Moher et al., 2009). PubMed, Scopus, and Web of Science were queried without date of publication restrictions. Search terms included: “free flap OR free tissue OR free tissue transfer OR flap reconstruction OR microvascular flap OR DIEP flap OR head and neck flap OR lower extremity free flap” AND “salvage OR salvage outcome OR take back OR take‐back OR compromise OR failure” AND “predictor OR risk factor OR factor OR influence OR comorbidity.”
2.2. Inclusion and exclusion criteria
Inclusion criteria were cohort studies and case series (greater than five patients) that included data involving FTT salvage procedures of any anatomic site and case‐ or patient‐specific factors impacting outcomes in humans. Exclusion criteria were studies reporting salvage rate without factors influencing outcomes, case reports, case series with less than five patients, studies in press or unpublished meeting proceedings, nonhuman studies, and non‐English studies.
2.3. Article selection
After obtaining records following our search query, all titles were screened independently by two authors (SKO, KRM) for relevance based on a priori criteria. Screened articles were then selected for full‐text review, which were completed by two authors (SKO, KRM) with additional screening from supporting authors. At every stage, articles required two authors to agree regarding inclusion. If a disagreement occurred, the senior author was consulted.
2.4. Data collection and analysis
Data extraction utilized a standardized form for accuracy. Data collection included authors, journal, publication year, total salvage attempts, total salvage success, and number of salvage attempts and success for each anatomic group (head/neck, extremity, and trunk/breast). Additionally, for categorical data, we collected the total number of successful or failed salvage attempts for patients with and without the following demographic information: gender (male/female), smoking status, hypertension (HTN), diabetes mellitus (DM), high cholesterol, peripheral vascular disease and coronary artery disease (PVD/CAD), hypercoagulability (defined as thrombophilia or previous thromboembolic event), previous radiation, previous chemotherapy, and previous takebacks. We collected the number of successful and failed salvages based on venous or arterial compromise, venous supercharging (>1 venous anastomosis), and tissue type—osteocutaneous, fasciocutaneous, or myocutaneous. For continuous data, we collected average study values for both successful and unsuccessful salvages for the following variables: age, body mass index (BMI), time from index operation to compromise detection (i.e., clinical identification, typical by Doppler probe), and total time from index operation to salvage.
Successful salvage was defined as both complete and partial salvages. As Mirzabeigi et al. described, partial flap loss and fat necrosis etiology are multifactorial, including factors like perforator selection, and not in the scope of this review (Mirzabeigi et al., 2012). Statistical analysis was completed in Microsoft Excel (Microsoft, Redmond, WA) using chi‐squared tests for categorical variables and correlation coefficients for continuous variables reported in Pearson's correlation coefficient and linear regression analysis p‐values. Statistical significance was set at p‐values <.05.
3. RESULTS
After our initial query, 9515 articles underwent title and abstract review (Figure 1). Deduplication occurred for 249 studies, and 153 records underwent full‐text review. After applying inclusion and exclusion criteria, 36 articles were included for analysis (Table 1). The date range for the studies included was June 2001 to March 2021. There were 1840 successful FTT salvages in 2735 total attempts, resulting in an average salvage rate of 67.28% (Table 2). While 11 studies (30.56%) did not mention any monitoring protocol, Doppler ultrasonography was used in 18 studies (50.00%) with 7 studies (19.44%) mentioning clinical monitoring without specific mention of ultrasonography. Overall, average salvage rates varied by anatomic location: head/neck (57.81%), trunk/breast (71.25%), extremity (50.76%). Salvage rates also varied by tissue type: fasciocutaneous (67.24%), osteocutaneous (58.97%), and myocutaneous (52.41%).
FIGURE 1.
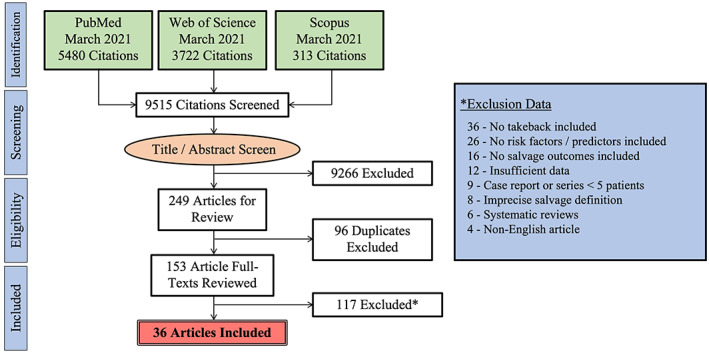
PRISMA guidelines for systematic review flow chart summarizing our initial article search and selection process.
TABLE 1.
Free flap salvage systematic review articles.
| Author | Year | Journal | N | Salvage rate | Author | Year | Journal | N | Salvage rate |
|---|---|---|---|---|---|---|---|---|---|
| Brown et al. | 2013 | Brit Journ of Oral Maxillofac Surg | 40 | 72.50% | Liu et al. | 2018 | Laryngoscope | 7 | 14.29% |
| Bui et al. (Bui et al., 2007) | 2007 | PRS | 38 | 63.16% | Mirzabeigi et al. (Mirzabeigi et al., 2012) | 2012 | PRS | 47 | 48.94% |
| Carney et al. (Carney et al., 2018) | 2018 | PRS | 70 | 65.71% | Nakamizo et al. | 2004 | Auris Nasus Larynx | 7 | 28.57% |
| Chang et al. (Chang, Zhang, et al., 2016) | 2016 | Head & Neck | 151 | 60.26% | Selber et al. (Selber et al., 2012) | 2012 | PRS | 157 | 57.96% |
| Chang et al. (Chang, Chang, et al., 2016) | 2016 | Ann of Plast Surg | 166 | 73.49% | Smit et al. (Smit et al., 2007) | 2007 | Microsurgery | 69 | 62.32% |
| Chen et al. (Chen et al., 2007) | 2007 | PRS | 113 | 84.07% | Stranix et al. | 2018 | PRS | 71 | 47.89% |
| Chen et al. | 2012 | Microsurgery | 9 | 77.78% | Sweeny et al. (Sweeny et al., 2020) | 2020 | Head & Neck | 162 | 45.68% |
| Chiu et al. (Chiu et al., 2017) | 2017 | Ann of Plast Surg | 150 | 72.67% | Tall et al.8 | 2015 | Ann of Plast Surg | 27 | 22.22% |
| Cho et al. (Cho et al., 2018) | 2018 | PRS | 70 | 51.43% | Wang et al. (Wang et al., 2012) | 2012 | PRS | 12 | 33.33% |
| Choi et al. | 2019 | Journ of Reconstr Microsurg | 36 | 77.78% | Wang et al. (Wang et al., 2019) | 2019 | Microsurgery | 21 | 61.90% |
| Dassonville et al. | 2008 | Euro Arch Oto | 32 | 65.63% | Wettstein et al. | 2008 | Journ Plast Reconstr Aesth Surg | 13 | 38.46% |
| Ho et al. (Ho et al., 2012) | 2012 | Brit Journ of Oral Maxillofac Surg | 72 | 59.72% | Winterton et al. (Winterton et al., 2010) | 2010 | Journ Plast Reconstr Aesth Surg | 327 | 88.99% |
| Hyodo et al. (Hyodo et al., 2007) | 2007 | Laryngoscope | 21 | 33.33% | Yang et al. (Yang et al., 2014) | 2014 | Int Journ Oral Maxillofac Surg | 47 | 55.32% |
| Joseph et al. | 2021 | Euro Journ of Plast Surg | 43 | 44.19% | Yang et al. | 2016 | Int Journ Oral Maxillofac Surg | 71 | 66.20% |
| Kamali et al. | 2021 | Journ Plast Reconstr Aesth Surg | 62 | 70.97% | Yii et al. (Yii et al., 2001) | 2001 | Ann of Plast Surg | 41 | 68.29% |
| Khansa et al. | 2013 | Microsurgery | 113 | 84.96% | Yim et al. | 2015 | Arch Plast Surg | 17 | 82.35% |
| Las et al. | 2016 | Journ Plast Reconstr Aesth Surg | 100 | 77.00% | Yu et al. | 2008 | Head & Neck | 49 | 44.90% |
| Lee et al. (Lee & Mun, 2013) | 2013 | Journ Plast Reconstr Aesth Surg | 31 | 58.06% | Zhao et al. (Zhao et al., 2020) | 2020 | Journ of Reconstr Microsurg | 273 | 74.73% |
Abbreviation: PRS: plastic and reconstructive surgery.
TABLE 2.
Free flap salvage rates summary.
| Successful/attempts (%) | ||
|---|---|---|
| Overall average salvage rate | 1840/2735 (67.28) | |
| Overall anatomic salvage rates | ||
| Head and neck | 596/1031 (57.81) | |
| Trunk/breast | 389/546 (71.25) | |
| Extremity | 100/197 (50.76) | |
| Overall flap type salvage rates | ||
| Fasciocutaneous | 78/116 (67.24) | |
| Osteocutaneous | 23/39 (58.97) | |
| Myocutaneous | 98/187 (52.41) | |
3.1. Patient‐specific factors
The presence of hypercoagulability in patients was associated with higher failure rates in free flap salvage procedures (Table 3). Radiation history was not associated with free flap salvage failure but was significantly associated with flap salvage failure in the head and neck portion of flaps (X 2 = 4.776, p = .028, N = 646; data not shown). Previous takebacks were significantly associated with free flap salvage failure. Patient age (R = −.5195) and BMI (R = −.6749) were both negatively correlated with salvage rate, but neither were statistically significant (p = .083 and p = .096, respectively) (Figure 2a,b). Other patient‐specific factors which were assessed but not significantly associated with salvage outcomes included HTN, gender, DM, smoking, PVD/CAD, prior chemotherapy, and high cholesterol (Tables 3 and 4).
TABLE 3.
Factors associated with salvage outcomes, part 1.
| Hypercoagulability | Previous takeback | ||||
|---|---|---|---|---|---|
| Success | Failure | Success | Failure | ||
| Hypercoagulability | 11 | 31 | Previous takeback | 25 | 54 |
| No hypercoagulability | 153 | 85 | No previous takeback | 157 | 72 |
| Chi‐squared stat | 21.3513 | Chi‐squared stat | 33.1085 | ||
| p‐value | <.00001 | p‐value | <.00001 | ||
| Significance level | .05 | Significance level | .05 | ||
| N | 280 | N | 308 | ||
| Radiation | Hypertension | ||||
|---|---|---|---|---|---|
| Success | Failure | Success | Failure | ||
| Radiation | 187 | 140 | Hypertension | 184 | 72 |
| No radiation | 381 | 221 | No hypertension | 322 | 162 |
| Chi‐squared stat | 3.3214 | Chi‐squared stat | 2.2132 | ||
| p‐value | .068384 | p‐value | .136835 | ||
| Significance level | .05 | Significance level | .05 | ||
| N | 929 | N | 740 | ||
FIGURE 2.
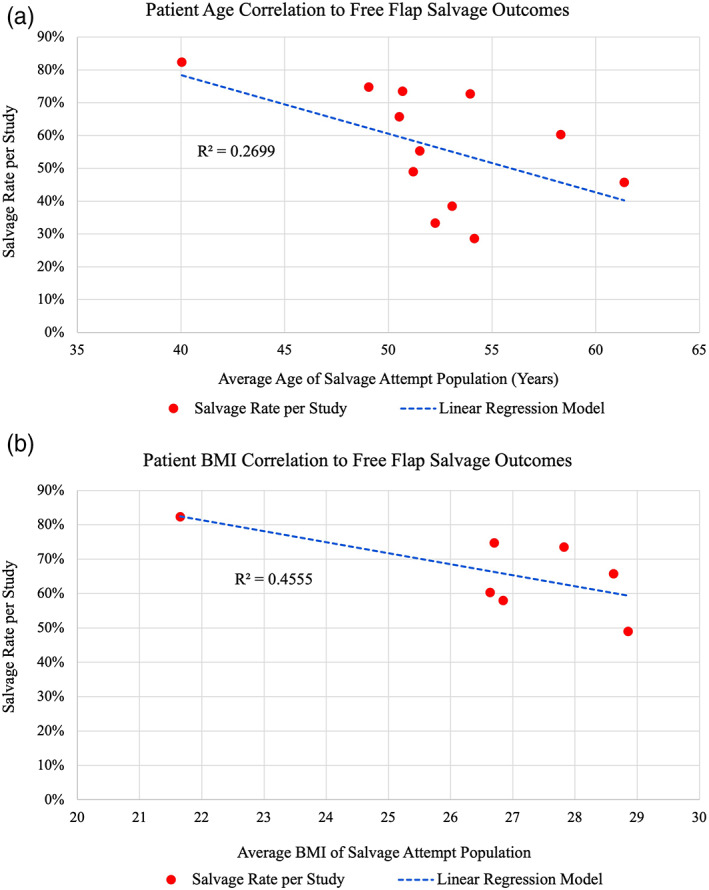
Linear regression model on scatter plots for average (a) patient age and (b) patient BMI per study correlated with study salvage rate. Age is negatively correlated with salvage rate (R = −.5195), as is BMI (R = −.6749), but neither are statistically significant (p = .083 and .096, respectively). Alpha was set at .05.
TABLE 4.
Factors associated with salvage outcomes, part 2.
| Gender | Diabetes mellitus | Smoking | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Success | Failure | Success | Failure | Success | Failure | |||||
| Male | 239 | 157 | DM | 48 | 30 | Smoking | 300 | 167 | ||
| Female | 109 | 90 | No DM | 423 | 251 | No smoking | 402 | 199 | ||
| Chi‐squared stat | 1.6983 | Chi‐squared stat | 0.0445 | Chi‐squared stat | 0.8185 | |||||
| p‐value | .192515 | p‐value | .832842 | p‐value | .365626 | |||||
| Significance level | .05 | Significance level | .05 | Significance level | .05 | |||||
| N | 595 | N | 752 | N | 1068 | |||||
| PVD/CAD | Prior chemotherapy | High cholesterol | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Success | Failure | Success | Failure | Success | Failure | |||||
| PVD/CAD | 55 | 24 | Chemo | 103 | 69 | High chol | 52 | 18 | ||
| No PVD/CAD | 319 | 201 | No chemo | 175 | 112 | No chol | 221 | 99 | ||
| Chi‐squared stat | 2.0019 | Chi‐squared stat | 0.0537 | Chi‐squared stat | 0.7462 | |||||
| p‐value | .157101 | p‐value | .81677 | p‐value | .38769 | |||||
| Significance level | .05 | Significance level | .05 | Significance level | .05 | |||||
| N | 599 | N | 459 | N | 390 | |||||
Abbreviations: Chemo: chemotherapy; Chol: cholesterol; PVD/CAD: peripheral vascular disease/coronary artery disease.
3.2. Surgery‐specific factors
For anatomic location of flaps, breast/trunk free flaps were significantly associated with successful salvage outcomes compared with both head/neck flaps, as well as extremity flaps (Table 5). When comparing head/neck free flaps to extremity free flaps, there was no significant difference in associated salvage rates. The time from index operation to salvage attempt was negatively correlated with salvage rate (R = −.8637, p = .012) (Figure 3a). The average times from index operation to salvage attempt for the failure and success groups were 78.19 h (56.1–100.8) and 37.75 h (19.4–46.5), respectively. A similar relationship was found when correlating time from index operation to compromise detection (R = −.6234, 59.08 h vs. 30.04 h for failure and success groups, respectively), but this was not significant (p = .38) (Figure 3b).
TABLE 5.
Factors associated with salvage outcomes, part 3.
| Breast versus H&N | Breast versus extremity | ||||
|---|---|---|---|---|---|
| Success | Failure | Success | Failure | ||
| Breast | 389 | 157 | Breast | 389 | 157 |
| H&N | 596 | 435 | Extremity | 100 | 97 |
| Chi‐squared stat | 27.4891 | Chi‐squared stat | 26.9982 | ||
| p‐value | <.00001 | p‐value | <.00001 | ||
| Significance level | .05 | Significance level | .05 | ||
| N | 1577 | N | 743 | ||
| Venous versus arterial | Venous versus combined | ||||
|---|---|---|---|---|---|
| Success | Failure | Success | Failure | ||
| Venous | 518 | 212 | Venous | 518 | 212 |
| Arterial | 259 | 198 | Combined | 36 | 100 |
| Chi‐squared stat | 25.3654 | Chi‐squared stat | 98.4481 | ||
| p‐value | <.00001 | p‐value | <.00001 | ||
| Significance level | .05 | Significance level | .05 | ||
| N | 1187 | N | 866 | ||
| H&N versus extremity | Fasciocutaneous versus myocutaneous | ||||
|---|---|---|---|---|---|
| Success | Failure | Success | Failure | ||
| H&N | 596 | 435 | Fasciocutaneous | 192 | 75 |
| Extremity | 100 | 97 | Myocutaneous | 171 | 115 |
| Chi‐squared stat | 3.3447 | Chi‐squared stat | 8.9937 | ||
| p‐value | .067423 | p‐value | .002709 | ||
| Significance level | .05 | Significance level | .05 | ||
| N | 1228 | N | 553 | ||
| Arterial versus combined | Myocutaneous versus osteocutaneous | ||||
|---|---|---|---|---|---|
| Success | Failure | Success | Failure | ||
| Arterial | 259 | 198 | Myocutaneous | 171 | 115 |
| Combined | 36 | 100 | Osteocutaneous | 33 | 20 |
| Chi‐squared stat | 38.2457 | Chi‐squared stat | 0.1142 | ||
| p‐value | <.00001 | p‐value | .7354 | ||
| Significance level | .05 | Significance level | .05 | ||
| N | 593 | N | 339 | ||
| Fasciocutaneous versus osteocutaneous | Myocutaneous versus osteocutaneous/fasciocutaneous | ||||
|---|---|---|---|---|---|
| Success | Failure | Success | Failure | ||
| Fasciocutaneous | 192 | 75 | Myocutaneous | 171 | 115 |
| Osteocutaneous | 33 | 20 | Osteo/fascio | 225 | 95 |
| Chi‐squared stat | 1.9712 | Chi‐squared stat | 7.3841 | ||
| p‐value | .160325 | p‐value | .00658 | ||
| Significance level | .05 | Significance level | .05 | ||
| N | 320 | N | 606 | ||
Abbreviation: H&N: head and neck.
FIGURE 3.
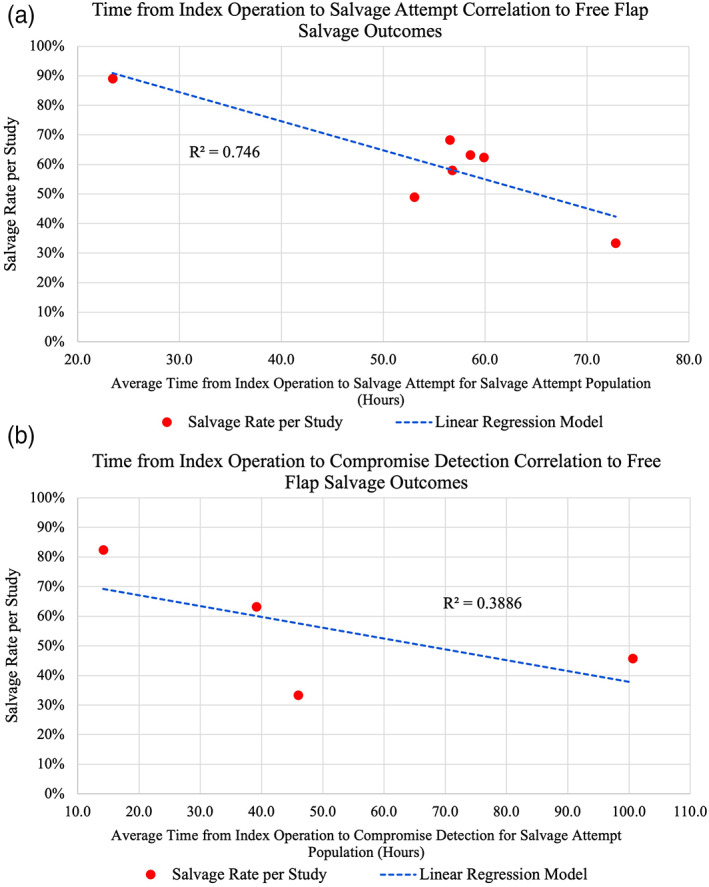
Linear regression model on scatter plots for average time from index operation to (a) salvage attempt and (b) compromise detection. Both are negatively correlated with salvage rates, but only time from index operation to salvage attempt was statistically significant (p = .012). Alpha was set at .05.
Venous compromise was associated with a higher percentage of free flap salvage when compared to arterial compromise (Table 5). When compared to a combined venous and arterial compromise etiology, both venous alone and arterial alone were associated with higher flap salvage rates. When analyzing flap tissue type, fasciocutaneous free flaps were associated with a significantly higher salvage rate when compared with myocutaneous FTT (Table 5). This relationship resembles when we combine osteocutaneous and fasciocutaneous flaps and compare this to muscle‐containing flaps, with muscle‐containing flaps being associated with lower salvage rates (Table 5). Lastly, venous supercharging was not associated with higher flap salvage rates (p = .122), regardless of the anatomic location (H/N: p = .105; trunk/breast: p = .262; data not shown).
4. DISCUSSION
The present systematic review is the first to summarize and analyze the current literature's data regarding both patient‐ and case‐specific factors impacting free flap salvage following FTT compromise. This investigation yielded 36 peer‐reviewed articles analyzing free flap salvage outcomes. Of the novel, patient‐specific findings assessed in our review, hypercoagulability and previous takeback attempts were most significantly associated with lower salvage rates. Of the case‐specific findings, increased time from index operation to salvage attempt and from index operation to detection were highly correlated with lower salvage rates. Lastly, free flaps to the breast/trunk, fasciocutaneous or osteocutaneous flaps, and those with venous compromise were most associated with higher salvage rates.
Individual factors correlated with salvage outcomes include age and BMI. Age is negatively correlated with FTT salvage outcomes (Figure 2a) but is not significant. This result can be expected, as age is a strong factor in atherosclerotic disease (Jaffer et al., 2002; Nozue et al., 2014). This intrinsic factor impacting vessel flow and clotting propensity is not affected by salvage attempts, and therefore was expected to be correlated to salvage rate in a negative fashion; one cannot simply undo this damage. Similarly, BMI is negatively correlated with many surgical outcomes, including FTT complication rate (Ozturk et al., 2014; Ri et al., 2015). While neither of these analyses were statistically significant, this data does trend in the direction of the established relationships with FTT salvage. Overall, linear regression analysis suggests patients with increased BMI and of greater age are associated with lower salvage rates, and thus nonideal candidates for salvage attempt.
History of previous takeback attempts is significantly associated with salvage failure (Table 3). There are likely many surgical factors involved in this, including accumulative vessel fragility and increased time from index operation. While a history of radiation therapy was not significantly correlated with failed salvage, it did approach significance (p = .06), and was significantly associated with failed salvage in the head and neck population (p = .028). The impact of radiation on free flap reconstruction is well‐documented (Onoda et al., 2014; Sweeny et al., 2020), and the association with failed salvage likely stems from recipient vessel friability and inflammation creating an overall pro‐thrombotic microenvironment. In general, patients with assumed vessel friability and fragility make nonideal candidates for salvage attempt.
Of the patient‐specific factors, hypercoagulability was the most significant, with patients lacking a history of hypercoagulability being associated with higher salvage rates (Table 3). Hypercoagulability has long been studied regarding FTT outcomes. While the impact is still debated, most studies report higher flap thrombosis rates among those with hypercoagulability (Biben & Atmodiwirjo, 2019; Wang et al., 2012). Therefore, it is natural to expect lower salvage rates in this population when handling microvasculature. Additionally, two studies included in this review (Carney et al., Mirzabeigi et al.) investigated the impact platelet count (PLT) has on FTT salvage outcomes (Carney et al., 2018; Mirzabeigi et al., 2012). The average PLT for free flap salvage failures and successes was 293 and 227, respectively (data not shown). Overall, this suggests that any predilection toward clotting increases one's risk for thrombosis, and therefore an increased risk of recurrent clotting with reexploration of FTT.
One significant finding was that breast free flaps were associated with higher salvage rates (p < .00001) (Table 5). Additionally, H&N flaps are closely associated with higher salvage rates compared to extremity flaps, but this was not significant. These relationships are likely due to the primary indication for the FTT and the subsequent physiologic anomalies. Trunk/breast flaps, almost exclusively breast flaps (i.e., DIEP), are either delayed or immediate reconstruction in cancer patients who are deemed medically cleared for this surgery; these procedures are planned (without concurrent systemic trauma) and patients are typically otherwise healthy (Chang, Chang, et al., 2016; Chang, Zhang, et al., 2016; Enajat et al., 2010; Mak & Kwong, 2020; Piwnica‐Worms et al., 2020). In comparison, H&N flaps are predominantly in older male patients with a history positive for tobacco use (Chiu et al., 2017). While these procedures are typically planned as well, either concurrent or prior radiation therapy is commonly used in this population (Onoda et al., 2014; Sweeny et al., 2020). As demonstrated in this review, radiation history is closely associated with FTT salvage failure. Lastly, extremity free flap salvage attempts yielded the lowest success rates (Table 2) when compared to both trunk/breast and H&N (Table 5). Similarly, this is also likely due to the primary population which undergoes extremity reconstruction – a younger male patient population following serious trauma (Cho et al., 2018; Piwnica‐Worms et al., 2020). These patients typically present with polytrauma and multiple concurrent injuries, and are thus likely in a hypovolemic, systemic inflammatory response state with large vessel injury, inducing hypercoagulability and higher PLT counts (Carney et al., 2018; Kloeters et al., 2017; Xiong et al., 2016). As we have shown, patients with a history of hypercoagulability are associated with decreased salvage rates. Additionally, these procedures are not as commonly planned, adding secondary factors impacting salvage, such as time of day, surgeon difference, or surgeon experience, which all likely have some, albeit smaller, effects on salvage outcomes (Chang, Chang, et al., 2016; Chang, Zhang, et al., 2016; Lee & Mun, 2013; Mirzabeigi et al., 2012; Zhao et al., 2020). Overall, these findings confirm the results of Shen et al., which demonstrated that complete failure rates vary by anatomic location, with salvage of breast flaps being the most successful (Shen et al., 2021).
FTT tissue type is associated with salvage procedure outcomes (Table 5). Muscle‐containing flaps (i.e., myocutaneous) are associated with lower FTT salvage rates compared to both osteocutaneous and fasciocutaneous. This was observed statistically when combining osteocutaneous and fasciocutaneous flaps together for analysis. Explanations for this finding are likely based on the metabolic requirements of muscle being higher than skin, fat, and bone (Rojdmark et al., 2002). With a higher metabolic requirement, there is a higher risk of injury with equivalent ischemia time. Additionally, muscle‐based flaps are more commonly used in H&N or extremity reconstruction (Dat et al., 2017); as we have seen, these anatomic locations are associated with lower salvage rates for previously discussed reasons.
Lastly, venous as opposed to arterial compromise and combined venous/arterial compromise is associated with higher FTT salvage rates (Table 5). One explanation for this finding is the sequence in which arterial and venous injury occur. In general, there are two etiologies of delayed (i.e., not during primary anastomosis) arterial thrombosis. One is intrinsic mechanisms related to clotting cascades or recipient vessel damage resulting in “white” (platelet) clot. The second is the more insidious mechanism related to arterial failure after venous outflow obstruction (Eriksson et al., 2005; Halle et al., 2010; Schwarzmaier et al., 2010). Given that arterial failure is often related to patient‐specific factors, (i.e., hypercoagulability, recipient vessel damage) it is assumed that these are not modifiable and thus not impacted by salvage attempt. Furthermore, venous outflow obstruction is often caused by modifiable factors – kinking, relative length mismatch, pedicle redundancy – and as such, they can frequently be corrected at the time of salvage. Interestingly, supercharging does not appear to be one of these modifiable factors associated with salvage success, thus requiring further study to define targets related to venous anastomosis.
Of all potential factors impacting salvage outcomes, time is perhaps the most well‐known and important. The only systematic review studying FTT salvage confirms this general knowledge, with declining salvage rates as time from index operation to compromise increases (Shen et al., 2021). This is well supported throughout the literature and further supported by our systematic analysis (Bui et al., 2007; Chang, Zhang, et al., 2016; Chen et al., 2007; Hyodo et al., 2007; Mirzabeigi et al., 2012; Smit et al., 2007; Winterton et al., 2010; Yii et al., 2001). Because of the way most studies reported data, we analyzed time through correlation plots, relating a study's average “time interval” with the overall study salvage rate (Figure 3). Those studies reporting in “time blocks” were not included in this analysis. Our findings mirror Shen et al: time from index operation to salvage attempt is strongly correlated with study salvage rate in a negative fashion. Explanations as to why time is an important factor impacting salvage outcomes is likely centered on ischemia time. It is well known that a higher ischemia time, such as in organ transplantation, is associated with increased risk of graft failure (Ballestin et al., 2018; Siemionow & Arslan, 2004). Additionally, flap monitoring is likely interlaced. Most centers have flap monitoring as standard of care, with ultrasound being a mainstay of this model. In the present review, 25 of 36 studies reported a monitoring protocol of some capacity, with the majority (18/36) including ultrasound. While multiple methods of monitoring have been studied, including implantable doppler probes and microdialysis, overall results suggest undifferentiating outcomes (Dakpe et al., 2020; Paydar et al., 2010; Rogers et al., 2013; Smit et al., 2010). Ultimately, high frequency clinical monitoring—despite its high cost (Jablonka et al., 2019)—improves accuracy of detecting flap compromise and likely shortens time from detection to salvage (Chae et al., 2015; Frost et al., 2015; Rozen et al., 2010; Soteropulos et al., 2019).
In summary, the failing FTT that is associated with the highest likelihood of salvage is one that is of the trunk/breast without muscle, with venous compromise, without hypercoagulability, without previous takeback attempts, and with a minimal time interval from index operation. Without odds ratios presented in most studies included, we can only offer correlations and associations between factors and salvage success; this is not a study of causality. In the future, studies investigating FTT salvage should summarize data through odds ratios to advance analytic potential. Another limitation of this data was a lack of stratification by salvage method, meaning whether thrombolysis, thrombectomy, or both were utilized. We chose not to include this in our analysis, as choice of method is predicated by surgeon‐perceived severity and other confounding factors—with highly varied responses—and was thus left out (Panchapakesan et al., 2003; Selber et al., 2012). Additionally, we recognize publication bias as potentially skewing these results. However, this is likely not overwhelming, as most factors reported were secondary results in studies, and thus reflect a diverse array of outcomes. Lastly, because we only included articles reporting factors impacting salvage outcomes, we naturally are missing a large sample of overall salvage rate.
Lastly, and in summary, this project was undertaken to provide a data set to serve as a guide in making difficult decisions in challenging circumstances and to add to the microsurgeon's arsenal of educational resources when setting expectations with patients. This is best recapitulated by the words of Professor Wayne Morrison when referring to the “theater of the absurd” that “Not all flaps will work, and futile added hours spent primarily to salvage the surgeon's ego must be resisted.” (Wei & Mardini, 2009) Our hope is that this data will save futile hours and will positively influence both patients and microsurgeons alike.
5. CONCLUSIONS
Free flap reconstruction is a versatile surgical modality for plastic and reconstructive surgeons. However, the most feared complication of flap compromise requires costly clinical monitoring and surgical revision through salvage procedures. The present data provides the first detailed summary of both patient‐ and case‐specific factors associated with salvage outcomes. Based on the 36 articles included, the presence of hypercoagulability, increased time from index operation to salvage, anatomic location of flap on the extremities, type of flap including muscle, and the presence of previous takebacks are the strongest factors associated with low salvage rates. Additionally, previous radiation has a significant impact on head and neck flaps salvage success. Salvage operations remain highly variable and create a feared and often misunderstood clinical picture for surgeons and patients alike, and require further data‐driven evaluations to determine guidelines.
AUTHOR CONTRIBUTIONS
Scott K. Odorico was involved in project inception, project development, article review, data acquisition, manuscript writing, and editing. Katie Reuter Muñoz was involved in project development, article review, data acquisition, and manuscript editing. Peter J. Nicksic was involved in project development, data acquisition, manuscript writing, and editing. Kirsten A. Gunderson was involved in article review, data acquisition, and project development. Kasey Wood was involved in article review, data acquisition, and project development. Zeeda H. Nkana was involved in article review, data acquisition, and project development. Evalina Bond was involved in project inception and development. Samuel O. Poore was involved in project inception, project development, article review, manuscript editing, and overall support.
ACKNOWLEDGMENTS
The authors would like to acknowledge the University of Wisconsin Department of Surgery Biostats group for their assistance (Glen Leverson and Lily Stalter). The authors would also like to thank Aaron Dingle, PhD for his continued mentorship.
Odorico, S. K. , Reuter Muñoz, K. , J. Nicksic, P. , Gunderson, K. A. , Wood, K. , H. Nkana, Z. , Bond, E. , & Poore, S. O. (2023). Surgical and demographic predictors of free flap salvage after takeback: A systematic review. Microsurgery, 43(1), 78–88. 10.1002/micr.30921
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abdel‐Galil, K. , & Mitchell, D. (2009). Postoperative monitoring of microsurgical free tissue transfers for head and neck reconstruction: A systematic review of current techniques – Part 1: Non‐invasive techniques. The British Journal of Oral & Maxillofacial Surgery, 47(5), 351–355. [DOI] [PubMed] [Google Scholar]
- Ballestin, A. , Casado, J. , Abellan, E. , Vela, F. J. , Alvarez, V. , Uson, A. , Lopez, E. , Marinaro, F. , Blazquez, R. , & Sanchez‐Margallo, F. (2018). Ischemia‐reperfusion injury in a rat microvascular skin free flap model: A histological, genetic, and blood flow study. PLoS One, 13(12), e0209624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biben, J. , & Atmodiwirjo, P. (2019). Free flap thrombosis in patients with hypercoagulability: A systematic review. Archives of Plastic Surgery, 46, 572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui, D. , Cordeiro, P. , Hu, Q. Y. , Disa, J. , Pusic, A. , & Mehrara, B. (2007). Free flap reexploration: Indications, treatment, and outcomes in 1193 free flaps. Plastic and Reconstructive Surgery, 119, 2092–2100. [DOI] [PubMed] [Google Scholar]
- Carney, M. , Weissler, J. , Tecce, M. , Mirzabeigi, M. , Wes, A. , Koltz, P. , Kanchwala, S. , Low, D. , Kovach, S. , Wu, L. , Serletti, J. , & Fosnot, J. (2018). 5000 free flaps and counting: A 10‐year review of a single academic institution's microsurgical development and outcomes. Plastic and Reconstructive Surgery, 141, 855–863. [DOI] [PubMed] [Google Scholar]
- Chae, M. , Rozen, W. , Whitaker, I. , Chubb, D. , Grinsell, D. , Ashton, M. , Hunter‐Smith, D. , & Lineaweaver, W. (2015). Current evidence for postoperative monitoring of microvascular free flaps. Annals of Plastic Surgery, 74, 621–632. [DOI] [PubMed] [Google Scholar]
- Chang, E. , Chang, E. , Soto‐Miranda, M. , Zhang, H. , Nosrati, N. , Crosby, M. , Reece, G. , Robb, G. , & Chang, D. (2016). Comprehensive evaluation of risk factors and management of impending loss in 2138 breast free flaps. Annals of Plastic Surgery, 77, 67–71. [DOI] [PubMed] [Google Scholar]
- Chang, E. , Zhang, H. , Liu, J. , Yu, P. , Skoracki, R. , & Hanasono, M. (2016). Analysis of risk factors for flap loss and salvage in free flap head and neck reconstruction. Head & Neck, 38(S1), e771–e775. [DOI] [PubMed] [Google Scholar]
- Chen, K. T. , Mardini, S. , Chuang, D. , Lin, C. H. , Cheng, M. H. , Lin, Y. T. , Huang, W. C. , Tsao, C. K. , & Wei, F. C. (2007). Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers. Plastic and Reconstructive Surgery, 120, 187–195. [DOI] [PubMed] [Google Scholar]
- Chiu, Y. H. , Chang, D. H. , & Perng, C. K. (2017). Vascular complications and free flap salvage in head and neck reconstructive surgery. Annals of Plastic Surgery, 78, s83–s88. [DOI] [PubMed] [Google Scholar]
- Cho, E. , Shammas, R. , Carney, M. , Weissler, J. , Bauder, A. , Glener, A. , Kovach, S. , Hollenbeck, S. , & Levin, L. S. (2018). Muscle versus fasciocutaneous free flaps in lower extremity traumatic reconstruction: A multicenter outcomes analysis. Plastic and Reconstructive Surgery, 141, 191–199. [DOI] [PubMed] [Google Scholar]
- Dakpe, S. , Colin, E. , Bettoni, J. , Davrou, J. , Diouf, M. , Devauchelle, B. , & Testelin, S. (2020). Intraosseous microdialysis for bone free flap monitoring in head and neck reconstructive surgery: A prospective pilot study. Microsurgery, 40, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat, A. , Loh, I. , & Bruscino‐Raiola, F. (2017). Free‐flap salvage: Muscle only versus skin paddle – An Australian experience. ANZ Journal of Surgery, 87, 1040–1043. [DOI] [PubMed] [Google Scholar]
- Disa, J. , Cordeiro, P. , & Hidalgo, D. (1999). Efficacy of conventional monitoring techniques in free tissue transfer: An 11‐year experience in 750 consecutive cases. Plastic and Reconstructive Surgery, 104(1), 97–101. [PubMed] [Google Scholar]
- Eckart, A. , & Fokas, K. (2003). Microsurgical reconstruction in the head and neck region: An 18‐year experience with 500 consecutive cases. Journal of Cranio‐Maxillofacial Surgery, 31, 197–201. [DOI] [PubMed] [Google Scholar]
- Enajat, M. , Rozen, W. , Whitaker, I. , Smit, J. , & Acosta, R. (2010). A single center comparison of one versus two venous anastomoses in 564 consecutive DIEP flaps: Investigating the effect on venous congestion and flap survival. Microsurgery, 30, 185–191. [DOI] [PubMed] [Google Scholar]
- Eriksson, E. , Karlof, E. , Lundmark, K. , Rotzius, P. , Hedin, U. , & Xie, X. (2005). Powerful inflammatory properties of large vein endothelium in vivo. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 723–728. [DOI] [PubMed] [Google Scholar]
- Frost, M. , Niumsawatt, V. , Rozen, W. , Eschen, G. , Damsgaard, T. , & Kiil, B. (2015). Direct comparison of postoperative monitoring of free flaps with microdialysis, implantable cook‐Swartz doppler probe, and clinical monitoring in 20 consecutive patients. Microsurgery, 35, 262–271. [DOI] [PubMed] [Google Scholar]
- Gill, P. , Hunt, J. , Guerra, A. , Sullivan, D. F. , Boraski, J. , Metzinger, S. , Dupin, C. , & Allen, R. (2004). A 10‐year retrospective review of 758 DIEP flaps for breast reconstruction. Plastic and Reconstructive Surgery, 113(4), 1153–1160. [DOI] [PubMed] [Google Scholar]
- Halle, M. , Ekstrom, M. , Farnebo, F. , & Tornvall, P. (2010). Endothelial activation with prothrombotic response in irradiated microvascular recipient veins. Journal of Plastic, Reconstructive & Aesthetic Surgery, 63, 1910–1916. [DOI] [PubMed] [Google Scholar]
- Ho, M. , Brown, J. , Magennis, P. , Bekiroglu, F. , Rogers, S. , Shaw, R. , & Vaughan, E. (2012). Salvage outcomes of free tissue transfer in liverpool: Trends over 18 years (1992‐2009). The British Journal of Oral & Maxillofacial Surgery, 50, 13–18. [DOI] [PubMed] [Google Scholar]
- Hyodo, I. , Nakayama, B. , Kato, H. , Hasegawa, Y. , Ogawa, T. , Terada, A. , & Torii, S. (2007). Analysis of salvage operation in head and neck microsurgical reconstruction. The Laryngoscope, 117, 357–360. [DOI] [PubMed] [Google Scholar]
- Jablonka, E. , Lamelas, A. , Kanchwala, S. , Rhemtulla, I. , & Smith, M. (2019). A simplified cost‐utility analysis of inpatient flap monitoring after microsurgical breast reconstruction and implications for hospital length of stay. Plastic and Reconstructive Surgery, 144, 540e–549e. [DOI] [PubMed] [Google Scholar]
- Jaffer, F. , O'Donnell, C. , Larson, M. , Chan, S. , Kissinger, K. , Kupka, M. , Salton, C. , Botnar, R. , Levy, D. , & Manning, W. (2002). Age and sex distribution of subclinical aortic atherosclerosis: A magnetic resonance imaging and examination of the Framingham heart study. Arteriosclerosis, Thrombosis, and Vascular Biology, 22, 849–854. [DOI] [PubMed] [Google Scholar]
- Kloeters, O. , Vasilic, D. , Hupkens, P. , & Ulrich, D. (2017). Markers of blood coagulation and fibrinolysis in patients with early and delayed microsurgical reconstructions in lower extremities. Journal of Plastic Surgery and Hand Surgery, 51(6), 420–426. [DOI] [PubMed] [Google Scholar]
- Lee, K. T. , & Mun, G. H. (2013). Is after‐hours free‐flap surgery associated with adverse outcomes? Journal of Plastic, Reconstructive & Aesthetic Surgery, 66, 460–466. [DOI] [PubMed] [Google Scholar]
- Mak, J. , & Kwong, A. (2020). Complications in post‐mastectomy immediate breast reconstruction: A ten‐year analysis of outcomes. Clinical Breast Cancer, 20(5), 402–407. [DOI] [PubMed] [Google Scholar]
- Mirzabeigi, M. , Wang, T. , Kovach, S. , Taylor, J. , Serletti, J. , & Wu, L. (2012). Free flap take‐back following postoperative microvascular compromise: Predicting salvage versus failure. Plastic and Reconstructive Surgery, 130, 579–589. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. (2009). PRISMA group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6(07), e1000097. [PMC free article] [PubMed] [Google Scholar]
- Nozue, T. , Yamamoto, S. , Tohyama, S. , Fukui, K. , Umezawa, S. , Onishi, Y. , Kunishima, T. , Sato, A. , Nozato, T. , Miyake, S. , Takeyama, Y. , Morino, Y. , Yamauchi, T. , Muramatsu, T. , Hirano, T. , Hibi, K. , Terashima, M. , & Michishita, I. (2014). Impacts of age on coronary atherosclerosis and vascular response to statin therapy. Heart and Vessels, 29, 456–463. [DOI] [PubMed] [Google Scholar]
- Onoda, S. , Kimata, Y. , Sugiyama, N. , Onoda, T. , & Mizukawa, N. (2014). Effects of radiation therapy on postoperative complications and adverse events in patients with head and neck reconstruction and flaps. Microsurgery, 34, 516–521. [DOI] [PubMed] [Google Scholar]
- Ozturk, C. , Kundu, N. , Bernard, S. , Cooper, K. , Ozturk, C. , & Djohan, R. (2014). Breast reconstruction with abdominal‐based free flaps in high body mass index population: Postoperative complications and impact of weight loss. Annals of Plastic Surgery, 72, 13–22. [DOI] [PubMed] [Google Scholar]
- Panchapakesan, V. , Addison, P. , Beausang, E. , Lipa, J. , Gilbert, R. , & Neligan, P. (2003). Role of thrombolysis in free‐flap salvage. Journal of Reconstructive Microsurgery, 19(8), 523–529. [DOI] [PubMed] [Google Scholar]
- Paydar, K. , Hansen, S. , Chang, D. , Hoffman, W. , & Leon, P. (2010). Implantable venous doppler monitoring in head and neck free flap reconstruction increases the salvage rate. Plastic and Reconstructive Surgery, 125, 1129–1134. [DOI] [PubMed] [Google Scholar]
- Piwnica‐Worms, W. , Stranix, J. , Othman, S. , Kozak, G. , Moyer, I. , Spencer, A. , Azoury, S. , Levin, L. S. , & Kovach, S. (2020). Risk factors for lower extremity amputation following attempted free flap limb salvage. Journal of Reconstructive Microsurgery, 36, 528–533. [DOI] [PubMed] [Google Scholar]
- Pu, L. , Medalie, D. , Rosenblum, W. , Lawrence, S. , & Vasconez, H. (2004). Free tissue transfer to a difficult wound of the lower extremity. Annals of Plastic Surgery, 53, 222–228. [DOI] [PubMed] [Google Scholar]
- Ri, M. , Miyata, H. , Aikou, S. , Seto, Y. , Akazawa, K. , Takeuchi, M. , Matsui, Y. , Konno, H. , Gotoh, M. , Mori, M. , Motomura, N. , Takamoto, S. , Sawa, Y. , Kuwano, H. , & Kokudo, N. (2015). Effects of body mass index (BMI) on surgical outcomes: A nationwide survey using a Japanese web‐based database. Surgery Today, 45, 1271–1279. [DOI] [PubMed] [Google Scholar]
- Rogers, M. , Brennan, P. , Leong, C. , Gowers, S. , Aldridge, T. , Mellor, T. , & Boutelle, M. (2013). Online rapid sampling microdialysis (rsMD) using enzyme‐based electroanalysis for dynamic detection of ischemia during free flap reconstructive surgery. Analytical and Bioanalytical Chemistry, 405, 3881–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojdmark, J. , Ungerstedt, J. , Blomqvist, L. , Ungerstedt, U. , & Heden, P. (2002). Comparing metabolism during ischemia and reperfusion in free flaps of different tissue composition. European Journal of Plastic Surgery, 24, 349–355. [Google Scholar]
- Rozen, W. , Chubb, D. , Whitaker, I. , & Acosta, R. (2010). The efficacy of postoperative monitoring: A single surgeon comparison of clinical monitoring and the implantable doppler probe in 547 consecutive free flaps. Microsurgery, 30, 105–110. [DOI] [PubMed] [Google Scholar]
- Saint‐Cyr, M. , Chang, D. , Robb, G. , & Chevray, P. (2007). Internal mammary perforator recipient vessels for breast reconstruction using free TRAM, DIEP, and SIEA flaps. Plastic and Reconstructive Surgery, 120, 1769–1773. [DOI] [PubMed] [Google Scholar]
- Schwarzmaier, S. , Kim, S. W. , Trabold, R. , & Plesnila, N. (2010). Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. Journal of Neurotrauma, 27, 121–130. [DOI] [PubMed] [Google Scholar]
- Selber, J. , Soto‐Miranda, M. , Liu, J. , & Robb, G. (2012). The survival curve: Factors impacting the outcome of free flap take‐backs. Plastic and Reconstructive Surgery, 130, 105–113. [DOI] [PubMed] [Google Scholar]
- Shen, A. , Lonie, S. , Lim, K. , Farthing, H. , Hunter‐Smith, D. , & Rozen, W. (2021). Free flap monitoring, salvage, and failure timing: A systematic review. Journal of Reconstructive Microsurgery, 37(3), 300–308. [DOI] [PubMed] [Google Scholar]
- Siemionow, M. , & Arslan, E. (2004). Ischemia/reperfusion injury: A review in relation to free tissue transfers. Microsurgery, 24, 468–475. [DOI] [PubMed] [Google Scholar]
- Smit, J. , Acosta, R. , Zeebregts, C. , Liss, A. , Anniko, M. , & Hartman, E. (2007). Early reintervention of compromised free flaps improves success rate. Microsurgery, 27(7), 612–616. [DOI] [PubMed] [Google Scholar]
- Smit, J. , Werker, P. , Liss, A. , Enajat, M. , de Bock, G. , Audolfsson, T. , & Acosta, R. (2010). Introduction of the implantable doppler system did not lead to an increased salvage rate of compromised flaps: A multivariate analysis. Plastic and Reconstructive Surgery, 125, 1710–1717. [DOI] [PubMed] [Google Scholar]
- Soteropulos, C. , Chen, J. , Poore, S. , & Garland, C. (2019). Postoperative management of lower extremity free tissue transfer: A systematic review. Journal of Reconstructive Microsurgery, 35, 1–7. [DOI] [PubMed] [Google Scholar]
- Sweeny, L. , Curry, J. , Crawley, M. , Cave, T. , Stewart, M. , Luginbuhl, A. , Heffelfinger, R. , Krein, H. , Petrisor, D. , Bender‐Heine, A. , & Wax, M. (2020). Factors impacting successful salvage of the failing free flap. Head & Neck, 42, 3568–3579. [DOI] [PubMed] [Google Scholar]
- Tall, J. , Bjorklund, T. , Skogh, A. C. , Arnander, C. , & Halle, M. (2015). Vascular complications after radiotherapy in head and neck free flap reconstruction. Annals of Plastic Surgery, 75, 309–315. [DOI] [PubMed] [Google Scholar]
- Wang, K. C. , Tsai, C. C. , Chang, C. H. , Tseng, W. L. , Hung, K. S. , Chang, T. Y. , Chen, S. H. , & Lee, Y. C. (2019). Comparison of flap outcomes between single‐ and multiple‐perforator‐based free anterolateral thigh flap in head and neck reconstruction. Microsurgery, 39, 150–155. [DOI] [PubMed] [Google Scholar]
- Wang, T. , Serletti, J. , Cuker, A. , McGrath, J. , Low, D. , Kovach, S. , & Wu, L. (2012). Free tissue transfer in the hypercoagulable patient: A review of 58 flaps. Plastic and Reconstructive Surgery, 129, 443–453. [DOI] [PubMed] [Google Scholar]
- Wei, F. C. , & Mardini, S. (2009). Flaps and reconstructive surgery. Saunders/Elsevier. [Google Scholar]
- Winterton, R. , Pinder, R. , Morritt, A. , Knight, S. , Batchelor, A. , Liddington, M. , & Kay, S. (2010). Long term study into surgical re‐exploration of the ‘free flap in difficulty’. Journal of Plastic, Reconstructive & Aesthetic Surgery, 63, 1080–1086. [DOI] [PubMed] [Google Scholar]
- Xiong, L. , Gazyakan, E. , Kremer, T. , Hernekamp, F. , Harhaus, L. , Saint‐Cyr, M. , Kneser, U. , & Hirche, C. (2016). Free flaps for reconstruction of soft tissue defects in lower extremity: A meta‐analysis on microsurgical outcome and safety. Microsurgery, 36, 511–524. [DOI] [PubMed] [Google Scholar]
- Yang, Q. , Ren, Z. , Chickooree, D. , Wu, H. , Tan, H. , Wang, K. , He, Z. , Gong, C. , Ram, V. , & Zhang, S. (2014). The effect of early detection of anterolateral thigh free flap crisis on the salvage success rate, based on 10 years of experience and 1072 flaps. International Journal of Oral and Maxillofacial Surgery, 43, 1059–1063. [DOI] [PubMed] [Google Scholar]
- Yii, N. , Evans, G. , Miller, M. , Reece, G. , Langstein, H. , Chang, D. , Kroll, S. , Wang, B. , & Robb, G. (2001). Thrombolytic therapy: What is its role in free flap salvage? Annals of Plastic Surgery, 46, 601–604. [DOI] [PubMed] [Google Scholar]
- Zhao, R. , Shammas, R. , Broadwater, G. , Le, E. , Hansen‐Estruch, C. , Kaakati, R. , Cason, R. , Lyes, M. , Orr, J. , & Hollenbeck, S. (2020). Assessing the influence of attending surgeon continuity on free flap outcomes following unplanned returns to the operating room. Journal of Reconstructive Microsurgery, 36, 583–591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


