Abstract
Background
Ultraviolet B (UVB) affects diverse pathways in skin cells, resulting in skin photoaging. Skin fibroblasts internalize and degrade elastin and collagen, playing prominent roles in photoaging. Green light is used in many fields of dermatology, but few studies have examined its role in photoaging. The present work aimed to assess low‐energy green light for its effects in a previously proposed cell model of photoaging and to explore the possible anti‐photoaging mechanism.
Methods
The stress‐induced premature senescence (SIPS) model was constructed via repeated treatment of MDFs with UVB. Senescence‐like phenotypes were compared among normal, low‐energy green light pretreatment and UVB groups, for example, cell morphological properties, senescence‐associated β‐galactosidase (SA‐β‐gal) amounts, extracellular matrix (ECM) biosynthesis and degradation, and autophagy.
Results
In comparison with the UVB group, the green light pretreatment group showed significantly decreased number of senescent mast cells and markedly declined signal intensity and amounts of SA‐β‐gal‐positive cells. Furthermore, green light pretreatment directly affected ECM by increasing type I and type III collagen production and decreasing MMP‐1 amounts. Moreover, changes in autophagy levels induced by green light pretreatment provided a potential mechanism underlying its anti‐aging property.
Conclusions
Low‐energy green light pretreatment improves senescence‐like phenotypes in vitro, indicating a possible application for anti‐aging in clinic after future research has uncovered the potential mechanism.
Keywords: autophagy, green light, photoaging, UVB
1. INTRODUCTION
Ultraviolet light, especially UVB and UVC, represents the top cause of extrinsic aging, also referred to as photoaging, 1 which is characterized by rhytids, decreased skin elasticity, lentigines, and mottled pigmentation. 2 Autophagic death represents an important and dynamic cellular event in which unwanted and impaired organelles/proteins are degraded. 3 Dermal fibroblasts biosynthesize collagen and perform extracellular matrix (ECM) remodeling. 4 The latter cells have critical functions in photoaging and anti‐aging, particularly in case of skin exposure to cumulative damage by ultraviolet exposure. The major aspects of photoaging comprise decreased ability to secrete collagen and increased matrix metalloproteinase (MMP) amounts resulting from ultraviolet exposure in fibroblasts. Therefore, many targets of photoaging therapy are hypothesized to play a role by acting on dermal fibroblasts, including intense pulsed light. 5 Although the specific mechanism of action is unclear, the change of cellular autophagy may be one of the mechanisms by which light plays its biological role. 6
Autophagy is an important biological process to maintain cell homeostasis. 7 Studies have shown autophagy contributes to UV‐induced skin light injury. 8 Autophagy induction protects cells against UV‐associated apoptosis and promotes the purge of oxidized phospholipids and protein aggregates. 9 Autophagy impairment was recently reported in chronologically aged skin fibroblasts, deteriorating skin integrity, and inducing skin fragility. 10
Green light is a visible light wave with a wavelength of 532 nm, whose effect on cell photoaging has not been reported. The current work aimed to examine the effect of green light treatment on photoaging in vitro and to explore the underpinning mechanism. A reproducible photodamage model of mouse dermal fibroblasts (MDFs) was recently established by our team via multiple UVB exposures, demonstrating diverse senescence‐like phenotypes such as flattened morphology, upregulation of MMPs, enhanced ECM degradation, and increased SA‐β‐gal staining signals. 11
2. MATERIALS AND METHODS
2.1. Cells and treatments
The Committee on the Ethics of Animal Experiments of Tongji University reviewed the experimental protocols, and the experiments were carried out in accordance with the Animal Experiment Guidelines of Tongji University. MDFs were obtained from neonate C57BL/6 mice following a recently described protocol. 12 Cell culture was carried out in DMEM containing 10% fetal bovine serum (FBS), glutamine, penicillin, and streptomycin at 37°C in a humid environment with 5% CO2. Subculture was performed at 80% confluence, and passage 1–2 cells were utilized in various arrays.
At 12 h post seeding, the medium in the green light group was changed with phosphate buffered saline (PBS). Cells underwent exposure to four doses of green light of 532 nm at 1.93 mJ/cm2 (Shanghai Intelligent Equipment Co, China) for 60 s before treatment with another four doses of UVB at 120 mJ/cm2 (Narrowband TL 20 W/01 RS lamp, Philips, The Netherlands) for 120 s as described in a previous study. 11 Immediately following irradiation, DMEM containing 1% FBS was added to replace PBS in the treatment group. The control group was handled similarly but with no UVB and green light exposure. Post‐exposure, cells were further incubated in DMEM containing 10% FBS for recovery.
2.2. Morphological analysis
At 24 h following the final UVB exposure, cells underwent seeding in 35‐mm plates and a 48‐h culture in DMEM containing 10% FBS. Then, fixation (4% paraformaldehyde) and permeabilization (0.3% Triton X‐100) were carried out for 15 min at ambient, before incubation with FITC‐Phalloidin (Sigma, USA) for 30 min. Finally, 4, 6‐diamidino‐2‐phenylindole (DAPI) counterstaining was performed, and data analysis utilized an Olympus IX70‐S1F2 fluorescence microscope (Olympus, Japan) at 200×.
2.3. SA‐β‐Gal detection
SA‐β‐Gal staining followed a previous protocol. 13 Briefly, following the final UVB irradiation, cells of each group were further cultured for 48 h in complete medium. Upon fixation, staining was carried out with a kit provided by CST (#9860, USA) as directed by the manufacturer. SA‐β‐Gal‐positive cells were counted for totally 400 cells/dish with Image‐Pro Plus (IPP) 6.0 (Media Cybernetics, USA). Data were provided as a percent of all cells counted.
2.4. Protein quantification and Western blotting
Cell collection was carried out 48 h following the final UVB irradiation to quantify soluble proteins. The number of cells was obtained for normalization, and cell lysates were prepared. Total protein was quantitated with the BCA kit (Pierce, USA).
Immunoblot was carried out as recently described. 14 Briefly, 12% SDS‐PAGE separation was followed by transfer onto PVDF membranes and incubation with primary antibodies targeting collagen I, collagen III, MMP‐1, and Beclin 1 (Abcam, UK). Detection was performed with horseradish peroxidase (HRP)‐linked secondary antibodies. Enhanced chemiluminescence was used for development, with a kit from Pierce (USA).
2.5. Transfection of GFP‐RFP‐LC3
Totally 48 h following the final UVB exposure, cells were transferred into 35‐mm plates for another 24‐h culture. Next, transfection was carried out with GFP‐RFP‐LC3 (Hanbio, China) at a multiplicity of infection (MOI) of 100 for 24 h. For assessment, GFP‐LC3 puncta in each cell were counted by microscopy. Thirty random cells were analysed, and data were provided as mean quantity of puncta per cell.
2.6. Statistical analysis
SPSS13.0 (SPSS, USA) was utilized for data analysis by paired Student's t‐test and one‐way ANOVA. Data are mean ± SD, and p < 0.05 indicated statistical significance.
3. RESULTS
3.1. Pretreatment with 532 nm green light improves the morphology of photoaging cells
Enlargement and flattening (senescence‐like morphology) were detected in UVB‐irradiated cells (Figure 1A) under an optical microscope. This result was more intuitive and straightforward after phalloidin staining (Figure 1B). Cells pretreated with 532 nm green light showed reduced lengths and widths compared with the UVB model group. Soluble protein content was assessed in each group per cell for quantifying the degree of cell hypertrophy. Protein amounts per cell were higher in the UVB group in comparison with control cells, while green light led‐pretreated cells markedly declined soluble protein content in each cell (Figure 1C).
FIGURE 1.
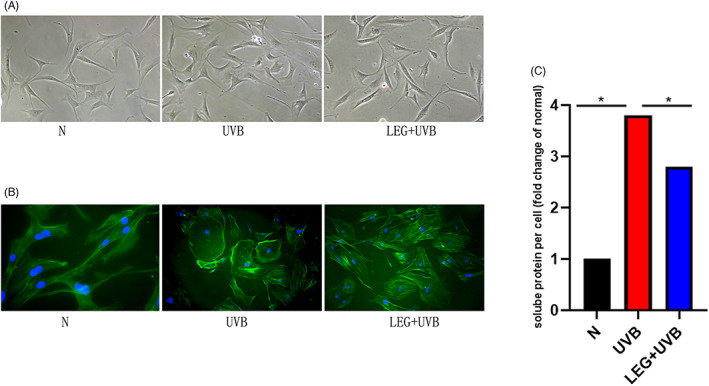
Effects of 532 nm green light on morphology in normal and photoaging MDFs. (A) The morphologies of control and photoaging cells incubated with or without green light are shown in Figure 1A. (B) Totally 72 h following the final irradiation, cell fixation (4% paraformaldehyde) was caried out with subsequent F‐actin staining with FITC phalloidin. The UVB group showed more diversified morphotypes. Cells pretreated with green light had reduced senescence features, with decreased amounts of flattened cells and some spindle shape preservation. Original magnification ×200. (C) At 72 h, cell trypsinization and counting were performed. Immunoblot was performed, and protein amounts were assessed per cell. Data are fold change versus control cells. Data are mean ± SD from triplicate assays (*p < 0.05)
3.2. Green light pretreatment reduces SA‐β‐Gal amounts
SA‐β‐Gal staining was carried out 72 h following the final exposure to UVB to examine if green light pretreatment prevents MDF senescence. The results demonstrated intense SA‐β‐Gal signals in UVB‐induced MDFs, which were reduced in green light‐pretreated cells (Figure 2A). Furthermore, the amounts of positive cells were effectively reduced by green light pretreatment (Figure 2B).
FIGURE 2.
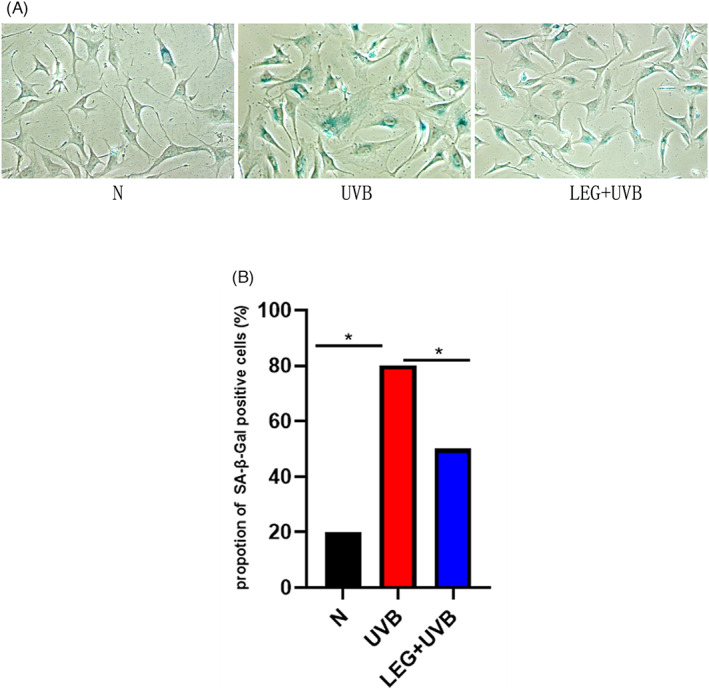
Senescence‐associated β‐galactosidase (SA‐β‐gal) amounts in MDFs. (A) Relatively higher number of SA‐β‐gal positive cells were found in the UVB group. The ratio of SA‐β‐gal positive cells was markedly reduced after green light pretreatment compared with the UVB group. SA‐β‐gal positive cells were rarely found in the normal group. (B) The proportions of SA‐β‐gal‐positive cells were based on 400 cells/specimen. Results are mean ± SD from three assays performed independently (*p < 0.05)
3.3. Green light pretreatment counteracts UVB‐induced alteration of cell secretory functions in MDFs
Reduced type I and III collagen amounts and MMP‐1 upregulation are characteristic changes occurring in photoaging. Therefore, the possible effects of green light intervention on collagen and MMP‐1 amounts were examined. Totally, 48 h following the final exposure, markedly increased MMP‐1 release and starkly reduced type I and III collagen protein amounts were found in the UVB group. Pre‐incubation with 532 nm green light resulted in decreased release of MMP‐1 and partially reduced UVB‐associated downregulation of type I and III collagen proteins (Figure 3).
FIGURE 3.

Effect of green light pretreatment on UVB‐triggered dermal matrix degradation in MDFs. Immunoblot analysis of MMP‐1, collagen I and collagen III at 72 h following the final UVB exposure. β‐actin expression was utilized for normalization. Data are mean ± SD from triplicate assays (*p < 0.05)
3.4. Green light pretreatment stimulates autophagy in photoaging MDFs
Following UVB exposure, both autophagosome and autolysosome (yellow and red puncta, respectively), amounts were reduced in photoaged cells, and these effects were alleviated by pre‐incubation with 532 nm green light (Figure 4A,B). Immunoblot revealed UVB downregulated the autophagy‐associated protein Beclin‐1, suggesting autophagy suppression in this photodamage model. Meanwhile, pre‐incubation with green light (532 nm) increased Beclin‐1 expression (Figure 4C). Therefore, pretreatment with green light markedly decreased the suppressive effects of UVB on autophagy.
FIGURE 4.
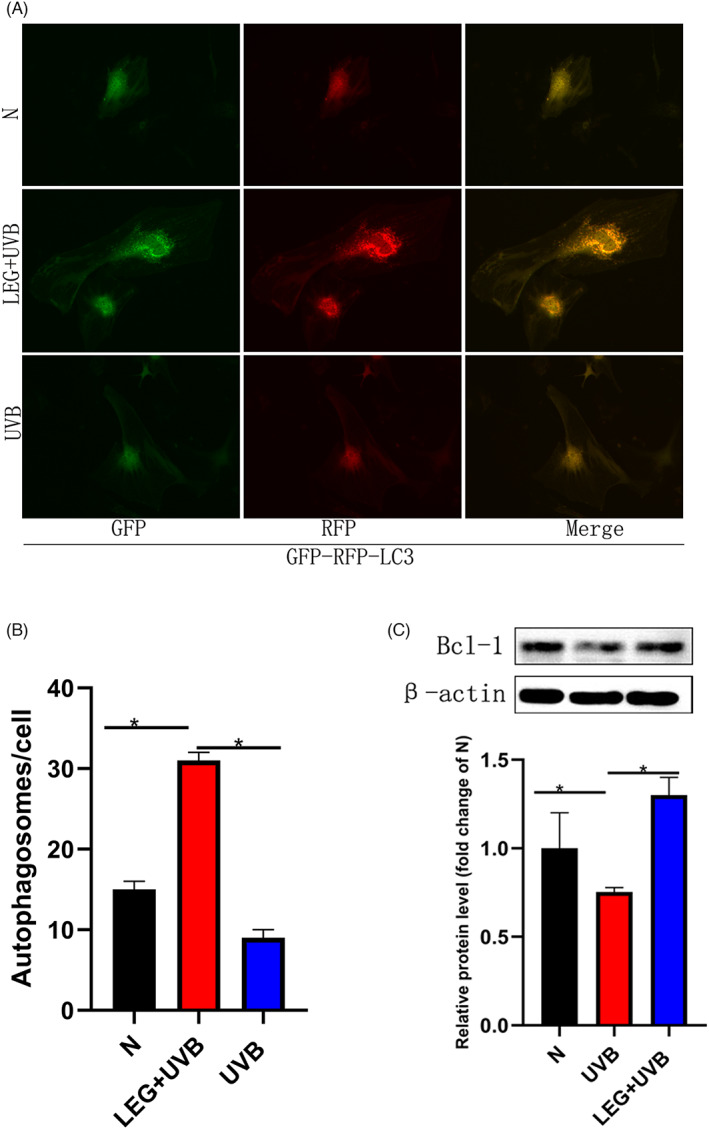
Green light pretreatment stimulates autophagy in UVB‐Induced MDFs. (A) Representative micrographs depicting LC3 signals after MDF infection with the GFP‐RFP‐LC3 adenovirus (24 h). Yellow and red puncta represent autophagosomes and autolysosomes, respectively. (B) The number of autophagosomes per cell in each group is shown. (C) Immunoblot demonstrated pre‐incubation with green light triggered autophagy, with Beclin‐1 upregulation. Each bar represents the mean ± SD of three samples (*p < 0.05)
4. DISCUSSION
Photoaging represents a skin degenerative disorder different from endogenous aging, regulated by genes. It is mostly caused by solar ultraviolet (UV) that induces MMPs and reduces collagen expression in skin fibroblasts, which have a critical function in ECM remodeling. 2 In addition, most physical blockers and sunscreens are widely applied to prevent UV penetration of skin cells, reducing its deleterious dermatological impact. 15 The low‐energy green laser is broadly utilized clinically, for example, for treating erythematous skin lesions, 16 tattoo pigment remonval,17 and managing retinopathy of prematurity. 18 Nevertheless, few studies have examined the effect of green light on photoaging.
This study found that pretreatment with 532 nm green light could counteract some UVB‐related senescence characteristics in MDFs. UVB‐exposed MDFs pretreated with low energy green light retained an elongated cell shape with markedly reduced signal intensity and rate of SA‐β‐Gal‐expressing cells. The aging skin commonly shows altered pigmentation, sallowness, and serious wrinkling. 19 The potential change increases collagen degradation by MMPs. 20 , 21 Multiple reports indicate the skin amounts of several MMPs in humans or mice, including MMP‐1, MMP‐2, MMP‐3, and MMP‐9, are increased upon UV irradiation. 22 , 23 The present study detected MMP‐1 (collagenase), collagen‐1, and collagen‐3. We here demonstrated that low‐energy green light (532 nm) suppressed collagen‐1 and collagen‐3 breakdown by UVB via MMP‐1 downregulation.
Next, the potential mechanism behind the anti‐photoaging effects of green light in MDFs after UVB irradiation was explored. Studies have shown autophagy contributes to UV‐induced skin light injury. 24 , 25
Autophagy is an essential biological process that maintains cell homeostasis by clearing damaged proteins. 7 It contributes to multiple pathologies such as neurodegenerative disorders, malignancies, infectious diseases, and particularly aging. 26 Other scholars have reported a complex role for autophagy in response to UV‐triggered oxidative stress injury, removing oxidized protein and lipid molecules, and reducing antioxidation in diverse cell types. 27 , 28 Based on the above findings, we explored whether low‐energy green light improves the basic autophagy level of cells and then showed its ability to resist cell photoaging. This work revealed starkly decreased cell autophagy after photoaging. However, pretreatment with low‐energy green light could increase the basic autophagy level of MDFs, which may alleviate the initial mitochondrial damage, protein aggregation, and ROS accumulation. Instead of dysregulating autophagy, which may result in exacerbated autophagic cell death as well as fibroblast dysfunction, 29 low‐energy green light seems to enhance the ability of cells to respond to UV light damage by enhancing their basic autophagy level. However, this study only verified the changes in the number of autophagosomes and the content of Bcl‐2 protein in each group. A more comprehensive exploration of the relevant indicators of autophagy to explain the mechanism of green light will be a further research direction, which is also the deficiency of this study.
Overall, this study showed pretreatment with green light ameliorates senescence‐like phenotypes such as flattened morphology and SA‐β‐gal biosynthesis. Besides, ECM degradation was prevented by low‐energy green light via MMP‐1 downregulation and collagen upregulation. Few studies have examined the anti‐aging property of green light in vitro, providing insights into its potential clinical use for rejuvenation. Naturally, further investigation is warranted to further examine the molecular pathways involved in this anti‐aging effect.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ETHICAL APPROVAL
The Committee on the Ethics of Animal Experiments of Tongji University reviewed the experimental protocols, and the experiments were carried out in accordance with the Animal Experiment Guidelines of Tongji University.
ACKNOWLEDGMENTS
This work was supported by grants from National Natural Science Foundation of China (No. 81773347).
Jia C, Gong C, Lu Y, Xu N. Low‐energy green light alleviates senescence‐like phenotypes in a cell model of photoaging. J Cosmet Dermatol. 2023;22:505‐511. doi: 10.1111/jocd.15175
Chuanlong Jia, Chengchen Gong and Yongzhou Lu equally contributed to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in [repository name] at [DOI].
REFERENCES
- 1. Wilson SR, Madronich S, Longstreth JD, Solomon KR. Interactive effects of changing stratospheric ozone and climate on tropospheric composition and air quality, and the consequences for human and ecosystem health. Photoch Photobio Sci. 2019;18(3):775‐803. [DOI] [PubMed] [Google Scholar]
- 2. Pka KG, Akubik AP, Kowska RM, Socha K. The impact of ultraviolet radiation on skin photoaging review of in vitro studies. J Cosmet Dermatol. 2021;20(11):3427‐3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vernon PJ, Tang D. Eat‐me: autophagy, phagocytosis, and reactive oxygen species signaling. Antioxid Redox Sign. 2013;18(6):677‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heo H, Lee H, Yang J, Sung J, Lee J. Protective activity and underlying mechanism of ginseng seeds against UVB‐induced damage in human fibroblasts. Antioxidants. 2021;10(3):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adel MM, Mahmoud E, Hussein EM. Evaluation of the efficacy of intense pulsed light in skin rejuvenation. QJM‐Int J Med. 2021;114(Supplement_1):1. [Google Scholar]
- 6. Bergamaschi D. Autophagy protects from photoageing in skin fibroblasts. Br J Dermatol. 2021;186(2):211‐212. [DOI] [PubMed] [Google Scholar]
- 7. Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. Embo J. 2021;40(19):e108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen LH, Chu PM, Lee YJ, et al. Targeting protective autophagy exacerbates UV‐triggered apoptotic cell death. IJMS. 2012;13:1209‐1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao Y, Zhang CF, Rossiter H, Eckhart L, Gruber F. Autophagy is induced by UVA and promotes removal of oxidized phospholipids and protein aggregates in epidermal keratinocytes. J Invest Dermatol. 2013;133(6):1629‐1637. [DOI] [PubMed] [Google Scholar]
- 10. Tashiro K, Shishido M, Fujimoto K, et al. Age‐related disruption of autophagy in dermal fibroblasts modulates extracellular matrix components. Biochem Biophys Res Commun. 2014;443(1):167‐172. [DOI] [PubMed] [Google Scholar]
- 11. Zeng JP, Bi B, Chen L, et al. Repeated exposure of mouse dermal fibroblasts at a sub‐cytotoxic dose of UVB leads to premature senescence: a robust model of cellular photoaging. J Dermatol Sci. 2014;73(1):49‐56. [DOI] [PubMed] [Google Scholar]
- 12. Fujisawa H, Nishikawa T, Zhu BH, Takeda N, Hosokawa M. Accelerated aging of dermal fibroblast‐like cells from the Senescence‐Accelerated Mouse (SAM): acceleration of changes in DNA ploidy associated with in vitro cellular aging. J Gerontol Biol Sci Med Sci. 1998;53(1):B11‐B17. [DOI] [PubMed] [Google Scholar]
- 13. Dimri GP, Lee XH, Basile G, et al. A biomarker that identifies senescent human‐cells in culture and in aging skin in‐vivo. Proc Natl Acad Sci USA. 1995;92(20):9363‐9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qin D, Ren R, Jia C, et al. Rapamycin protects skin fibroblasts from ultraviolet B‐induced photoaging by suppressing the production of reactive oxygen species. Cell Physiol Biochem. 2018;46(5):1849‐1860. [DOI] [PubMed] [Google Scholar]
- 15. Kostrzewska P, Mandera A, Pawlikowska A, Szuster E. Sunscreens as a prevention of the photoaging. J Educ Health Sport. 2020;10(8):11‐16. [Google Scholar]
- 16. Nam CH, Kim MH, Hong SP, Park BC. Fractional 532‐nm KTP diode laser and 595‐nm pulsed dye laser in treatment of facial telangiectatic erythema. J Cosmet Dermatol. 2019;18(3):783‐787. [DOI] [PubMed] [Google Scholar]
- 17. Kurniadi I, Tabri F, Madjid A, Anwar AI, Widita W. Laser tattoo removal: fundamental principles and practical approach. Dermatol Ther. 2021;34(1):e14418. [DOI] [PubMed] [Google Scholar]
- 18. Singh SR, Katoch D, Handa S, et al. Safety and efficacy of 532 nm frequency‐doubled Nd‐YAG green laser photocoagulation for treatment of retinopathy of prematurity. Indian J Ophthalmol. 2019;67(6):860‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Csekes E, Rackova L. Skin aging, cellular senescence and natural polyphenols. Int J Mol Sci. 2021;22(23):12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen X, Yang C, Jiang G. Research progress on skin photoaging and oxidative stress. Postepy Dermatol Alergol. 2021;38(6):931‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poon F, Kang S, Chien AL. Mechanisms and treatments of photoaging. Photodermatol Photoimmunol Photomed. 2015;31(2):65‐74. [DOI] [PubMed] [Google Scholar]
- 22. Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix‐degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14(1):20‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17(6):868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cadet J, Douki T. Formation of UV‐induced DNA damage contributing to skin cancer development. Photochem Photobiol Sci. 2018;17:1816‐1841. [DOI] [PubMed] [Google Scholar]
- 25. Cadet J, Grand A, Douki T. Solar UV radiation‐induced DNA bipyrimidine photoproducts: formation and mechanistic insights. Topics Curr Chem. 2014;356:249‐275. [DOI] [PubMed] [Google Scholar]
- 26. Wang M, Lei M, Chang L, Xing Y, Zhong JL. Bach2 regulates autophagy to modulate UVA‐induced photoaging in skin fibroblasts. Free Radical Bio Med. 2021;169(22):304‐316. [DOI] [PubMed] [Google Scholar]
- 27. Hammouda MB, Ford AE, Liu Y, Zhang JY. The JNK signaling pathway in inflammatory skin disorders and cancer. Cells. 2020;9(4):857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao G, Gong L, Su D, et al. Cullin5 deficiency promotes small‐cell lung cancer metastasis by stabilizing integrin β1. J Clin Invest. 2019;129(3):972‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Condello M, Pellegrini E, Caraglia M, Meschini S. Targeting autophagy to overcome human diseases. Int J Mol Sci. 2019;20(3):725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in [repository name] at [DOI].


