Intracranial mesenchymal tumor, FET::CREB fusion-positive, is a recently recognized provisional entity in the 2021 WHO Classification of Tumors of the Central Nervous System.1 These are mesenchymal, nonmeningothelial tumors, with a wide morphologic spectrum.2–4 Mitotic activity is usually low (<5 mitoses/mm2). Architectural patterns vary from syncytial or sheet-like to reticular cord-like structures. The stroma can be collagenous or myxoid. A fusion between a FET RNA-binding protein family gene (EWSR1 and FUS) and a member of the CREB family of transcription factors (ATF1, CREB, and CREM) is diagnostic.1 Specifically, EWSR1::CREB fusions have been shown in angiomatoid fibrous histiocytoma and clear cell sarcoma.5,6 Prior nosological designations “intracranial myxoid variant of angiomatoid fibrous histiocytoma,” “intracranial myxoid mesenchymal tumor with EWSR1::CREB family gene fusions” are no longer recommended for this entity.7,8 We report a case of intracranial mesenchymal tumor, FET::CREB fusion-positive, in lateral ventricle of an adult patient.
Case
A 37-year-old woman presented to medical attention during a regular follow-up appointment with her ophthalmologist, who noted papilledema and signs of increased intracranial pressure. She reported strong headaches that she attributed to clomiphene and letrozole which she was taking for infertility, as well as right facial spasms of one-month duration. She was referred to an emergency room. Brain MR imaging demonstrated a lobulated heterogeneously enhancing mass with central hypoenhancement, without restricted diffusion, arising in the atrium of the left lateral ventricle. There was associated surrounding vasogenic edema involving the posterior frontal, parietal, temporal, and occipital lobes accompanied by a 3-mm left-to-right midline shift (Figure 1). Based on appearance, location, female gender, and age of nearly 40-years-old, the mass was considered most typical for an intraventricular meningioma. Other considerations such as ependymoma, choroid plexus papilloma, central neurocytoma, and metastasis were thought to be less likely given patient demographics, tumor location, and imaging characteristics.9,10 She was initiated on dexamethasone corticosteroids, with improvement of headaches. During the neurosurgery consultation, she experienced lightheadedness upon standing up, occasional right facial spasms, and occasional headaches. The neurologic exam was unremarkable except for face spasms. The patient underwent left parietal craniotomy with gross total tumor resection.
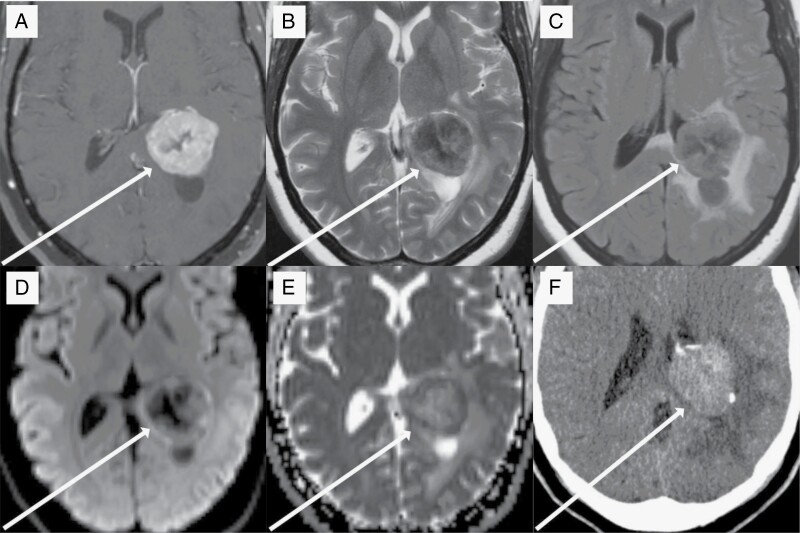
Figure 1.
37-year-old woman with intraventricular intracranial mesenchymal tumor. T1-postcontrast imaging (a) reveals a strongly enhancing lobulated mass within the atrium of the left lateral ventricle. There is a central region of nonenhancing tumor that is more hypointense on T2-weighted imaging (b) compared to the periphery of the tumor. T2-FLAIR imaging (c) reveals moderate surrounding vasogenic edema within the surrounding brain parenchyma. Diffusion-weighted imaging (d) shows hyperintensity in the periphery of the tumor, which, along with the hypointensity seen on apparent diffusion coefficient imaging (e), represents decreased diffusivity and is suggestive of a high cellularity. A noncontrast CT study (f) shows that the tumor is hyperdense compared to normal brain parenchyma and contains course areas of calcification.
Intraoperatively, the tumor was well delineated and lightly attached to the walls of the lateral ventricle with arterial feeding vessels originating from the choroid plexus. The consistency of the tumor was firm and rubbery with calcifications. Its overall gross appearance was consistent with meningioma as suspected from MRI and location.
Hematoxylin and Eosin (H&E)-stained tissue sections show a tumor with good demarcation from the adjacent brain tissue (Figure 2). Tumor cells do not show classical meningothelial features such as whorls and psammoma bodies. Instead, the tumor consisted of sheets and cords of epithelioid and clear cells (Figure 2). Hyalinization and calcifications were present. Necrosis was not identified. Mitotic figures were manually quantified using an M-phase-specific anti-phosphohistone H3 (pHH3) antibody, with a maximum of 5 mitoses per 10 high-power-fields (HPF; 1 HPF = 0.23 mm2). Computer-assisted automated quantitation yielded an elevated Ki67 antigen labeling index of 11.7% (Figure 2). The tumor cells were positive for EMA and CD99. Desmin and cytokeratin were negative. There was no nuclear expression of STAT6, and progesterone receptor (PR) was focally positive. A diagnosis of atypical meningioma, WHO grade 2, was initially rendered under the 2016 WHO classification of CNS tumors. Postoperative imaging showed that a radiographically complete resection had been achieved. The patient was dispositioned to imaging surveillance.
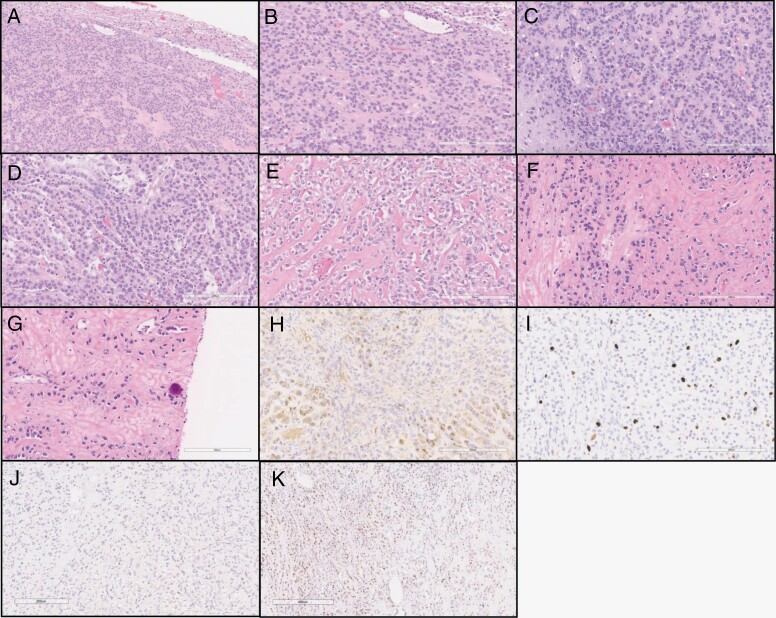
Figure 2.
Histologic features of the tumor. (a) Tumor with good demarcation from the adjacent brain tissue. (b–c) Tumor cells arranged in trabecular/sheet-like pattern. (d) Epithelioid tumor cells organized in cords. (e) Epithelioid, almost clear cell morphology of tumor cells infiltrating fibrous stroma. (f) Tumor cells are dispersed as single-file-like cords in the dense stroma. (g) Fibrous stroma and infiltrating tumor cells in single cell appearance resembling carcinoma and focal calcification. (h) EMA expression in tumor cells. (i) Ki-67 index, quantitated as 11.7% by automated analysis. (j) No nuclear staining detected with STAT6. (k) Focal progesterone receptor (PR) expression was noted.
Approximately 3 years after the initial surgery, follow-up MR imaging demonstrated a 2.1 cm enhancing mass in the atrium of the left lateral ventricle with transependymal flow of CSF, and left temporal horn entrapment, rightward midline shift, and left uncal herniation. The patient endorsed mild headache, confusion, and blurry vision. She underwent a second left parietal craniotomy and gross total resection of tumor. The histology of the tumor was similar to that of the original resection. She was dispositioned to imaging surveillance. Subsequently, next generation sequencing (NGS) was performed with an assay interrogating 610 cancer-associated genes for single nucleotide variants (SNV) and insertion/deletions, copy number variants in 583 genes and rearrangements in 34 genes. An EWSR1::CREB3L3 fusion, LRP1B p.V4264L mutation, and amplification of BRD4, CHEK2, FH, and NOTCH3 were detected (Table 1). The in-frame fusion involved intron 8 of EWSR1 and intron 3 of CREB3L3 (transcript IDs NM_013986.4 and NM_032607.3). The histologic features and molecular alterations of the tumor support a diagnosis of intracranial mesenchymal tumor, FET::CREB fusion-positive under the 2021 WHO classification on CNS tumors.
Table 1.
Gene Alterations Detected by Next Generation Sequencing
| Gene | Alteration | Details |
|---|---|---|
| EWSR1::CREB3L3 | Fusion | EWSR1(exon 8)::CREB3L3(exon 4) |
| LRP1B | SNV-Missense | c.12790G > C p.V4264L |
| BRD4 | Amplification | Cytoband 19p13.12 |
| CHEK2 | Amplification | Cytoband 22q12.1 |
| FH | Amplification | Cytoband 1q43 |
| NOTCH3 | Amplification | Cytoband 19p13.12 |
MR imaging 4 months after the second surgery demonstrated a new nodular focus of enhancement within the body of the left lateral ventricle concerning recurrent tumor. She was dispositioned to Gamma knife stereotactic radiosurgery (15 Gy). Subsequently, MRI demonstrated enhancement around the left temporal surgical cavity, and she was dispositioned to fractionated proton radiation (54 Gy in 30 fractions). Surveillance MRI revealed progressive nodular enhancement at the left cerebellopontine angle, with a new focus at the right internal auditory canal, concerning tumor deposit. There was also progressive nodular enhancement in the right inferior cerebellum with an exophytic component. Given concern for disseminated disease (LM), lumbar puncture, MRI of the spine, and PET/CT imaging were recommended to evaluate further.
MR spine imaging demonstrated a 5 mm enhancing focus at the T1 level dorsal within the thecal sac, concerning drop metastasis. F-18 fluorodeoxyglucose (FDG) PET/CT imaging showed focal tracer uptake in the right paramedian inferior cerebellum that could be related to recurrent tumor and did not suggest extra-CNS involvement. Lumbar puncture was negative for tumor cells. The patient was dispositioned to craniospinal irradiation (CSI) with protons and carveout of the previously irradiated left parietal region. She completed proton CSI therapy and received 4 cycles of octreotide/everolimus. The patient developed sensorineural hearing loss and received a cochlear implant. The most recent MRI showed stable findings in the brain, and a slowly progressive intradural spinal lesion at the T1 level; continuation of systemic therapy was recommended.
Discussion
The constellation of clinicopathologic features seen in this case, including anatomic location, imaging data, morphologic features, immunophenotype, and molecular signature alteration is consistent with the newly recognized entity intracranial mesenchymal tumor, FET::CREB fusion-positive. These tumors show fusion between a FET RNA-binding protein family gene (primarily EWSR1) and a CREB transcription factors family member (CREM, ATF1, CREB).8 The tumors are commonly extra-axial and supratentorial, intraventricular, or attached to dura or meninges.2 The intracranial myxoid mesenchymal tumor with EWSR1 fusion with CREB family transcription factors nomenclature was first introduced by Kao et al., who reported 4 intracranial tumors with a characteristic fusion, ages 12–23.4 The controversy at the time was whether this was a new entity, sui generis, or an intracranial variant of myxoid angiomatoid fibrous histiocytoma (AFH). AFH had not been previously described in the central nervous system. Bale et al. reported 3 pediatric intracranial cases, ages 12–18, all of whom had EWSR1::CREM fusion.8 The tumors in these two series mostly showed myxoid and/or fibro-myxoid background stroma, with a variety of tumor architectural patterns, including cords and sheets, and diverse cell morphology, including epithelioid, rhabdoid, and spindle cell. Additional cases of intracranial myxoid mesenchymal tumors with EWSR1::CREB family fusions have been reported in the pediatric and adult population.3,7,8
Specifically, EWSR1::CREB3L3 fusion has been reported in 3 cases. First, Dewaele et al. reported 18-year-old male with intraabdominal mesenteric sclerosing epithelioid fibrosarcoma.11 They isolated total RNA from frozen tumor tissue samples that previously stored at −80°C and performed RT-PCR and Sanger sequencing.11 Subsequently, in-frame fusion between exon 14 of EWSR1 (nucleotide 1759, accession number NM_013986.3) and the last nucleotide of exon 3 of CREB3L3 (nucleotide 604, accession number NM_032607) was detected.11 Dong et al. reported 40- year-old female with a pelvic mass.12 Along with histologic and immunohistochemical studies, they extracted DNA from FFPE tumor tissue and performed targeted next-generation sequencing. They identified rearrangement with breakpoints involving EWSR1 intron 8 and CREB3L3 intron 3.12 Also, an unbalanced EWSR1 rearrangement was detected by fluorescent in situ hybridization (FISH) and the tumor was diagnosed as sclerosing epithelioid fibrosarcoma.12 Shenoy et al. reported a 16-year-old female who had right mandibular mass.13 They performed FISH on frozen tumor tissue and detected EWSR1 rearrangement.13 Subsequently, using a targeted next-generation sequencing panel, they detected an EWSR1::CREB3L3 fusion, and the tumor was diagnosed as sclerosing epithelioid fibrosarcoma of the bone.13
Although the FET::CREB fusion is diagnostic of this entity, imaging, and histological features can be misleading. In the present patient, MR imaging was highly suspicious for choroid plexus (glomus choroideum) meningioma. The tumor did not show characteristic histologic features of meningioma, it was composed of epithelioid/clear cells organized in cords and sheets.
Previously reported histologic features such as myxoid stroma, internodular septae, lymphoplasmacytic cuffing, hemangioma-like, or staghorn/HPC-like vasculature were absent in the current case.2 Instead of myxoid stroma, there was a fibrous collagen-rich background with occasional regenerative changes, such as hyalinization and focal calcification. Necrosis was not observed. Due to the wide spectrum of morphology seen in the entity, histologic features can be difficult to distinguish from rhabdoid or chordoid meningioma. EMA positivity can also be misleading, biasing the observer towards a diagnosis of meningioma. In that scenario, Desmin and CD99 expression can be useful in parsing the differential diagnosis. In the present case, some of the areas showed poorly cohesive and individually dispersed tumor cells arranged in single-file or cords, resembling invasive lobular carcinoma morphology, which was excluded due to absence of cytokeratin expression. NGS analysis provided the key data, detecting a EWSR1::CREB3L3 fusion, and a diagnosis of intracranial mesenchymal tumor, FET::CREB fusion-positive, was rendered.
The type of surgical resection (en bloc vs. piecemeal) could influence the chance of CSF dissemination. Even though we have no data for this particular tumor, given its rarity, data exists for parenchymal brain metastases showing that the risk of local recurrence and of LMD are increased by a piecemeal removal.14 However, during removal of an intraventricular tumor, a piecemeal approach is often needed to do the resection with acceptable levels of safety, given the tumor’s proximity to the ventricular walls and the various nerve tracts that run adjacent to them.15 If the risk of CSF dissemination is considered high despite the extent of resection, craniospinal irradiation may be the preferred adjuvant approach. However, more clinical data is needed to guide treatment decisions in patients with this tumor type.
Sloan et al. reported the clinical outcomes for 20 patients diagnosed as intracranial myxoid mesenchymal tumor with FET::CREB fusion.2 Clinical behavior includes local recurrences, dissemination, or metastasis, with better outcomes in patients who have gross total resection. Adjuvant radiotherapy can be an option for locally recurrent and incompletely resected tumors. In our case, even though the patient had a gross total resection, the tumor recurred after 3 years. Additional investigation is needed to fully elucidate the behavior of this entity.1
Conclusion
Intracranial mesenchymal tumor, FET::CREB fusion-positive is a recently described, molecular signature-defined, WHO provisional entity that can be seen in both pediatric and adult populations. It is crucial to be aware of the full clinicopathologic spectrum, as the differential diagnosis can be challenging.
Contributor Information
Hanim I Ozkizilkaya, Department of Translational Molecular Pathology, The University of Texas MD Anderson Cancer Center, Houston, USA.
Jason M Johnson, Division of Diagnostic Imaging, Department of Neuroradiology, The University of Texas MD Anderson Cancer Center, Houston, USA.
Barbara J O’Brien, Division of Cancer Medicine, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, USA.
Ian E McCutcheon, Division of Surgery, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, USA.
Sujit S Prabhu, Division of Surgery, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, USA.
Amol J Ghia, Division of Radiation Oncology, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, USA.
Gregory N Fuller, Division of Diagnostic Imaging, Department of Neuroradiology, The University of Texas MD Anderson Cancer Center, Houston, USA; Division of Pathology and Lab Medicine, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, USA.
Jason T Huse, Department of Translational Molecular Pathology, The University of Texas MD Anderson Cancer Center, Houston, USA; Division of Pathology and Lab Medicine, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, USA.
Leomar Y Ballester, Department of Translational Molecular Pathology, The University of Texas MD Anderson Cancer Center, Houston, USA; Division of Pathology and Lab Medicine, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, USA.
Funding
This work was partly supported by the National Cancer Institute of the National Institutes of Health under Award Number K08CA241651 (LYB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Statement
The authors have no relevant financial or non-financial interests to disclose.
Authors Contributions
Manuscript preparation (HIO, LYB, GNF, JMJ, BJO, SSP, IEM, AJG, JTH), neuroradiology images (JMJ), neuro-oncology treatment information (BJB), neurosurgical aspects (IEM, SSP), radiation treatment information (AJG), neuropathology aspects (GNF, JTH, LYB). All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of University of Texas MD Anderson Cancer Center (PA17-0216).
References
- 1. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncology. 2021; 23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sloan EA, Chiang J, Villanueva-Meyer JE, et al. Intracranial mesenchymal tumor with FET-CREB fusion—a unifying diagnosis for the spectrum of intracranial myxoid mesenchymal tumors and angiomatoid fibrous histiocytoma-like neoplasms. Brain Pathol. 2021; 31(4):e12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Komatsu M, Yoshida A, Tanaka K, et al. Intracranial myxoid mesenchymal tumor with EWSR1–CREB1 gene fusion: a case report and literature review. Brain Tumor Pathol. 2020; 37(2):76–80. [DOI] [PubMed] [Google Scholar]
- 4. Kao YC, Sung YS, Zhang L, et al. EWSR1 fusions with CREB family transcription factors define a novel myxoid mesenchymal tumor with predilection for intracranial location. Am J Surg Pathol. 2017;41(4):482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonescu CR, Cin PD, Nafa K, et al. EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosom Cancer. 2007; 46(12):1051–1060. [DOI] [PubMed] [Google Scholar]
- 6. Li BX, David LL, Davis LE, Xiao X.. Protein arginine methyltransferase 5 is essential for oncogene product EWSR1-ATF1-mediated gene transcription in clear cell sarcoma. J Biol Chem. 2022;298(10):102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu C, Liu Y, Zhao Y, et al. Primary intracranial mesenchymal tumor with EWSR1-CREM gene fusion: a case report and literature review. World Neurosurg. 2020; 142:318–324. [DOI] [PubMed] [Google Scholar]
- 8. Bale TA, Oviedo A, Kozakewich H, et al. Intracranial myxoid mesenchymal tumors with EWSR1–CREB family gene fusions: myxoid variant of angiomatoid fibrous histiocytoma or novel entity? Brain Pathol. 2017; 28(2):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith AB, Smirniotopoulos JG, Horkanyne-Szakaly I.. From the radiologic pathology archives1: intraventricular neoplasms: radiologic-pathologic correlation. Radiographics. 2013; 33(1):21–43. [DOI] [PubMed] [Google Scholar]
- 10. Pereira BJA, de Almeida AN, Paiva WS, et al. Natural history of intraventricular meningiomas: systematic review. Neurosurg Rev. 2020; 43(2):513–523. [DOI] [PubMed] [Google Scholar]
- 11. Dewaele B, Libbrecht L, Levy G, et al. A novel EWS-CREB3L3 gene fusion in a mesenteric sclerosing epithelioid fibrosarcoma. Genes Chromosom Cancer. 2017; 56(9):695–699. [DOI] [PubMed] [Google Scholar]
- 12. Dong F, Quade BJ, Cin PD, Jo VY.. Expanding the spectrum of translocations in sclerosing epitheloid fibrosarcoma: a new case with EWSR1-CREB3L3 fusion. Genes Chromosom Cancer. 2018; 57(12):675–677. [DOI] [PubMed] [Google Scholar]
- 13. Shenoy A, Surrey L, Jain P, et al. Sclerosing epithelioid fibrosarcoma of the bone with rare EWSR1-CREB3L3 translocation driving upregulation of the PI3K/mTOR signaling pathway. Pediatr Dev Pathol. 2019; 22(6):594–598. [DOI] [PubMed] [Google Scholar]
- 14. Patel AJ, Suki D, Hatiboglu MA, et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis: clinical article. J Neurosurg. 2010; 113(2):181–189. [DOI] [PubMed] [Google Scholar]
- 15. Schroeder HWS. Intraventricular tumors. World Neurosurg. 2013; 79(2):S17.e15–S17.e19. [DOI] [PubMed] [Google Scholar]