Abstract
Background:
Spontaneous coronary artery dissection (SCAD) is an increasingly recognized cause of acute coronary syndrome. Guidance regarding the optimal management of patients with SCAD has been published over the past 10 years, but the impact on clinical practice has not been evaluated. The present study aims to examine if approaches to invasive management, medical therapy, and vascular imaging have changed over time.
Methods:
This is a retrospective cohort study of 157 patients treated for SCAD between 2005 and 2019 at an academic health system in Philadelphia, Pennsylvania. We aimed to examine change in management over time, including rates of coronary revascularization, discharge medications, and vascular imaging.
Results:
Conservative management of SCAD increased over time from 35% before 2013 to 89% in 2019, p < 0.001. Revascularization was associated with younger age, pregnancy-associated SCAD, and lesions of the left main artery, left anterior descending artery, and multiple vessels, p < 0.05 for all. Partial imaging for extracoronary vascular abnormalities ranged from 33% before 2013 to 71% in 2018, p = 0.146. The rate of comprehensive vascular imaging (cross-sectional head to pelvis imaging) remained low in all time categories (10–18%) and did not change over time. Patients who underwent comprehensive imaging were more likely to be diagnosed with fibromuscular dysplasia (FMD) compared to those with partial imaging (63% vs 15%, p < 0.001).
Conclusion:
Management of spontaneous coronary artery dissection has changed over time. More patients are being managed conservatively and undergo screening for extracoronary vascular abnormalities such as FMD. Future efforts should focus on improving rates of comprehensive vascular screening.
Keywords: cardiovascular disease, coronary artery disease, fibromuscular dysplasia (FMD), spontaneous coronary artery dissection (SCAD)
See Commentary: Henkin SH, Gornik HL, Kim ESH. Improving the care of patients with spontaneous coronary artery dissection (SCAD): Are we doing enough? Vasc Med 2023; 28: 139–140.
Background
In recent years, spontaneous coronary artery dissection (SCAD) has been increasingly recognized as an important cause of acute coronary syndrome (ACS), especially in women. SCAD accounts for up to 35% of ACS in women aged under 50 years and is a leading cause of pregnancy-associated myocardial infarction.1–4 SCAD results from the formation of an intramural hematoma with or without intimal tear in a coronary artery, leading to occlusion and ischemia. In the past 5 years, the American Heart Association (AHA), American College of Cardiology (ACC), and European Society of Cardiology (ESC) have published expert consensus statements on SCAD management advising a conservative approach rather than revascularization for stable patients without high-risk features, as well as screening for extracoronary vascular abnormalities (EVAs).5–7 Though these statements have been widely circulated, the adoption of these recommendations over time has not been well studied.
There have been no randomized controlled trials of SCAD management, but an increasing number of cohort studies published between 2012 and 2022 demonstrate that SCAD may be optimally managed via a conservative approach rather than coronary revascularization (percutaneous intervention or coronary artery bypass graft surgery [CABG]) in cases without ongoing chest pain, cardiogenic shock, arrhythmia, or left main artery dissection.1–3,8–12 A conservative approach is further supported by high rates of spontaneous dissection healing and a higher failure rate from PCI in SCAD compared to atherosclerotic ACS.3,9,13,14 However, optimal medical therapy is not well defined. One study suggested that beta-blockers decrease the risk of recurrence, but this has not been shown in other cohorts.12,15,16 Similarly, the benefit of dual antiplatelet therapy (DAPT) is unclear given the difference in pathophysiologic mechanisms between SCAD and atherosclerotic ACS and lack of clinical trials demonstrating benefit. 6
The etiology of SCAD is not clearly defined; however, fibromuscular dysplasia (FMD) is the most commonly associated vascular condition and is diagnosed in 30–60% of patients with SCAD who undergo comprehensive vascular imaging.6,12,17 FMD is a noninflammatory vascular condition that predominately affects women and can lead to arterial stenosis, dissection, and/or aneurysm. Additionally, cerebral aneurysms are diagnosed in 7–14% of patients with SCAD and may need intervention.3,17,18 Expert consensus statements recommend that all patients with SCAD be screened for FMD and other extracoronary vascular abnormalities, but previous studies have shown rates of vascular imaging are as low as 30–50% in clinical practice.10,11,19
Given the increasing recognition of SCAD clinically and expanding literature regarding preferred management strategies, we hypothesize that trends in acute management and subsequent vascular imaging have changed over time. In this retrospective cohort study, we aim to describe changes in SCAD management over time at a single health system, including revascularization practices, medication use, and screening for extracoronary vascular abnormalities, as well as to describe factors associated with management strategies.
Methods
Study population
The study was approved by the University of Pennsylvania Institutional Review Board. We identified patients with a history of SCAD either diagnosed or subsequently treated at an inpatient or outpatient clinical site affiliated with Penn Medicine. Penn Medicine is a six-hospital quaternary care academic medical center which serves a racially and socioeconomically diverse population reflective of the greater Philadelphia area. We identified patients in the electronic health record (EHR) with an encounter for coronary artery dissection, as identified by ICD-9 (International Classification of Diseases, 9th revision) code 414.12 and ICD-10 (10th revision) code I25.42, between 2005 and 2019. We excluded patients with iatrogenic, atherosclerotic, or traumatic coronary artery dissection. Medical records were manually reviewed by one physician (EF) to assess plausibility of SCAD diagnosis. All records with possible SCAD diagnosis were reviewed by a second physician (JL, RW, or PF), with review of cardiac catheterization when available, and SCAD diagnosis was reached by consensus. Patients were categorized as having definite SCAD, possible SCAD, or uncertain SCAD, defined as follows.
Definite SCAD: Catheterization film or report available with description of SCAD and subsequent evaluation by cardiologist who documented the diagnosis.
Possible SCAD: Catheterization film or report available that was equivocal between SCAD and an alternate diagnosis, such as vasospasm or plaque erosion. In these cases, either additional testing to clarify diagnosis was not performed (e.g., intracoronary imaging, repeat catheterization to assess for healing) or did not clarify the diagnosis.
Uncertain SCAD: Diagnosis of SCAD was documented in clinical notes but cardiac catheterization report and cardiology follow-up notes were not available for review.
Cases were indexed by date of SCAD event. If patients had more than one SCAD event treated in our system, the first one was considered the index event. Time categories of the index event were calculated by quintiles: before 2013, 2013–2016, 2017, 2018, and 2019. The two most recent time periods (2018 and 2019) represent practice after the AHA Scientific Statement on SCAD was published in February 2018. 5 Time periods were chosen to have a roughly similar number of index SCAD cases.
Covariates and outcomes
Patient demographics, comorbidities, clinical presentation, angiographic findings, and discharge medications were abstracted from index hospitalization if documentation was available, or the first follow-up visit after the index SCAD event if hospital records were unavailable. Presence of comorbid conditions and family history including dyslipidemia, diabetes, tobacco use, hypertension, headaches, connective tissue disease, autoimmune disease, depression, and family history of premature coronary artery disease were abstracted from the EHR. Dyslipidemia was defined as being on a lipid-lowering agent prior to the index event, low-density lipoprotein (LDL) > 130 mg/dL or triglycerides > 150 mg/dL. Pregnancy-associated SCAD was defined as SCAD in a patient who was pregnant or ⩽ 1 year postpartum. This category was further divided into early postpartum (within 6 weeks of delivery) and late postpartum (6 weeks to 1 year after delivery). Revascularization was defined as coronary artery balloon angioplasty, coronary stent placement, or CABG. Complication from percutaneous intervention (PCI) was defined as any of the following: propagation of dissection requiring additional stenting, iatrogenic dissection of another vessel not affected by SCAD, emergent CABG, or death.
Data on extracoronary vascular imaging were obtained by reviewing EHR notes and radiology reports. We assessed extracoronary vascular imaging according to vascular bed imaged and type of imaging modality. Cerebrovascular imaging included computed tomography angiography (CTA) or magnetic resonance angiography (MRA) of the head. Neck imaging included carotid artery duplex ultrasound or neck CTA or MRA. Abdominal imaging included catheter-based angiography of the renal arteries, renal artery duplex ultrasound, or abdominal CTA or MRA. A patient was considered to have partial extracoronary vascular imaging if one or more vascular bed was imaged. Comprehensive extracoronary vascular imaging was defined as brain-to-pelvis cross-sectional imaging with CTA or MRA of the head, neck, and abdomen/pelvis, in accordance with the 2018 AHA Scientific Statement on SCAD, which recommends using CT or MR angiography due to higher sensitivity for diagnosing vascular abnormalities. 5
Statistical analysis
Statistical analysis was performed with SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). Continuous data were summarized as mean ± SD. Discrete variables were expressed as frequencies and percentages. Comparisons for categorical variables were performed using the chi-squared test and Fisher’s exact test. The Cochran–Armitage trend test was used to compare trends over time. We conducted a multivariable analysis to evaluate factors associated with revascularization, including the following variables: year of index event, age, pregnancy or postpartum SCAD, SCAD diagnosed in the left main artery or left anterior descending artery, and SCAD in multiple territories. A value of p < 0.05 was considered statistically significant. We conducted several sensitivity analyses. First, we restricted our analysis of treatment strategies to patients with a definite SCAD diagnosis. Next, we restricted our analysis of extracoronary vascular imaging to patients with two or more cardiology follow-up visits after their SCAD event, or one cardiology follow-up visit at ⩾ 1 year after their SCAD event to allow for adequate follow-up time.
Results
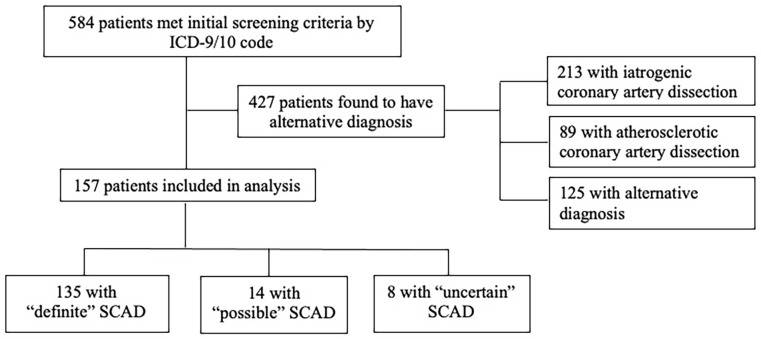
Of the 584 patients initially identified as having coronary artery dissection, 157 patients were included in the analysis (Figure 1). The majority of patients were excluded for having a diagnosis of atherosclerotic coronary artery disease (n = 89) or iatrogenic dissection (n = 213). Mean (SD) age was 47.0 (11.4) years. The majority of patients identified as White (81.8%), and 13.5% identified as Black. Overall, 145 patients were women (92.4%), of whom 18.6% were pregnant or postpartum at the time of the index SCAD event. At the time of the index SCAD event, 40.8% of patients had dyslipidemia and 34.6% had hypertension (Table 1). Thirty-nine patients presented with STEMI (24.8%) and the majority had involvement of a single vessel (144/157, 91.6%), with the left anterior descending coronary artery being the most common location of dissection (61.9%).
Figure 1.
Study flow diagram.
The final cohort included 157 patients with SCAD. Of these, 135 (85.9%) had ‘definite’ SCAD diagnosed by their primary cardiologist and subsequently adjudicated by two physician reviewers; 14 (8.9%) had ‘possible’ SCAD with an alternate diagnosis (i.e., vasospasm, plaque erosion) equally likely; eight (5.0%) had ‘uncertain’ SCAD, which was documented in the EHR but further clinical information was not available for review.
EHR, electronic health record; ICD, International Classification of Diseases; SCAD, spontaneous coronary artery dissection.
Table 1.
Patient characteristics, clinical presentation, and vascular distribution of index SCAD event (N = 157).
| Patients with available data, n | Mean ± SD or n (%) | |
|---|---|---|
| Baseline characteristics | ||
| Age, years | 157 | 47.0 ± 11.4 |
| Body mass index, kg/m2 | 131 | 27.8 ± 6.8 |
| Women | 157 | 145 (92.4) |
| Race | 148 | |
| White | 121 (81.8) | |
| Black | 20 (13.5) | |
| Hispanic/Latina | 3 (2.0) | |
| Asian | 4 (2.7) | |
| Insurance status | 148 | |
| Medicaid | 11 (7.4) | |
| Medicare | 17 (11.5) | |
| Private | 120 (81.1) | |
| Pregnant/postpartum a | 143 | |
| Early postpartum | 17 (11.8) | |
| Late postpartum | 7 (4.9) | |
| Pregnant | 3 (2.0) | |
| Precipitating factor | 114 | |
| Physical activity | 68 (59.6) | |
| Emotional stress | 18 (15.8) | |
| Past medical history | ||
| Family history of premature CAD | 149 | 13 (8.7) |
| Previous MI | 157 | 6 (3.8) |
| Dyslipidemia | 142 | 58 (40.8) |
| Diabetes | 153 | 13 (8.5) |
| Tobacco use | 151 | 38 (25.1) |
| Connective tissue disease | 157 | 9 (5.7) |
| Autoimmune disease | 156 | 12 (7.7) |
| Hypertension | 153 | 53 (34.6) |
| Depression | 157 | 28 (17.8) |
| Headaches | 156 | 35 (22.4) |
| Clinical presentation | ||
| STEMI | 157 | 39 (24.8) |
| Ejection fraction at time of diagnosis | 133 | |
| ⩾ 50% | 92 (69.2) | |
| < 50% | 41 (30.8) | |
| Location of dissection | 155 | |
| L main | 8 (5.2) | |
| LAD | 96 (61.9) | |
| LCx | 45 (29.0) | |
| RCA | 22 (14.2) | |
| Multiple territories | 13 (8.4) | |
Early postpartum = within 6 weeks of delivery; late postpartum = 6 weeks to 1 year after delivery.
CAD, coronary artery disease; LAD, left anterior descending artery; LCx, left circumflex artery; L main, left main coronary artery; MI, myocardial infarction; RCA, right coronary artery; SCAD, spontaneous coronary artery dissection; STEMI, ST elevation myocardial infarction.
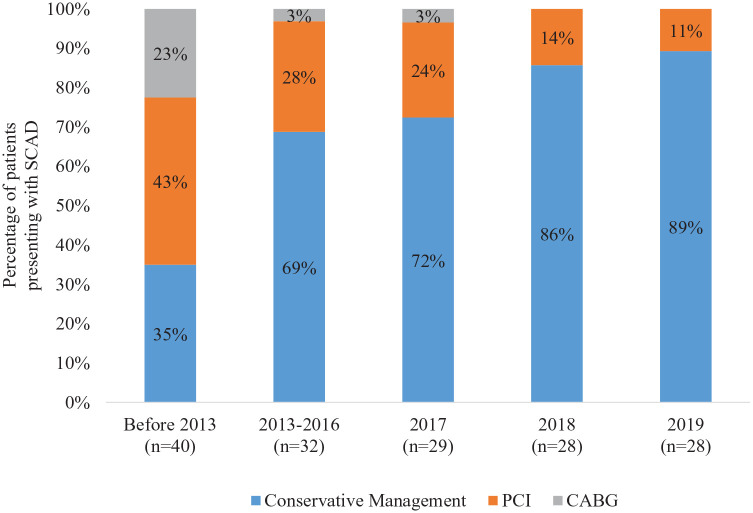
The proportion of patients treated with conservative management increased over time; test for trend p < 0.001 (Figure 2). Prior to 2013, 35% of patients were treated conservatively compared to 89% of patients in 2019. The proportion of patients undergoing revascularization with CABG decreased over time: 23% of patients were revascularized with CABG prior to 2013, whereas no patients underwent CABG in 2018–2019; test for trend p < 0.001. Patients undergoing revascularization with PCI or CABG were more likely to be younger and have pregnancy-associated SCAD, dissection of the left main or left anterior descending artery, and multivessel involvement; p < 0.05 for all (Table 2). In multivariable analysis, only year of the index SCAD event, age, and SCAD in multiple territories remained statistically significantly associated with revascularization (online Supplemental Table 1). Among 39 patients who presented with STEMI, 24 (62%) were treated conservatively, 13 (33%) underwent PCI, and two (5%) underwent CABG. This was not significantly different from patients who presented with non-ST elevation myocardial infarction (NSTEMI). Of the 40 patients who underwent PCI, 11 (27.5%) had complications. The rate of PCI complication did not change over time. Sensitivity analysis restricted to patients with definite SCAD showed similar findings (online Supplemental Figure 1).
Figure 2.
Management of SCAD over time.
Prior to 2013, 35% of patients with SCAD were managed conservatively compared to 89% in 2019; test for trend p < 0.001.
CABG, coronary artery bypass graft surgery; PCI, percutaneous intervention; SCAD, spontaneous coronary artery dissection.
Table 2.
Factors associated with revascularization after SCAD.
| Revascularization (n = 51) |
Conservative management (n = 106) |
p-value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, years | 42.3 ± 10.2 | 49.2 ± 11.4 | < 0.001 |
| Women | 48 (94.1) | 97 (91.5) | 0.56 |
| White race | 39 (79.6) | 82 (82.8) | 0.63 |
| BMI, kg/m2 | 27.1 ± 6.8 | 28.1 ± 6.8 | 0.45 |
| PSCAD | 14 (28.0) | 13 (12.4) | 0.02 |
| Medicaid insurance | 7 (15.2) | 4 (3.9) | 0.015 |
| Clinical presentation | |||
| STEMI | 15 (29.4) | 24 (22.6) | 0.36 |
| EF < 50% a | 18 (47.4) | 23 (24.2) | 0.09 |
| Regional wall motion abnormalities b | 22 (64.7) | 66 (71.0) | 0.50 |
| Coronary territory | |||
| L main | 8 (16.3) | 0 (0.0) | < 0.001 |
| LAD | 37 (75.5) | 59 (55.7) | 0.02 |
| LCx | 9 (18.4) | 36 (34.0) | 0.05 |
| RCA | 7 (14.3) | 15 (14.2) | 0.98 |
| Multiple territories | 9 (18.4) | 4 (3.8) | 0.002 |
Data presented as n (%) or mean ± SD.
Bold p-values were statistically significant (p<0.05)
Ejection fraction data available for 133 patients.
Wall motion abnormality data available for 127 patients.
BMI, body mass index; EF, ejection fraction; LAD, left anterior descending artery; LCx, left circumflex artery; L main, left main coronary artery; PSCAD, pregnancy-associated SCAD; RCA, right coronary artery; SCAD, spontaneous coronary artery dissection; STEMI, ST elevation myocardial infarction.
Prescribed medications at hospital discharge were available for 145 (92.3%) patients (Table 3). Aspirin and beta-blocker use was high prior to 2013 and did not significantly change over time. The proportion of patients treated with statins increased significantly over time, from 47% prior to 2013 to 89% in 2019; test for trend p < 0.001. Of patients treated with statins, only 45% had documented dyslipidemia. The proportion of patients discharged on DAPT increased from 56% prior to 2013 to 82% in 2019, whereas angiotensin-converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB) usage did not significantly change over time. Sensitivity analysis restricted to patients with definite SCAD showed similar findings (online Supplemental Table 2). After the index SCAD event, recurrent major adverse cardiovascular events occurred in 10/157 (6.4%) patients and recurrent SCAD occurred in 4/157 (2.5%) patients.
Table 3.
Discharge medications after SCAD event by time quintiles.
| Medications | Before 2013 (n = 32) |
2013–2016 (n = 29) |
2017 (n = 29) |
2018 (n = 28) |
2019 (n = 27) |
p-value |
|---|---|---|---|---|---|---|
| ASA | 29 (91) | 28 (97) | 29 (100) | 28 (100) | 27 (100) | 0.105 |
| DAPT | 18 (56) | 19 (66) | 21 (72) | 22 (79) | 22 (82) | 0.203 |
| Statins | 15 (47) | 21 (72) | 24 (83) | 23 (82) | 24 (89) | 0.002 |
| Beta-blockers | 25 (78) | 25 (86) | 26 (90) | 23 (82) | 25 (93) | 0.529 |
| ACEi/ARB | 12 (38) | 10 (34) | 10 (34) | 11 (39) | 13 (48) | 0.831 |
Data presented as n (%).
Bold p-values were statistically significant (p<0.05)
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, aspirin; DAPT, dual antiplatelet therapy; SCAD, spontaneous coronary artery dissection.
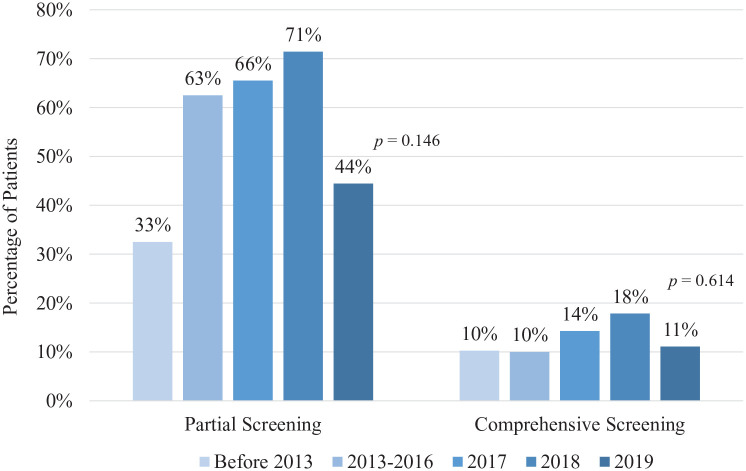
The proportion of patients undergoing partial extracoronary vascular imaging varied over time, though the trend was not significant; test for trend p = 0.146 (Figure 3). Prior to 2013, 33% of patients underwent imaging of one or more vascular beds compared to 71% of patients in 2018. The proportion of patients undergoing imaging in 2019 was notably lower at 44%. The proportion of patients undergoing comprehensive extracoronary vascular imaging (defined as cross-sectional imaging of the head, neck, and abdominal vascular beds) remained low (10–18%) and did not change over time; test for trend p = 0.614. Among patients who underwent any extracoronary vascular imaging (n = 84), patients with comprehensive imaging had higher rates of FMD diagnosis compared to those who had partial imaging (63% vs 15%, p < 0.001). In patients diagnosed with FMD, renal arteries were affected in 56.5% of cases, carotid and/or vertebral arteries in 30.4% of cases, and intracranial vessels in 8.7% of cases. Female sex was associated with undergoing extracoronary vascular imaging (96.4% vs 87.5%, p = 0.037) (Table 4). Of patients who did not undergo any extracoronary vascular imaging, 6.9% had imaging ordered by a provider that was not completed. Sensitivity analysis restricted to patients who had two or more cardiology follow-up visits or one cardiology follow-up visit at ⩾ 1 year after their SCAD event showed similar findings (online Supplemental Figure 2).
Figure 3.
Fibromuscular dysplasia screening rates over time.
The p-value represents test for trend using Cochrane–Armitage trend test.
Table 4.
Factors associated with FMD screening.
| No FMD screening (n = 72) |
FMD screening (n = 84) |
p-value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, years | 47.4 ± 13.2 | 46.8 ± 9.8 | 0.751 |
| Women | 63 (87.5) | 81 (96.4) | 0.037 |
| White | 55 (82.1) | 66 (82.5) | 0.948 |
| PSCAD | 11 (15.7) | 16 (19.0) | 0.588 |
| Medicaid insurance | 5 (7.7) | 5 (6.1) | 0.703 |
| Clinical presentation | |||
| STEMI | 19 (26.4) | 19 (22.6) | 0.584 |
| EF < 50% a | 15 (26.8) | 26 (33.8) | 0.389 |
| Regional wall motion abnormalities b | 35 (67.3) | 53 (70.7) | 0.687 |
| Management | |||
| Underwent revascularization | 27 (37.5) | 23 (27.4) | 0.177 |
Data presented as n (%) or mean ± SD.
Bold p-values were statistically significant (p<0.05)
Ejection fraction data available for 133 patients.
Wall motion abnormality data available for 127 patients.
EF, ejection fraction; FMD, fibromuscular dysplasia; PSCAD, pregnancy-associated spontaneous coronary artery dissection; STEMI, ST elevation myocardial infarction.
Discussion
This retrospective cohort study of a single health system demonstrates that the management of SCAD has significantly changed over time, with a majority of patients being managed medically. More than 85% of patients were managed conservatively, without revascularization, in recent years. Patients who underwent revascularization were more likely to be younger, have pregnancy-associated SCAD, or SCAD of the left main artery, left anterior descending artery, or multiple territories. This is consistent with previous studies suggesting that patients with SCAD who are peripartum or who have high-risk lesions in the left main artery or proximal left anterior descending artery have more severe presentations and are more likely to undergo revascularization.20–22
The overall rate of revascularization (32%) in our cohort was similar to that of previous SCAD cohorts. 9–11,15,19,23 Prior to the present study, the trend in revascularization over time had not been described. Our results demonstrating decreasing rates of revascularization over time reflect the increasing evidence that supports spontaneous arterial healing after SCAD, good clinical outcomes with conservative management, and an increased complication rate in patients undergoing PCI for SCAD compared to those undergoing PCI for atherosclerotic ACS.2,3,9,14 Further, as SCAD recognition increases, we are likely identifying less severe cases of SCAD which do not require intervention, thus contributing to the decrease in revascularization rates over time.
Though the rate of partial imaging for extracoronary vascular abnormalities ranged from 33% to 71% in this cohort, the rate of comprehensive imaging was consistently less than 20% during all time periods. Comprehensive imaging led to more frequent diagnosis of FMD compared to partial imaging (63% vs 15%), suggesting that FMD is underdiagnosed in patients who undergo partial vascular imaging.
Compared to previous SCAD cohort studies, which report a comprehensive extracoronary vascular imaging rate of 30–60%, our rate of 12.5% was substantially lower.10,11,19,23 There are several possible explanations for this difference in vascular screening rates. First, much of the previous data come from prospective studies and SCAD registries in which patients are routinely followed by SCAD experts, who are more likely to order comprehensive vascular imaging.11,12,19,23 The present study more accurately reflects real-world clinical practice because it includes any patient treated within Penn Medicine, some of whom were subsequently followed outside of the health system at community practices. The lower rate of vascular imaging in this study may be compounded by loss to follow-up. Many patients were seen for referral at Penn Medicine and then continued to follow with their local clinicians, whose records we may not be able to access. Among patients who did not undergo vascular screening, the leading cause of patients not undergoing screening (93%) was the physician not ordering testing. This represents a large knowledge gap that needs to be addressed by continuing medical education directed towards providers caring for patients with SCAD, including cardiologists, vascular medicine specialists, and women’s health providers.
In addition to a lack of familiarity with SCAD management, some providers feel that the diagnosis of vascular anomalies may cause patient stress without impacting management; however, patients with FMD can have vascular anomalies that require intervention. In one large study of FMD patients, 12.9% of patients who underwent intracranial imaging were found to have at least one intracranial aneurysm, 43.2% of which were > 5 mm in size and required increased surveillance or intervention. 24 A recent large, prospective, cohort study also demonstrated that FMD is a predictor of future major adverse cardiac events in patients with SCAD. 12 Diagnosing patients with FMD and other extracoronary vascular abnormalities may impact prognosis, medical management, lifestyle counseling, and suggested family screening.
Interventions to improve extracoronary vascular imaging could include physician education, patient education, and care coordination. Increasing graduate or continuing medical education about nonatherosclerotic ACS may improve physician comfort with acute and longitudinal care of patients with SCAD. Patient registries, such as the Mayo Clinic SCAD registry, 25 the multicenter iSCAD registry, 26 and the Canadian SCAD registry, 27 continue to provide important opportunities for patients to receive second opinions and access evolving research in this field. In addition, referring patients to online SCAD support groups facilitates patient education and advocacy. 28 Cross-sectional imaging does not need to be urgently completed at the time of SCAD diagnosis; however, vascular imaging during the index hospital admission may reduce patient burden, as outpatient vascular imaging typically takes 2 days. Adopting protocols to complete imaging of multiple vascular beds during one session may also improve screening rates. 29
Study limitations
Our study has several limitations. Our cohort includes patients who were seen within a single academic medicine health system, which may limit the generalizability of our findings. Our sample size is small and many patients did not follow-up at Penn Medicine after their index admission, which limited our ability to capture clinical events after SCAD and may partially account for the low rates of recurrent major adverse cardiovascular events and comprehensive vascular imaging. We did not include chest imaging as part of the extracoronary vascular imaging so our ‘comprehensive screening’ does not include screening for abnormalities of the thoracic aorta. We chose time periods to achieve a relatively even distribution of patients across categories, but patients diagnosed in the earliest time period had their index SCAD event between 1992 and 2012. There was likely considerable variability in practice among those years, which is not fully captured by collapsing those patients into one time category. Lastly, there were considerably lower rates of extracoronary vascular imaging seen in the 2019 cohort compared to 2018. Reasons for this are not completely clear but we hypothesize that this is related to the COVID-19 pandemic, which has been shown to obstruct the delivery of preventive care.30,31 Specialized SCAD-focused care has since been implemented at Penn and we expect that this will greatly improve our rates of comprehensive extracoronary vascular imaging as more patients are referred to this clinic.
Conclusion
In summary, management of SCAD has advanced significantly over time, with an increased number of patients being managed conservatively and an increased focus on screening for extracoronary vascular abnormalities. Though many patients undergo partial extracoronary vascular imaging, this study suggests that rates of comprehensive vascular imaging are low and should be a topic of further investigation. Future studies should focus on elucidating the reasons for low screening rates and proposing methods of improvement.
Supplemental Material
Supplemental material, sj-pdf-1-vmj-10.1177_1358863X231155305 for Management of spontaneous coronary artery dissection: Trends over time by Elliot Feldbaum, Elizabeth W Thompson, Tessa S Cook, Monika Sanghavi, Robert L Wilensky, Paul N Fiorilli and Jennifer Lewey in Vascular Medicine
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was funded in part by a grant from the National Institutes of Health to Dr Lewey (K23HL153667). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
ORCID iD: Elliot Feldbaum  https://orcid.org/0000-0003-4954-4191
https://orcid.org/0000-0003-4954-4191
Supplementary material: The supplementary material is available online with the article.
References
- 1.Rashid HNZ, Wong DTL, Wijesekera H, et al. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome—A single-centre Australian experience. Int J Cardiol 2016; 202: 336–338. [DOI] [PubMed] [Google Scholar]
- 2.Nakashima T, Noguchi T, Haruta S, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the Angina Pectoris–Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol 2016; 207: 341–348. [DOI] [PubMed] [Google Scholar]
- 3.Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: Association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7: 645–655. [DOI] [PubMed] [Google Scholar]
- 4.Havakuk O, Goland S, Mehra A, Elkayam U. Pregnancy and the risk of spontaneous coronary artery dissection. Circ Cardiovasc Interv 2017; 10: e004941. [DOI] [PubMed] [Google Scholar]
- 5.Hayes SN, Kim ESH, Saw J, et al. Spontaneous coronary artery dissection: Current state of the science: A Scientific Statement from the American Heart Association. Circulation 2018; 137: e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayes SN, Tweet MS, Adlam D, et al. Spontaneous coronary artery dissection. J Am Coll Cardiol 2020; 76: 961–984. [DOI] [PubMed] [Google Scholar]
- 7.Adlam D, Alfonso F, Maas A, et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group: A position paper on spontaneous coronary artery dissection. Eur Heart J 2018; 39: 3353–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126: 579–588. [DOI] [PubMed] [Google Scholar]
- 9.Tweet MS, Eleid MF, Best PJM, et al. Spontaneous coronary artery dissection: Revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7: 777–786. [DOI] [PubMed] [Google Scholar]
- 10.Clare R, Duan L, Phan D, et al. Characteristics and clinical outcomes of patients with spontaneous coronary artery dissection. J Am Heart Assoc 2019; 8: e012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saw J, Starovoytov A, Humphries K, et al. Canadian spontaneous coronary artery dissection cohort study: In-hospital and 30-day outcomes. Eur Heart J 2019; 40: 1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saw J, Starovoytov A, Aymong E, et al. Canadian spontaneous coronary artery dissection cohort study. J Am Coll Cardiol 2022; 80: 1585–1597. [DOI] [PubMed] [Google Scholar]
- 13.Hassan S, Prakash R, Starovoytov A, Saw J. Natural history of spontaneous coronary artery dissection with spontaneous angiographic healing. JACC Cardiovasc Interv 2019; 12: 518–527. [DOI] [PubMed] [Google Scholar]
- 14.Alfonso F, Paulo M, Lennie V, et al. Spontaneous coronary artery dissection: Long-term follow-up of a large series of patients prospectively managed with a ‘conservative’ therapeutic strategy. JACC Cardiovasc Interv 2012; 5: 1062–1070. [DOI] [PubMed] [Google Scholar]
- 15.Saw J, Humphries K, Aymong E, et al. Spontaneous coronary artery dissection. J Am Coll Cardiol 2017; 70: 1148–1158. [DOI] [PubMed] [Google Scholar]
- 16.Tweet MS, Olin JW. Insights into spontaneous coronary artery dissection. J Am Coll Cardiol 2017; 70: 1159–1161. [DOI] [PubMed] [Google Scholar]
- 17.Persu A, Lopez-Sublet M, Al-Hussaini A, et al. Prevalence and disease spectrum of extracoronary arterial abnormalities in spontaneous coronary artery dissection. JAMA Cardiol 2022; 7: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saw J, Ricci D, Starovoytov A, et al. Spontaneous coronary artery dissection. JACC Cardiovasc Interv 2013; 6: 44–52. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Kaadan MI, Duran JM, et al. Risk factors, imaging findings, and sex differences in spontaneous coronary artery dissection. Am J Cardiol 2019; 123: 1783–1787. [DOI] [PubMed] [Google Scholar]
- 20.Lobo AS, Cantu SM, Sharkey SW, et al. Revascularization in patients with spontaneous coronary artery dissection and ST-segment elevation myocardial infarction. J Am Coll Cardiol 2019; 74: 1290–1300. [DOI] [PubMed] [Google Scholar]
- 21.Tweet MS, Hayes SN, Codsi E, et al. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol 2017; 70: 426–435. [DOI] [PubMed] [Google Scholar]
- 22.García-Guimaraes M, Bastante T, Macaya F, et al. Spontaneous coronary artery dissection in Spain: Clinical and angiographic characteristics, management, and in-hospital events. Rev Esp Cardiol (Engl Ed) 2021; 74: 15–23. [DOI] [PubMed] [Google Scholar]
- 23.Kok SN, Hayes SN, Cutrer FM, et al. Prevalence and clinical factors of migraine in patients with spontaneous coronary artery dissection. J Am Heart Assoc 2018; 7: e010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lather HD, Gornik HL, Olin JW, et al. Prevalence of intracranial aneurysm in women with fibromuscular dysplasia: A report from the US Registry for Fibromuscular Dysplasia. JAMA Neurol 2017; 74: 1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayes SN. The “Virtual” Multicenter Spontaneous Coronary Artery Dissection (SCAD) Registry. ClinicalTrials.gov Identifier: NCT01429727, https://clinicaltrials.gov/ct2/show/NCT01429727 (2022, accessed 12 January 2023).
- 26.PERFUSE Study Group. SCAD Alliance. International Spontaneous Coronary Artery Dissection (SCAD) “iSCAD” Registry. ClinicalTrials.gov Identifier: NCT04496687, https://clinicaltrials.gov/ct2/show/NCT04496687 (2022, accessed 12 January 2023).
- 27.Saw J, Mancini JGB, Humphries K. Cardiology Research UBC. Canadian SCAD Study. ClinicalTrials.gov Identifier: NCT04906356, https://clinicaltrials.gov/ct2/show/NCT04906356 (2021, accessed 12 January 2023).
- 28.Laupacis A. I had to learn about SCAD by Googling it. Can Med Assoc J 2022; 194: E562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abozeed M, Bolen MA. Screening CT angiography in patients with suspected fibromuscular dysplasia: Improved patient care with single-session skull vertex to pelvis coverage. Cardiovasc Diagn Ther 2020; 10: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayo M, Potugari B, Bzeih R, et al. Cancer screening during the COVID-19 pandemic: A systematic review and meta-analysis. Mayo Clin Proc Innov Qual Outcomes 2021; 5: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander GC, Tajanlangit M, Heyward J, et al. Use and content of primary care office-based vs telemedicine care visits during the COVID-19 pandemic in the US. JAMA Netw Open 2020; 3: e2021476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vmj-10.1177_1358863X231155305 for Management of spontaneous coronary artery dissection: Trends over time by Elliot Feldbaum, Elizabeth W Thompson, Tessa S Cook, Monika Sanghavi, Robert L Wilensky, Paul N Fiorilli and Jennifer Lewey in Vascular Medicine