Abstract
Background
Caspase-8 is a molecule in the FAS pathway that initiates apoptosis. One of the rarest autoimmune lymphoproliferative syndromes is caspase-8 deficiency. Immunodeficiency, splenomegaly, and lymphadenopathy are the common symptoms of this condition.
Case Presentation
A two-year-old boy entered this study with a fever of unknown origin (FUO) and dysentery. Moreover, he suffered from failure to thrive and was allergic to the cow's milk protein. His fever and dysentery did not respond to antibiotic therapy. The colonoscopy revealed diffuse ulcerations regions in the sigmoid along with skipped areas, mimicking Crohn's disease aphthous lesions. He represented very early-onset inflammatory bowel disease (IBD) and was diagnosed with the caspase-8 deficiency.
Conclusion
There can be diarrhea or dysentery as the first or main symptoms of inborn errors of immunity (IEIs). The cause of diarrhea and dysentery in this case was early-onset IBD. One of the symptoms of IEIs such as caspase-8 deficiency is early-onset of IBD. Patients with early-onset had normal T cell count and low or normal immunoglobulin levels with insufficient immune response.
Introduction
Caspase-8, encoded by the CASP8 gene, initiates apoptosis’s extrinsic pathway. Caspase-8 is downstream of cell surface death receptors like FAS [1]. FAS receptors have extracellular, intracellular and transmembrane domains. Upon activation, the intracellular domain recruits FAS-associated death domain (FADD), which is made up of two domains including, the death domain (DD) and death effector domain (DED) [1, 2]. The DED heterodimer formation resulted in the recruitment of additional procaspase-8. The procaspase 8 interacts with the DED of the previously formed procaspase-8 [3].
Procaspase-8 proteins undergo autoproteolytic cleavage once their proteolytic domains homodimerize. Caspase-8 is ultimately activated as a result of the autoproteolytic cleavage [4].
Caspase-8 deficiency is an autosomal recessive subtype of the broad-spectrum autoimmune lymphoproliferative syndrome (ALPS) [5]. Embryonic lethality, abnormal heart muscle, and decreased hematopoietic precursors have all been observed in homozygous caspase-8 deficient mice [6]. Impairment of lymphocytic apoptosis in humans leads to accumulation of lymphocytes in the lymphatic organs. Splenomegaly and lymphadenopathy may ensue as a result [5, 7]. Immunodeficiency is another manifestation of caspase-8 disease since the enzyme is important for lymphocyte activation [1, 7]. Several treatment modalities are used for ALPS. Immunosuppressive agents like mycophenolate mofetil and sirolimus are used as treatments for this illness. A definite therapy for ALPS is hematopoietic stem cell transplantation (HSCT) [8].
Caspase-8 deficiency is a rare and often fatal immunological disorder, it is crucial to diagnose patients suffering from this disease in order to appropriately manage their symptoms..
The purpose of this study was to report a caspase-8 deficient patient and review other cases who have been reported in the literature.
Material and methods
Subject
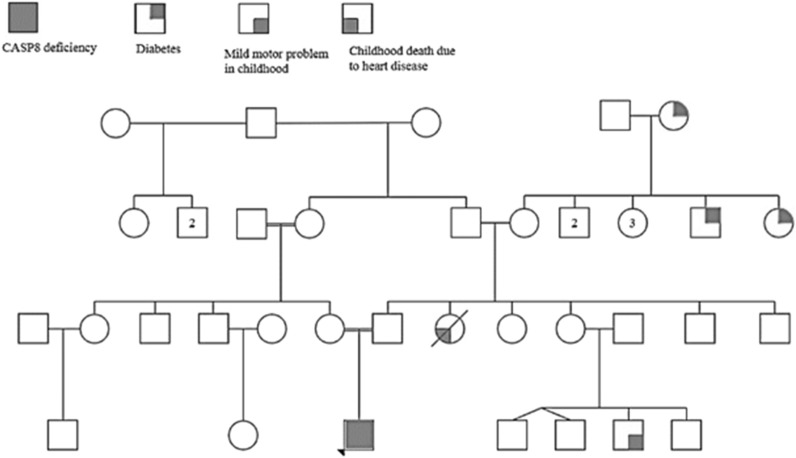
A two-year-old boy was hospitalized at Mofid Children's hospital with a history of prolonged fever and dysentery for 50 days. His parents were consanguineous, and he was the sole child (Fig. 1). He underwent physical and comprehensive paraclinical evaluations.
Fig. 1.
The pedigree of the proband and his family. The proband is represented by a filled square and other conditions in pedigree has been shown with other symbols
Genetic analysis
Written informed consent was received from the parents of the patient. Afterward, blood samples were taken from the patient, his mother and father in the tubes containing 0.5 mmol ethylene diamine tetra acetic acid (EDTA). The ExgeneTM Blood SV DNA purification kit (GeneAll Biotechnology, Korea) was utilized to isolate the genomic DNA from whole blood according to the manufacturer's instructions [9].
The patient’s whole exome sequencing (WES) was performed by CeGaT GmbH (Tübingen, Germany) with an average coverage of roughly 100 × . Several public databases, including ANNOVAR [10], were used to annotate variants with an observed frequency of the alternative allele (OFA) of > 0.02. Subsequently, variants were filtered based on population allele frequency < 0.05 in the Exome Aggregation Consortium (ExAC [11]) Genome Aggregation Database (gnomAD [12]) and an in-house database of over 2000 Iranian exomes. Variants located in exonic regions and flanking ten base pairs (bp) were included for further analysis. In addition, synonymous variants located outside of splicing regions were excluded. Included variants were prioritized based on the correlation of the associated gene with the patient's phenotype. Multiple lines of in silico prediction tools, namely MutationTaster [13], Combined Annotation-Dependent Depletion (CADD [14]), and Deleterious Annotation of genetic variants using Neural Networks [15] PhyloP, PhastCons and GERP [16] were used to evaluate the impact of the identified variants. Furthermore, Human Gene Mutation Database (HGMD), ClinVar [17], and literature were searched to find previous reports of the variant. Finally, the considered variants were classified based on the American College of Medical Genetics and Genomics (ACMG) standards and guidelines for interpreting sequence variants [18].
Sanger sequencing was conducted using ABI 3500 Genetic Analyzer to validate the detected CASP8 variant in the patient and confirm the carrier status of his parents.
Results
Case description
The patient was full-term at the time of vaginal delivery. He had a history of repeated hospitalizations for fever of unknown origin (FUO) and dysentery. Despite antibiotic therapy throughout, no remission had been observed. In addition to minor thalassemia, he had a cow's milk allergy, as well as multiple anal fissures that were surgically repaired. He was vaccinated according to the national vaccination protocol with no adverse reactions. Though underweight, he was neurologically and developmentally normal with a Z score of -3.31 (0.093 percentile). The physical examination revealed cervical lymphadenopathy, splenomegaly, and anal fissure. He underwent a complete workup to rule out infectious diseases. Table 1 shows the results of different para-clinical evaluations performed on the patient. Moreover, the results of flow cytometry and lymphocyte transformation tests are also shown in Table 1.
Table 1.
The immunological and laboratory findings for the patient in this study
| Diagnostic workup | Results | Normal range |
|---|---|---|
| WBC (*103/μL) | 9.3 (neutrophil = 33, lymph = 59) | 5.5–15.5 |
| Hb (g/dL) | 6.6 | 10.5–14.5 |
| PLT (*103/μL) | 810 | 150–450 |
| BC | Negative | |
| UA | Normal | |
| S/E | Normal | |
| S/C | Normal | |
| CRP (mg/L) | 75 | < 10 |
| Bone marrow PCR | Normal | |
| HIV Ab | 0.2 (Negative) | < 1 |
| PBS Malaria | Negative | |
| PBS Borrelia | Negative | |
| ESR (mm/hr) | 31 | < 10 |
| Wright | Negative | |
| CMV PCR | Negative | |
| Fungi PCR | Negative | |
| TB PCR | Negative | |
| Mycobactrium PCR | Negative | |
| Anti Diphtheria Ab (IgG) | > 1 (Positive) |
< 0.1: No response 0.1–1: Poor response >1: Normal response |
| Anti-Tetanus Ab (IgG) | 3.3 (Positive) |
< 0.1: No response 0.1–1: Poor response >1: Normal response |
| Bacteriology culture | Negative | |
| BACTEC | Negative | |
| Na (mEq/L) | 132 | 135–145 |
| K (mEq/L) | 5.1 | 3.5–5 |
| Sweat chloride test | Negative | 0–40: Negative |
| Stool calprotectin | 1012 | 50–200 |
| BUN (mg/dL) | 10.2 | 5–18 |
| Cr (mg/dL) | 1.5 | 0.5–1 |
| Ph (mg/dL) | 4.9 | 4–7 |
| AST (U/L) | 24 | 10–40 |
| ALT (U/L) | 14 | 10–40 |
| ALP (U/L) | 417 | < 350 |
| WBC (*103/μL) | 10 | 5.5–15.5 |
| Hb (g/dL) | 7.4 | 10.5–14.5 |
| PLT (*103/μL) | 860 | 150–450 |
| C-ANCA | Negative | |
| P-ANCA | Negative | |
| Anti-TTG |
IgA:0.1 IgG:2.6 |
IgA:0–4 IgG:0–5 |
| CD3 (%) | 52 (decreased) | 57–75% |
| CD4 (%) | 10 (decreased) | 28—47% |
| CD16 (%) | 10 (normal) | 3–15% |
| CD19 (%) | 27 (normal) | 14–33% |
| CD8 (%) | 22 (normal) | 18–35% |
| CD56 (%) | 10.7 (normal) | 3–15% |
| PHA | 4.1 | > 3 |
| BCG | 3.6 | > 2.5 |
| Candida | 2.5 | > = 2.5 |
| NBT | 100 | > 95 |
WBC white blood cell, Hb hemoglobin, PLT platelet, BC blood culture, UA urinary analysis, S/E stool exam, S/C stool culture, CRP C-reactive protein, HIV ab HIV antibody, PBS peripheral blood smear, ESR erythrocyte sedimentation rate, BUN blood urine nitrogen, Cr creatinine, Ph phosphorus, AST aspartate transaminase, ALT alanine transaminase
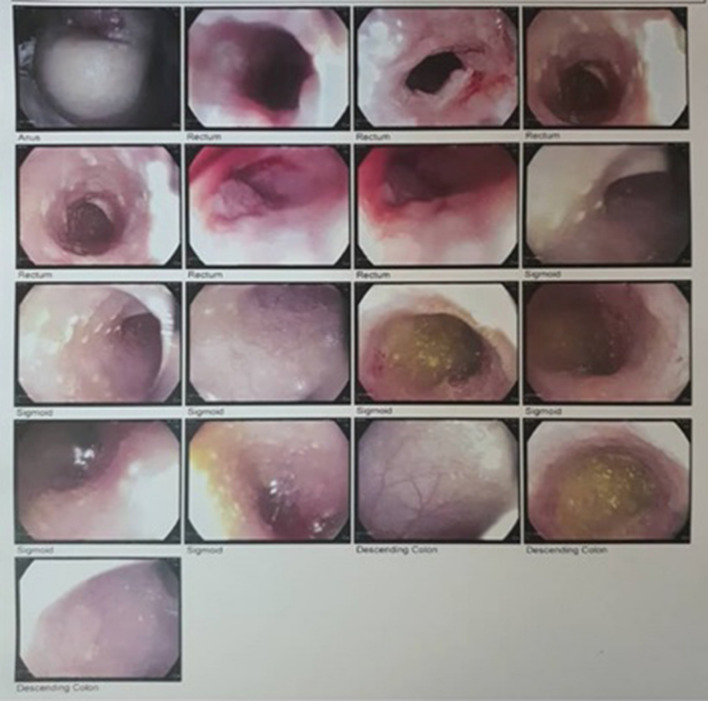
Multiple polyploidy lesions with dispersed ulcers and hemorrhage were discovered during his colonoscopy. The diffuse ulceration region in the sigmoid with skipped areas mimics Crohn-like aphthous lesion (Fig. 2). A 1.5-cm-long stenotic area was seen 0.5 cm away from the surgical margin. As a result, he underwent an ileostomy and colostomy.
Fig. 2.
The colonoscopy of the patient. Rectum had mucosal erythema with diffuse ulcerative lesion and mucosal friability. Diffuse ulcerative lesions with skipped area mimic Crohn like aphthous lesion were evident in sigmoid. One deep ulcerative lesion with active bleeding in proximal descending colon was observed
During endoscopy, reflux esophagitis grade A, gastritis erythematous, and nodularity were observed. His H. Pylori test result was negative. On superficial soft tissue sonography of the perianal region, there were no evidence of collection or abscess.
A hematology consultation ruled out malignancy and hemophagocytic lymphohistiocytosis (HLH). Bone survey and bone marrow biopsy were normal.
The symptoms of FUO, dysentery and FTT suggest he may have immunodeficiency. The results of his immunological tests revealed normal lymphocyte transformation tests (LTT) and decreases in CD3, CD4, and CD16 populations.
The results of immunological tests revealed normal lymphocyte transformation test (LTT) and decrease in CD3, CD4, and CD16 populations.
During his hospitalization, he received IVIG three times. He experienced frequent episodes of fever and diarrhea despite being given IVIG. He was discharged from the hospital with antibiotics, omeprazole, and sachet granule Mesalazine. A monthly IVIG was also administered to him.
Genetic findings
A homozygous missense variant (c.1572G > T, p.Q524H) was detected in exon 9 of the CASP8 gene (NM_001080125) out of a total of 55,356 variants (Table 2). This variant was not found in 1000G, ExAC, GnomAD, and the Iranian local database. Furthermore, in silico analysis predicted that this variant is deleterious (scores for CADD = 22.4, SIFT = 0, and PolyPhen = 0.944; MutationTaster predicts that as a disease causing variant) and affects a conserved amino acid. To the best of our knowledge, this variant has not yet been reported in constitutional (inherited) status.
Table 2.
Genetic result of the patient
| Gene/transcript | Variation location | Variant | Chromosome position (GRCh37) | Relationships | Zygosity | Variation classificationa |
|---|---|---|---|---|---|---|
| CASP8, ENST00000358485.4 and NM_001080125 | Exon 9 | c.1572G > T, p.Q524H | Chr2:202,151,272 | Patient | Homozygous | Likely Pathogenic |
| Father | Heterozygous | |||||
| Mother | Heterozygous |
aBased on American College of Genetics and Genomics (ACMG) standards and guidelines for the interpretation of sequence variants, 2015
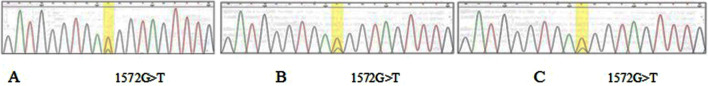
Based on ACMG guidelines, this variant met PS3 (Well-established functional study, as mentioned above), PM2 (absent in population databases), and PP3 (computational evidence) criteria. This variant would therefore be categorized as likely pathogenic. Sanger sequencing confirmed the homozygosity of the case and the heterozygosity of his parents. Figure 3 displays the zygosity status of the patient and his parents.
Fig. 3.
The chromatograph of the P8 mutation in caspase-8 gene. Illustration of the homozygosity in patient 8 (1572G > T) (A), the heterozygosity in the patient's father (B), the heterozygosity in the patient's mother (C)
The patient had a pathogenic variant in the HBB gene, considering the minor thalassemia status of the patient (NM_000518; c.93-21G > A).
III. Literature review
Clinical manifestations
Herein, we reviewed eight patients with caspase-8 deficiency. The first two early-onset patients in Table 3 (P1, P2) had lymphadenopathy and splenomegaly. They both showed sinopulmonary herpetic infections and did not respond well to immunization [19]. Although recurrent mucocutaneous herpetic infection is a common feature of caspase-8 deficiency [19], herpetic infection was not observed in the case of this study.
Table 3.
The demograghic data, immunological and genetic findings of 8 patients with caspase-8 deficiency
| Subject | Patient 1a [19] | Patient 2a [19] | Patient 3b [20] | Patient 4b[20] |
Patient 5 [21] |
Patient 6 [21] |
Patient 7 [21] |
Patient 8 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Age of onset (Year) |
12y | 11y | 38y | 37y | Whitin the first years of life | Within the first years of life | Within the first years of life | 2y | ||||
| Gender | Female | Male | Female | Male | NA | NA | NA | Male | ||||
| First presentation | LAP and splenomegaly | LAP and splenomegaly | Acute shortness of breath | Complex neurological syndrome | Recurrent fever | Bloody diarrhea, recurrent infections and fever | Non-bloody diarrhea, recurrent infections | FUO, dysentery | ||||
| Symptoms | Poor response to immunization, organomegaly, sinopulmonary and herpes simplex virus infection | Poor response to immunization, organomegaly, sinopulmonary and herpes simplex virus infection |
Pneumonia, Pulmonary hypertension, Interstitial lung disease, high grade fever, pancytopenia, multiple necrotizing and non-necrotizing granulomas of lung |
1 cm3 mass at the Meckel’s cave(necrotizing granuloma) aspiration pneumonia, bronchiectasis, organomegaly Multinodular lesions in liver and spleen, cranial nerve palsy/paresis |
FTT, diarrhea, perianal disease, proctocolitis, refractory colitis increased susceptibility to bacterial and viral infections, multiple food allergy with mild peripheral eosinophilia and chronic eosinophilic infiltration antrum and duodenum Chronic eczema, pityriasis amiantacea, and hypothyroidism |
FTT, diarrhea, perianal disease, proctocolitis, increased susceptibility to bacterial and viral infections, Refractory colitis |
FTT, perianal disease, proctocolitis. increased susceptibility to bacterial and viral infections, intestinal obstruction, Refractory colitis |
FUO, dysentry, FTT, lymphadenopathy, allergy to cow's milk protein, splenomegaly, Perianal fissures, Crohn's disease | ||||
| Failure to thrive | + | + | − | − | + | + | + | + | ||||
| Lymphadenopathy | + | + | − | − | + | − | − | − | ||||
| Splenomegaly | + | + | − | + | + | − | − | + | ||||
| Eczema | + | + | − | − | + | − | − | − | ||||
| Reactive airway disease | + | + | − | − | − | − | − | − | ||||
| Pneumonia | + | + | + | + | + | Not mentioned | + | − | ||||
| HSV labialis | + | + | − | − | − | − | − | − | ||||
| Chronic diarrhea | − | + | − | − | + | + | + | + | ||||
| Food allergy | − | − | − | − | + | − | − | + (bovine protein allergy) | ||||
| Infections | sinopulmonary and herpes simplex virus infection | sinopulmonary and herpes simplex virus infection | Acute EBV infection, Nocardia asteroids meningoencephalitis, pneumonia | Aspiration pneumonia | Pneumonia, otitis media, conjuctivitis, purulent dermatitis, molluscum contagiosum, and local infections | |||||||
| Cause of death | Nocardia asteroids meningoencephalitis | Progressive neurological and pulmonary complications | Alive | Septic complications | Alive | Alive | ||||||
| Genetic test |
homozygous CASP 8 mutation c.919C > T; p.R307W |
homozygous CASP 8 mutation c.919C > T; p.R307W |
homozygous CASP 8 mutation c.919C > T; p.R307W |
homozygous CASP 8 mutation c.919C > T; p.R307W |
homozygous CASP8 mutation c.836A>G, p.Q279R |
homozygous CASP8 mutation c.836A > G, p.Q279R |
homozygous CASP 8 mutation c.919C > T; p.R307W |
homozygous CASP8 mutation c.1572G > T p.Q524H |
||||
| Immunoglobulin concentration |
IgG: normal IgA: normal IgM: normal IgE: normal |
IgG: low IgA: normal IgM: low IgE:normal |
IgG: normal IgA: normal IgM: Low IgE: normal |
IgG: normal IgA: normal IgM: Low IgE: normal |
Not available | Not available | Not available |
IgG: normal IgA: low IgM: normal |
||||
| Total lymphocytes |
1510 (normal) |
2362 (normal) |
990 (low) |
3710 (high) |
Not available | Not available | Not available |
5487 (high) |
||||
| CD4% |
23.1 (low) |
25 (low) |
23.5 (low) |
9.3 (low) |
Not available | Not available | Not available | 10 | ||||
| CD8% |
49.6 (normal) |
46.2 (normal) |
65.6 (normal) |
87.6 (high) |
Not available | Not available | Not available | 22 | ||||
| CD4/CD8 ratio |
0.5 (low) |
0.5 (low) |
0.35 (low) |
0.1 (low) |
Not available | Not available | Not available |
0.45 (normal) |
||||
| B Lymphocytes | 361 | 570 | Not available | Not available | Not available | Not available | Not available | Not available | ||||
(Patient number 8 is the case presented in this study)
FTT failure to thrive, LAP lymphadenopathy, FUO fever of unknown origin, NA Not Available
aPatient number 1 and 2 were siblings
bPatients number 3 and 4 were siblings
The third case (P3) began to develop symptoms two months after giving birth to her baby. She experienced severe pulmonary involvement, a high fever, and pancytopenia. She underwent a lung transplant as a result of organ failure. The most incapacitating symptoms were recurring infections at different sites. She eventually died of meningoencephalitis caused by Nocardia. The complex neurological syndrome was the first manifestation and the cause of death in the fourth case (P4) [20].
The three other patients (P5, P6, P7) from unrelated consanguineous parents developed chronic diarrhea and failure to thrive and were eventually diagnosed with very early-onset IBD. Aside from having perianal disease, severe structuring, and fistulizing proctocolitis, they were prone to bacterial and viral infections [21]. Despite IBD being a frequent manifestation of caspase-8 deficiency, the mechanism is still unknown [21].
The clinical manifestations of caspase-8 deficiency can vary by age of onset, as shown in Table 3.
Multiple organ failure, organomegaly, and lymphocyte infiltrations are common symptoms of adult-onset disorders [20]. Furthermore, the nervous system was involved in all adult-onset cases [20]. In contrast, early-onset patients typically suffer from FTT, organomegaly, and infection. In all cases, the development was normal [5, 19, 21]. Hypersensitivity is common among individuals with early-onset disease, but none of the adult-onset patients exhibited with hypersensitivity [19]. In both early-onset and adult-onset patients, the only common symptom was recurrent infections.
Diarrhea and dysentery may be the first or main symptoms in patients with inborn errors of immunity [21]. The initial symptom in our case was a bloody stool, which was misdiagnosed as cow's milk allergy and infectious gastroenteritis, but elemental formula and antibiotics were ineffective. Consequently, a pediatric gastroenterologist suspected IBD. In light of this, it is relevant to note that very early-onset IBD (VEO-IBD) is an unusual condition, so these patients must be screened for inborn errors of immunity. IBD that appears under the age of 6 is called VEO-IBD [22]. In most cases of monogenic IBD, children younger than six years old are affected. The monogenic IBD is more prevalent among infants less than two years old, who are classified as infantile-onset IBD [22, 23]. The underlying causes of monogenic IBD are often PIDs, which indicates the importance of immune system dysregulation such as caspase-8 deficiency in VEO-IBD [22]. In our case as well as other reports, FTT, chronic diarrhea, eczema, other allergic disorders, lymphadenopathy, and splenomegaly were observed as the most common manifestations of early-onset caspase-8 deficiency.
Laboratory findings
As shown in Table 3, our case had a higher number of lymphocytes than the two previous early-onset cases. Early-onset cases had CD4/CD8 ratio of around 0.5.
In both cases of late-onset disease, the immunoglobulin levels changed in a similar way. Low level of IgM was observed in both cases [20].
Serum immunoglobulins level could be in the normal range or low, but the humoral response was insufficient. Despite normal T-cell counts, pediatric patients had reverse CD4 + /CD8 + ratios.
Genetic abnormalities
In eight patients with caspase-8 deficiency, three different homozygous variants including (NM_001080125): c.919C > T (p.R307W), c.836A > G (p.Q279R), and c.1572G > T (p.Q524H) were found.
Potential effects of the different mutations on molecular mechanisms
Two mutations p.R307W and p.Q279R are located in the large catalytic p18 domain of caspase-8 [24] so they might affect the catalytic activity of the protein. It has been reported that the variant p.R307W causes the protein to lose its enzymatic properties, as well as reducing its stability [19].
The germline homozygous Q524H variant (they named this variant as the Q482H, based on NM_001228.4 transcript) detected in our patient has been previously reported in 7.53% acute myeloid leukemia (AML) cases with somatic status. They found that the Q524H variant hindered the dimerization of procaspase 8. Consequently, caspase-8 mediated apoptotic signaling is impaired, and chemotherapy resistance may eventually develop. Anti-apoptotic effect was functionally confirmed [25], and it is consistent with the underlying ALPS-IIB mechanism.
A number of patients have reportedly been impacted by ALPS-IIB. In a recent study, a patient with similar phenotypes to our patient was reported to have another homozygote missense variant (c.1358C > T;p.Pro453Leu)[26]. The authors found impaired cleavage of procaspase 8 and decreased apoptosis induced by FAS in effector memory T cells.
In conclusion, caspase-8 deficiency is a rare immunological disorder with various clinical presentations. It is recommended that patients with recurrent infections and atypical immunological symptoms be evaluated for this deficiency.
This manuscript presented the clinical manifestations of the eighth caspase-8 deficiency with IBD-like syndrome. We also reviewed the previous 7 caspase-8 deficiency cases and compared early-onset and adult-onset cases.
Acknowledgements
Not applicable.
Author contributions
NB and AT contributed to the acquisition of data, drafting the article. ZG, MK and AK contributed to genetic analysis, intrepretation and revising the manuscript. DN, AK, MF, MJ contributed to the acquisition of data, managing the patient, revising the manuscript. ZCH and SSH contributed to the acquisition of data, managing the patient, intrepretation of results, revising the article. All authors read and approved the final version of manuscript and have agreed to be accountable for all aspects of the study.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The written informed consent was obtained from the parents of the patient for publication.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Narges Bazgir and Azin Tahvildari contributed equally to this study and are the first authors on this article
References
- 1.Oliveira JB, Fleisher T. Autoimmune lymphoproliferative syndrome. Curr Opin Allergy Clin Immunol. 2004;4(6):497–503. doi: 10.1097/00130832-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Fritsch M, Günther SD, Schwarzer R, Albert MC, Schorn F, Werthenbach JP, et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575(7784):683–687. doi: 10.1038/s41586-019-1770-6. [DOI] [PubMed] [Google Scholar]
- 3.Schleich K, Buchbinder JH, Pietkiewicz S, Kähne T, Warnken U, Öztürk S, et al. Molecular architecture of the DED chains at the DISC: regulation of procaspase-8 activation by short DED proteins c-FLIP and procaspase-8 prodomain. Cell Death Differ. 2016;23(4):681–694. doi: 10.1038/cdd.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91(4):443–446. doi: 10.1016/S0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 5.Grzela T, Krauze A, Grzela K, Lazarczyk M, Niderla J, Brawura-Biskupski-Samaha R, et al. Impaired apoptosis of lymphocytes derived from patient with decreased expression of caspase-8 results in Alps-like phenotype. Int J Mol Med. 2004;14(5):937–942. [PubMed] [Google Scholar]
- 6.Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9(2):267–276. doi: 10.1016/S1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 7.Puck JM, Zhu S. Immune disorders caused by defects in the caspase cascade. Curr Allergy Asthma Rep. 2003;3(5):378–384. doi: 10.1007/s11882-003-0070-1. [DOI] [PubMed] [Google Scholar]
- 8.Hafezi N, Zaki-Dizaji M. Clinical, immunological, and genetic features in 780 patients with autoimmune lymphoproliferative syndrome (ALPS) and ALPS-like diseases: a systematic review. Pediatr Allergy Immunol. 2021;32(7):1519–1532. doi: 10.1111/pai.13535. [DOI] [PubMed] [Google Scholar]
- 9.Saberi M, Golchehre Z, Salmani H, Karamzade A, Tabatabaie SZ, Keramatipour M. First report of Klein-Waardenburg Syndrome in Iran and a novel pathogenic splice site variant in PAX3 gene. Int J Pediatr Otorhinolaryngol. 2018;113:229–233. doi: 10.1016/j.ijporl.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Chang X, Wang K. wANNOVAR: annotating genetic variants for personal genomes via the web. J Med Genet. 2012;49(7):433–436. doi: 10.1136/jmedgenet-2012-100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karczewski KJ, Weisburd B, Thomas B, Solomonson M, Ruderfer DM, Kavanagh D, et al. The ExAC browser: displaying reference data information from over 60 000 exomes. Nucleic Acids Res. 2017;45(D1):D840–D845. doi: 10.1093/nar/gkw971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.gnomAD. https://gnomad.broadinstitute.org00000000/.
- 13.MutationTaster. https://www.mutationtaster.org/.
- 14.CADD—Combined Annotation Dependent Depletion. https://cadd.gs.washington.edu/.
- 15.Quang D, Chen Y, Xie X. DANN: a deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics. 2015;31(5):761–763. doi: 10.1093/bioinformatics/btu703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S. Identifying a high fraction of the human genome to be under selective constraint using GERP++ PLoS Comput Biol. 2010;6(12):e1001025. doi: 10.1371/journal.pcbi.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ClinVar. https://www.ncbi.nlm.nih.gov/clinvar.
- 18.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419(6905):395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 20.Niemela J, Kuehn HS, Kelly C, Zhang M, Davies J, Melendez J, et al. Caspase-8 deficiency presenting as late-onset multi-organ lymphocytic infiltration with granulomas in two adult siblings. J Clin Immunol. 2015;35(4):348–355. doi: 10.1007/s10875-015-0150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehle AS, Farin HF, Marquardt B, Michels BE, Magg T, Li Y, et al. Intestinal inflammation and dysregulated immunity in patients with inherited caspase-8 deficiency. Gastroenterology. 2019;156(1):275–278. doi: 10.1053/j.gastro.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 22.Ouahed J, Spencer E, Kotlarz D, Shouval DS, Kowalik M, Peng K, et al. Very early onset inflammatory bowel disease: a clinical approach with a focus on the role of genetics and underlying immune deficiencies. Inflamm Bowel Dis. 2020;26(6):820–842. doi: 10.1093/ibd/izz259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147(5):990–1007.e3. doi: 10.1053/j.gastro.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandal R, Barrón JC, Kostova I, Becker S, Strebhardt K. Caspase-8: the double-edged sword. Biochimica et Biophysica Acta (BBA) Rev Cancer. 2020;1873(2):188357. doi: 10.1016/j.bbcan.2020.188357. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Yao J, Zhang X, Chen X, Chen J, Guan Y, et al. Q482H mutation of procaspase-8 in acute myeloid leukemia abolishes caspase-8-mediated apoptosis by impairing procaspase-8 dimerization. Biochem Biophys Res Commun. 2018;495(1):1376–1382. doi: 10.1016/j.bbrc.2017.11.168. [DOI] [PubMed] [Google Scholar]
- 26.Kanderova V, Grombirikova H, Zentsova I, Reblova K, Klocperk A, Fejtkova M, et al. Lymphoproliferation, immunodeficiency and early-onset inflammatory bowel disease associated with a novel mutation in Caspase 8. Haematologica. 2019;104(1):e32–e34. doi: 10.3324/haematol.2018.201673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.