Abstract
Relapse is a major limitation of chimeric antigen receptor (CAR) T-cell therapy. Here, we speculated that decitabine (DAC) in combination with fludarabine and cyclophosphamide (FC) as a lymphodepletion regimen may improve the efficacy of CD19/CD22 CAR T-cell therapy. Fourteen of 26 patients with relapsed/refractory B cell acute lymphoblastic leukemia (r/r B-ALL) without remission before lymphodepletion treatment were treated with DAC (total dose 100 mg/m2 in 3 days) followed by the FC regimen (DAC group), while twelve patients received the FC regimen (CON group). On Day 28 after CAR T-cells infusion, no significant differences in complete remission (CR) and minimal residual disease negative CR rates were found between both groups. However, there were significant differences in overall survival (OS) and leukemia-free survival (LFS) between two groups: 3-year OS, 92.3% (DAC) versus 41.7% (CON), P = 0.005 and 3-year LFS, 92.9% (DAC) versus 27.3% (CON), P < 0.001. There was no significant difference in the incidence of cytokine release syndrome between both groups. Median time to platelet and neutrophil counts recovery was similar in both groups. All adverse events were reversible and manageable. In conclusion, DAC in combination with the FC lymphodepletion regimen may be a new treatment option that can improve the efficacy of CAR T-cell therapy in r/r B-ALL.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40164-023-00397-z.
Keywords: Decitabine, Chimeric antigen receptor T cell, Relapsed/refractory, Acute lymphoblastic leukemia, Lymphodepletion
To the editor
Antigen escape-mediated relapse is a major limitation of chimeric antigen receptor (CAR) T-cell therapy. One strategy to overcome antigen escape following CAR T-cell therapy is to generate T cells simultaneously targeting both CD19 and CD22. Although some clinical trials, using CD19/CD22 bispecific CAR T-cell therapy, have demonstrated promising therapeutic efficacy. CD19/CD22 CAR T-cell therapy has not induced durable remissions or reduced the relapse rate in patients with relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL) [1, 2]. Relapse after CD19/CD22 CAR T-cell therapy is often associated with poor persistence of CAR T- cells in part caused by T cell exhaustion and the immunosuppressive microenvironment, which suggests the need for novel strategies to improve CD19/CD22 CAR T-cell therapy.
The hypomethylating agent decitabine (DAC) has been demonstrated to reverse T cell exhaustion, increase antigen expression, enhance T cell activation and modify the tumor microenvironment [3–7]. In addition, DAC combined with cytotoxic chemotherapy represents a promising strategy for the treatment of patients with high tumor burden [8], which is a significant predictor of poor prognosis in B-ALL. Therefore, we speculated that DAC in combination with fludarabine and cyclophosphamide (FC) as a lymphodepletion regimen may synergize and improve the efficacy of CAR T-cell therapy.
We retrospectively analyzed 26 r/r B-ALL patients without remission before lymphodepletion treatment who were enrolled in a phase 1/2 clinical trial of CD19/CD22 CAR T-cell therapy (NCT03614858) from October 2017 to May 2021 at the First Affiliated Hospital of Soochow University (Additional file 1: Figure S1). Fourteen patients received DAC combined with the FC regimen (DAC group) while twelve patients were treated with FC alone (CON group) followed by CAR T-cell therapy. The patients received DAC combined with FC depending on disease characteristics such as TP53 mutation, comorbidities, patients' wishes, and economic burden. Patients received the following lymphodepletion regimen: FC (fludarabine 30 mg/m2/day and cyclophosphamide 300 mg/m2/day) on days -5 to -3, with or without DAC (total dose 100 mg/m2 from day -6 to -4; Additional file 2: Figure S2).
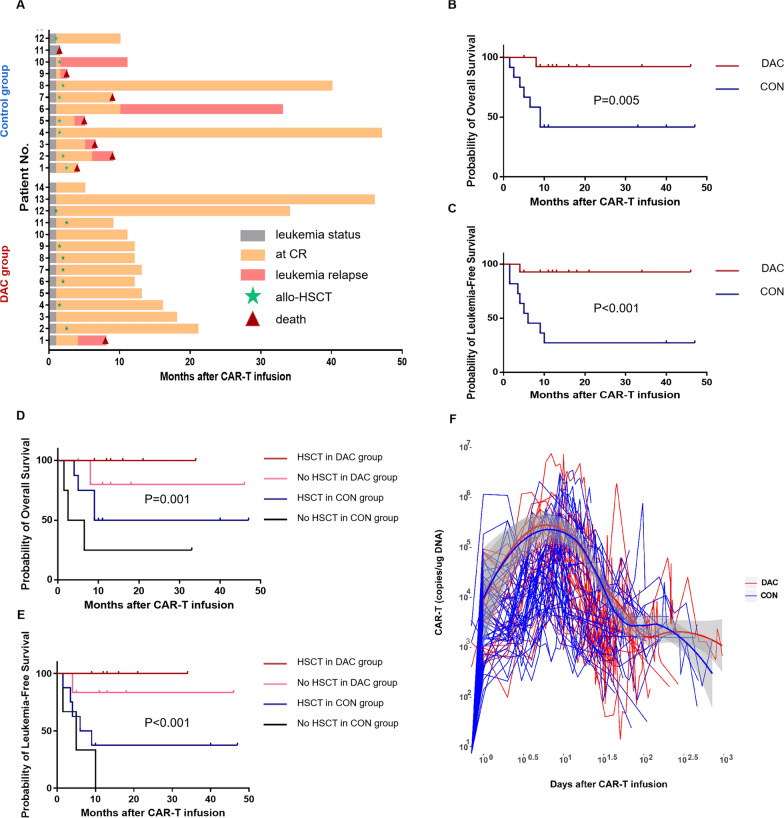
There were more patients (42.9%) who relapsed after allogeneic hematopoietic stem cell transplantation (allo-HSCT) prior to CAR T-cell therapy in the DAC group than in the CON group (P = 0.017) (Table 1). On Day 28 after CAR T-cells infusion, no significant difference in minimal residual disease negative CR rates was found between both groups (Additional file 3: Table S1). Among the nontransplant patients in the DAC group, only one patient (16.7%, 1/6) relapsed. However, 1 of four nontransplant patients in the CON group had no response after CAR T-cell therapy and 3 patients relapsed after CAR T-cell treatment (Fig. 1A).
Table 1.
Baseline Characteristics of Patients
| Baseline characteristics of patients | |||
|---|---|---|---|
| Characteristic | DAC group (14) | Control group (12) | P value |
| Gender | |||
| Male | 10 (71.4%) | 5 (41.7%) | 0.233 |
| Female | 4 (28.6%) | 7 (58.3%) | |
| Age, median | 26.5 (8–52) | 31 (16–74) | 0.226 |
| Ph + ALL, n (%) | 2 (14.3%) | 5 (41.7%) | 0.190 |
| Ph-like ALL, n (%) | 3 (21.4%) | 2 (16.7%) | 1.000 |
| White blood cell ≥ 50 × 10^9/L, n (%) | 6 (42.9%) | 2 (16.7%) | 0.216 |
| Extramedullary leukemia, n (%) | 2 (14.3%) | 0 (0%) | 0.483 |
| Monosomal karyotype, n (%) | 0 (0%) | 1 (8.3%) | 0.462 |
| Complex karyotype, n (%) | 2 (14.3%) | 2 (16.7%) | 1.000 |
| KMT2A rearranged, n (%) | 1 (7.1%) | 0 (0%) | 1.000 |
| TP53 mutation or deletion, n (%) | 2 (14.3%) | 0 (0%) | 0.483 |
| T315I mutation, n (%) | 1 (7.1%) | 2 (16.7%) | 0.580 |
| Poor-risk cytogenetics, n (%) | 8 (57.1%) | 8 (66.7%) | 0.701 |
| Prior cycles of therapy, median (range) | 4 (2–16) | 6.5 (1–20) | 0.251 |
| < 5 cycles of therapy, n (%) | 9 (64.3%) | 4 (33.3%) | 0.235 |
| ≥ 5 and < 10 lines of therapy, n (%) | 2 (14.3%) | 5 (41.7%) | |
| ≥ 10 cycles of therapy, n (%) | 3 (21.4%) | 3 (25%) | |
| Primary refractory to chemotherapy, n (%) | 2 (14.3%) | 3 (25%) | 0.635 |
| Numbers of relapses, median (range) | 1 (0–2) | 1 (0–2) | 0.685 |
| Relapse after previous HSCT, n (%) | |||
| Yes | 6 (42.9%) | 0 (0%) | 0.017 |
| No | 8 (57.1%) | 12 (100%) | |
| Blasts in BM before lymphodepletion treatment, median (range) | 35.75 (5–82) | 43.75 (6–85.5) | 0.487 |
| LDH, pre-lymphodepletion, median (range) | 173.8 (106.5–644) | 168.7 (83.6–722.2) | 0.956 |
| Ferritin, pre-lymphodepletion, median (range) | 774.945 (234.06–2718.84) | 1614.66 (411.63–2663.69) | 0.140 |
| CRP, pre-lymphodepletion, median (range) | 5.115 (0.28–15.36) | 1.195 (0.12–15.36) | 0.170 |
| ECOG, pre-lymphodepletion, median (range) | 2 (1–3) | 2 (1–3) | 0.609 |
| CAR T-cell dose, 10^7/kg, median (range) | 1 (0.5–2) | 1 (0.5–2.5) | 0.950 |
| Source of CAR T-cells, n (%) | |||
| Autologous | 10 (71.4%) | 12 (100%) | 0.100 |
| Donor-derived allogenic | 4 (28.6%) | 0 (0%) | |
| Transduction rate (%), median | 31.435 (13.02–64.35) | 44.685 (6.07–63.92) | 0.643 |
Fig. 1.
Treatment response, overall survival probability, long-term prognosis and CAR T-cells persistence. A Clinical outcomes of patients treated with CD19/CD22 CAR T-cells. Clinical outcomes, treatment response of each patient after CD19/CD22 CAR T-cell therapy and the duration of response. Patient number is shown to the left. B–C Survival analysis. OS and LFS from the day of CAR T-cells infusion are shown for patients who received FC lymphodepletion with DAC (DAC, n = 14) compared with those who received FC alone (CON, n = 12). D–E Survival analysis of 4 subgroups. The OS and LFS were prolonged by allo-HSCT in all subgroups. F The expansion and persistence of CAR T-cells. The presence of CD19/CD22 CAR T-cells in the peripheral blood as assessed by quantitative real-time polymerase chain reaction (PCR) assay. Time points after a second CAR T-cells infusion or allo-HSCT are excluded. We analyzed the kinetics of CAR T-cell counts by using LOESS (Local Polynomial Regression) curve fitting. Bold line represents the averaged data using LOESS curve fitting approximation with the standard error in grey
With a median follow-up of 13 months, there were significant differences in overall survival (OS) and leukemia-free survival (LFS) between both groups (Fig. 1B, C). Patients who underwent allo-HSCT after CAR T-cell therapy in the DAC or the CON group had higher OS and LFS than those without allo-HSCT, although the number of patients in some subgroups was relatively small (Fig. 1D-E). CAR T- cells copies in the peripheral blood were detected by qPCR at several indicated time points after infusion in all patients (Fig. 1F).
There was no statistically significant difference in the incidence of cytokine release syndrome between both groups (Additional file 4: Table S2). All adverse events were reversible and manageable. The medians of peak type 1 helper T (Th1) cytokines (IL-2 and IFN-γ) concentrations were higher in the DAC group than in the CON group. However, in regard to type 2 helper T (Th2) cytokines, the peak value of serum IL-4 after CAR T-cells infusion was significantly higher in the CON group than in the DAC group (P = 0.029; Additional file 5: Table S3).
There are several potential mechanisms underlying the therapeutic benefit of DAC-based lymphodepletion prior to CAR T-cell therapy: 1. DAC can upregulate CD19 expression to make leukemia cells more susceptible to CAR T-cell therapy [5]. 2. DAC can inhibit the methylation of tumor suppressor genes associated with B-ALL and induce leukemia cell apoptosis at high doses. 3. DAC pretreatment can modify the immunosuppressive tumor microenvironment to enhance CAR T-cell efficacy and endogenous immunity, leading to long-term antileukemia immunity [9]. In our study, an increased level of IL-4 was detected in the CON group compared with the DAC group, which suggested that DAC depolarized Th2 cells and inhibited tumor growth [10].
In summary, our data demonstrated that the combination of DAC and FC as a conditioning regimen was safe and effective for Chinese patients with r/r B-ALL. DAC in combination with the FC lymphodepletion regimen may be a new treatment option that can improve the efficacy of CAR T-cell therapy in r/r B-ALL. Moreover, CD19/CD22 CAR T-cell therapy as a bridge to allo-HSCT could be a promising strategy for r/r B-ALL patients to achieve prolonged OS and LFS. However, due to the small sample size and retrospective nature of this study, large-scale randomized controlled clinical trials should be prospectively conducted to confirm our results. Further studies are warranted to determine the key factors and pathways that underlie the synergistic antitumor effect of DAC and CAR T-cells.
Supplementary Information
Additional file 1: Figure S1. A schematic diagram of patient allocation, selection and exclusion. Patients were enrolled in the phase 1/2 clinical trial of CD19/CD22 CAR T-cell therapy (NCT03614858) from October 2017 to May 2021 at the First Affiliated Hospital of Soochow University. The patients received DAC combined with FC depending on disease characteristics such as TP53 mutation, comorbidities, patients' wishes.
Additional file 2: Figure S2. Schematic diagram of anti-CD19/CD22 CAR and the clinical protocol procedures. (A) Schematic diagram of anti-CD19/CD22 CAR. The third-generation CAR used in this study was composed of single-chain variable fragments derived from murine monoclonal antibodies against human CD19 and CD22, 2 costimulatory domains from CD28 and OX40, and the CD3-ζchain as the activation domain. SP, signal peptide; VL, variable L chain; VH, variable H chain; TM, transmembrane. (B) Schematic diagram of study procedures. After providing written informed consent, patients with r/r B-ALL underwent leukapheresis, lymphodepleting chemotherapy: FC (fludarabine 30 mg/m2/day and cyclophosphamide 300 mg/m2/day) on days -5 to -3, with or without DAC (total dose 100 mg/m2 in 3 days from day -6 to -4). CD19/CD22 CAR T-cells were infused on successive days from day zero. Bone marrow aspiration was performed for response assessment every month for half a year and every 3 months thereafter. All patients were followed up until they died, lost to follow-up, or withdrew consent. Suitable patients received allo-HSCT within 3 months after CAR T-cell therapy.
Additional file 5. Peak numbers of CAR T-cells and cytokine levels.
Acknowledgements
The authors would like to thank all members of the study team, the patient and their family, and Shanghai Unicar-Therapy Bio-medicine Technology Co., Ltd.
Author contributions
YM collected, analyzed research data, performed statistical analyses, wrote and edited the manuscript; SL, XL, MC and LL collected research data; XT, HD, QC, WC and JY treated the patients; LK and LY designed the clinical CAR vector, supervised the production of CAR T-cell Product; DW and XT conceived of the study and revised the paper. All authors have read and approved the final manuscript.
Funding
This work was supported by research grants from National Natural Science Foundation of China (81873443, 82070162), The Key Science Research Project of Jiangsu Commission of Health (K2019022), Translational Research Grant of NCRCH (2020ZKZC04) and Natural Science Foundation of Jiangsu Province (BK20201169), Bethune Charitable Foundation (BCF-IBW-XY-20220930-08, BCF-IBW-XY-20220930-13), Suzhou diagnosis and treatment project of Clinical Key Diseases (LCZX202201), Frontier Clinical Technical Project of Suzhou Science and Technology plan (SKY2022001), Natural Science Foundation Boxi Training Plan of The First Affiliated Hospital of Soochow University (BXQN202206), China International Medical Foundation (Z-2018-31-2102-4), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Availability of data and materials
The datasets supporting the conclusions are included within this article.
Declarations
Ethics approval consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (2022445) and was conducted in accordance with the Declaration of Helsinki principles. All patients provided written informed consent.
Consent for publication
Written informed consent was obtained from the patient and his parents.
Competing interests
All authors declare there are no competing interest to report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yunju Ma, Haiping Dai, Qingya Cui have contributed equally to this work.
Contributor Information
Depei Wu, Email: drwudepei@163.com.
Xiaowen Tang, Email: xwtang1020@163.com.
References
- 1.Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):30–39. doi: 10.1186/s13045-020-00856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Zhou Y, Zhang M, Ge W, Li Y, Yang L, et al. CRISPR/Cas9-engineered universal CD19/CD22 dual-targeted CAR-T cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia. Clin Cancer Res. 2021;27(10):2764–2772. doi: 10.1158/1078-0432.CCR-20-3863. [DOI] [PubMed] [Google Scholar]
- 3.Nahas MR, Stroopinsky D, Rosenblatt J, Cole L, Pyzer AR, Anastasiadou E, et al. Hypomethylating agent alters the immune microenvironment in acute myeloid leukaemia (AML) and enhances the immunogenicity of a dendritic cell/AML vaccine. Br J Haematol. 2019;185(4):679–690. doi: 10.1111/bjh.15818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Tong C, Dai H, Wu Z, Han X, Guo Y, et al. Low-dose decitabine priming endows CAR T cells with enhanced and persistent antitumour potential via epigenetic reprogramming. Nat Commun. 2021;12(1):409. doi: 10.1038/s41467-020-20696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Xue L, Wang M, Qiang P, Xu H, Zhang X, et al. Decitabine enhances cytotoxic effect of T cells with an anti-CD19 chimeric antigen receptor in treatment of lymphoma. Onco Targets Ther. 2019;12:5627–5638. doi: 10.2147/OTT.S198567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.You L, Han Q, Zhu L, Zhu Y, Bao C, Yang C, et al. Decitabine-mediated epigenetic reprograming enhances anti-leukemia efficacy of CD123-targeted chimeric antigen receptor T-cells. Front Immunol. 2020;11:1787. doi: 10.3389/fimmu.2020.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prinzing B, Zebley CC, Petersen CT, Fan Y, Anido AA, Yi Z, et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med. 2021;13(620):272. doi: 10.1126/scitranslmed.abh0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benton CB, Thomas DA, Yang H, Ravandi F, Rytting M, O'Brien S, et al. Safety and clinical activity of 5-aza-2'-deoxycytidine (decitabine) with or without Hyper-CVAD in relapsed/refractory acute lymphocytic leukaemia. Br J Haematol. 2014;167(3):356–365. doi: 10.1111/bjh.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Zhang Y, Chen M, Mei Q, Liu Y, Feng K, et al. Increased IFNgamma(+) T cells are responsible for the clinical responses of low-dose DNA-Demethylating agent decitabine antitumor therapy. Clin Cancer Res. 2017;23(20):6031–6043. doi: 10.1158/1078-0432.CCR-17-1201. [DOI] [PubMed] [Google Scholar]
- 10.Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19(11):776–800. doi: 10.1038/s41573-020-0077-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. A schematic diagram of patient allocation, selection and exclusion. Patients were enrolled in the phase 1/2 clinical trial of CD19/CD22 CAR T-cell therapy (NCT03614858) from October 2017 to May 2021 at the First Affiliated Hospital of Soochow University. The patients received DAC combined with FC depending on disease characteristics such as TP53 mutation, comorbidities, patients' wishes.
Additional file 2: Figure S2. Schematic diagram of anti-CD19/CD22 CAR and the clinical protocol procedures. (A) Schematic diagram of anti-CD19/CD22 CAR. The third-generation CAR used in this study was composed of single-chain variable fragments derived from murine monoclonal antibodies against human CD19 and CD22, 2 costimulatory domains from CD28 and OX40, and the CD3-ζchain as the activation domain. SP, signal peptide; VL, variable L chain; VH, variable H chain; TM, transmembrane. (B) Schematic diagram of study procedures. After providing written informed consent, patients with r/r B-ALL underwent leukapheresis, lymphodepleting chemotherapy: FC (fludarabine 30 mg/m2/day and cyclophosphamide 300 mg/m2/day) on days -5 to -3, with or without DAC (total dose 100 mg/m2 in 3 days from day -6 to -4). CD19/CD22 CAR T-cells were infused on successive days from day zero. Bone marrow aspiration was performed for response assessment every month for half a year and every 3 months thereafter. All patients were followed up until they died, lost to follow-up, or withdrew consent. Suitable patients received allo-HSCT within 3 months after CAR T-cell therapy.
Additional file 5. Peak numbers of CAR T-cells and cytokine levels.
Data Availability Statement
The datasets supporting the conclusions are included within this article.