Abstract
Objective:
Vasomotor symptoms (VMS) are prevalent symptoms that can have a negative impact on quality of life. VMS have also been linked to cardiovascular disease risk, yet the mechanisms underlying these associations have not been elucidated. Some initial work links VMS to adverse adipokine profiles, or cytokines produced by adipose tissue. However, results are not entirely consistent and are based entirely on self-report VMS, which is influenced by a range of memory and reporting biases. The aim of this work is to test whether physiologically-assessed VMS are associated with lower adiponectin, the most abundant adipokine in the body, controlling for confounding factors. We also consider whether adiponectin explains previously-documented relationships between VMS and carotid atherosclerosis.
Methods:
300 peri- and postmenopausal nonsmoking women ages 40–60 enrolled in the MsHeart Study comprised the analytic sample. Women were free of hormone therapy or other medications impacting VMS, insulin-dependent diabetes, and cardiovascular disease. Participants underwent ambulatory physiologic VMS monitoring, physical measures, a carotid ultrasound, and fasting phlebotomy.
Results:
More frequent physiologically-assessed VMS were associated with lower adiponectin [b(SE)=−.081 (.028), p=.004; or .081 lower ug/mL in adiponectin for each additional VMS over 24 hours], controlling for age, race/ethnicity, education, insulin resistance, and waist circumference. Associations were not explained by endogenous estradiol. Adiponectin did not explain associations between VMS and carotid atherosclerosis.
Conclusions:
Physiologic VMS were associated with lower adiponectin after considering potential confounders. The role of adipokines in VMS as well as in links between VMS and health warrant further attention.
Keywords: Vasomotor symptoms, menopause, hot flashes, adipokines, adiponectin, women
Introduction
Vasomotor symptoms (VMS), also known as hot flashes and night sweats, are a prevalent menopausal symptom1 that are well-documented to have a significant negative impact on quality of life and functioning for women in the menopause transition.2 For many women, VMS last a decade or more.3 In addition to their impact on quality of life and functioning, an emerging body of research also links VMS to indicators of cardiovascular disease (CVD) risk, including greater CVD risk factors, subclinical atherosclerosis, and incident clinical CVD events.4,5 However, the mechanisms underlying these relationships remain unclear.
Adipokines are cytokines produced by adipose tissue, with adiponectin being the most abundant adipokine in the body. Adiponectin is an anti-inflammatory adipokine that has a range of salubrious physiologic actions, including suppressing hepatic gluconeogenesis, promoting insulin sensitivity, inhibiting inflammation, and enhancing cell survival.6 Multiple studies show higher adiponectin to be associated with better cardiovascular health, including lower risk of CVD events.7,8
Emerging studies indicate that VMS may be associated with lower adiponectin. We previously found that self-reported VMS were linked to lower adiponectin among 536 midlife women participating the longitudinal cohort study the Study of Women’s Health Across the Nation.9 Another cross-sectional study of 151 midlife women found self-reported VMS linked to lower adiponectin and higher leptin levels.10 Finally, a cross-sectional analysis of 898 postmenopausal women found self-reported VMS associated with higher levels of ghrelin, but not adiponectin.11 Thus, there appear to be links between VMS and adiponectin, but studies are few and associations are not entirely consistent.
A limitation to the existing work is the reliance on self-reported VMS. Whereas self-report VMS measures are critical to understand the subjective experience of VMS, these measures are well-documented to incorporate multiple memory and reporting biases; for example, poor sleep or negative mood affects VMS recall and reporting.12,13 Further, self-report measures, particularly diary measures, require adherence, and non-adherence can masquerade as the absence of VMS. Moreover, self-report measures are not ideal for events occurring during sleep. Physiologic measures of VMS exist. These measures assess VMS physiologically as a woman goes about her daily activities and during wake and sleep.14 Physiologic measures avoid the memory, reporting, and adherence influences inherent in self-report.
In a sample of 300 midlife women who underwent ambulatory physiologic VMS monitoring, we tested the relationship between physiologically-assessed VMS and adiponectin. We hypothesized that more frequent VMS would be associated with lower adiponectin levels; we considered a range of factors in these associations, including age, race, adiposity, insulin resistance, and endogenous estradiol. As we previously documented associations between VMS and carotid atherosclerosis,15 we further tested whether adiponectin accounted for the associations between VMS and carotid atherosclerosis. Finally, given our prior work showing that the relationship between VMS and adiponectin varied by menopause stage,9 we tested whether associations between VMS varied by the time since the last menstrual period.
Methods
Sample
The study sample included 304 late perimenopausal (2–12 months amenorrhea)16 and postmenopausal (≥12 months amenorrhea)17 nonsmoking women aged 40–60. Participants were enrolled in MsHeart, a study about hot flashes and CVD risk, the methods of which have been published previously.15 Briefly, by design, half of the women reported daily VMS, and half reported no VMS in the past three months. Exclusion criteria included hysterectomy and/or bilateral oophorectomy; history of heart disease, stroke, arrhythmia, ovarian/gynecological cancer, pheochromocytoma, pancreatic tumor, kidney failure, seizures, Parkinson’s disease, Raynaud’s Phenomenon; current pregnancy; or having used select medications in the past 3 months (oral/transdermal estrogen or progesterone, selective estrogen receptor modulators, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, gabapentin, insulin, beta blockers, calcium channel blockers, and alpha-2 adrenergic agonists). Women who had undergone endometrial ablation, endarterectomy, or who were undergoing dialysis or chemotherapy were also excluded. All 304 women had usable physiologic VMS and adiponectin data. However, one woman was excluded due to an extreme adiponectin value (>7 standard deviations from the mean). An additional three women were excluded from multivariable models that included homeostatic model assessment (HOMA) due to missing data; thus, primary multivariable adjusted models included 300 women. In secondary models that considered carotid atherosclerosis, an additional 4 additional women were excluded due to missing ultrasound data, yielding 296 women in secondary models.
Design and Procedures
Women were recruited from the community via advertisements, mailings, and message boards. Once enrolled, they underwent physical measurements, ambulatory VMS monitoring, phlebotomy, and carotid artery ultrasound. Procedures were approved by the University of Pittsburgh Institutional Review Board. Participants provided written, informed consent.
Measures
VMS
Participants completed three days of ambulatory VMS monitoring, which included 24 hours of physiologic VMS monitoring and three days of electronic VMS diary completion. During the first laboratory visit, participants were equipped with a VMS monitor (VU-AMS, VU University Amsterdam, Netherlands), a wearable monitor that quantifies VMS via sternal skin conductance, a validated physiologic measure of VMS.14 The VMS includes an event mark button. In addition, participants were provided with an electronic VMS diary and instructed on its use. Participants reported VMS in two ways, which collectively provide date and time-stamped VMS reports: 1) by pressing the event mark button on the VMS monitor (first 24 hours only), and 2) by completing an electronic VMS diary entry (all three days). After being equipped with the monitor and diary, participants wore the VU-AMS monitor for 24 hours, after which time they removed it and stored it in a carrying case. For the remaining two days, women carried the VMS diary only. At the end of the three days, participants returned to the laboratory and returned study equipment. Physiologic VMS data were downloaded and scored via UFI software (DPS; Morro Bay, CA) according to validated methods that have demonstrated reliability, including in the present laboratory (ĸ=.86). Only VU-AMS files with ≥70% of usable data were included. For both self-reported and physiologic VMS, VMS rates were calculated as number of VMS/monitoring time and standardized to a 24-hr day to account for variations in monitoring durations.
Adiponectin
Phlebotomy was performed after a 12-hr fast. Phlebotomy was performed after a 12-hr fast. Adiponectin [total and high molecular weight (HMW)] was assessed in serum via a multimeric assay based on a sandwich ELISA format using reagents obtained from ALPCO Diagnostics (Salem, NH). Blanks, standards (0.075 to 4.8 ng/ml), control pools and samples (in duplicate) are run during each assay. The intra and inter-assay coefficients of variation for total adiponectin were 6.6% and 9.7%, respectively, and for HMW adiponectin, 7.2% and 12.1%, respectively.
Other Measures
Height and weight were measured via a fixed stadiometer and balance beam scale. Body mass index (BMI) was calculated (kg/m2). Waist circumference was measured at the natural waist (the narrowest part of the torso as seen from the anterior aspect). If a waist narrowing was difficult to identify, the measure was taken at the smallest circumference in the area between the ribs and the iliac crest. Demographics, medical, and reproductive history were assessed by standard instruments. Time since the last menstrual period was time in months between the last reported menstrual period and the study visits. Glucose and insulin were measured enzymatically. HOMA, reflecting insulin resistance, was calculated.18 Estradiol (E2) was assessed via liquid chromatography-tandem mass spectrometry (LC-MS/MS), the gold standard method to measure estradiol at low postmenopausal levels (lower limit of detection=2.5pg/mL).19 Intra- and inter coefficients of variation (CV) were, respectively: glucose: 1.9% and 2.4%; insulin: 4.8% and 10.5%; E2: 5.0% and 8.1%. Carotid intima media thickness (IMT) was measured via carotid artery ultrasound, details of which have been published previously.15 Briefly, sonographers obtained bilateral carotid images via B-mode ultrasound using a Sonoline Antares (Siemens, Malvern, PA) high resolution duplex scanner. Images were obtained from eight locations from the carotid artery (four locations each from the left and right carotid arteries: near and far walls of the distal common carotid artery, far walls of the carotid bulb, and internal carotid artery) and read using semi-automated reading software.20 Average values were recorded for each of the eight locations; the mean of the average readings across the locations comprised mean IMT. Reproducibility of IMT measures was excellent [intraclass correlation coefficient between sonographers 0.87–0.94, between readers=0.94–0.99].
Data analysis
All variables were examined for distributions, cell sizes, and outliers. HOMA and E2 were log transformed for analysis. Associations between VMS and adiponectin or HMW adiponectin were evaluated using linear regression, with physiologic and self-reported VMS considered separately. Covariates (age, race/ethnicity, waist circumference, education, HOMA) were selected on an a priori basis. E2 was added in a separate step. We considered the role of adiponectin in VMS-IMT relationships by first testing the relationships between adiponectin and IMT in linear regression models and next adding adiponectin to linear regression models of relationships between VMS and IMT; models were also adjusted for age, race, education, waist circumference, and HOMA. Separate models were estimated for adiponectin and HMW adiponectin. Interactions by continuous time since the last menstrual period were tested by cross product terms in multivariable models. We also considered adiponectin as the predictor and VMS as the outcome in linear regression models in secondary/exploratory analyses. Residual analysis and diagnostic plots were conducted to verify model assumptions. Analyses were performed with SAS v9.4 (SAS Institute, Cary, NC). Models were 2-sided at α=0.05.
Results
Women were on average 54 years old, overweight, and with low levels of E2 (Table 1). Approximately 28% of the sample was nonwhite, primarily African American. The correlation between physiologic and self-reported VMS was r=.61 (p<.0001), and the correlation between adiponectin and HMW adiponectin was r=.93 (p<.0001).
Table 1.
Sample characteristics
| N | 300 |
| Age, M (SD) | 54.06 (4.00) |
| Race/ethnicity, N (%) | |
| White | 216 (72.00) |
| Black | 67 (22.33) |
| Other race/ethnicitya | 17 (5.67) |
| Education, N (%) | |
| <College | 129 (43.00) |
| College degree or higher | 171 (57.00) |
| Time since last menstrual period, years, M (SD) | 4.47 (4.02) |
| Menopause stage, N (%) | |
| Perimenopausal | 48 (16.00) |
| Postmenopausal | 252 (84.00) |
| Estradiol, pg/mL, Median (IQR) | 4.95 (2.00, 11.00) |
| Body mass index, M (SD) | 29.08 (6.75) |
| Waist circumference, inches, M (SD) | 34.95 (5.73) |
| HOMA, Median (IQR) | 2.20 (1.67, 3.19) |
| Physiologic VMS, number/24 hours, M (SD)b | 8.75 (8.90) |
| Self-reported VMS, number/24 hours, M (SD)b | 2.68 (3.72) |
| Adiponectin, μg/mL, Mean (SD) | 9.75 (4.68) |
| HMW Adiponectin, μg/mL, M (SD) | 4.67 (3.07) |
| Intima media thickness, mm, M (SD) | 0.68 (0.11) |
HMW, high molecular weight; HOMA, homeostatic model assessment; VMS, vasomotor symptoms
Other race/ethnicities includes 11 Asian women, 2 Hispanic women, and 4 women who identify as mixed race
Number of VMS/monitoring time (hours)*24
We examined relationships between VMS and adiponectin, finding that more VMS were associated with lower adiponectin (Table 2, Figure 1). Associations were not explained by factors such as waist circumference or HOMA scores, nor by endogenous E2. Associations were apparent for both physiologic VMS and self-reported VMS and were similar for both adiponectin and HMW adiponectin.
Table 2.
Associations of VMS to adiponectin and HMW adiponectin
| Adiponectin | HMW Adiponectin | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| B(SE) | B(SE) | B(SE) | B(SE) | |
| Physiologic VMS | −.081 (.028)a | −.083 (.029)a | −.048 (.018)a | −.049 (.019)a |
| Self-report VMS | −.207 (.066)a | −.206 (.067)a | −.119 (.043)a | −.0119 (.043)a |
Model 1: age, race, education; waist circumference, HOMA (log), Model 2: Model 1 + estradiol (log)
p<.01
HMW, high molecular weight; HOMA, homeostatic model assessment; VMS, vasomotor symptoms
Note: Coefficients represent the differences in ug/mL adiponectin associated with each additional VMS/24 hours.
Figure 1.
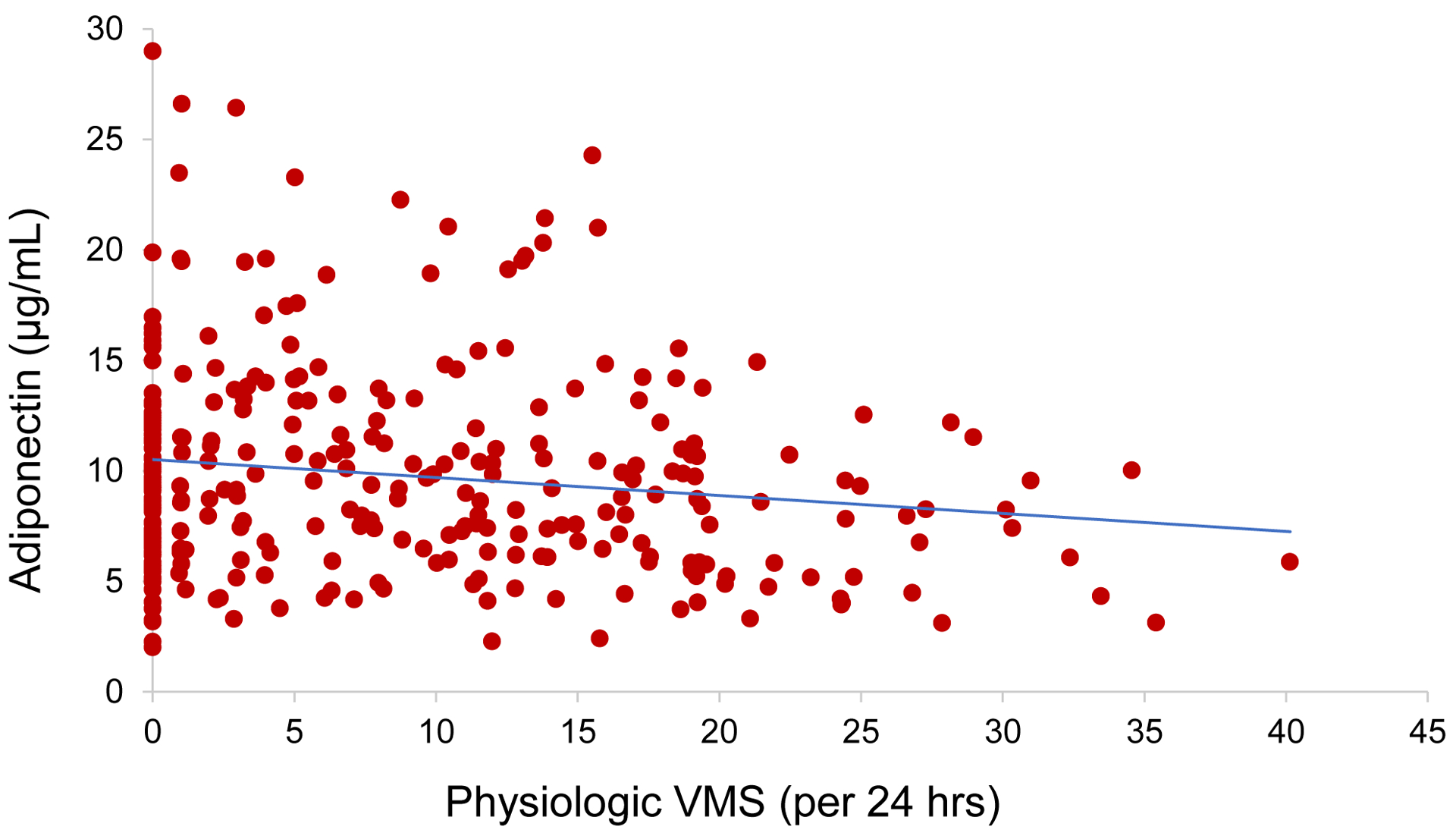
Scatterplot of association between physiologic vasomotor symptoms (VMS) and adiponectin
We next considered whether adiponectin explained previously-documented relationships between VMS and IMT among women reporting VMS.15 We found that adiponectin was marginally inversely associated with IMT [B(SE)=−.005 (.003), p=.08, adjusted for age, race, education, waist circumference, HOMA] and that adiponectin did not explain relationships between VMS and IMT [e.g., physiologic VMS in relation to IMT: b(SE)=.003 (.001), p=.007, models adjusted for age, race, education, waist circumference, HOMA, adiponectin].
In an exploratory fashion, we tested interactions between VMS and the time since the last menstrual period; these interactions were not significant (p’s>.25), indicating no effect modification of relationships by time since the last menstrual period. Finally, since the directionality of relationships between VMS and adiponectin are uncertain, we explored the predictor of adiponectin in relation to the outcome of VMS; adiponectin was significantly associated with VMS, similar to primary results (Supplemental Table).
Discussion
In this study of 300 midlife women who underwent physiologic ambulatory VMS monitoring, we found more VMS related to lower adiponectin and HMW adiponectin. These associations were not explained by key covariates such as adiposity, insulin resistance, or endogenous E2. These findings indicate an unfavorable adiponectin profile among women with more frequent VMS.
Prior work has linked VMS to lower adiponectin, yet studies are few and findings have not been entirely consistent. Further, these studies relied upon self-report VMS measures, which have known memory and reporting biases. With its use of ambulatory VMS monitoring, the present study represents an advancement over prior work, demonstrating links between physiologic VMS and adiponectin that were robust to adjustment for multiple covariates.
Given the well-documented links between adiponectin to CVD risk,7,8 and work linking VMS to CVD risk,4,5 low adiponectin has been conceptualized as one mechanism by which VMS may be linked to CVD risk.10 Accordingly, we considered adiponectin in associations between VMS and carotid atherosclerosis, yet adiponectin did not explain these relationships. While more research is warranted, the present data do not support a role for adiponectin in relationships between VMS and carotid atherosclerosis.
Alternatively, adiponectin has been conceptualized as involved in the physiology of VMS. Adiponectin has pleiotropic properties and acts on a range of organs including the arcuate nucleus of the hypothalamus.6,21 Importantly, a subpopulation of neurons in the arcuate nucleus of the hypothalamus that express estrogen receptor α, neurokinin 3 receptor, kisspeptin, neurokinin B (NKB), and dynorphin (known as KNDy neurons) appear central to the etiology of VMS.22 These neurons project to thermoregulatory centers in the median preoptic area of the hypothalamus, where they co-express NKB. In the face of E2 withdrawal, these neurons undergo a somatic hypertrophy and show increased kisspeptin and NKB gene expression. Activation of these neurons can induce VMS-type responses in rodent models, and blockade of NKB receptors can reduce VMS in women. Notably, adiponectin receptors have been found in gonadotropin hormone releasing hormone (GnRH)-secreting nuclei of the arcuate nucleus.21,23 Administration of adiponectin can inhibit kisspeptin transcription, the upstream signaler of GnRH release.24 Thus, adiponectin may act at KNDy neurons to inhibit kisspeptin and reduce VMS. In short, adiponectin may influence the central regulation of VMS.
Several other findings warrant mention. We considered both total and HMW adiponectin. Adiponectin is made up of three subspecies, and the HMW subspecies is thought to be the most metabolically active form most linked to CVD risk.6,25 Findings were consistent between adiponectin and HMW adiponectin, underscoring the consistency of these findings. Moreover, our prior research found associations between VMS and adipokines largely for women early in the menopause transition.9 However, here we observed associations among a largely postmenopausal sample, and did not find evidence of effect modification by time since the last menstrual period, indicating that associations extend to postmenopausal women.
This study had several limitations. First, only adiponectin and HMW adiponectin were assessed, and future work should expand this work to include other adipokines including leptin. Whereas non-Hispanic white and African American women were well-represented, other racial ethnic groups were under-represented here. The study enrolled women who were non-smokers and without clinical CVD and who were late peri- or postmenopausal; results may not be generalized to nonsmokers, women with CVD, or early peri- or premenopausal women.
The study had several strengths. In the largest study to date to implement physiologic VMS measures, we evaluated the relationships between physiologic VMS and adiponectin. We were able to consider a range of potential confounders or pathways, including adiposity and LC-MS/MS-assessed E2. We were able to consider the role of adiponectin in relations between VMS and carotid atherosclerosis.
Conclusion
In summary, VMS are a prevent midlife symptom associated with impairments in quality of life. We found more physiologically-assessed VMS associated with lower adiponectin levels, an anti-inflammatory cytokine linked to CVD risk that can regulate the reproductive axis. Future work should continue to consider the role of a wider range adipokines in both links between VMS and CVD, as well as in the regulation of VMS.
Supplementary Material
Funding
This work was supported by the National Institutes of Health, National Heart Lung and Blood Institute (R01HL105647, 2K24123565 to Thurston) and the University of Pittsburgh Clinical and Translational Science Institute (NIH Grant UL1TR000005). This project used the services of the University of Pittsburgh Small Molecule Biomarker Core, which was partially funded by NIH through S10RR023461 and S10OD028540.
Footnotes
Conflict of Interest Disclosure
Thurston is a consultant for Bayer, Astellas, and Happify Health, and past consultant for Pfizer, P&G, Vira Health, and Virtue Health. Chang has nothing to disclose.
References
- 1.Gold E, Colvin A, Avis N, et al. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Am J Public Health. 2006;96(7):1226–1235. doi: 10.2105/AJPH.2005.066936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation. Menopause. 2009;16(5):860–869. doi: 10.1097/gme.0b013e3181a3cdaf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–1104. doi: 10.1097/AOG.0b013e318214f0de [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurston RC. Vasomotor symptoms: natural history, physiology, and links with cardiovascular health. Climacteric. 2018;21(2):96–100. doi: 10.1080/13697137.2018.1430131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thurston RC, Aslanidou Vlachos HE, Derby CA, et al. Menopausal vasomotor symptoms and risk of incident cardiovascular disease events in SWAN. J Am Heart Assoc. 2021:e017416. doi: 10.1161/JAHA.120.017416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8(2):93–100. doi: 10.1093/jmcb/mjw011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao G, Li W, Guo R, et al. Serum total adiponectin level and the risk of cardiovascular disease in general population: a meta-analysis of 17 prospective studies. Atherosclerosis. 2013;228(1):29–35. doi: 10.1016/j.atherosclerosis.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Mo X, Hao Y, et al. Adiponectin levels and risk of coronary heart disease: a meta-analysis of prospective studies. Am J Med Sci. 2013;345(6):455–461. doi: 10.1097/MAJ.0b013e318262dbef [DOI] [PubMed] [Google Scholar]
- 9.Thurston RC, Chang Y, Mancuso P, Matthews KA. Adipokines, adiposity, and vasomotor symptoms during the menopause transition: findings from the Study of Women’s Health Across the Nation. Fertil Steril. 2013;100(3):793–800. doi: 10.1016/j.fertnstert.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang WY, Chang CC, Chen DR, Kor CT, Chen TY, Wu HM. Circulating leptin and adiponectin are associated with insulin resistance in healthy postmenopausal women with hot flashes. PLoS One. 2017;12(4):e0176430. doi: 10.1371/journal.pone.0176430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karim R, Dang HM, Hodis HN, Stanczyk FZ, Brinton RD, Mack WJ. Association of hot flushes with ghrelin and adipokines in early versus late postmenopausal women. Menopause. 2020;27(5):512–518. doi: 10.1097/GME.0000000000001508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu PB, Matthews KA, Thurston RC. How well do different measurement modalities estimate the number of vasomotor symptoms? Findings from the Study of Women’s Health Across the Nation FLASHES Study. Menopause. 2014;21(2):124–130. doi: 10.1097/GME.0b013e318295a3b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Emotional antecedents of hot flashes during daily life. Psychosom Med. 2005;67(1):137–146. doi: 10.1097/01.psy.0000149255.04806.07 [DOI] [PubMed] [Google Scholar]
- 14.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6(3):209–215. doi: 10.1097/00042192-199906030-00006 [DOI] [PubMed] [Google Scholar]
- 15.Thurston RC, Chang Y, Barinas-Mitchell E, et al. Menopausal hot flashes and carotid intima media thickness among midlife women. Stroke. 2016;47(12):2910–2915. doi: 10.1161/STROKEAHA.116.014674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlow SD, Mitchell ES, Crawford S, Nan B, Little R, Taffe J. The ReSTAGE Collaboration: defining optimal bleeding criteria for onset of early menopausal transition. Fertil Steril. 2008;89(1):129–140. doi: 10.1016/j.fertnstert.2007.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Park City, Utah, July, 2001. Menopause. 2001;8(6):402–407. doi: 10.1097/00042192-200111000-00004 [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Teacher DF, Turner RC. Homeostasis model assessment: insulin resistance and B cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 19.Santen RJ, Lee JS, Wang S, et al. Potential role of ultra-sensitive estradiol assays in estimating the risk of breast cancer and fractures. Steroids. 2008;73(13):1318–1321. doi: 10.1016/j.steroids.2008.05.008 [DOI] [PubMed] [Google Scholar]
- 20.Wendelhag I, Gustavsson T, Suurkula M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol. 1991;11(6):565–577. doi: 10.1111/j.1475-097x.1991.tb00676.x [DOI] [PubMed] [Google Scholar]
- 21.Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165(2):313–327. doi: 10.1111/j.1476-5381.2011.01560.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211–227. doi: 10.1016/j.yfrne.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rak A, Mellouk N, Froment P, Dupont J. Adiponectin and resistin: potential metabolic signals affecting hypothalamo-pituitary gonadal axis in females and males of different species. Reproduction. 2017;153(6):R215–R226. doi: 10.1530/REP-17-0002 [DOI] [PubMed] [Google Scholar]
- 24.Wen JP, Liu C, Bi WK, et al. Adiponectin inhibits KISS1 gene transcription through AMPK and specificity protein-1 in the hypothalamic GT1–7 neurons. J Endocrinol. 2012;214(2):177–189. doi: 10.1530/JOE-12-0054 [DOI] [PubMed] [Google Scholar]
- 25.Simpson F, Whitehead JP. Adiponectin--it’s all about the modifications. Int J Biochem Cell Biol. 2010;42(6):785–788. doi: 10.1016/j.biocel.2009.12.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


