Abstract
We report the results from a multicenter retrospective study of 69 adult patients who underwent haploidentical blood or marrow transplantation (haplo-BMT) with post-transplantation cyclophosphamide (PTCy) for chronic phase myelofibrosis. The median age at BMT was 63 years (range, 41–74). Conditioning regimens were reduced intensity in 54% and nonmyeloablative in 39%. Peripheral blood grafts were used in 86%. The median follow-up was 23.1 months (range, 1.6–75.7). At 3 years, the overall survival, relapse-free survival (RFS), and graft-versus-host-disease (GVHD)-free-RFS were 72% (95% CI 59–81), 44% (95% CI 29–59), and 30% (95% CI 17–43). Cumulative incidences of non-relapse mortality and relapse were 23% (95% CI 14–34) and 31% (95% CI 17–47) at 3 years. Spleen size ≥22 cm or prior splenectomy (HR 6.37, 95% CI 2.02–20.1, P = 0.002), and bone marrow grafts (HR 4.92, 95% CI 1.68–14.4, P = 0.004) were associated with increased incidence of relapse. Cumulative incidence of acute GVHD grade 3–4 was 10% at 3 months and extensive chronic GVHD was 8%. Neutrophil engraftment was reported in 94% patients, at a median of 20 days (range, 14–70). In conclusion, haplo-BMT with PTCy is feasible in patients with myelofibrosis. Splenomegaly ≥22 cm and bone marrow grafts were associated with a higher incidence of relapse in this study.
INTRODUCTION
Myelofibrosis, either primary or post-essential thrombocythemia (ET)/ polycythemia vera (PV), is a clonal hematopoietic neoplasm marked by constitutive JAK-STAT activation, bone marrow fibrosis, blood count aberrations, and constitutional symptoms [1]. Overall survival (OS) can vary significantly based on the Dynamic International Prognostic Scoring System (DIPSS) plus score, ranging from 185 months in the lowest risk group to 16 months in the highest risk group [2]. Despite the recent approval of drugs that target the JAK-STAT pathway [3–6], allogeneic blood or bone marrow transplantation (BMT) remains the only potentially curative option in this disease [7, 8]. Limited comparative data of BMT and non-transplantation treatment options suggest that BMT may provide better survival for patients with intermediate-1 and higher DIPSS risk groups, but this comes at the cost of early non-relapse mortality (NRM) [9–11].
Several important issues arise when contemplating BMT for myelofibrosis. First, the median age at diagnosis is ~67 years, and age-related comorbidities need to be considered [12]. Second, graft failure, perhaps in part related to splenomegaly and a hostile marrow micro-environment, has historically been seen more often in myelofibrosis than other transplant indications [13, 14]. Third, previous reports have suggested inferior outcomes with matched or mismatched unrelated donors compared to matched sibling donors in hematological malignancies, including myelofibrosis [15–17]. Older patient age often poses challenges to finding a suitable and available matched sibling donor. While haploidentical donor (haplo)-BMT has been known for a long time, its use was not widely adopted given difficulties with bidirectional alloreactivity leading to high incidence of graft failure and graft versus host disease (GVHD) [18–20]. In the current era, with the advent of post-transplantation cyclophosphamide (PTCy), haplo-BMT has improved the feasibility of BMT by mitigating the historically high incidences of GVHD [21–24].
While now considered standard-of-care for many hematological malignancies, data on outcomes with haplo-BMT in myelofibrosis are limited. There is only one report from the European Society for Blood and Marrow Transplantation (EBMT) on a cohort of 56 patients, of whom 79% received PTCy [25]. At 2 years, OS and progression-free survival were 56 and 43%. At 2 years, relapse incidence was 19%, while graft failure and NRM were 9% and 38%, respectively. Here, we report the results of a retrospective, multicenter study across North America to describe clinical outcomes of patients with chronic phase myelofibrosis who underwent haplo-BMT with PTCy.
METHODS
Data collection/patient selection
Thirteen centers collaborated to conduct a retrospective study of patients who underwent first haplo-BMT for myelofibrosis in chronic phase (<10% blasts in peripheral blood or marrow) as defined by WHO [26]. Each center obtained approval from its respective institutional review boards. All patients (age ≥ 18 years) who underwent first haplo-BMT with PTCy for primary or post-ET/PV myelofibrosis in chronic phase between 1 January 2000 and 31 December 2019 were included. A haploidentical donor was defined as a related donor mismatched for one haplotype. Donor selection was performed per institutional protocol. Patients with accelerated or blast phase myelofibrosis were excluded [27]. Patient, disease, treatment, and BMT characteristics were recorded by chart review along with clinical outcomes of interest.
Definitions
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) ≥ 0.5 × 109/L. Platelet engraftment was defined as the first of 3 consecutive days at which the platelet count was 20 × 109/L without transfusion support. Graft failure was defined as lack of hematopoietic cell engraftment following BMT, without evidence of disease relapse [28]. For this study, relapse was determined by the treating physician. GVHD grading was recorded per standard definitions [29, 30]. GVHD-free, relapse-free survival (GRFS) was defined as a composite endpoint of one of the following events: death, relapse, grade 3 or 4 acute GVHD or chronic GVHD requiring systemic treatment [31]. Conditioning regimens were classified as myeloablative (MAC), nonmyeloablative (NMA), or reduced-intensity conditioning (RIC) per previously published criteria [32].
Statistical analysis
Clinical outcomes of interest included OS, relapse-free survival (RFS), GRFS, NRM, relapse, acute and chronic GVHD, and graft function. OS, RFS, and GRFS were estimated with the Kaplan–Meier method. Competing risks were used for NRM, relapse, and GVHD, and their outcomes were estimated with cumulative incidence. Outcome estimates are given at specified time points with 95% confidence intervals (CI) for the estimates.
Univariate analyses were conducted to assess the association of baseline characteristics with OS, RFS, GRFS, NRM, and relapse. Univariate prognostic factors for OS, RFS, and GRFS were assessed with Cox regression, while risk factors for NRM and relapse with assessed with Fine and Gray regression. Results are reported as hazard ratios (HR) with 95% CI. Due to the lack of existing consensus regarding the prognostic effect of spleen size, exploratory analyses were conducted among patients who had a measurable spleen. We used spleen size cut-offs at 20, 21, 22, 23, 24, and 25 cm, and also analyzed spleen size as a continuous variable. Another assessment was done combining spleen size ≥22 and prior splenectomy in one group since all splenectomies were done for advanced disease. This group was compared to the cohort with spleen size <22 cm at BMT. CD34 + dose had one large outlier and hence, was analyzed categorically by splitting into two groups near the median dose. Exploratory analyses were conducted to assess the prognostic effect of the most recent treatment received prior to BMT (categorized as JAK inhibitor alone, or a hypomethylating agent [HMA] with or without JAK inhibitor) and the conditioning regimen intensity. When conditioning intensity was assessed using all three types of conditioning, the HR could not be calculated because there were a limited number of patients who received MAC regimens. Most of them did not have any of the events of clinical interest. Hence, log-rank or Gray tests were used instead. Because of the limited number of events for each outcome and missing data on key variables, we were unable to conduct a traditional multivariate analysis. Instead, focused multivariate analyses were done for OS, RFS, NRM, and relapse using all pairs of statistically significant variables in univariate analysis.
Data were analyzed with SAS® software (SAS Institute, Inc., Cary, NC, USA). All P values were two-sided, and P ≤ 0.05 was considered statistically significant.
RESULTS
Baseline patient, disease, treatment, and BMT characteristics
We identified 69 consecutive patients who underwent haplo-BMT with PTCy for primary or post-ET/PV myelofibrosis. Patient demographics, disease, treatment, and BMT-specific characteristics are detailed in Table 1. Five patients had undergone a splenectomy prior to BMT, all in response to myelofibrosis-related symptomatic splenomegaly. Spleen size at the time of BMT was available for 62 of the remaining 64 patients and measured by imaging in 50 (81%), or by physical examination in 12 (19%) patients. The median size was 18 cm (range 9.4–28.1) in these 62 patients. The most recent treatments prior to BMT and details of the conditioning regimens are shown in Supplementary Tables 1 and 2. Forty patients (58%) received a JAK inhibitor prior to BMT. Fourteen patients (20%) received HMA with or without a JAK inhibitor [33]. One patient received induction chemotherapy per the institution’s former practice to induce immunosuppression prior to BMT. One patient who had low-risk disease by DIPSS plus, proceeded with BMT given young age at diagnosis and donor availability. Another patient who also had low-risk disease by DIPSS plus, proceeded with BMT due to the presence of 6% myeloid blasts in the bone marrow. Two additional patients had DIPSS plus intermediate-1 disease, and proceeded to BMT due of the presence of high-risk somatic mutations. Fludarabine, cyclophosphamide, with total body irradiation 2 Gy was the most common conditioning regimen used (N = 27 [39%]). GVHD prophylaxis included PTCy in all patients. Fifty-one (74%) patients additionally received a combination of calcineurin inhibitors (CNI) plus mycophenolate mofetil (MMF). Eight (12%) received an mTOR inhibitor plus MMF with PTCy. The remaining 10 (14%) used a combination of anti-thymocyte globulin (ATG) plus CNI with PTCy.
Table 1.
Baseline characteristics of patients, disease, treatment details and BMT.
| Characteristics | Results in N (%) unless otherwise specified | |
|---|---|---|
| Age at diagnosis, years, median (range) | 59 (33–71) | |
| Patient gender | Men | 44 (64) |
| Women | 25 (36) | |
| Age at BMT, years, median (range) | 63 (41–74) | |
| Time from diagnosis to BMT, months, median (range) | 20.5 (1.8–143.1) | |
| Year of BMT | 2010–2015 | 14 (20) |
| 2016–2019 | 55 (80) | |
| Disease type | Primary MF | 35 (51) |
| Post-ET MF | 19 (28) | |
| Post-PV MF | 15 (22) | |
| Grade of fibrosis at diagnosis (N = 66) | MF 0 | 2 (3) |
| MF 1 | 8 (12) | |
| MF 2 | 30 (46) | |
| MF 3 | 26 (39) | |
| DIPSS plus at BMT | Low | 2 (3) |
| Intermediate-1 | 2 (3) | |
| Intermediate-2 | 48 (69) | |
| High | 17 (25) | |
| High risk cytogeneticsa (N = 66) | 10 (15) | |
| Driver Mutation | JAK2 (N = 66) | 45 (68) |
| CALR (N = 64) | 11 (17) | |
| MPL (N = 64) | 4 (6) | |
| Prior splenectomy (N = 67) | 5 (7) | |
| Spleen size at BMT (N = 62), cm, median (range) | 18.0 (9.4–28.1) | |
| JAK inhibitor use prior to BMT (N = 68) | 59 (87) | |
| HCT-CI ≥ 3 (N = 64) | 28 (44) | |
| Graft source | PB | 59 (86) |
| BM | 10 (14) | |
| Donor to recipient gender | F to F | 12 (17) |
| F to M | 12 (17) | |
| M to F | 13 (19) | |
| M to M | 32 (46) | |
| Donor to recipient CMV status | D+/R+ | 29 (42) |
| D+/R− | 6 (9) | |
| D−/R+ | 15 (22) | |
| D−/R− | 19 (28) | |
| CD34+ cell dose, ×106/kg, median (range) | 5.80 (1.79–28.60) | |
| Conditioning regimen intensity | NMA | 27 (39) |
| RIC | 37 (54) | |
| MAC | 5 (7) | |
| TBI | 50 (72) | |
| GVHD prophylaxis | CNI/MMF/PTCy | 51 (74) |
| CSA/ATG/PTCy | 10 (14) | |
| mTORi/MMF/PTCy | 8 (12) |
ATG antithymocyte globulin, BM bone marrow, BMT blood or marrow transplantation, cGy centigrays, CMV cytomegalovirus, CNI calcineurin inhibitor, ET essential thrombocythemia, DIPSS Dynamic International Prognostic Scoring System, D/R donor/recipient, FK tacrolimus, GVHD graft versus host disease, HCT-CI Hematopoietic Cell Transplantation-specific Comorbidity Index, HMA hypomethylating agent, JAK Janus kinase, MAC myeloablative conditioning, MF myelofibrosis, MMF mycophenolate mofetil, mTORi mTOR inhibitor, NMA non-myeloablative, PB peripheral blood, PTCy post-transplantation cyclophosphamide, PV polycythemia vera, RIC reduced intensity conditioning, TBI total body irradiation.
High-risk cytogenetics was defined as a complex karyotype or abnormalities including +8, −7/7q−, i(17q), −5/5q−, 12p, inv(3) or 11q23 rearrangement.
Survival outcomes
Forty-nine (71%) patients were alive at last follow-up with a median follow-up of 23.1 months (range 1.6–75.7). OS at 1 year was 74% (95% CI 61–83) and at 3 years was 72% (95% CI 59–81) (Table 2 and Fig. 1A). Hematopoietic cell transplantation-specific Comorbidity Index (HCT-CI) ≥ 3 was associated with inferior OS (HR 3.97, 95% CI 1.51–10.4, P = 0.005), while the use of a male donor was associated with improved OS (HR 0.42, 95% CI 0.17–1.00, P = 0.05). RFS at 1 year was 72% (95% CI 60–82) and at 3 years was 44% (95% CI 29–59) (Table 2 and Fig. 1A). HCT-CI ≥ 3 was associated with inferior RFS (HR 2.16, 95% CI 1.04–4.52, P = 0.04), while recipient cytomegalovirus (CMV)-positivity exhibited improved RFS (HR 0.46, 95% CI 0.22–0.96, P = 0.038). GRFS at 1 year was 55% (95% CI 42–66) and at 3 years was 30% (95% CI 17–43) (Table 2 and Fig. 1A). Recipient CMV-positivity was associated with improved GRFS (HR 0.52, 95% CI 0.28–0.97, P = 0.039). A detailed univariate analysis is shown in Table 3a. No differences in OS, RFS, or GRFS were noted on univariate analysis with the spleen size at BMT, most recent treatment received prior to BMT, the intensity of conditioning regimens, DIPSS Plus at BMT, or driver mutations (Table 3a and Supplementary Table 3).
Table 2.
BMT outcomes.
| Outcomes | Time after BMT | Outcome estimate (95% CI) unless otherwise specified |
|---|---|---|
| OS | 1 year | 74 (61–83) |
| 3 years | 72 (59–81) | |
| RFS | 1 year | 72 (60–82) |
| 3 years | 44 (29–59) | |
| GRFS | 1 year | 55 (42–66) |
| 3 years | 30 (17–43) | |
| NRM | 1 year | 21 (12–32) |
| 3 years | 23 (14–34) | |
| Relapse | 1 year | 5 (1–12) |
| 3 years | 31 (17–47) | |
| Acute GVHD, all grades | 3 months | 36 (25–48) |
| 6 months | 48 (36–60) | |
| Acute GVHD, Grades 3–4 | 3 months | 10 (4–19) |
| 6 months | 13 (6–22) | |
| Chronic GVHD, all grades | 1 year | 22 (13–33) |
| 2 years | 29 (17–41) | |
| Chronic GVHD, extensive grade | 1 and 2 years | 8 (3–16) |
| Days to neutrophil engraftment (N = 65), median (range) | 20 (14–70) | |
| Days to platelet engraftment (N = 56), median (range) | 34 (15–224) |
BMT blood or marrow transplantation, CI 95% confidence intervals, GRFS GVHD-free relapse-free survival, GVHD graft versus host disease, NRM non-relapse mortality, OS overall survival, RFS relapse free survival
Fig. 1. Estimates of outcomes.
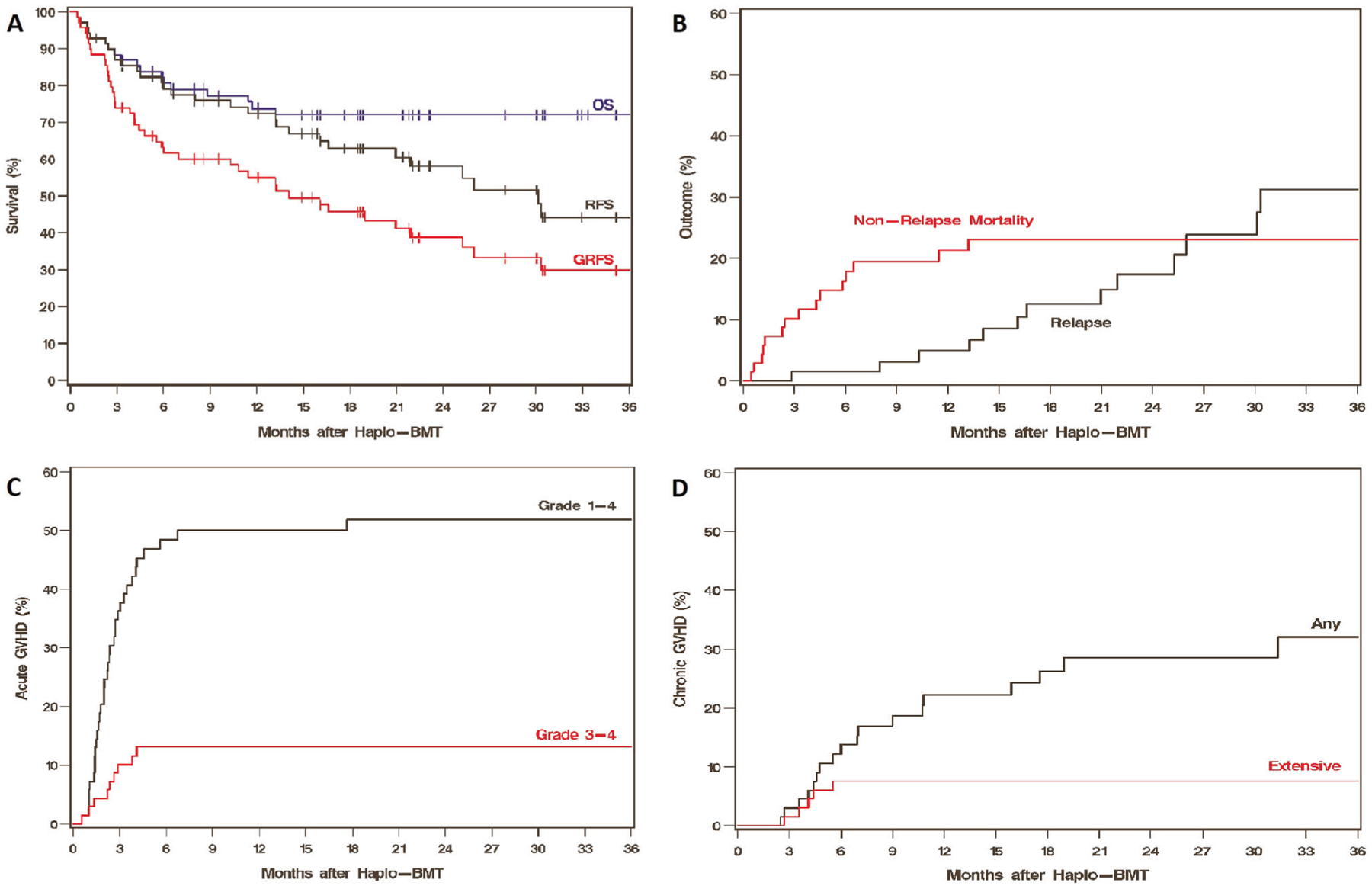
Kaplan-Meier estimates of OS, RFS and GRFS (A), Cumulative incidences of NRM and relapse (B), acute GVHD (C) and chronic GVHD (D).
Table 3.
a Univariate analysis of factors affecting OS, RFS, and GRFS. b Univariate analysis of factors affecting NRM and relapse.
| Variable | OS | RFS | GRFS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | CI | P-value | HR | CI | P-value | HR | CI | P-value | |
| a | |||||||||
| Age at BMT per 10-year increase | 1.27 | 0.70–2.33 | 0.44 | 0.90 | 0.55–1.48 | 0.68 | 0.96 | 0.63–1.46 | 0.86 |
| Year of BMT per 1-year increase | 1.15 | 0.87–1.50 | 0.32 | 0.94 | 0.78–1.13 | 0.51 | 1.06 | 0.89–1.25 | 0.52 |
| Gender (men vs women) | 0.66 | 0.27–1.60 | 0.36 | 0.82 | 0.39–1.73 | 0.61 | 0.84 | 0.45–1.58 | 0.59 |
| HCT-CI (≥3 vs <3) N = 64 | 3.97 | 1.51–10.4 | 0.005 | 2.16 | 1.04–4.52 | 0.040 | 1.48 | 0.78–2.79 | 0.23 |
| High risk cytogenetics (yes vs no) N = 66 | 1.02 | 0.30–3.50 | 0.97 | 1.31 | 0.50–3.47 | 0.58 | 1.52 | 0.67–3.48 | 0.32 |
| JAK2 mutated (yes vs no) N = 66 | 1.35 | 0.49–3.75 | 0.57 | 1.01 | 0.44–2.32 | 0.99 | 0.58 | 0.30–1.14 | 0.11 |
| CALR mutated (yes vs no) N = 64 | 1.00 | 0.29–3.44 | 0.99 | 1.56 | 0.58–4.22 | 0.38 | 1.00 | 0.41–2.42 | 0.99 |
| MPL mutated (yes vs no) N = 64 | 0.74 | 0.10–5.60 | 0.77 | 1.06 | 0.25–4.48 | 0.94 | 2.53 | 0.88–7.31 | 0.09 |
| Prior JAK inhibitor use (yes vs no) N = 68 | 2.82 | 0.38–21.1 | 0.31 | 2.07 | 0.49–8.73 | 0.32 | 2.10 | 0.65–6.81 | 0.22 |
| HMA+/− JAKi vs JAKi alone N = 54 | 1.69 | 0.58–4.90 | 0.33 | 1.69 | 0.66–4.35 | 0.28 | 1.41 | 0.66–3.04 | 0.38 |
| Spleen size at BMT (≥22 vs <22 cm) N = 62 | 0.71 | 0.20–2.58 | 0.62 | 1.40 | 0.58–3.39 | 0.45 | 0.69 | 0.30–1.59 | 0.38 |
| Spleen size at BMT (≥22 + prior splenectomy vs <22 cm) N = 67 | 1.01 | 0.38–2.67 | 0.99 | 1.52 | 0.69–3.32 | 0.30 | 0.85 | 0.42–1.70 | 0.64 |
| DIPSS plus at BMT (high vs <high) | 0.87 | 0.32–2.40 | 0.79 | 0.72 | 0.31–1.69 | 0.45 | 0.58 | 0.27–1.21 | 0.14 |
| Donor gender (men vs women) | 0.42 | 0.17–1.00 | 0.05 | 0.48 | 0.22–1.02 | 0.06 | 0.59 | 0.31–1.11 | 0.10 |
| Conditioning regimen MAC vs NMA vs RIC | P = 0.22; log-rank test | P = 0.32; log-rank test | P = 0.31; log-rank test | ||||||
| Graft source (BM vs PB) | 0.22 | 0.03–1.67 | 0.14 | 1.07 | 0.41–2.83 | 0.89 | 0.57 | 0.22–1.47 | 0.25 |
| CD34+ cell dose, ×106/kg (>6 vs ≤6) | 0.67 | 0.27–1.64 | 0.38 | 0.82 | 0.39–1.71 | 0.59 | 1.09 | 0.59–2.01 | 0.79 |
| Recipient CMV (positive vs negative) | 0.71 | 0.30–1.72 | 0.45 | 0.46 | 0.22–0.96 | 0.038 | 0.52 | 0.28–0.97 | 0.039 |
| Variable | NRM | Relapse | ||||
|---|---|---|---|---|---|---|
| HR | CI | P-value | HR | CI | P-value | |
| b | ||||||
| Age at BMT per 10-year increase | 1.88 | 1.08–3.26 | 0.025 | 0.36 | 0.16–0.81 | 0.014 |
| Year of BMT per 1-year increase | 1.13 | 0.84–1.52 | 0.40 | 0.73 | 0.61–0.87 | <0.001 |
| Gender (men vs women) | 0.65 | 0.24–1.76 | 0.39 | 1.56 | 0.51–4.79 | 0.44 |
| HCT-CI (≥3 vs <3) N = 64 | 4.07 | 1.32–12.5 | 0.014 | 0.54 | 0.17–1.76 | 0.31 |
| High risk cytogenetics (yes vs no) N = 66 | 0.78 | 0.20–3.09 | 0.73 | 1.24 | 0.30–5.08 | 0.77 |
| JAK2 mutated (yes vs no) N = 66 | 1.21 | 0.39–3.79 | 0.74 | 0.70 | 0.22–2.25 | 0.55 |
| CALR mutated (yes vs no) N = 64 | 0.91 | 0.20–4.12 | 0.90 | 2.80 | 0.85–9.25 | 0.09 |
| MPL mutated (yes vs no) N = 64 | 1.11 | 0.17–7.35 | 0.91 | 1.13 | 0.13–9.86 | 0.91 |
| Prior JAK inhibitor use (yes vs no) N = 68 | 2.08 | 0.25–17.5 | 0.50 | 1.55 | 0.19–12.5 | 0.68 |
| HMA+/− JAKi vs JAKi alone (N = 54) | 2.33 | 0.78–7.02 | 0.13 | 0.48 | 0.06–3.85 | 0.49 |
| Spleen size at BMT (≥22 vs <22 cm) N = 62 | 0.57 | 0.12–2.73 | 0.48 | 4.57 | 1.31–16.0 | 0.017 |
| Spleen size at BMT (≥22 + prior splenectomy vs <22 cm) N = 67 | 0.40 | 0.08–1.89 | 0.25 | 6.37 | 2.02–20.1 | 0.002 |
| DIPSS plus at BMT (high vs <high) | 1.01 | 0.33–3.07 | 0.99 | 0.64 | 0.19–2.14 | 0.47 |
| Donor gender (men vs women) | 0.40 | 0.15–1.08 | 0.07 | 1.39 | 0.40–4.86 | 0.61 |
| Conditioning regimen MAC vs NMA vs RIC | P = 0.51; Gray test | P = 0.57; Gray test | ||||
| Graft source (BM vs PB) | P = 0.08; Gray test | 4.92 | 1.68–14.4 | 0.004 | ||
| CD34+ cell dose, ×106/kg (>6 vs ≤6) | 0.70 | 0.25–1.96 | 0.49 | 1.43 | 0.49–4.15 | 0.51 |
| Recipient CMV (positive vs negative) | 0.87 | 0.31–2.42 | 0.79 | 0.32 | 0.11–0.94 | 0.038 |
BM bone marrow, BMT blood or marrow transplantation, CI 95% confidence intervals, CMV cytomegalovirus, DIPSS Dynamic International Prognostic Scoring System, GRFS GVHD-free relapse-free survival, GVHD graft versus host disease, HCT-CI Hematopoietic Cell Transplantation-specific Comorbidity Index, JAK Janus kinase, NMA non-myeloablative conditioning, NRM non-relapse mortality, OS overall survival, PB peripheral blood, RFS relapse free survival, RIC reduced-intensity conditioning.
Statistically significant values have been highlighted as bold.
A focused multivariate analysis was conducted for OS and RFS using the 2-variable model (Table 4). HCT-CI ≥ 3 remained independently associated with an inferior OS (HR 6.71, 95% CI 2.35–19.2, P < 0.001), and male donor with improved OS (HR 0.21, 95% CI 0.08–0.56, P = 0.002). Similarly, on the multivariate analysis for RFS, HCT-CI ≥ 3 remained independently associated with inferior RFS (HR 2.36, 95% CI 1.12–4.99, P = 0.024) while recipient CMV-positivity was associated with improved RFS (HR 0.46, 95% CI 0.22–0.97, P = 0.041). A multivariate analysis was not conducted for GRFS as only one variable (recipient CMV serostatus) was statistically significant in the univariate analysis.
Table 4.
Multivariate analysis using 2-variable models.
| Model | Variable | HR | CI | P-value |
|---|---|---|---|---|
| Model for OS | HCT-CI (≥3 vs <3) | 6.71 | 2.35–19.2 | <0.001 |
| Donor gender (Men vs Women) | 0.21 | 0.08–0.56 | 0.002 | |
| Model for RFS | HCT-CI (≥3 vs <3) | 2.36 | 1.12–4.99 | 0.024 |
| Recipient CMV (Positive vs Negative) | 0.46 | 0.22–0.97 | 0.041 | |
| Model for NRM | Age at BMT per 10 year increase | 2.30 | 1.22–4.34 | 0.010 |
| HCT-CI (≥3 vs <3) | 5.11 | 1.55–16.9 | 0.007 | |
| Model 1 for relapse | Spleen Size (≥22 cm + prior splenectomy vs <22 cm) | 6.46 | 1.85–22.6 | 0.004 |
| Age at BMT per 10 year increase | 0.39 | 0.18–0.81 | 0.012 | |
| Model 2 for relapse | Spleen Size (≥22 cm + prior splenectomy vs <22 cm) | 6.04 | 1.93–18.9 | 0.002 |
| Recipient CMV serostatus (Positive vs Negative) | 0.28 | 0.09–0.90 | 0.032 | |
| Model 3 for relapse | Spleen Size (≥22 cm + prior splenectomy vs <22 cm) | 4.73 | 1.18–18.9 | 0.028 |
| Graft Source (BM vs PB) | 3.34 | 0.90–12.4 | 0.07 | |
| Model 4 for relapse | Age at BMT per 10 year increase | 0.29 | 0.14–0.62 | 0.001 |
| Recipient CMV serostatus (Positive vs Negative) | 0.23 | 0.07–0.70 | 0.010 | |
| Model 5 for relapse | Age at BMT per 10 year increase | 0.34 | 0.16–0.73 | 0.006 |
| Graft Source (BM vs PB) | 5.38 | 1.57–18.4 | 0.007 | |
| Model 6 for relapse | Recipient CMV serostatus (Positive vs Negative) | 0.44 | 0.14–1.38 | 0.16 |
| Graft Source (BM vs PB) | 3.71 | 1.31–10.5 | 0.014 | |
| Model 7 for relapse | Spleen Size (≥22 cm + prior splenectomy vs <22 cm) | 5.47 | 1.58–18.9 | 0.007 |
| Year of BMT per 1 year increase | 0.80 | 0.66–0.98 | 0.028 | |
| Model 8 for relapse | Age at BMT per 10 year increase | 0.34 | 0.14–0.80 | 0.013 |
| Year of BMT per 1 year increase | 0.69 | 0.54–0.87 | 0.002 | |
| Model 9 for relapse | Recipient CMV serostatus (Positive vs Negative) | 0.33 | 0.11–0.97 | 0.043 |
| Year of BMT per 1 year increase | 0.76 | 0.64–0.89 | 0.001 | |
| Model 10 for relapse | Graft Source (BM vs PB) | 3.50 | 1.16–10.6 | 0.026 |
| Year of BMT per 1 year increase | 0.82 | 0.68–0.98 | 0.028 |
BM bone marrow, BMT blood or marrow transplantation, CI 95% confidence intervals, CMV cytomegalovirus, HCT-CI Hematopoietic Cell Transplantation-specific Comorbidity Index, HR Hazard ratio, NRM non-relapse mortality, OS overall survival, PB peripheral blood, RFS relapse free survival.
NRM and relapse
NRM incidence at 1 year was 21% (95% CI 12–32), and 3 at years was 23% (95% CI 14–34) (Table 2 and Fig. 1B). Among the 15 patients who experienced NRM, the causes were infection in 8 (53%) patients, end-organ toxicity in 4 (27%), GVHD in 2 (13%), and intracranial hemorrhage in 1 (7%). Univariate analysis demonstrated a higher risk of NRM with increasing age (HR 1.88, 95% CI 1.08–3.26, P = 0.025) and HCT-CI ≥ 3 (HR 4.07, 95% CI 1.32–12.5, P = 0.014). Additional details of this univariate analysis are shown in Table 3b. In the 2-variable model for multivariate analyses (Table 4), increasing age and HCT-CI ≥ 3, both remained independently associated with higher NRM (HR 2.30, 95% CI 1.22–4.34, P = 0.010 for age, and HR 5.11, 95% CI 1.55–16.9, P = 0.007 for HCT-CI).
Incidence of relapse at 1 year was 5% (95% CI 1–12) and at 3 years was 31% (95% CI 17–47) (Table 2 and Fig. 1B). Spleen size, analyzed as a continuous variable (per 5 cm increase), had a statistically significant association with relapse (HR 1.90, 95% CI 1.00–3.58, P = 0.049). A larger spleen was associated with a higher risk of relapse for a cut-off of 22 cm (HR 4.57, 95% CI 1.31–16.0, P = 0.017; Fig. 2A), and for all cut-offs above 22 cm (Table 3b and Supplementary Table 3). Patients who had undergone a splenectomy prior to BMT, when included with patients with spleen size ≥22 cm (as all splenectomies were performed due to myelofibrosis-related symptomatic splenomegaly), had a significantly higher incidence of relapse (HR 6.37, 95% CI 2.02–20.1, P = 0.002) (Table 3b and Fig. 2B) compared to patients with a spleen size <22 cm at BMT. We also conducted this analysis by only including patients in whom the spleen size was available by imaging (N = 50). Incidence of relapse remained high in patients with a spleen size ≥22 cm (data not shown). Compared to peripheral blood, bone marrow grafts were associated with an increased risk of relapse (HR 4.92, 95% CI 1.68–14.4, P = 0.004) (Table 3b and Fig. 2C). Older age at BMT (HR 0.36, 95% CI 0.16–0.81, P = 0.014), recipient CMV-positivity (HR 0.32, 95% CI 0.11–0.94, P = 0.038; Fig. 2D), and a later year of BMT (HR 0.73, 95% CI 0.61–0.87, P < 0.001) were associated with a lower risk of relapse. No differences in NRM or relapse were noted on univariate analysis with the most recent treatment received prior to BMT, the intensity of conditioning regimens, DIPSS Plus at BMT, or driver mutations (Table 3b).
Fig. 2. Patient and BMT characteristics associated with relapse.
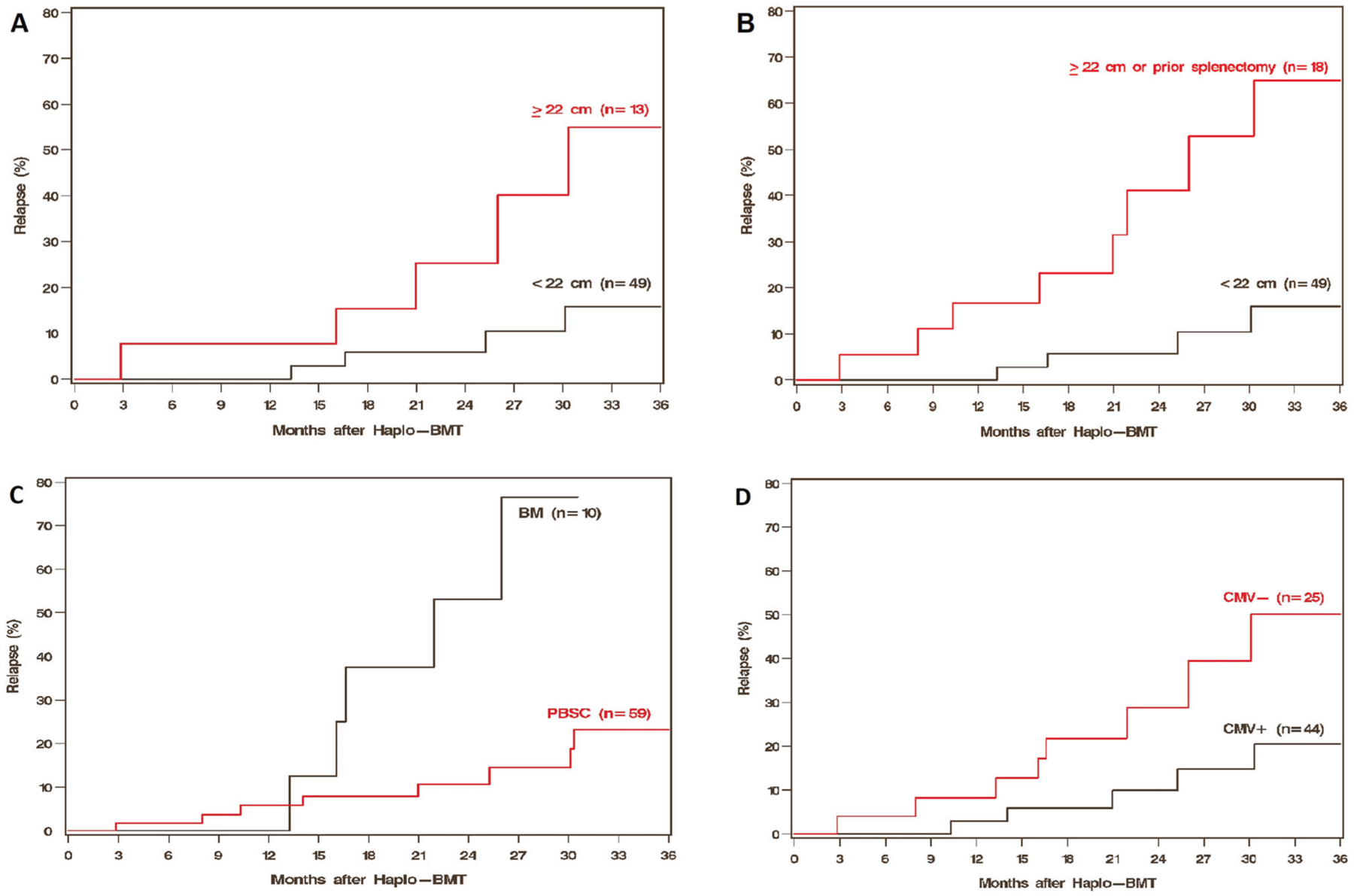
Effect of spleen size (A, B), graft source (C) and recipient CMV status (D) on relapse.
As noted above, multiple variables were significantly associated with relapse on the univariate analysis. In the two-variable model for multivariable analysis (Table 4), splenomegaly ≥22 cm or prior splenectomy remained independently associated with a higher risk of relapse after adjustment for recipient age (HR 6.46, 95% CI 1.85–22.6, P = 0.004), year of BMT (HR 5.47, 95% CI 1.58–18.9, P = 0.007), recipient CMV serostatus (HR 6.04, 95% CI 1.93–18.9, P = 0.002), or graft source (HR 4.73, 95% CI 1.18–18.9, P = 0.028). Similarly, bone marrow grafts remained independently associated with a higher incidence of relapse despite adjustment for recipient age (HR 5.38, 95% CI 1.57–18.4, P = 0.007), year of BMT (HR 3.50, 95% CI 1.16–10.6, P = 0.026), and CMV serostatus (HR 3.71, 95% CI 1.31–10.5, P = 0.014).
Two patients (3%) received a donor lymphocyte infusion after relapse on days +351 and +891, and remained alive at last follow-up with persistent relapsed disease in chronic phase.
GVHD
The incidence of all grade acute GVHD at 3 months was 36% (95% CI 25–48), and that for grades 3–4 was 10% (95% CI 4–19) (Table 2 and Fig. 1C). The median time to all grade acute GVHD was 67 days (range, 17–537), and that for grade 3–4 acute GVHD and 72 days (range, 17–125). The incidence of all grades chronic GVHD at 1 was 22% (95% CI 13–33) and at 2 years was 29% (95% CI 17–41) (Table 2 and Fig. 1D). The incidence of extensive chronic GVHD at both 1 and 2 years was 8% (95% CI 3–16). Chronic GVHD needing systemic treatment was reported in 10 (14%) patients, at a median of 134 days (range, 82–329).
Graft function
Neutrophil engraftment (Table 2) was reported in 65 (94%) patients at a median of 20 days (range, 14–70). Platelet engraftment was observed in 56 (81%) patients at a median of 34 days (range, 15–224).
Four patients (6%) had a graft failure. Of these, 3 had known measurable spleens of sizes 16, 19, and 23 cm. The spleen status of the remaining patient was unavailable. The conditioning intensity was RIC and NMA in two patients each. Peripheral blood grafts were used in all four patients. The median CD34+ cell dose was 9.9 × 106/kg (range, 3.2–10.96). All patients died of a complication of BMT, 2 each from infection and end-organ toxicity.
Prior splenectomy outcomes
Out of 67 patients who had spleen data available, 5 (7%) patients had undergone a prior splenectomy due to symptomatic splenomegaly secondary to underlying myelofibrosis. All five patients were treated with a JAK inhibitor prior to BMT. All patients engrafted neutrophils, with a median time of engraftment of 20 days (range, 15–30). With peripheral blood graft use in four of the five (80%) patients, three patients experienced relapse and subsequent death secondary to relapse. The remaining two patients were alive at the last follow-up, with a median follow-up of 16 months in remission.
DISCUSSION
BMT remains the only potentially curative option for patients with myelofibrosis with improved survival in intermediate-1 or higher DIPSS risk myelofibrosis [9]. For patients lacking matched donors, safe and effective alternative donor options are of particular importance. To our knowledge, this is the largest retrospective cohort in North America describing outcomes for haplo-BMT with PTCy in myelofibrosis. We demonstrate low rate of graft failure (6%) with haploidentical donors and PTCy in myelofibrosis, along with survival outcomes that appear overall comparable to those reported previously with matched donors [16, 34]. For reference, engraftment after BMT for myelofibrosis, using a variety of donors and conditioning regimens, has ranged anywhere from a low of 76% to a high of 97% [16, 17, 25, 34–36]. We were unable to evaluate clinical factors associated with graft failure due to a low number of events. Nevertheless, the high rates of engraftment with use of a haploidentical donor, predominantly RIC or NMA conditioning, and PTCy are encouraging in myelofibrosis, where the disease features adversely affect engraftment following BMT.
NRM, relapse, and GVHD outcomes in this study are comparable to prior reports using alternative and unrelated donors [16, 25]. The study of family mismatched BMT in chronic myelofibrosis by EBMT noted a relapse incidence of 19% (95% CI 7–31) at 2 years, which was comparable to our study results [25]. In a large Center for International Blood and Marrow Transplant Research (CIBMTR) study, the cumulative incidence of relapse was reported at 47% (CI, 40–53) at 3 years [16]. Similarly, the incidence of NRM was reported as 38% at 2 years in the EBMT study, and 22% at 3 years in the CIBMTR study [16, 25]. We observed that older age at BMT was associated with higher NRM as expected, but also fewer relapses. The latter is potentially due to selection bias amongst older patients taken to BMT.
Prior studies have been inconclusive regarding the impact of spleen size on outcomes of BMT for myelofibrosis [13, 34, 37]. In a previous single-center study from Italy, a spleen size of >22 cm was noted to be associated with higher NRM, worse OS (statistically significant), and higher relapse-related death (not statistically significant) [35]. In this study, the spleen size of 22 cm was used as it was the median value in the sample, which can be an arbitrary selection for clinical use. Hence, we attempted to explore this in our cohort as a continuous variable as well as by a cut-off at every centimeter size above 20 cm, to obtain clinically meaningful information. We demonstrated that a spleen size ≥22 cm or an increase by every 5 cm increases the risk of relapse. No statistically significant difference was seen in the OS or RFS. We surmise that the large spleen size suggests advanced disease biology of myelofibrosis, and hence, explains the higher relapse. This is also evident from the higher relapse incidence in the combined cohort of patients with a spleen size of ≥22 cm and those who underwent a splenectomy for disease-related symptomatic splenomegaly in this study. Taken together with the Italian study, a spleen size of over 22 cm at BMT can be estimated to be associated with an inferior outcome overall [35]. In this study, we could not evaluate the specific role of JAK inhibitors in spleen size reduction due to sample size limitations. Another retrospective study has suggested higher OS in patients who respond to JAK inhibitors prior to BMT than patients who have progressive splenomegaly on JAK inhibitors [38].
Bone marrow grafts were associated with significantly higher relapses in our study compared to peripheral blood grafts, along with a suggestion of lower NRM. However, survival outcomes do not appear statistically different. Prior larger studies comparing graft sources with matched unrelated donors have shown a higher probability of engraftment and higher rates of chronic GVHD with peripheral blood grafts without differences in relapse or survival [39–41]. None of these studies were representative of myelofibrosis, as they mostly included patients with acute leukemia or myelodysplastic syndrome. These differences in the role of peripheral blood and bone marrow grafts will need further exploration in a larger study focused on myelofibrosis due to its unique aspects related to graft failure, enlarged spleens, and fibrotic marrow milieu.
Our study revealed no statistical difference in outcomes based on conditioning regimen intensity. In prior studies, conditioning regimen intensity has shown decreased risks of relapse and RFS with MAC, but with a comparable OS [42]. We did not see such a difference, perhaps due to a small number of patients receiving MAC. In addition, given the different RIC regimens used at various centers and low numbers with each regimen, we did not compare the individual RIC regimens. Previously, however, one study compared 3 RIC regimens (fludarabine plus melphalan, busulfan plus melphalan, and fludarabine plus carmustine plus melphalan), and showed no statistically significant difference in clinical outcomes, although a lower relapse was suggested with fludarabine plus melphalan, while lower NRM was suggested with fludarabine plus busulfan [43]. Similar results were demonstrated in another study comparing fludarabine plus melphalan versus busulfan plus fludarabine [44].
Recipient CMV-positivity was another factor associated with a lower risk of relapse and improved RFS and GRFS in the univariate analysis. While we did not look at CMV reactivation directly, recipient CMV seropositive status is an established risk factor for CMV reactivation [45]. In that regard, data suggest that CMV reactivation facilitates T cell reconstitution and is associated with a lower risk of relapse [46–49]. This needs to be explored further, especially in times of consideration of CMV prophylaxis [50]. We also observed that the year of BMT, when analyzed as an increase by 1 year, was associated with a lower risk of relapse. This is likely an effect of evolving clinical practices for disease control prior to BMT, over the years.
There are several limitations to our study, mainly attributed to its retrospective nature and limited sample size as noted above. Given patients included are from 13 BMT centers, there is heterogeneity in BMT platforms used and institutional practices. Molecular data beyond driver mutations, which have been associated with outcomes, were not available for most patients [51–53]. We did not have data on molecular clonal evolution or serial fibrosis grading. However, a previously published study has shown that improvement in marrow fibrosis at day +100 following BMT did not correlate with outcomes, while the absence of molecular evidence of disease (detectable driver mutation or incomplete donor chimerism) at day +100 following BMT was associated with improved RFS [54]. Despite these limitations, this descriptive exploratory study provides essential data on the outcomes for haplo-BMT with PTCy for patients with chronic myelofibrosis. We conclude that this is a feasible option in patients with myelofibrosis, with encouraging clinical outcomes. While a direct comparison is lacking in our study, overall outcomes appear similar to those previously reported with matched sibling or unrelated donors. Additional studies, possibly using CIBMTR registry data, are ongoing to identify the optimal donor type for these patients.
Supplementary Material
COMPETING INTERESTS
M.R.G. has received consulting fees from Abbvie, Agios, Amgen, Astellas, Blueprint Medicines, Bristol Myers Squibb, Cardinal Health, Daiichi Sankyo, Gilead, Incyte, Karius, Pfizer, Premier, Sierra Oncology, Stemline, and Trovagene; research support from Incyte, Genentech/Roche, and Janssen; and owns stock in Medtronic. B.D. reports institutional research support from Takeda, Janssen, Angiocrine, Pfizer, and Poseida, and serves on the advisory board of Jazz. S.A. reports research funding through Helsinn Healthcare, Actinium Pharmaceuticals, and Pfizer and has received consulting fees from Abbvie and Agios. A.D. reports honoraria through Abbvie, Taiho, and Novartis. V.G. reports institutional research funding through Novartis and honoraria through Novartis, BMS-Celgene, Abbvie, Constellation Pharmaceuticals, and Sierra Oncology. A.T.G. reports research funding through Sierra Oncology, Pfizer, Celgene, CTI Biopharma, Incyte Corporation, Roche/Genentech, Imago Biosciences, and Gilead Sciences, and has received consulting fees from Celgene, CTI Biopharma, AstraZeneca/MedImmune, Incyte Corporation, and Apexx Oncology. T.J. reports institutional research support from CTI Biopharma, Incyte and Syneos Health, Consultancy with Targeted Healthcare Communications, advisory board with Care Dx, and Bristol Myers Squibb. The remaining authors do not have any conflicts of interest.
Footnotes
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41375-021-01449-1.
REFERENCES
- 1.Cervantes F How I treat myelofibrosis. Blood. 2014;124:2635–42. [DOI] [PubMed] [Google Scholar]
- 2.Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–7. [DOI] [PubMed] [Google Scholar]
- 3.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl J Med 2012;366:787–98. [DOI] [PubMed] [Google Scholar]
- 4.Harrison CN, Schaap N, Vannucchi AM, Kiladjian JJ, Tiu RV, Zachee P, et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017;4:e317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D, et al. Safety and Efficacy of Fedratinib in Patients With Primary or Secondary Myelofibrosis: A Randomized Clinical Trial. JAMA Oncol. 2015;1:643–51. [DOI] [PubMed] [Google Scholar]
- 6.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl J Med 2012;366:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain T, Mesa RA, Palmer JM. Allogeneic stem cell transplantation in myelofibrosis. Biol Blood Marrow Transpl. 2017;23:1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLornan DP, Yakoub-Agha I, Robin M, Chalandon Y, Harrison CN, Kroger N. State-of-the-art review: allogeneic stem cell transplantation for myelofibrosis in 2019. Haematologica. 2019;104:659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gowin K, Ballen K, Ahn KW, Hu ZH, Ali H, Arcasoy MO, et al. Survival following allogeneic transplant in patients with myelofibrosis. Blood Adv. 2020;4:1965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015;29:2126–33. [DOI] [PubMed] [Google Scholar]
- 11.Robin M, de Wreede LC, Wolschke C, Schetelig J, Eikema DJ, Van Lint MT, et al. Long-term outcome after allogeneic hematopoietic cell transplantation for myelofibrosis. Haematologica. 2019;104:1782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–95. Am J Hematol. 1999;61:10–5. [DOI] [PubMed] [Google Scholar]
- 13.Keyzner A, Han S, Shapiro S, Moshier E, Schorr E, Petersen B, et al. Outcome of allogeneic hematopoietic stem cell transplantation for patients with chronic and advanced phase myelofibrosis. Biol Blood Marrow Transpl. 2016;22:2180–6. [DOI] [PubMed] [Google Scholar]
- 14.Slot S, Smits K, van de Donk NW, Witte BI, Raymakers R, Janssen JJ, et al. Effect of conditioning regimens on graft failure in myelofibrosis: a retrospective analysis. Bone Marrow Transpl. 2015;50:1424–31. [DOI] [PubMed] [Google Scholar]
- 15.Ballen KK, Shrestha S, Sobocinski KA, Zhang MJ, Bashey A, Bolwell BJ, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transpl. 2010;16:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta V, Malone AK, Hari PN, Ahn KW, Hu ZH, Gale RP, et al. Reduced-intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transpl. 2014;20:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rondelli D, Goldberg JD, Isola L, Price LS, Shore TB, Boyer M, et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood. 2014;124:1183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale GA. Perspective on the role of haploidentical transplantation in the management of hematologic malignancies: why do it? Curr Hematol Malig Rep. 2007;2:202–7. [DOI] [PubMed] [Google Scholar]
- 19.Henslee-Downey PJ. Allogeneic transplantation across major HLA barriers. Best Pr Res Clin Haematol. 2001;14:741–54. [DOI] [PubMed] [Google Scholar]
- 20.Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLAidentical siblings. J Clin Oncol. 1997;15:1767–77. [DOI] [PubMed] [Google Scholar]
- 21.Bolanos-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6: e132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones RJ. Haploidentical transplantation: repurposing cyclophosphamide. Biol Blood Marrow Transpl. 2012;18:1771–2. [DOI] [PubMed] [Google Scholar]
- 23.Kasamon YL, Bolanos-Meade J, Prince GT, Tsai HL, McCurdy SR, Kanakry JA, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33:3152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLAhaploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raj K, Eikema DJ, McLornan DP, Olavarria E, Blok HJ, Bregante S, et al. Family Mismatched Allogeneic Stem Cell Transplantation for Myelofibrosis: Report from the Chronic Malignancies Working Party of European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2019;25:522–8. [DOI] [PubMed] [Google Scholar]
- 26.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. [DOI] [PubMed] [Google Scholar]
- 27.Jain T, Rampal RK. Accelerated and blast phase myeloproliferative neoplasms. Hematol Oncol Clin North Am. 2021;35:325–35. [DOI] [PubMed] [Google Scholar]
- 28.Lowsky R, Messner H. Mechanisms and treatment of graft failure. In: (eds Forman SJ, Negrin RS, Antin JH, & Appelbaum FR) Thomas’ hematopoietic cell transplantation: stem cell transplantation, I, 5th edn. Thomas’ hematopoietic cell transplantation. Chichester, UK, John Wiley & Sons, Ltd., 2015. p. 944–58. [Google Scholar]
- 29.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11:945–56. [DOI] [PubMed] [Google Scholar]
- 30.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–64. [DOI] [PubMed] [Google Scholar]
- 31.Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl. 2009;15:1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masarova L, Verstovsek S, Hidalgo-Lopez JE, Pemmaraju N, Bose P, Estrov Z, et al. A phase 2 study of ruxolitinib in combination with azacitidine in patients with myelofibrosis. Blood. 2018;132:1664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bregante S, Dominietto A, Ghiso A, Raiola AM, Gualandi F, Varaldo R, et al. Improved outcome of alternative donor transplantations in patients with myelofibrosis: from unrelated to haploidentical family donors. Biol Blood Marrow Transpl. 2016;22:324–9. [DOI] [PubMed] [Google Scholar]
- 35.Bacigalupo A, Soraru M, Dominietto A, Pozzi S, Geroldi S, Van Lint MT, et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transpl. 2010;45:458–63. [DOI] [PubMed] [Google Scholar]
- 36.Lwin Y, Kennedy G, Gottlieb D, Kwan J, Ritchie D, Szer J, et al. Australasian trends in allogeneic stem cell transplantation for myelofibrosis in the molecular era: a retrospective analysis from the Australasian bone marrow transplant recipient registry. Biol Blood Marrow Transpl. 2020;26:2252–61. [DOI] [PubMed] [Google Scholar]
- 37.Ciurea SO, Sadegi B, Wilbur A, Alagiozian-Angelova V, Gaitonde S, Dobogai LC, et al. Effects of extensive splenomegaly in patients with myelofibrosis undergoing a reduced intensity allogeneic stem cell transplantation. Br J Haematol. 2008;141:80–3. [DOI] [PubMed] [Google Scholar]
- 38.Shanavas M, Popat U, Michaelis LC, Fauble V, McLornan D, Klisovic R, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with myelofibrosis with prior exposure to Janus kinase 1/2 inhibitors. Biol Blood Marrow Transpl. 2016;22:432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheralblood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eapen M, Logan BR, Confer DL, Haagenson M, Wagner JE, Weisdorf DJ, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transpl. 2007;13:1461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ringden O, Labopin M, Beelen DW, Volin L, Ehninger G, Finke J, et al. Bone marrow or peripheral blood stem cell transplantation from unrelated donors in adult patients with acute myeloid leukaemia, an Acute Leukaemia Working Party analysis in 2262 patients. J Intern Med. 2012;272:472–83. [DOI] [PubMed] [Google Scholar]
- 42.McLornan D, Szydlo R, Koster L, Chalandon Y, Robin M, Wolschke C, et al. Myeloablative and Reduced-Intensity Conditioned Allogeneic Hematopoietic Stem Cell Transplantation in Myelofibrosis: A Retrospective Study by the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2019;25:2167–71. [DOI] [PubMed] [Google Scholar]
- 43.Jain T, Kunze KL, Temkit M, Partain DK, Patnaik MS, Slack JL, et al. Comparison of reduced intensity conditioning regimens used in patients undergoing hematopoietic stem cell transplantation for myelofibrosis. Bone Marrow Transpl. 2019;54:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robin M, Porcher R, Wolschke C, Sicre de Fontbrune F, Alchalby H, Christopeit M, et al. Outcome after transplantation according to reduced-intensity conditioning regimen in patients undergoing transplantation for myelofibrosis. Biol Blood Marrow Transpl. 2016;22:1206–11. [DOI] [PubMed] [Google Scholar]
- 45.George B, Pati N, Gilroy N, Ratnamohan M, Huang G, Kerridge I, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010;12:322–9. [DOI] [PubMed] [Google Scholar]
- 46.Ito S, Pophali P, Co W, Koklanaris EK, Superata J, Fahle GA, et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transpl. 2013;48:1313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manjappa S, Bhamidipati PK, Stokerl-Goldstein KE, DiPersio JF, Uy GL, Westervelt P, et al. Protective effect of cytomegalovirus reactivation on relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients is influenced by conditioning regimen. Biol Blood Marrow Transpl. 2014;20:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peric Z, Wilson J, Durakovic N, Ostojic A, Desnica L, Vranjes VR, et al. Early human cytomegalovirus reactivation is associated with lower incidence of relapse of myeloproliferative disorders after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 201853:1450–6. [DOI] [PubMed] [Google Scholar]
- 49.Jain T, Cho C, Hilden P, Politikos I, Borrill T, Giralt SA, et al. Cytomegalovirus reactivation promotes CD8+ T cell subset recovery after unmodified allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25:S326–7. [Google Scholar]
- 50.Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N. Engl J Med 2017;377:2433–44. [DOI] [PubMed] [Google Scholar]
- 51.Ali H, Aldoss I, Yang D, Mokhtari S, Khaled S, Aribi A, et al. MIPSS70+ v2.0 predicts long-term survival in myelofibrosis after allogeneic HCT with the Flu/Mel conditioning regimen. Blood Adv. 2019;3:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroger N, Panagiota V, Badbaran A, Zabelina T, Triviai I, Araujo Cruz MM, et al. Impact of molecular genetics on outcome in myelofibrosis patients after allogeneic stem cell transplantation. Biol Blood Marrow Transpl. 2017;23:1095–101. [DOI] [PubMed] [Google Scholar]
- 53.Tamari R, Rapaport F, Zhang N, McNamara C, Kuykendall A, Sallman DA, et al. Impact of high-molecular-risk mutations on transplantation outcomes in patients with myelofibrosis. Biol Blood Marrow Transpl. 2019;25:1142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jain T, Kunze KL, Mountjoy L, Partain DK, Kosiorek H, Khera N, et al. Early post-transplantation factors predict survival outcomes in patients undergoing allogeneic hematopoietic cell transplantation for myelofibrosis. Blood Cancer J. 2020;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


