Abstract
The alkaloid derivatives of Mitragyna speciosa, commonly known as kratom, pose a threat to society due to its potential for abuse, adverse reactions and tendency to be used as self-medication for opioid withdrawal, pain and mood disorders. A number of deaths have been reported along with complications such as respiratory depression, cardiopulmonary arrest, torsade de pointes and seizures. Its various effects and potential are yet to be fully studied. We describe the case of a healthy young male who presented with progressive respiratory failure requiring mechanical ventilation. Imaging revealed multifocal lung infiltrates while extensive infectious and cardiac work-up was negative. Based on the clinical course, a diagnosis of acute respiratory distress syndrome (ARDS) caused by kratom was made. The patient showed gradual clinical improvement and was weaned off supplemental oxygen. This case highlights yet another adverse reaction to kratom and the growing threat posed by its use.
LEARNING POINTS
Kratom is a herbal supplement with opioid-like effects at high doses and stimulant effects at low doses.
It is most commonly used to self-treat opioid withdrawal, mood disorders and pain.
Acute respiratory distress syndrome (ARDS) is one of the adverse effects of kratom, which also include kratom withdrawal syndrome, seizures, rhabdomyolysis, torsades de pointes and sudden death.
Kratom has growing abuse potential; the FDA is acting to prevent its use and recommends healthcare professionals voluntarily report any adverse reactions.
Keywords: Acute respiratory distress syndrome, kratom, Mitragyna speciosa
INTRODUCTION
Kratom is an unregulated herbal psychogenic substance which originated in southeast Asia[1]. It is an extract prepared from the leaves of Mitragyna speciosa, an evergreen tree belonging to the coffee family. Use of this product has increased in the USA due to its availability and dual effect of a stimulant effect at low doses and an opioid-like effect at high doses[2]. It is also used for pain control, mood disorders and as a cost-effective self-medicating substitute for opioids to treat withdrawal[3–5]. An increasing number of overdoses and deaths from kratom have been reported. However, the side effects have not all been determined and there are few studies about its effects on the lungs and respiratory system. Given this background, we describe a case of respiratory complications induced by kratom.
CASE DESCRIPTION
A 36-year-old man with medical history of remote polysubstance use who was a current tobacco smoker with a 7.5-pack year history presented to the emergency department with a 3-day history of progressive pleuritic chest pain and shortness of breath. The patient reported sharp pain across his chest with an associated non-productive cough. Further history was negative for intravenous drug use, relevant work or inhalational exposure, a history of hunting or animal exposure, sick contacts, a history of a lymphoproliferative disorder or malignancy, chronic medication use, a recent history of choking or aspiration events, or alcohol use. On presentation, he was normotensive with a blood pressure of 130/73 mmHg, heart rate of 128 beats per minute, respiratory rate of 22 breaths per minute, and oxygen saturation of 87% on room air. Physical examination was significant for coarse breath sounds without wheezes or rhonchi, and no jugular venous distention or pedal oedema. His respiratory status progressively worsened requiring intubation and mechanical ventilator support with a PaO2:FiO2 ratio of 166 on a PEEP of 7 mmHg within 72 hours of presentation.
Chest radiography revealed extensive bilateral infiltrates (Fig. 1). CT angiography of the chest showed no evidence of pulmonary embolism but revealed extensive bilateral multifocal consolidative changes (Fig. 2). Laboratory studies showed leukopenia with a white blood cell count of 200/μl, haemoglobin of 11.0 g/dl, and brain natriuretic peptide (BNP) of 24.5 pg/ml. An extensive infectious work-up was negative for urine legionella antigen, mycoplasma PCR, chlamydia PCR, HIV, toxoplasma Ig, COVID, syphilis, cryptococcus, Fungitell, urine histoplasmosis antigen, urine blastomycosis antigen, cytomegalovirus PCR, bartonella antibody, parvovirus B19 IgM, pneumocystis PCR, legionella PCR and culture, and bronchoalveolar lavage study for histoplasmosis antigen, aspergillus antigen, blastomycosis antigen and cytology. Transthoracic echocardiography revealed a normal ejection fraction and absence of valvular abnormalities. The initial working diagnosis was sepsis due to suspected multifocal pneumonia. However, the extensive infectious work-up returned negative and the patient remained unresponsive to antibiotic treatment with worsening symptoms, ruling out infection. Cardiogenic pulmonary oedema was unlikely due to the absence of signs of heart failure, and normal BNP and echocardiography.
Figure 1.
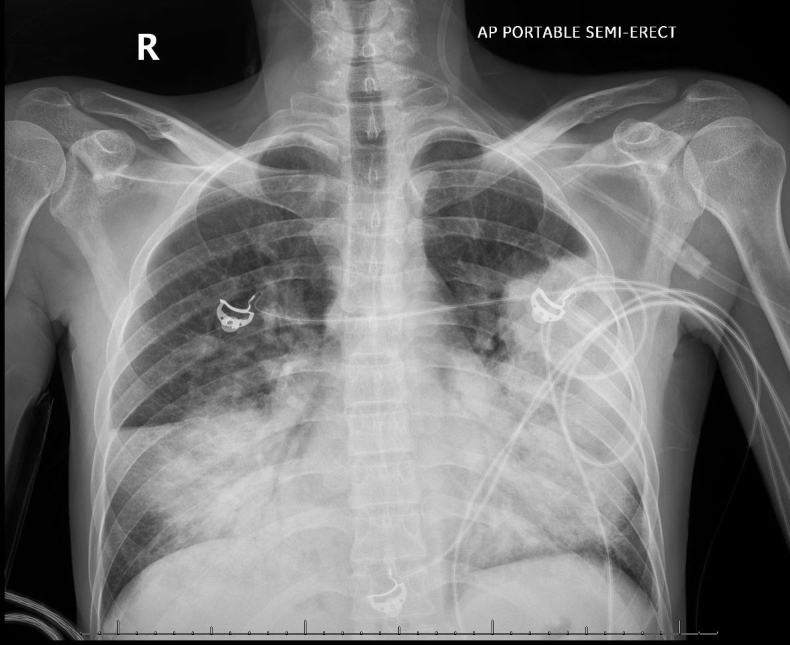
Chest x-ray (PA view) showing extensive bilateral pulmonary consolidation without cardiomegaly
Figure 2.
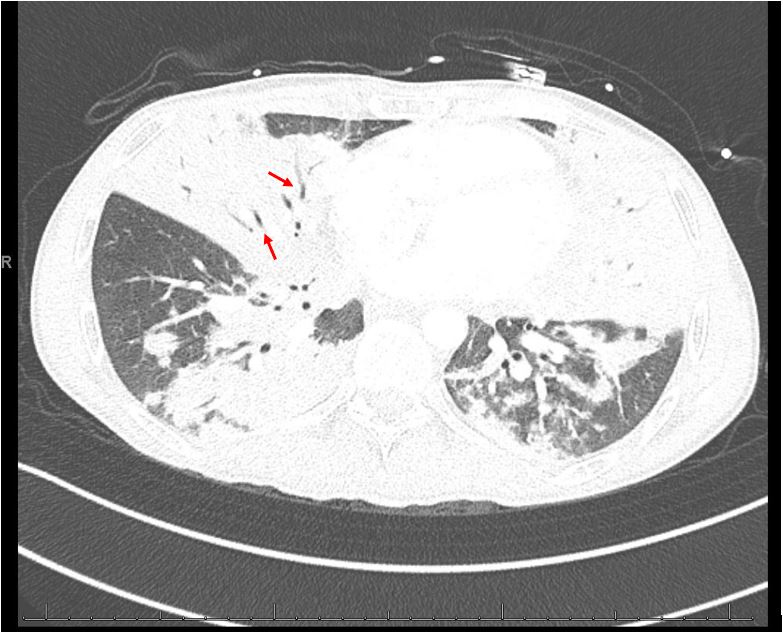
Computed tomography angiography (CTA) of the chest (axial view) showing diffuse consolidation with air bronchogram (red arrows)
Further discussions with the patient’s family revealed that he used kratom to combat his opioid cravings. Given the rapidly progressive dyspnoea and respiratory failure requiring mechanical ventilation, with no other triggering events identified, negative infectious and cardiology work-up, and imaging findings of worsening bilateral lung opacities, with a PaO2:FiO2 ratio <300 on a PEEP of >5 mmHg, a final diagnosis of kratom-induced acute respiratory distress syndrome (ARDS) was made.
Given the initial suspicion of sepsis due to multifocal pneumonia, the patient was empirically initiated on broad-spectrum antibiotics. Following intubation, he continued to be hypoxic and refractory to change in ventilator setting while under deep sedation with dyssynchrony and double triggering. Hence, he was paralyzed with a neuromuscular blocking agent cisatracurium for 24 hours. He was also placed in a prone position with a better respiratory response and started on intravenous methylprednisolone 40 mg four times daily which he was weaned off over 3 weeks.
He subsequently showed improvement on chest radiography and his respiratory status improved on mechanical ventilation. The patient was extubated 4 days after starting steroid therapy with a total of 11 days on mechanical ventilation. He was weaned down to 2–3 litres of supplemental oxygen and discharged home in a stable condition. On a follow-up visit 6 days later, his respiratory status remained stable with 3 litres of supplemental oxygen without complaints of chest pain, shortness of breath, fever or cough. He is currently off the supplemental oxygen after 3 months.
DISCUSSION
Although the prevalence of kratom use in the USA is not clearly known, a study conducted using an online survey reported 6.1% of a representative population of US adults reported having used kratom during their lifetime[6]. It is available as capsules, liquids, is smoked or the leaves are chewed[7]. Mitragynine (MG) and 7-hydroxymitragynine (7-OHMG) are the two active major and minor alkaloid components of kratom, respectively. They act as partial agonists on μ-opioid receptors through the G-protein signalling pathway and are competitive antagonists of κ- and δ-opioid receptors. The 7-OHMG is more potent than morphine, while MG is less potent. Given the partial agonist effect, it has a low tendency to cause respiratory depression compared with opioids. Mitragynine also stimulates alpha 2 adrenergic receptors which increases the sedative and hypnotic effects without causing respiratory depression. However, when combined with other sedatives such as opioids, benzodiazepines or alcohol, it can cause significant central nervous system depression[8]. In 2018, the FDA reported 44 deaths associated with kratom use. Kratom is not a part of standard drug screens and only 40% of users inform their healthcare provider of its consumption. The various adverse effects of kratom use include kratom withdrawal syndrome, seizures, rhabdomyolysis and torsades de pointes, which can all lead to the death of the patient[9–13]. However, the pharmacological profile and a complete list of adverse reactions have not yet been reported.
An extensive literature search revealed only two abstracts describing patients who developed ARDS following kratom ingestion, and no published case reports[14,15]. Further in-depth review revealed that mitragynine causes apoptotic cell death by releasing oxygen free radicals and also enhances endothelial barrier permeability. These mechanisms are thought to play a major role in the pathogenesis of ARDS[16].
A retrospective analysis found that pulmonary oedema was one of the most common findings in autopsy reports of mitragynine-positive cases[17]. The sudden death of a 17-year-old white male with a history of kratom use was reported. The autopsy revealed pulmonary oedema and the medical examiner certified the cause of death as possible kratom use[18]. Another case of pulmonary oedema and congestion caused by kratom was reported in a 24-year-old man with a history of alcohol use and depression who was found unresponsive in bed. Therapeutic concentrations of mitragynine along with venlafaxine, mirtazapine and diphenhydramine were detected at autopsy[19].
There is growing concern about the various adverse effects of kratom. It is rapidly becoming a drug of abuse and the FDA warns against its use given its potential for abuse and unknown pharmacological profile with multiple reported deaths. With this case report, we hope to raise the awareness of physicians about this evolving threat and highlight another complication of kratom and its derivatives.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
REFERENCES
- 1.Demick DS, Lee TT, Summers AT, El-Mallakh RS. Kratom: a growing substance of abuse in the United States. Ann Clin Psychiatry. 2020;32:275–280. doi: 10.12788/acp.0012. [DOI] [PubMed] [Google Scholar]
- 2.Ya KM, Methaneethorn JP, Tran QBP, Trakulsrichai SM, Wananukul WM, Lohitnavy MP. Development of a physiologically based pharmacokinetic model of mitragynine, psychoactive alkaloid in kratom (Mitragyna Speciosa Korth.), in rats and humans. J Psychoactive Drugs. 2021;53:127–139. doi: 10.1080/02791072.2020.1849877. [DOI] [PubMed] [Google Scholar]
- 3.Stanciu C, Ahmed S, Gnanasegaram S, Gibson S, Penders T, Grundmann O, et al. Kratom as an opioid alternative: harm, or harm reduction? A systematic review of literature. Am J Drug Alcohol Abuse. 2022;48:509–528. doi: 10.1080/00952990.2022.2111685. [DOI] [PubMed] [Google Scholar]
- 4.Grundmann O. Patterns of kratom use and health impact in the US-Results from an online survey. Drug Alcohol Depend. 2017;176:63–70. doi: 10.1016/j.drugalcdep.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Wilson LL, Harris HM, Eans SO, Brice-Tutt AC, Cirino TJ, Stacy HM, et al. Lyophilized kratom tea as a therapeutic option for opioid dependence. Drug Alcohol Depend. 2020;216:108310. doi: 10.1016/j.drugalcdep.2020.108310. [DOI] [PubMed] [Google Scholar]
- 6.Covvey JR, Vogel SM, Peckham AM, Evoy KE. Prevalence and characteristics of self-reported kratom use in a representative US general population sample. J Addict Dis. 2020;38:506–513. doi: 10.1080/10550887.2020.1788914. [DOI] [PubMed] [Google Scholar]
- 7.White CM. Pharmacologic and clinical assessment of kratom. Am J Health Syst Pharm. 2018;75:261–267. doi: 10.2146/ajhp161035. [DOI] [PubMed] [Google Scholar]
- 8.Sethi R, Hoang N, Ravishankar DA, McCracken M, Manzardo AM. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507. doi: 10.4088/PCC.19nr02507. [DOI] [PubMed] [Google Scholar]
- 9.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] [Google Scholar]
- 10.Afzal H, Esang M, Rahman S. A case of kratom-induced seizures. Cureus. 2020;12:e6588. doi: 10.7759/cureus.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botejue M, Walia G, Shahin O, Sharma J, Zackria R. Kratom-induced liver injury: a case series and clinical implications. Cureus. 2021;13:e14679. doi: 10.7759/cureus.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangani V, Sunnoqrot N, Gargis K, Ranabhotu A, Mubasher A, Pokal M. Unusual presentation of kratom overdose with rhabdomyolysis, transient hearing loss, and heart failure. J Investig Med High Impact Case Rep. 2021;9:23247096211005069. doi: 10.1177/23247096211005069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76:1915–1025. doi: 10.1093/ajhp/zxz221. [DOI] [PubMed] [Google Scholar]
- 14.Pathak V, Hahn C, Cabellon M, Aris R. Adult respiratory distress syndrome secondary to the use of herbal drug kratom. Am J Resp Crit Care Med. 2014;189:A6492. [Google Scholar]
- 15.Jaliawala HA, Abdo T, Carlile PV. Kratom; a potential cause of acute respiratory distress syndrome. Am J Resp Crit Care Med. 2018;197:A6604. [Google Scholar]
- 16.Matsunaga T, Morikawa Y, Kamase K, Horinouchi M, Sasajima Y, Suenami K, et al. Enhancement of endothelial barrier permeability by mitragynine. Biol Pharm Bull. 2017;40:1779–1783. doi: 10.1248/bpb.b17-00117. [DOI] [PubMed] [Google Scholar]
- 17.Jittasopa W, Srisont S. The causes of death and pathological findings of kratom users: a 5-year retrospective analysis. Am J Forensic Med Pathol. 2021;42:335–340. doi: 10.1097/PAF.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 18.Neerman MF, Frost RE, Deking J. A drug fatality involving kratom. J Forensic Sci. 2013;58(Suppl 1):S278–279. doi: 10.1111/1556-4029.12009. [DOI] [PubMed] [Google Scholar]
- 19.McIntyre IM, Trochta A, Stolberg S, Campman SC. Mitragynine ‘kratom’ related fatality: a case report with postmortem concentrations. J Anal Toxicol. 2015;39:152–155. doi: 10.1093/jat/bku137. [DOI] [PubMed] [Google Scholar]


