Abstract
There is co-morbidity between post-traumatic stress disorder (PTSD) and opioid use disorder (OUD), perhaps because PTSD-like stressful experiences early in life alter the hypothalamic-pituitary stress axis to increase risk for OUD. The current study determined if the glucocorticoid receptor antagonist PT150 reduces the escalation of fentanyl intake in rats exposed to a “two-hit” model of early life stress (isolation rearing and acute stress). Male and female rats were raised during adolescence in either isolate or social housing and then were given either a single acute stress (restraint and cold-water swim) or control treatment in young adulthood. Rats were then treated daily with PT150 (50 mg/kg, oral) or placebo and were tested for acquisition of fentanyl self-administration in 1-hr sessions, followed by escalation across 6-hr sessions. Regardless of PT150 treatment or sex, acquisition of fentanyl self-administration in 1-hr sessions was greater in isolate-housed rats compared to social-housed rats; the acute stress manipulation did not have an effect on self-administration even though it transiently increased plasma corticosterone levels. During the 6-hr sessions, escalation of fentanyl was observed across all treatment groups; however, there was a significant PT150 treatment x sex interaction. While males self-administered more than females overall, PT150 decreased intake in males and increased intake in females, thus negating the sex difference. Although PT150 may serve as an effective treatment for reducing risk of OUD following early life stress in males, further work is needed to determine the mechanism underlying the differential effects of PT150 in males and females.
Keywords: fentanyl, post-traumatic stress disorder (PTSD), corticosterone, PT150, social isolation
Introduction
There is high morbidity between post-traumatic stress disorder (PTSD) and opioid use disorder (OUD) in various populations (Dahlby & Kerr, 2020; López-Martínez et al., 2019). Extensive preclinical evidence shows that stressful PTSD-like events experienced during development increase drug self-administration and this increase in self-administration involves, at least in part, alterations in corticotrophin-releasing factor (CRF) regulation of the hypothalamic-pituitary (HPA) axis (Baracz et al., 2020; Bardo et al., 2021; Burke & Miczek, 2014; Walters & Kosten, 2019). This suggests the possibility that the increased risk for OUD resulting from early life stressful events may be ameliorated by medications that normalize function of the HPA axis.
The glucocorticoid receptor (GR) may be a target for the treatment of PTSD-related increases in vulnerability to substance use disorders (Kim et al., 2017). In support of this, the GR antagonist mifepristone ameliorates opioid withdrawal signs in rats following a stress challenge (McNally & Lam, 2005). Mifepristone also reduces stress-induced opioid seeking (Karimi et al., 2014) and blocks the effect of morphine withdrawal on signaling pathways thought to be involved in substance use disorders (Navarro-Zaragoza et al., 2017). However, in terms of selectivity, the therapeutic use of mifepristone is limited due to off-target actions, most notably at progesterone receptors (Sun et al., 2014). An alternative potential therapeutic is PT150 (formerly known as ORG-34517), an orally available GR antagonist with reduced affinity for progesterone receptors relative to mifepristone (Bachmann et al., 2003; Peeters et al., 2004). PT150 is a selective and competitive GR antagonist, preventing translocation of the GR to the cell nucleus (Peeters et al., 2008). PT150 reduces stress-induced increases in fentanyl seeking in male rats (Hammerslag et al., 2021) and is known to be safe and well-tolerated in humans (Morice et al., 2021).
The purpose of the present study was to determine if PT150 would ameliorate the effect of early-life stress on escalation of fentanyl self-administration. Toward this goal, we used a “two-hit” model of PTSD in male and female rats. The first “hit” was a chronic stressor applied during adolescence by isolating rats in the home cage beginning at 21 postnatal days of age; social-caged rats served as controls. Our laboratory and others have shown that chronic social isolation during adolescence has profound detrimental effects on the trajectory of drug self-administration vulnerability assessed in emerging adulthood (Bardo et al., 2013; Deehan et al., 2011; Stairs & Bardo, 2009). Compared to rats raised in enriched social conditions, rats raised in chronic social isolation show enhanced sensitivity of the HPA stress axis as measured by plasma corticosterone levels (Stairs et al., 2011), as well as greater self-administration of various drugs, including the short-acting μ opioid agonist remifentanil (Hofford et al., 2017). The second “hit” was an intense acute stressor (restraint and cold water swim) applied during young adulthood (postnatal day 62); non-stressed rats served as controls. Acute stress manipulations are widely used as a model of PTSD (Liberzon et al., 1997) and they possess validity because animals exposed to acute stress demonstrate fear extinction retention deficits and enhanced negative feedback of the HPA axis, both common in patients with PTSD (Dayan Knox et al., 2012; D. Knox et al., 2012).
We hypothesized that two-hit stress treatment would increase fentanyl self-administration later in life and that this increase in self-administration would be ameliorated by PT150. Fentanyl self-administration began using 1-hr daily sessions, but then was switched to 6-hr sessions because extended access schedules are thought to more closely model the escalation of intake that characterizes substance use disorders, including OUD (Ahmed & Koob, 1998; Wade et al., 2015). The dose of PT150 selected (50 mg/kg, oral, once daily) was chosen based on previous work showing that this dose reduces stress-induced reinstatement of fentanyl seeking, with no greater effect observed using 100 mg/kg (Hammerslag et al., 2021).
Methods
We report how we determined our sample size, all data exclusions, all manipulations, and all measures in the study.
Animals
A total of 96 male (n=48) and female (n=48) Sprague Dawley rats (Envigo-Harlan, Indianapolis, IN, USA) arrived at postnatal day (PND) 21 and were randomly divided into isolate or social housing conditions, with food and water available ad libitum in the home cage throughout the experiment, except where noted. The beginning sample size was determined based on the effect size from a group comparison showing that PT150 significantly reduces fentanyl seeking in rats (Hammerslag et al., 2021). Rats were housed in a temperature- and humidity-controlled room that was maintained on a 12 hr light-dark cycle (lights on at 07:00). Four separate squads of 24 rats were run through each phase of the experiment in succession. Each squad consisted of 6 isolate-housed males, 6 isolate-housed females, 6 social-housed males, and 6 social-housed females. Isolate-housed rats were housed individually in small stainless-steel cages (17 × 24 × 20 cm) with grid metal floors, and no bedding. Social-housed rats were housed in groups of 6 same-sex peers in large custom-built stainless-steel cages (122 × 61 × 45.5 cm), with bedding. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky and the Animal Care and Use Review Office of US Army Medical Research and Development Command, and they conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th Edition). Of the 96 rats that started the study, 19 were omitted in the analysis due to anesthetic overdose during surgery or failed catheter patency during behavioral testing, thus leaving a total of 77 rats completing all phases of the study.
Apparatus
Fentanyl self-administration was conducted in an operant conditioning chamber (28 × 24 × 25 cm; ENV-001; MED Associates, St. Albans VT, USA) housed in a sound-attenuating cabinet containing a fan to dampen extraneous noise (ENV-018M). The chamber was equipped with two retractable levers (ENV-112CM), a 28-V white stimulus light (ENV-211M) located 6 cm above each lever, a 28-V hooded house light with diffuser (ENV-227M) centered on the opposite wall, and a syringe pump for drug delivery (PHM-100). A recessed food tray was present between the levers but was not used in this experiment. For the self-administration sessions, rats were connected to the syringe pump via tubing passed through a metal leash (C313CS; Plastics One, Roanoke VA, USA) that was attached to a swivel (375/22PS; Instech, Plymouth Meeting, PA, USA) located above the chamber.
Drugs
Fentanyl HCl was obtained through the NIDA drug supply program (Bethesda, MD, USA) and prepared at a concentration of 2.5 μg/kg for each 0.1 ml infusion, with dose expressed as salt weight. For PT150 (Pop Test Oncology LLC, aka Palisades Therapeutics, Cliffside Park, NJ, USA), the contents of 150 mg tablets of PT150 or placebo were dissolved in 8 drops of peanut oil and mixed into Nutella® to create a homogenous suspension containing placebo or PT150 (50 mg/g).
Surgical procedures
On PND 55 or 56, rats underwent jugular catheter implantation surgery. Rats were treated with carprofen (5 mg/kg, s.c.) the day before surgery and for 2 days after surgery. Male rats were anesthetized with a combination of ketamine (Butler Schein, Dublin, OH, USA), xylazine (Akorn, Inc., Decatur, IL, USA) and acepromazine (Boehringer Ingelheim, St. Joseph, MO, USA), which were mixed together to yield a single cocktail (75/7.5/0.75 mg/kg; 0.15 ml/100 g body weight; i.p.); female rats received a ketamine and xylazine cocktail diluted with sterile water (55/7.5 mg/kg; i.p.), but they did not receive acepromazine based on veterinary consultation. During surgery, a silastic catheter was inserted into the right jugular vein and threaded under the skin to an incision on the scalp. A cannula was connected to the end of the catheter and was secured to the skull with dental acrylic and jeweler’s screws. Following surgery, rats stayed in individual polycarbonate cages overnight, then returned to their original housing assignment and recovered for 7 days before the start of self-administration training.
Nutella Pre-Exposure
Following catheter surgery (PND 58), isolate- and social-housed male and female rats were subdivided into a drug treatment group receiving either PT150 or placebo, as well as a stress treatment group receiving either acute stress or control treatment. From PND 58–62, rats were moved daily to polycarbonate transfer cages for Nutella pre-exposure. In each transfer cage, rats were given 0.1 g/100 g body weight of Nutella spread on the inside wall and remained in the transfer cage for 1 hr to ensure Nutella consumption. Across these 5 days, all rats learned to consume the entire Nutella mixture during the time allotment.
Acute Stress
On PND 62, after 5 days of Nutella pre-exposure, and following 6 or 7 days of recovery from surgery, rats were exposed to acute stress or control treatment. On test days, squads of rats (each squad = 11 or 12 rats, counterbalanced for sex and housing condition) were placed singly in a polycarbonate transfer cage with water in a designated procedure room, except for being removed for an acute stress test or a blood draw. Acute stress and control rats were assigned into pairs, with each pair consisting of one acute stress and one control rat to account for the changing conditions and procedural timing. One hr prior to the acute stress procedure, 0.2 ml of blood was collected from the saphenous vein, with the blood draw from the control rat occurring 5 min after the blood drawn from the acute stress rat within each pair. This first blood draw occurred between 08:00 and 10:30 h for all rats, with the time of first draw counterbalanced for sex and housing condition. Rats were then moved to a procedure room and were administered their final Nutella pre-exposure allotment in their transfer cages.
Sixty min after the first blood draw, restraint stress commenced in a separate procedure room; acute stress rats within each pair were restrained in plastic restrainers (DecapiCone, Braintree Scientific, Braintree MA, model DC-200, with nose portion enlarged for breathing) for 2 hr. Immediately following restraint stress, acute stress rats underwent cold swim stress by being placed in a pool of cold water (24–26° C) for 15 min or until physically exhausted, whichever came first. The cold-water swim was conducted under constant surveillance and, upon completion, rats were removed from the water, lightly dried, and transferred to a separate procedure room for an immediate blood draw. A blood sample was also taken from the control rat 5 min after the blood draw from the acute stress rat within the pair. Acute stress and control rats were returned to the original procedure room and heating pads were placed under the cages of acute stress rats for at least 10 min. The final blood draw occurred 60 min after completion of the cold swim stress. After the final blood draw, rats returned to the original procedure room until all rats in the squad completed the acute stress procedure. Once the final blood draw was completed for all rats, they were returned to their original housing assignment in the colony room. All blood samples were allowed to coagulate for 30–60 min before they were centrifuged at 2,000 g for 15 min and the supernatant was collected and stored at −20° C to await ELISA assay.
PT150 Treatment
Beginning on PND 63 (one day after acute stress), rats from each housing and acute stress condition were randomly assigned to drug treatment groups, receiving either 50 mg/kg PT150 or placebo. PT150 or placebo were administered in a Nutella mixture in individual transfer cages. From PND 63–64, rats were exposed to the Nutella mixture until it was consumed completely, or for a maximum of 1 hr, before they were returned to their original housing assignments. Prior to each daily fentanyl self-administration session (PND 65 – 92), the Nutella and PT150 or placebo mixture was administered in the transfer cages 1 hr prior to the session start.
Self-administration training
Beginning on PND 65, all rats were trained to self-administer fentanyl (2.5 μg/kg/infusion) on a continuous reinforcement schedule (FR1) through a 7-day autoshaping procedure similar to that described previously (Hofford et al., 2017). The unit dose of fentanyl was chosen because it elicits robust responding during 1- and 6-hr sessions (Wade et al., 2015). On each of 7 consecutive days, rats had 2 different 1-hr sessions separated by 2 hr. The first session was an autoshaping session and the second was an operant conditioning session. During the autoshaping session, the house light was illuminated and an inactive lever (no programmed consequence for pressing; position counterbalanced across rats) was extended continuously. The active lever extended on a random time 6-min interval for the first 25–35 min of the session. The active lever remained extended for 15 sec or until it was pressed, then it retracted, an infusion of fentanyl was delivered (0.1 ml over 3.4 sec), and the cue lights above the levers illuminated for 20 sec. A total of 5 infusions were delivered in each autoshaping session, and then the active lever remained retracted until the end of the session. The subsequent operant conditioning session was like the autoshaping session, except that (1) the active lever was available throughout the session, (2) each infusion required an active lever press, and (3) the 20-sec illumination of the cue lights was also a timeout period, where lever presses had no programmed consequence.
After 7 days of autoshaping and 1-hr operant conditioning sessions, rats next completed 21 days of 6-hr operant conditioning sessions; the autoshaping session was omitted in this phase. The 6-hr sessions were identical to the 1-hr sessions, except for the longer duration. Because previous research in our laboratory indicated that some rats develop self-injurious behavior during 21 6-hr sessions, we provided all rats with an aspen wood chew block (AC-S03, Lomir Biomedical Inc) on the first day of 6-hr self-administration. Chew blocks accompanied the rats in both their home cage and operant chamber. To manage any self-injury, we treated individual rats with a mixture of liquid bandage (New-Skin) and metronidazole before each session. Wounds were cleansed with dilute hibiclens and saline, then treated with antibiotic ointment at the end of each session. The chamber walls, levers, and floors were sanitized using MB-10 spray after the session.
Among all of the rats given PT150 treatment in Nutella that completed the study, 5 (3 male and 2 female) failed to consume the entire mixture early in the initial exposures. In these cases, the rat was maintained on food restriction in the home cage until the mixture was consumed completely during the allotted time prior to the self-administration session. By the seventh day of fentanyl self-administration (end of autoshaping phase), all rats consumed the entire mixture.
A summary of the procedural timeline, from initial housing at PND 21 to the end of fentanyl self-administration, is depicted in Figure 1.
Figure 1. Schematic of Timeline.
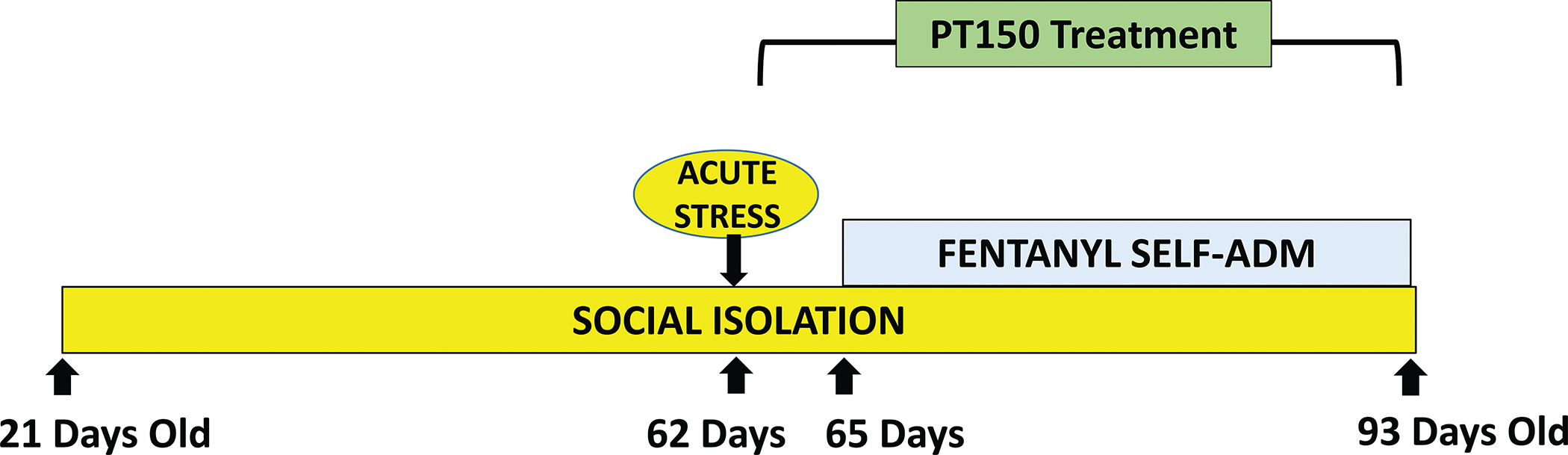
Timeline depicting sequence of social isolation and acute stress treatments, followed by PT150 treatment and assessment of fentanyl self-administration.
Corticosterone assay
Plasma was collected following blood sample centrifugation at 2,000 g for 15 min. Plasma was diluted 1:500 for corticosterone ELISA (Cayman Chemical #501320, Ann Arbor MI) following assay instructions. Samples were run in triplicate and absorbance was read at 405 nm after 2 hr incubation with provided Ellman’s solution.
Data Analysis
Plasma data collected from the corticosterone assay were analyzed using a mixed linear regression model that included sex, housing condition and acute stress treatment as between-subject factors, as well as timepoint of blood draw (pre-stress, immediately post-stress, and 1 hr post-stress) as a within-subject factor. Planned comparisons were conducted to examine the effect of housing, acute stress and sex on the observed corticosterone levels.
Data from 1- and 6-hr self-administration session phases of the experiment, including active and inactive lever presses, were analyzed using separate mixed, Poisson regression models that included sex, housing condition, acute stress treatment and PT150 treatment as between-subject factors and session as a within-subject; the model also included a linear and quadratic effect for session to account for potential curvature in the data. Active lever presses for data analyses were only those that occurred in the absence of the cue-signaled timeout and thus active lever presses during acquisition and escalation are equivalent to the number of infusions. Planned comparisons were conducted to examine the effect of PT150, housing, acute stress and sex on performance during each self-administration phase.
All analyses were conducted using SAS 9.4 utilizing the GENMOD and MIXED procedures. The alpha level was set at p ≤ 0.05 for all measures, and results are presented as mean ± S.E.M. The data that support the findings of this study are available from the corresponding author upon request. This study was not preregistered.
Results
Acute Stress-Induced Change in Corticosterone
Figure 2 illustrates the time course effect of acute stress on plasma corticosterone levels in social- and isolate-housed males and females prior to any PT150 treatment. The repeated measure mixed model collapsed across PT150 treatment revealed a main effect of time [F(2,131) = 46.29; p < 0.0001] and an acute stress × time interaction [F(2,131) = 18.14; p < 0.0001]; there was no main effect or interaction involving the housing condition. Subsequent least squares mean comparisons revealed that corticosterone levels immediately following acute stress were elevated relative to the pre-stress and 1-hr post-stress timepoints. As expected, comparisons between the acute stress and control groups at the pre-stress timepoint showed no differences. Further comparison between the pre-stress and 1-hr post-stress timepoints, after excluding the immediate post-stress timepoint, also revealed a significant difference (p < 0.05) between the acute stress and control groups, indicating that corticosterone levels, while elevated most dramatically immediately after acute stress application, continued to stay modestly elevated 1 hr after stress termination. Finally, while females tended to show greater levels of corticosterone relative to males, this difference was not statistically significant.
Figure 2. Corticosterone Levels.
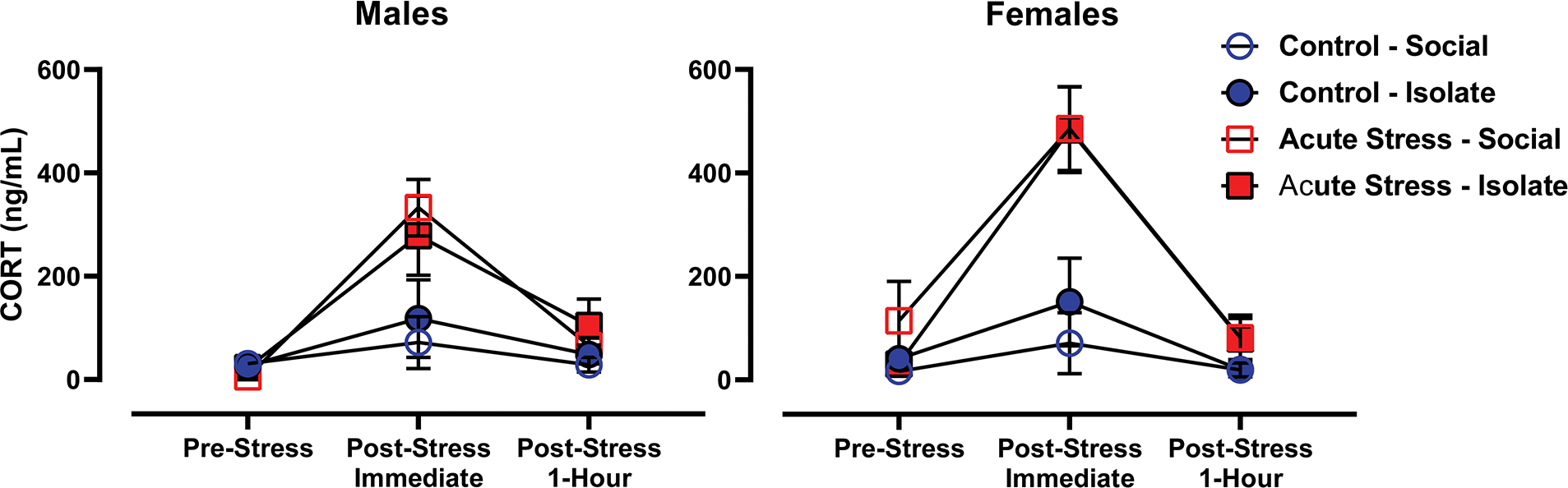
Mean (±SEM) plasma corticosterone levels for males (left panel) and females (right panel) measured prior to acute stress, immediately after acute stress and 1 hr after termination of acute stress.
Fentanyl Self-administration in 1-hr Sessions
Figure 3 displays fentanyl self-administration in social- and isolate-housed males and females treated with either PT150 or placebo across the seven 1-hr sessions that followed autoshaped training sessions. The Poisson regression model revealed significant overall linear and quadratic effects across sessions (χ2 = 22.06, p < 0.0001 and χ2 = 11.59, p < 0.001, respectively), indicative of acquisition of lever pressing. There was also a significant main effect of housing (χ2 = 8.36, p < 0.01), with isolate rats self-administering more than social-housed rats. There was no significant effect of PT150 treatment or sex, although the sex × PT150 treatment interaction approached significance (p = 0.069). The number of inactive lever presses was low for all groups (mean number <10) with no significant increase across sessions (results not shown).
Figure 3. Acquisition of Fentanyl Self-Administration During 1-hr Sessions.
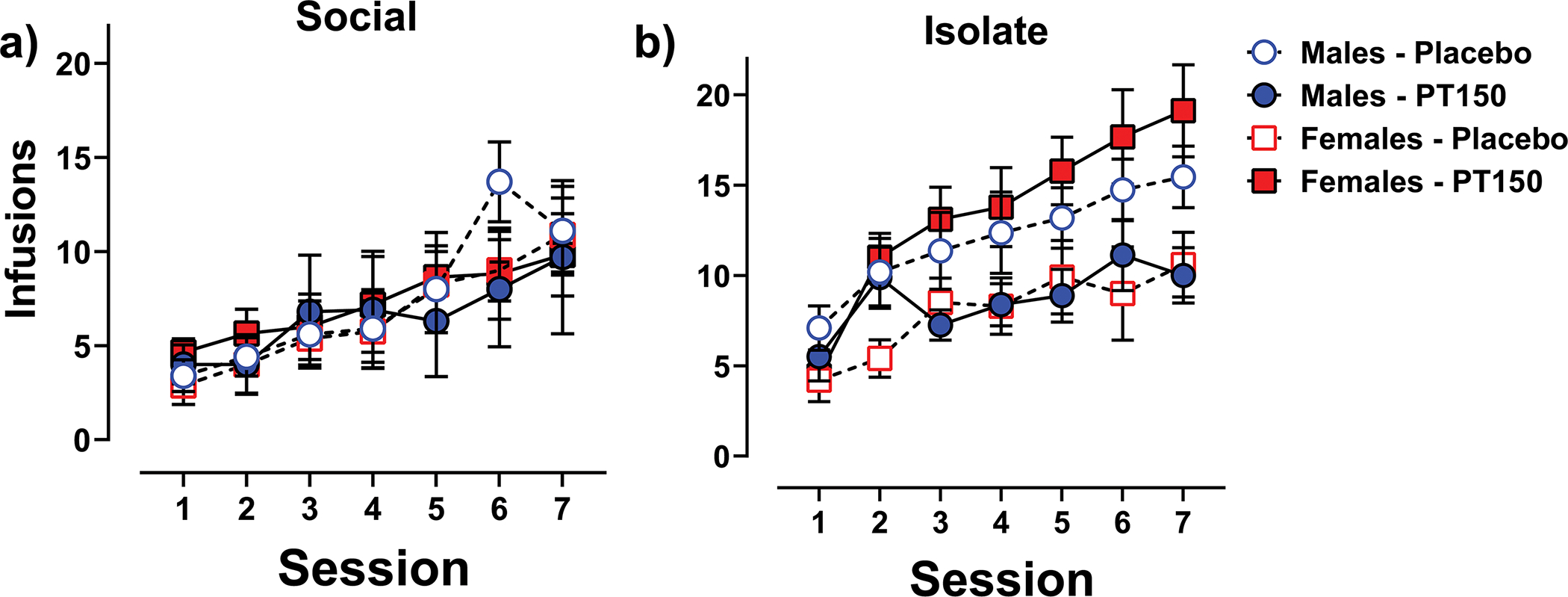
Mean (±SEM) number of fentanyl infusions earned in 1-hr self-administration sessions for social-housed males and females given either PT150 or placebo (panel a) and isolate-housed males and females given either PT150 or placebo (panel b). For clarity, data are collapsed across the acute stress treatment, as that factor yielded no significant effect in the statistical model.
Fentanyl Self-administration in 6-hr Sessions
Figure 4 displays fentanyl self-administration in social- and isolate-housed males and females treated with either PT150 or placebo across the 21 6-hr sessions. The Poisson regression model revealed significant overall linear and quadratic effects across sessions (χ2 = 18.37, p < 0.0001 and χ2 = 9.10, p < 0.01, respectively), indicative of escalation of intake. Similar to initial 1-hr sessions, isolate rats self-administered more than social-housed rats, although the effect in this phase only approached significance (p = 0.069). More important, the Poisson regression model revealed a significant sex × PT150 treatment interaction (χ2 = 4.97, p < 0.05). Subsequent least squares mean comparisons revealed that males self-administered more than females within the placebo condition (z = −3.14, p < 0.01), while there was no significant sex difference within the PT150 condition. Further comparisons indicated that PT150 significantly increased fentanyl self-administration in females (z = 2.08, p < 0.05), but not in males. Thus, collapsed across housing and acute stress conditions, males self-administered more fentanyl than females during the 6-hr sessions, and this sex difference was negated by PT150.
Figure 4. Escalation of Fentanyl Self-Administration During 6-hr Sessions.
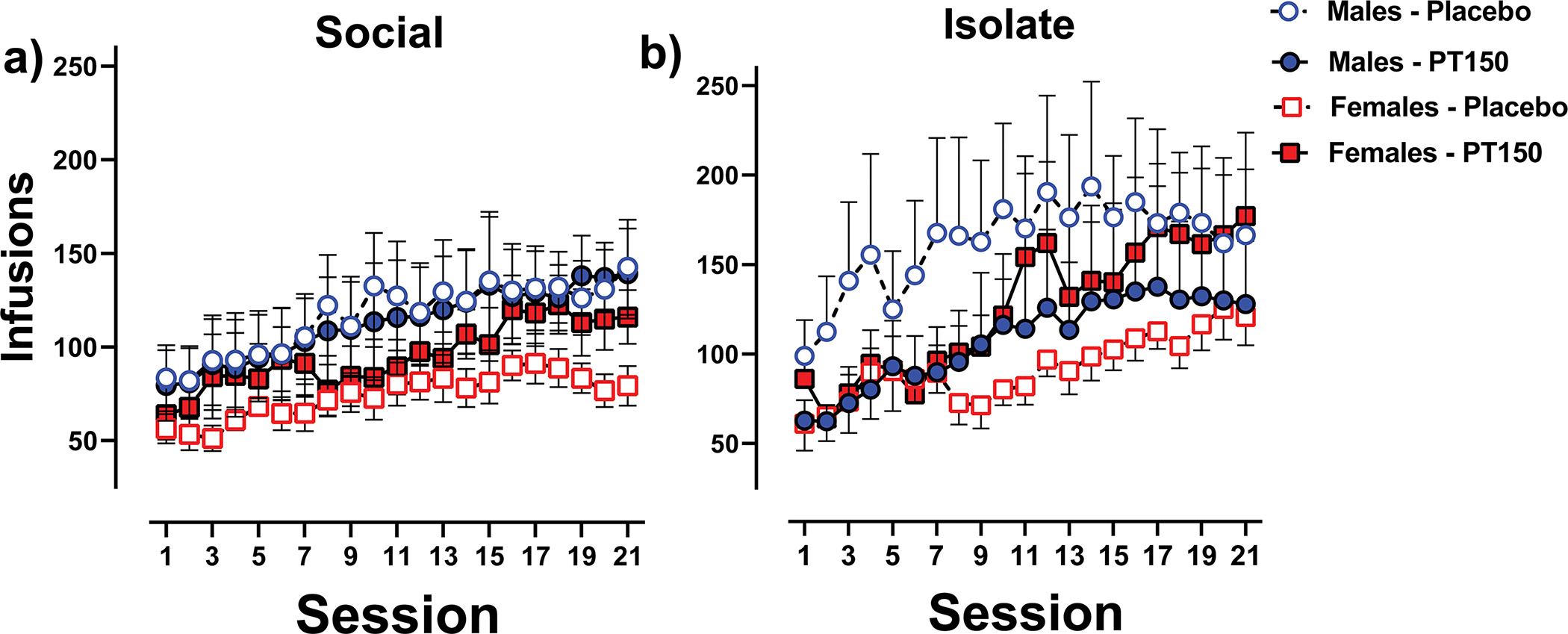
Mean (±SEM) number of infusions earned in 6-hr self-administration sessions for social-housed males and females given either PT150 or placebo (panel a) and isolate-housed males and females given either PT150 or placebo (panel b). For clarity, data are collapsed across the acute stress treatment, as that factor yielded no significant effect in the statistical model.
Discussion
Since early life adversity is known to increase opioid self-administration, at least in part, by strengthening CRF modulation of HPA activity (Baracz et al., 2020; Bardo et al., 2021; Burke & Miczek, 2014; Walters & Kosten, 2019), the current project determined if intervention with PT150 following a two-hit model of developmental adversity (social isolation and acute stress) would reduce subsequent risk. As expected, in the absence of PT150 treatment, social isolation initiated at PND 21 increased overall adult fentanyl intake during initial 1-hr sessions, as well as during 6-hr sessions; however, the acute stress treatment did not add to risk, even though it was shown to robustly increase plasma corticosterone levels. There was also an unexpected sex difference, with males showing greater overall fentanyl intake than females regardless of the early life stress treatments. Most important, during the 6-hr sessions, PT150 treatment negated the sex difference by decreasing intake in males and increasing intake in females.
The two-hit model has been used previously in our laboratory to assess the effect of early life adversity on risk for cocaine use (Hofford et al., 2018). Rats in that study were given chronic social isolation and acute stress (restraint/cold swim) and then were tested for cocaine self-administration in 1-hr sessions in adulthood. Results revealed that acute stress initially decreased cocaine self-administration regardless of housing condition (isolate-, pair- or social enriched-housing), but that two-hit rats (isolation and acute stress) showed greater acceleration of responding for cocaine across repeated sessions, as well as greater sensitivity to changes in the cocaine unit dose, compared to pair- and social enriched-housed rats. In contrast to that study, the current study found that while social isolation increased fentanyl self-administration during 1-hr sessions, this housing effect dissipated during subsequent 6-hr sessions. Moreover, application of the second hit (restraint/cold swim) did not add to the effect of social isolation alone. The finding that social isolation increased overall fentanyl self-administration during initial 1-hr sessions is consistent with previous studies reporting similar isolation-induced increases in self-administration of remifentanil and sufentanil (Hofford et al., 2017; Weinhold et al., 1993). The current results also extend that previous work by showing that the isolation-induced increase in acquisition of fentanyl self-administration observed during initial 1-hr sessions may not be preserved when switching to long access sessions that model OUD (Wade et al., 2015).
The current study showed that fentanyl intake was greater in males than females during 6-hr sessions. This finding contrasts with reports showing that, compared to females, males show less acquisition of heroin self-administration (Carroll et al., 2002), lower progressive ratio breakpoints for heroin or morphine (Cicero et al., 2003), less escalation of oxycodone self-administration with long access (12-hr) sessions (Kimbrough et al., 2020), and less reinforcer effectiveness for fentanyl using either FR5 or PR schedules (Townsend et al., 2019). However, other reports have found no reliable sex difference in heroin self-administration assessed with various unit doses (12.5–50 μg/kg/infusion) in 3-hr sessions (Stewart et al., 1996) or with a single low or high unit dose (2.5 or 100 μg/kg/infusion) in 6-hr sessions (Hammerslag et al., 2021; Venniro et al., 2017); another report found no reliable sex difference in fentanyl self-administration using a food-drug choice procedure (Townsend et al., 2019). Moreover, a recent report found that males may self-administer more morphine than females when tested at a low unit dose (0.25 mg/kg/infusion) using either a FR1 or progressive ratio schedule across 6-hr sessions (Sanchez et al., 2021). While various methodological differences prevent a firm explanation for these mixed results, the current finding of greater fentanyl intake in males than females during 6-hr sessions should be tempered because only a single unit dose was assessed using a simple FR1 schedule.
Regardless of the explanation for males having higher fentanyl intake than females during the 6-hr sessions, the most important finding from this study is that PT150 negated the sex difference. That is, in males, PT150 produced a non-significant decrease in fentanyl intake, whereas in females, PT150 produced a significant increase in fentanyl intake. In a previous report from our laboratory (Hammerslag et al., 2021), we also found that the same dose of PT150 used here (50 mg/kg daily) decreased stress-induced reinstatement of fentanyl seeking; however, this effect was driven primarily by males in the sample. Taken together, these results suggest that males may be more responsive than females to the ameliorative effect of PT150 in reducing fentanyl intake and seeking associated with stress.
One potential explanation for the sex difference in response to PT150 may be related to differential effects of PT150 on hormonal systems in males and females. Although PT150 is known as a GR antagonist, it also has a weak off-target effect on progesterone receptors (Peeters et al., 2004). PT150 may also interact with estrogen receptors, as there is cross-talk between progesterone and estrogen receptors (Thomas & Gustafsson, 2015). Mifepristone, an analog of PT150, has activity as a non-competitive antagonist at estrogen receptors (McDonnell & Goldman, 1994) and it has been shown to down-regulate both progesterone and estrogen receptors (Jiang et al., 2002). This raises the possibility that off-target inhibition of progesterone and/or estrogen function may underline the PT150-induced increase in fentanyl self-administration observed in females. Consistent with this, evidence suggests that high levels of progesterone and estrogen are associated with decreased opioid intake. For example, heroin self-administration in female rats is highest during the non-proestrus phases of the ovarian cycle (Lacy et al., 2016; Schmidt et al., 2021), when levels of both estrogen and progesterone are relatively low. In addition, heroin and remifentanil self-administration is reduced by administration of estrogen, but not by progesterone, indicating the estrogen receptor is critically involved (Sharp et al., 2021; Smith et al., 2020); however, also see (Roth et al., 2002). Thus, to the extent that PT150 may inhibit estrogen receptor function in females, it may explain the PT150-induced increase in fentanyl intake in females in the current study. Alternatively, since the closely related analog mifepristone also binds to the mu opioid receptor (Maggi et al., 1996) and there is a sex difference in opiate receptors in brain (Limonta et al., 1991), we cannot rule out the possibility of a sex-dependent difference in PT150 interaction at this other non-GR target. Regardless of the explanation, however, the current results suggest that PT150 may have efficacy in reducing risk for elevated fentanyl intake in males, but not in females, Future work is needed to understand this sex difference, perhaps by focusing more closely on hormonal cycling in females.
Public Significance:
The effect of the glucocorticoid receptor antagonist PT150 on fentanyl self-administration in male and female rats subjected to early life stress was examined. Males self-administered more than females, but PT150 eliminated this sex difference, with intake decreasing in males and increasing in females. PT150 may be useful to treat opioid use disorder in males, but not females.
Acknowledgement of funding and grants:
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Alcohol and Substance Abuse Research Program under Award No. W81XWH-18-2-0044. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014, USA was the awarding and administering acquisition office. In addition, CMC was supported by NIH training grant T32 DA035200 and MTB was supported by NIH grant R01 DA053070.
Footnotes
Disclosure of potential conflicts of interest: MTB and MAP serve as scientific advisors for and have stock options with Pop Test Oncology LLC, aka Palisades Therapeutics, Cliffside Park, NJ, USA. Any potential royalty stream would be consistent with University of Kentucky policy. A conflict of interest management plan was used to mitigate the risk, with raw data transferred to Research Triangle Institute and all analyses conducted by BAC.
Prior dissemination: These results were presented virtually at the MOMRP Alcohol & Substance Use Progress Review meeting of US Army, September 22–23, 2021.
Statement on Data Sharing:
The data that support the findings of this study are available from the corresponding author upon reasonable request. This study was not preregistered.
References
- Ahmed SH, & Koob GF (1998, Oct 9). Transition from moderate to excessive drug intake: change in hedonic set point. Science, 282(5387), 298–300. 10.1126/science.282.5387.298 [DOI] [PubMed] [Google Scholar]
- Bachmann CG, Linthorst AC, Holsboer F, & Reul JM (2003, Jun). Effect of chronic administration of selective glucocorticoid receptor antagonists on the rat hypothalamic-pituitary-adrenocortical axis. Neuropsychopharmacology, 28(6), 1056–1067. 10.1038/sj.npp.1300158 [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, & Cornish JL (2020, 2020/03/01/). The impact of early life stress on the central oxytocin system and susceptibility for drug addiction: Applicability of oxytocin as a pharmacotherapy. Neuroscience & Biobehavioral Reviews, 110, 114–132. 10.1016/j.neubiorev.2018.08.014 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Hammerslag LR, & Malone SG (2021). Effect of early life social adversity on drug abuse vulnerability: focus on corticotropin-releasing factor and oxytocin. Neuropharmacology, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, & Kelly TH (2013, Jan). Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev, 65(1), 255–290. 10.1124/pr.111.005124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, & Miczek KA (2014, Apr). Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology (Berl), 231(8), 1557–1580. 10.1007/s00213-013-3369-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, & Dess NK (2002). Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: Phenotype and sex differences. Psychopharmacology (Berl), 161(3), 304–313. 10.1007/s00213-002-1030-5 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, & Meyer ER (2003). Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacology, biochemistry and behavior, 74(3), 541–549. 10.1016/S0091-3057(02)01039-0 [DOI] [PubMed] [Google Scholar]
- Dahlby L, & Kerr T (2020, Mar 16). PTSD and opioid use: implications for intervention and policy. Subst Abuse Treat Prev Policy, 15(1), 22. 10.1186/s13011-020-00264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA Jr., Palmatier MI, Cain ME, & Kiefer SW (2011, Apr). Differential rearing conditions and alcohol-preferring rats: consumption of and operant responding for ethanol. Behav Neurosci, 125(2), 184–193. 10.1037/a0022627 [DOI] [PubMed] [Google Scholar]
- Hammerslag LR, Denehy ED, Carper B, Nolan TL, Prendergast MA, & Bardo MT (2021). Effects of the glucocorticoid receptor antagonist PT150 on stress-induced fentanyl seeking in male and female rats. Psychopharmacology (Berl), in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Chow JJ, Beckmann JS, & Bardo MT (2017, Dec). Effects of environmental enrichment on self-administration of the short-acting opioid remifentanil in male rats. Psychopharmacology (Berl), 234(23–24), 3499–3506. 10.1007/s00213-017-4734-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Prendergast MA, & Bardo MT (2018, Feb 15). Modified single prolonged stress reduces cocaine self-administration during acquisition regardless of rearing environment. Behav Brain Res, 338, 143–152. 10.1016/j.bbr.2017.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Wu R. f., Wang Z. h., Sun H. c., Xu Z, & Xiu H. m. (2002). Effect of mifepristone on estrogen and progesterone receptors in human endometrial and endometriotic cells in vitro. Fertility and sterility, 77(5), 995–1000. 10.1016/S0015-0282(02)03081-9 [DOI] [PubMed] [Google Scholar]
- Karimi S, Attarzadeh-Yazdi G, Yazdi-Ravandi S, Hesam S, Azizi P, Razavi Y, & Haghparast A (2014, May 1). Forced swim stress but not exogenous corticosterone could induce the reinstatement of extinguished morphine conditioned place preference in rats: involvement of glucocorticoid receptors in the basolateral amygdala. Behav Brain Res, 264, 43–50. 10.1016/j.bbr.2014.01.045 [DOI] [PubMed] [Google Scholar]
- Kim S, Kwok S, Mayes LC, Potenza MN, Rutherford HJV, & Strathearn L (2017, Apr). Early adverse experience and substance addiction: dopamine, oxytocin, and glucocorticoid pathways. Ann N Y Acad Sci, 1394(1), 74–91. 10.1111/nyas.13140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, Kononoff J, Simpson S, Kallupi M, Sedighim S, Palomino K, Conlisk D, Momper JD, de Guglielmo G, & George O (2020). Oxycodone self-administration and withdrawal behaviors in male and female Wistar rats. Psychopharmacology (Berl), 237(5), 1545–1555. 10.1007/s00213-020-05479-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, & Liberzon I (2012, February 1, 2012). Single prolonged stress disrupts retention of extinguished fear in rats. Learning & Memory, 19(2), 43–49. 10.1101/lm.024356.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Nault T, Henderson C, & Liberzon I (2012, October/25/). Glucocorticoid receptors and extinction retention deficits in the single prolonged stress model. Neuroscience, 223, 163–173. 10.1016/j.neuroscience.2012.07.047 [DOI] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Feinstein MA, Robinson AM, & Smith MA (2016). The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology (Berl), 233(17), 3201–3210. 10.1007/s00213-016-4368-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Krstov M, & Young EA (1997, 8//). Stress-restress: Effects on ACTH and fast feedback. Psychoneuroendocrinology, 22(6), 443–453. 10.1016/S0306-4530(97)00044-9 [DOI] [PubMed] [Google Scholar]
- Limonta P, Dondi D, Maggi R, & Piva F (1991). TESTOSTERONE AND POSTNATAL ONTOGENY OF HYPOTHALAMIC-MU ([H-3]DIHYDROMORPHINE) OPIOID RECEPTORS IN THE RAT. Brain research. Developmental brain research, 62(1), 131–136. 10.1016/0165-3806(91)90198-R [DOI] [PubMed] [Google Scholar]
- López-Martínez AE, Reyes-Pérez Á, Serrano-Ibáñez ER, Esteve R, & Ramírez-Maestre C (2019, Dec 26). Chronic pain, posttraumatic stress disorder, and opioid intake: A systematic review. World J Clin Cases, 7(24), 4254–4269. 10.12998/wjcc.v7.i24.4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi R, Pimpinelli F, Casulari LA, Piva F, & Martini L (1996). Antiprogestins inhibit the binding of opioids to μ-opioid receptors in nervous membrane preparations. European journal of pharmacology, 301(1), 169–177. 10.1016/0014-2999(96)00003-9 [DOI] [PubMed] [Google Scholar]
- McDonnell DP, & Goldman ME (1994). RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. The Journal of biological chemistry, 269(16), 11945–11949. [PubMed] [Google Scholar]
- McNally GP, & Lam S (2005, Oct). Altered vulnerability to acute opiate withdrawal following stress: roles of N-methyl-D-aspartate and glucocorticoid receptors. Behav Neurosci, 119(5), 1215–1221. 10.1037/0735-7044.119.5.1215 [DOI] [PubMed] [Google Scholar]
- Morice C, Baker DG, Patel MM, Nolen TL, Nowak K, Hirsch S, Kosten TR, & Verrico CD (2021, May 10). A randomized trial of safety and pharmacodynamic interactions between a selective glucocorticoid receptor antagonist, PT150, and ethanol in healthy volunteers. Sci Rep, 11(1), 9876. 10.1038/s41598-021-88609-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Zaragoza J, Laorden ML, & Milanes MV (2017, Mar). Glucocorticoid receptor but not mineralocorticoid receptor mediates the activation of ERK pathway and CREB during morphine withdrawal. Addict Biol, 22(2), 342–353. 10.1111/adb.12328 [DOI] [PubMed] [Google Scholar]
- Peeters BW, Ruigt GS, Craighead M, & Kitchener P (2008, Dec). Differential effects of the new glucocorticoid receptor antagonist ORG 34517 and RU486 (mifepristone) on glucocorticoid receptor nuclear translocation in the AtT20 cell line. Ann N Y Acad Sci, 1148, 536–541. 10.1196/annals.1410.072 [DOI] [PubMed] [Google Scholar]
- Peeters BW, Tonnaer JA, Groen MB, Broekkamp CL, van der Voort HA, Schoonen WG, Smets RJ, Vanderheyden PM, Gebhard R, & Ruigt GS (2004, Dec). Glucocorticoid receptor antagonists: new tools to investigate disorders characterized by cortisol hypersecretion. Stress, 7(4), 233–241. 10.1080/10253890400019672 [DOI] [PubMed] [Google Scholar]
- Roth ME, Casimir AG, & Carroll ME (2002). Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacology, biochemistry and behavior, 72(1), 313–318. 10.1016/S0091-3057(01)00777-8 [DOI] [PubMed] [Google Scholar]
- Sanchez EO, Bavley CC, Deutschmann AU, Carpenter R, Peterson DR, Karbalaei R, Flowers J, Rogers CM, Langrehr MG, Ardekani CS, Famularo ST, Bongiovanni AR, Knouse MC, Floresco SB, Briand LA, Wimmer ME, & Bangasser DA (2021). Early life adversity promotes resilience to opioid addiction-related phenotypes in male rats and sex-specific transcriptional changes. Proceedings of the National Academy of Sciences - PNAS, 118(8), e2020173118. 10.1073/pnas.2020173118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KT, Sharp JL, Ethridge SB, Pearson T, Ballard S, Potter KM, & Smith MA (2021). The effects of strain and estrous cycle on heroin- and sugar-maintained responding in female rats. Behavioural brain research, 409, 113329–113329. 10.1016/j.bbr.2021.113329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp JL, Ethridge SB, Ballard SL, Potter KM, Schmidt KT, & Smith MA (2021). The effects of chronic estradiol treatment on opioid self-administration in intact female rats. Drug and alcohol dependence, 225, 108816–108816. 10.1016/j.drugalcdep.2021.108816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ethridge SB, Gibson AN, Schmidt KT, & Sharp JL (2020). The Effects of Artificially Induced Proestrus on Heroin Intake: A Critical Role for Estradiol. Experimental and clinical psychopharmacology. 10.1037/pha0000428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, & Bardo MT (2009, May). Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav, 92(3), 377–382. 10.1016/j.pbb.2009.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Prendergast MA, & Bardo MT (2011, Nov). Environmental-induced differences in corticosterone and glucocorticoid receptor blockade of amphetamine self-administration in rats. Psychopharmacology (Berl), 218(1), 293–301. 10.1007/s00213-011-2448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Woodside B, & Shaham Y (1996). Ovarian hormones do not affect the initiation and maintenance of intravenous self-administration of heroin in the female rat. Psychobiology (Austin, Tex.), 24(2), 154–159. [Google Scholar]
- Sun Y, Fang M, Davies H, & Hu Z (2014, Mar). Mifepristone: a potential clinical agent based on its anti-progesterone and anti-glucocorticoid properties. Gynecol Endocrinol, 30(3), 169–173. 10.3109/09513590.2013.856410 [DOI] [PubMed] [Google Scholar]
- Thomas C, & Gustafsson J (2015, Sep). Progesterone receptor-estrogen receptor crosstalk: a novel insight. Trends Endocrinol Metab, 26(9), 453–454. 10.1016/j.tem.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Caine SB, Thomsen M, & Banks ML (2019). Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology (New York, N.Y.), 44(12), 2022–2029. 10.1038/s41386-019-0356-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Shaham Y, & Caprioli D (2017). Incubation of Methamphetamine but not Heroin Craving After Voluntary Abstinence in Male and Female Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 42(5), 1126–1135. 10.1038/npp.2016.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, & Koob GF (2015, Jan). Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology, 40(2), 421–428. 10.1038/npp.2014.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters H, & Kosten TA (2019, 2019/11/01/). Early life stress and the propensity to develop addictive behaviors. International Journal of Developmental Neuroscience, 78, 156–169. 10.1016/j.ijdevneu.2019.06.004 [DOI] [PubMed] [Google Scholar]
- Weinhold LL, Sharpe LG, & Jaffe JH (1993). Housing conditions influence acquisition of sufentanil aerosol self-administration in rats. Pharmacology, biochemistry and behavior, 44(1), 141–144. 10.1016/0091-3057(93)90291-Z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. This study was not preregistered.


